Treatment of Dairy Wastewater Retentate After Microfiltration: Evaluation of the Performance of the System Based on Activated Sludge and Activated Carbon
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
- I—activated sludge index, (cm3 × g−1);
- VOS—sediment volume after 30 min of thickening (cm3);
- SS—activated sludge concentration in the cylinder, (gsm/cm3);
- V—initial sludge volume (cm3).
2.2. Methods
2.2.1. Three-Step Process of Retentate Treatment
- JA is the permeate flux (at constant temperature and pressure; mL (min × m2)−1);
- V is the volume of filtrate (dm3);
- A is the effective area of the flat sheet membrane (m2);
- t is the sampling time (min).
2.2.2. Analytics Methods
- Characteristics of the physicochemical properties of process media
- R—retention of a component at a given stage of purification [%];
- Cx—concentration of individual components at the entrance to each purification stage [mg/dm3];
- C0—concentration of individual components after each purification stage [mg/dm3].
- All analysed indicators were measured three times.
- Characteristics of powdered activated carbon (PAC)
3. Results and Discussion
3.1. First Stage of Purification—MF
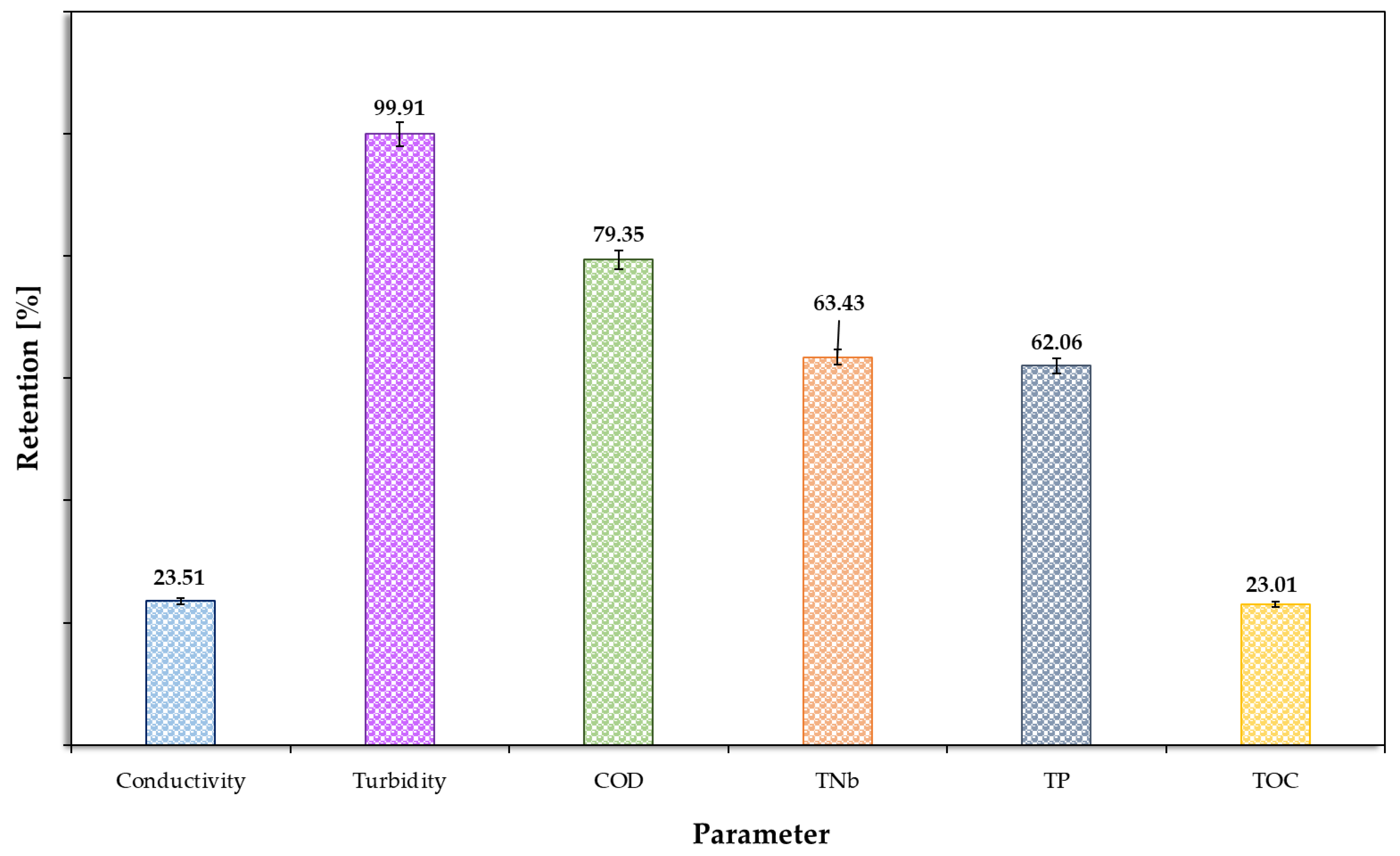
3.2. Second Step of the Treatment Process—Biological Treatment
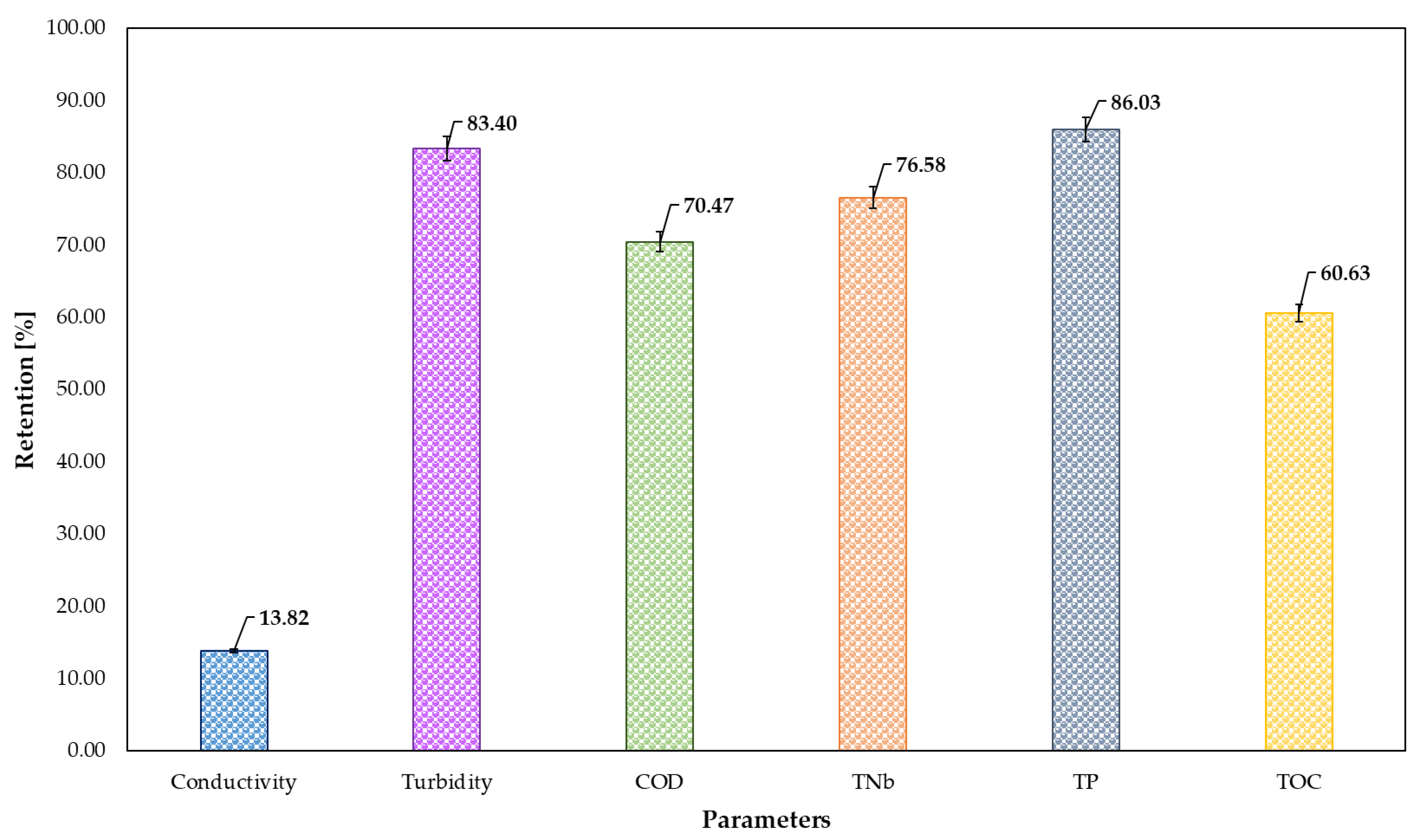
3.3. Third Step of the Treatment Process—Adsorption of PAC
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Główny Urząd Statystyczny. Rocznik Statystyczny Rolnictwa 2023, GUS 2024; Główny Urząd Statystyczny: Warsaw, Poland, 2023.
- Steinfoff-Wrześniewska, A.; Rajmund, A. Zużycie wody w wybranych branżach przemysłu spożywczego. Inżynieria Ekol. 2013, 32, 164–171. [Google Scholar]
- Joshiba, G.J.; Kumar, P.S.; Femina, C.C.; Jayashree, E.; Racchana, R.; Sivanesan, S. Critical review on biological treatment strategies of dairy wastewater. Desalination Water Treat. 2016, 160, 94–109. [Google Scholar] [CrossRef]
- Nadais, M.H.G.A.G.; Capela, M.I.A.P.F.; Arroja, L.M.G.A.; Hung, Y.T. Anaerobic treatment of milk processing wastewater. In Handbook of Environmental Engineering Vol. 11. Environmental Bioengineering; Wang, L.K., Tay, J.H., Tay, S.T.L., Hung, Y.T., Eds.; Springer Humana Press: New York, NY, USA, 2010; pp. 555–618. [Google Scholar]
- Demirel, B.; Yenigun, O.; Onay, T.T. Anaerobic treatment of dairy waste waters: A review. Process Biochem. 2005, 40, 2583–2595. [Google Scholar] [CrossRef]
- Karadag, D.; Köroğlu, O.E.; Ozkaya, B.; Cakmakci, M. A review on anaerobic biofilm reactors for the treatment of dairy industry wastewater. Process Biochem. 2015, 50, 262–271. [Google Scholar] [CrossRef]
- Cristian, O. Characteristics of the untreated wastewater produced by the food industry. An. Univ. Oradea Fasc. Prot. Med. 2010, 15, 709–714. [Google Scholar]
- Slavov, A.K. General Characteristics and Treatment Possibilities of Dairy Wastewater—A Review. Food Technol. Biotechnol. 2017, 55, 14–28. [Google Scholar] [CrossRef]
- Rodziewicz, J.; Mielcarek, A.; Kłobukowska, K.; Jóźwiakowski, K.; Siwiec, T.; Bugajski, P.; Janczukowicz, W. Water Consumption, Quantity and Quality of Wastewater and Sewage Sludge from Polish Dairies. Appl. Sci. 2025, 15, 1525. [Google Scholar] [CrossRef]
- Sinha, S.; Srivastava, A.; Mehrotra, T.; Singh, R. A Review on the Dairy Industry Waste Water Characteristics, Its Impact on Environment and Treatment Possibilities. In Emerging Issues in Ecology and Environmental Science. Springer Briefs in Environmental Science; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Yonar, T.; Sivrioğlu, Ö.; Özengin, N. Physico-chemical treatment of dairy industry wastewaters: A review. In Technological Approaches for Novel Applications in Dairy Processing; Koca, N., Ed.; IntechOpen: Rijeka, India, 2018. [Google Scholar] [CrossRef]
- Ahmad, T.; Aadil, R.M.; Ahmed, H.; Rahman, U.U.; Soares, B.C.; Souza, S.L.; Pimentel, T.C.; Scudino, H.; Guimarães, J.T.; Esmerino, E.A.; et al. Treatment and utilization of dairy industrial waste: A review. Trends Food Sci. Technol. 2019, 88, 361–372. [Google Scholar] [CrossRef]
- Cecconet, D.; Molognoni, D.; Callegari, A.; Capodaglio, A.G. Agro-food industry wastewater treatment with microbial fuel cells: Energetic recovery issues. Int. J. Hydrogen Energy 2018, 48, 500–511. [Google Scholar] [CrossRef]
- Sar, T.; Harirchi, S.; Ramezani, M.; Bulkan, G.; Akbas, M.Y.; Pandey, A.; Taherzadeh, M.J. Potential utilization of dairy industries by-products and wastes through microbial processes: A critical review. Sci. Total Environ. 2022, 810, 152253. [Google Scholar] [CrossRef] [PubMed]
- Shams, D.F.; Singhal, N.; Elefsiniotis, P. Effect of feed characteristics and operational conditions on treatment of dairy farm wastewater in a coupled anoxic-up flow and aerobic system. Biochem. Eng. J. 2018, 133, 186–195. [Google Scholar] [CrossRef]
- Kaewsuk, J.; Thorasampan, W.; Thanuttamavong, M.; Seo, G. Kinetic development and evaluation of membrane sequencing batch reactor (msbr) with mixed cultures photosynthetic bacteria for dairy wastewater treatment. J. Environ. Manag. 2010, 91, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Sivrioğlu, Ö.; Yonar, T. Determination of the acute toxicities of physicochemical pretreatment and advanced oxidation processes applied to dairy effluents on activated sludge. J. Dairy Sci. 2015, 98, 2337–2344. [Google Scholar] [CrossRef]
- Zieliński, M.; Dębowski, M.; Kazimierowicz, J. Performance of an innovative low-cost recycled filling (lcrf) in anaerobic treatment of dairy effluent—A pilot-scale study. Materials 2022, 15, 7815. [Google Scholar] [CrossRef]
- Mekuria, G. Dairy wastewater treatment through synergies of the biological and hybrid membrane: A systematic review. J. Environ. Inform. Lett. 2022, 8, 31–50. [Google Scholar] [CrossRef]
- Lima, L.K.; Santana, L.N.; Lira, H.L.; Rodríguez, M.A.; Souza, M.Y.; Júnior, M.G.; Lira, B.S. Development of asymmetric ceramic membranes for dairy wastewater treatment—A comparison between co-sintering and conventional firing process. J. Water Process Eng. 2024, 57, 104611. [Google Scholar] [CrossRef]
- Kertész, S.; Gulyás, N.S.; Al-Tayawi, A.N.; Huszár, G.; Lennert, J.R.; Csanádi, J.; Beszédes, S.; Hodúr, C.; Szabó, T.; László, Z. Modeling of organic fouling in an ultrafiltration cell using different three-dimensional printed turbulence promoters. Membranes 2023, 13, 262. [Google Scholar] [CrossRef] [PubMed]
- Zinadini, S.; Vatanpour, V.; Zinatizadeh, A.A.; Rahimi, M.; Rahimi, Z.; Kian, M. Preparation and characterization of antifouling graphene oxide/polyethersulfone ultrafiltration membrane: Application in mbr for dairy wastewater treatment. J. Water Process Eng. 2015, 7, 280–294. [Google Scholar] [CrossRef]
- Al-Shammari, S.; Bou-Hamad, S.; Al-Saffar, A.; Salman, M.; Al-Sairafi, A. Treatment of dairy wastewater effluent using submerged membrane microfiltration system. J. Environ. Sci. Eng. A 2015, 4, 107–118. [Google Scholar] [CrossRef]
- Kertész, S.; Al-Tayawi, A.N.; Gergely, G.; Ott, B.; Gulyás, N.S.; Jákói, Z.; Beszédes, S.; Hodúr, C.; Szabó, T.; László, Z. Investigation of different pre-treatment techniques and 3D printed turbulence promoter to mitigate membrane fouling in dairy wastewater module. Materials 2023, 16, 3117. [Google Scholar] [CrossRef]
- Kacprzyńska-Gołacka, J.; Łożyńska, M.; Barszcz, W.; Sowa, S.; Wieciński, P.; Woskowicz, E.; Życki, M. In-fluence of deposition parameters of tio2 + cuo coating on the membranes surface used in the filtration process of dairy wastewater on their functional properties. Membranes 2021, 11, 290. [Google Scholar] [CrossRef]
- Mishra, S.; Kumar Bindhani, S.; Jena, H. Adsorption Isotherm Studies for COD-BOD Removal from Dairy Waste Water Using Freshly Prepared Adsorbent. Int. J. Environ. Sci. 2024, 13, 71–75. [Google Scholar] [CrossRef]
- Hammam, R.; Shaltout, F.; Mansour, S.; Hassanain, A. Chemical treatment of industrial wastewater using fenton reaction. Eng. Res. J. 2022, 173, 219–231. [Google Scholar] [CrossRef]
- Sarghini, F.; Sorrentino, A.; Pierro, P. An integrated mechanical-enzymatic reverse osmosis treatment of dairy industry wastewater and milk protein recovery as a fat replacer: A closed loop approach. J. Agric. Eng. 2013, 44 (Suppl. S2), e57. [Google Scholar] [CrossRef]
- Kowalik-Klimczak, A.; Gajewska-Midziałek, A.; Buczko, Z.; Łożyńska, M.; Życki, M.; Barszcz, W.; Ciciszwili, T.; Dąbrowski, A.; Kasierot, S.; Charasińska, J.; et al. Circular Economy Approach in Treatment of Galvanic Wastewater Em-ploying Membrane Processes. Membranes 2023, 13, 325. [Google Scholar] [CrossRef]
- Mestawet, A.T.; France, T.C.; Mulcahy, P.G.J.; O’Mahony, J.A. Microfiltration retentate co-product from whey protein isolate production—Composition, processing, applications and potential for value addition. Trends Food Sci. Technol. 2024, 153, 104739. [Google Scholar] [CrossRef]
- FAO. Regulation of the Minister of Maritime Economy and Inland Navigation of 12 July 2019 on Substances Particularly Harmful to the Aquatic Environment and the Conditions that Must be Met when Discharging Sewage into Water or Soil, as well as when Discharging Rainwater or Meltwater into Water or Water Facilities. Water Law, 12 July 2019. [Google Scholar]
- Kowalik-Klimczak, A. Influence of NF Membrane Properties on Water Recovery From the Dairy Industry Wastewater. J. Membr. Sci. Res. 2022, 8, 530129. [Google Scholar] [CrossRef]
- Tikariha, A.; Sahu, O. Study of characteristics and treatments of dairy industry waste water. J. Appl. Environ. Microbiol. 2014, 2, 16–22. [Google Scholar]
- Kühnl, W.; Piry, A.; Kaufmann, V.; Grein, T.; Ripperger, S.; Kulozik, U. Impact of colloidal interactions on the flux in cross-flow microfiltration of milk at different pH values: A surface energy approach. J. Membr. Sci. 2010, 352, 107–115. [Google Scholar] [CrossRef]
- Rozporządzenie Ministra Zdrowia z dnia 7 Grudnia 2017 r. w Sprawie Jakości Wody Przeznaczonej do Spożycia Przez Ludzi, Dz.U. 2017 poz. 2294. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20170002294 (accessed on 29 July 2025).
- Sibhatu, H.; Zielińska, M. Post-treatment of dairy wastewater by activated sludge–ultrafiltration for water reuse. Desal. Water Treat 2018, 115, 24–32. [Google Scholar] [CrossRef]
- Das, A.; Kundu, P.; Adhikari, S. Biological treatment of dairy industry wastewater in a suspended growth batch reactor: Performance evaluation and biodegradation kinetics. Bioremediation J. 2021, 26, 341–359. [Google Scholar] [CrossRef]
- Zielińska, M.; Galik, M. Use of ceramic membranes in a membrane filtration supported by coagulation for the treatment of dairy wastewater. Water Air Soil Pollut. 2017, 228, 173. [Google Scholar] [CrossRef] [PubMed]
- Ramsuroop, J.; Gutu, L.; Ayinde, W.B.; Basitere, M.; Manono, M.S. A Review of Biological Processes for Dairy Wastewater Treatment and the Effect of Physical Parameters Which Affect Their Efficiency. Water 2024, 16, 537. [Google Scholar] [CrossRef]
- Palela, M.; Ifrim, G.; Barbu, M.; Bahrim, G.; Caraman, S. Strategies for the aerobic bio-logical treatment of the dairy wastewaters in controlled conditions. Environ. Eng. Manag. J. 2010, 9, 399–405. [Google Scholar] [CrossRef]
- Bokobza, L.; Bruneel, J.L.; Couzi, M. Raman Spectra of Carbon-Based Materials (from Graphite to Carbon Black) and of Some Silicone Composites. C 2015, 1, 77–94. [Google Scholar] [CrossRef]
- Jawad, A.H.; Abdulhameed, A.S.; Bahrudin, N.N.; Hum, N.N.M.F.; Surip, S.N.; Syed-Hassan, S.S.A.; Yousif, E.; Sabar, S. Microporous activated carbon developed from KOH activated bio-mass waste: Surface mechanistic study of methylene blue dye adsorption. Water Sci. Technol. 2021, 84, 1858–1872. [Google Scholar] [CrossRef]
- Tan, K.L.; Hameed, B.H. Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions. J. Taiwan Inst. Chem. Eng. 2017, 74, 25–48. [Google Scholar] [CrossRef]
- Streit, A.F.; Pereira, H.A.; Moreno-Pérez, J.; Mendoza-Castillo, D.I.; Reynel-Ávila, H.E.; Martinello, K.d.B.; Silva, L.F.; Ahmad, N.; Mohandoss, S.; Bonilla-Petriciolet, A.; et al. New study of the adsorption mechanism of different dye molecules by high porous sludge activated carbon produced from a dairy-treatment effluent plant. J. Environ. Chem. Eng. 2024, 12, 113745. [Google Scholar] [CrossRef]
- Kuśmierek, K.; Dąbek, L.; Świątkowski, A. The influence of the shape and grain size of commercial activated carbons on their sorption efficiency towards organic water pollutants. Desalin. Water Treat. 2025, 321, 100996. [Google Scholar] [CrossRef]
- Hanchao, L.; Suping, F.; Xiaolin, D.; Nannan, Z.; Yongli, L. Comparison of three sorbents for organic pollutant removal in drinking water. Energy Procedia 2011, 5, 985–990. [Google Scholar] [CrossRef][Green Version]
- Ozcan, D.O.; Hendekci, M.C.; Ovez, B. Enhancing the adsorption capacity of organic and inorganic pollutants onto impregnated olive stone derived activated carbon. Heliyon 2024, 10, e32792. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Yu, X.; Wang, X.; Hafida, W.; Okonkwo, C.E.; Wang, Y.; Huang, C.; Zhou, C. Covalent organic framework adsorption separation of the mother liquor sugars from industrial ste-viol: A combination of experimental and theoretical analysis. Sep. Purif. Technol. 2024, 347, 127608. [Google Scholar] [CrossRef]
- Wang, M.; Hu, S.; Wang, Q.; Liang, Y.; Liu, C.; Xu, H.; Ye, Q. Enhanced nitrogen and phosphorus adsorption performance and stabilization by novel panda manure biochar modified by CMC stabilized nZVZ composite in aqueous solution: Mechanisms and application potential. J. Clean. Prod. Vol. 2021, 291, 125221. [Google Scholar] [CrossRef]
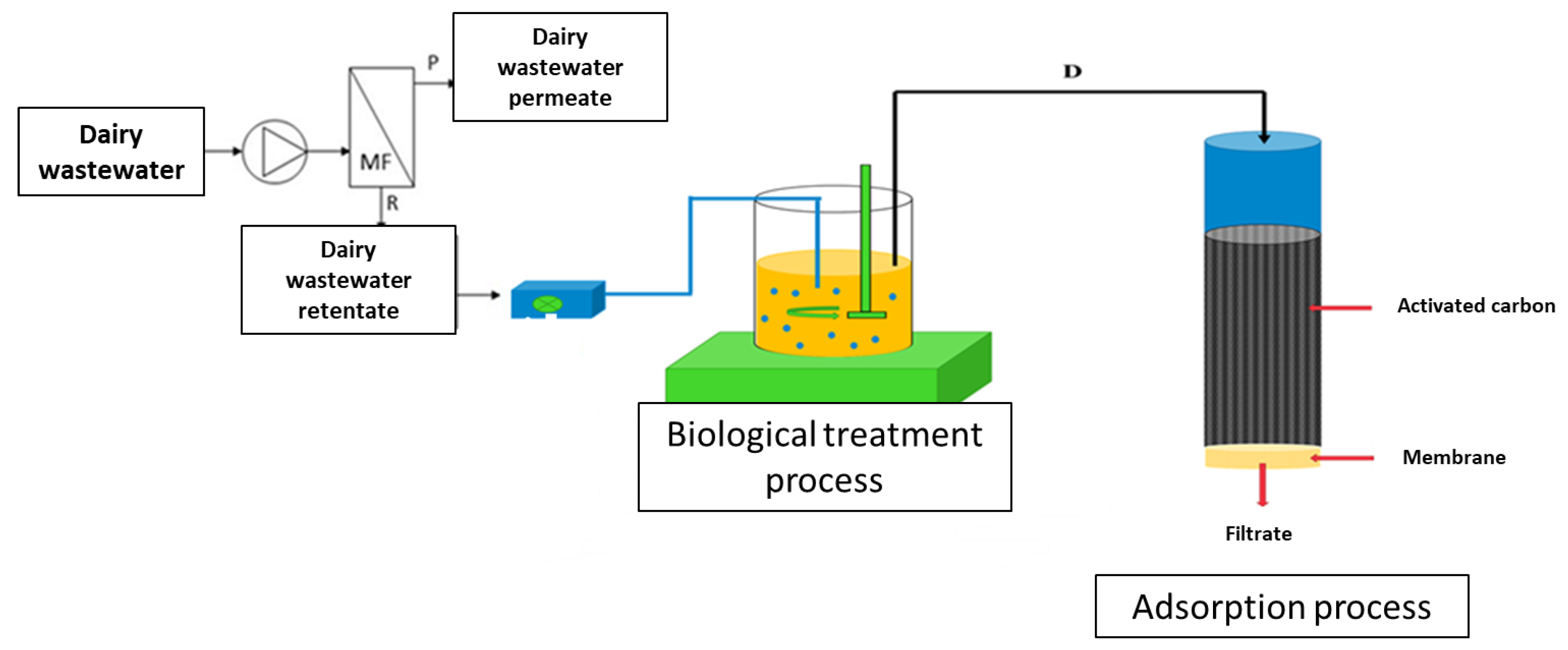


| Parameter | Unit | Raw Sewage |
|---|---|---|
| pH | - | 6.76 ± 0.05 |
| Conductivity | µS/cm | 1785.10 ± 18.42 |
| Turbidity | NTU | 1740.87 ± 21.12 |
| COD | mg/dm3 | 3616.00 ± 26.4 |
| TNb | mg/dm3 | 83.40 ± 4.1 |
| TP | mg/dm3 | 16.23 ± 0.31 |
| TOC | mg/dm3 | 644.70 ± 0.08 |
| Parameters | MF Membrane |
|---|---|
| Manufacturer | TriSept Membranes |
| Model | TM10-QXF |
| Polymer | PVDF |
| Area, m2 | 5.8 |
| Pore size, µm | 0.2 |
| Molecular weight cut-off, kDa | - |
| Max. pressure, bar | 21 |
| Max. temperature, °C | 45 |
| Parameter | Unit | Permeate | 2 Times Conc. Retentate | Required Levels [31] |
|---|---|---|---|---|
| pH | - | 7.10 ± 0.13 | 6.96 ± 0.07 | 6.5–9.0 |
| Conductivity | µS/cm | 1356.40 ± 34.58 | 1602.80 ± 23.61 | - |
| Turbidity | NTU | 1.47 ± 0.06 | 1259.00 ± 39.88 | - |
| COD | mg/dm3 | 758.67 ± 21.55 | 3454.00 ± 34.01 | 125 |
| TNb | mg/dm3 | 31.67 ± 3.21 | 86.00 ± 3.07 | 30 |
| TP | mg/dm3 | 6.04 ± 0.21 | 17.74.00 ± 0.44 | 2 |
| TOC | mg/dm3 | 493.07 ± 8.46 | 648.07 ± 4.99 | 30 |
| Parameter | Unit | Permeate | Required Levels [35] |
|---|---|---|---|
| pH | - | 7.10 ± 0.13 | 6.5–9.5 |
| Conductivity | µS/cm | 1356.40 ± 34.58 | 2.500 |
| Turbidity | NTU | 1.47 ± 0.06 | Up to 1.0 |
| COD | mg/dm3 | 758.67 ± 21.55 | - |
| TNb | mg/dm3 | 31.67 ± 3.21 | - |
| Pog. | mg/dm3 | 6.04 ± 0.21 | - |
| TOC | mg/dm3 | 493.07 ± 8.46 | No abnormal changes |
| Parameter | Unit | 2-Times Conc. Retentate | Filling | Reaction 3 | Sedimentation | Decanting |
|---|---|---|---|---|---|---|
| pH | - | 6.96 ± 0.07 | 7.15 ± 0.07 | 7.39 ± 0.02 | 7.30 ± 0.03 | 7.30 ± 0.02 |
| Conductivity | µS/cm | 1602.80 ± 23.61 | 1621.03 ± 34.76 | 1573.83 ± 28.10 | 1576.33 ± 18.58 | 1561.27 ± 39.11 |
| Turbidity | NTU | 1259.00 ± 39.88 | 847.33 ± 29.57 | 365.63 ± 10.65 | 315.93 ± 11.76 | 311.60 ± 8.03 |
| COD | mg/dm3 | 3454.00 ± 34.01 | 2651.33 ± 34.78 | 1348.33 ± 21.77 | 1343.67 ± 12.58 | 1319.67 ± 24.01 |
| TNb | mg/dm3 | 86.00 ± 3.07 | 85.77 ± 0.42 | 55.77 ± 1.27 | 59.43 ± 0.91 | 56.50 ± 0.95 |
| TP. | mg/dm3 | 17.74.00 ± 0.44 | 14.96 ± 0.49 | 8.21 ± 0.13 | 9.53 ± 0.11 | 9.40 ± 0.17 |
| TOC | mg/dm3 | 648.07 ± 4.99 | 630.57 ± 1.02 | 494.19 ± 0.41 | 427.33 ± 0.59 | 456.65 ± 0.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Życki, M.; Barszcz, W.; Łożyńska, M. Treatment of Dairy Wastewater Retentate After Microfiltration: Evaluation of the Performance of the System Based on Activated Sludge and Activated Carbon. Membranes 2025, 15, 237. https://doi.org/10.3390/membranes15080237
Życki M, Barszcz W, Łożyńska M. Treatment of Dairy Wastewater Retentate After Microfiltration: Evaluation of the Performance of the System Based on Activated Sludge and Activated Carbon. Membranes. 2025; 15(8):237. https://doi.org/10.3390/membranes15080237
Chicago/Turabian StyleŻycki, Maciej, Wioletta Barszcz, and Monika Łożyńska. 2025. "Treatment of Dairy Wastewater Retentate After Microfiltration: Evaluation of the Performance of the System Based on Activated Sludge and Activated Carbon" Membranes 15, no. 8: 237. https://doi.org/10.3390/membranes15080237
APA StyleŻycki, M., Barszcz, W., & Łożyńska, M. (2025). Treatment of Dairy Wastewater Retentate After Microfiltration: Evaluation of the Performance of the System Based on Activated Sludge and Activated Carbon. Membranes, 15(8), 237. https://doi.org/10.3390/membranes15080237






