Membrane Distillation for Water Desalination: Assessing the Influence of Operating Conditions on the Performance of Serial and Parallel Connection Configurations
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Distillation System Setup
3. Results and Discussion
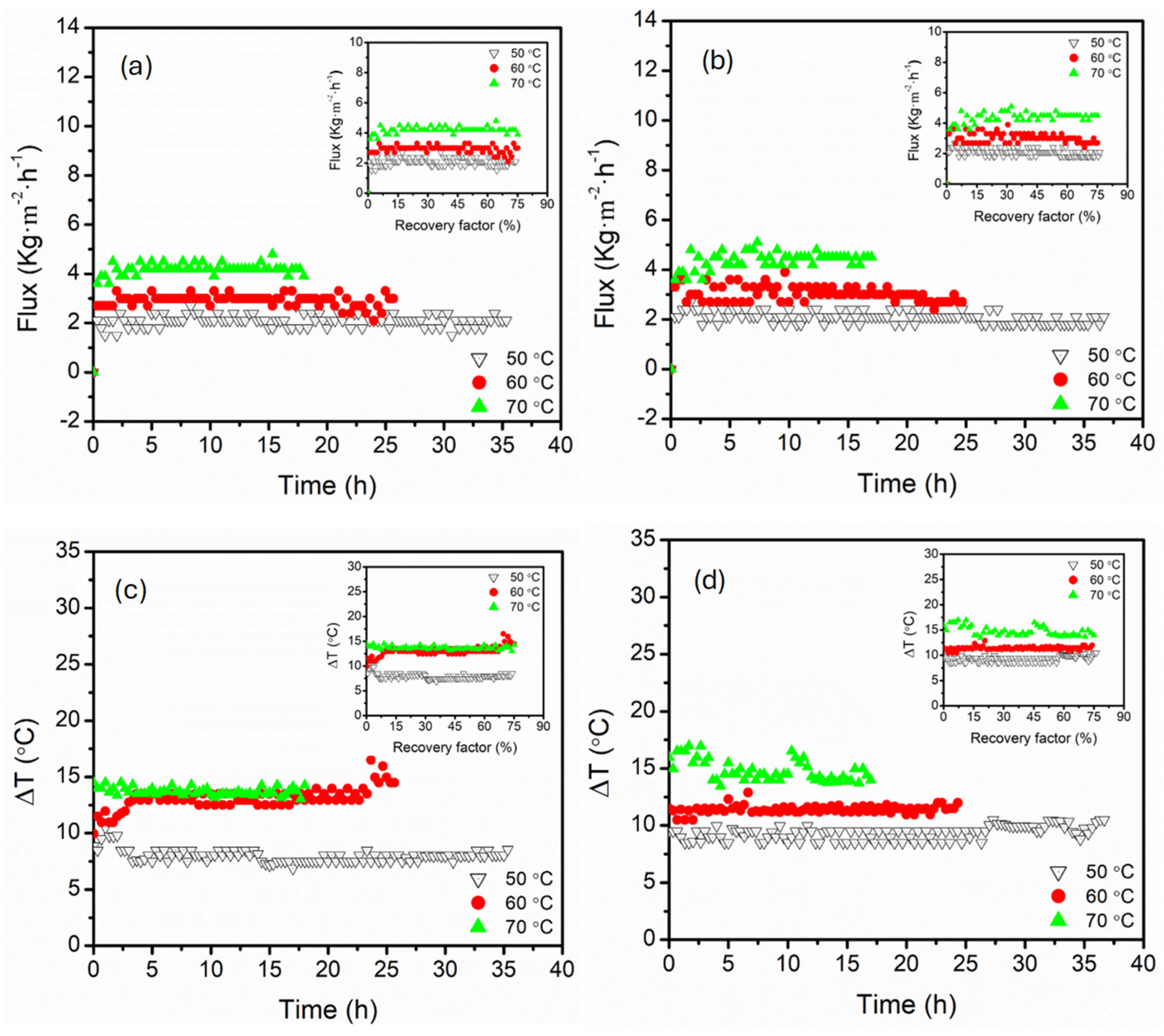
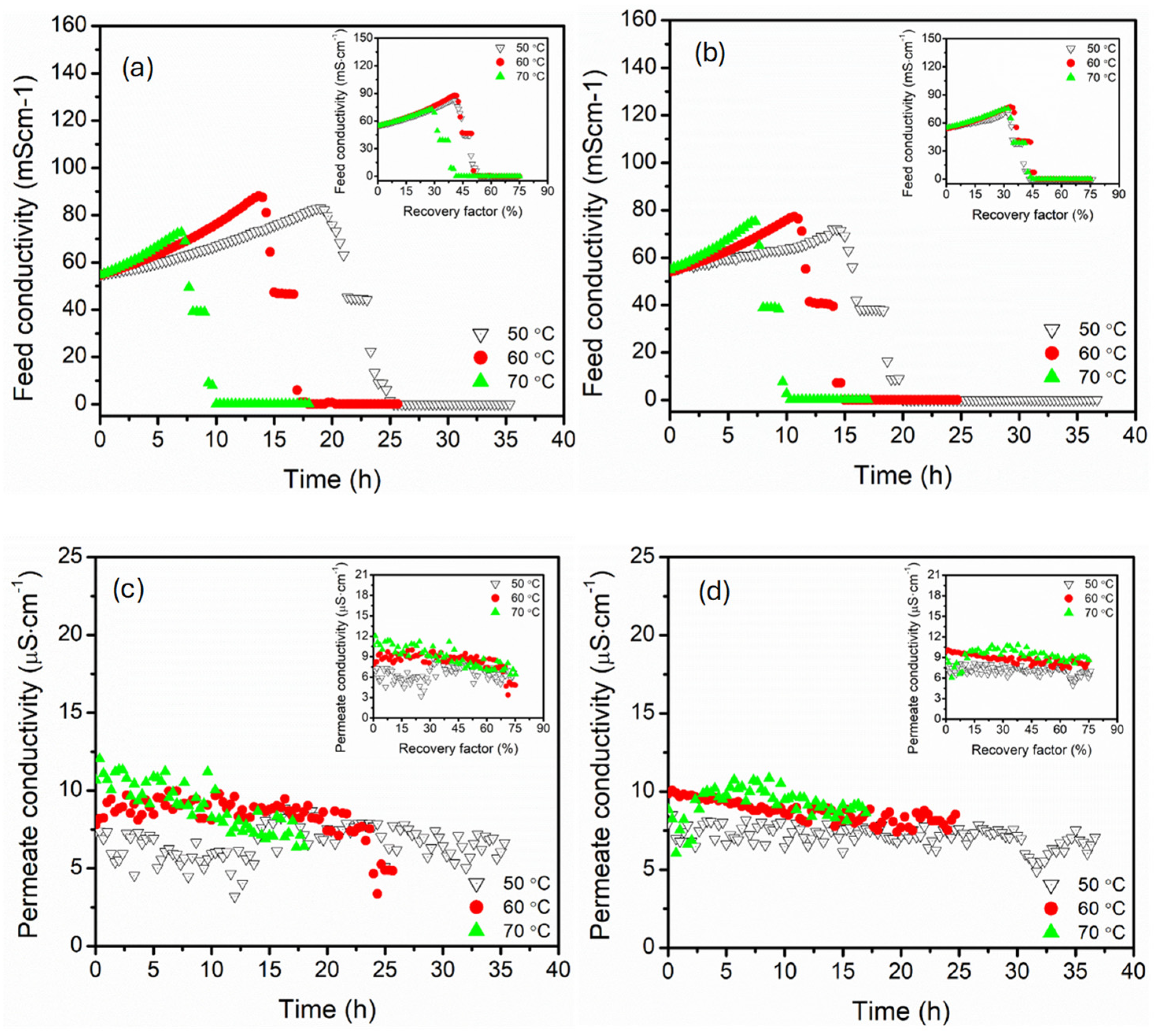
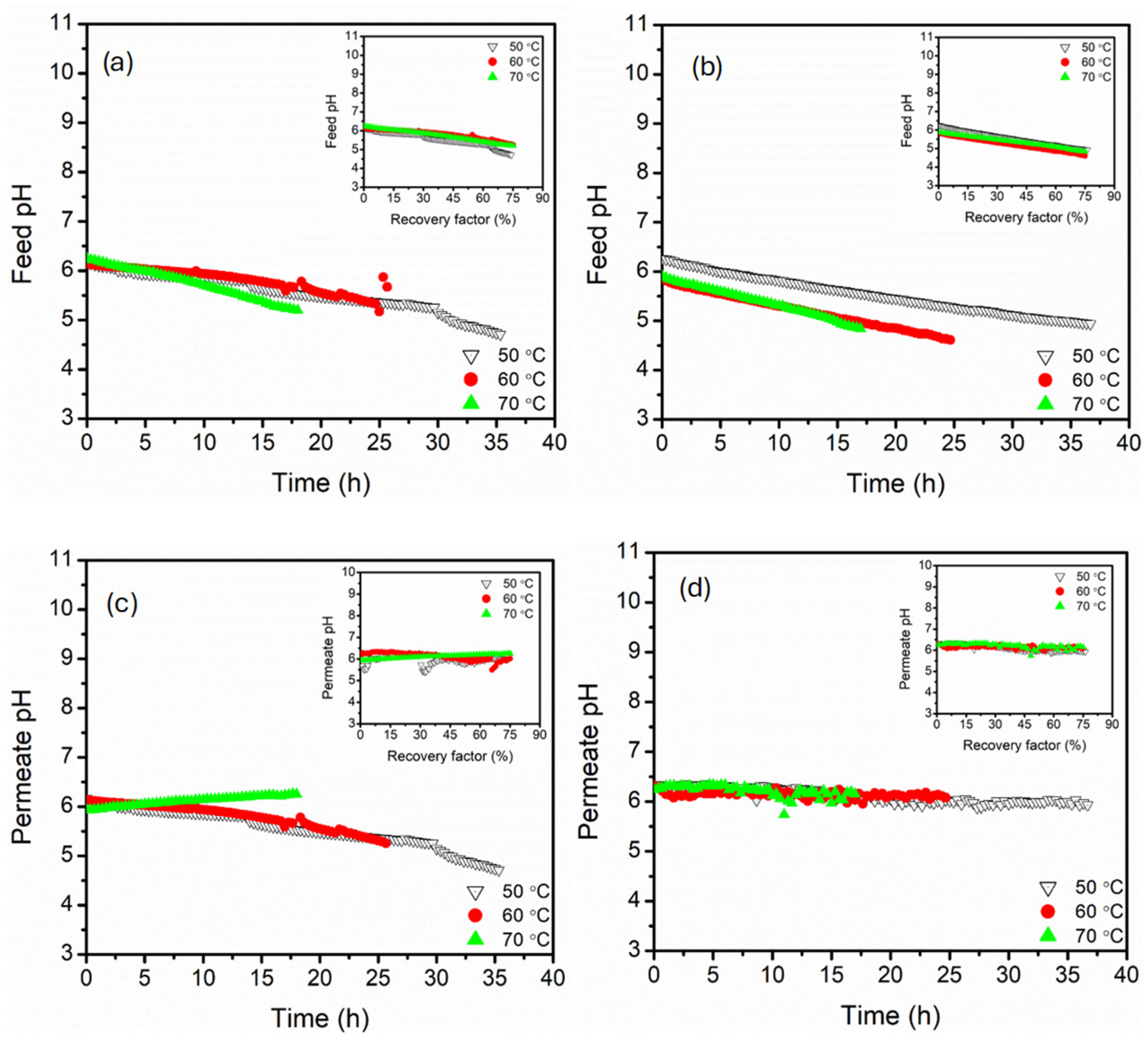
3.1. Effect of Feed Temperature (50, 60, 70 °C)
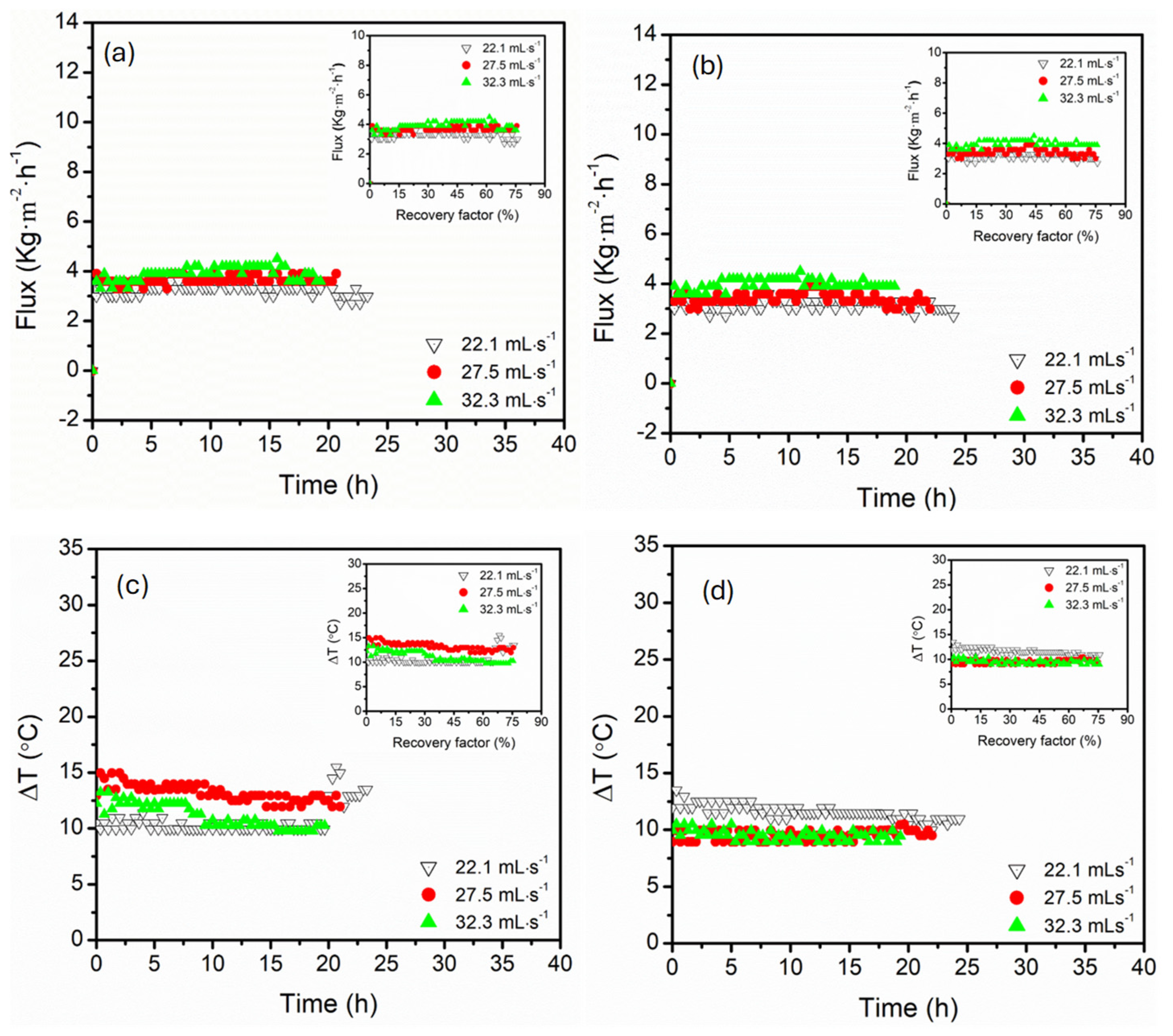
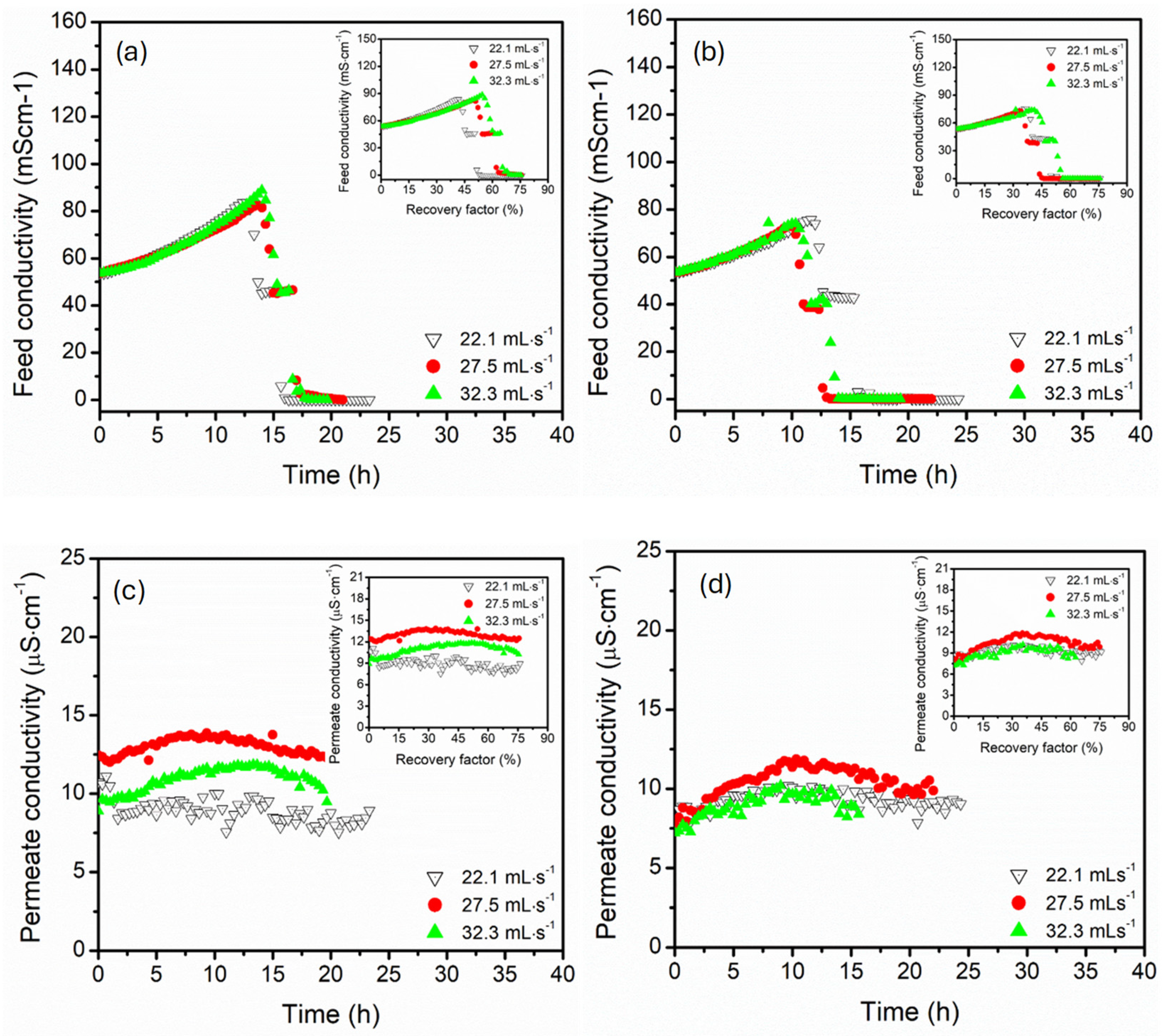
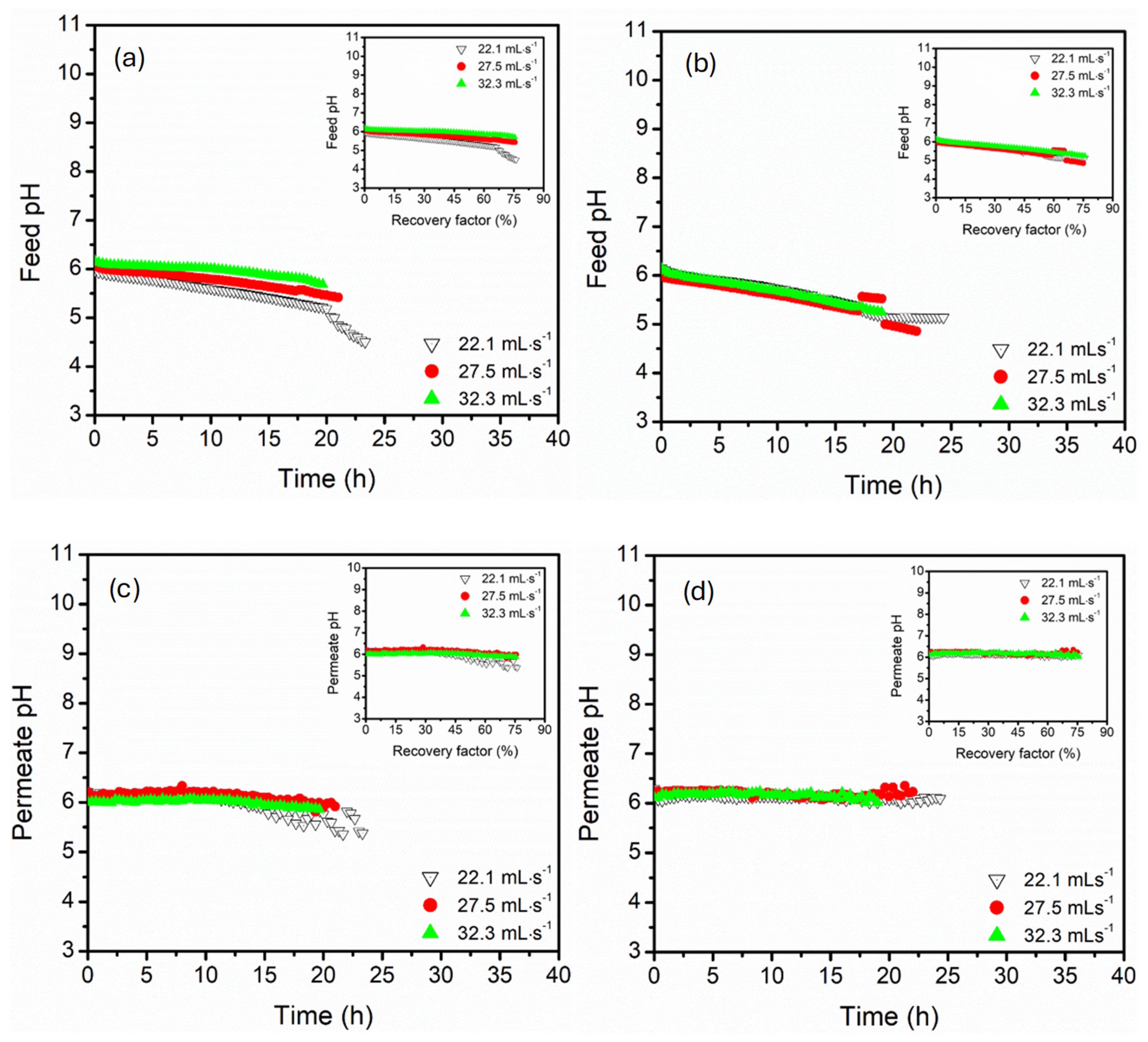
3.2. Effect of Circulation Flow Rate (22, 27, 32 mL·s−1)
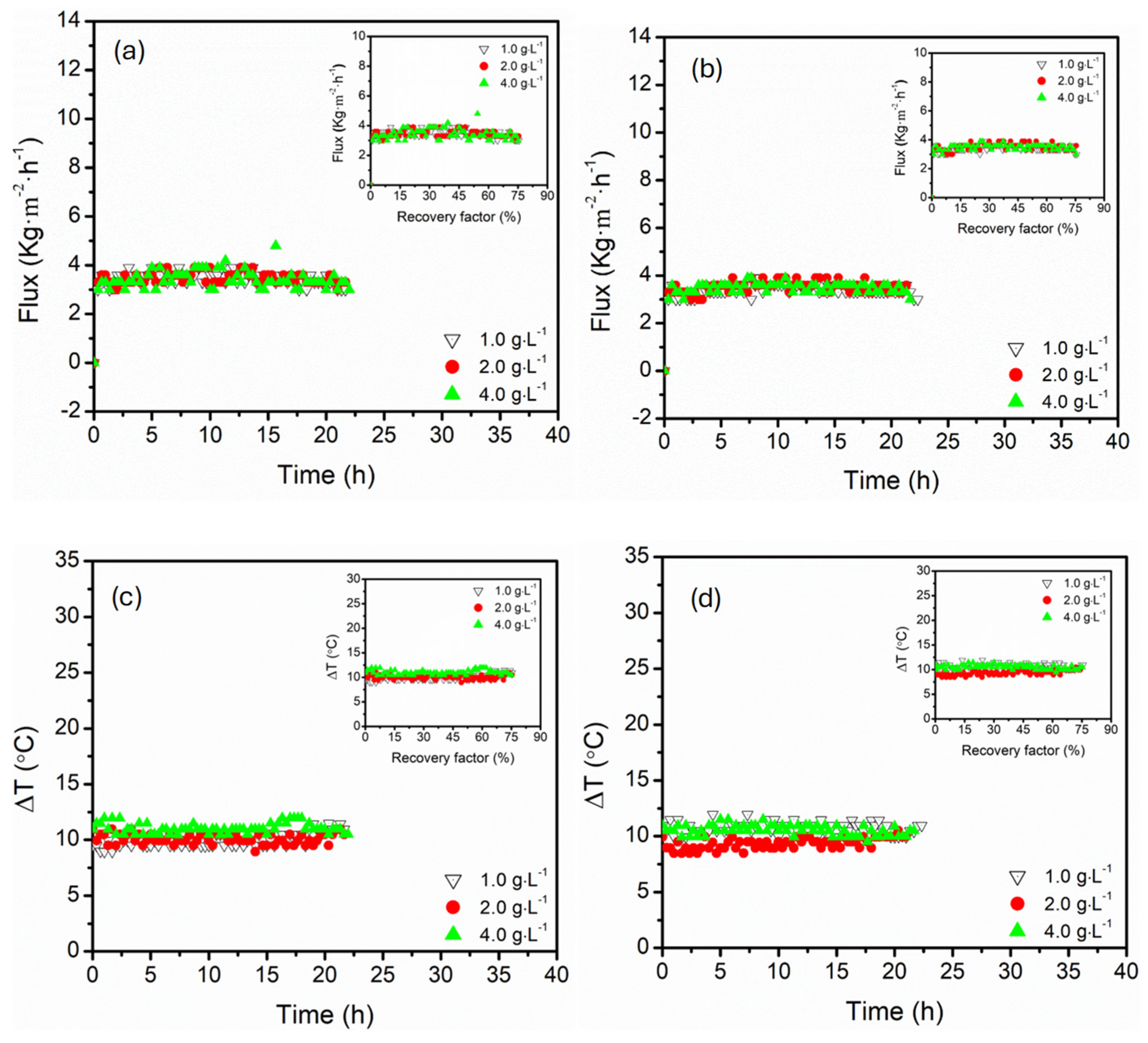
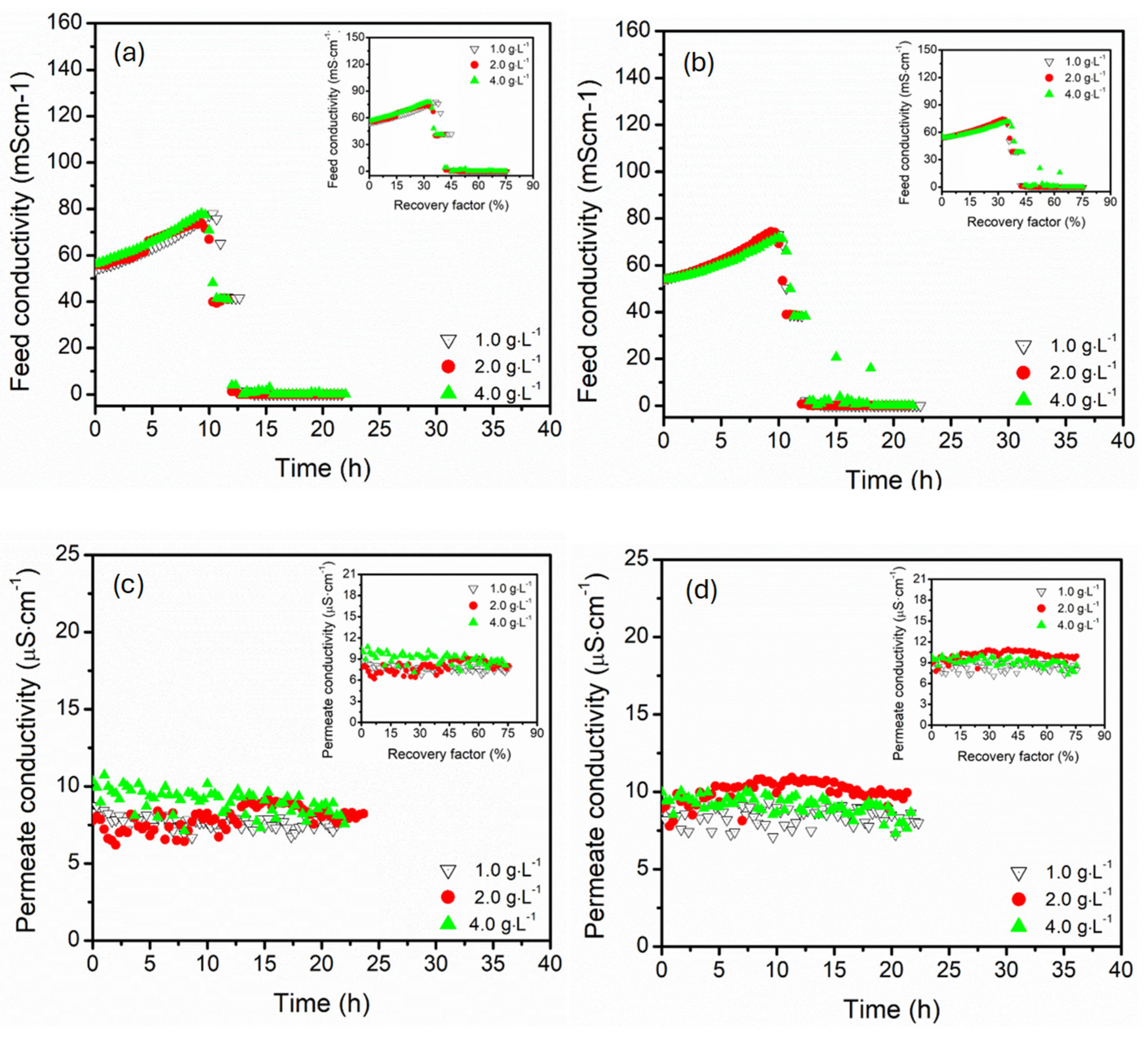
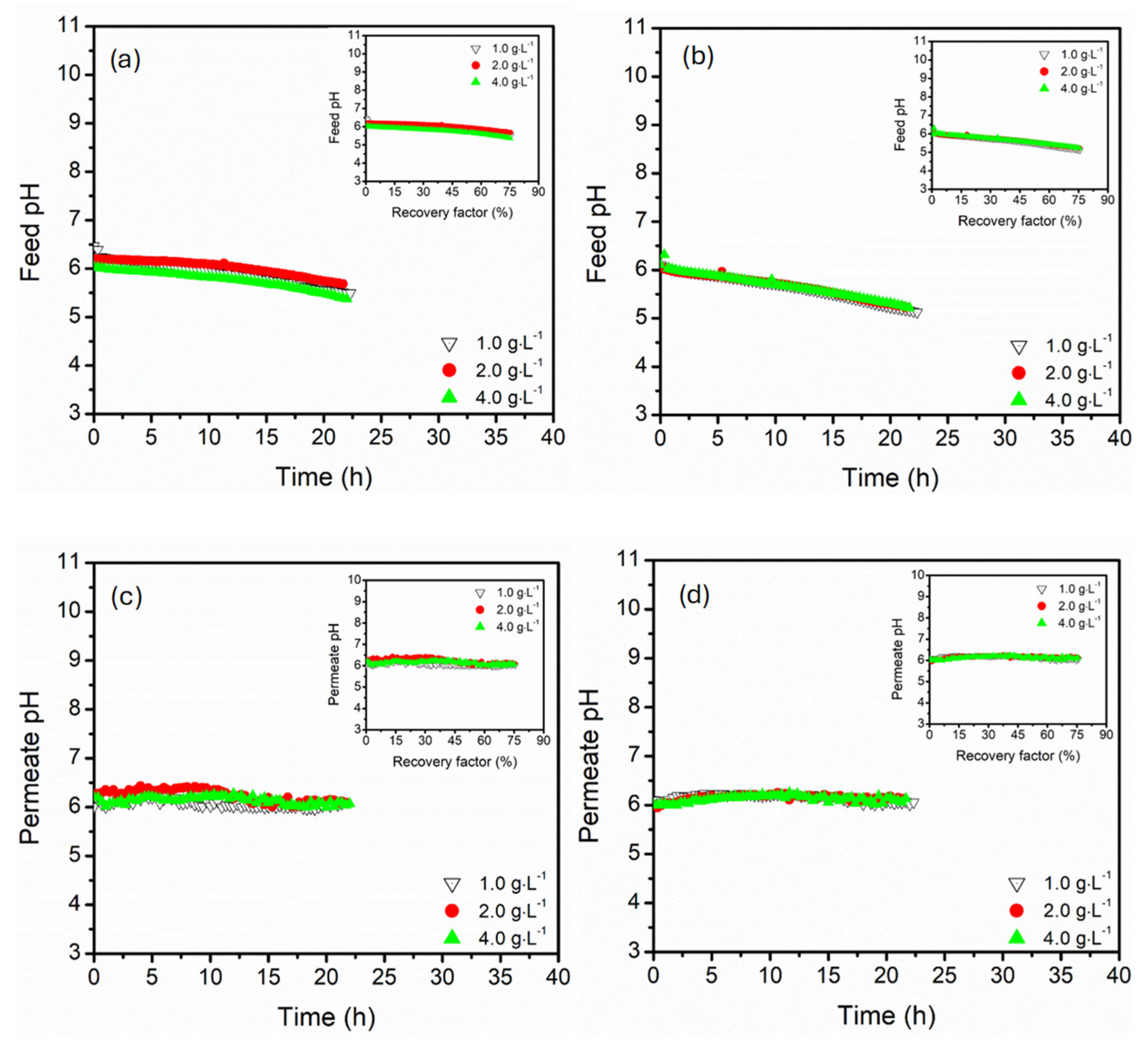
3.3. Effect of Magnesium Sulphate Concentration (1.0, 2.0, 4.0 g·L−1)
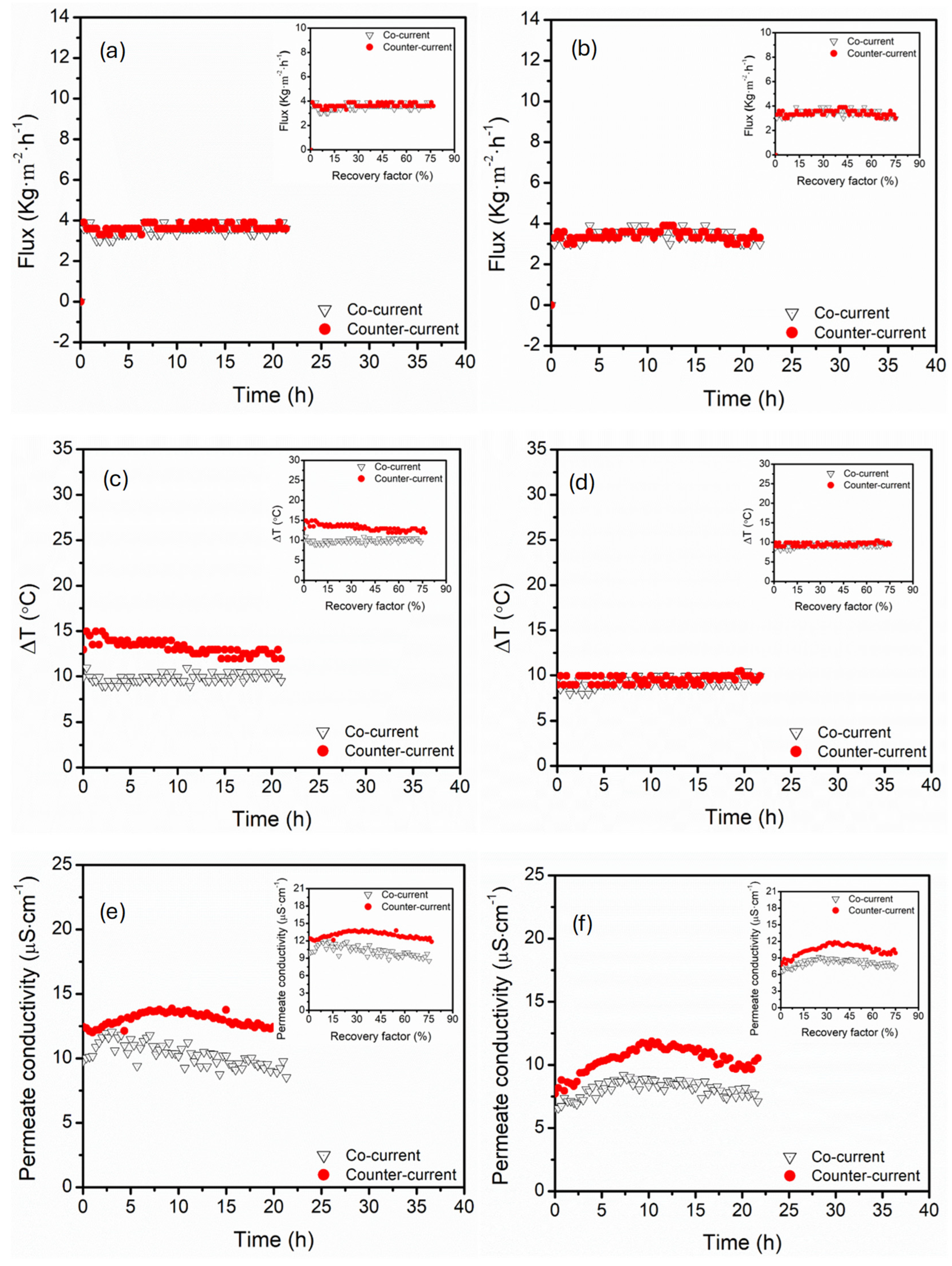
3.4. Effect of Flow Direction (Co-Current vs. Counter-Current)
3.5. Thermal Efficiency of Hollow Fibre DCMD Under Varying Operational Conditions in Different Module Connection Configurations
| Process Conditions | Connection Configuration | Parameters | Thermal Efficiency (%) |
|---|---|---|---|
| Effect of feed temperature (°C) | Serial | 50 | 26.4 |
| 60 | 24.2 | ||
| 70 | 21.7 | ||
| Parallel | 50 | 30.8 | |
| 60 | 20.5 | ||
| 70 | 22.3 | ||
| Effect of circulation flow rate (mL·s−1) | Serial | 22.1 | 25.1 |
| 27.5 | 26.4 | ||
| 32.3 | 26.1 | ||
| Parallel | 22.1 | 27.9 | |
| 27.5 | 21.0 | ||
| 32.3 | 22.0 | ||
| Effect of MgSO4 concentration (g·L−1) | Serial | 1.0 | 28.9 |
| 2.0 | 27.8 | ||
| 4.0 | 20.8 | ||
| Parallel | 1.0 | 32.0 | |
| 2.0 | 25.5 | ||
| 4.0 | 19.6 | ||
| Circulation flow configuration | Serial | Co-current | 34.0 |
| Counter-current | 28.9 | ||
| Parallel | Co-current | 32.9 | |
| Counter-current | 32.0 |
4. Conclusions
- Parallel configuration exhibited high operational stability, controlled polarisation effects, and delayed flux decline, even at high recovery factors (74%). This process architecture enhanced hydrodynamic distributions with supported uniform thermal and mass transfer, suggesting maintenance of long-term stable flux even at high recovery factors.
- In contrast, the serial configuration presented intense supersaturation at lower recovery factors, facilitating the earlier onset of crystallisation. Despite the great advantage of serial configuration in crystallisation processes, the process suffered from thermal and concentration polarisation, particularly at elevated temperatures and low flow rates, causing flux instabilities and the risk of scaling along the sequential module path, especially in long-term operations. Upon increasing the circulation flow rates, thermal efficiency was maintained in this configuration, indicating resistance to thermal losses.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- He, C.; Liu, Z.; Wu, J.; Pan, X.; Fang, Z.; Li, J.; Bryan, B.A. Future global urban water scarcity and potential solutions. Nat. Commun. 2021, 12, 4667. [Google Scholar] [CrossRef]
- Salehi, M. Global water shortage and potable water safety; Today’s concern and tomorrow’s crisis. Environ. Int. 2022, 158, 106936. [Google Scholar] [CrossRef]
- Mcdonald, R.I.; Green, P.; Balk, D.; Fekete, B.M.; Revenga, C.; Todd, M.; Montgomery, M. Urban growth, climate change, and freshwater availability. Proc. Natl. Acad. Sci. USA 2011, 108, 6312–6317. [Google Scholar] [CrossRef] [PubMed]
- Wee, S.Y.; Aris, A.Z. Revisiting the “forever chemicals”, PFOA and PFOS exposure in drinking water. npj Clean Water 2023, 6, 57. [Google Scholar] [CrossRef]
- Lee, B.X.; Kjaerulf, F.; Turner, S.; Cohen, L.; Donnelly, P.D.; Muggah, R.; Davis, R.; Realini, A.; Kieselbach, B.; MacGregor, L.S.; et al. Transforming Our World: Implementing the 2030 Agenda Through Sustainable Development Goal Indicators. J. Public Health Policy 2016, 37, S13–S31. [Google Scholar] [CrossRef] [PubMed]
- van Vliet, M.T.H.; Jones, E.R.; Flörke, M.; Franssen, W.H.P.; Hanasaki, N.; Wada, Y.; Yearsley, J.R. Global water scarcity including surface water quality and expansions of clean water technologies. Environ. Res. Lett. 2021, 16, 024020. [Google Scholar] [CrossRef]
- Alenezi, A.; Alabaiadly, Y. Emerging technologies in water desalination: A review and future outlook. Energy Nexus 2025, 17, 100373. [Google Scholar] [CrossRef]
- Moreira, V.R.; Raad, J.V.; Lazarini, J.X.; Santos, L.V.S.; Amaral, M.C.S. Recent progress in membrane distillation configurations powered by renewable energy sources and waste heat. J. Water Process Eng. 2023, 53, 103816. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, Q.; Song, N.; Chen, J.; Ding, M.; Mei, C.; Zong, Y.; Chen, X.; Gao, L. Membrane distillation by novel Janus-enhanced membrane featuring hydrophobic-hydrophilic dual-surface for freshwater recovery. Sep. Purif. Technol. 2022, 302, 122036. [Google Scholar] [CrossRef]
- Gryta, M. Effectiveness of water desalination by membrane distillation process. Membranes 2012, 2, 415–429. [Google Scholar] [CrossRef]
- Nthunya, L.N.; Bopape, M.F.; Mahlangu, O.T.; Mamba, B.B.; Van der Bruggen, B.; Quist-Jensen, C.A.; Richards, H. Fouling, performance and cost analysis of membrane-based water desalination technologies: A critical review. J. Environ. Manag. 2022, 301, 113922. [Google Scholar] [CrossRef] [PubMed]
- Gontarek-castro, E.; Castro-Muñozb, R. How to make membrane distillation greener: A review of environmentally friendly and sustainable aspects. Green Chem. 2024, 26, 164–185. [Google Scholar] [CrossRef]
- Abdelgaied, M.; Seleem, M.F.; Bassuoni, M.M. Recent technological advancements in membrane distillation and solar stills: Preheating techniques, heat storage materials, and nanomaterials—A detailed review. Environ. Sci. Pollut. Res. 2022, 29, 38879–38898. [Google Scholar] [CrossRef]
- Zaragoza, G.A.; Andrés-Mañas, J.; Ruiz-Aguirre, A. Commercial scale membrane distillation for solar desalination. npj Clean Water 2018, 1, 20. [Google Scholar] [CrossRef]
- Yadav, A.; Labhasetwar, P.K.; Shahi, V.K. Membrane distillation using low-grade energy for desalination: A review. J. Environ. Chem. Eng. 2021, 9, 105818. [Google Scholar] [CrossRef]
- Ho, C.; Chen, L.; Lim, J.; Lin, P.; Lu, P. Distillate Flux Enhancement of Direct Contact Membrane Distillation Modules with Inserting Cross-Diagonal Carbon-fiber Spacers. Membranes 2021, 11, 973. [Google Scholar] [CrossRef]
- Ezugbe, E.O.; Rathilal, S. Membrane technologies in wastewater treatment: A review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef]
- Nthunya, L.N.; Chong, K.C.; Lai, S.O.; Lau, W.J.; López-Maldonado, E.A.; Camacho, L.M.; Shirazi, M.M.A.; Ali, A.; Mamba, B.B.; Osial, M.; et al. Progress in membrane distillation processes for dye wastewater treatment: A review. Chemosphere 2024, 360, 142347. [Google Scholar] [CrossRef]
- Tai, Z.S.; Othman, M.H.D.; Koo, K.N.; Jaafar, J. Critical review on membrane designs for enhanced flux performance in membrane distillation. Desalination 2023, 553, 116484. [Google Scholar] [CrossRef]
- Liu, J.; Xie, B.; Mushtaq, N.; Xu, G.; Bar-Zeev, E.; Hu, Y. New insights into the role of carbon nanotubes spray-coated on both sides of the PTFE membrane in suppressing temperature polarization and enhancing water flux in direct contact membrane distillation. J. Membr. Sci. 2024, 689, 122184. [Google Scholar] [CrossRef]
- Ali, A.; Shirazi, M.M.A.; Nthunya, L.; Castro-Muñoz, R.; Ismail, N.; Tavajohi, N.; Zaragoza, G.; Quist-Jensen, C.A. Progress in module design for membrane distillation. Desalination 2024, 581, 117584. [Google Scholar] [CrossRef]
- Camacho, L.M.; Dumée, L.; Zhang, J.; de Li, J.; Duke, M.; Gomez, J.; Gray, S. Advances in membrane distillation for water desalination and purification applications. Water 2013, 5, 94–196. [Google Scholar] [CrossRef]
- Chen, L.; Xu, P.; Wang, H. Interplay of the factors affecting water flux and salt rejection in membrane distillation: A state-of-the-art critical review. Water 2020, 12, 2841. [Google Scholar] [CrossRef]
- Ho, C.D.; Wang, Y.W.; Chao, Y.; Chew, T.L.; Jiang, M.S.; Chen, J.H.; Li, C.Y. Enhancing the Permeate Flux Improvement of Direct Contact Membrane Distillation Modules with Inserted S-Ribs Carbon-Fiber Filaments. Membranes 2024, 14, 98. [Google Scholar] [CrossRef]
- Francis, L.; Ahmed, F.E.; Hilal, N. Advances in Membrane Distillation Module Configurations. Membranes 2022, 12, 81. [Google Scholar] [CrossRef]
- Lawson, K.W.; Lloyd, D.R. Membrane distillation. J. Membr. Sci. 2021, 124, 1–25. [Google Scholar] [CrossRef]
- Lawson, K.W.; Lloyd, D.R. Membrane distillation. I. Module design and performance evaluation using vacuum membrane distillation. J. Membr. Sci. 1996, 120, 111–121. [Google Scholar] [CrossRef]
- Andersson, S.-I.; Kjellander, N.; Rodesjö, B. Design and field tests of a new membrane distillation desalination process. Desalination 1985, 56, 345–354. [Google Scholar] [CrossRef]
- Bin Abid, M.; Wahab, R.A.; Salam, M.A.; Moujdin, I.A.; Gzara, L. Desalination technologies, membrane distillation, and electrospinning, an overview. Heliyon 2023, 9, e12810. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, A.E.; Alawad, S.M. Air gap and water gap multistage membrane distillation for water desalination. Desalination 2018, 437, 175–183. [Google Scholar] [CrossRef]
- Alawad, S.M.; Khalifa, A.E. Case Studies in Thermal Engineering Development of an efficient compact multistage membrane distillation module for water desalination. Case Stud. Therm. Eng. 2021, 25, 100979. [Google Scholar] [CrossRef]
- Alawad, S.M.; Khalifa, A.E. Performance and energy evaluation of compact multistage air gap membrane distillation system: An experimental investigation. Sep. Purif. Technol. 2021, 268, 118594. [Google Scholar] [CrossRef]
- Aryapratama, R.; Koo, H.; Jeong, S.; Lee, S. Performance evaluation of hollow fiber air gap membrane distillation module with multiple cooling channels. Desalination 2016, 385, 58–68. [Google Scholar] [CrossRef]
- Ali, A.; Aimar, P.; Drioli, E. Effect of module design and flow patterns on performance of membrane distillation process. Chem. Eng. J. 2015, 277, 368–377. [Google Scholar] [CrossRef]
- Meindersma, G.W.; Guijt, C.M.; de Haan, A.B. Desalination and water recycling by air gap membrane distillation. Desalination 2006, 187, 291–301. [Google Scholar] [CrossRef]
- Kariman, H.; Mohammed, H.A.; Zargar, M.; Khiadani, M. Performance comparison of flat sheet and hollow fibre air gap membrane distillation: A mathematical and simulation modelling approach. J. Membr. Sci. 2025, 721, 123836. [Google Scholar] [CrossRef]
- Dong, Y.; Dai, X.; Zhao, L.; Gao, L.; Xie, Z.; Zhang, J. Review of transport phenomena and popular modelling approaches in membrane distillation. Membranes 2021, 11, 122. [Google Scholar] [CrossRef] [PubMed]
- Herwig, H. What exactly is the Nusselt number in convective heat transfer problems and are there alternatives? Entropy 2016, 18, 198. [Google Scholar] [CrossRef]
- Cancilla, N.; Tamburini, A.; Tarantino, A.; Visconti, S.; Ciofalo, M. Friction and Heat Transfer in Membrane Distillation Channels: An Experimental Study on Conventional and Novel Spacers. Membranes 2022, 12, 1029. [Google Scholar] [CrossRef]
- Ali, E. Optimal Control of Direct Contact Membrane Distillation Operated under Fluctuating Energy Source. Membranes 2022, 12, 628. [Google Scholar] [CrossRef] [PubMed]
- Nthunya, L.N.; Ali, A.; Richards, H.; Chimuka, L.; Quist-Jensen, C.; Mamba, B.B. Innovative dual-purpose remediation of acid mine drainage and resource recovery through membrane distillation crystallization. npj Clean Water 2025, 8, 61. [Google Scholar] [CrossRef]
- Nthunya, L.N.; Setati, B.; Richards, H.; Chimuka, L.; Mamba, B.B. From extract to crystals: Unlocking green nanotechnology towards recovery of bioactive compounds from Moringa oleifera via membrane distillation crystallization. Sep. Purif. Technol. 2025, 373, 133552. [Google Scholar] [CrossRef]
- McGaughey, A.L.; Childress, A.E. Wetting indicators; modes, and trade-offs in membrane distillation. J. Membr. Sci. 2022, 642, 119947. [Google Scholar] [CrossRef]
- Silva, R.; Cadorin, L.; Rubio, J. Sulphate ions removal from an aqueous solution: I. Co-precipitation with hydrolysed aluminum-bearing salts. Miner. Eng. 2010, 23, 1220–1226. [Google Scholar] [CrossRef]
- Tomaszewska, M.; Gryta, M.; Morawski, A. Study on the concentration of acids by membrane distillation. J. Membr. Sci. 1995, 102, 113–122. [Google Scholar] [CrossRef]
- Wang, F.; Liu, J.; Li, D.; Liu, Z.; Zhang, J.; Ding, P.; Liu, G.; Feng, Y. High-Efficiency Water Recovery from Urine by Vacuum Membrane Distillation for Space Applications: Water Quality Improvement and Operation Stability. Membranes 2022, 12, 629. [Google Scholar] [CrossRef]
- Alkhatib, A.; Ayari, M.A.; Hawari, A.H. Fouling mitigation strategies for different foulants in membrane distillation. Chem. Eng. Process. Process. Intensif. 2021, 167, 108517. [Google Scholar] [CrossRef]
- Moujdin, I.A.; Totah, H.S.; Allaf, I.; Abulkhair, H.; Fayed, M. Evaluation of swirling flow phenomenon in direct contact membrane distillation. Front. Mech. Eng. 2025, 11, 1569918. [Google Scholar] [CrossRef]
- Ve, Q.L.; Rahaoui, K.; Bawahab, M.; Faqeha, H.; Date, A.; Akbarzadeh, A.; Do, M.C.; Nguyen, Q.L.; Ve, L.; Do, C.; et al. Experimental Investigation of Heat Transfer Correlation for Direct Contact Membrane Distillation. J. Heat Transf. 2019, 142, 012001. [Google Scholar] [CrossRef]
- Ali, K.; Abdelsamie, M.M.; Ali, M.I.H. Toward sustainable energy resource: Integrated concentrated photovoltaic and membrane distillation systems for fresh water and electricity production. Energy 2024, 308, 132998. [Google Scholar] [CrossRef]
- Blankert, B.; Vrouwenvelder, J.S.; Witkamp, G.-J.; Ghaffour, N. Minimum net driving temperature concept for membrane distillation. Membranes 2020, 10, 100. [Google Scholar] [CrossRef]
- Abu-Zeid, M.A.E.-R.; Lu, X.; Zhang, S. Developing symmetrical traditional double-stage membrane distillation system based on air-gap and water-gap configurations. Desalination Water Treat. 2020, 202, 121–132. [Google Scholar] [CrossRef]
- Chimanlal, I.; Nthunya, L.N.; Quist-Jensen, C.; Richards, H. Membrane distillation crystallization for water and mineral recovery: The occurrence of fouling and its control during wastewater treatment. Front. Chem. Eng. 2022, 4, 1066027. [Google Scholar] [CrossRef]
- Nthunya, L.N.; Pinier, J.; Ali, A.; Quist-jensen, C.; Richards, H. Valorization of acid mine drainage into potable water and valuable minerals through membrane distillation crystallization. Sep. Purif. Technol. 2024, 334, 126084. [Google Scholar] [CrossRef]
- Balis, E.; Griffin, J.C.; Hiibel, S.R. Membrane Distillation-Crystallization for inland desalination brine treatment. Sep. Purif. Technol. 2022, 29, 4–11. [Google Scholar] [CrossRef]
- Li, X.; Pan, J.; Macedonio, F.; Ursino, C.; Carraro, M.; Bonchio, M.; Drioli, E.; Figoli, A.; Wang, Z.; Cui, Z. Fluoropolymer Membranes for Membrane Distillation and Membrane Crystallization. Polymers 2022, 14, 5439. [Google Scholar] [CrossRef]
- Rezaei, M.; Warsinger, D.M.; Duke, M.C.; Matsuura, T.; Samhaber, W.M. Wetting phenomena in membrane distillation: Mechanisms, reversal, and prevention. Water Res. 2018, 139, 329–352. [Google Scholar] [CrossRef] [PubMed]
- Nthunya, L.N.; Gutierrez, L.; Lapeire, L.; Verbeken, K.; Zaouri, N.; Nxumalo, E.N.; Mamba, B.B.; Verliefde, A.R.; Mhlanga, S.D. Fouling resistant PVDF nanofibre membranes for the desalination of brackish water in membrane distillation. Sep. Purif. Technol. 2019, 228, 115793. [Google Scholar] [CrossRef]
- Schilling, S.; Glade, H. Review and Analysis of Heat Transfer in Spacer-Filled Channels of Membrane Distillation Systems. Membranes 2023, 13, 842. [Google Scholar] [CrossRef]
- Khalifa, A.E.; Alawad, S.M.; Antar, M.A. Parallel and series multistage air gap membrane distillation. Desalination 2017, 417, 69–76. [Google Scholar] [CrossRef]
- Chou, I.-M.; Seal, R.R. Determination of Epsomite-Hexahydrite Equilibria by the Humidity-Buffer Technique at 0.1 MPa with Implications for Phase Equilibria in the System MgSO4-H2O. Astrobiology 2003, 3, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Soumbati, Y.; Bouatou, I.; Abushaban, A.; Belmabkhout, Y.; Necibi, M.C. Review of membrane distillation for desalination applications: Advanced modeling, specific energy consumption, and water production cost. J. Water Process. Eng. 2025, 71, 107296. [Google Scholar] [CrossRef]
- Meo, R.R.; Craveri, L.; Bertozzi, E.; Malaguti, M.; Tiraferri, A.; Morciano, M.; Fasano, M. Systematic exploration of direct solar absorption potential to enhance direct contact membrane distillation. Desalination 2025, 606, 118740. [Google Scholar] [CrossRef]
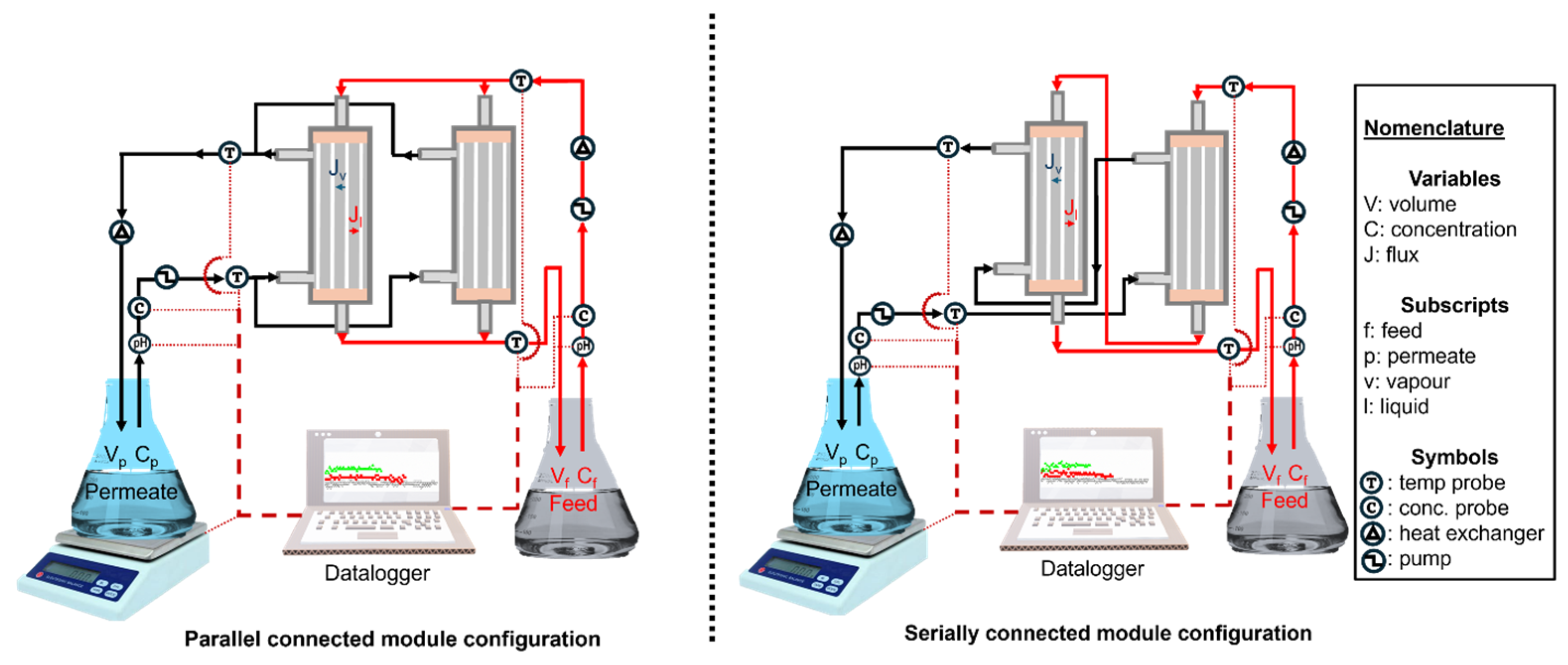
| Connection Configuration | Feed Temperature (°C) | Feed Inlet Temperature (°C) | Feed Outlet Temperature (°C) | Permeate Inlet Temperature (°C) | Permeate Outlet Temperature (°C) |
|---|---|---|---|---|---|
| Serial | 50 | 33.2 | 29.1 | 19.9 | 23.1 |
| 60 | 37.8 | 34.2 | 23.1 | 26.2 | |
| 70 | 44.3 | 38.8 | 23.6 | 28.5 | |
| Parallel | 50 | 32.5 | 28.7 | 20.1 | 23.3 |
| 60 | 37.2 | 33.8 | 23.2 | 27.1 | |
| 70 | 43.5 | 37.9 | 24.1 | 29.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nthunya, L.N.; Mamba, B.B. Membrane Distillation for Water Desalination: Assessing the Influence of Operating Conditions on the Performance of Serial and Parallel Connection Configurations. Membranes 2025, 15, 235. https://doi.org/10.3390/membranes15080235
Nthunya LN, Mamba BB. Membrane Distillation for Water Desalination: Assessing the Influence of Operating Conditions on the Performance of Serial and Parallel Connection Configurations. Membranes. 2025; 15(8):235. https://doi.org/10.3390/membranes15080235
Chicago/Turabian StyleNthunya, Lebea N., and Bhekie B. Mamba. 2025. "Membrane Distillation for Water Desalination: Assessing the Influence of Operating Conditions on the Performance of Serial and Parallel Connection Configurations" Membranes 15, no. 8: 235. https://doi.org/10.3390/membranes15080235
APA StyleNthunya, L. N., & Mamba, B. B. (2025). Membrane Distillation for Water Desalination: Assessing the Influence of Operating Conditions on the Performance of Serial and Parallel Connection Configurations. Membranes, 15(8), 235. https://doi.org/10.3390/membranes15080235






