Abstract
In this work, for the first time, the stability of the TiO2 suspensions used for the photocatalytic membrane preparation was studied by considering the Turbiscan Stability Index (TSI). The use of a stable suspension during the membrane preparation (by the dip-coating method) permitted a better dispersion of the TiO2 nanoparticles into the membrane structure due to a reduction of agglomerates formation. The dip-coating was performed on the macroporous structure (external surface) of the Al2O3 membrane to avoid large reduction of the permeability. In addition, the reduction of the suspension infiltration along the membrane’s cross-section allowed us to preserve the separative layer of the modified membrane. The water flux was reduced by about 11% after the dip-coating. The photocatalytic performance of the prepared membranes was evaluated using the methyl orange as a model pollutant. The reusability of the photocatalytic membranes was also demonstrated.
1. Introduction
Today, the discharge of wastewater, produced by textile, paper and food industries [1], causes serious health problems for humans and aquatic life, mainly due to the presence of synthetic dye in the effluents [2]. These chemical compounds are divided into different categories: acid, basic, direct, azoic, reactive and disperse dyes [3]. The azo dyes are widely used in the textile industry [4] and are very resistant. The traditional technologies used for their treatment are ineffective due to their elevated chemical stability [5]. Advanced oxidation processes (AOPs) represent an alternative route for the effective removal of pollutants present in the wastewater [6]. These processes produce highly oxidative radicals that are capable of degrading, in a non-selective way, organic pollutants, and converting them to mineralized species [7]. They can be divided into different classes: chemical (ozonation, peroxidation, Fenton), physical (sonolysis, cavitation), photochemical, photocatalytic (UV/solar/semi-conductors, UV/H2O2, UV/O3, etc.), and electrochemical processes (electrooxidation, electro-Fenton, etc.) [8]. The photo-processes favor the excitation of the electrons in a semiconductor by using UV and UV-Vis light [9]. In particular, the absorption of a photon, with energy equal or greater than the band gap, permits the electron’s excitation from the valance band to conduction band, leaving a positive hole (h+) in the valance band [9]. The electrons can reduce the pollutants or react with electron acceptors (as, for example, with O2 present in the water), reducing it to a superoxide radical anion (O2−). The surface of the photocatalyst at the valence band is positively charged and takes electrons from water to create hydroxyl radicals (OH˙) [10]. The radicals formed can degrade the pollutants present in the wastewater. Today, the photocatalyst deeply used is titanium oxide (TiO2), being non-toxic, inexpensive and having high efficiency [11]. This process exhibits some limitations, with the formation of agglomerates during the reaction with a decrease of the catalytic activity (reduction of the active sites) and a reduction of the incident light [12]. In addition, the recovery of the TiO2 nanoparticles (NPs) from the reaction medium makes the process expensive [13]. These drawbacks can be overcome with a photocatalytic membrane reactor (PMR) that combines the photocatalytic technology with membrane processes. A PMR can be designed in two main configurations: with suspended catalysts or ones immobilized in/on the membrane structure [14]. In the first configuration, the photocatalytic particles degrade the pollutants, and the membrane exhibits activities of confining the catalyst, the pollutant, and the intermediate species formed during the process [15]. This configuration ensures good contact between the catalytic sites and reactants, presenting higher efficiency than the immobilized one. However, the light penetration is reduced when the catalyst concentration is too high [15]. In addition, considering that the suspension is an unstable system, the NPs’ precipitation on the membrane surface causes a decline in the permeate flux and a decrease in the catalytic activity [16]. The PMR with the catalyst immobilized on/in the membrane permits an easy recovery of the catalyst and so its reuse. With this configuration, the fouling is mitigated, due to the self-cleaning property of the membrane surface [16]. However, with an immobilized catalyst, a reduction of the catalytic activity is detected, due to a reduction of the surface area [16]. Zhang et al. [17] studied the degradation of the reactive brilliant red by means of a suspended photocatalytic membrane reactor. The rejection of dye markedly depends on the deposition of TiO2 aggregate on the membrane surface. A comparison between the two different configurations of the PMR for the degradation of two azo dyes was achieved by Molinari and coworkers [18]. The experimental data evidenced that the PMR configuration with suspended photocatalytic nanoparticles was significantly more efficient than the configuration with the photocatalyst entrapped in the membrane.
Polymeric membranes are used in various applications, such as in gas separation processes, as well as water purification, desalination, and dialysis, being cheap and simply manufactured and processed [18]. Different papers have been published concerning wastewater treatment, and the effect of catalyst and pollutant concentrations, pH, light intensity, and membrane pore size on the catalytic activity has been deeply investigated [19,20,21,22,23,24]. Ceramic membranes seem more appropriate for wastewater treatment than polymeric ones, owing to their higher hydrophilicity that implies lower fouling and higher fluxes at low trans-membrane pressures [25], even if their application at the industrial scale is limited by high capital costs [26]. Deepracha et al. [27] demonstrated the possibility of removing phenol (21%) from water with a TiO2-Al2O3 membrane. A recent study used a TiO2–alumina membrane prepared with sol-gel spin coating method [28]. The dye degradation after 120 min was found to be equal to 36% [28].
In this study, high-flux ceramic photocatalytic membranes having a tubular configuration were prepared by using the dip-coating method. For the first time, the Turbiscan Stability Index was used for evaluating the stability of the suspensions employed for the photocatalytic membrane preparation. The photocatalytic activity of the prepared membranes was tested in a PMR for the degradation of methyl orange (MO), a recalcitrant azo dye. The reusability of the photocatalytic membranes during four successive reactions was also evaluated. Finally, the kinetics of MO degradation have been evaluated.
2. Materials and Methods
2.1. Materials
Titanium dioxide (TiO2; Honeywell Fluka, Seelze, Germany) was used as a photocatalyst. The additives used for the slurry stabilization were nitric acid (HNO3, Honeywell Fluka, pure) and polyvinylpyrrolidone (PVP, Carl Erba reagents, average molecular weight 17,000 Da). A tubular α-Al2O3 membrane (pore size: 200 nm, internal diameter.: 7 mm, outer diameter.: 10 mm; length: 10 cm; IKTS, Hermsdorf (Thuringia), Germany) was utilized for the preparation of the photocatalytic membranes. Methyl orange (Titolchimica S.p.a., Pontecchio Polesine (Ro) ITALY) was employed as a recalcitrant model dye. Distilled water was used to prepare the suspensions and for the membrane washing.
2.2. Suspension Preparation and Characterization
The morphology and size of the commercial TiO2 nanoparticles were observed by scanning electron microscope (SEM) using a Cambridge Zeiss LEO 400 microscope. The TiO2 powder was analyzed by X-ray diffractometry, using a Rigaku MiniFlex 600 X-ray diffractometer (Rigaku Corporation, Tokyo, Japan) with CuKα (wavelength of 1.5406 Å) radiation generated at 20 mA and 40 KV. The samples were scanned at 0.02 2θ at a rate of 1°/min, between 5°and 60° (2θ angle range).
To prepare the suspensions, an appropriate amount of TiO2 nanoparticles was dispersed in 30 mL of distilled water and stirred magnetically for 30 min at 30 °C (see Table 1). Subsequently, the effect of sonication and some additives on suspension stability was evaluated. In particular, the suspension after the magnetic stir (for 30 min) was sonicated for 20 min at 30 °C.

Table 1.
Composition of the titanium dioxide suspensions.
To obtain a suspension containing the PVP, used as adsorbate, 5 mL of the polymeric solution (two concentrations were considered: 0.2 wt. % and 0.8 wt. % [29]) was mixed with 25 mL of the TiO2 suspension (see Table 1), and stirred for 30 min at 30 °C. Finally, the effect of the nitric acid was evaluated by adding some drops of nitric acid solution (pH: 2.0) to the suspension containing titanium dioxide nanoparticles.
The suspension stability was studied by Turbiscan LAB® (Formulation SAS, Versailles, France) at room temperature for 5 h, and the coating was carried out in the same time interval.
This instrument works by the use of light-scattering to detect particle migration and the formation of clusters in the liquid dispersion. It exhibits two detectors, working in transmission (T) and backscattering (BS) mode (λ = 880 nm), and, therefore, transparent and opaque samples were analyzed [30]. The equation used for calculating T is the following [7].
and λ is given by:
where T is the transmittance, λ the mean free-path of the photon, ri is the inner radius of the sample vial, d is the average size of the particle, Φ is the volume concentration of the dispersion phase, and Qs is an optical parameter. The backscattering is influenced by the size of the particles and volume concentration as reported in Equation (3).
Turbiscan LAB® calculates the TSI, a parameter directly related to transmission and backscattering signals. The instrument calculates the TSI value by comparing each scan to the previous one at a selected height, and dividing the result by the total height (see Equation (4)) [31].
where scani (h) and scani-1(h) are the values of the profile for a given scan “i” and the previous one “i-1” obtained at a given height “h”. H is the total height of the sample. The TSI values change in the range 0–100. The increase of TSI value indicates a loss of suspension stability.
Some suspensions’ zeta potential was measured using a Malvern Mastersizer 2000, Malvern Instruments. Before the analysis, particles were dispersed with an ultrasonication bath for 5 min.
2.3. Photocatalytic Membranes: Preparation and Characterization
Photocatalytic membranes were prepared by dipping the alumina membrane into the more stable TiO2 suspension. The coating occurred on the external surface of the ceramic membrane. In addition, the photocatalytic nanoparticles’ presence in the support’s internal surface was avoided by sealing the ends of the alumina tubular membranes with Teflon tape. The laboratory scale plant is schematized in Figure 1.
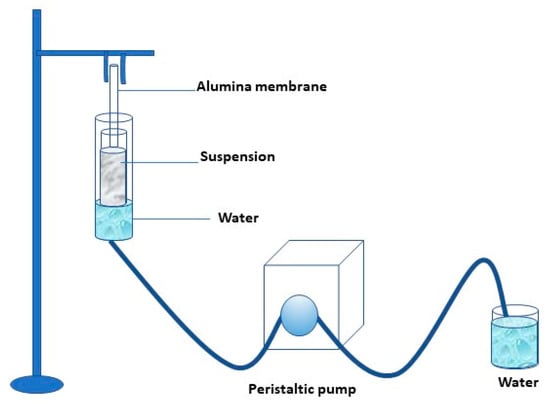
Figure 1.
Scheme of the lab plant used for the preparation of the photocatalytic membrane.
The membranes were dip-coated for 5 h, and the removal of the water from the glass tube was realized with the aid of a peristaltic pump at a feed flow rate of 0.2 mLs−1.The morphology of both pristine and photocatalytic membranes (indicated as TiO2-Al2O3 membrane) was observed by scanning electron microscope. Elemental analysis of the prepared membranes has been performed using energy dispersive X-ray (EDX) using a ZEISS crossbeam 350 instrument. The water flux through pristine and coated membranes has been measured under different trans-membrane pressures (0.2–1.6 bar), and the permeate flux was calculated by using Equation (5):
where J is the permeate flux (Lm−2 h−1), V is the volume of the accumulated permeate, A the membrane area, and t is the filtration time [32]. The hydraulic permeance was determined from the slope of water flux versus transmembrane pressure difference. Each experiment was carried out at least in triplicate.
2.4. Photocatalytic Membrane Tests
The laboratory scale plant used for assessing he photocatalytic activity of the TiO2-Al2O3 membranes is reported in Figure 2.
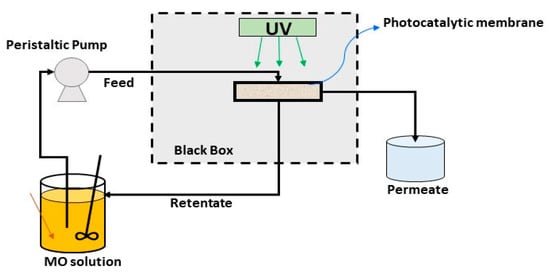
Figure 2.
Scheme of the PMR used for MO degradation.
The TiO2–Al2O3 membranes (area = 17.59 cm2) were housed in a tubular glass membrane module (as shown in Figure 2), and placed in a light exposure chamber. The distance between the UV light and the membrane module was about 12 cm. The feed was 1 L of an aqueous solution of methyl orange (concentration = 1 mgL−1) and was recirculated through the system by a peristaltic pump. The PMR, operated in crossflow filtration mode, and the methyl orange degradation was performed continuously. In particular, the retentate has been collected in the feed tank, and the permeate in a separate container. The dye solution was exposed to ultraviolet (UV) light (λ = 365 nm) for 4 h.
The stability and reusability of the photocatalytic membranes were tested by reusing the same membranes (four reactions). The MO concentration was determined by measuring the absorbance at a wavelength of 464 nm, by using a UV-visible Lambda EZ201 spectrophotometer (PerkinElmer).
The degradation percentage of MO was calculated by using the Equation (6):
where C0 is the in initial concentration and Ct the residual concentration after time t.
The kinetics of dye degradation was assessed considering the zero-order, first-order, and second-order models, and the Equations are reported below.
Zero-order kinetics:
First order kinetics:
Second order kinetics:
where k0, k1, and k2 are the kinetic rate constants for zero-order, first-order, and second-order reaction kinetics, respectively.
3. Results and Discussions
TiO2 is largely used as a photocatalyst for the degradation of organic materials, due to its high superior photocatalytic activity, low cost, and elevated chemical stability [33]. However, the use of titanium dioxide in the industrial process presents different disadvantages; the first is TiO2’s separation from the slurry after the water treatment. For this reason, a separation/recovery step is required. Another problem is represented by the tendency of the TiO2 particle to form agglomerates, with a consequent reduction of its surface area [34]. The combination of the membrane separation with the photocatalytic process represents an efficient, low-cost, eco-friendly technology, with great potential in wastewater treatment. In this work, high-flux inorganic photocatalytic membranes have been developed using the dip-coating method. The novelty of the work is represented by the study of the TiO2 suspension stability, considering the Turbiuscan Stability Index. The use of stable suspensions for the photocatalytic membrane preparation permits a reproducible method and, at the same time, a better distribution of the titanium dioxide nanoparticles in the membrane structure (due to a reduction of the agglomerate formation). This last aspect ensures an improvement of the photocatalytic activity, for better interactions between the active catalytic sites and the molecules of dye.
Initially, the TiO2 powder was characterized by SEM analysis, and an average particle size of 150 nm was measured (see Figure 3a). TiO2 in nature exhibits different crystal structures: rutile, anatase, brookite, and srilankite [35]. When compared with the XRD reference patterns, the X-ray diffraction pattern of the TiO2 commercial powder was shown to be in the anatase state (typical peaks at 25° and 48°) [36] (see Figure 3b).
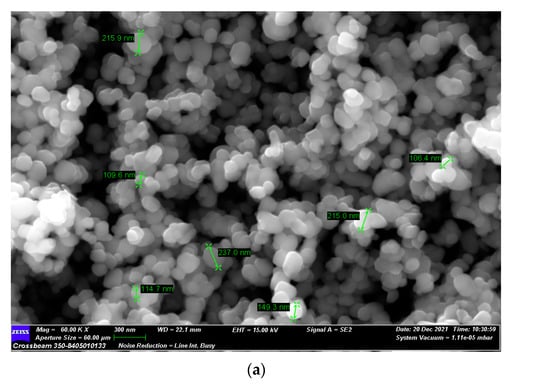
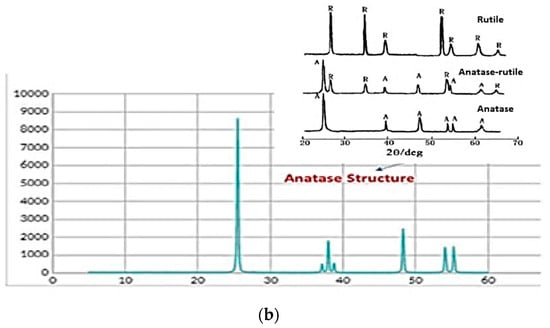
Figure 3.
The structure analysis of commercial TiO2: (a) SEM picture and (b) XRD diffraction pattern.
The titanium dioxide powder was used to prepare the suspensions for the dip-coating of the commercial tubular alumina membranes. The preparation of a stable suspension is very difficult, because the particles in the aqueous medium tend to aggregate, owing to van der Waals attractive forces. This determines the formation of clusters and their subsequent precipitation. There are several methods to suppress the agglomeration of the nanoparticles in a suspension, such as electrostatic or steric stabilization [37,38]. The electrostatic stabilization is obtained by changing the pH, and in this case, the van der Waals force attractions are counterbalanced by the repulsive Coulomb forces. The steric stabilization method involves using some additives (e.g., polymers) and their adsorption on the surface of the NPs prevents their agglomeration [39]. Considering these aspects, different suspensions were prepared (see Table 1), and the TSI trend over time for each of them was evaluated. The TSI values obtained for the suspensions prepared without additives are reported in Table 2.

Table 2.
TSI for the TiO2 suspensions prepared without the additive.
TSI values decreased with decreasing titanium dioxide concentrations, indicating higher stability, due to a reduction of TiO2 agglomerate formation (sedimentation and clarification phenomena suppression) [40,41]. Therefore, the suspension with a TiO2 concentration of 0.025 wt. % was the most stable. The stability of this suspension was further improved with the addition of nitric acid and PVP. The addition of the acid permitted an electrostatic stabilization, and the PVP addition permitted steric stabilization. The lowest TSI value was found for the suspension having a titanium dioxide concentration of 0.025 wt. %, and containing nitric acid (see Figure 4). The improved stability is related to the zeta potential (ZP) value of the suspension [42]. In particular, for ZP values close to zero (isoelectric point), the particles form agglomerates [43]. In contrast, for highly negative or positive values of ZP (more than +30 mV or less than −30 mV), the formation of agglomerates is avoided [43,44]. The zeta potential values for the suspensions (TiO2: 0.025 wt. %) prepared with and without additives are reported in Table 3. It is possible to observe that the acid’s presence determined a lowering of the zeta potential. In fact, the zeta potential value of this suspension was lower than −30 mV, and so the nanoparticles (negatively charged) repelled each other by electrostatic repulsion.
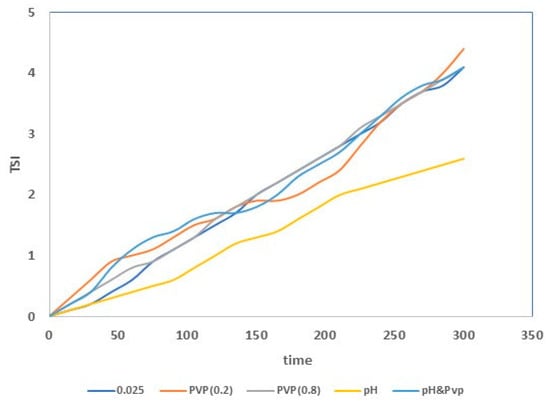
Figure 4.
TSI values for the different prepared suspensions.

Table 3.
Zeta potential values for TiO2 suspensions with and without additives.
In addition, considering that the zero charge point of α-Al2O3 is 9.0 [45], the use of a suspension having a pH of four enabled the electrostatic interaction between alumina and titanium dioxide during the dip-coating. The SEM pictures of the pristine and photocatalytic membranes are reported in Figure 5.
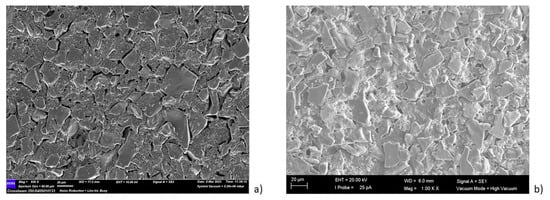
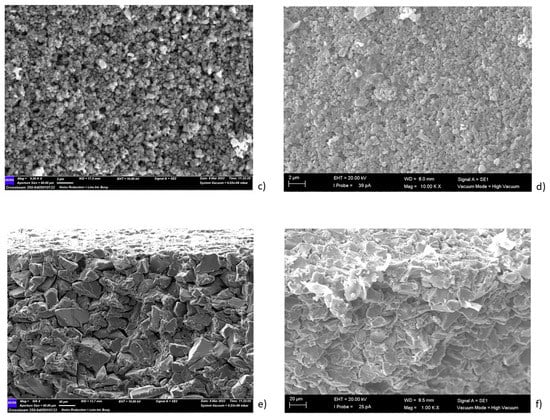
Figure 5.
Morphological analysis of the (a) external surface, (c) internal layer and (e) cross-section of the pristine membrane; (b) the external surface, (d) internal layer and (f) cross-section of the photocatalytic membranes.
The tubular α-Al2O3 membranes exhibited an asymmetric structure. In particular, an external layer with an average pore size of 3 μm and a separate layer with a pore size of about 0.2 µm were detected. The SEM analyses of the photocatalytic membranes showed the presence of the TiO2 nanoparticles, which were mainly on the external surface. The EDX analyses confirmed this result. In fact, it was shown that the titanium dioxide was mainly present on the surface, and decreased along the cross-section (see Figure 6).
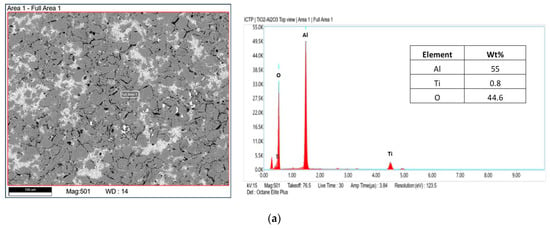
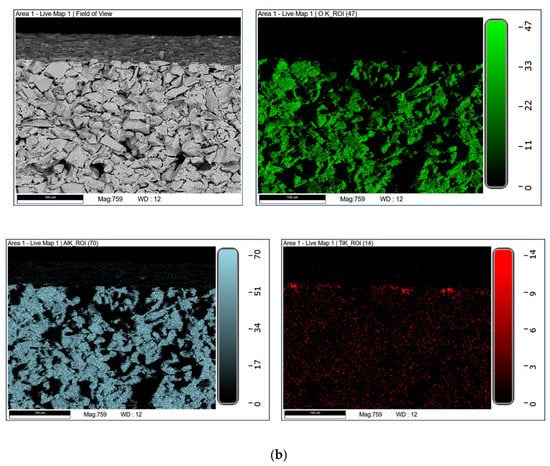
Figure 6.
EDX analysis of the photocatalytic membrane; (a) top-view and (b) cross-section.
The hydraulic permeance of pristine membranes is about 1500 Lm−2 h−1 bar−1, and after the dip coating, the membranes’ flux decreased by about 11%. The dip-coating method permitted the formation of the photocatalytic layer, mainly on the surface of the macroporous alumina membrane, with the possibility of avoiding a large reduction of the water permeability. A comparison with literature data, in terms of photocatalytic inorganic membranes prepared with the dip-coating method, is reported in Table 4.

Table 4.
Comparison with the water permeabilities of different inorganic photocatalytic membranes.
A significant loss of permeability indicates the plugging of titanium dioxide into the pores of the alumina membranes [46,47,48] (coating of the surface is characterized by a small pore size). A smaller permeability decrease was observed when coating the membrane’s external surface (macroporus structure). In this case, it is possible to minimize the porosity reduction of the membrane (ensuring high fluxes), and, at the same, to preserve its separative layer.
The photocatalytic activity of the prepared membranes was studied using the methyl orange a recalcitrant and carcinogenic azo dye. In Figure 7a, the MO degradation is shown as a function of time, obtained using a PMR operating in a continuous cross-flow mode. It is possible to observe that the degradation percentage increased by enhancing the UV exposure time. The dye degradation was 36% across the interval of time considered. The photocatalytic reaction was performed without UV irradiation for the first 30 min. In this case, a slight decrease in the MO concentration was detected due to the adsorption of MO on the surface of the alumina membrane. The adsorption between the MO and the alumina is favored, considering their zero charge points (MO = 2.5 [49] and α-Al2O3 = 9.0 [45]), and the pH (6.5) of the feed solution. The decrease of the MO concentration, achieved by turning on the UV light, indicates the positive effect of the generation of reactive species by the photocatalyst under UV irradiation. No photocatalytic degradation of MO was observed when a pristine membrane was used. The reusability of the photocatalytic membranes was tested by comparing the MO degradation during four successive reactions, each 4 h in duration; after each reaction, the plant was washed with distilled water. The obtained results are reported in Figure 7b. The experimental data showing the photocatalytic activity slightly decreased, which was probably due to the accumulation of organic materials (formed during the reaction) on the active sites of the TiO2 nanoparticles. Each repeated experiment exhibited a loss of efficiency of about 1%.
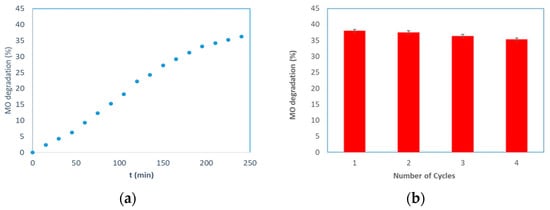
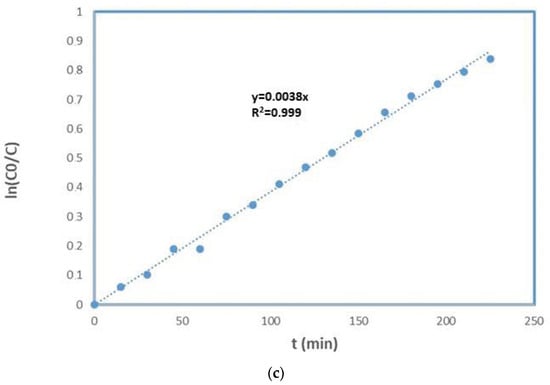
Figure 7.
MO degradation by using the TiO2–Al2O3 membranes: (a) MO degradation versus time. (b) Reusability of the TiO2–Al2O3 membrane. (b) MO degradation after four successive reactions. Kinetics of methyl orange photodegradation: (c) ln(C0/C) versus time for the MO degradation. Operating conditions: C0 = 1 mgL−1; Volume of MO solution = 1 L; Stirring rate = 3 rpm; T = 25 °C; UV energy = 365 nm.
For evaluating the kinetic degradation of the MO, the experimental data were plotted versus time considering the Equations (7)–(9). A linear plot was observed by plotting the ln(C0/Ct) versus time (R21), indicating that the degradation reaction of methyl orange followed the pseudo-first-order kinetic model [50,51] (see Figure 7c). In different papers, the same result was also found [52,53,54]. Finally, a comparison with literature data is reported in Table 5.

Table 5.
Comparison with literature data.
A comparison with literature data showed that the amount of TiO2 immobilized into the porous structure of the alumina tubular membrane influenced the performance of the process. Small or large amounts of photocatalytic particles negatively influenced the degradation process. This is explained, considering that a higher amount determines a reduction of both surface area and light absorption capacity, due to the formation of agglomerates [55,56]. In addition, if the amount of photocatalytic NPs is small, the numbers of active sites decrease, with a reduction of the MO degradation [57].
This work presents a key factor in the preparation of the photocatalytic membranes, by using the dip-coating to improve the stability of the suspension. In fact, the use of a stable suspension (in the interval time used for the membrane preparation) permits us to reduce the agglomerate formation and to achieve a better dispersion of the TiO2 NPs in the porous structure of the membranes ensure a better contact between dyes and catalytic sites.
4. Conclusions
In this work, the Turbiscan stability index was used for the first time to evaluate the stability of the suspensions used for preparing the photocatalytic membranes by the dip-coating method. The dip-coating, performed on the external surface of the alumina membranes, permitted the preparation of high-flux photocatalytic membranes. The photocatalytic performance of the membranes has been examined in a photocatalytic membrane reactor (PMR) under UV irradiation, and using methyl orange as a model recalcitrant dye. A degradation of 36% was detected after 4 h of reaction. The reusability of the photocatalytic membranes was also studied, and each repeated experiment exhibited a low-efficiency loss. Photodegradation of MO followed the pseudo-first-order kinetic model.
Author Contributions
Methodology, M.B. and C.A.; investigation, M.B. and C.A.; conceptualization, C.A. and V.C.; data curation, C.A., S.C. and V.C.; Supervision, C.A. and S.C.; writing-original draft preparation, C.A. and S.C.; review and editing: C.A. and S.C.; funding acquisition, V.C. and S.C.; project admistration: V.C. and S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by PRIMA Foundation under grant agreement no. [2024].
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data will be available on request.
Acknowledgments
This work has received funding from PRIMA Foundation under grant agreement no. Grant Agreement number: [2024] [TRUST]-Call 2020 Section 1 Water IA.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Sharma, S.; Bhattacharya, A. Drinking water contamination and treatment techniques. Appl. Water Sci. 2017, 7, 1043–1067. [Google Scholar] [CrossRef]
- Adams, J.B.; Rubidge, G. The state of persistent organic pollutants in South African estuaries: A review of environmental exposure and source. Ecotoxicol. Environ. Saf. 2021, 219, 112316. [Google Scholar] [CrossRef]
- Rafaqata, S.; Ali, N.; Torres, C.; Rittmann, B. Recent progress in treatment of dyes wastewater using microbial-electro-Fenton technology. RSC Adv. 2022, 12, 17104–17137. [Google Scholar] [CrossRef] [PubMed]
- Eltaboni, F.; Bader, N.; El-Kailany, R.; Elsharif, N.; Ahmida, A. Chemistry and applications of azo dyes: A comprehensive review. J. Chem. Rev. 2022, 4, 313–330. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef]
- Kurian, M. Advanced oxidation processes and nanomaterials-a review. Clean. Eng. Technol. 2021, 2, 100090. [Google Scholar] [CrossRef]
- García-Rodríguez, O.; Banuelos, J.A.; Rico-Zavala, A.; Godínez, L.A.; Rodríguez-Valadez, F.J. Electrocatalytic activity of three carbon materials for the In-situ production of hydrogen peroxide and Its application to the electro-Fenton heterogeneous process. Int. J. Chem. React. Eng. 2016, 14, 843–850. [Google Scholar] [CrossRef]
- Titchou, F.E.; Zazou, H.; Afanga, H.; Gaayda, J.E.; Akbour, R.A.; Nidheesh, P.V.; Hamdan, M. Removal of organic pollutants from wastewater by advanced oxidation processes and its combination with membrane processes. Chem. Eng. Process. Process Intensif. 2021, 169, 108631. [Google Scholar] [CrossRef]
- Molinari, R.; Lavorato, C.; Argurio, P. Photocatalytic reduction of acetophenone in membrane reactors under UV and visible light using TiO2 and Pd/TiO2 catalysts. Chem. Eng. J. 2015, 274, 307–316. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations: A review. Appl. Catal. B 2004, 491, 1–14. [Google Scholar] [CrossRef]
- Dave, S.; Das, J. Technological model on advanced stages of oxidation of wastewater effluent from food industry. In Advanced Oxidation Processes for Effluent Treatment Plants; Shah, M.P., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 33–49. [Google Scholar]
- Gao, B.; Yap, P.S.; Lim, T.M.; Lim, T.T. Adsorption-photocatalytic degradation of Acid Red 88 by supported TiO2: Effect of activated carbon support and aqueous anions. Chem. Eng. 2011, 171, 1098–1107. [Google Scholar] [CrossRef]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, P.; Wang, H. Photocatalytic membrane reactors for produced water treatment and reuse: Fundamentals, affecting factors, rational design, and evaluation metrics. J. Hazard. Mater. 2022, 15, 127493. [Google Scholar] [CrossRef] [PubMed]
- Ashley, A.; Thrope, B.; Choudhury, M.R.; Pinto, A.H. Emerging investigator series: Photocatalytic membrane reactors: Fundamentals and advances in preparation and application in wastewater treatment. Environ. Sci. Water Res. Technol. 2022, 8, 22–46. [Google Scholar] [CrossRef]
- Mozia, S. Photocatalytic membrane reactors (PMRs) in water and wastewater treatment. A review. Sep. Purif. Technol. 2010, 73, 71–91. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Zhang, J.; Wang, Z.; Xu, L.; Fan, Z. Influence of azo dye-TiO2 interactions on the filtration performance in a hybrid photocatalysis/ultrafiltration process. J. Colloid Interface Sci. 2013, 389, 273–283. [Google Scholar] [CrossRef]
- Molinari, R.; Lavorato, C.; Argurio, P. The Evolution of Photocatalytic Membrane Reactors over the Last 20 Years: A State of the Art Perspective. Catalysts 2021, 11, 775. [Google Scholar] [CrossRef]
- Algieri, C.; Drioli, E. Zeolite membranes: Synthesis and applications. Sep. Purif. Technol. 2022, 278, 119295. [Google Scholar] [CrossRef]
- Singh, R.; Hankins, N.P. Introduction to Membrane Processes for Water Treatment. In Emerging Membrane Technology for Sustainable Water Treatment; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Athanasekou, C.P.; Romanos, G.E.; Katsaros, F.K.; Kordatos, K.; Likodimos, V.; Falaras, P. Very efficient composite titania membranes in hybrid ultrafiltration/photocatalysis water treatment processes. J. Membr. Sci. 2012, 392–393, 192–203. [Google Scholar] [CrossRef]
- Pereira, V.R.; Isloor, A.M.; Zulhairun, A.K.; Subramaniam, M.N.; Lau, W.J.; Ismail, A.F. Preparation of polysulfone-based PANI–TiO2 nanocomposite hollow fiber membranes for industrial dye rejection applications. RSC Adv. 2016, 6, 99764–99773. [Google Scholar] [CrossRef]
- Erdei, L.; Arecrachakul, N.; Vigneswaran, S. A combined photocatalytic slurry reactor-immersed membrane module system for advanced wastewater treatment. Sep. Purif. Technol. 2008, 62, 382–388. [Google Scholar] [CrossRef]
- Romanos, G.E.; Athanasekou, C.P.; Katsaros, F.K.; Kanellopoulos, N.K.; Dionysiou, D.D.; Likodimos, V.; Falaras, P. Double-side active TiO2-modified nanofiltration membranes in continuous flow photocatalytic reactors for effective water purification. J. Hazard. Mater. 2012, 211–212, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.B.; Zhang, Z. Ceramic membrane technology for water and wastewater treatment: A critical review of performance, full-scale applications, membrane fouling and prospects. Chem. Eng. J. 2021, 418, 129481. [Google Scholar] [CrossRef]
- Algieri, C.; Macedonio, F.; Drioli, E. Zeolite Membranes for Desalination in Sustainable Materials and Systems for Water Desalination; Inamuddin, A.K., Ed.; Springer: Cham, Switzerland, 2021; ISBN 978-3-030-72873-1. [Google Scholar]
- Deepracha, S.; Atfane, L.; Ayral, A.; Ogawa, M. Simple and efficient method for functionalizing photocatalytic ceramic membranes and assessment of its applicability for wastewater treatment in up-scalable membrane reactors. Sep. Purif. Technol. 2021, 262, 118307. [Google Scholar] [CrossRef]
- Phattepur, H.; Hiremath, P.G. Fabrication of Al2O3 supported TiO2 membranes for photocatalytic applications. Mater. Today Proc. 2002, 65, 3694–3699. [Google Scholar] [CrossRef]
- Ting, P.H.; Jing, J.F.; Zhenglong, Y.; Biao, Y.; Xin, L. Effects of polyvinylpyrrolidone and carbon nanotubes on magnetorheological properties of iron-based magnetorheological fluids. J. Appl. Polym. Sci. 2006, 102, 1653–1657. [Google Scholar] [CrossRef]
- De Paola, M.G.; Paletta, R.; Lopresto, C.G.; Lio, G.E.; De Luca, A.; Chakraborty, S.; Calabrò, V. Stability of film-forming dispersions: Affects the morphology and optical properties of polymeric films. Polymers 2021, 13, 1464. [Google Scholar] [CrossRef]
- De Paola, M.G.; Arcuri, N.; Calabro, V.; De Simone, M. Thermal and stability investigation of phase change material dispersions for thermal energy storage by T-history and optical methods. Energies 2017, 10, 354. [Google Scholar] [CrossRef]
- Asiri, A.M.; Petrosino, F.; Pugliese, V.; Khan, S.B.; Alamry, K.A.; Alfifi, S.Y.; Marwani, H.M.; Alotaibi, M.M.; Algieri, C.; Chakraborty, S. Synthesis and characterization of blended cellulose acetate membranes. Polymers 2022, 14, 4. [Google Scholar] [CrossRef]
- Kanan, S.; Moyet, M.A.; Arthur, R.B.; Patterson, H.H. Recent advances on TiO2-based photocatalysts toward the degradation of pesticides and major organic pollutants from water bodies. Catal. Rev. Sci. Eng. 2020, 62, 1–65. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Jedrzejczak, A.; Batory, D.; Cichomski, M.; Miletic, A.; Czerniak-Reczulska, M.; Niedzielski, P.; Dudeka, M. Formation of anatase and srilankite mixture as a result of the thermally induced transformation of the a-C:H:TiOx coating. Surf. Coat. Technol. 2020, 400, 126230. [Google Scholar] [CrossRef]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why is anatase a better photocatalyst than rutile?–Model studies on epitaxial TiO2 films. Sci. Rep. 2014, 4, 44043. [Google Scholar] [CrossRef] [PubMed]
- Satoru, I.; Chu, S.Z.; Wada, K.; Li, D.; Haneda, H. New roots to formation of nanostructures on glass surface through anodic oxidation of sputtered aluminum. Sci. Technol. Adv. Mater. 2003, 4, 269–276. [Google Scholar] [CrossRef]
- Othman, H.; Rashid, S.A.; Ghazi, T.I.M.; Abdullah, N. Dispersion and stabilization of photocatalytic TiO2 nanoparticles in aqueous suspension for coatings applications. J. Nanomater. 2012, 718214. [Google Scholar] [CrossRef]
- Huertas, S.P.; Terpiłowski, K.; Wiśniewska, M.; Zarko, V. Influence of polyvinylpyrrolidone adsorption on stability of silica aqueous suspension–effects of polymer concentration and solid content. Physicochem. Probl. Miner. Process. 2017, 53, 121–135. [Google Scholar] [CrossRef]
- Candamano, S.; Sgambitterra, E.; Lamuta, C.; Pagnotta, L.; Chakraborty, S.; Crea, F. Graphene nanoplatelets in geopolymeric systems: A new dimension of nanocomposites. Mater. Lett. 2019, 236, 550–553. [Google Scholar] [CrossRef]
- Jiang, J.; Oberdörster, G.; Biswas, P. Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J. Nanopart. Res. 2009, 11, 77–89. [Google Scholar] [CrossRef]
- De Paola, M.G.; Lopresto, C.G. Waste oils and their transesterification products as novel bio-based phase change materials. J. Phase Change Mater. 2021, 1, 1. [Google Scholar] [CrossRef]
- Rangan, K.; Forbesa, H.; Kumar, N. Inorganic salt hydrates-hydrogel composites as phase change materials for energy storage in buildings. J. Phase Change Mater. 2022, 2, 25–40. [Google Scholar] [CrossRef]
- Meißner, T.; Potthoff, A.; Richter, V. Suspension characterization as important key for toxicological investigations. J. Phys. Conf. Ser. 2009, 170, 012012. [Google Scholar] [CrossRef]
- Franksm, G.V.; Meagher, L. The isoelectric points of sapphire crystals and alpha-alumina powder. Coll. Surf. A Physicochem. Eng. Asp. 2003, 214, 99–110. [Google Scholar] [CrossRef]
- Djafer, L.; Ayral, A.; Ouagued, A. Robust synthesis and performance of a titania based ultrafiltration membrane with photocatalytic properties. Sep. Purif. Technol. 2010, 75, 198–203. [Google Scholar] [CrossRef]
- Grilli, R.; Di Camillo, D.; Lozzi, L.; Horovitz, I.; Mamane, H.; Avisar, D.; Baker, M.A. Surface Characterization and photocatalytic performance of N-doped TiO2 thin films deposited onto 200 nm pore size alumina membranes by sol–gel methods. Mater. Chem. Phys. 2015, 159, 25–37. [Google Scholar] [CrossRef]
- Ma, N.; Quan, X.; Zhang, Y.; Chen, S.; Zhao, H. Integration of separation and photocatalysis using an inorganic membrane modified with Si-doped TiO2 for water purification. J. Membr. Sci. 2009, 335, 58–67. [Google Scholar] [CrossRef]
- Lau, Y.Y.; Wong, Y.S.; Teng, T.T.; Morad, N.; Rafatullah, M.; Ong, S.A. Degradation of cationic and anionic dyes in coagulation–flocculation process using bi-functionalized silica hybrid with aluminum-ferric as auxiliary agent. RSC Adv. 2015, 42, 34206. [Google Scholar] [CrossRef]
- Hir, Z.A.M.; Moradihamedani, P.; Abdullah, A.H.; Azuwa, M. Immobilization of TiO2 into polyethersulfone matrix as hybrid film photocatalyst for effective degradation of methyl orange dye. Mater. Sci. Semicond. Process. 2017, 57, 157. [Google Scholar] [CrossRef]
- Romanos, G.E.; Athanasekou, C.P.; Likodimos, V.; Aloupogiannis, P.; Falaras, P. Hybrid Ultrafiltration/Photocatalytic Membranes for Efficient Water Treatment. Ind. Eng. Chem. Res. 2013, 52, 13938–13947. [Google Scholar] [CrossRef]
- Moustakas, N.G.; Katsaros, F.K.; Kontos, A.G.; Romanos, G.E.; Dionysiou, D.D.; Falaras, P. Visible light active TiO2 photocatalytic filtration membranes with improved permeability and low energy consumption. Catal. Today 2014, 224, 56–69. [Google Scholar] [CrossRef]
- Curcio, S. Applications of ANNs on photocatalytic reactors: A brief overview. J. Phase Change Mater. 2022, 2, 1–6. [Google Scholar] [CrossRef]
- Wang, X.; Shi, F.; Huang, W.; Fan, C. Synthesis of high quality TiO2 membranes on alum.ina supports and their photocatalytic activity. Thin Solid Films 2012, 520, 2488. [Google Scholar] [CrossRef]
- Mathumba, P.; Maziya, K.; Kuvarega, A.T.; Dlamini, L.N.; Malinga, S.P. Photocatalytic degradation of a basic dye in water by nanostructured HPEI/TiO2 containing membranes. Water SA 2020, 46, 500–505. [Google Scholar] [CrossRef]
- Lei, P.; Wang, F.; Gao, X.; Ding, Y.; Zhang, S.; Zhao, J.; Liu, S.; Yang, M. Immobilization of TiO2 nanoparticles in polymeric substrates by chemical bonding for multi-cycle photodegradation of organic pollutants. J. Hazard. Mater. 2012, 227–228, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Ouyang, L. Photocatalytic activity and hydroxyl radical formation of carbon-doped TiO2 nanocrystalline: Effect of calcination temperature. Chem. Eng. J. 2009, 148, 248–253. [Google Scholar] [CrossRef]
- Canute, S.; Nieto-Maestre, J.; Kanel, S. Developing Novel Electro-catalysts for Green Hydrogen Generation. J. Phase Change Mater. 2022, 2, 52–58. [Google Scholar] [CrossRef]
- Das, P.; Saha, S. Characterization of CdS, CdXZn1-XS and ZnS Nanocrystallites Grown by Chemical Route. J. Phase Change Mater. 2022, 2, 4–11. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).