Fabrication of a Zircon Microfiltration Membrane for Culture Medium Sterilization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
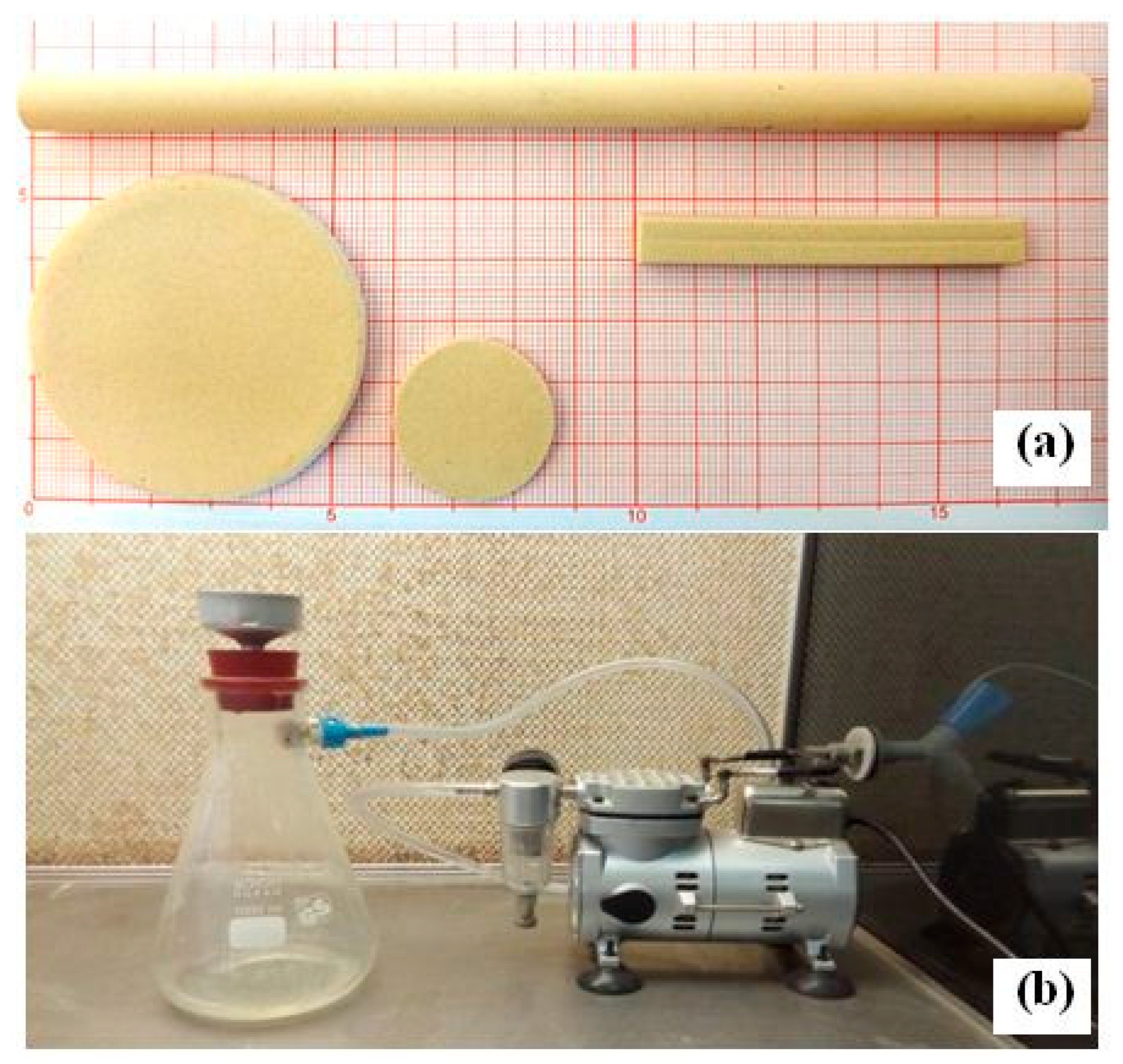
2.2. Production of the Supports
- -
- Tubular and flat rectangular supports were made by pressing the paste in a die, using a hydraulic press. The extruded tubes were placed on rotating rollers to cause the support to rotate so that they dried up and stayed straight. Tubular supports had the following dimensions: 6 mm (I.D.) and 10 mm (O.D.) for the inner and outer diameters, respectively, while the length of the supports was chosen according to our needs. They were used to study membrane features.
- -
- Rectangular samples (with 40 × 8 × 6 mm) were prepared using the extrusion technique. These samples were used to estimate mechanical properties.
- -
- Flat disc samples (with a diameter of 50 mm and a thickness of 2 mm) were prepared by hydrostatic pressure. They were used in the filtration tests.
2.3. Membranes Preparation
- Crushing of the powder and calibration at 40 μm by sieving;
- Dispersing the mineral powder (20 g) in distilled water (50 mL);
- Adding an aqueous solution of hydroxyethyl cellulose (30 g);
- Homogenizing by magnetic stirring followed by the deposition on a silica layer by using the slip casting method (the time of deposition is about 5 min);
- Drying followed by sintering at 1200 °C for 1 h at a heating rate of 5 °C/min.
2.4. Culture Medium Preparation and Sterilization
2.5. Characterization Techniques
3. Results and Discussion
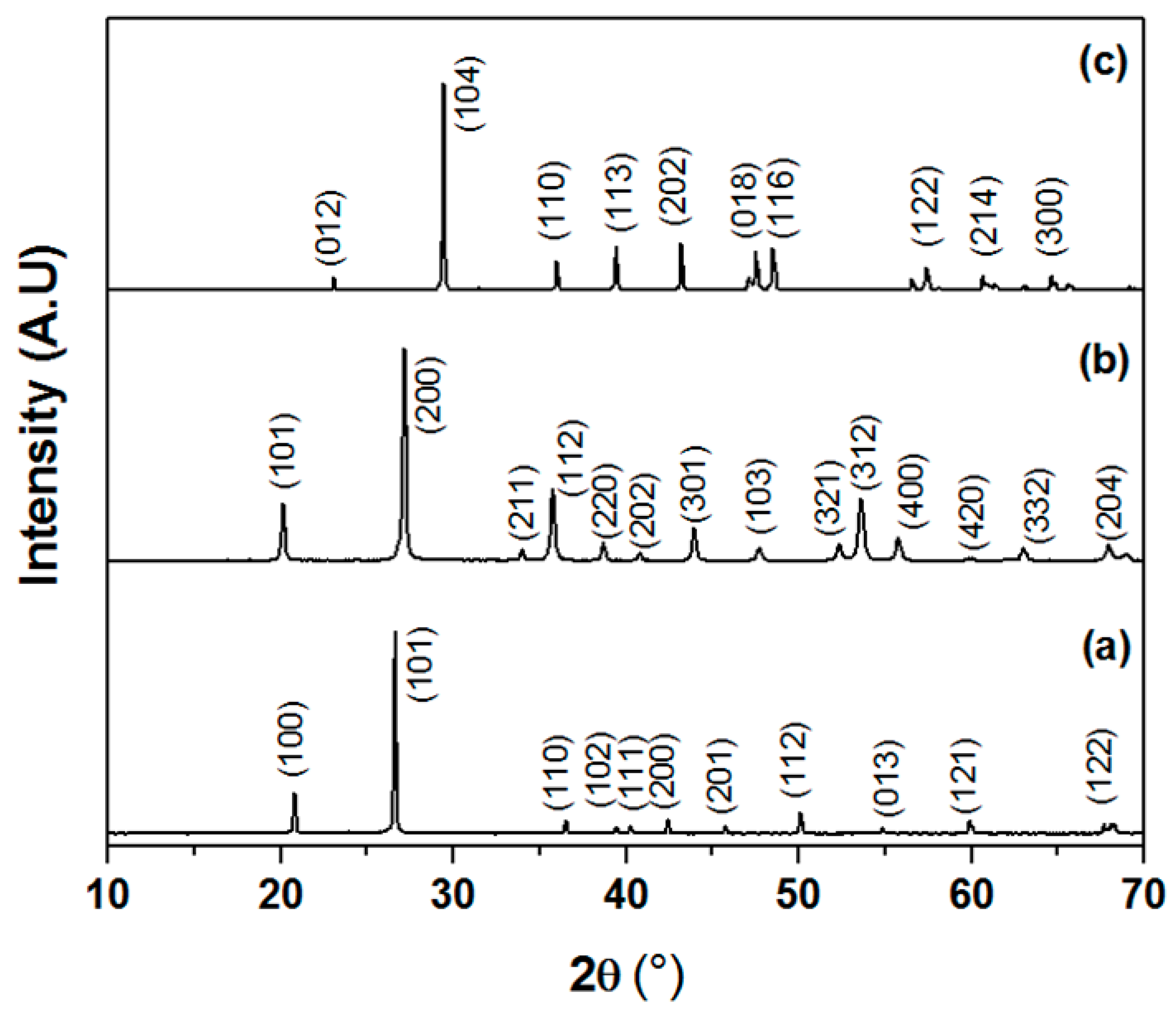
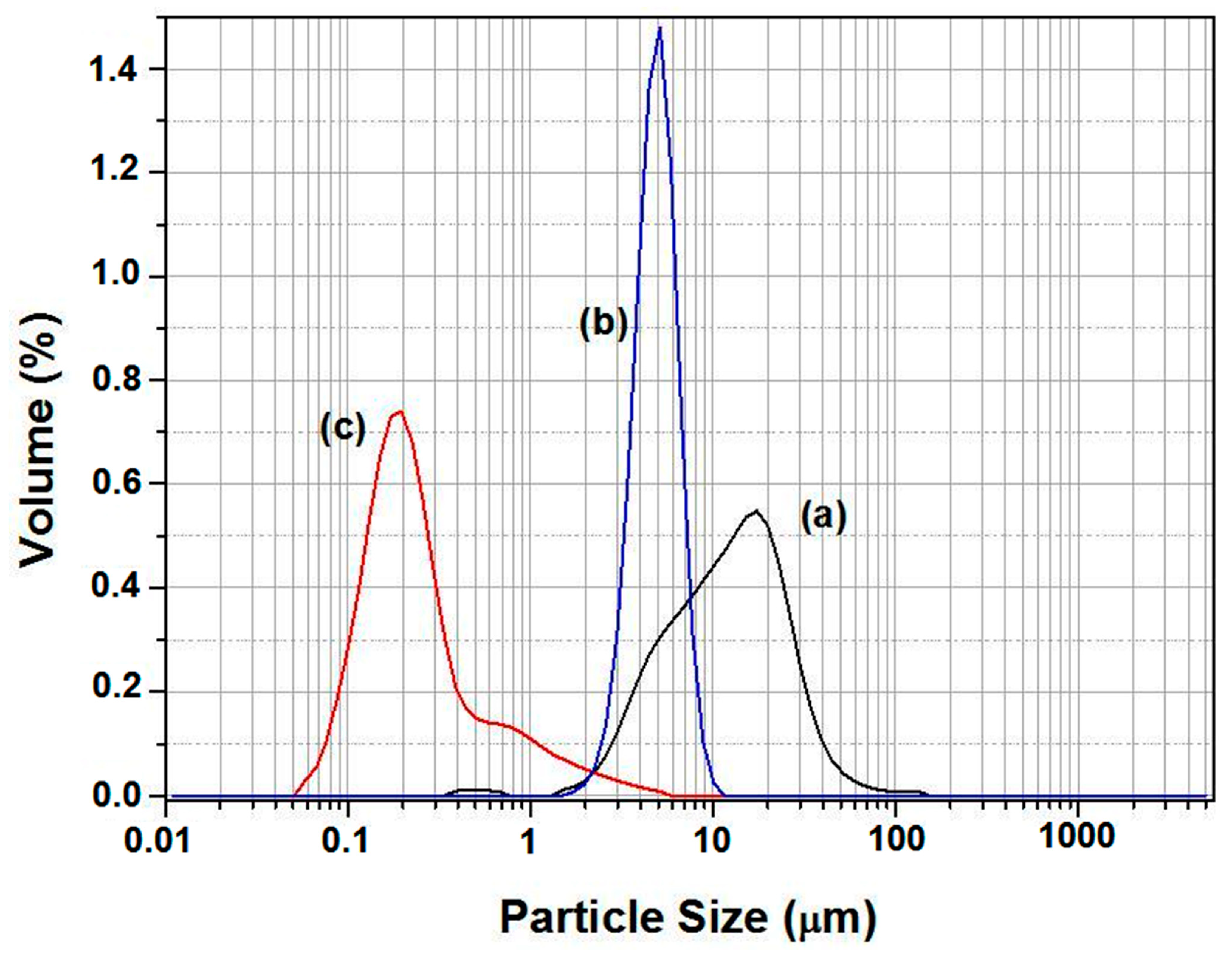
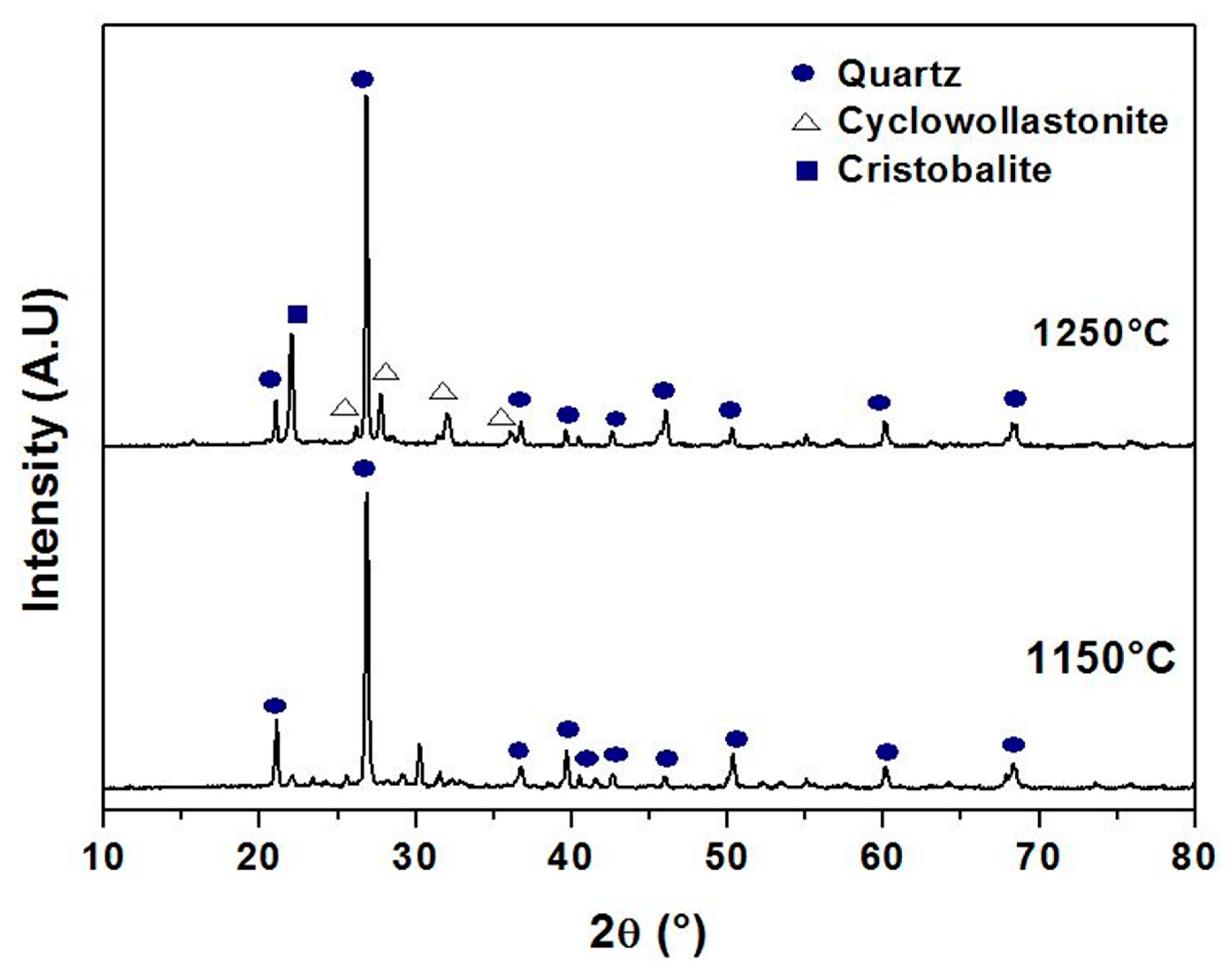
3.1. Raw Materials Characterization
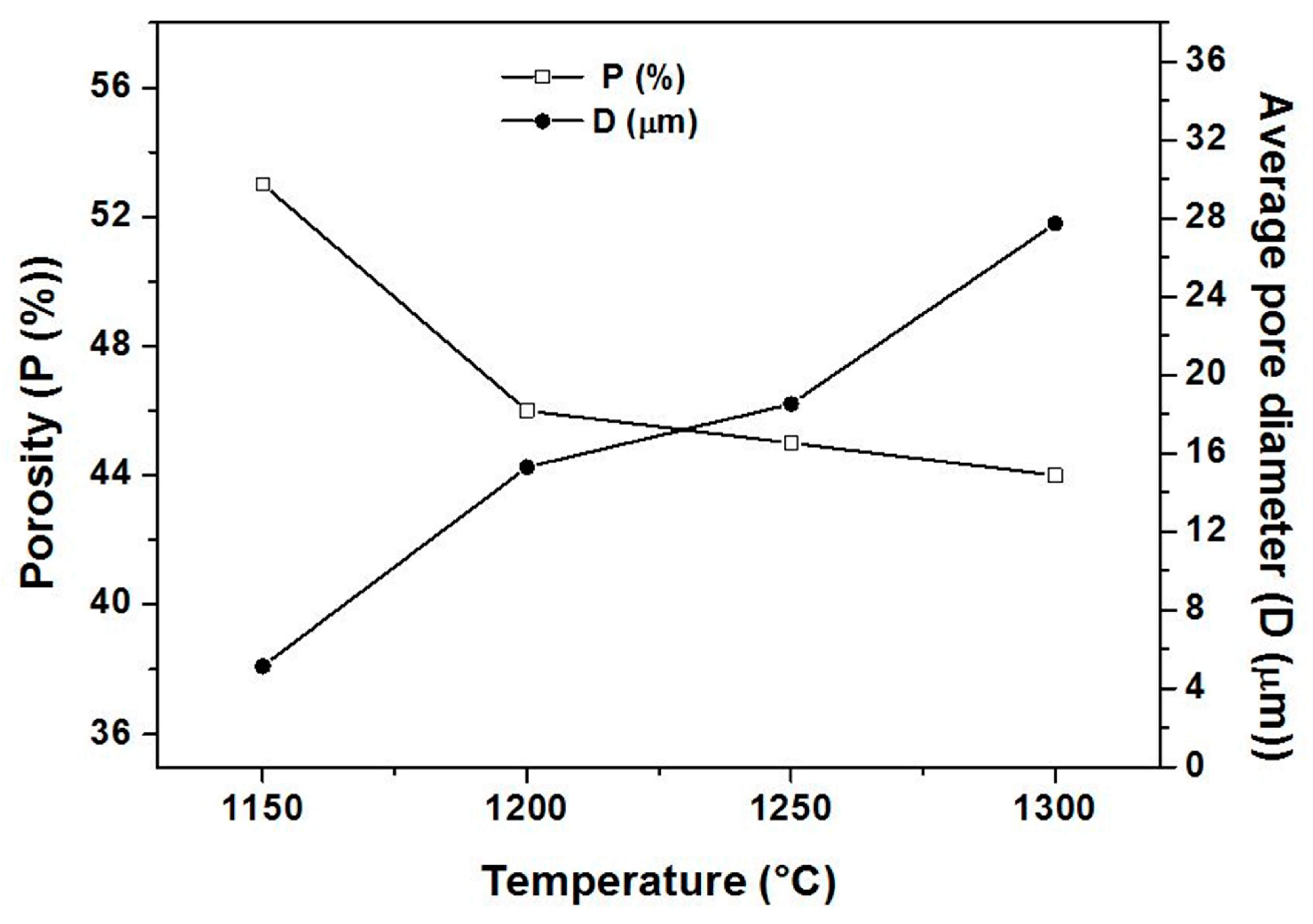
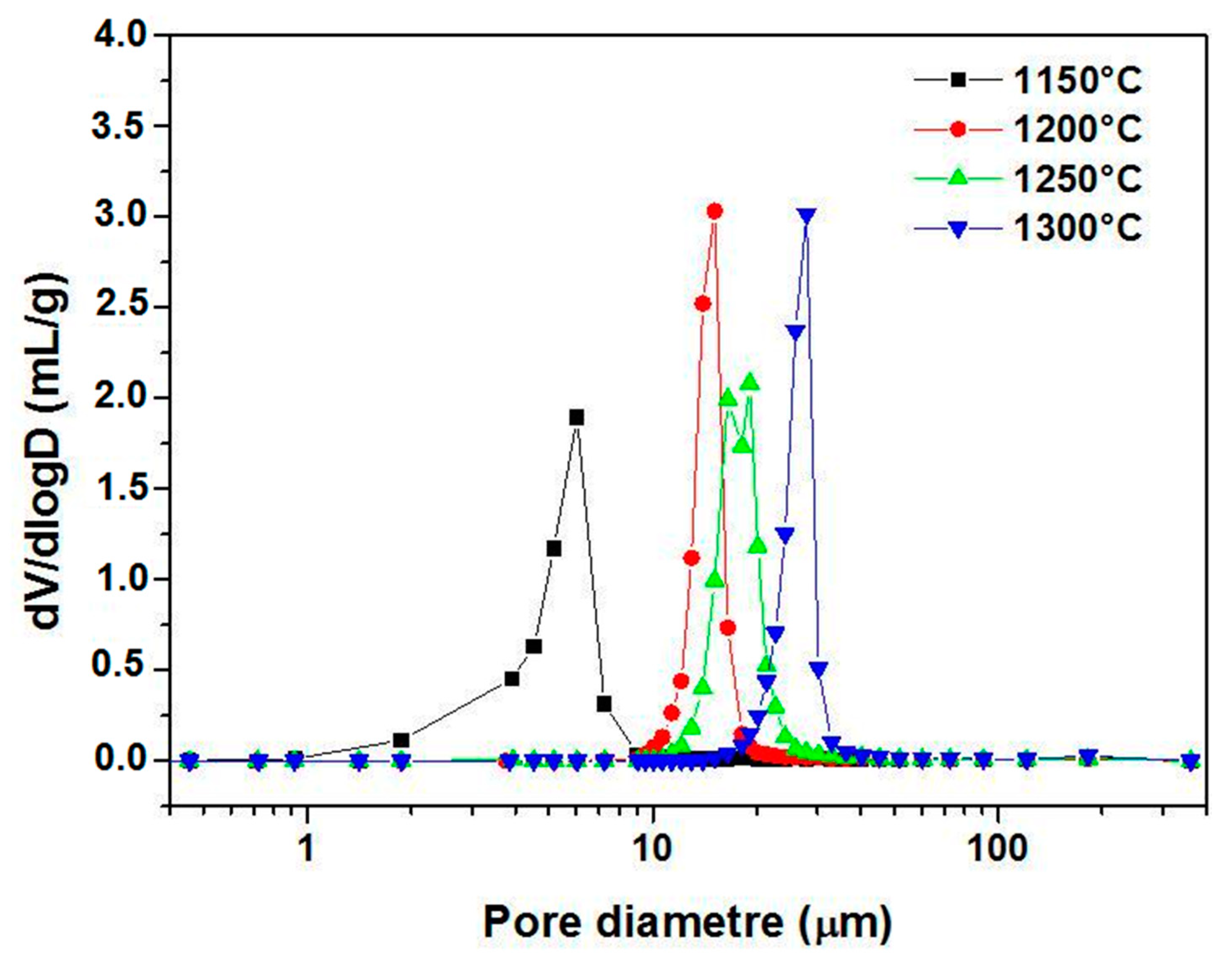
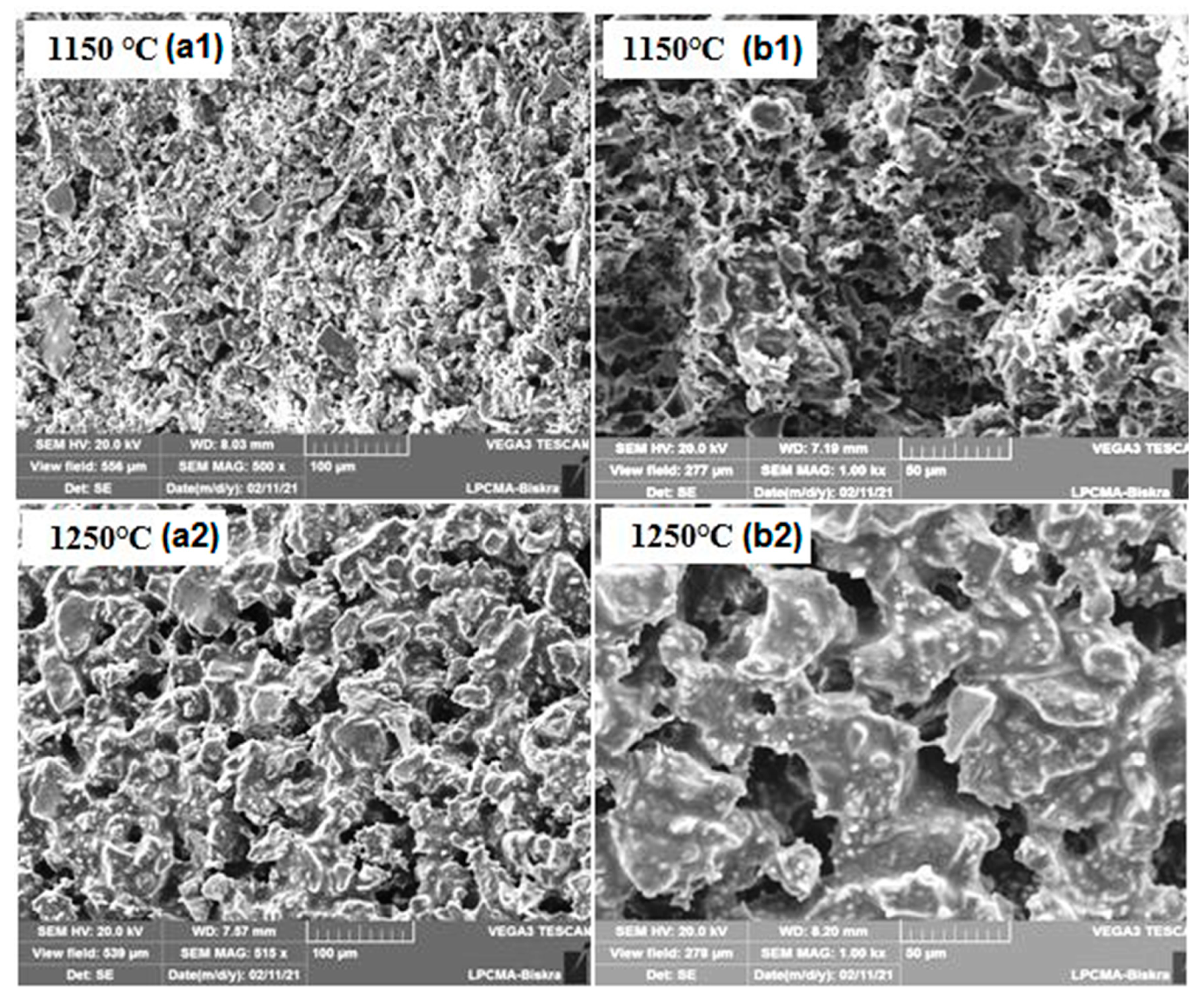
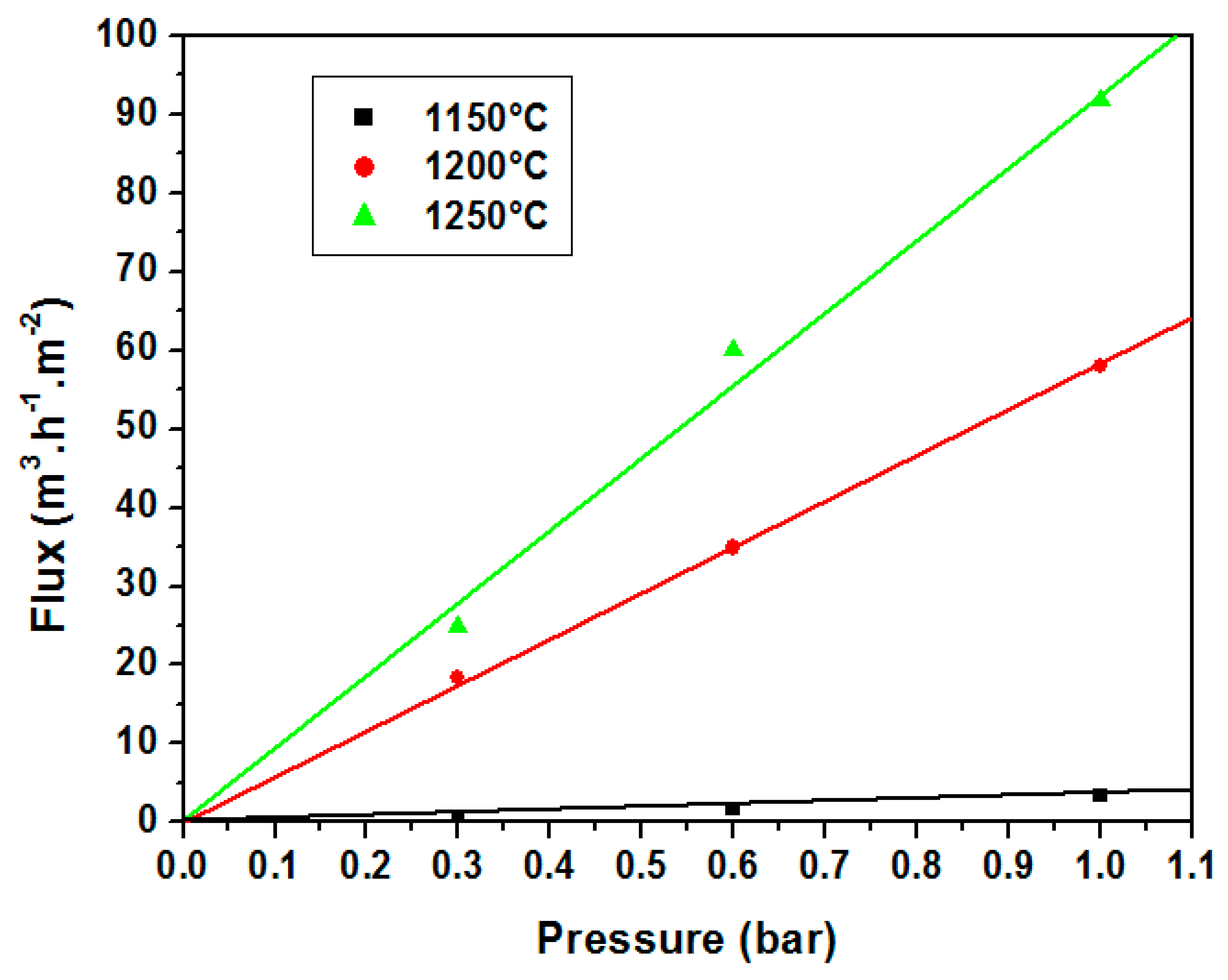
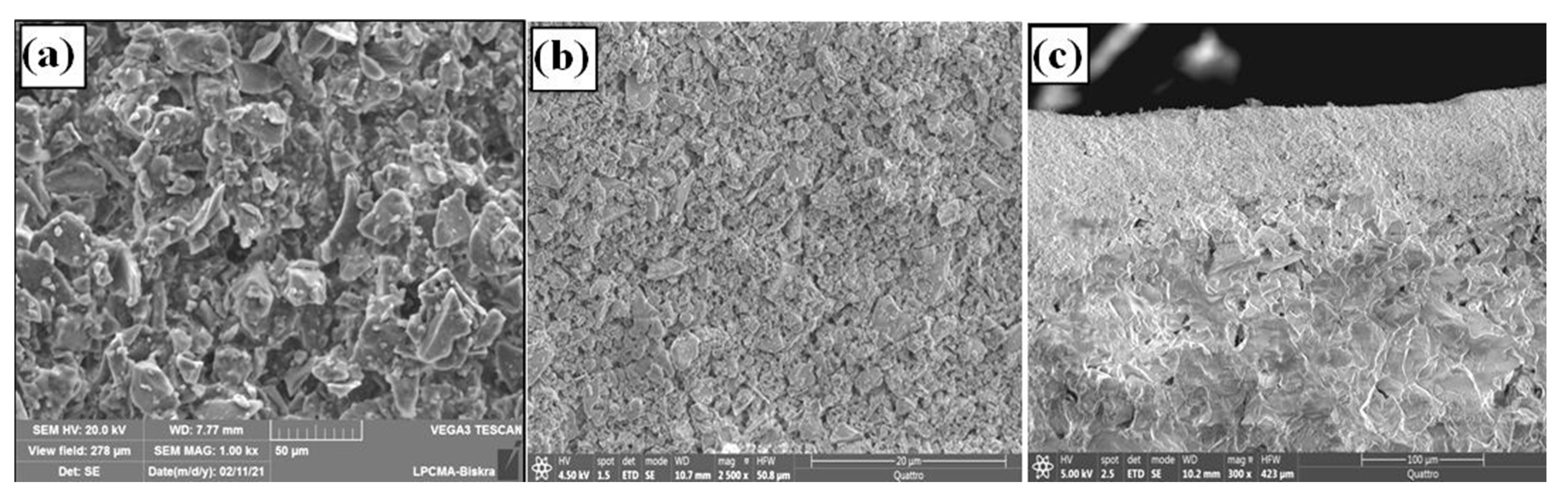
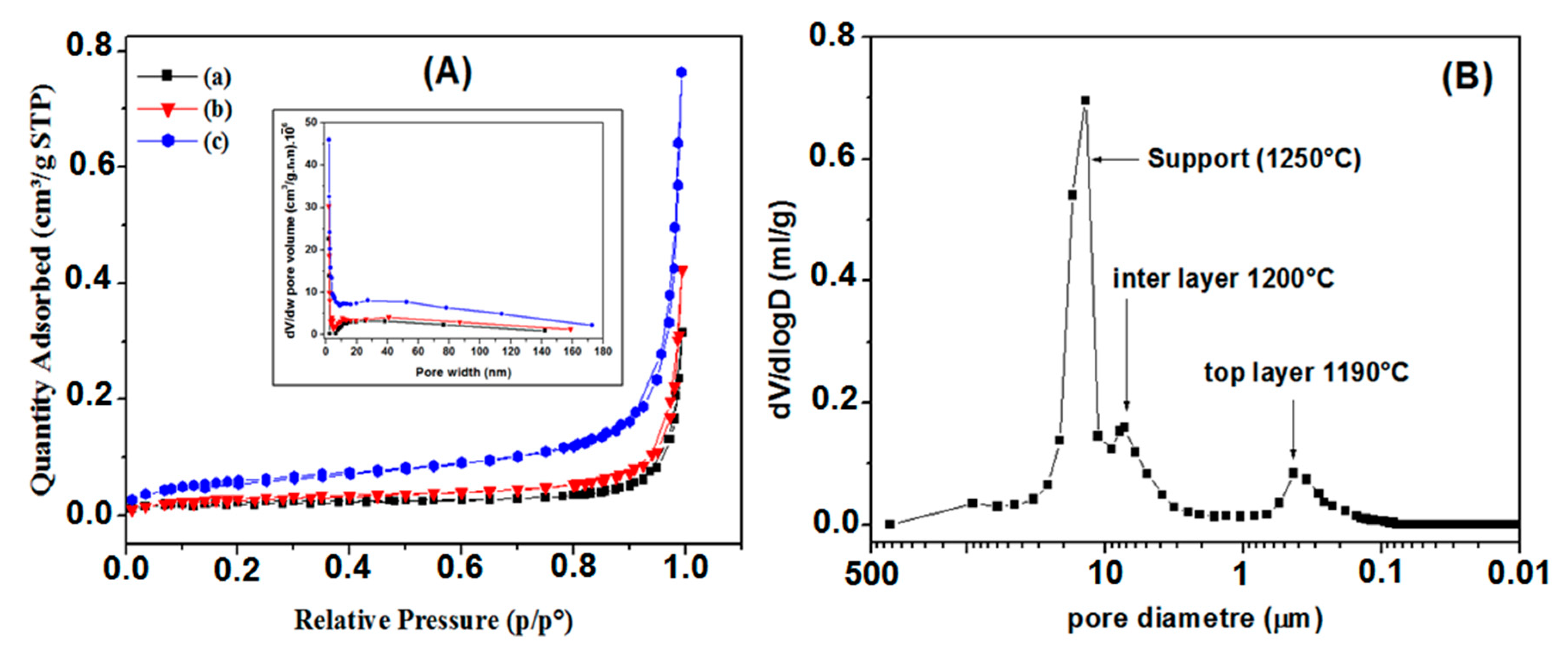
3.2. Supports Characterizations
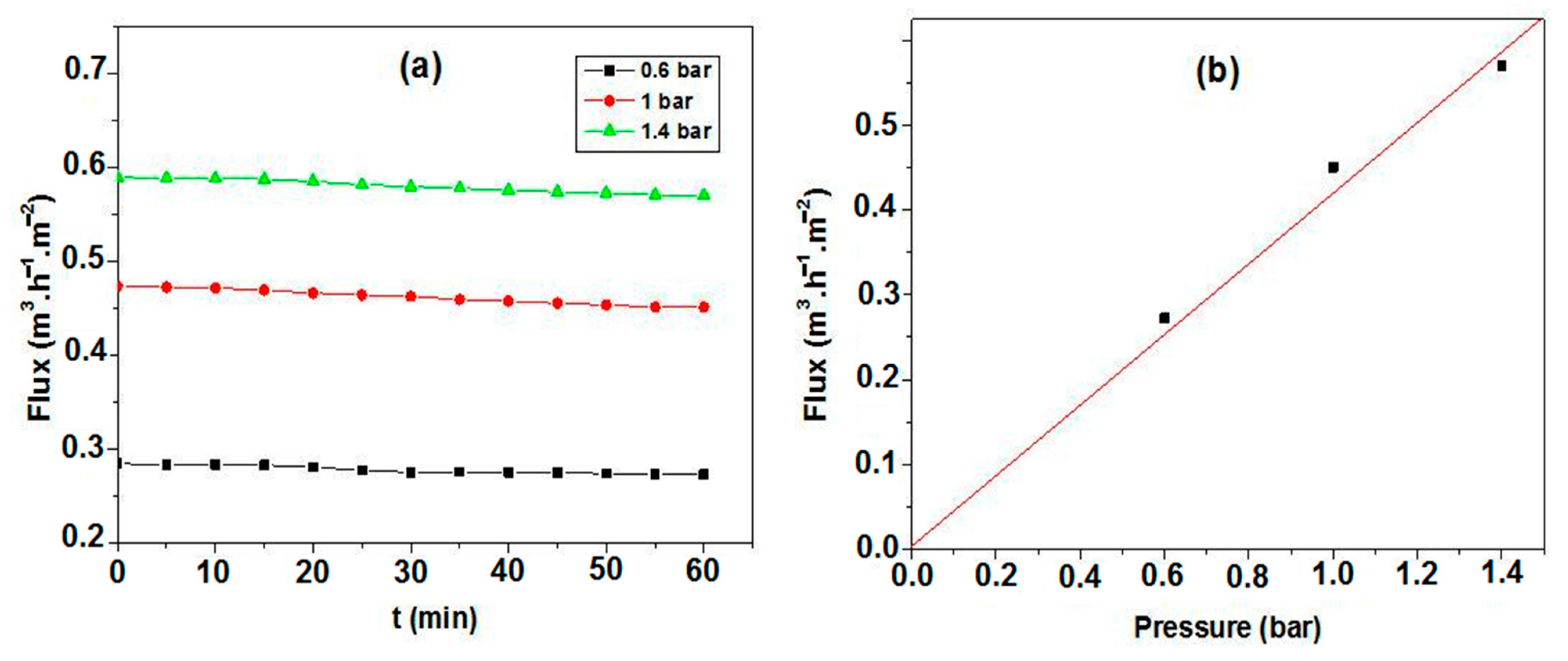
3.3. Membranes Characterization
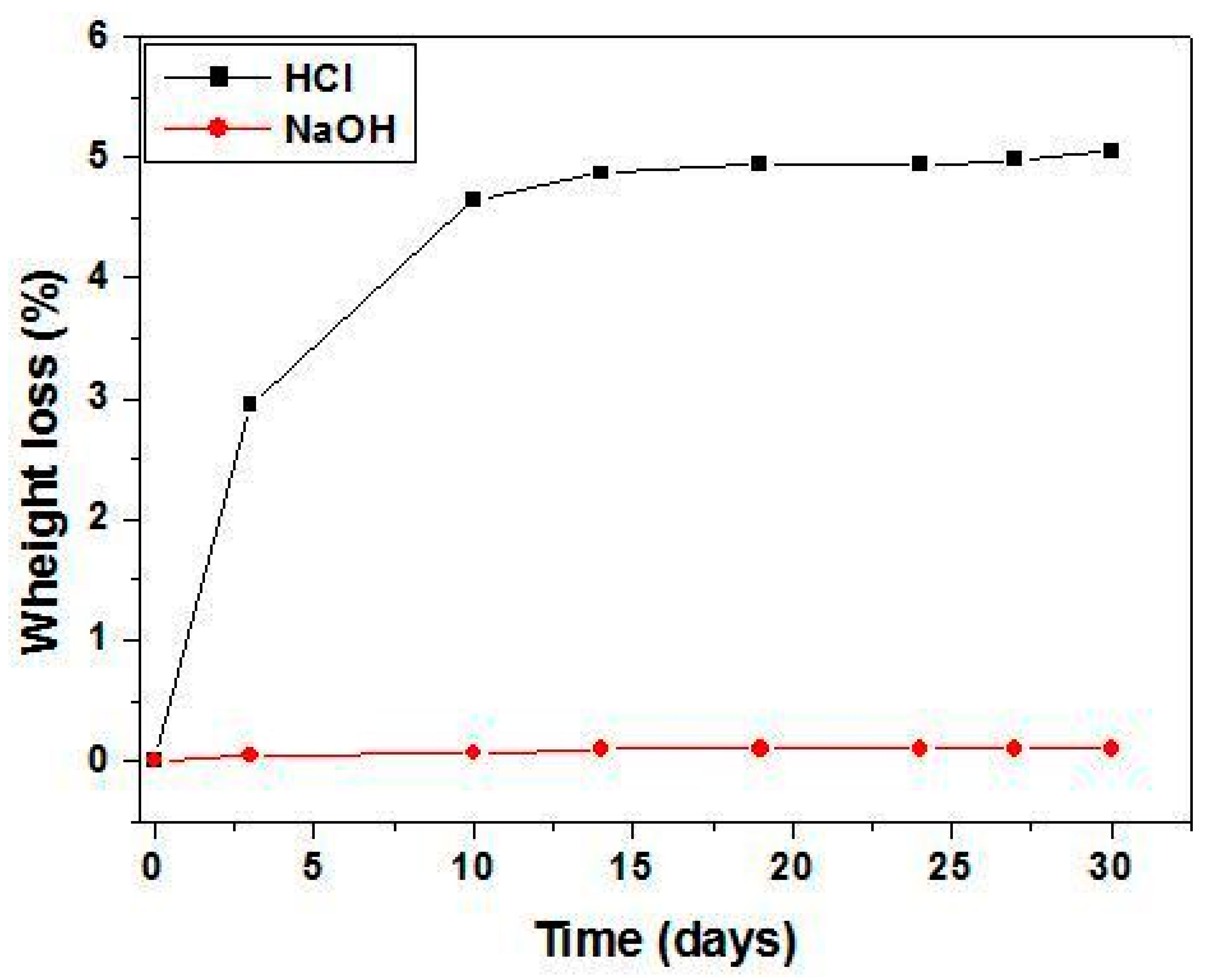
3.4. Efficient Sterilization by Membrane Filtration
4. Conclusions
- Average zircon layer thickness: 70 μm;
- Average pore size: 0.3 μm (suitable for MF application);
- Porous volume: 43%;
- Water permeability: 440 lh−1m−2bar−1;
- Good stability in aqueous solutions.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Li, W.; Ling, G.Q.; Huang, P.; Li, K.; Lu, H.Q.; Hang, F.X.; Zhang, Y.; Xie, C.F.; Lu, D.J.; Li, H.; et al. Performance of ceramic microfiltration membranes for treating carbonated and filtered remelt syrup in sugar refinery. J. Food Eng. 2016, 170, 41–49. [Google Scholar] [CrossRef]
- Ravanchi, M.T.; Kaghazchi, T.; Kargari, A. Application of membrane separation processes in petrochemical industry: A review. Desalination 2009, 235, 199–244. [Google Scholar] [CrossRef]
- Iulianelli, A.; Drioli, E. Membrane engineering: Latest advancements in gas separation and pre-treatment processes, petrochemical industry and refinery, and future perspectives in emerging applications. Fuel Process. Technol. 2020, 206, 106464. [Google Scholar] [CrossRef]
- Chen, D.; Sirkar, K.K.; Jin, C.; Singh, D.; Pfeffer, R. Membrane-Based Technologies in the Pharmaceutical Industry and Continuous Production of Polymer-Coated Crystals/Particles. Curr. Pharm. Des. 2017, 23, 242–249. [Google Scholar] [CrossRef]
- Goh, K.S.; Chen, Y.; Ng, D.Y.F.; Chew, J.W.; Wang, R. Organic solvent forward osmosis membranes for pharmaceutical concentration. J. Membr. Sci. 2022, 642, 119965. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, G.; Xu, N.; Shi, J. Preparation and application in oil–water separation of ZrO2/α-Al2O3 MF membrane. J. Membr. Sci. 1998, 142, 235–243. [Google Scholar] [CrossRef]
- Kanagaraj, P.; Nagendran, A.; Rana, D.; Matsuura, T.; Neelakandan, S. Separation of macromolecular proteins and rejection of toxic heavy metal ions by PEI/cSMM blend UF membranes. Int. J. Biol. Macromol. 2015, 72, 223–229. [Google Scholar] [CrossRef]
- Zhang, R.; Ma, H.; Wang, B. Removal of Chromium(VI) from Aqueous Solutions Using Polyaniline Doped with Sulfuric Acid. Ind. Eng. Chem. Res. 2010, 49, 9998–10004. [Google Scholar] [CrossRef]
- Karthik, R.; Meenakshi, S. Removal of hexavalent chromium ions using polyaniline/silica gel composite. J. Water Process Eng. 2014, 1, 37–45. [Google Scholar] [CrossRef]
- Rojas, G.; Silva, J.; Flores, J.A.; Rodriguez, A.; Ly, M.; Maldonado, H. Adsorption of chromium onto cross-linked chitosan. Sep. Purif. Technol. 2005, 44, 31–36. [Google Scholar] [CrossRef]
- Chitrakar, H.; Arun, M.I.; Mahesh, P.; Ahmad, F.I.; Lau, W. New CPS-PPEES blend membranes for CaCl2 and NaCl2 rejection. Membr. Water Treat. 2012, 3, 25–34. [Google Scholar] [CrossRef]
- Boulkrinat, A.; Bouzerara, F.; Harabi, A.; Harrouche, K.; Stelitano, S.; Russo, F.; Galiano, F.; Figoli, A. Synthesis and characterization of ultrafiltration ceramic membranes used in the separation of macromolecular proteins. J. Eur. Ceram. Soc. 2020, 40, 5967–5973. [Google Scholar] [CrossRef]
- van der Laan, H.; van Halem, D.; Smeets, P.; Soppe, A.; Kroesbergen, J.; Wubbels, G.; Nederstigt, J.; Gensburger, I.; Heijman, B. Bacteria and virus removal effectiveness of ceramic pot filters with different silver applications in a long term experiment. Water Res. 2014, 51, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Medjemem, N.; Harabi, A.; Bouzerara, F.; Foughali, L.; Boudaira, B.; Guechi, A.; Brihi, N. Elaboration and characterization of low cost ceramics microfiltration membranes applied to the sterilization of plant tissue culture media. J. Taiwan Inst. Chem. Eng. 2016, 59, 79–85. [Google Scholar] [CrossRef]
- Majewska-Nowak, K.M. Application of ceramic membranes for the separation of dye particles. Desalination 2010, 254, 185–191. [Google Scholar] [CrossRef]
- Vetten, M.A.; Yah, C.S.; Singh, T.; Gulumian, M. Challenges facing sterilization and depyrogenation of nanoparticles: Effects on structural stability and biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1391–1399. [Google Scholar] [CrossRef]
- Hussain, A.; Ahmed, I.; Nazir, H.; Ullah, I. Plant Tissue Culture: Current Status and Opportunities. In Recent Advances in Plant In Vitro Culture; Annarita, L., Ed.; Intech Open: London, UK, 2012. [Google Scholar] [CrossRef]
- Thomas, P.; Prabhakara, B.S.; Pitchaimuthu, M. Cleansing the long-term micropropagated triploid watermelon cultures from covert bacteria and field testing the plants for clonal fidelity and fertility during the 7–10 year period in vitro. Plant Cell Tissue Organ Cult. 2006, 85, 317–329. [Google Scholar] [CrossRef]
- Georg, E.F.; Hall, M.A.; De Klerk, G.-J. (Eds.) The Components of Plant Tissue Culture Media I: Macro- and Micro-Nutrients. In Plant Propagation by Tissue Culture, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 65–113. [Google Scholar] [CrossRef]
- Gago, J.; Pérez-Tornero, O.; Landín, M.; Burgos, L.; Gallego, P.P. Improving knowledge of plant tissue culture and media formulation by neurofuzzy logic: A practical case of data mining using apricot databases. J. Plant Physiol. 2011, 168, 1858–1865. [Google Scholar] [CrossRef]
- Odutayo, O.I.; Oso, R.T.; Akinyemi, B.O.; Amusa, N.A. Microbial contaminants of cultured Hibiscus cannabinus and Telfaria occidentalis tissues. Afr. J. Biotechnol. 2004, 3, 473–476. [Google Scholar] [CrossRef][Green Version]
- Wang, C.-Y.; Wang, F.; Wang, T.; Yang, X.-L.; Bian, Y.-R.; Kengara, F.; Li, Z.-B.; Jiang, X. Effects of Autoclaving and Mercuric Chloride Sterilization on PAHs Dissipation in a Two-Liquid-Phase Soil Slurry. Pedosphere 2011, 21, 56–64. [Google Scholar] [CrossRef]
- Shi, Y.; Xu, L.; Gong, D.; Lu, J. Effects of sterilization treatments on the analysis of TOC in water samples. J. Environ. Sci. 2010, 22, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Davis, T.A.; Matthews, M.A.; Drews, M.J.; LaBerge, M.; An, Y.H. Sterilization using high-pressure carbon dioxide. J. Supercrit. Fluids 2006, 38, 354–372. [Google Scholar] [CrossRef]
- Faria, L.F.F.; Di Luccio, M.; Nobrega, R.; Borges, C.P. Developement and characterization of microfiltration Hollow—Fiber modules for sterilization of fermentation media, Braz. J. Chem. Eng. 2002, 19, 141–150. [Google Scholar] [CrossRef]
- Cruz, R.M.S.; Vieira, M.C.; Silva, C.L. Effect of heat and thermosonication treatments on watercress (Nasturtium officinale) vitamin C degradation kinetics. Innov. Food Sci. Emerg. Technol. 2008, 9, 483–488. [Google Scholar] [CrossRef]
- Perrut, M. Sterilization and virus inactivation by supercritical fluids. J. Supercrit. Fluids 2012, 66, 359–371. [Google Scholar] [CrossRef]
- Leitzen, S.; Vogel, M.; Steffens, M.; Zapf, T.; Müller, C.E.; Brandl, M. Quantification of Degradation Products Formed during Heat Sterilization of Glucose Solutions by LC-MS/MS: Impact of Autoclaving Temperature and Duration on Degradation. Pharmaceuticals 2021, 14, 1121. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Heijman, S.G.; Luiten-Olieman, M.W.; Rietveld, L.C. Oil-in-water emulsion separation: Fouling of alumina membranes with and without a silicon carbide deposition in constant flux filtration mode. Water Res. 2022, 216, 118267. [Google Scholar] [CrossRef] [PubMed]
- Wehling, J.; Köser, J.; Lindner, P.; Lüder, C.; Beutel, S.; Kroll, S.; Rezwan, K. Silver nanoparticle-doped zirconia capillaries for enhanced bacterial filtration. Mater. Sci. Eng. C 2015, 48, 179–187. [Google Scholar] [CrossRef]
- Kouras, N.; Harabi, A.; Bouzerara, F.; Foughali, L.; Policicchio, A.; Stelitano, S.; Galiano, F.; Figoli, A. Macro-porous ceramic supports for membranes prepared from quartz sand and calcite mixtures. J. Eur. Ceram. Soc. 2017, 37, 3159–3165. [Google Scholar] [CrossRef]
- Malekshahi, M.; Sabbaghi, S.; Rasouli, K. Preparation of α-alumina/γ-alumina/γ-alumina-titania ceramic composite membrane for chloride ion removal. Mater. Chem. Phys. 2022, 287, 126218. [Google Scholar] [CrossRef]
- Boulkrinat, A.; Bouzerara, F. Elaboration of tubular titania microfiltration membranes for wastewater treatment. Desalination Water Treat. 2021, 211, 319–322. [Google Scholar] [CrossRef]
- Bouzerara, F.; Boulanacer, S.; Harabi, A. Shaping of microfiltration (MF) ZrO2 membranes using a centrifugal casting method. Ceram. Int. 2015, 41, 5159–5163. [Google Scholar] [CrossRef]
- Liu, C.; Wang, L.; Ren, W.; Rong, Z.; Wang, X.; Wang, J. Synthesis and characterization of a mesoporous silica (MCM-48) membrane on a large-pore α-Al2O3 ceramic tube. Microporous Mesoporous Mater. 2007, 106, 35–39. [Google Scholar] [CrossRef]
- Fan, P.M.; Zhen, K.F.; Zan, Z.Y.; Chao, Z.; Jian, Z.; Yun, J.Z. Preparation and development of porous ceramic membrane supports fabricated by extrusion technique. Chem. Eng. Trans. 2016, 55, 277–282. [Google Scholar] [CrossRef]
- Almandoz, M.; Pagliero, C.; Ochoa, N.; Marchese, J. Composite ceramic membranes from natural aluminosilicates for microfiltration applications. Ceram. Int. 2015, 41, 5621–5633. [Google Scholar] [CrossRef]
- Issaoui, M.; Limousy, L. Low-cost ceramic membranes: Synthesis, classifications, and applications. Comptes Rendus Chim. 2019, 22, 175–187. [Google Scholar] [CrossRef]
- Fang, J.; Qin, G.; Wei, W.; Zhao, X. Preparation and characterization of tubular supported ceramic microfiltration membranes from fly ash. Sep. Purif. Technol. 2011, 80, 585–591. [Google Scholar] [CrossRef]
- Biesheuvel, P.; Breedveld, V.; Higler, A.P.; Verweij, H. Graded membrane supports produced by centrifugal casting of a slightly polydisperse suspension. Chem. Eng. Sci. 2001, 56, 3517–3525. [Google Scholar] [CrossRef]
- Nijmeijer, A.; Huiskes, C.; Sibelt, N.G.M.; Kruidhof, H.; Verweij, H. Centrifugal casting of tubular mem-brane supports. Am. Ceram. Soc. Bull. 1998, 77, 95–98. [Google Scholar]
- Chen, C.-H.; Takita, K.; Ishiguro, S.; Honda, S.; Awaji, H. Fabrication on porous alumina tube by centrifugal molding. J. Eur. Ceram. Soc. 2005, 25, 3257–3264. [Google Scholar] [CrossRef]
- de la Rocha, M.R.; Virginie, M.; Khodakov, A.; Pollo, L.D.; Marcílio, N.R.; Tessaro, I.C. Preparation of alumina based tubular asymmetric membranes incorporated with coal fly ash by centrifugal casting. Ceram. Int. 2021, 47, 4187–4196. [Google Scholar] [CrossRef]
- Jedidi, I.; Saïdi, S.; Khmakem, S.; Larbot, A.; Elloumi-Ammar, N.; Fourati, A.; Charfi, A.; Ben Amar, R. New ceramic microfiltration membranes from mineral coal fly ash. Arab. J. Chem. 2009, 2, 31–39. [Google Scholar] [CrossRef]
- Khemakhem, S.; Ben Amar, R.; Ben Hassen, R.; Larbot, A.; Medhioub, M.; Ben Salah, A.; Cot, L. New ceramic membranes for tangential waste-water filtration. Desalination 2004, 167, 19–22. [Google Scholar] [CrossRef]
- Masmoudi, S.; Ben Amar, R.; Larbot, A.; El Feki, H.; Salah, A.; Cot, L. Elaboration of inorganic microfiltration membranes with hydroxyapatite applied to the treatment of wastewater from sea product industry. J. Membr. Sci. 2005, 247, 1–9. [Google Scholar] [CrossRef]
- Bagdassarov, N.; Delépine, N. α–β Inversion in quartz from low frequency electrical impedance spectroscopy. J. Phys. Chem. Solids 2004, 65, 1517–1526. [Google Scholar] [CrossRef]
- Kadiri, C.; Harabi, A.; Bouzerara, F.; Foughali, L.; Brihi, N.; Hallour, S.; Guechi, A.; Boudaira, B. Preparation and properties of tubular macroporous ceramic membrane supports based on natural quartz sand and dolomite. J. Aust. Ceram. Soc. 2020, 56, 379–387. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, X.; Wang, Y.; Larbot, A.; Hu, X. Elaboration and characterization of tubular macroporous ceramic support for membranes from kaolin and dolomite. J. Porous Mater. 2010, 17, 1–9. [Google Scholar] [CrossRef]
- Kaur, H.; Bulasara, V.K.; Gupta, R.K. Effect of carbonates composition on the permeation characteristics of low-cost ceramic membrane supports. J. Ind. Eng. Chem. 2016, 44, 185–194. [Google Scholar] [CrossRef]
- Rouquerol, J.; Avnir, D.; Fairbridge, C.W.; Everett, D.H.; Haynes, J.M.; Pernicone, N.; Ramsay, J.D.F.; Sing, K.S.W.; Unger, K.K. International Union of Pure and Applied Chemistry Physical Chemistry Division Commission on Colloid and Surface Chemistry, Subcommittee on Characterization of Porous Solids: “Recommendations for the characterization of porous solids (Technical Report)”. Pure Appl. Chem. 1994, 66, 1739–1758. [Google Scholar] [CrossRef]
- Bazin, M.M.; Ahmad, N.; Nakamura, Y. Preparation of porous ceramic membranes from Sayong ball clay. J. Asian Ceram. Soc. 2019, 7, 417–425. [Google Scholar] [CrossRef]
- Vasanth, D.; Pugazhenthi, G.; Uppaluri, R. Fabrication and properties of low cost ceramic microfiltration membranes for separation of oil and bacteria from its solution. J. Membr. Sci. 2011, 379, 154–163. [Google Scholar] [CrossRef]
- Kumar, C.M.; Roshni, M.; Vasanth, D. Treatment of aqueous bacterial solution using ceramic membrane prepared from cheaper clays: A detailed investigation of fouling and cleaning. J. Water Process Eng. 2019, 29, 100797. [Google Scholar] [CrossRef]
- Kroll, S.; Treccani, L.; Rezwan, K.; Grathwohl, G. Development and characterisation of functionalised ceramic microtubes for bacteria filtration. J. Membr. Sci. 2010, 365, 447–455. [Google Scholar] [CrossRef]



| SiO2 | CaO | Al2O3 | K2O | SO3 | MgO | ZrO2 | Fe2O3 | Na2O | TiO2 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Silica sand | 85.17 | 2.50 | 6.04 | 0.31 | 0.06 | 0.63 | - | 1.69 | 0.05 | 0.03 |
| Calcite | 0.06 | 55.95 | 0.06 | 0.01 | 0.04 | 0.33 | - | 0.02 | 0.06 | - |
| Zircon | 34.00 | - | - | - | - | - | 65.5 | 0.12 | - | 0.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khebli, Z.; Bouzerara, F.; Brihi, N.; Figoli, A.; Russo, F.; Galiano, F.; Chahredine, S. Fabrication of a Zircon Microfiltration Membrane for Culture Medium Sterilization. Membranes 2023, 13, 399. https://doi.org/10.3390/membranes13040399
Khebli Z, Bouzerara F, Brihi N, Figoli A, Russo F, Galiano F, Chahredine S. Fabrication of a Zircon Microfiltration Membrane for Culture Medium Sterilization. Membranes. 2023; 13(4):399. https://doi.org/10.3390/membranes13040399
Chicago/Turabian StyleKhebli, Zineb, Ferhat Bouzerara, Nourddine Brihi, Alberto Figoli, Francesca Russo, Francesco Galiano, and Sadek Chahredine. 2023. "Fabrication of a Zircon Microfiltration Membrane for Culture Medium Sterilization" Membranes 13, no. 4: 399. https://doi.org/10.3390/membranes13040399
APA StyleKhebli, Z., Bouzerara, F., Brihi, N., Figoli, A., Russo, F., Galiano, F., & Chahredine, S. (2023). Fabrication of a Zircon Microfiltration Membrane for Culture Medium Sterilization. Membranes, 13(4), 399. https://doi.org/10.3390/membranes13040399









