Sodium Super Ionic Conductor-Type Hybrid Electrolytes for High Performance Lithium Metal Batteries
Abstract
:1. Introduction
2. Experimental
2.1. Fabrication of CSEs
2.2. Assembly of Li-Metal Batteries
2.3. Materials and Electrochemical Characterization
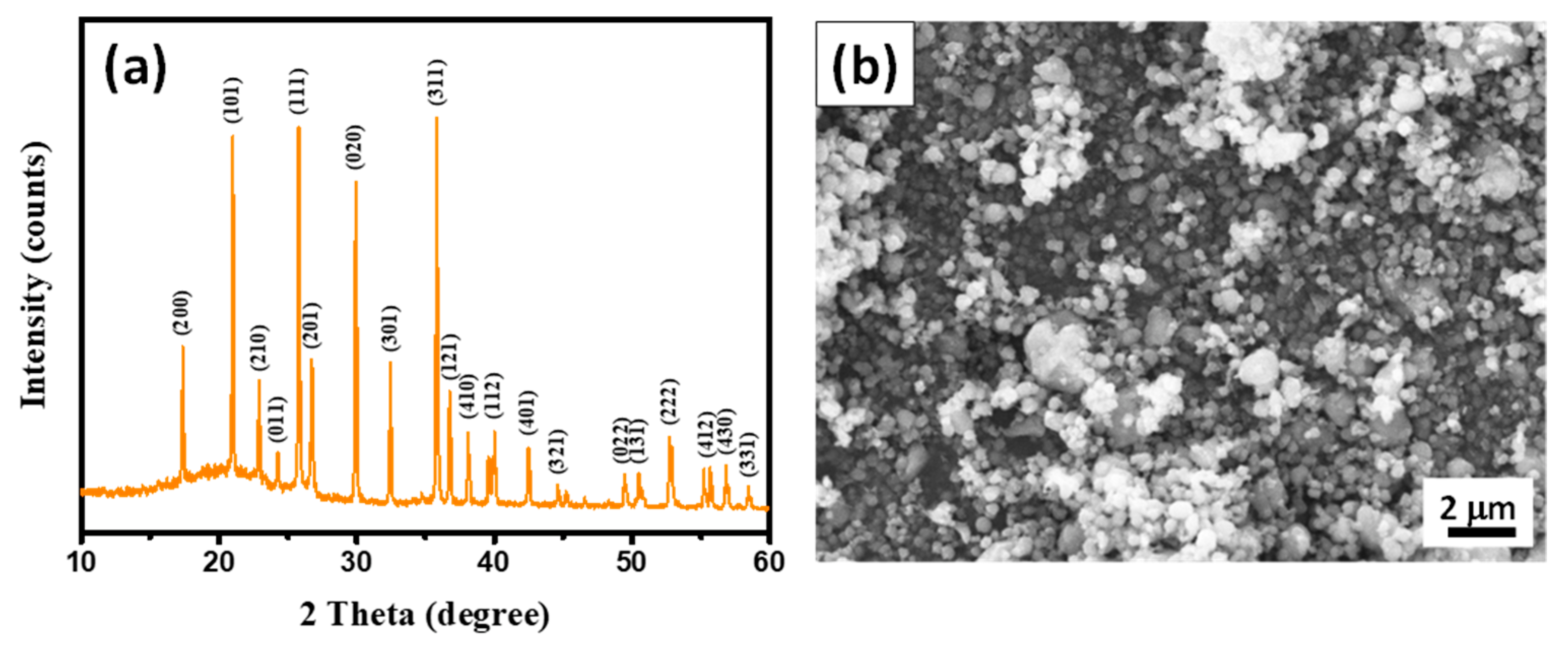
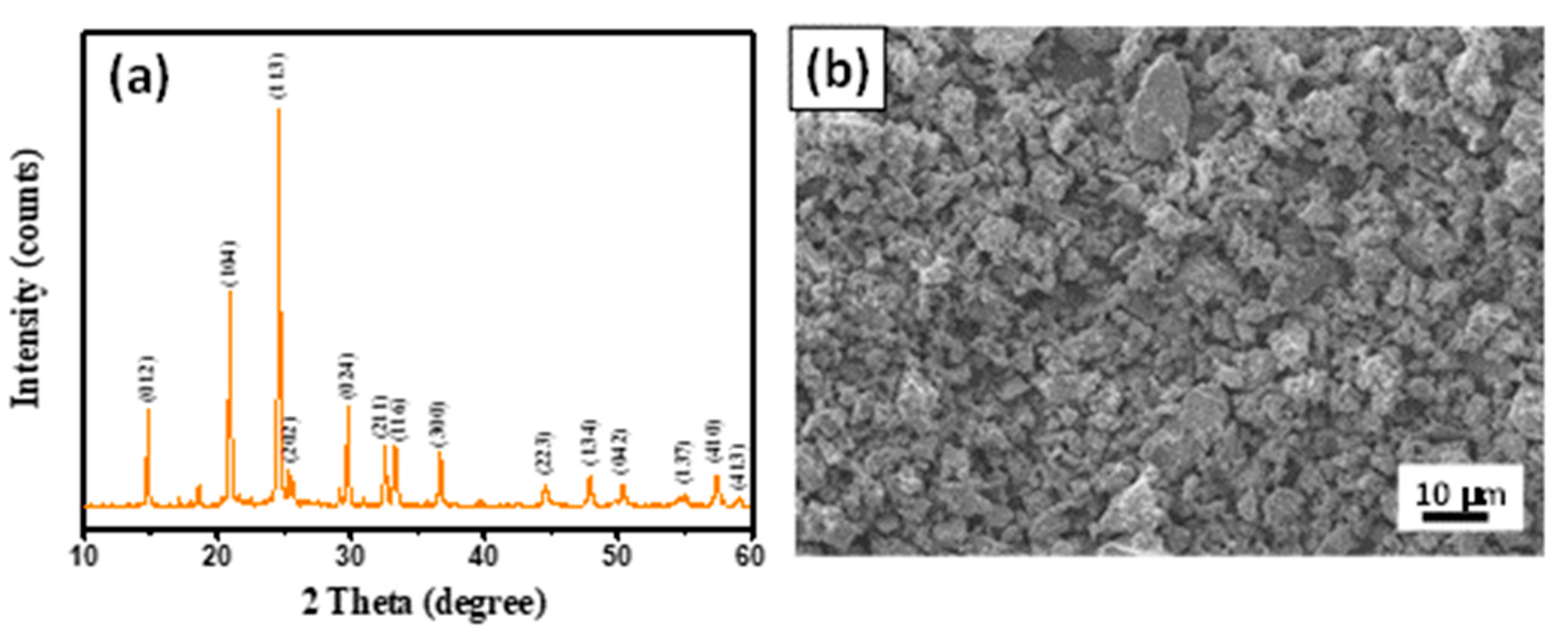
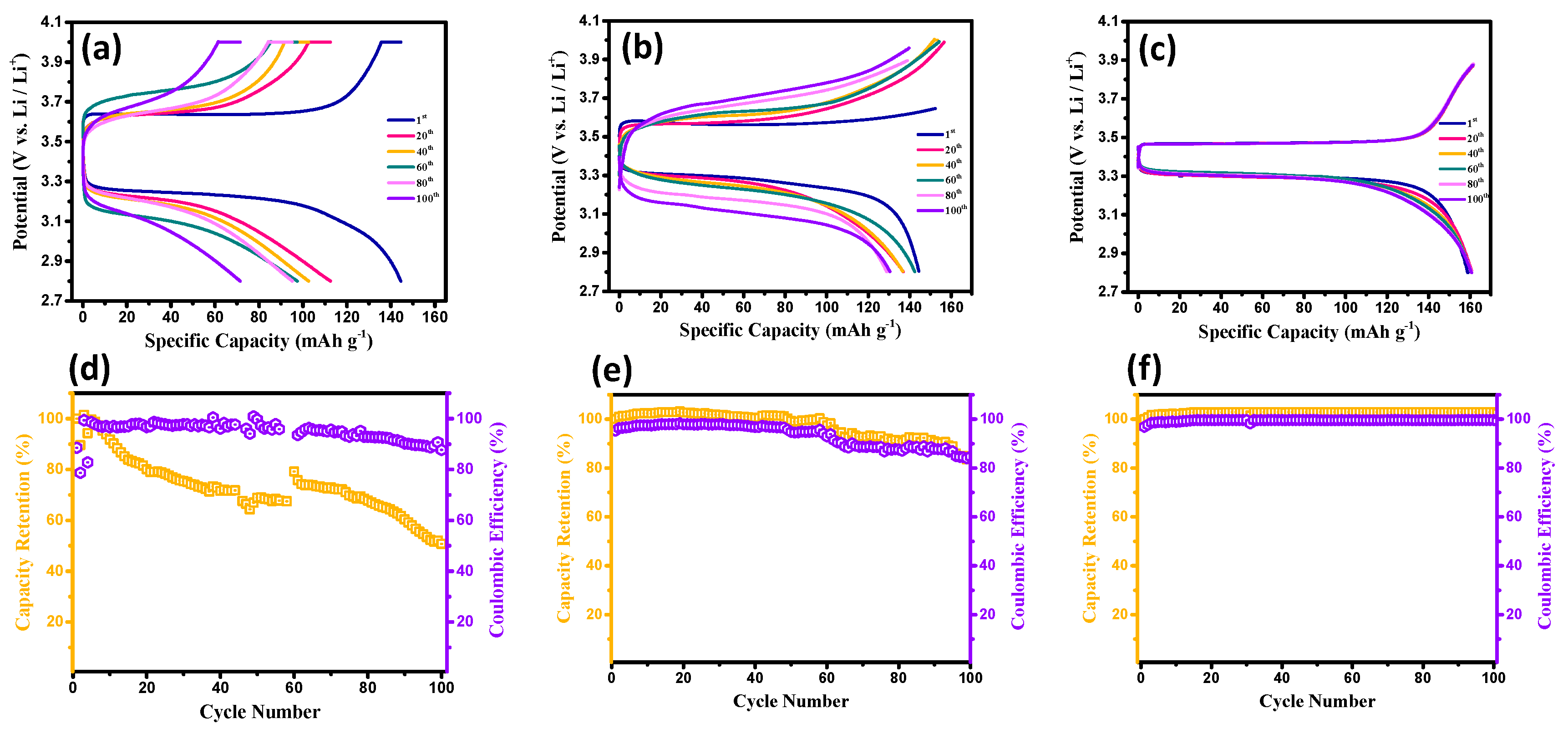
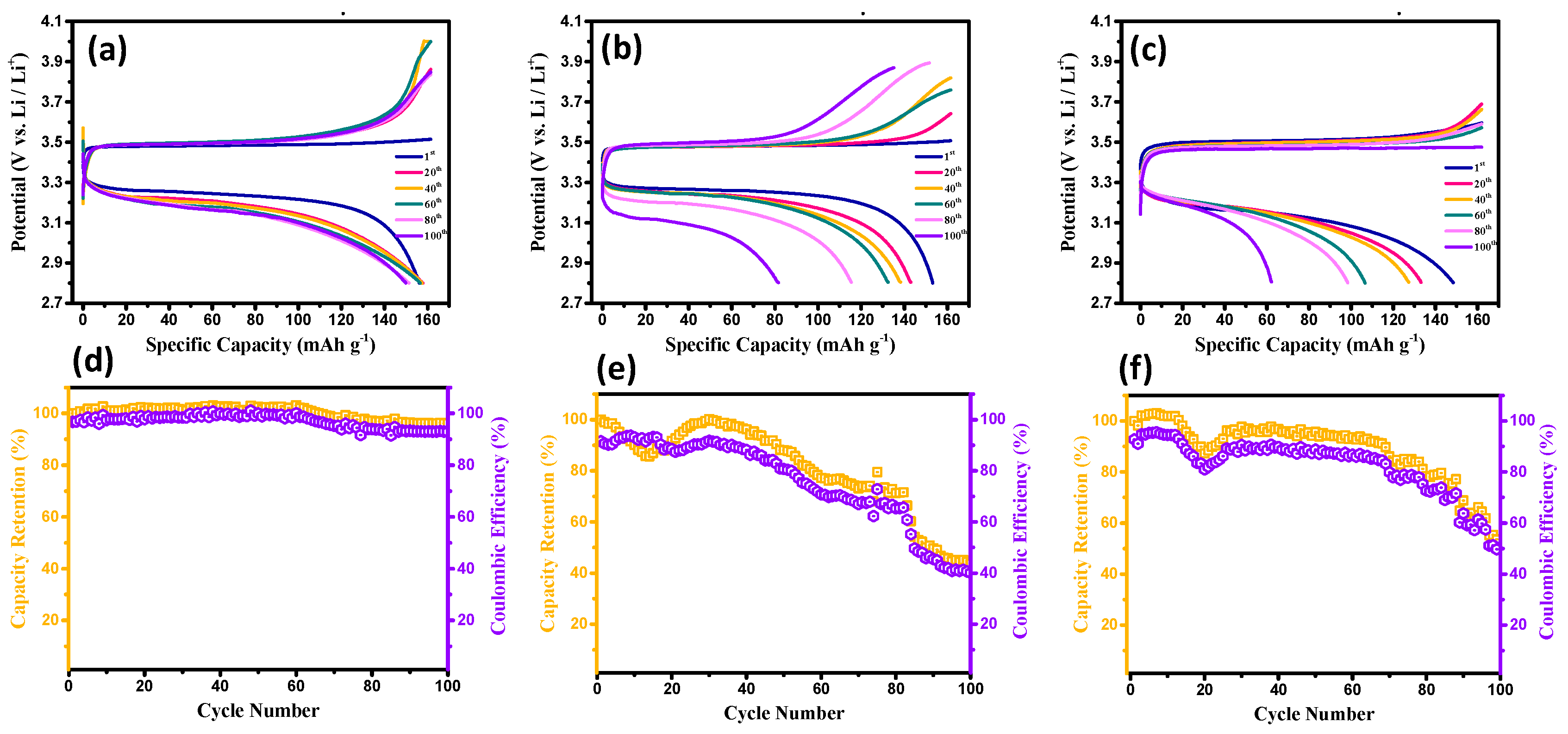
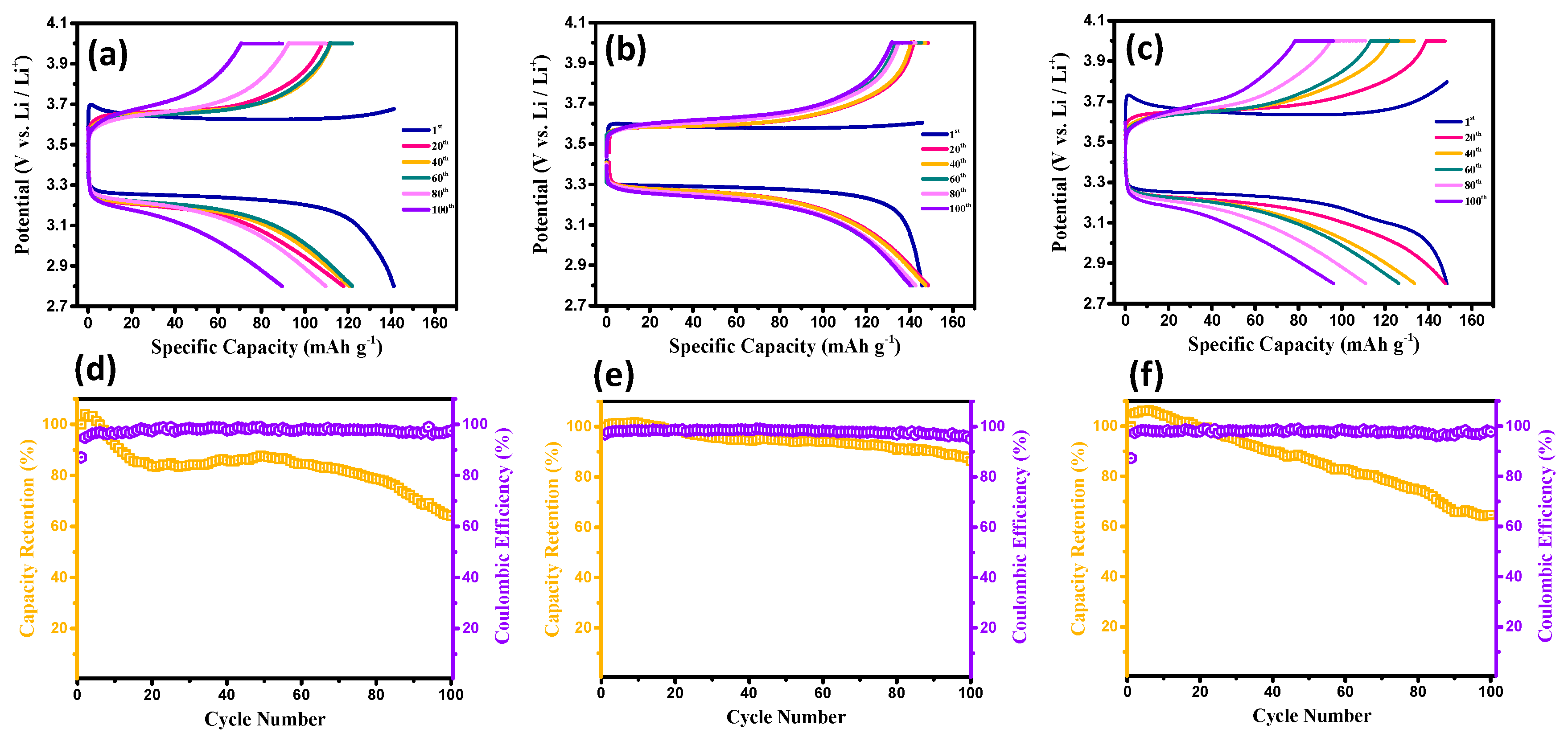
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siller, V.; Morata, A.; Eroles, M.N.; Arenal, R.; Gonzalez-Rosillo, J.C.; López del Amo, J.M.; Tarancon, A. High performance LATP thin film electrolytes for all-solid-state microbattery applications. J. Mater. Chem. A 2021, 9, 17760–17769. [Google Scholar] [CrossRef]
- Liao, G.; Mahrholz, T.; Geier, S.; Wierach, P.; Wiedemann, M. Nanostructured all-solid-state supercapacitors based on NASICON-type Li1.4Al0.4Ti1.6(PO4)3 electrolyte. J. Solid State Electrochem. 2018, 22, 1055–1061. [Google Scholar] [CrossRef]
- Soavi, F.; Bettini, L.G.; Piseri, P.; Milani, P.; Santoro, C.; Atanassov, P.; Arbizzani, C. Miniaturized supercapacitors: Key materials and structures towards autonomous and sustainable devices and systems. J. Power Sources 2016, 326, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, H. Composite solid electrolytes with NASICON-type LATP and PVdF-HFP for solid-state lithium batteries. Ind. Eng. Chem. Res. 2021, 60, 1494–1500. [Google Scholar] [CrossRef]
- Li, J.; Ma, C.; Chi, M.; Liang, C.; Dudney, N.J. Solid electrolyte: The key for high-voltage lithium batteries. Adv. Energy Mater. 2015, 5, 1401408. [Google Scholar] [CrossRef]
- Zheng, F.; Kotobuki, M.; Song, S.; Lai, M.O.; Lu, L. Review on solid electrolytes for all-solid-state lithium-ion batteries. J. Power Sources 2018, 389, 198–213. [Google Scholar] [CrossRef]
- Mauger, A.; Julien, C.M.; Paolella, A.; Armand, M.; Zaghib, K. Building better batteries in the solid state: A review. Materials 2019, 12, 3892. [Google Scholar] [CrossRef]
- Murugan, R.; Thangadurai, V.; Weppner, W. Fast lithium ion conduction in garnet-type Li7La3Zr2O12. Angew. Chem. Int. Ed. 2007, 46, 7778–7781. [Google Scholar] [CrossRef]
- Fu, K.; Gong, Y.; Liu, B.; Zhu, Y.; Xu, S.; Yao, Y.; Luo, W.; Wang, C.; Lacey, S.D.; Dai, J.; et al. Toward garnet electrolyte−based Li metal batteries: An ultrathin, highly effective, artificial solid-state electrolyte/metallic Li interface. Sci. Adv. 2017, 3, e1601659. [Google Scholar] [CrossRef]
- Morimoto, H.; Awano, H.; Terashima, J.; Shindo, Y.; Nakanishi, S.; Ito, N.; Ishikawa, K.; Tobishima, S.I. Preparation of lithium ion conducting solid electrolyte of NASICON-type Li1+xAlxTi2−x(PO4)3 (x = 0.3) obtained by using the mechanochemical method and its application as surface modification materials of LiCoO2 cathode for lithium cell. J. Power Sources 2013, 240, 636–643. [Google Scholar] [CrossRef]
- DeWees, R.; Wang, H. Synthesis and properties of NASICON type LATP and LAGP solid electrolytes. ChemSusChem 2019, 12, 3713–3725. [Google Scholar] [CrossRef]
- Paolella, A.; Zhu, W.; Xu, G.-L.; La Monaca, A.; Savoie, S.; Girard, G.; Vijh, A.; Demers, H.; Perea, A.; Delaporte, N.; et al. Understanding the reactivity of a thin Li1.5Al0.5Ge1.5(PO4)3 solid-state electrolyte toward metallic lithium anode. Adv. Energy Mater. 2020, 10, 2001497. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Hsieh, C.-T.; Zhang, R.-S.; Mohanty, D.; Ashraf Gandomi, Y.; Hung, I.-M. Hybrid solid state electrolytes blending NASICON-type Li1+xAlxTi2–x(PO4)3 with poly(vinylidene fluoride-co-hexafluoropropene) for lithium metal batteries. Electrochim. Acta 2022, 427, 140903. [Google Scholar] [CrossRef]
- Liu, Z.; Fu, W.; Payzant, E.; Yu, X.; Wu, Z.; Dudney, N.; Kiggans, J.; Hong, K.; Rondinone, A.; Liang, C. Anomalous high ionic conductivity of nanoporous beta-Li3PS4. J. Am. Chem. Soc. 2013, 135, 975–978. [Google Scholar] [CrossRef]
- Yu, C.; Ganapathy, S.; de Klerk, N.J.; Roslon, I.; van Eck, E.R.; Kentgens, A.P.; Wagemaker, M. Unravelling Li-ion transport from picoseconds to seconds: Bulk versus interfaces in an argyrodite Li6PS5Cl-Li2S all-solid-state Li-ion battery. J. Am. Chem. Soc. 2016, 138, 11192–11201. [Google Scholar] [CrossRef]
- Ziolkowska, D.A.; Arnold, W.; Druffel, T.; Sunkara, M.; Wang, H. Rapid and economic synthesis of a Li7PS6 solid electrolyte from a liquid approach. ACS Appl. Mater. Interfaces 2019, 11, 6015–6021. [Google Scholar] [CrossRef]
- Arnold, W.; Buchberger, D.A.; Li, Y.; Sunkara, M.; Druffel, T.; Wang, H. Halide doping effect on solvent-synthesized lithium argyrodites Li6PS5X (X= Cl, Br, I) superionic conductors. J. Power Sources 2020, 464, 228158. [Google Scholar] [CrossRef]
- Han, L.; Hsieh, C.-T.; Mallick, B.; Li, J.; Ashraf Gandomi, Y. Recent progress and future perspective on atomic layer deposition to prepare/modify solid-state electrolytes and interface between electrodes for next-generation lithium batteries. Nanoscale Adv. 2021, 3, 2728–2740. [Google Scholar] [CrossRef]
- Key, B.; Schroeder, D.J.; Ingram, B.J.; Vaughey, J.T. Solution-based synthesis and characterization of lithium-ion conducting phosphate ceramics for lithium metal batteries. Chem. Mater. 2012, 24, 287–293. [Google Scholar] [CrossRef]
- Liu, Y.; Li, B.; Kitaura, H.; Zhang, X.; Han, M.; He, P.; Zhou, H. Fabrication and performance of all-solid-state Li-air battery with SWCNTs/LAGP cathode. ACS Appl. Mater. Interfaces 2015, 9, 17307–17310. [Google Scholar] [CrossRef]
- Zhang, P.; Matsui, M.; Takeda, Y.; Yamamoto, O.; Imanishi, N. Water-stable lithium ion conducting solid electrolyte of iron and aluminum doped NASICON-type LiTi2(PO4)3. Solid State Ion. 2014, 263, 27–32. [Google Scholar] [CrossRef]
- Mertens, A.; Yu, S.; Schon, N.; Gunduz, D.C.; Tempel, H.; Schierholz, R.; Hausen, F.; Kungl, H.; Granwehr, J.; Eichel, R. Superionic bulk conductivity in Li1.3Al0.3Ti1.7(PO4)3 solid electrolyte. Solid State Ion. 2017, 309, 180–186. [Google Scholar] [CrossRef]
- Commarieu, B.; Paolella, A.; Daigle, J.-C.; Zaghib, K. Toward high lithium conduction in solid polymer and polymer-ceramic batteries. Curr. Opin. Electrochem. 2018, 9, 56–63. [Google Scholar] [CrossRef]
- Li, Y.; Arnold, W.; Thapa, A.; Jasinski, J.B.; Sumanasekera, G.; Sunkara, M.; Druffel, T.; Wang, H. Stable and flexible sulfide composite electrolyte for high-performance solid-state lithium batteries. ACS Appl. Mater. Interfaces 2020, 12, 42653–42659. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Liu, W.; Liu, Y.; Lee, H.R.; Hsu, P.-C.; Liu, K.; Cui, Y. High ionic conductivity of composite solid polymer electrolyte via in situ synthesis of monodispersed SiO2 nanospheres in poly(ethylene oxide). Nano Lett. 2016, 16, 459–465. [Google Scholar] [CrossRef]
- Choudhury, S.; Stalin, S.; Vu, D.; Warren, A.; Deng, Y.; Biswal, P.; Archer, L.A. Solid-state polymer electrolytes for high-performance lithium metal batteries. Nat. Commun. 2019, 10, 4398. [Google Scholar] [CrossRef]
- Han, L.; Lehmann, M.; Zhu, J.; Liu, T.; Zhou, Z.; Tang, X.; Hsieh, C.-T.; Sokolov, A.P.; Cao, P.; Chen, X.; et al. Recent developments and challenges in hybrid solid electrolytes for lithium-ion batteries. Front. Energy Res. 2020, 8, 202. [Google Scholar] [CrossRef]
- Subadevi, R.; Sivakumar, M.; Rajendran, S.; Wu, H.C.; Wu, N.L. Development and characterizations of PVdF-PEMA gel polymer electrolytes. Ionics 2011, 18, 283–289. [Google Scholar] [CrossRef]
- Karuppasamy, K.; Reddy, P.A.; Srinivas, G.; Tewari, A.; Sharma, R.; Shajan, X.S.; Gupta, D. Electrochemical and cycling performances of novel nonafluorobutanesulfonate (nonaflate) ionic liquid based ternary gel polymer electrolyte membranes for rechargeable lithium ion batteries. J. Membr. Sci. 2016, 514, 350–357. [Google Scholar] [CrossRef]
- Wang, M.; Xue, Y.; Zhang, K.; Zhang, Y. Synthesis of FePO4·2H2O nanoplates and their usage for fabricating superior high-rate performance LiFePO4. Electrochim. Acta 2011, 56, 4294–4298. [Google Scholar] [CrossRef]
- Hsieh, C.-T.; Pai, C.-T.; Chen, Y.-F.; Chen, I.-L.; Chen, W.-Y. Preparation of lithium iron phosphate cathode materials with different carbon contents using glucose additive for Li-ion batteries. J. Taiwan Inst. Chem. Eng. 2014, 45, 1501–1508. [Google Scholar] [CrossRef]
- Liang, Y.; Lin, Z.; Qiu, Y.; Zhang, X. Fabrication and characterization of LATP/PAN composite fiber-based lithium-ion battery separators. Electrochim. Acta 2011, 56, 6474–6480. [Google Scholar] [CrossRef]
- Kumar, J.; Kichambare, P.; Rai, A.K.; Bhattacharya, R.; Rodrigues, S.; Subramanyam, G.A. A high performance ceramic-polymer separator for lithium batteries. J. Power Sources 2016, 301, 194–198. [Google Scholar] [CrossRef]
- Watzig, K.; Rost, A.; Langklotz, U.; Matthey, B.; Schilm, J. An explanation of the microcrack formation in Li1.3Al0.3Ti1.7(PO4)3 ceramics. J. Eur. Ceram. Soc. 2016, 36, 1995–2001. [Google Scholar]
- Arbi, K.; Mandal, S.; Rojo, J.M.; Sanz, J. Dependence of ionic conductivity on composition of fast ionic conductors Li1+xTi2-xAlx(PO4)3, 0 ≤ x ≤ 0.7. A parallel NMR and electric impedance study. Chem. Mater. 2002, 14, 1091–1097. [Google Scholar] [CrossRef]
- Xu, G.; Li, F.; Tao, Z.; Wei, X.; Liu, Y.; Li, X.; Ren, Z.; Shen, G.; Han, G. Monodispersed LiFePO4@C core–shell nanostructures for a high power Li-ion battery cathode. J. Power Sources 2014, 246, 696–702. [Google Scholar] [CrossRef]
- Hsieh, C.-T.; Liu, J.-R.; Juang, R.-S.; Lee, C.-E.; Chen, Y.-F. Microwave synthesis of copper network onto lithium iron phosphate cathode materials for improved electrochemical performance. Mater. Chem. Phys. 2015, 103, 153–159. [Google Scholar] [CrossRef]
- Hsieh, C.-T.; Chen, I.-L.; Chen, W.-Y.; Wang, J.-P. Synthesis of iron phosphate powders by chemical precipitation route for high-power lithium iron phosphate cathodes. Electrochim. Acta 2012, 83, 202–208. [Google Scholar] [CrossRef]
- Hsieh, C.-T.; Pai, C.-T.; Chen, Y.-F.; Yu, P.-Y.; Juang, R.-S. Electrochemical performance of lithium iron phosphate cathodes at various temperatures. Electrochim. Acta 2014, 115, 96–102. [Google Scholar] [CrossRef]
- Ahsan, Z.; Ding, B.; Cai, Z.; Wen, C.; Yang, W.; Ma, Y.; Zhang, S.; Song, G.; Javed, M.S. Recent progress in capacity enhancement of LiFePO4 cathode for Li-ion batteries. J. Electrochem. Energy Convers. Storage 2021, 18, 010801. [Google Scholar] [CrossRef]
- Hsieh, C.-T.; Chen, W.-Y.; Wu, F.-L. Fabrication and superhydrophobicity of fluorinated carbon fabrics with micro/nanoscaled two-tier roughness. Carbon 2008, 46, 1218–1224. [Google Scholar] [CrossRef]
- Hsieh, C.-T.; Teng, H.; Chen, W.-Y.; Cheng, Y.-S. Synthesis, characterization, and electrochemical capacitance of amino-functionalized carbon nanotube/carbon paper electrodes. Carbon 2010, 48, 4219–4229. [Google Scholar] [CrossRef]
- Nien, Y.H.; Carey, J.R.; Chen, J.S. Physical and electrochemical properties of LiFePO4/C composite cathode prepared from various polymer-containing precursors. J. Power Sources 2009, 193, 822–827. [Google Scholar] [CrossRef]
- Wu, H.; Wu, H.; Lee, E.; Wu, N. High-temperature carbon-coated aluminum current collector for enhanced power performance of LiFePO4 electrode of Li-ion batteries. Electrochem. Commun. 2010, 12, 488–491. [Google Scholar] [CrossRef]
- Chang, H.H.; Chang, C.C.; Su, C.Y.; Wu, H.C.; Yang, M.H.; Wu, N.L. Effects of TiO2 coating on high-temperature cycle performance of LiFePO4-based lithium-ion batteries. J. Power Sources 2008, 185, 466–472. [Google Scholar] [CrossRef]
- Jaumaux, P.; Liu, Q.; Zhou, D.; Xu, X.; Wang, T.; Wang, Y.; Kang, F.; Li, B.; Wang, G. Deep-eutectic-solvent-based self-healing polymer electrolyte for safe and long-life lithium-metal batteries. Angew. Chem. 2020, 59, 9134–9142. [Google Scholar] [CrossRef]
- Sun, J.; Yao, X.; Li, Y.; Zhang, Q.; Hou, C.; Shi, Q.; Wang, H. Facilitating interfacial stability via bilayer heterostructure solid electrolyte toward high-energy, safe and adaptable lithium batteries. Adv. Energy Mater. 2020, 10, 2000709. [Google Scholar] [CrossRef]
- Mishra, M.; Hsu, C.W.; Rath, P.C.; Patra, J.; Lai, H.Z.; Chang, T.L.; Wang, C.-Y.; Wu, T.Y.; Lee, T.-C.; Chang, J.K. Ga-doped lithium lanthanum zirconium oxide electrolyte for solid-state Li batteries. Electrochim. Acta 2020, 353, 136536. [Google Scholar] [CrossRef]
- Chao, C.H.; Hsieh, C.T.; Ke, W.J.; Lee, L.W.; Lin, Y.F.; Liu, H.W.; Gu, S.; Fu, C.C.; Juang, R.S.; Mallick, B.C.; et al. Roll-to-roll atomic layer deposition of titania coating on polymeric separators for lithium ion batteries. J. Power Sources 2021, 482, 228896. [Google Scholar] [CrossRef]
- Prasanna, K.; Subburaj, T.; Lee, W.J.; Lee, C.W. Polyethylene separator: Stretched and coated with porous nickel oxide nanoparticles for enhancement of its efficiency in Li-ion batteries. Electrochim. Acta 2014, 137, 273–279. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, P.-Y.; Lu, M.; Hsieh, C.-T.; Ashraf Gandomi, Y.; Gu, S.; Liu, W.-R. Sodium Super Ionic Conductor-Type Hybrid Electrolytes for High Performance Lithium Metal Batteries. Membranes 2023, 13, 201. https://doi.org/10.3390/membranes13020201
Sung P-Y, Lu M, Hsieh C-T, Ashraf Gandomi Y, Gu S, Liu W-R. Sodium Super Ionic Conductor-Type Hybrid Electrolytes for High Performance Lithium Metal Batteries. Membranes. 2023; 13(2):201. https://doi.org/10.3390/membranes13020201
Chicago/Turabian StyleSung, Po-Yu, Mi Lu, Chien-Te Hsieh, Yasser Ashraf Gandomi, Siyong Gu, and Wei-Ren Liu. 2023. "Sodium Super Ionic Conductor-Type Hybrid Electrolytes for High Performance Lithium Metal Batteries" Membranes 13, no. 2: 201. https://doi.org/10.3390/membranes13020201
APA StyleSung, P.-Y., Lu, M., Hsieh, C.-T., Ashraf Gandomi, Y., Gu, S., & Liu, W.-R. (2023). Sodium Super Ionic Conductor-Type Hybrid Electrolytes for High Performance Lithium Metal Batteries. Membranes, 13(2), 201. https://doi.org/10.3390/membranes13020201








