Novel Pilot-Scale Photocatalytic Nanofiltration Reactor for Agricultural Wastewater Treatment
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Physicochemical Properties of the PNFR’s Photoactive Components
3.1.1. Nanoparticulate Titania Photocatalysts
3.1.2. Asymmetric PVDF/TiO2 Porous Hollow Fibers (PHFs)
3.2. Design and Construction of PNFR Unit
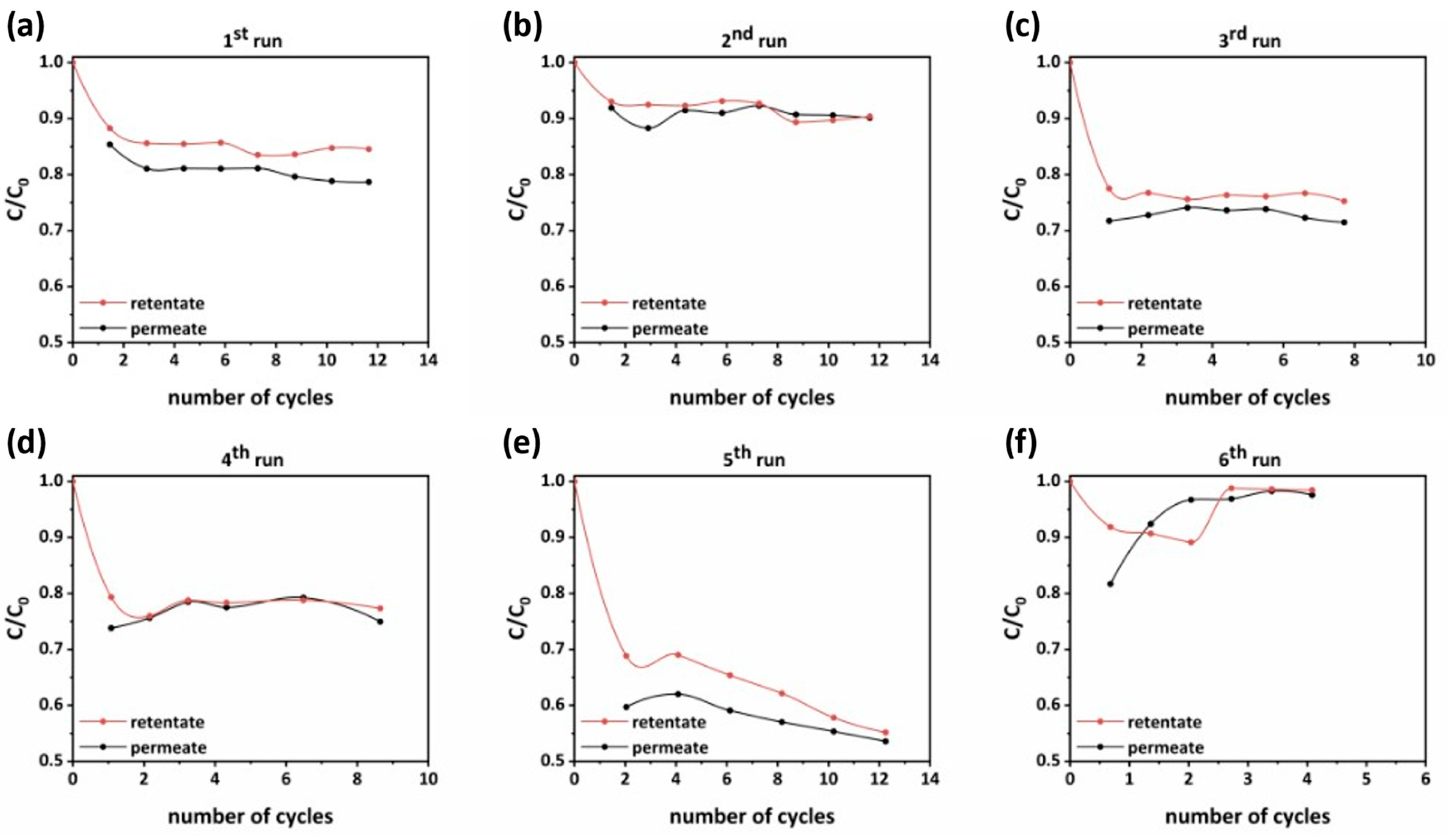
3.3. Operation and Photocatalytic Performance of PNFR Unit
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saleh, I.; Zouari, N.; Al-Ghouti, M. Removal of pesticides from water and wastewater: Chemical, physical and biological treatment approaches. Environ. Technol. Innov. 2020, 19, 101026. [Google Scholar] [CrossRef]
- Oller, I.; Malato, S.; Sánchez-Pérez, J. Combination of Advanced Oxidation Processes and biological treatments for wastewater decontamination—A review. Sci. Total Environ. 2011, 409, 4141–4166. [Google Scholar] [CrossRef] [PubMed]
- Falaras, P.; Romanos, G.; Aloupogiannis, P. Photocatalytic Purification Device. EU Patent EP2409954, 25 January 2012. [Google Scholar]
- Pelaez, M.; Nolan, N.; Pillai, S.; Seery, M.; Falaras, P.; Kontos, A.; Dunlop, P.; Hamilton, J.; Byrne, J.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B-Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Athanasekou, C.; Romanos, G.; Katsaros, F.; Kordatos, K.; Likodimos, V.; Falaras, P. Very efficient composite titania membranes in hybrid ultrafiltration/photocatalysis water treatment processes. J. Mem. Sci. 2012, 392–393, 192–203. [Google Scholar] [CrossRef]
- Athanasekou, C.; Moustakas, N.; Morales-Torres, S.; Pastrana-Martínez, L.; Figueiredo, J.; Faria, J.; Silva, A.; Dona-Rodriguez, J.M.; Romanos, G.; Falaras, P. Ceramic photocatalytic membranes for water filtration under UV and visible light. Appl. Catal. B-Environ. 2015, 178, 12–19. [Google Scholar] [CrossRef]
- Zada, A.; Ali, N.; Subhan, F.; Anwar, N.; Shah, M.; Ateeq, M.; Hussain, Z.; Zaman, K.; Khan, M. Suitable energy platform significantly improves charge separation of g-C3N4 for CO2 reduction and pollutant oxidation under visible-light. Prog. Nat. Sci. Mat. Int. 2019, 29, 138–144. [Google Scholar] [CrossRef]
- Xu, B.; Zada, A.; Wang, G.; Qu, Y. Boosting the visible-light photoactivities of BiVO4 nanoplates by doping Eu and coupling CeOx nanoparticles for CO2 reduction and organic oxidation. Sust. Energy Fuels 2019, 3, 3363–3369. [Google Scholar] [CrossRef]
- Moustakas, N.; Katsaros, F.; Kontos, A.; Romanos, G.; Dionysiou, D. Visible light active TiO2 photocatalytic filtration membranes with improved permeability and low energy consumption. Catal. Today 2014, 224, 56–69. [Google Scholar] [CrossRef]
- Romanos, G.; Athanasekou, C.; Katsaros, F.; Kanellopoulos, N.; Dionysiou, D.; Likodimos, V.; Falaras, P. Double-side active TiO2-modified nanofiltration membranes in continuous flow photocatalytic reactors for effective water purification. J. Hazard. Mater. 2012, 211-212, 304–316. [Google Scholar] [CrossRef]
- European Commission Implementing Regulation (EU) 2018/783. Off. J. Eur. Union. 2018, 132, 31–33.
- European Commission Implementing Regulation (EU) 2018/784. Off. J. Eur. Union. 2018, 132, 35–39.
- European Commission Implementing Regulation (EU) 2018/785. Off. J. Eur. Union. 2018, 132, 40–44.
- Phogat, A.; Singh, J.; Kumar, V.; Malik, V. Toxicity of the acetamiprid insecticide for mammals: A review. Environ. Chem. Lett. 2022, 20, 1453–1478. [Google Scholar] [CrossRef]
- Venancio, W.L.; Rodrigues-Silva, C.; Spina, M.; Diniz, V.; Guimarães, J.R. Degradation of benzimidazoles by photoperoxidation: Metabolites detection and ecotoxicity assessment using Raphidocelis subcapitata microalgae and Vibrio fischeri. Environ. Sci. Pollut. Res. 2021, 28, 23742–23752. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, L. Influence of sublethal doses of acetamiprid and halosulfuron-methyl on metabolites of zebra fish (Brachydanio rerio). Aquat. Toxicol. 2017, 19, 85–94. [Google Scholar] [CrossRef]
- Tanaka, T.; Suzuki, T.; Inomata, A.; Moriyasu, T. Combined effects of maternal exposure to fungicides on behavioral development in F1-generation mice: 2. Fixed dose study of thiabendazole. Birth Defects Res. 2020, 20, 1809–1824. [Google Scholar] [CrossRef]
- Athanasiou, D.; Romanos, G.; Falaras, P. Design and optimization of a photocatalytic reactor for water purification combining optical fiber and membrane technologies. Chem. Eng. J. 2016, 305, 92–103. [Google Scholar] [CrossRef]
- Papageorgiou, S.; Katsaros, F.; Favvas, E.; Romanos, G.E.; Athanasekou, C.; Beltsios, K.; Tzialla, O.; Falaras, P. Alginate fibers as photocatalyst immobilizing agents applied in hybrid photocatalytic/ultrafiltration water treatment Processes. Water Res. 2012, 46, 1858–1872. [Google Scholar] [CrossRef]
- Du, P.; Carneiro, J.; Moulijn, J.; Mul, G. A novel photocatalytic monolith reactor for multiphase heterogeneous photocatalysis. Appl. Catal. A Gen. 2008, 334, 119–128. [Google Scholar] [CrossRef]
- Chatzidaki, E.; Favvas, E.; Papageorgiou, S.; Kanellopoulos, N.; Theophilou, N.V. New polyimide-polyaniline hollow fibers: Synthesis, characterization and behavior in gas separation. Eur. Polym. J. 2007, 43, 5010–5016. [Google Scholar] [CrossRef]
- Dzinun, H.; Othman, M.D.; Ismail, A.; Puteh, M.; Rahman, M.; Jaafar, J.; Adrus, N.; Hashim, N. Antifouling Behavior and Separation Performance of Immobilized TiO2 in Dual Layer Hollow Fiber Membranes. Polym. Eng. Sci. 2018, 58, 1636–1643. [Google Scholar] [CrossRef]
- Dzinun, H.; Othman, M.D.; Ismail, A.F.; Puteh, M.; Rahman, M.A.; Jaafar, J. Morphological study of co-extruded dual-layer hollow fiber membranes incorporated with different TiO2 loadings. J. Membr. Sci. 2015, 479, 123–131. [Google Scholar] [CrossRef]
- Theodorakopoulos, G.; Katsaros, F.; Papageorgiou, S.; Beazi-Katsioti, M.; Romanos, G. Engineering commercial TiO2 powder into tailored beads for efficient water purification. Materials 2022, 15, 326. [Google Scholar] [CrossRef] [PubMed]
- Arfanis, M.; Athanasekou, C.; Sakellis, E.; Boukos, N.; Ioannidis, N.; Likodimos, V.; Sygellou, L.; Bouroushian, M.; Kontos, A.; Falaras, P.J. Photocatalytic properties of copper—Modified core-shell titania nanocomposites. Photochem Photobiol. A 2019, 370, 145–155. [Google Scholar] [CrossRef]
- Arfanis, M.; Adamou, P.; Moustakas, N.; Triantis, T.M.; Kontos, A.G.; Falaras, P. Photocatalytic degradation of salicylic acid and caffeine emerging contaminants using titania nanotubes. Chem Eng. J. 2017, 310, 525. [Google Scholar] [CrossRef]
- Lagopati, N.; Tsilibary, E.; Falaras, P.; Papazafiri, P.; Pavlatou, E.; Kotsopoulou, E.; Kitsiou, P. Effect of nanostructured TiO2 crystal phase on photoinduced apoptosis of breast cancer epithelial cells. Int. J. Nanomed. 2014, 9, 3219–3230. [Google Scholar]
- Ayinla, R.; Dennis, J.; Zaid, H.; Sanusi, Y.; Usman, F.; Adebayo, L. A review of technical advances of recent palm bio-waste conversion to activated carbon for energy storage. J. Clean. Prod. 2019, 229, 1427–1442. [Google Scholar] [CrossRef]
- Chen, L.; Graham, M.; Li, G.; Gray, K. Fabricating highly active mixed phase TiO2 photocatalysts by reactive DC magnetron sputter deposition. Thin Solid Film. 2006, 515, 1176–1181. [Google Scholar] [CrossRef]
- Jiang, X.; Manawan, M.; Feng, T.; Qian, R.; Zhao, T.; Zhou, G.; Kong, F.; Wang, Q.; Dai, S.; Pan, J. Anatase and rutile in evonik aeroxide P25: Heterojunctioned or individual nanoparticles? Catal. Today 2018, 300, 12–17. [Google Scholar] [CrossRef]
- Li, G.; Ciston, S.; Saponjic, Z.; Chen, L.; Dimitrijevic, N.; Rajh, T.; Gray, K. Synthesizing mixed-phase TiO2 nanocomposites using a hydrothermal method for photo-oxidation and photoreduction applications. J. Catal. 2008, 253, 105–110. [Google Scholar] [CrossRef]
- Li, G.; Richter, C.; Milot, R.; Cai, L.; Schmuttenmaer, C.; Crabtree, R.H.; Brudvig, G.W.; Batista, V. Synergistic effect between anatase and rutile TiO2 nanoparticles in dye-sensitized solar cells. Dalton Trans. 2009, 45, 10078–10085. [Google Scholar] [CrossRef]
- Habisreutinger, S.; Schmidt-Mende, L.; Stolarczyk, J. Photocatalytic Reduction of CO2 on TiO2 and other semiconductors. Angew. Chem. Int. Ed. 2013, 52, 2–39. [Google Scholar] [CrossRef]
- Papailias, I.; Todorova, N.; Giannakopoulou, T.; Ioannidis, N.; Dallas, P.; Dimotikali, D.; Trapalis, C. Novel torus shaped g-C3N4 photocatalysts. Appl. Cat. B Env. 2020, 268, 118733. [Google Scholar] [CrossRef]
- Theodorakopoulos, G.; Karousos, D.; Mansouris, K.; Sapalidis, A.; Kouvelos, E.; Favvas, E. Graphene nanoplatelets based polyimide/Pebax dual-layer mixed matrix hollow fiber membranes for CO2/CH4 and He/N2 separations. Int. J. Greenh. Gas Control 2022, 114, 103588. [Google Scholar] [CrossRef]
- Fang, C.; Jeon, S.; Rajabzadeh, S.; Cheng, L.; Fang, L.; Matsuyama, H. Tailoring the surface pore size of hollow fiber membranes in the TIPS process. J. Mater. Chem. A 2018, 6, 535–547. [Google Scholar] [CrossRef]
- Elashmawi, I.; Gaabour, L. Raman, morphology and electrical behavior of nanocomposites based on PEO/PVDF with multi-walled carbon nanotubes. Results Phys. 2015, 5, 105–110. [Google Scholar] [CrossRef]
- Kaspar, P.; Sobola, D.; Částková, K.; Dallaev, R.; Št’astná, E.; Sedlák, P.; Knápek, A.; Trčka, T.; Holcman, V. Case study of polyvinylidene fluoride doping by carbon nanotubes. Materials 2021, 14, 1428. [Google Scholar] [CrossRef]
- Kobayashi, M.; Tashiro, K.; Tadokoro, H. Molecular vibrations of three crystal forms of poly(vinylidene fluoride). Macromolecules 1975, 8, 158–171. [Google Scholar] [CrossRef]
- Harstad, S.; D’Souza, N.; Soin, N.; El-Gendy, A.; Gupta, S.; Pecharsky, V.; Shah, T.; Siores, E.; Hadimani, R. Enhancement of β-phase in PVDF films embedded with ferromagnetic Gd5Si4 nanoparticles for piezoelectric energy harvesting. AIP Adv. 2017, 7, 056411. [Google Scholar] [CrossRef]
- Athanasekou, C.; Sapalidis, A.; Katris, I.; Savopoulou, E.; Beltsios, K.; Tsoufis, T.; Kaltzoglou, A.; Falaras, P.; Bounos, G.; Antoniou, M.; et al. Mixed Matrix PVDF/graphene and composite-skin PVDF/graphene oxide membranes applied in membrane distillation. Polym. Eng. Sci. 2019, 59, E262–E278. [Google Scholar] [CrossRef]
- Theodorakopoulos, G.; Romanos, G.; Katsaros, F.; Papageorgiou, S.; Kontos, A.; Spyrou, K.; Beazi-Katsioti, M.; Falaras, P. Structuring efficient photocatalysts into bespoke fiber shaped systems for applied water treatment. Chemosphere 2021, 277, 130253. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Han, Z.; Da, X.; Yang, Z.; Zhang, D.; Hong, R.; Tao, C.; Lin, H.; Huang, Y. Preparation and photocatalytic activity of red light-emitting carbon dots/P25 heterojunction photocatalyst with ultra-wide absorption spectrum. Mater. Res. Express 2021, 8, 025002. [Google Scholar] [CrossRef]
- You-ji, L.; Wei, C. Photocatalytic degradation of Rhodamine B using nanocrystalline TiO2-zeolite surface composite catalysts: Effects of photocatalytic condition on degradation efficiency. Catal. Sci. Technol. 2011, 1, 802–809. [Google Scholar] [CrossRef]
- Krishnan, R.; Kesavamoorthy, R.; Dash, S.; Tyagi, A.; Raj, B. Raman spectroscopic and photoluminescence investigations on laser surface modified α-Al2O3 coatings. Scr. Mater. 2003, 48, 1099–1104. [Google Scholar] [CrossRef]
- Pezzotti, G.; Zhu, W. Resolving stress tensor components in space from polarized Raman spectra: Polycrystalline alumina. Phys. Chem. Chem. Phys. 2015, 17, 2608–2627. [Google Scholar] [CrossRef]
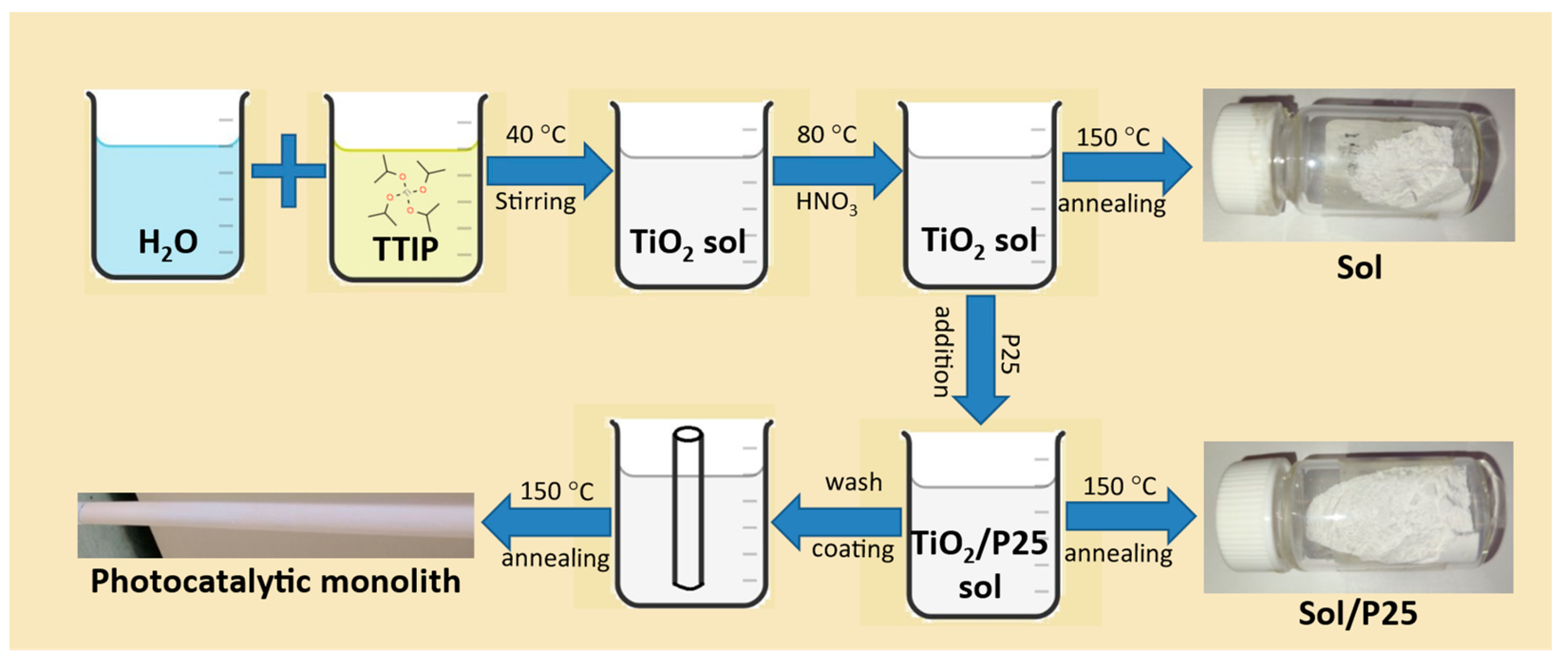
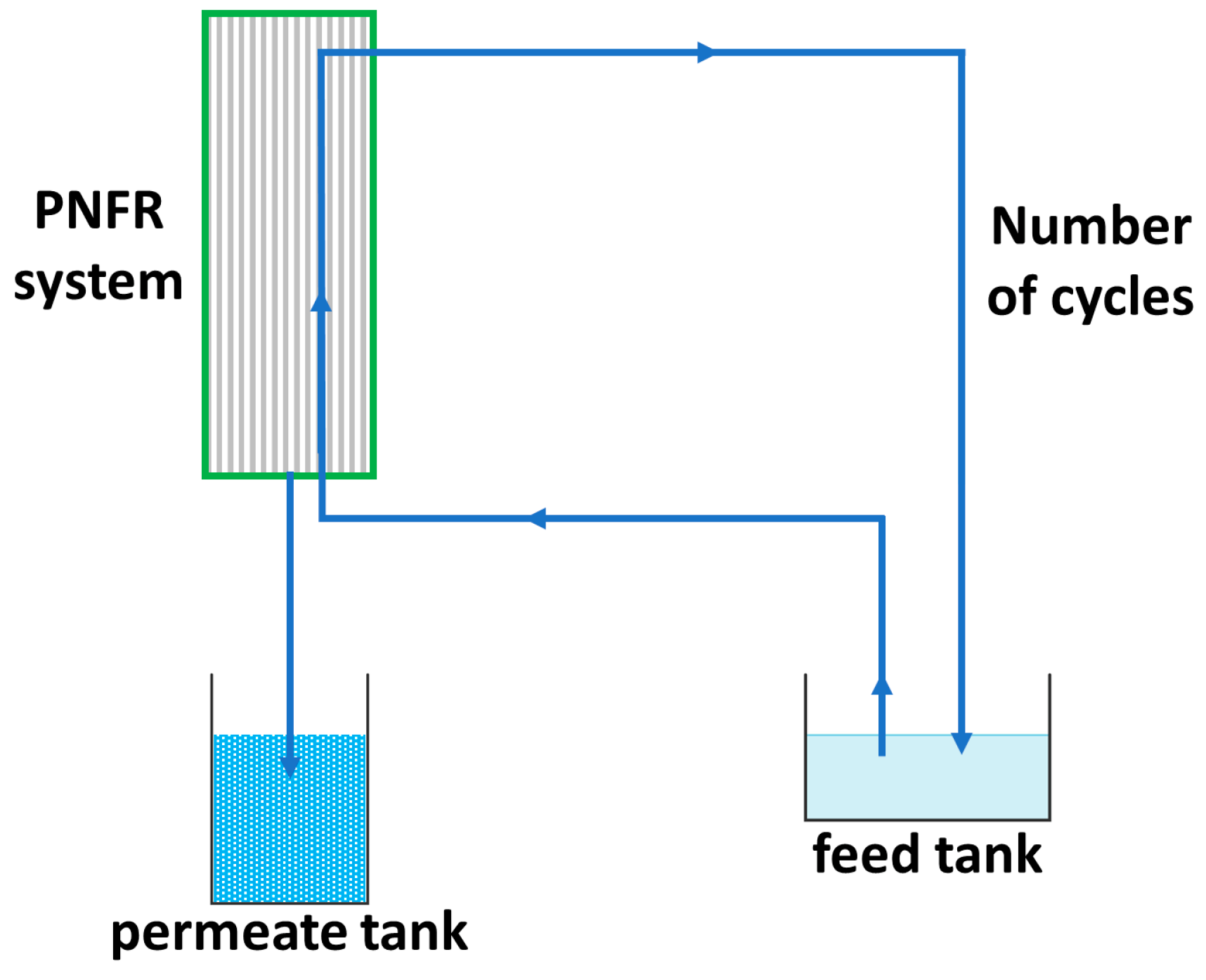
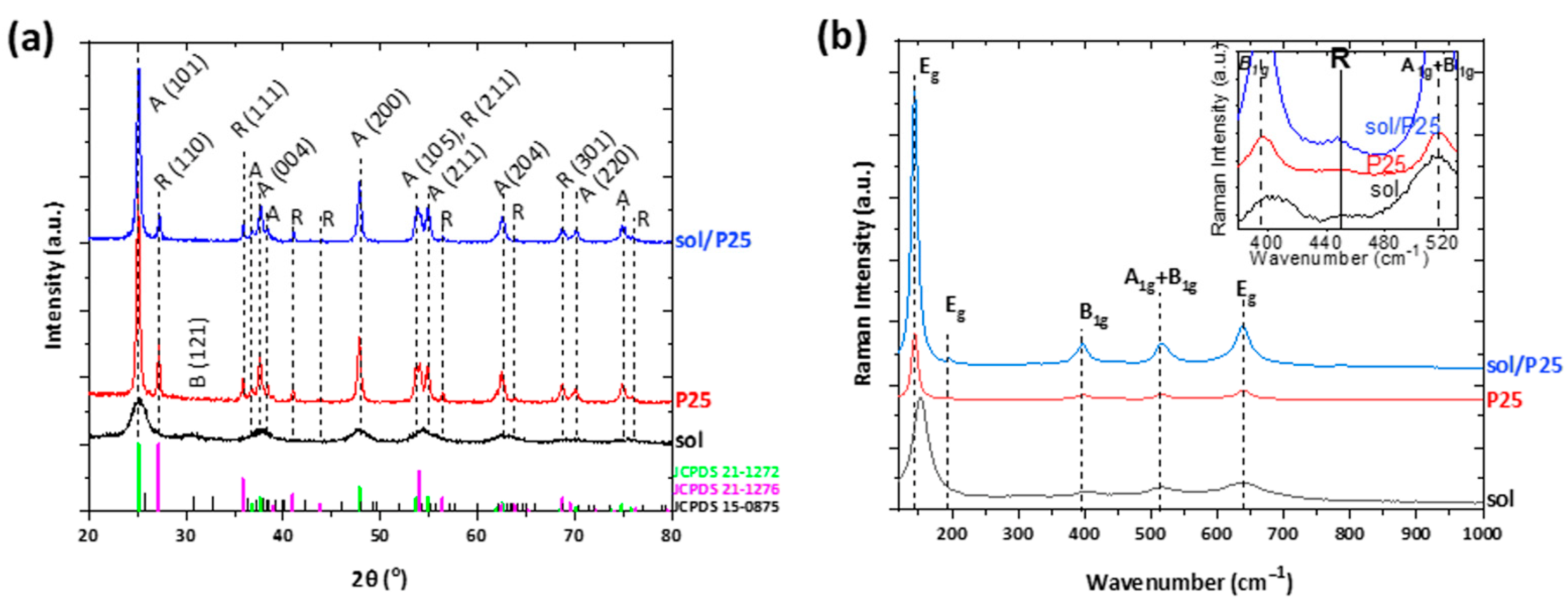
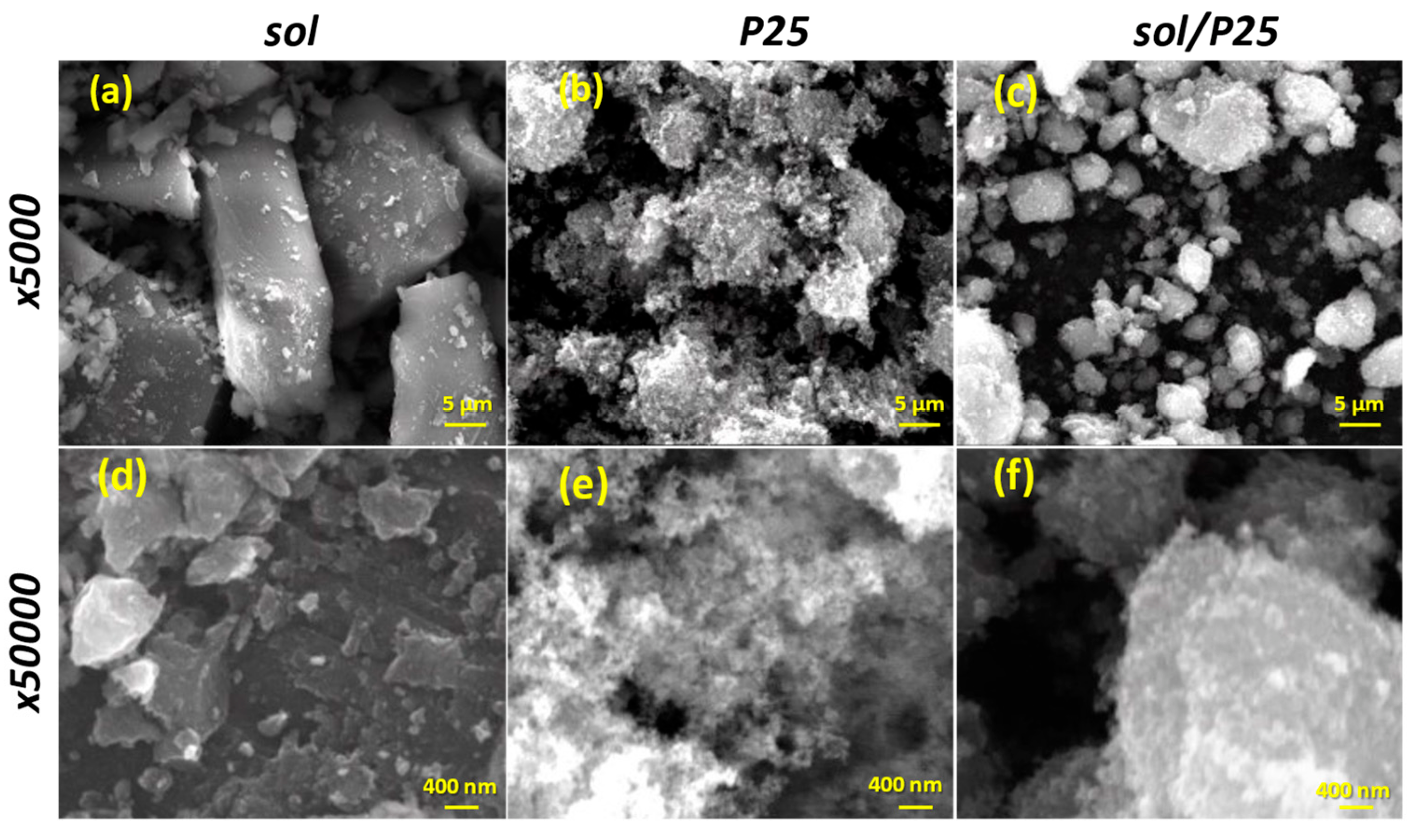
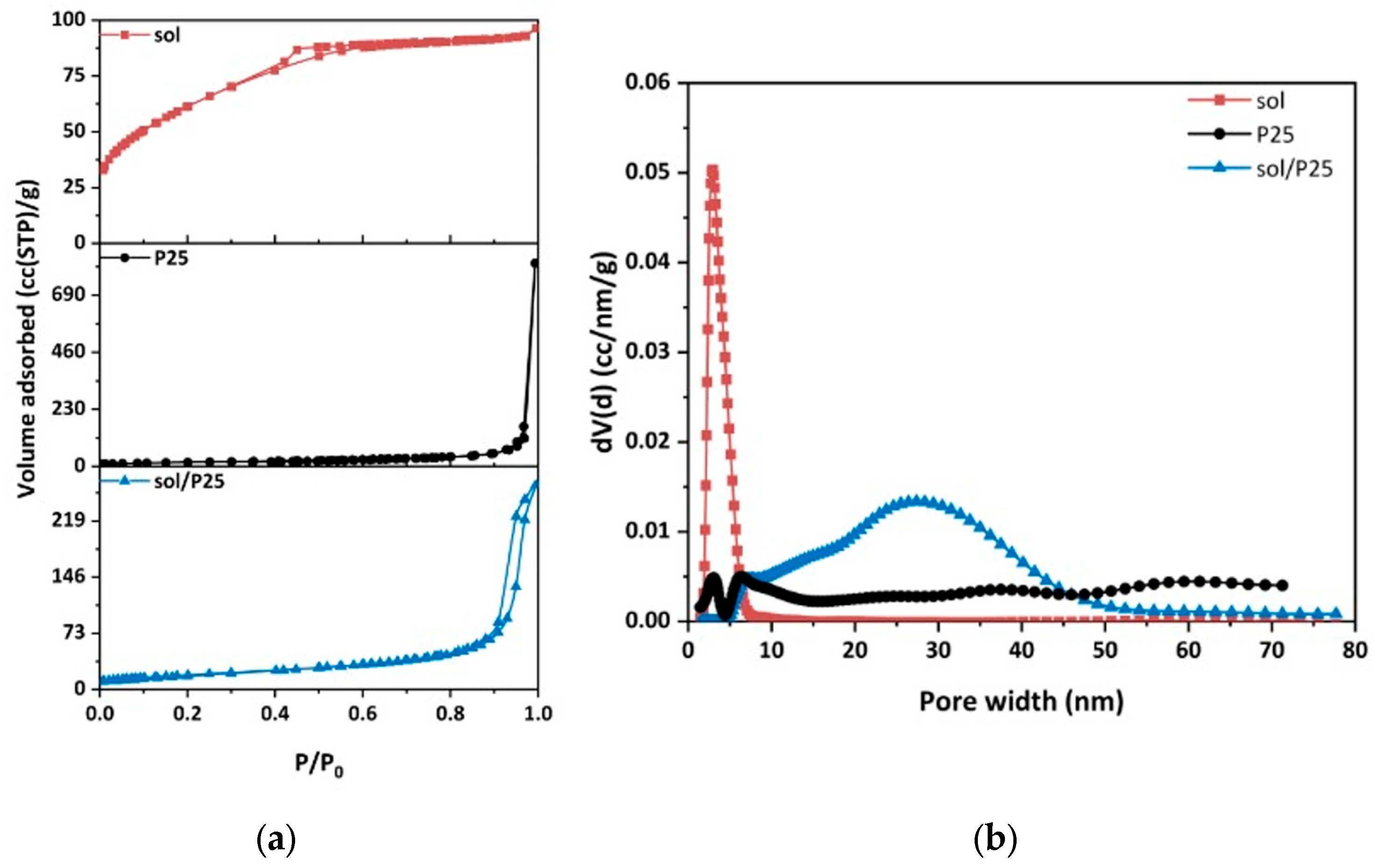
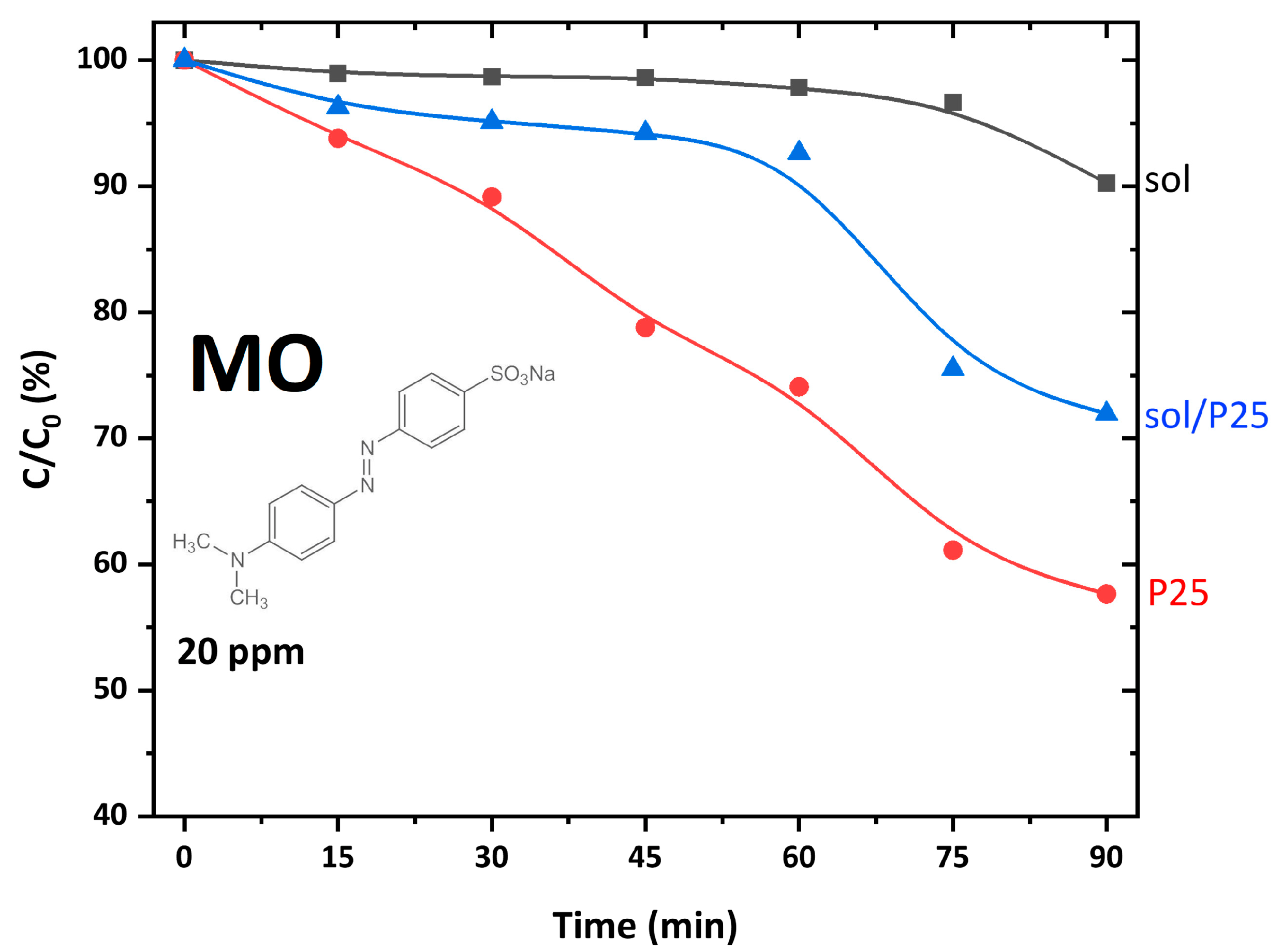
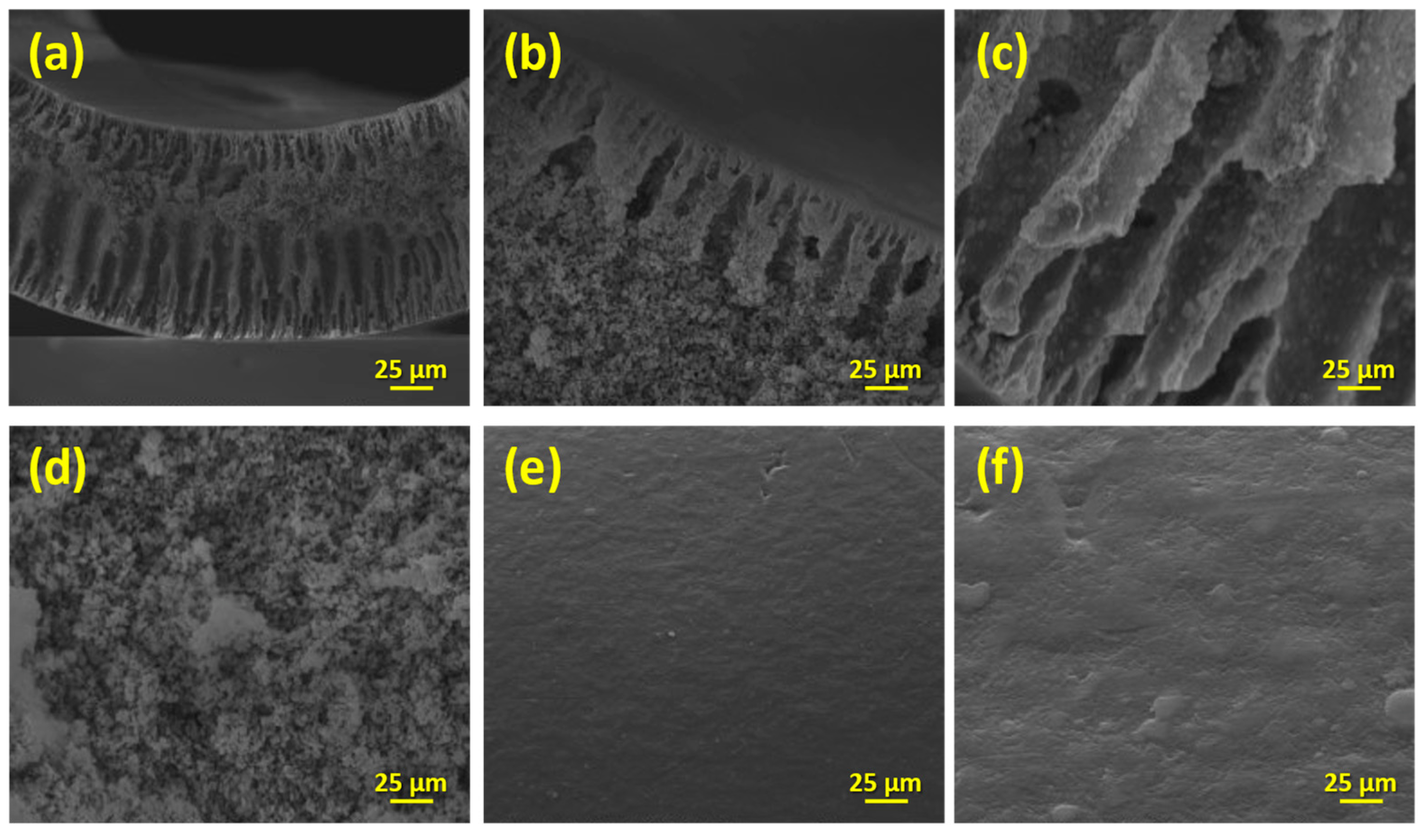

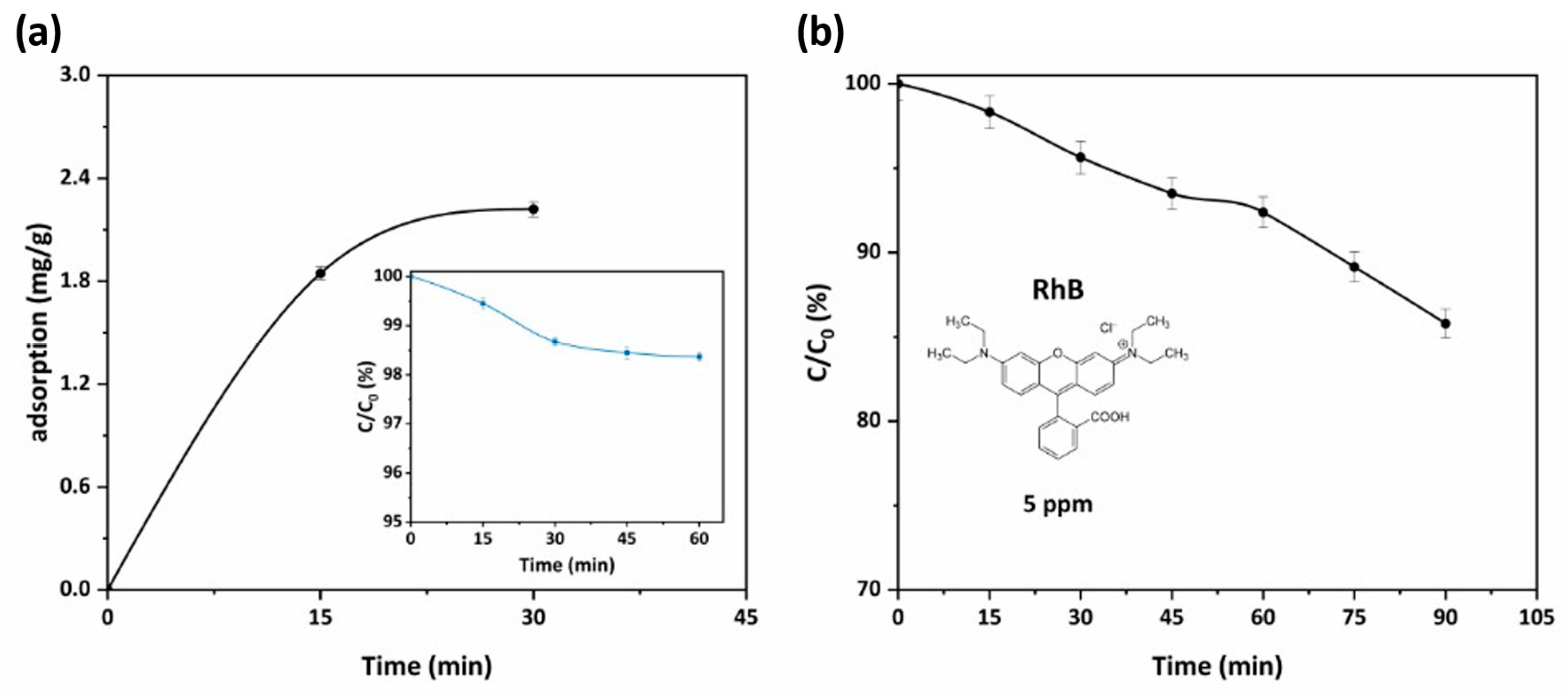
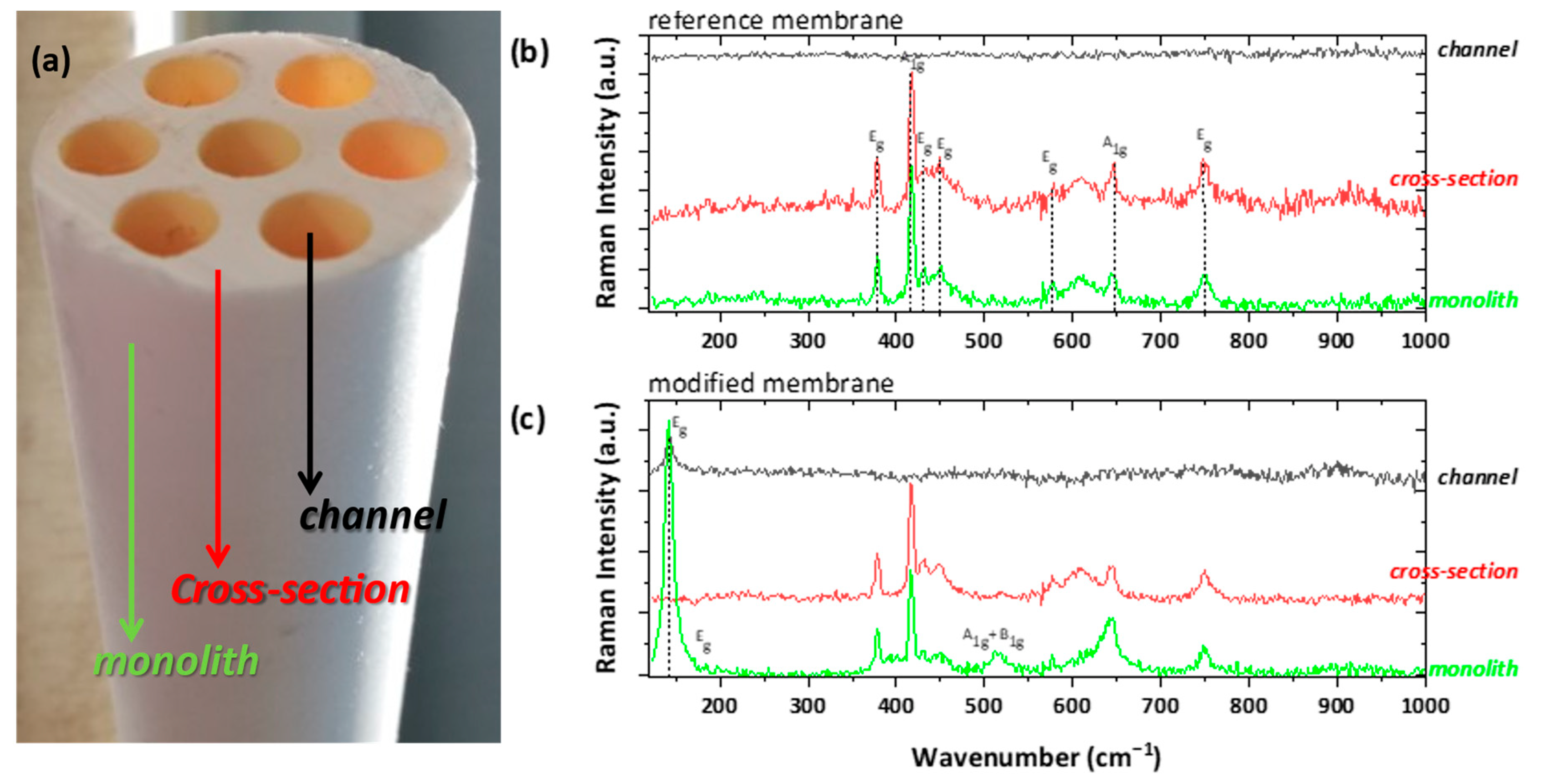
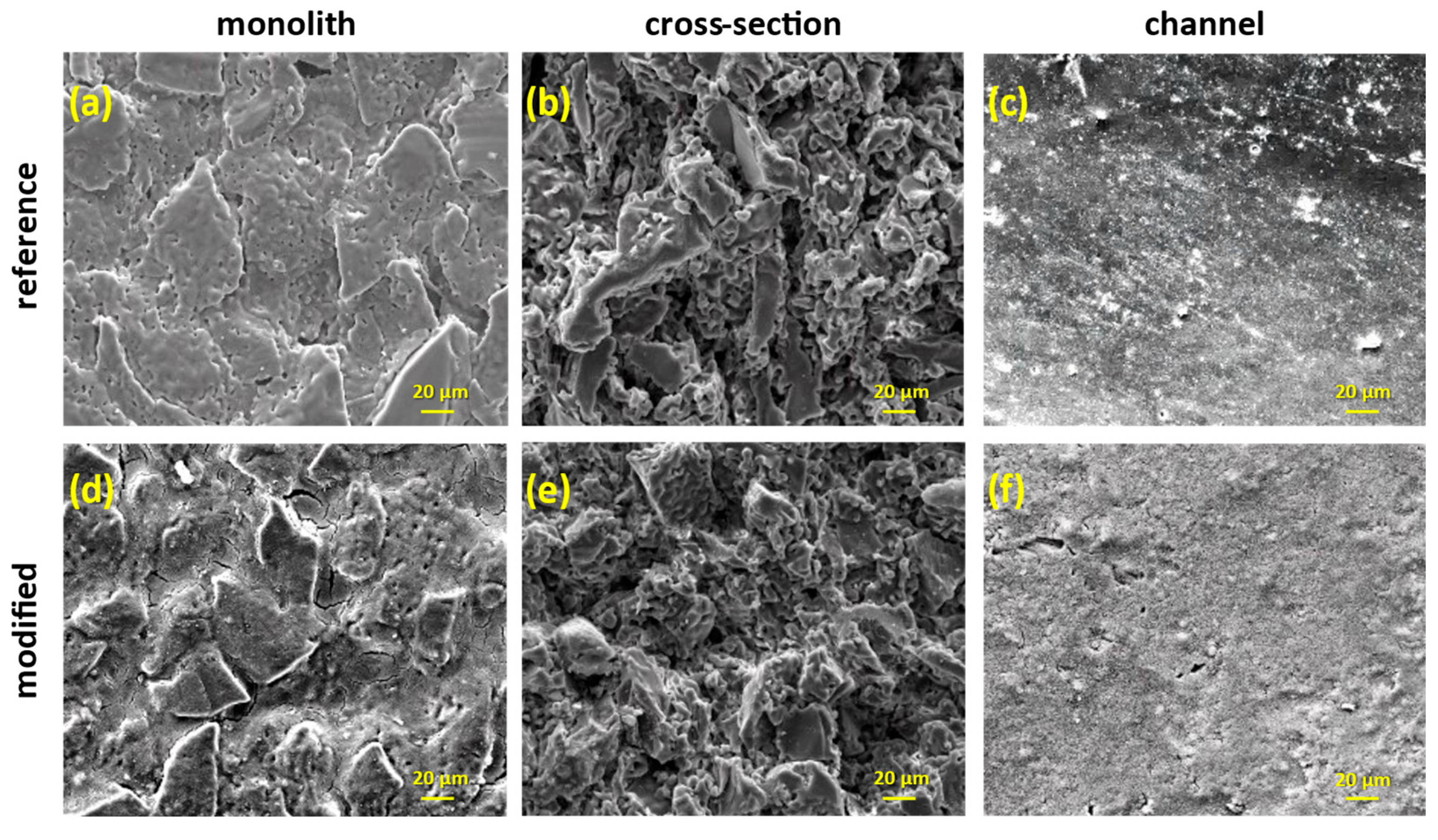

| Sample | Anatase (nm) | Rutile (nm) | wa (%) | wr (%) |
|---|---|---|---|---|
| sol/P25 | 18.5 | 27.6 | 84.7 | 15.3 |
| P25 | 20.0 | 27.6 | 76.8 | 23.2 |
| sol | 4.7 | - | - | - |
| Sample | TPV 1 | SBET | Porosity ε | dmean 2 | dBJH 3 | Dparticle 4 |
|---|---|---|---|---|---|---|
| (ml/g) | (m2/g) | (%) | (nm) | (nm) | (nm) | |
| sol/P25 | 0.342 | 68.6 | 56.8 | 19.9 | 31.6 | 22.7 |
| P25 | 0.176 | 62.0 | 40.7 | 11.3 | 51.2 | 24.8 |
| Sol | 0.144 | 224.9 | 35.2 | 2.6 | 3.5 | 7.1 |
| PHFs | 0.137 | 29.8 | 22.6 | 18.4 | 16.1 | 94.5 |
| Element | Cross-Section | External Surface | ||
|---|---|---|---|---|
| Near the Shell Surface | Intermediate Region | Near the Lumen Surface | ||
| C (wt.%) | 52.52 | 53.61 | 58.67 | 53.77 |
| O (wt.%) | 6.13 | 6.12 | 6.53 | 5.67 |
| F (wt.%) | 32.23 | 32.03 | 24.32 | 34.22 |
| Ti (wt.%) | 9.12 | 8.24 | 10.48 | 6.34 |
| Element | Monolith | Channel | ||||
|---|---|---|---|---|---|---|
| Upper | Middle | Bottom | Upper | Middle | Bottom | |
| Al (wt.%) | 64.81 | 65.6 | 9.20 | 11.34 | 9.15 | 21.32 |
| Ti (wt.%) | 6.38 | 6.63 | 77.99 | 51.27 | 51.97 | 34.44 |
| Run | Pressure | Feed Flow | Pollutant | UV Illumination |
|---|---|---|---|---|
| (bar) | (L/min) | (ppb) | ||
| 1 | 4.1 | 3.3 | ACT (50.0) | on |
| 2 | 4.4 | 3.3 | ACT (54.6) | on |
| 3 | 3.0 | 2.5 | ACT (54.8) | on |
| 4 | 3.1 | 2.4 | ACT (48.0) | off |
| 5 | 3.3 | 2.3 | TBZ (7.6) | on |
| 6 | 3.3 | 2.3 | TBZ (5.8) | off |
| Run | Cycle Time | Total Permeate Volume | Initial Amount of Pollutant | Total Amount of Pollutant Removed | Rejection |
|---|---|---|---|---|---|
| (min) | (L) | (mg) | (mg) | (%) | |
| 1 | 10.30 | 46.8 | 3.880 | 0.676 | 17.43 |
| 2 | 10.32 | 52.2 | 4.528 | 0.403 | 8.89 |
| 3 | 13.63 | 13.2 | 4.109 | 1.017 | 24.76 |
| 4 | 13.88 | 12.4 | 3.600 | 0.810 | 22.49 |
| 5 | 14.69 | 8.1 | 0.570 | 0.236 | 41.32 |
| 6 | 14.69 | 2.7 | 0.434 | 0.014 | 3.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theodorakopoulos, G.V.; Arfanis, M.K.; Sánchez Pérez, J.A.; Agüera, A.; Cadena Aponte, F.X.; Markellou, E.; Romanos, G.E.; Falaras, P. Novel Pilot-Scale Photocatalytic Nanofiltration Reactor for Agricultural Wastewater Treatment. Membranes 2023, 13, 202. https://doi.org/10.3390/membranes13020202
Theodorakopoulos GV, Arfanis MK, Sánchez Pérez JA, Agüera A, Cadena Aponte FX, Markellou E, Romanos GE, Falaras P. Novel Pilot-Scale Photocatalytic Nanofiltration Reactor for Agricultural Wastewater Treatment. Membranes. 2023; 13(2):202. https://doi.org/10.3390/membranes13020202
Chicago/Turabian StyleTheodorakopoulos, George V., Michalis K. Arfanis, José Antonio Sánchez Pérez, Ana Agüera, Flor Ximena Cadena Aponte, Emilia Markellou, George Em. Romanos, and Polycarpos Falaras. 2023. "Novel Pilot-Scale Photocatalytic Nanofiltration Reactor for Agricultural Wastewater Treatment" Membranes 13, no. 2: 202. https://doi.org/10.3390/membranes13020202
APA StyleTheodorakopoulos, G. V., Arfanis, M. K., Sánchez Pérez, J. A., Agüera, A., Cadena Aponte, F. X., Markellou, E., Romanos, G. E., & Falaras, P. (2023). Novel Pilot-Scale Photocatalytic Nanofiltration Reactor for Agricultural Wastewater Treatment. Membranes, 13(2), 202. https://doi.org/10.3390/membranes13020202









