Analysis of Operational Parameters in Acid and Base Production Using an Electrodialysis with Bipolar Membranes Pilot Plant
Abstract
:1. Introduction
2. Materials and Methods
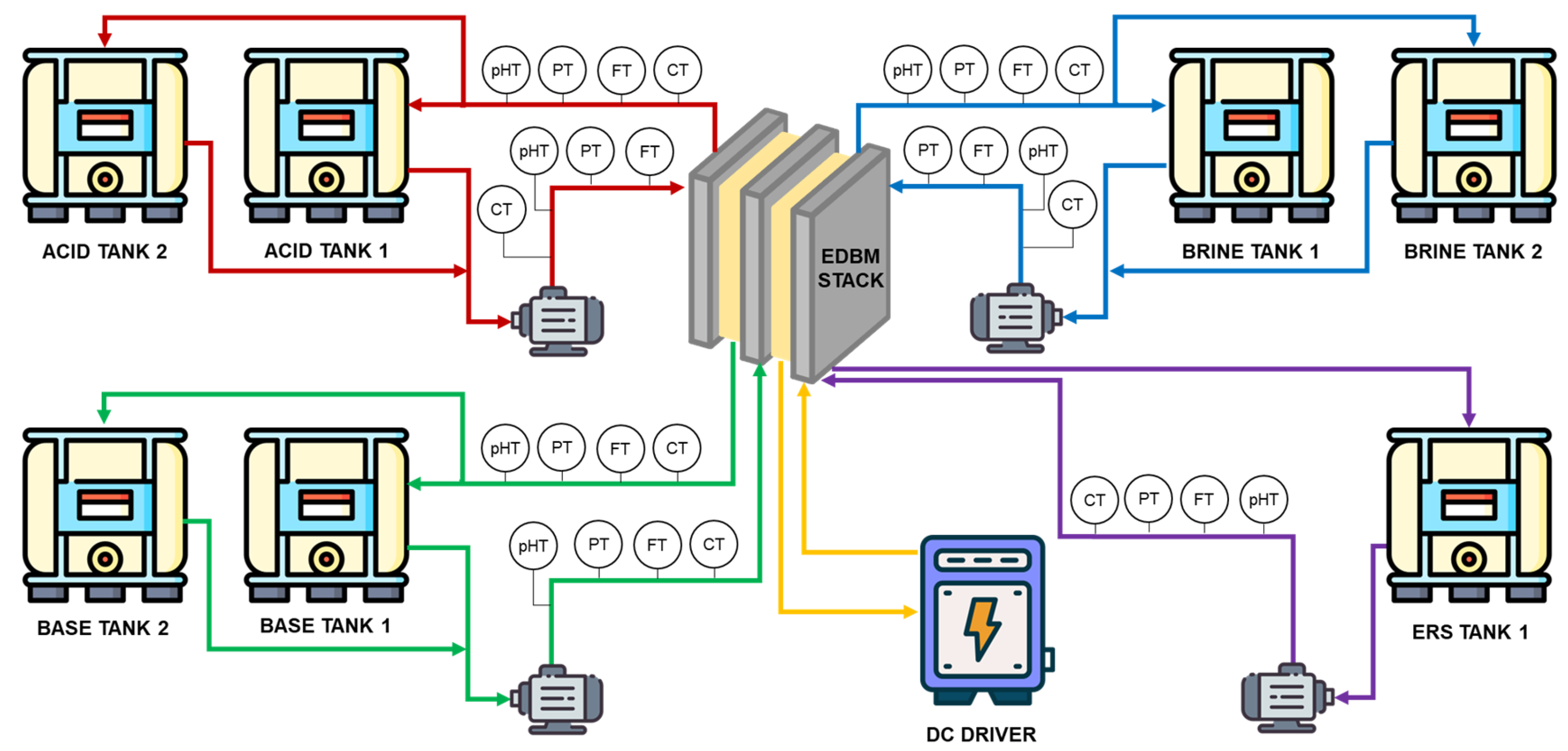
2.1. Experimental Set-Up
2.2. Experiments Design
2.3. Experimental Procedure
2.4. Calculation of Performance Parameters
3. Results and Discussion
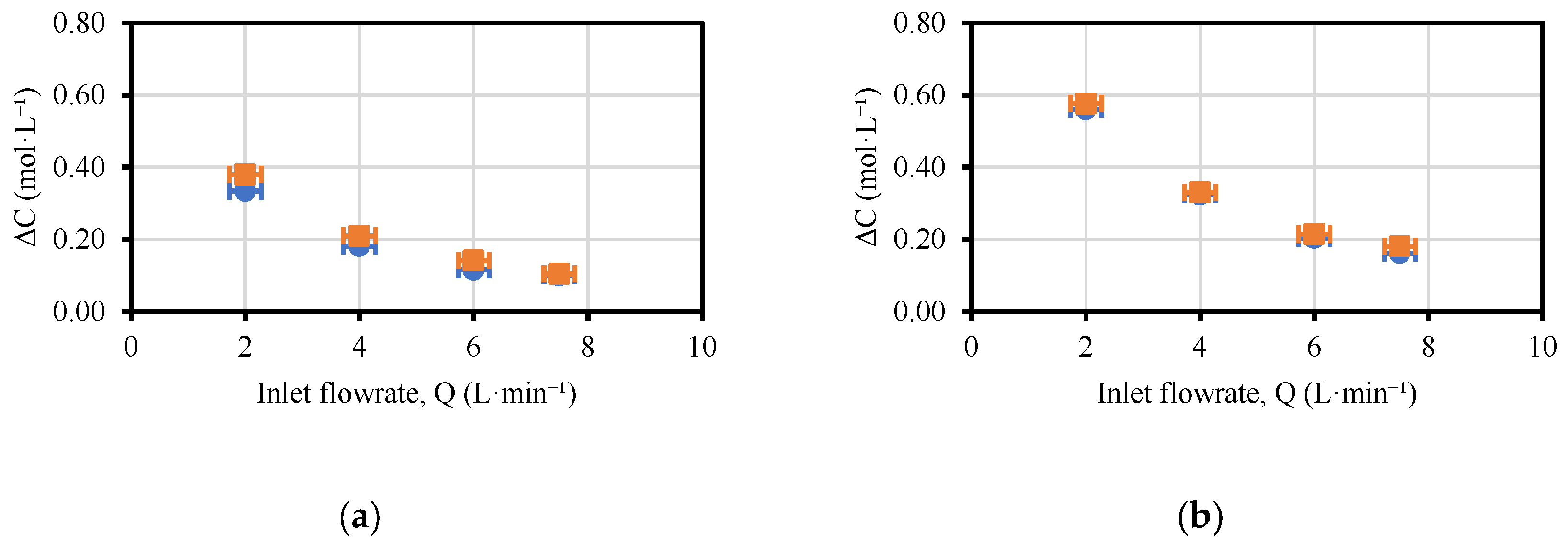
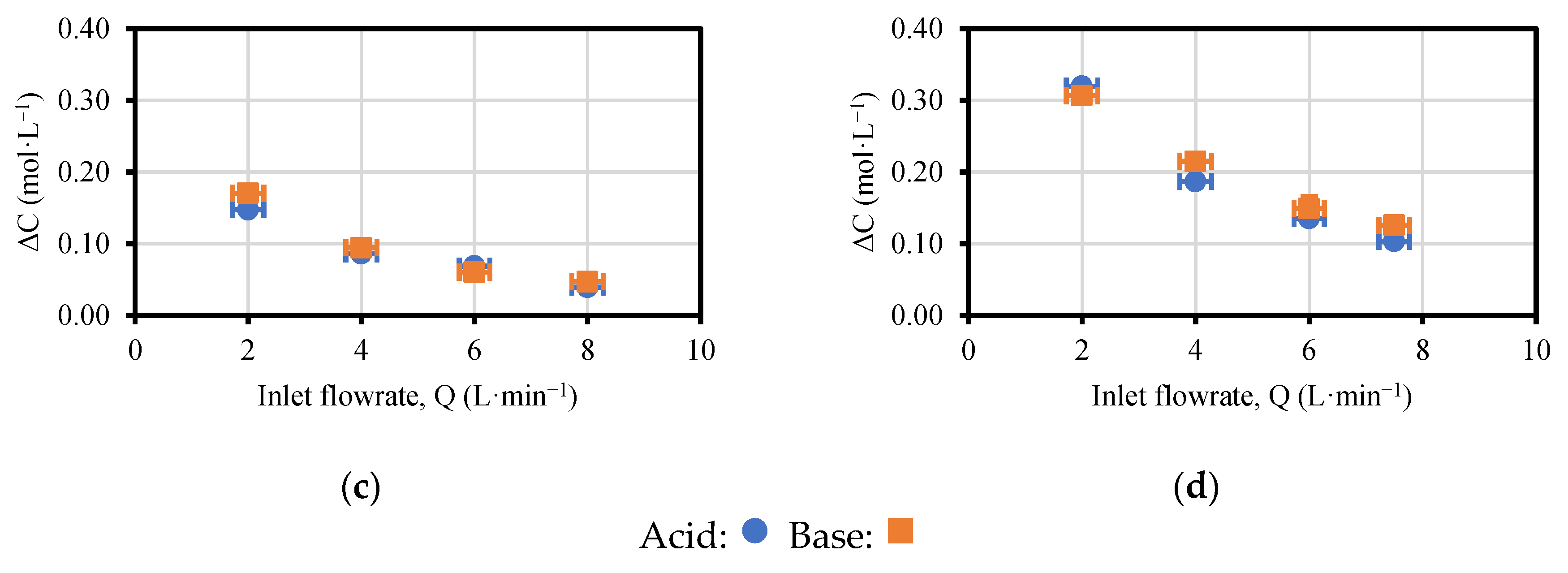
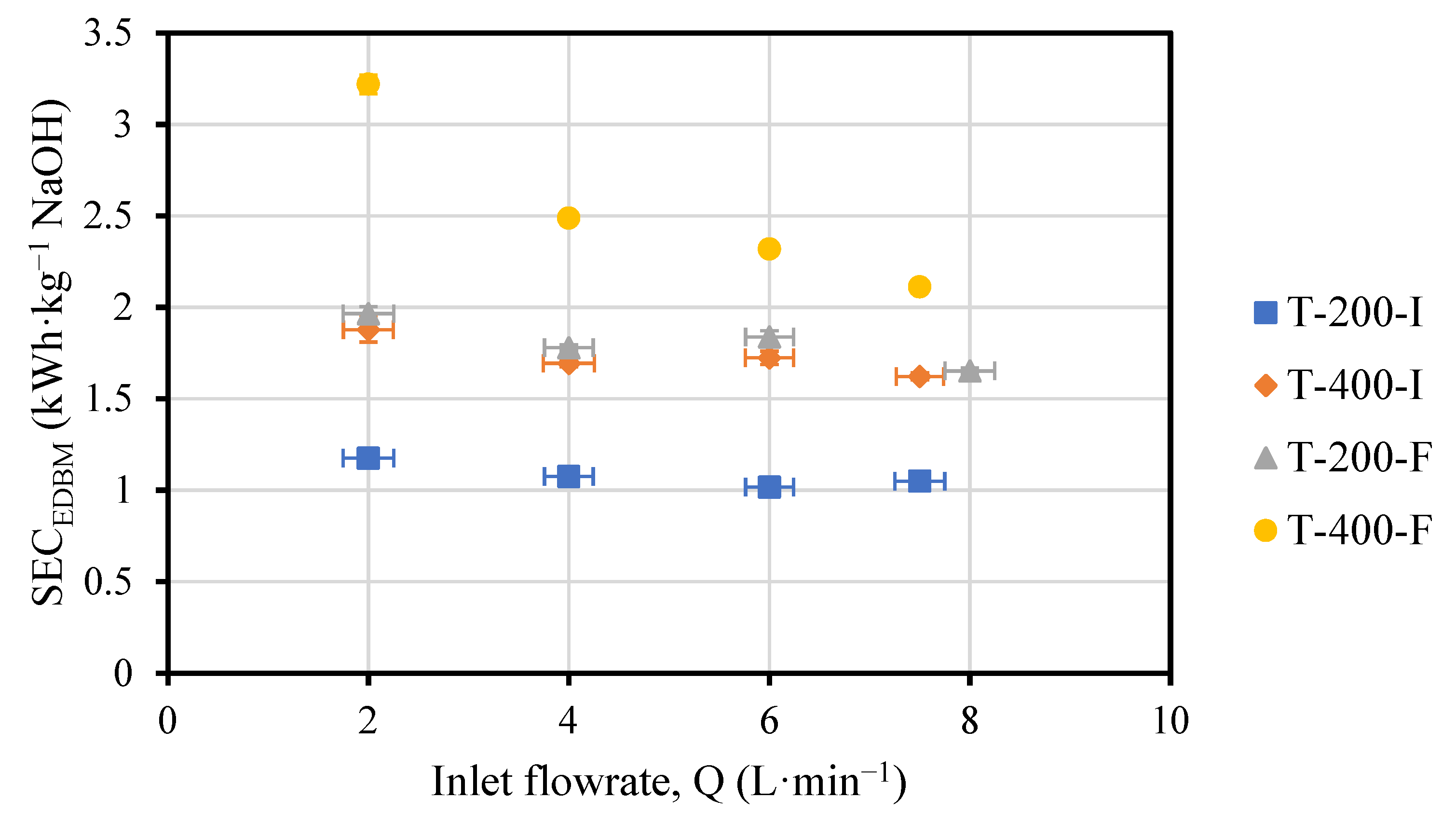
3.1. Effect of Acid, Base and Saline Flowrates on the Performance of the EDBM Unit
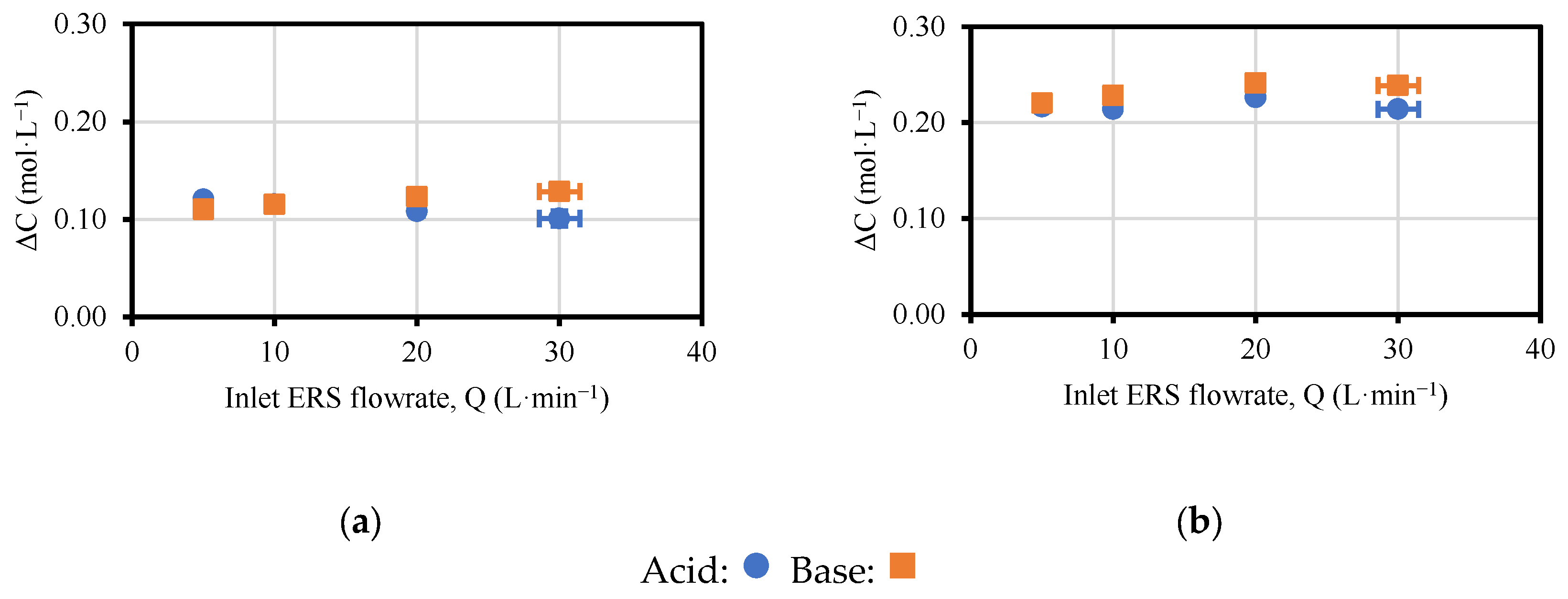
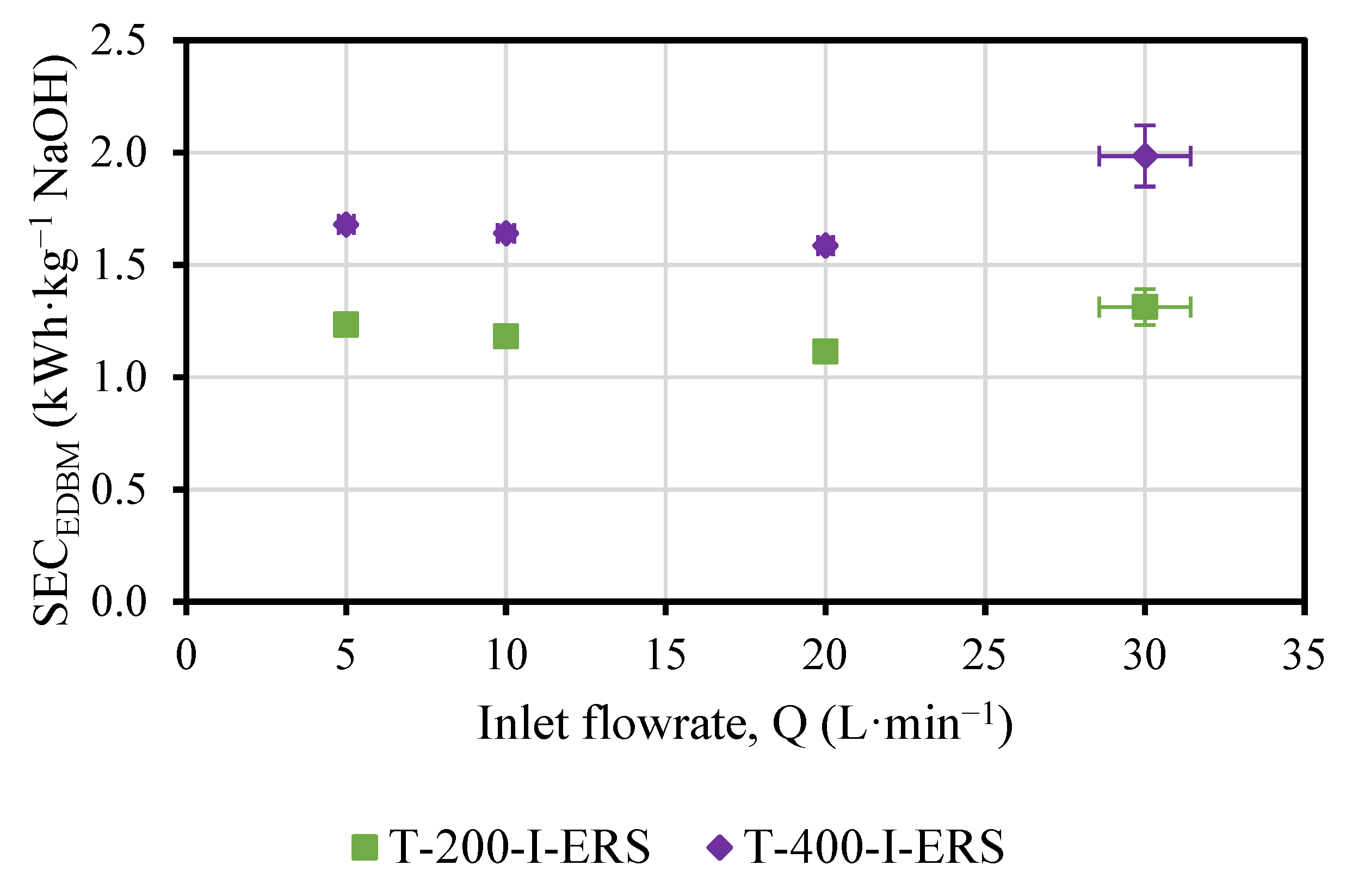
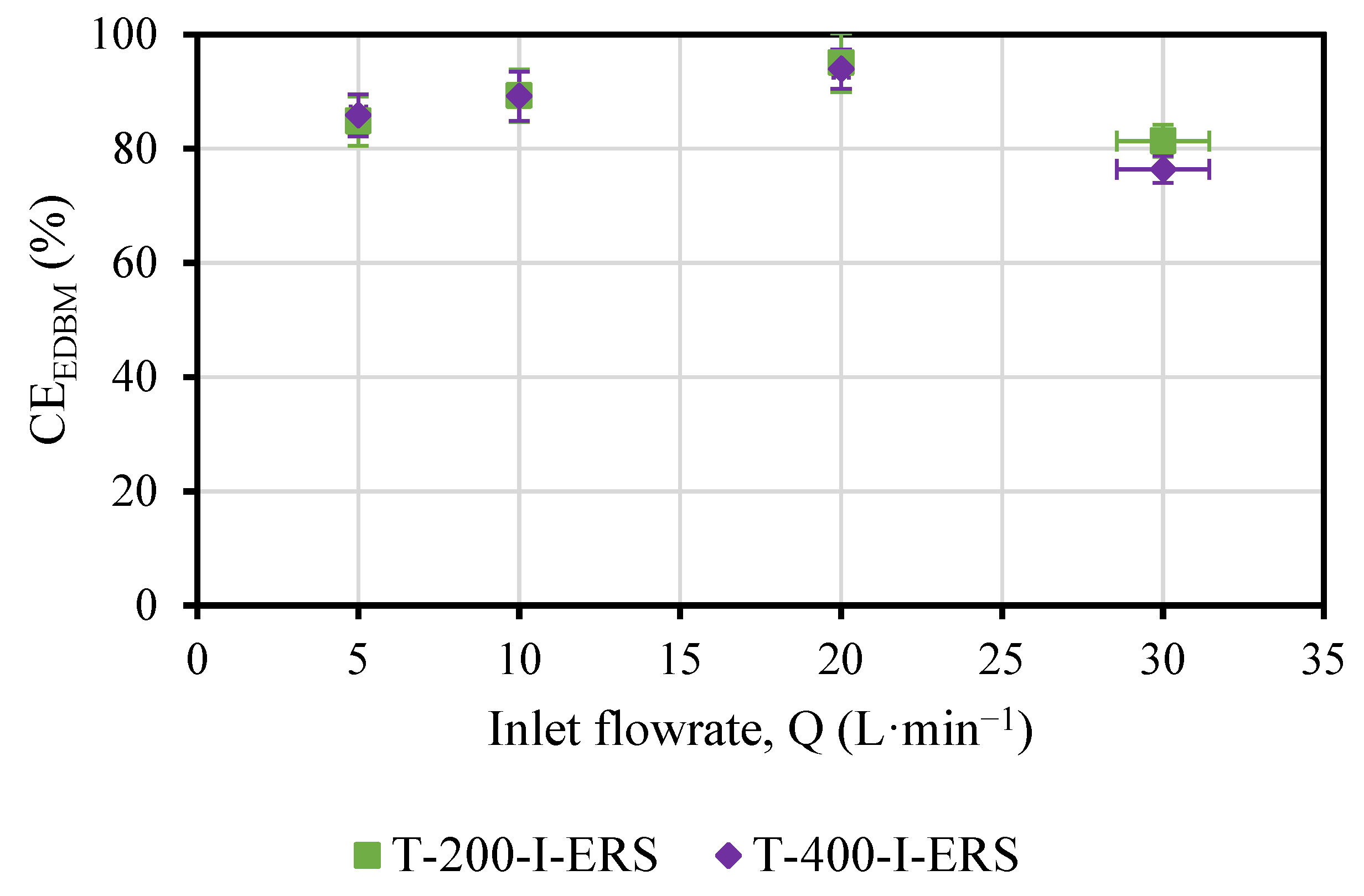
3.2. Effect of ERS Flowrate on the Performance of the EDBM Unit
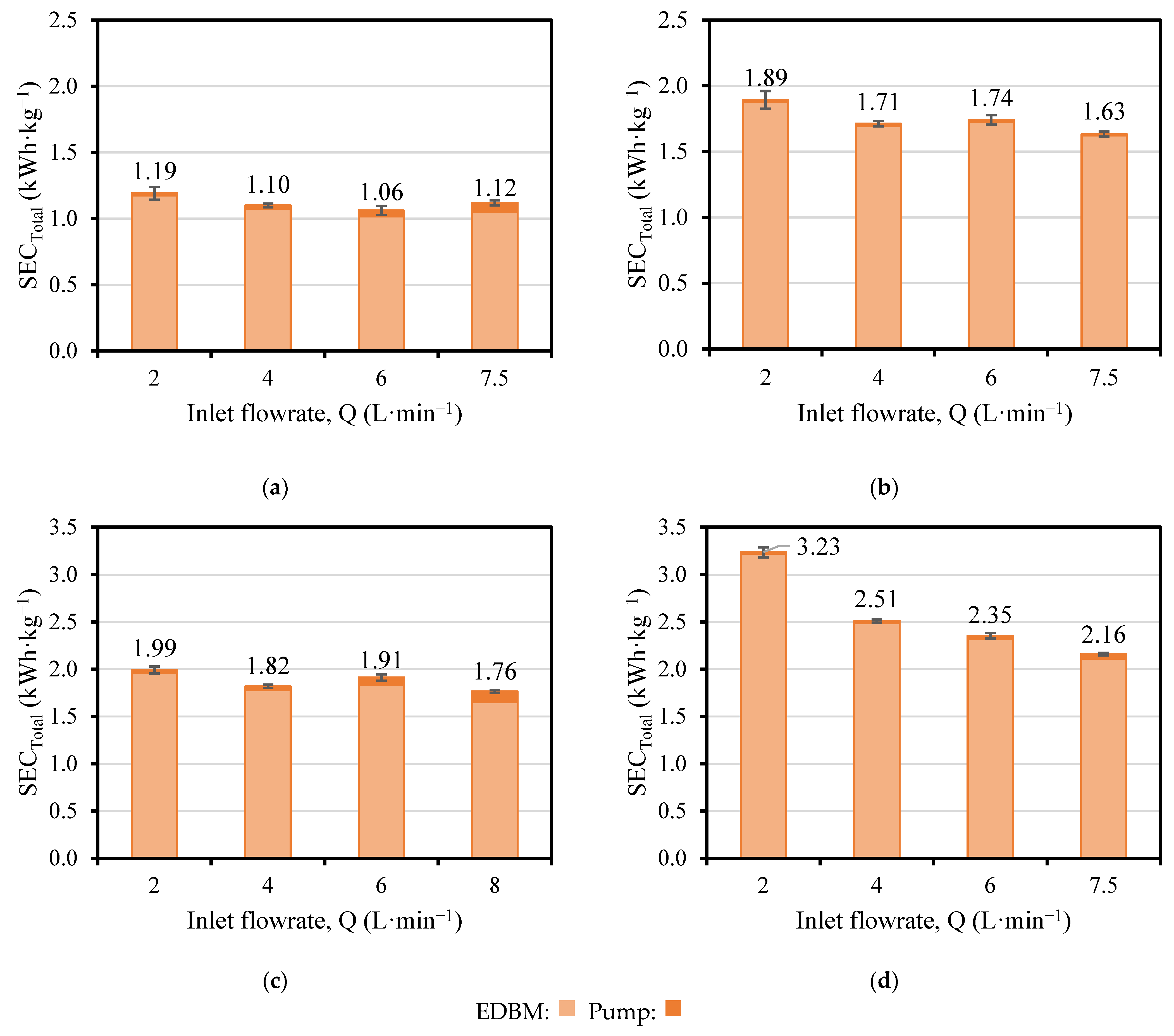
3.3. Evaluation of Pumping Contribution to SECTotal
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Acronyms/Abbreviations | |
| AEM | Anion exchange membrane |
| BPM | Bipolar membrane |
| CEM | Cation exchange membrane |
| EDBM | Electrodialysis with bipolar membranes |
| ERS | Electrode rinse solution |
| PP | Polypropylene |
| RO | Reverse osmosis |
| SWRO | Sea water reverse osmosis |
| TRL | Technology readiness level |
| ZLD | Zero liquid discharge |
Nomenclature
| Symbols | |
| C | Molar concentrations in the channel |
| CE | Current efficiency |
| F | Faraday constant |
| I | Current |
| MW | Molecular weight |
| n | Number of triplets |
| η | Pump efficiency |
| P | Pressure in the stack |
| Pcons | Energy consumed by the pumping system |
| Q | Volumetric flowrate in the stack |
| SEC | Specific energy consumption |
| V | Voltage |
| z | Ion valence |
| Subscripts/Superscripts | |
| EDBM | Related to the electrodialysis with bipolar membranes |
| in | Inlet |
| out | Outlet |
| pump | Related to pumping |
| Total | Related to both EDBM and pumping |
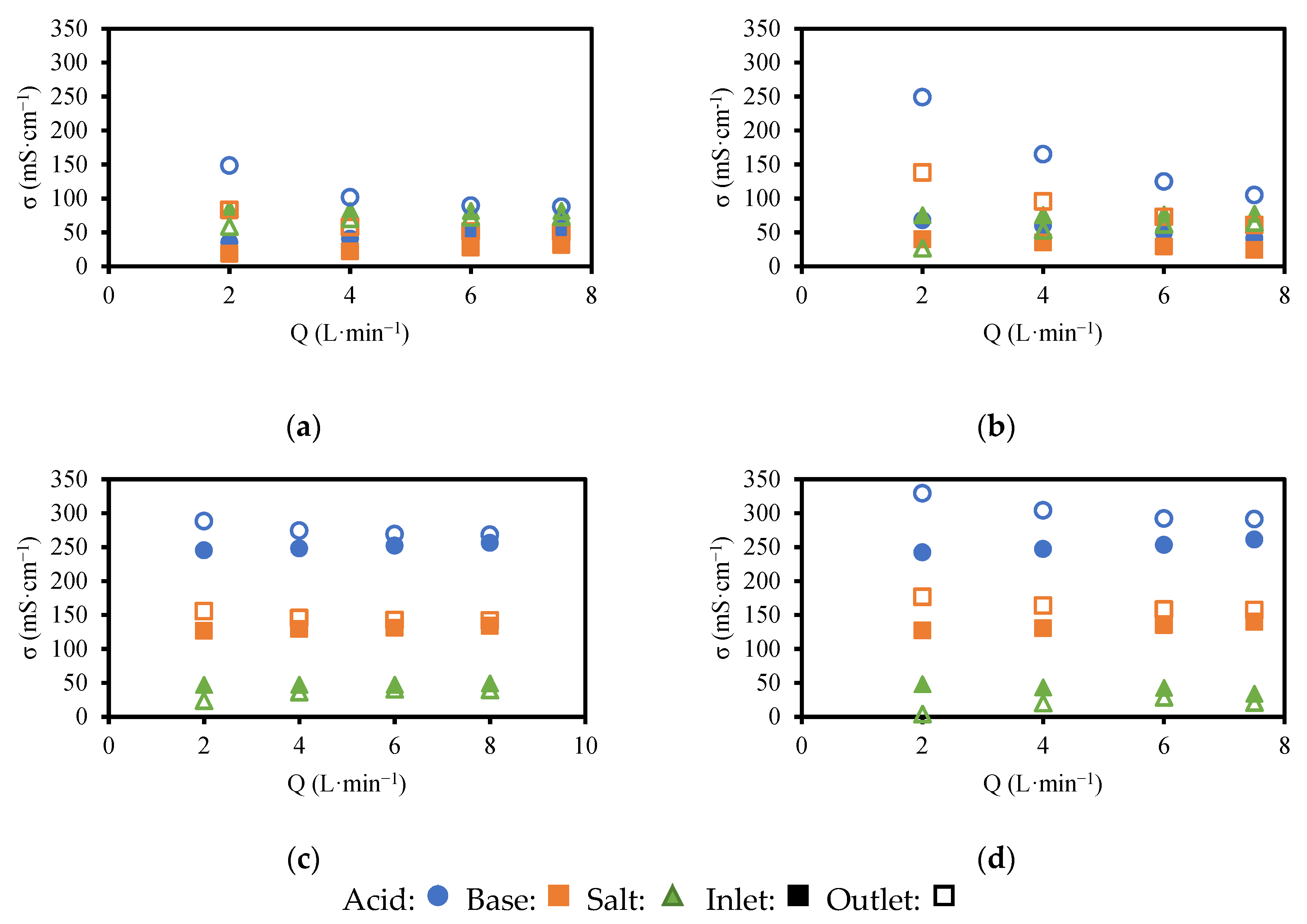
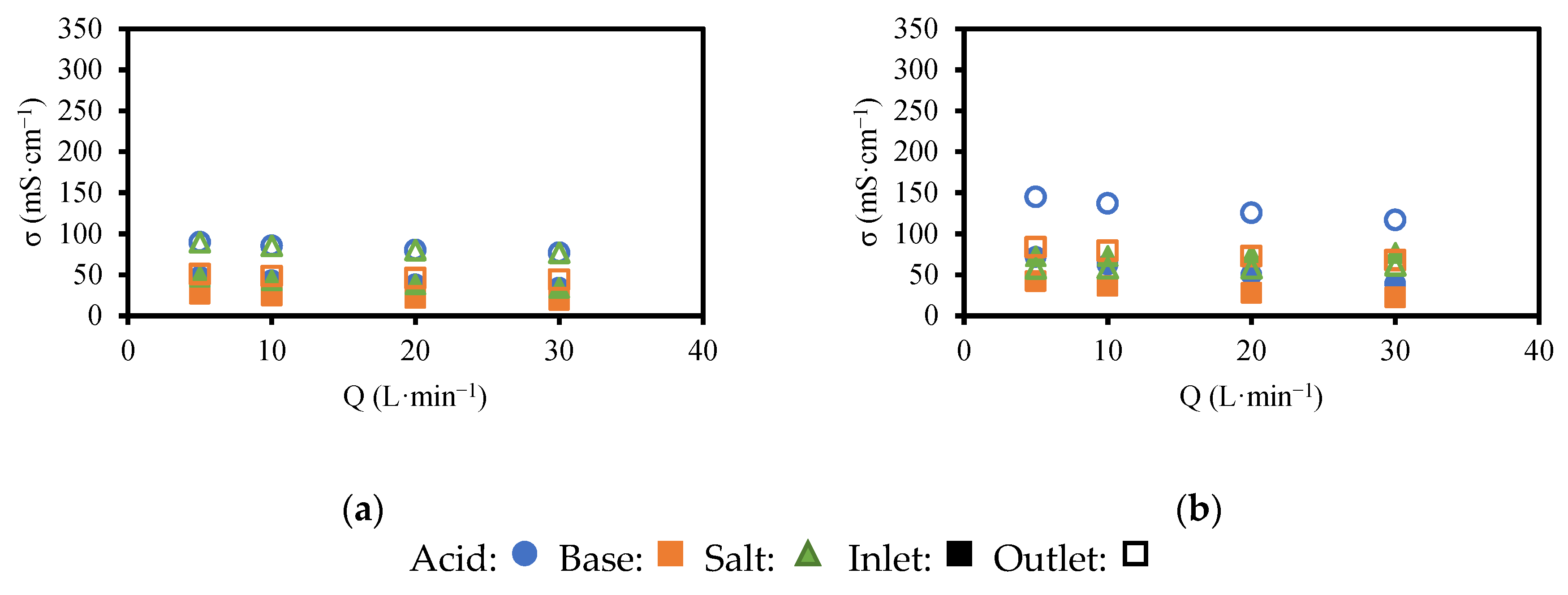
Appendix A. Electrical Conductivity Profiles
References
- Mekonnen, M.M.; Hoekstra, A.Y. Four billion people facing severe water scarcity. Sci. Adv. 2016, 2, e1500323. [Google Scholar] [CrossRef]
- He, C.; Liu, Z.; Wu, J.; Pan, X.; Fang, Z.; Li, J.; Bryan, B.A. Future global urban water scarcity and potential solutions. Nat. Commun. 2021, 12, 4667. [Google Scholar] [CrossRef]
- van Vliet, M.T.H.; Jones, E.R.; Flörke, M.; Franssen, W.H.P.; Hanasaki, N.; Wada, Y.; Yearsley, J.R. Global water scarcity including surface water quality and expansions of clean water technologies. Environ. Res. Lett. 2021, 16, 024020. [Google Scholar] [CrossRef]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Mariñas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef]
- Elimelech, M.; Phillip, W.A. The Future of Seawater Desalination: Energy, Technology, and the Environment. Science 2011, 333, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Department of Economic and Social Affairs, U.N. Ensure Availability and Sustainable Management of Water and Sanitation for All. Available online: https://sdgs.un.org/goals/goal6 (accessed on 11 October 2022).
- Panagopoulos, A. Water-energy nexus: Desalination technologies and renewable energy sources. Environ. Sci. Pollut. Res. 2021, 28, 21009–21022. [Google Scholar] [CrossRef]
- Elsaid, K.; Kamil, M.; Sayed, E.T.; Abdelkareem, M.A.; Wilberforce, T.; Olabi, A. Environmental impact of desalination technologies: A review. Sci. Total Environ. 2020, 748, 141528. [Google Scholar] [CrossRef] [PubMed]
- Mavukkandy, M.O.; Chabib, C.M.; Mustafa, I.; al Ghaferi, A.; AlMarzooqi, F. Brine Management in Desalination Industry: From Waste to Resources Generation. Desalination 2019, 472, 114187. [Google Scholar] [CrossRef]
- Morillo, J.; Usero, J.; Rosado, D.; El Bakouri, H.; Riaza, A.; Bernaola, F.-J. Comparative study of brine management technologies for desalination plants. Desalination 2014, 336, 32–49. [Google Scholar] [CrossRef]
- Gacia, E.; Invers, O.; Manzanera, M.; Ballesteros, E.; Romero, J. Impact of the brine from a desalination plant on a shallow seagrass (Posidonia oceanica) meadow. Estuar. Coast. Shelf Sci. 2007, 72, 579–590. [Google Scholar] [CrossRef]
- Meneses, M.; Pasqualino, J.; Céspedes-Sánchez, R.; Castells, F. Alternatives for Reducing the Environmental Impact of the Main Residue From a Desalination Plant. J. Ind. Ecol. 2010, 14, 512–527. [Google Scholar] [CrossRef]
- Oren, Y.; Korngold, E.; Daltrophe, N.; Messalem, R.; Volkman, Y.; Aronov, L.; Weismann, M.; Bouriakov, N.; Glueckstern, P.; Gilron, J. Pilot studies on high recovery BWRO-EDR for near zero liquid discharge approach. Desalination 2010, 261, 321–330. [Google Scholar] [CrossRef]
- Pérez-González, A.; Urtiaga, A.; Ibáñez, R.; Ortiz, I. State of the art and review on the treatment technologies of water reverse osmosis concentrates. Water Res. 2012, 46, 267–283. [Google Scholar] [CrossRef] [PubMed]
- Zarzo, D. Beneficial Uses and Valorization of Reverse Osmosis Brines; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128167120. [Google Scholar]
- Revolve Water-Mining. Available online: https://watermining.eu/ (accessed on 11 October 2022).
- Morgante, C.; Vassallo, F.; Battaglia, G.; Cipollina, A.; Vicari, F.; Tamburini, A.; Micale, G. Influence of Operational Strategies for the Recovery of Magnesium Hydroxide from Brines at a Pilot Scale. Ind. Eng. Chem. Res. 2022, 61, 15355–15368. [Google Scholar] [CrossRef] [PubMed]
- Culcasi, A.; Ktori, R.; Pellegrino, A.; Rodriguez-Pascual, M.; van Loosdrecht, M.; Tamburini, A.; Cipollina, A.; Xevgenos, D.; Micale, G. Towards sustainable production of minerals and chemicals through seawater brine treatment using Eutectic freeze crystallization and Electrodialysis with bipolar membranes. J. Clean. Prod. 2022, 368, 133143. [Google Scholar] [CrossRef]
- Fernandez-Gonzalez, C.; Dominguez-Ramos, A.; Ibañez, R.; Irabien, A. Electrodialysis with Bipolar Membranes for Valorization of Brines. Sep. Purif. Rev. 2016, 45, 275–287. [Google Scholar] [CrossRef]
- Thiel, G.P.; Kumar, A.; Gómez-González, A.; Lienhard, V.J.H. Utilization of Desalination Brine for Sodium Hydroxide Production: Technologies, Engineering Principles, Recovery Limits, and Future Directions. ACS Sustain. Chem. Eng. 2017, 5, 11147–11162. [Google Scholar] [CrossRef]
- Campione, A.; Gurreri, L.; Ciofalo, M.; Micale, G.; Tamburini, A.; Cipollina, A. Electrodialysis for water desalination: A critical assessment of recent developments on process fundamentals, models and applications. Desalination 2018, 434, 121–160. [Google Scholar] [CrossRef]
- Ibáñez, R.; Pérez-González, A.; Gómez, P.; Urtiaga, A.; Ortiz, I. Acid and base recovery from softened reverse osmosis (RO) brines. Experimental assessment using model concentrates. Desalination 2013, 309, 165–170. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, X.; Fan, A.; Fu, L.; Gao, C. An innovative beneficial reuse of seawater concentrate using bipolar membrane electrodialysis. J. Membr. Sci. 2014, 449, 119–126. [Google Scholar] [CrossRef]
- Davis, J.R.; Chen, Y.; Baygents, J.C.; Farrell, J. Production of Acids and Bases for Ion Exchange Regeneration from Dilute Salt Solutions Using Bipolar Membrane Electrodialysis. ACS Sustain. Chem. Eng. 2015, 3, 2337–2342. [Google Scholar] [CrossRef]
- Reig, M.; Casas, S.; Gibert, O.; Valderrama, C.; Cortina, J. Integration of nanofiltration and bipolar electrodialysis for valorization of seawater desalination brines: Production of drinking and waste water treatment chemicals. Desalination 2016, 382, 13–20. [Google Scholar] [CrossRef]
- Herrero-Gonzalez, M.; Diaz-Guridi, P.; Dominguez-Ramos, A.; Irabien, A.; Ibañez, R. Highly concentrated HCl and NaOH from brines using electrodialysis with bipolar membranes. Sep. Purif. Technol. 2020, 242, 116785. [Google Scholar] [CrossRef]
- Gazigil, L.; Er, E.; Kestioğlu, O.E.; Yonar, T. Pilot-Scale Test Results of Electrodialysis Bipolar Membrane for Reverse-Osmosis Concentrate Recovery. Membranes 2022, 12, 83. [Google Scholar] [CrossRef] [PubMed]
- Blommaert, M.A.; Aili, D.; Tufa, R.A.; Li, Q.; Smith, W.A.; Vermaas, D.A. Insights and Challenges for Applying Bipolar Membranes in Advanced Electrochemical Energy Systems. ACS Energy Lett. 2021, 6, 2539–2548. [Google Scholar] [CrossRef]
- Cassaro, C.; Virruso, G.; Culcasi, A.; Cipollina, A.; Tamburini, A.; Micale, G. Electrodialysis with Bipolar Membranes for the Sustainable Production of Chemicals from Seawater Brines at Pilot Plant Scale. ACS Sustain. Chem. Eng. 2023. [Google Scholar] [CrossRef]
- FuMa-Tech BWT GmbH Membranes for Water Treatment Processes. Available online: https://www.fumatech.com/en/products/membranes-water-treatment-processes/ (accessed on 13 January 2023).
- Culcasi, A.; Gurreri, L.; Cipollina, A.; Tamburini, A.; Micale, G. A comprehensive multi-scale model for bipolar membrane electrodialysis (BMED). Chem. Eng. J. 2022, 437, 135317. [Google Scholar] [CrossRef]
- Herrero-Gonzalez, M.; Ibañez, R. Technical and Environmental Feasibilities of the Commercial Production of NaOH from Brine by Means of an Integrated EDBM and Evaporation Process. Membranes 2022, 12, 885. [Google Scholar] [CrossRef]
- Micari, M.; Cipollina, A.; Tamburini, A.; Moser, M.; Bertsch, V.; Micale, G. Combined membrane and thermal desalination processes for the treatment of ion exchange resins spent brine. Appl. Energy 2019, 254, 113699. [Google Scholar] [CrossRef]
- Mareev, S.; Evdochenko, E.; Wessling, M.; Kozaderova, O.; Niftaliev, S.; Pismenskaya, N.; Nikonenko, V. A comprehensive mathematical model of water splitting in bipolar membranes: Impact of the spatial distribution of fixed charges and catalyst at bipolar junction. J. Membr. Sci. 2020, 603, 118010. [Google Scholar] [CrossRef]


| Membrane | FAB-PK-130 | FKB-PK-130 | FBM |
|---|---|---|---|
| Membrane type | AEM | CEM | BPM |
| Reinforcement | PEEK woven web | PEEK woven web | PEEK woven web |
| Thickness (µm) | 130 | 130 | 150 |
| Resistance (mΩ·cm2) 1 | 8 | 6.5 | 110 |
| Selectivity (%) 1 | >95 | >97 | >95 |
| Swelling at 80 °C per dimension (%) | <4 | < 4 | <5 |
| E-Modulus (MPa) | >1.500 | >1.500 | >1.500 |
| Code | Current Density (A·m−2) | Chamber | Concentrations (mol·L−1) | Flowrate (L·min−1) | Chamber Flow Velocity (cm·s−1) |
|---|---|---|---|---|---|
| T-200-I | 200 | Acid (HCl) | 0.1 | 2.0–7.5 | 0.88–3.30 |
| Base (NaOH) | 0.1 | 2.0–7.5 | 0.88–3.30 | ||
| Saline (NaCl) | 1.0 | 2.0–7.5 | 0.88–3.30 | ||
| ERS (Na2SO4) | 0.25 | 20 | 4.40 | ||
| T-400-I | 400 | Acid (HCl) | 0.1 | 2.0–7.5 | 0.88–3.30 |
| Base (NaOH) | 0.1 | 2.0–7.5 | 0.88–3.30 | ||
| Saline (NaCl) | 1.0 | 2.0–7.5 | 0.88–3.30 | ||
| ERS (Na2SO4) | 0.25 | 20 | 4.40 | ||
| T-200-F | 200 | Acid (HCl) | 0.7 | 2.0–8.0 | 0.88–3.52 |
| Base (NaOH) | 0.7 | 2.0–8.0 | 0.88–3.52 | ||
| Saline (NaCl) | 0.5 | 2.0–8.0 | 0.88–3.52 | ||
| ERS (Na2SO4) | 0.25 | 20 | 4.40 | ||
| T-400-F | 400 | Acid (HCl) | 0.7 | 2.0–7.5 | 0.88–3.30 |
| Base (NaOH) | 0.7 | 2.0–7.5 | 0.88–3.30 | ||
| Saline (NaCl) | 0.5 | 2.0–7.5 | 0.88–3.30 | ||
| ERS (Na2SO4) | 0.25 | 20 | 4.40 | ||
| T-200-I-ERS | 200 | Acid (HCl) | 0.1 | 6.0 | 2.64 |
| Base (NaOH) | 0.1 | 6.0 | 2.64 | ||
| Saline (NaCl) | 1.0 | 6.0 | 2.64 | ||
| ERS (Na2SO4) | 0.25 | 5.0–30 | 1.10–6.60 | ||
| T-400-I-ERS | 400 | Acid (HCl) | 0.1 | 6.0 | 2.64 |
| Base (NaOH) | 0.1 | 6.0 | 2.64 | ||
| Saline (NaCl) | 1.0 | 6.0 | 2.64 | ||
| ERS (Na2SO4) | 0.25 | 5.0–30 | 1.10–6.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrero-Gonzalez, M.; López, J.; Virruso, G.; Cassaro, C.; Tamburini, A.; Cipollina, A.; Cortina, J.L.; Ibañez, R.; Micale, G. Analysis of Operational Parameters in Acid and Base Production Using an Electrodialysis with Bipolar Membranes Pilot Plant. Membranes 2023, 13, 200. https://doi.org/10.3390/membranes13020200
Herrero-Gonzalez M, López J, Virruso G, Cassaro C, Tamburini A, Cipollina A, Cortina JL, Ibañez R, Micale G. Analysis of Operational Parameters in Acid and Base Production Using an Electrodialysis with Bipolar Membranes Pilot Plant. Membranes. 2023; 13(2):200. https://doi.org/10.3390/membranes13020200
Chicago/Turabian StyleHerrero-Gonzalez, Marta, Julio López, Giovanni Virruso, Calogero Cassaro, Alessandro Tamburini, Andrea Cipollina, Jose Luis Cortina, Raquel Ibañez, and Giorgio Micale. 2023. "Analysis of Operational Parameters in Acid and Base Production Using an Electrodialysis with Bipolar Membranes Pilot Plant" Membranes 13, no. 2: 200. https://doi.org/10.3390/membranes13020200
APA StyleHerrero-Gonzalez, M., López, J., Virruso, G., Cassaro, C., Tamburini, A., Cipollina, A., Cortina, J. L., Ibañez, R., & Micale, G. (2023). Analysis of Operational Parameters in Acid and Base Production Using an Electrodialysis with Bipolar Membranes Pilot Plant. Membranes, 13(2), 200. https://doi.org/10.3390/membranes13020200