Obtaining Electrospun Membranes of Chitosan/PVA and TiO2 as a Solid Polymer Electrolyte with Potential Application in Ion Exchange Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
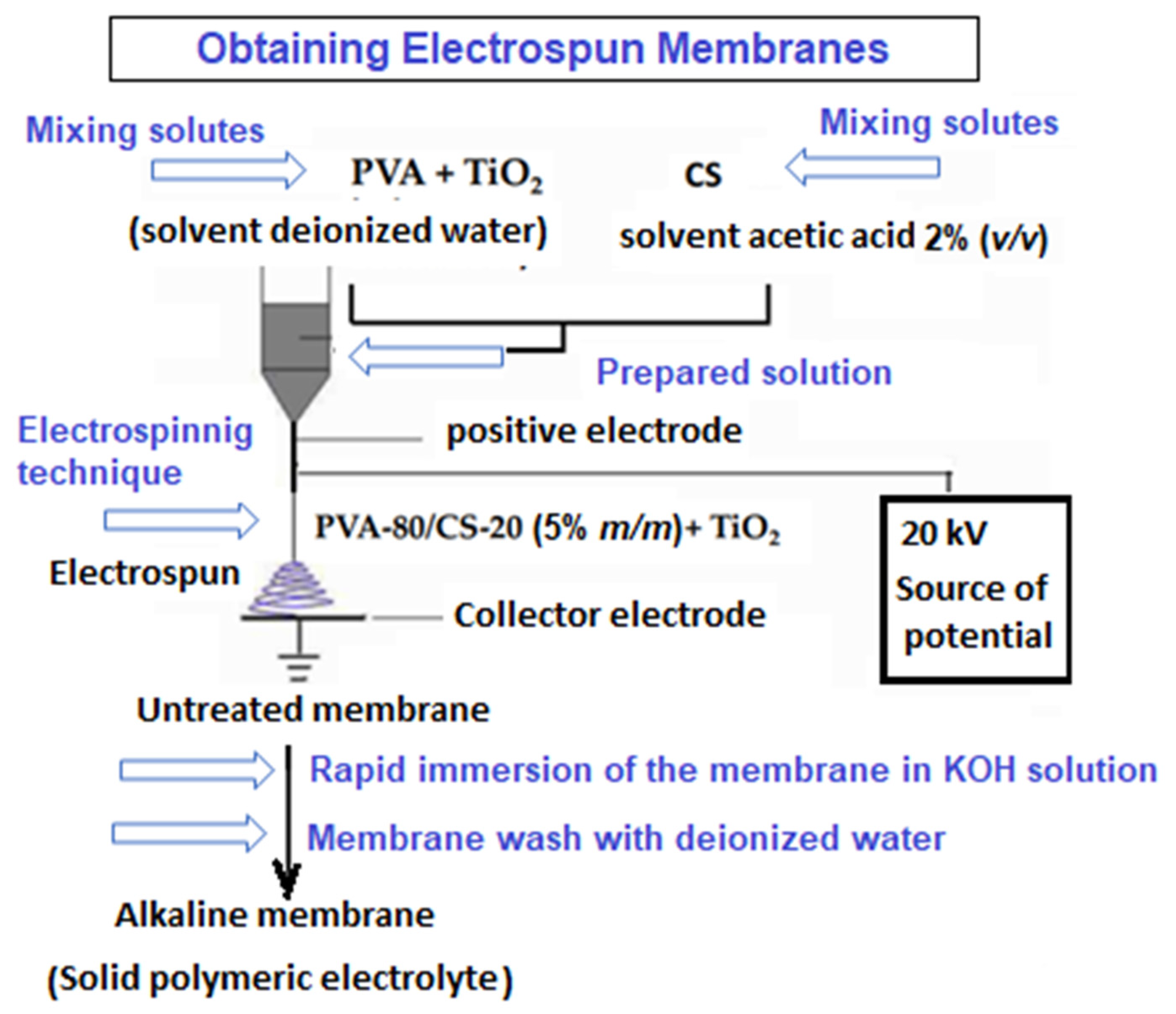
2.2. Preparation of Polymeric Nanofibers PVA/CS-x TiO2
2.3. Morphological Characterization
2.4. Thermal Characterization (DSC and TGA)
2.5. Moisture Absor Ption
2.6. Complex Impedance Spectroscopy
3. Results and Discussion
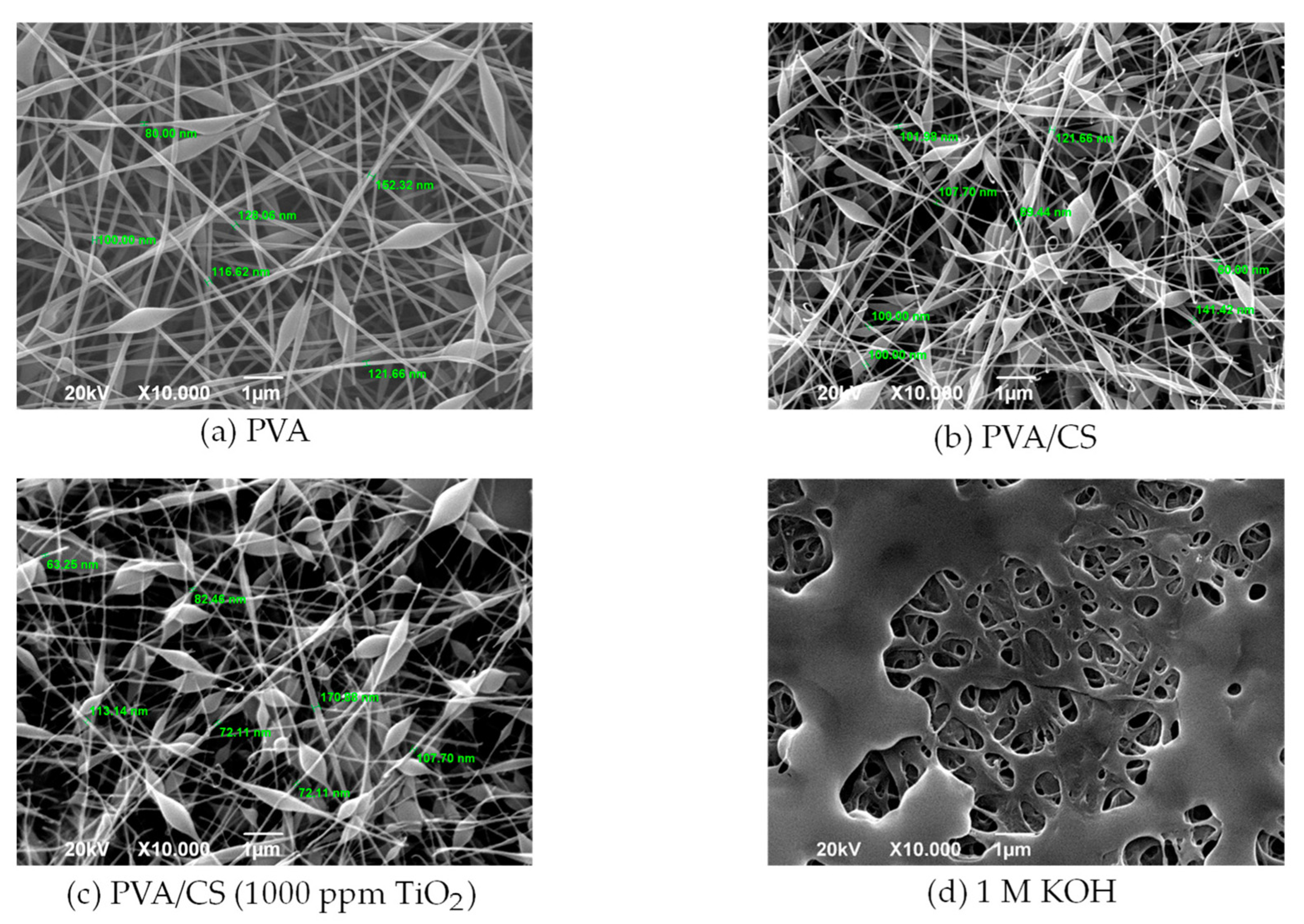
3.1. SEM Morphology
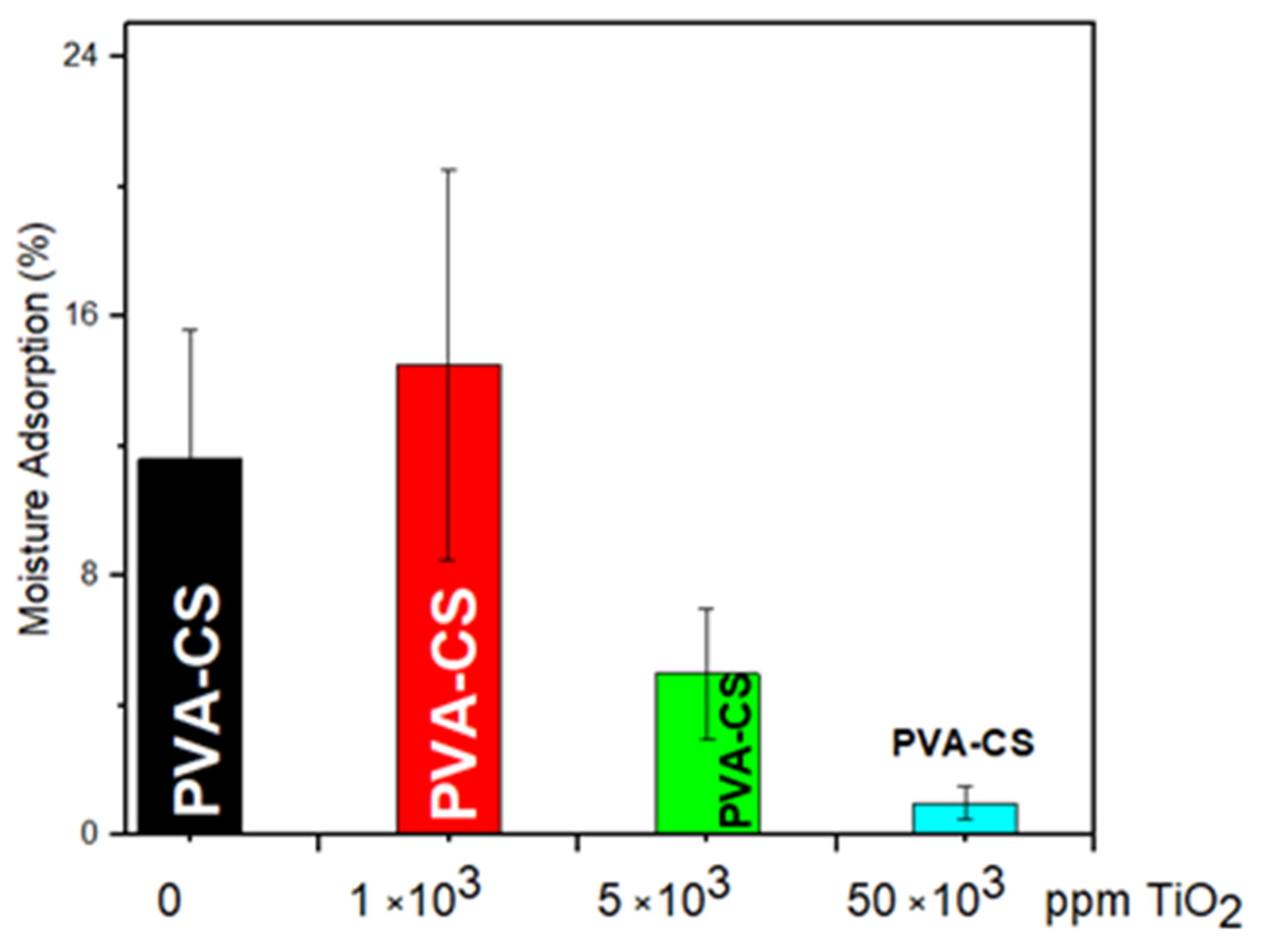
3.2. Moisture Absorption of PVA/CS-TiO2 (1000 to 50,000 ppm)
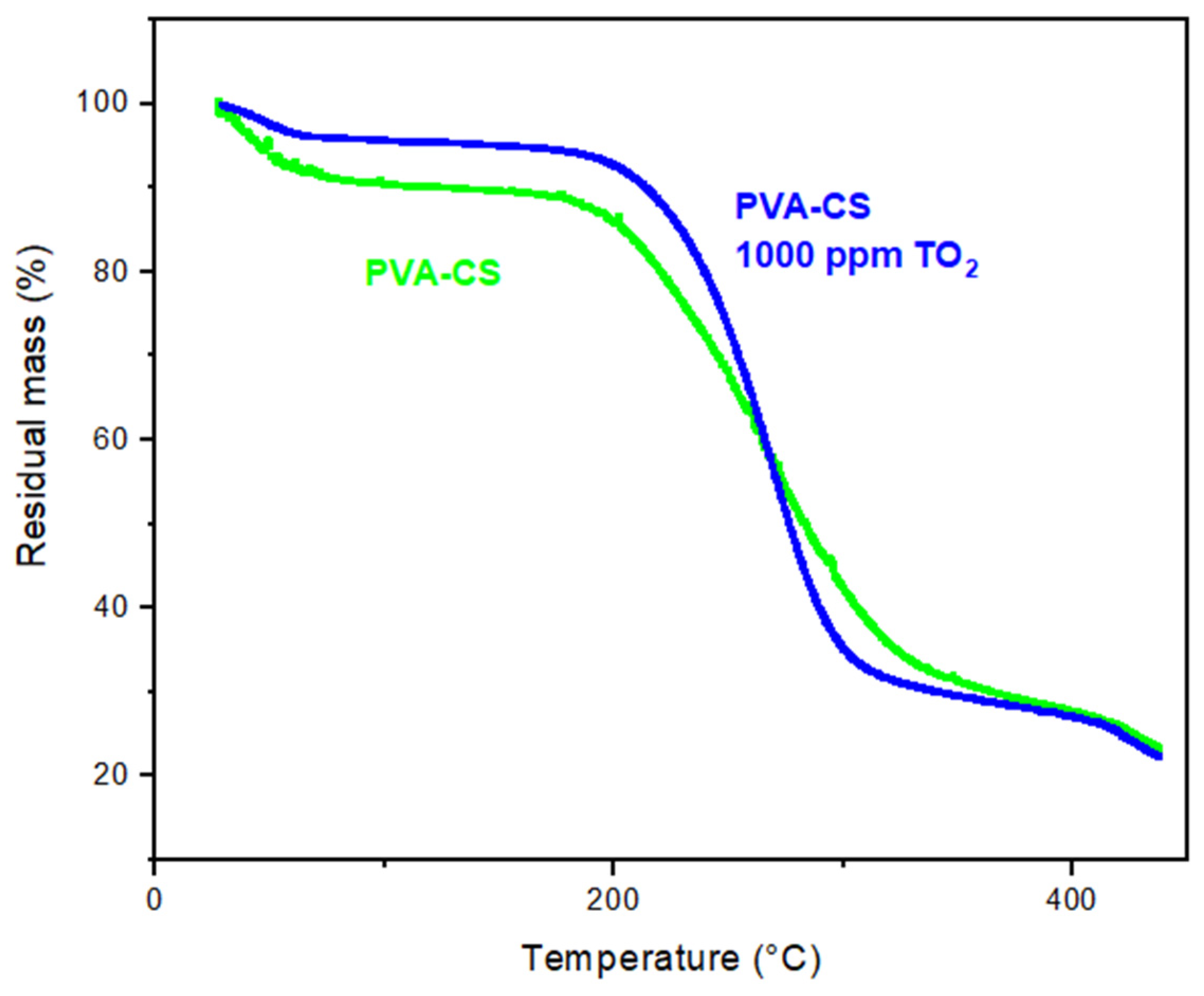
3.3. Thermogravimetric Analysis (TGA)
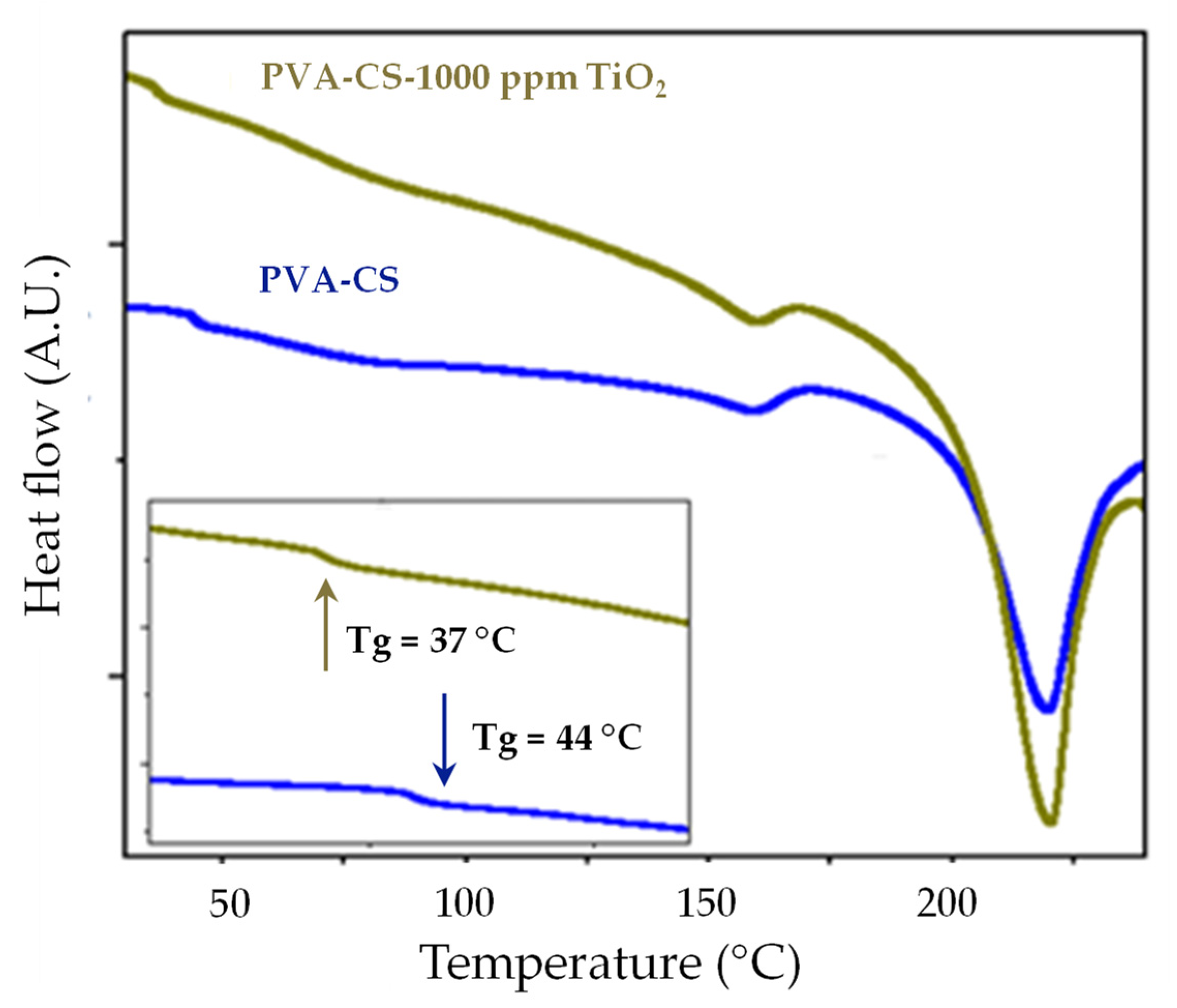
3.4. Differential Scanning Calorimetry (DSC)
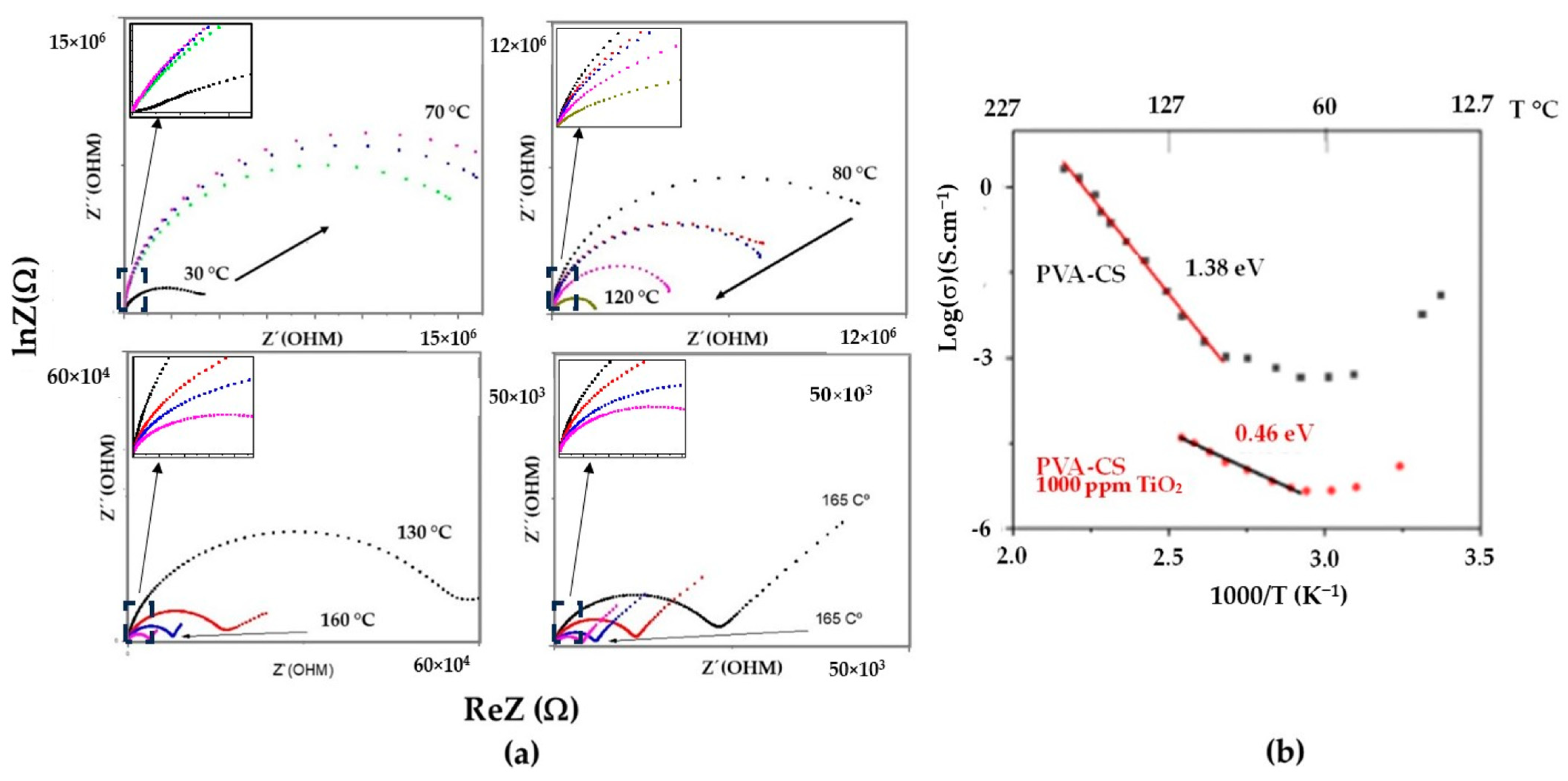
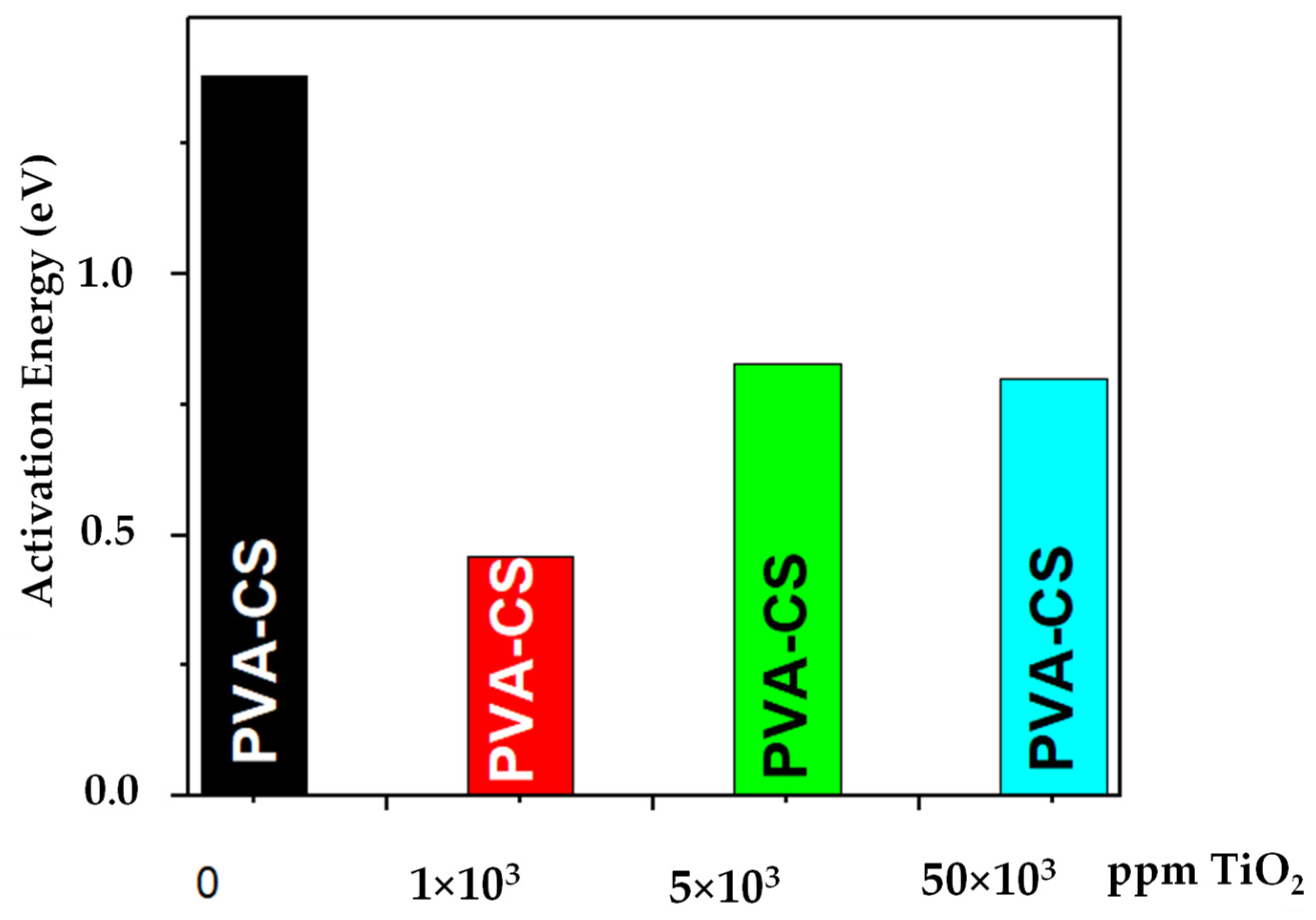
3.5. Complex Impedance Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atapour, M.; Rajaei, V.; Trasatti, S.; Casaletto, M.P.; Chiarello, G.L. Thin Niobium and Niobium Nitride PVD Coatings on AISI 304 Stainless Steel as Bipolar Plates for PEMFCs. Coatings 2020, 10, 889. [Google Scholar] [CrossRef]
- Tripathi, B.P.; Shahi, V.K. Organic-Inorganic nanocomposite polymer electrolyte membranes for fuel cell applications. Prog. Polym. Sci. 2020, 36, 945–979. [Google Scholar] [CrossRef]
- Ruiz, E.; Mina, J.; Diosa, J.E. Development of a Chitosan/PVA/TiO2 Nanocomposite for Application as a Solid Polymeric Electrolyte in Fuel Cells. Polymers 2020, 12, 1691. [Google Scholar] [CrossRef] [PubMed]
- Ayse, A.; Bozkurt, A. Nanocomposite polymer electrolyte membranes based on poly (vinyl phosphonic acid)/sulfated nano-titania. J. Power Sources 2012, 21, 158–163. [Google Scholar]
- Wang, J.; Wang, L. Preparation and properties of organic–inorganic alkaline hybrid membranes for direct methanol fuel cell application. Solid State Ion. 2013, 255, 96–103. [Google Scholar] [CrossRef]
- González, Y.F.; Vargas, R.A. Estudio de las Propiedades Termodinámicas y Eléctricas de Materiales Compuestos Poliméricos Basados En El Poli (Vinil Alcohol) (PVA) + H3PO2 + TiO2. Rev. Iberoam. Polím. 2011, 12, 64–75. [Google Scholar]
- Ali, M.; Gherissi, A. Synthesis and characterization of the composite material PVA/chitosan/5% sorbitol with different ratio of chitosan. Int. J. Mech. Mechatron. Eng. 2017, 17, 15–28. [Google Scholar]
- Aziz, S.B.; Abdullah, O.G.; Hussein, S.A.; Ahmed, H.M. Effect of PVA blending on structural and ion transport properties of CS:AgNt-Based polymer electrolyte membrane. Polymers 2017, 9, 622. [Google Scholar] [CrossRef]
- Benítez, M.; Diosa, J.E.; Vargas, R.A. Effect of H3PO2 on the mechanical, thermal, and electrical properties of polymers based on poly (vinyl alcohol) (PVA) and chitosan (CS). Ionics 2018, 24, 2029–2034. [Google Scholar] [CrossRef]
- Gonçalves, R.P.; Ferreira, W.H.; Gouvêa, R.F.; Andrade, C.T. Effect of chitosan on the properties of electrospun fibers from mixed poly(vinyl alcohol)/chitosan solutions. Mater. Res. 2017, 20, 984–993. [Google Scholar] [CrossRef]
- Quintana, D.A.; Baca, E.; Mosquera, E.; Vargas, R.A.; Diosa, J.E. Improving the ionic conductivity in nanostructured membranes based on poly(vinyl alcohol) (PVA), chitosan (CS), phosphoric acid (H3PO4), and niobium oxide (Nb2O5). Ionics 2019, 25, 1131–1136. [Google Scholar] [CrossRef]
- Jia, Y.T.; Gong, J.; Gu, X.H.; Kim, H.Y.; Dong, J.; Shen, X.Y. Fabrication and characterization of poly (vinyl alcohol)/chitosan blend nanofibers produced by electrospinning method. Carbohydr. Polym. 2007, 67, 403–409. [Google Scholar] [CrossRef]
- Xiao-Xiong, W.; Gui-Feng, Y.; Jun, Z.; Miao, Y.; Seeram, R.; Yun-Ze, L. Conductive polymer ultrafine fibers via electrospinning: Preparation, physical properties and applications. Prog. Mater. Sci. 2021, 115, 100704. [Google Scholar]
- Habiba, U.; Islam, M.S.; Siddique, T.A.; Afifi, A.M.; Ang, B.C. Adsorption and photocatalytic degradation of anionic dyes on Chitosan/PVA/Na–Titanate/TiO2 composites synthesized by solution casting method. Carbohydr. Polym. 2016, 149, 317–331. [Google Scholar] [CrossRef]
- Yang, J.M.; Fan, C.S.; Wang, N.C.; Chang, Y.H. Evaluation of membrane preparation method on the performance of alkaline polymer electrolyte: Comparison between poly(vinyl alcohol)/chitosan blended membrane and poly(vinyl alcohol)/chitosan electrospun nanofiber composite membranes. Electrochim. Acta 2018, 266, 332–340. [Google Scholar] [CrossRef]
- Yang, C.C.; Chiu, S.J.; Lee, K.T.; Chien, W.C.; Lin, C.T.; Huang, C.A. Study of poly (vinyl alcohol)/titanium oxide composite polymer membranes and their application on alkaline direct alcohol fuel cell. J. Power Sources 2008, 184, 44–51. [Google Scholar] [CrossRef]
- Guan, Y.; Li, W.; Zhang, Y.; Shi, Z.; Tan, J.; Wang, F.; Wang, Y. Aramid nanofibers and poly (vinyl alcohol) nanocomposites for ideal combination of strength and toughness via hydrogen bonding interactions. Compos. Sci. Technol. 2017, 144, 193–201. [Google Scholar] [CrossRef]
- Fernández, M.A.; Castillo, J.E.; Bedoya, F.; Diosa, J.E.; Vargas, R.A. Dependence of the mechanical and electrical properties on the acid content in PVA + H3PO2 + H2O membranes. Rev. Mex. De Física 2014, 60, 249–252. [Google Scholar]
- Yang, C.C.; Li, Y.J.; Liou, T.H. Preparation of novel poly (vinyl alcohol)/SiO2 Nanocomposite membranes by a sol–gel process and their application on alkaline DMFCs. Desalination 2011, 276, 366–372. [Google Scholar] [CrossRef]
- Vargas, M.A.; Vargas, R.A.; Mellander, B.E. More studies on the PVAl_H3PO2 + H2O proton conductor gels. Electrochim. Acta 2000, 45, 1399–1403. [Google Scholar] [CrossRef]
- Mollá, S.; Campañ, V. Performance of composite Nafion/PVA membranes for direct methanol fuel cells. J. Power Sources 2011, 196, 2699–2708. [Google Scholar] [CrossRef]
- González-Campos, J.; Betzabe del Río Rosa, E. Compuestos de quitosano/nanopartículas de Ag: Conductividad y mecanismos de relajación y su relación con sus propiedades macroscópicas. Sociedad Mexicana de Ciencia y Tecnología de Superficies y Materiales. Superficies y Vacío 2012, 25, 43–48. [Google Scholar]
- Wan, Y.; Creber, K.A.; Peppley, B.; Tam Bui, V. Chitosan-based solid electrolyte composite membranes I. Preparation and characterization. J. Membr. Sci. 2006, 280, 666–674. [Google Scholar] [CrossRef]
- Permana, D.; Ilimu, E.; Faariu, N.M.; Setyawati, A.; Kadidae, L.O.; Ramadhan, L.O.A.N. Synthesis and characterization of chitosan-polyvinyl alcohol-Fe2O3 composite membrane for DMFC application. Makara J. Sci. 2020, 24, 1–9. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez, E.E.R.; Hernandez, J.H.M. Obtaining Electrospun Membranes of Chitosan/PVA and TiO2 as a Solid Polymer Electrolyte with Potential Application in Ion Exchange Membranes. Membranes 2023, 13, 862. https://doi.org/10.3390/membranes13110862
Gómez EER, Hernandez JHM. Obtaining Electrospun Membranes of Chitosan/PVA and TiO2 as a Solid Polymer Electrolyte with Potential Application in Ion Exchange Membranes. Membranes. 2023; 13(11):862. https://doi.org/10.3390/membranes13110862
Chicago/Turabian StyleGómez, Elio Enrique Ruiz, and Jose Herminsul Mina Hernandez. 2023. "Obtaining Electrospun Membranes of Chitosan/PVA and TiO2 as a Solid Polymer Electrolyte with Potential Application in Ion Exchange Membranes" Membranes 13, no. 11: 862. https://doi.org/10.3390/membranes13110862
APA StyleGómez, E. E. R., & Hernandez, J. H. M. (2023). Obtaining Electrospun Membranes of Chitosan/PVA and TiO2 as a Solid Polymer Electrolyte with Potential Application in Ion Exchange Membranes. Membranes, 13(11), 862. https://doi.org/10.3390/membranes13110862






