Prolonged Anesthesia Effects of Locally Administered Ropivacaine via Electrospun Poly(caprolactone) Fibrous Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PCL Fibrous Membranes via Electrospinning
2.3. Preparation of Ropivacaine-Loaded PCL Fibrous Membranes via Electrospinning
2.4. Preparation of Ropivacaine-Loaded PCL Dense Membranes
2.5. Characterizations
2.6. Ropivacaine Release In Vitro Experiments
2.7. In Vivo Experiments
2.8. Anesthesia Effects Evaluation
2.9. Local Inflammatory Response and Wound Healing Assessment
2.10. Statistical Analysis
3. Results and Discussion
3.1. Microstructures and Ropivacaine Integration
3.2. Ropivacaine Release from PCL Carriers In Vitro
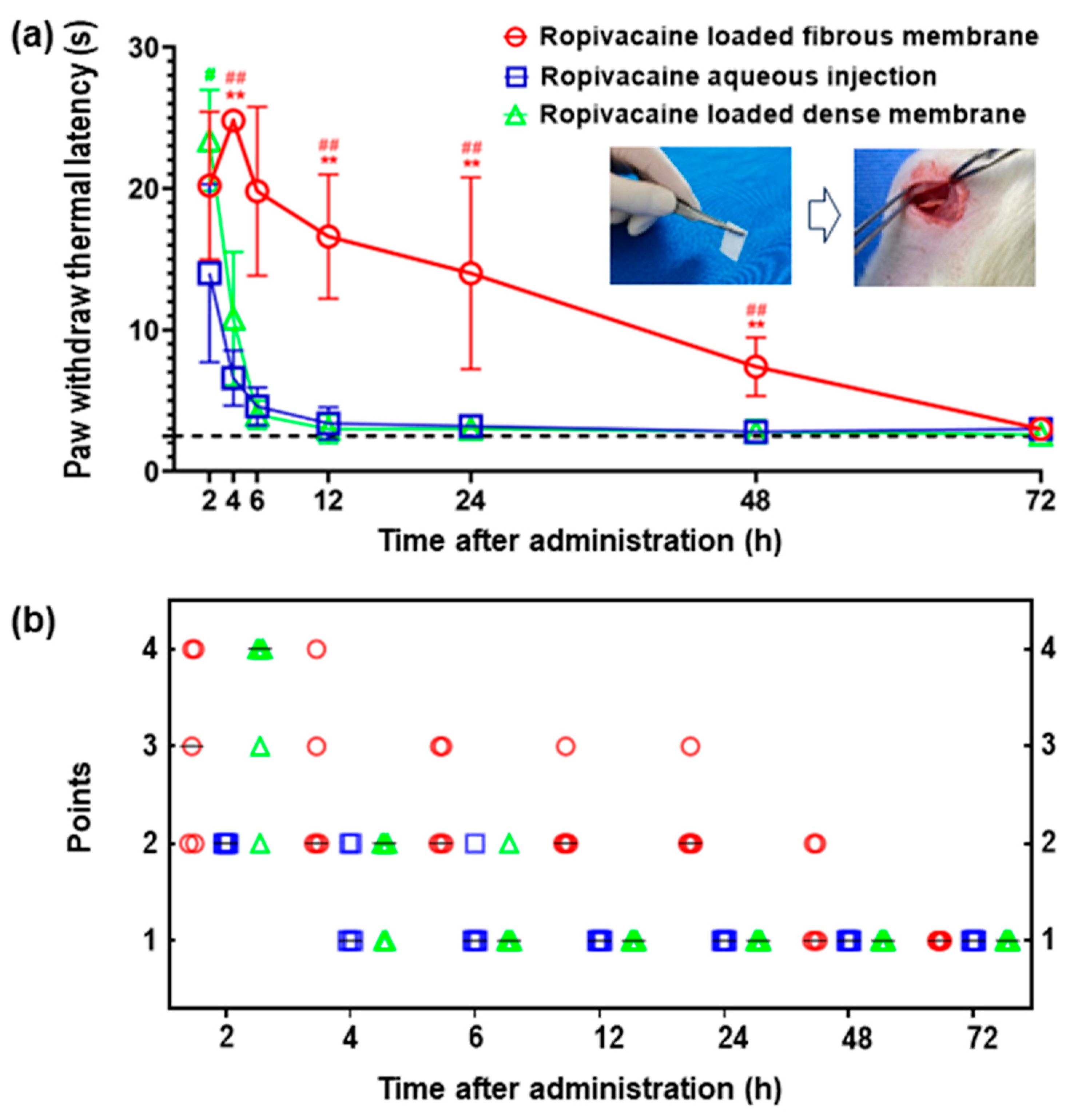
3.3. Prolonged Anesthesia Effects
3.4. Characterization of PCL Fibrous Membranes after Ropivacaine Release In Vivo
3.5. Biocompatibility and Safety Assessment of PCL Fiber Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kehlet, H.; Jensen, T.S.; Woolf, C.J. Persistent postsurgical pain: Risk factors and prevention. Lancet 2006, 367, 1618–1625. [Google Scholar] [CrossRef]
- Glare, P.; Aubrey, K.R.; Myles, P.S. Transition from acute to chronic pain after surgery. Lancet 2019, 393, 1537–1546. [Google Scholar] [CrossRef]
- Volkow, N.D.; Blanco, C. The changing opioid crisis: Development, challenges and opportunities. Mol. Psychiatry 2021, 26, 218–233. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, A.J.R.; Gitman, M.; Bornstein, K.J.; El-Boghdadly, K.; Weinberg, G. Updates in our understanding of local anaesthetic systemic toxicity: A narrative review. Anaesthesia 2021, 76 (Suppl. S1), 27–39. [Google Scholar] [CrossRef]
- Desai, N.; Kirkham, K.R.; Albrecht, E. Local anaesthetic adjuncts for peripheral regional anaesthesia: A narrative review. Anaesthesia 2021, 76 (Suppl. S1), 100–109. [Google Scholar] [CrossRef] [PubMed]
- Lirk, P.; Hollmann, M.W.; Strichartz, G. The Science of Local Anesthesia: Basic Research, Clinical Application, and Future Directions. Anesth. Analg. 2018, 126, 1381–1392. [Google Scholar] [CrossRef]
- Santamaria, C.M.; Woodruff, A.; Yang, R.; Kohane, D.S. Drug delivery systems for prolonged duration local anesthesia. Mater. Today 2017, 20, 22–31. [Google Scholar] [CrossRef]
- San Diego: Pacira Pharmaceuticals, I. Exparel [Package Insert]. 2014. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/022496s005lbl.pdf (accessed on 14 September 2023).
- Vyas, K.S.; Rajendran, S.; Morrison, S.D.; Shakir, A.; Mardini, S.; Lemaine, V.; Nahabedian, M.Y.; Baker, S.B.; Rinker, B.D.; Vasconez, H.C. Systematic Review of Liposomal Bpivacaine (Exparel) for Postoperative Analgesia. Plast. Reconstr. Surg. 2016, 138, 748e–756e. [Google Scholar] [CrossRef]
- Aggarwal, N. Local anesthetics systemic toxicity association with exparel (bupivacaine liposome)-A pharmacovigilance evaluation. Expert Opin. Drug Saf. 2018, 17, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Okoroha, K.R.; Lynch, J.R.; Keller, R.A.; Korona, J.; Amato, C.; Rill, B.; Kolowich, P.A.; Muh, S.J. Liposomal bupivacaine vs. interscalene nerve block for pain control after shoulder arthroplasty: A prospective randomized trial. J. Shoulder Elb. Surg. 2016, 25, 1742–1748. [Google Scholar] [CrossRef]
- Hussain, N.; Brull, R.; Sheehy, B.; Essandoh, M.K.; Stahl, D.L.; Weaver, T.E.; Abdallah, F.W. Perineural Liposomal Bupivacaine Is Not Superior to Nonliposomal Bupivacaine for Peripheral Nerve Block Analgesia. Anesthesiology 2021, 134, 147–164. [Google Scholar] [CrossRef] [PubMed]
- Ilfeld, B.M.; Eisenach, J.C.; Gabriel, R.A. Clinical Effectiveness of Liposomal Bupivacaine Administered by Infiltration or Peripheral Nerve Block to Treat Postoperative Pain. Anesthesiology 2021, 134, 283–344. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, T.W.; Knight, R.; Stokes, J.R.; Rombach, I.; Cooper, C.; Davies, L.; Dutton, S.J.; Barker, K.L.; Cook, J.; Lamb, S.E.; et al. Efficacy of Liposomal Bupivacaine and Bupivacaine Hydrochloride vs Bupivacaine Hydrochloride Alone as a Periarticular Anesthetic for Patients Undergoing Knee Replacement: A Randomized Clinical Trial. JAMA Surg. 2022, 157, 481–489. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, K.; Yang, X.; Chen, W.; Wang, T.; Kang, Y.; Gong, D.; Qian, Z.; Zhang, W. Sustained release of levobupivacaine from temperature-sensitive injectable hydrogel for long-term local anesthesia in postoperative pain management. Biomaterials 2023, 299, 122129. [Google Scholar] [CrossRef]
- Tang, L.; Qin, F.; Gong, D.; Dong, Y.; Pan, L.; Zhou, C.; Yin, Q.; Song, X.; Ling, R.; Huang, J.; et al. Long-term sciatic nerve block led by a supramolecular arrangement of self-delivery local a nesthetic nano systems. Chem. Commun. 2023, 59, 8400–8403. [Google Scholar] [CrossRef]
- Fu, J.; Yu, X.; Jin, Y. 3D printing of vaginal rings with personalized shapes for controlled release of progesterone. Int. J. Pharm. 2018, 539, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Shetty, K.; Bhandari, A.; Yadav, K.S. Nanoparticles incorporated in nanofibers using electrospinning: A novel nano-in-nano delivery system. J. Control. Release 2022, 350, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.Y.; Al-Salami, H.; Dass, C.R. Microparticles, microcapsules and microspheres: A review of recent developments and prospects for oral delivery of insulin. Int. J. Pharm. 2018, 537, 223–244. [Google Scholar] [CrossRef] [PubMed]
- Zhan, C.; Santamaria, C.M.; Wang, W.; McAlvin, J.B.; Kohane, D.S. Long-acting liposomal corneal anesthetics. Biomaterials 2018, 181, 372–377. [Google Scholar] [CrossRef]
- Hu, J.-W.; Yen, M.-W.; Wang, A.-J.; Chu, I.M. Effect of oil structure on cyclodextrin-based Pickering emulsions for bupivacaine topical application. Colloids Surf. B Biointerfaces 2018, 161, 51–58. [Google Scholar] [CrossRef]
- Weiniger, C.F.; Golovanevski, M.; Sokolsky-Papkov, M.; Domb, A.J. Review of prolonged local anesthetic action. Expert Opin. Drug Deliv. 2010, 7, 737–752. [Google Scholar] [CrossRef] [PubMed]
- Sokolsky-Papkov, M.; Golovanevski, L.; Domb, A.J.; Weiniger, C.F. Prolonged local anesthetic action through slow release from poly (lactic acid co castor oil). Pharm. Res. 2009, 26, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, R.; Li, X.; Xie, J. Electrospinning: An enabling nanotechnology platform for drug delivery and regenerative medicine. Adv. Drug Deliv. Rev. 2018, 132, 188–213. [Google Scholar] [CrossRef]
- Dorati, R.; Chiesa, E.; Pisani, S.; Genta, I.; Modena, T.; Bruni, G.; Brambilla, C.R.M.; Benazzo, M.; Conti, B. The Effect of Process Parameters on Alignment of Tubular Electrospun Nanofibers for Tissue Regeneration Purposes. J. Drug Deliv. Sci. Technol. 2020, 58, 101781. [Google Scholar] [CrossRef]
- Ramos Carriles, Y.; Suetel, M.; Henze, S.; Álvarez Brito, R.; Mueller, W.-D. Electrospun meshes of poly (n-butyl cyanoacrylate) and their potential applications for drug delivery and tissue engineering. Int. J. Pharm. 2021, 606, 120735. [Google Scholar] [CrossRef] [PubMed]
- Brennan, D.A.; Flamini, M.D.; Posselt, J.; Wagner, C.T.; Beachley, V. Effect of post-drawing and tension on enzymatic degradation of electrospun polycaprolactone nanofibers. Mater. Today Commun. 2023, 34, 104990. [Google Scholar] [CrossRef]
- Busuioc, C.; Alecu, A.E.; Costea, C.C.; Beregoi, M.; Bacalum, M.; Raileanu, M.; Jinga, S.I.; Deleanu, I.M. Composite Fibers Based on Polycaprolactone and Calcium Magnesium Silicate Powders for Tissue Engineering Applications. Polymers 2022, 14, 4611. [Google Scholar] [CrossRef]
- Fu, Y.; Li, X.; Ren, Z.; Mao, C.; Han, G. Multifunctional Electrospun Nanofibers for Enhancing Localized Cancer Treatment. Small 2018, 14, e1801183. [Google Scholar] [CrossRef] [PubMed]
- Mather, L.E.; Chang, D.H. Cardiotoxicity with modern local anaesthetics: Is there a safer choice? Drugs 2001, 61, 333–342. [Google Scholar] [CrossRef]
- Sun, H.; Mei, L.; Song, C.; Cui, X.; Wang, P. The in vivo degradation, absorption and excretion of PCL-based implant. Biomaterials 2006, 27, 1735–1740. [Google Scholar] [CrossRef]
- Chen, S.; Yao, W.; Wang, H.; Wang, T.; Xiao, X.; Sun, G.; Yang, J.; Guan, Y.; Zhang, Z.; Xia, Z.; et al. Electrospinning: Injectable electrospun fiber-hydrogel composite sequentially releasing clonidine and ropivacaine for prolonged and walking regional analgesia. Theranostics 2022, 12, 4904–4921. [Google Scholar] [CrossRef] [PubMed]




| Parameters | Unit | Electrospun PCL Fibrous Membrane | Electrospun PCL Fibrous Membrane with Ropivacaine |
|---|---|---|---|
| Solution Composition | wt% | PCL/DCM (20/80) | Ropivacaine/PCL/DCM (2/20/78) |
| Flow Rate | mL/min | 0.02 | 0.02 |
| Voltage | kV | +12.8/−3.8 | +11.2/−3.1 |
| Rotating Speed | rpm | 30 | 30 |
| Receiving Distance | cm | 20 | 15 |
| Porosity | % | 80 ± 5 | 72 ± 6 |
| WCA | ° | 121 ± 5 | 125 ± 5 |
| Thickness | μm | 70 ± 10 | 90 ± 10 |
| Fiber Diameter | μm | 4 | 3.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Chen, J.; Li, Z.; Guo, F. Prolonged Anesthesia Effects of Locally Administered Ropivacaine via Electrospun Poly(caprolactone) Fibrous Membranes. Membranes 2023, 13, 861. https://doi.org/10.3390/membranes13110861
Wang L, Chen J, Li Z, Guo F. Prolonged Anesthesia Effects of Locally Administered Ropivacaine via Electrospun Poly(caprolactone) Fibrous Membranes. Membranes. 2023; 13(11):861. https://doi.org/10.3390/membranes13110861
Chicago/Turabian StyleWang, Li, Jiaming Chen, Zicen Li, and Fei Guo. 2023. "Prolonged Anesthesia Effects of Locally Administered Ropivacaine via Electrospun Poly(caprolactone) Fibrous Membranes" Membranes 13, no. 11: 861. https://doi.org/10.3390/membranes13110861
APA StyleWang, L., Chen, J., Li, Z., & Guo, F. (2023). Prolonged Anesthesia Effects of Locally Administered Ropivacaine via Electrospun Poly(caprolactone) Fibrous Membranes. Membranes, 13(11), 861. https://doi.org/10.3390/membranes13110861







