Experimental Proof of a Transformation Product Trap Effect with a Membrane Photocatalytic Process for VOC Removal
Abstract
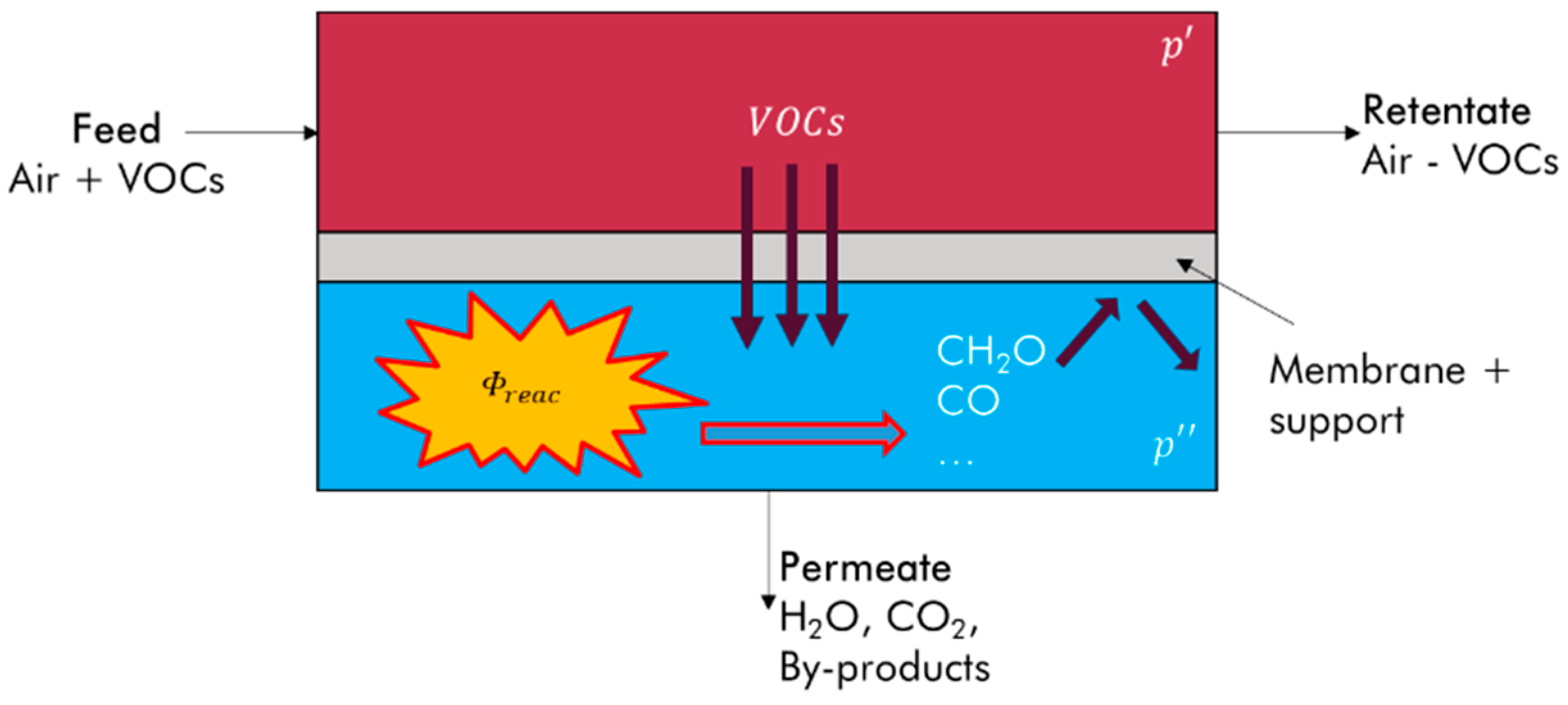
:1. Introduction
2. Materials and Method
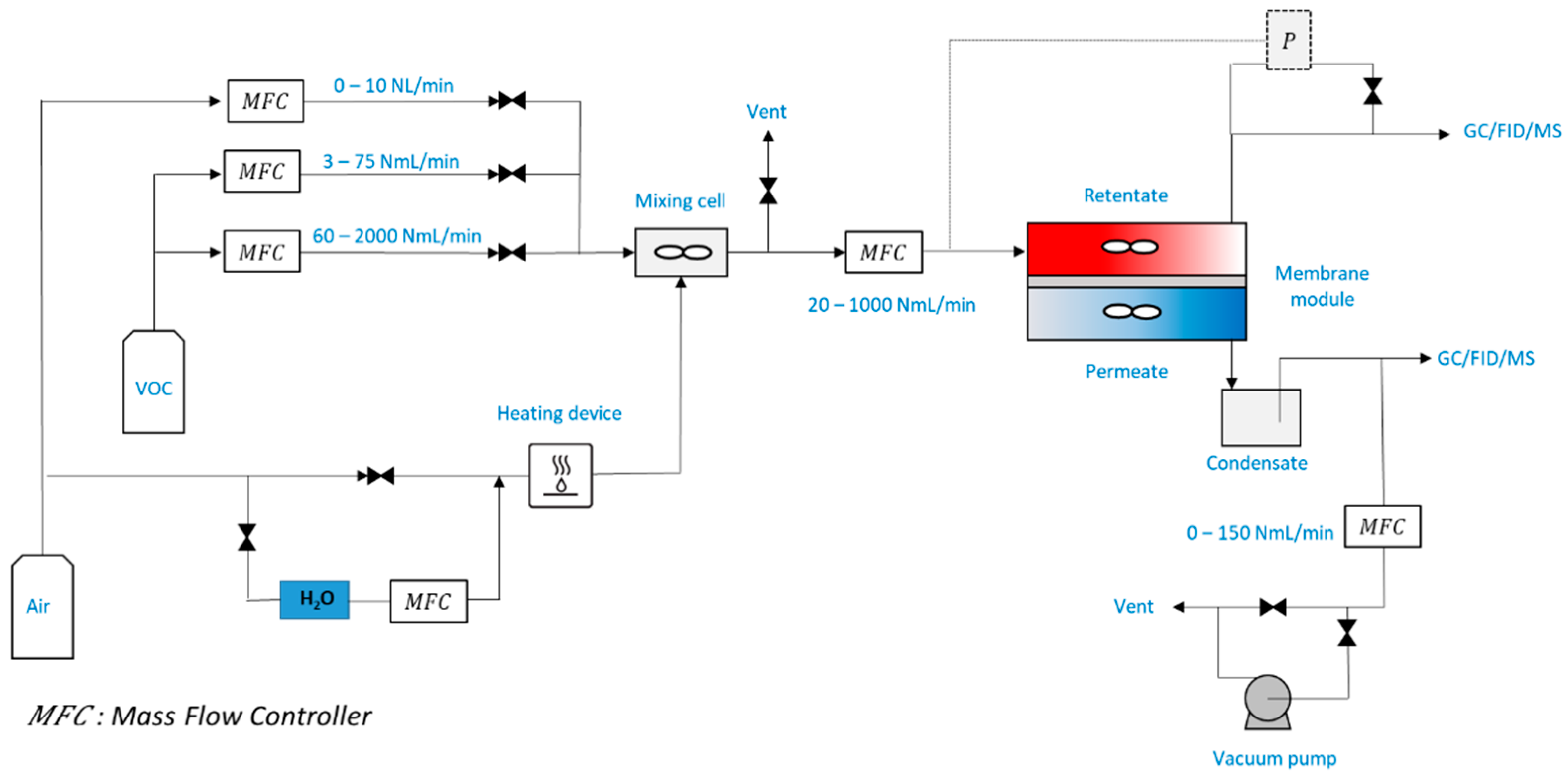
2.1. Experiment
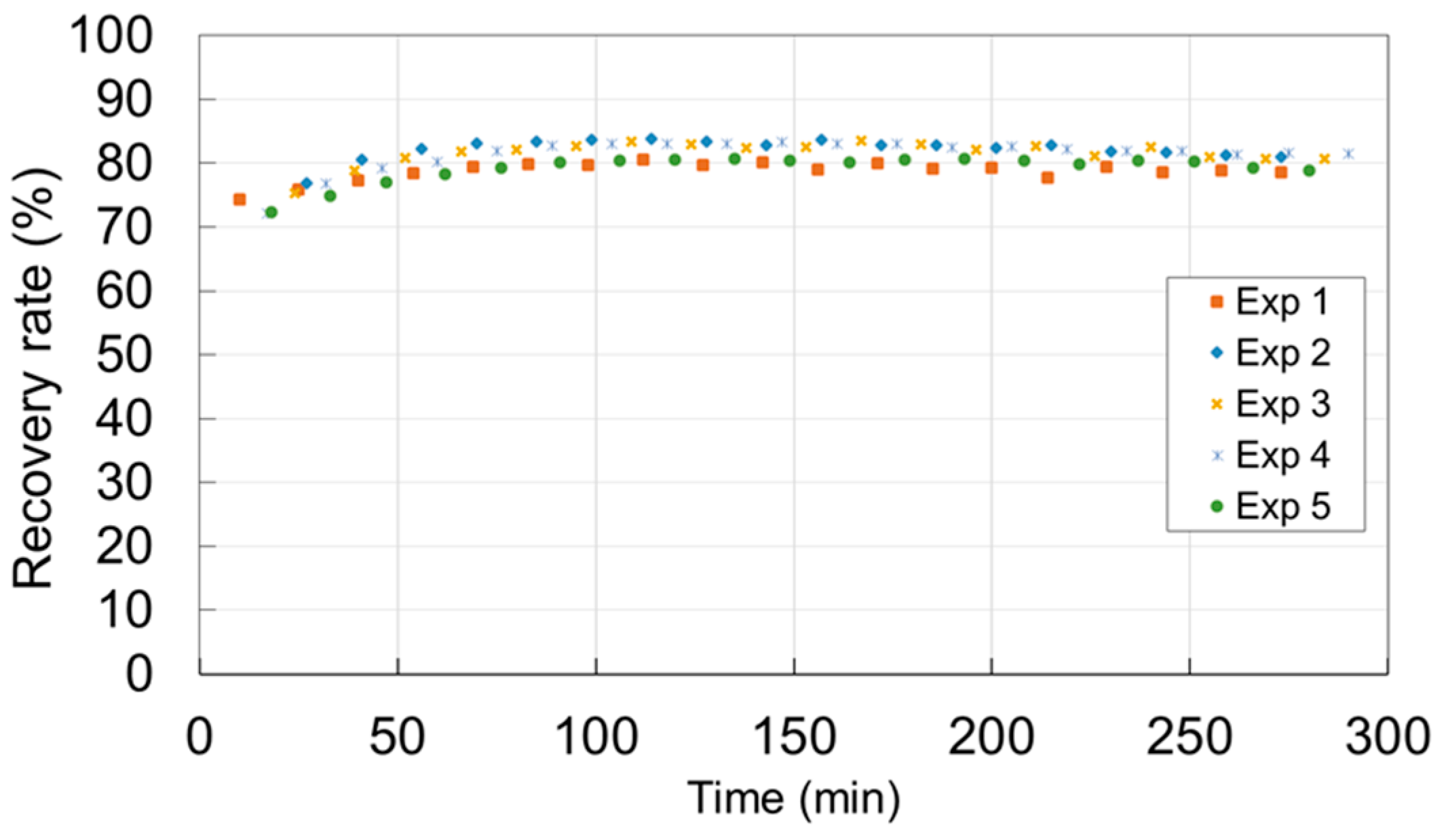
- -
- Membrane diameter 70 mm (membrane surface area = 38.5 cm2);
- -
- VOC inlet concentration: 2 and 10 ppm of toluene;
- -
- Feed flow rate: 100 NmL min−1, i.e., 7.42 × 10−5 mol s−1;
- -
- Pressure (p″: permeate pressure/p′: retentate pressure): 0.98 bar/1.3 bar–0.98 bar/2 bar;
- -
- Sweep gas flow rate: 0%, 10% and 30% of the feed flow rate value;
- -
- Temperature: 24 °C;
- -
- Relative humidity: 1% and 50% (in the feed flow);
- -
- Irradiance: 0.04, 0.3, 1.2, 2.8, 7 and 11 W m−2;
- -
- Catalyst mass: m0 = 0.02, m1 = 0.15 and m2 = 0.41 g.
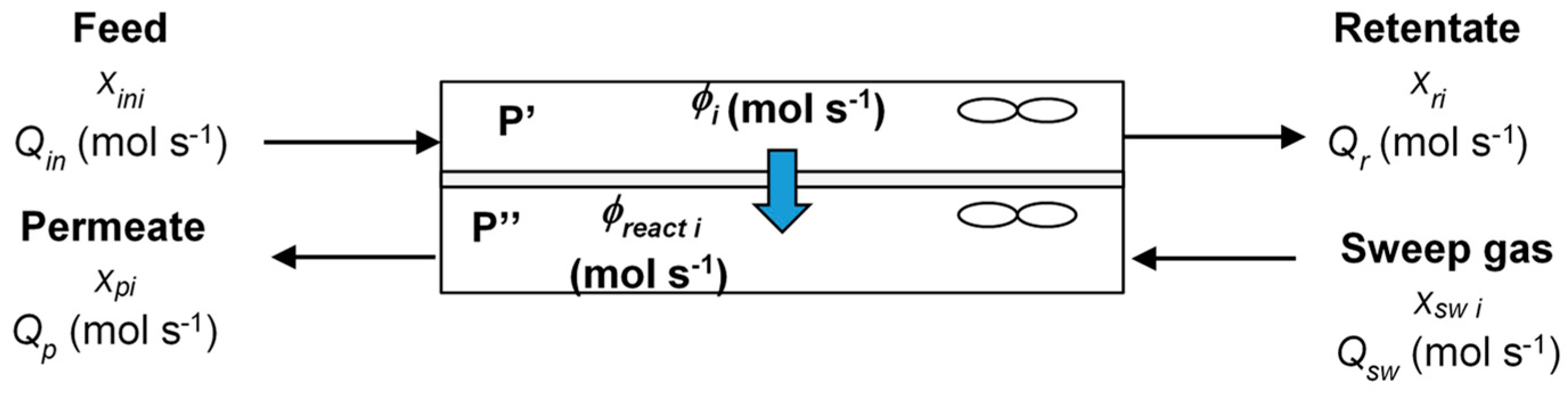
- The pressure ratio represents the ratio of the pressures on the permeate and retentate sides:
- The stage cut (%) is the ratio of the permeate flow rate to the feed flow rate:
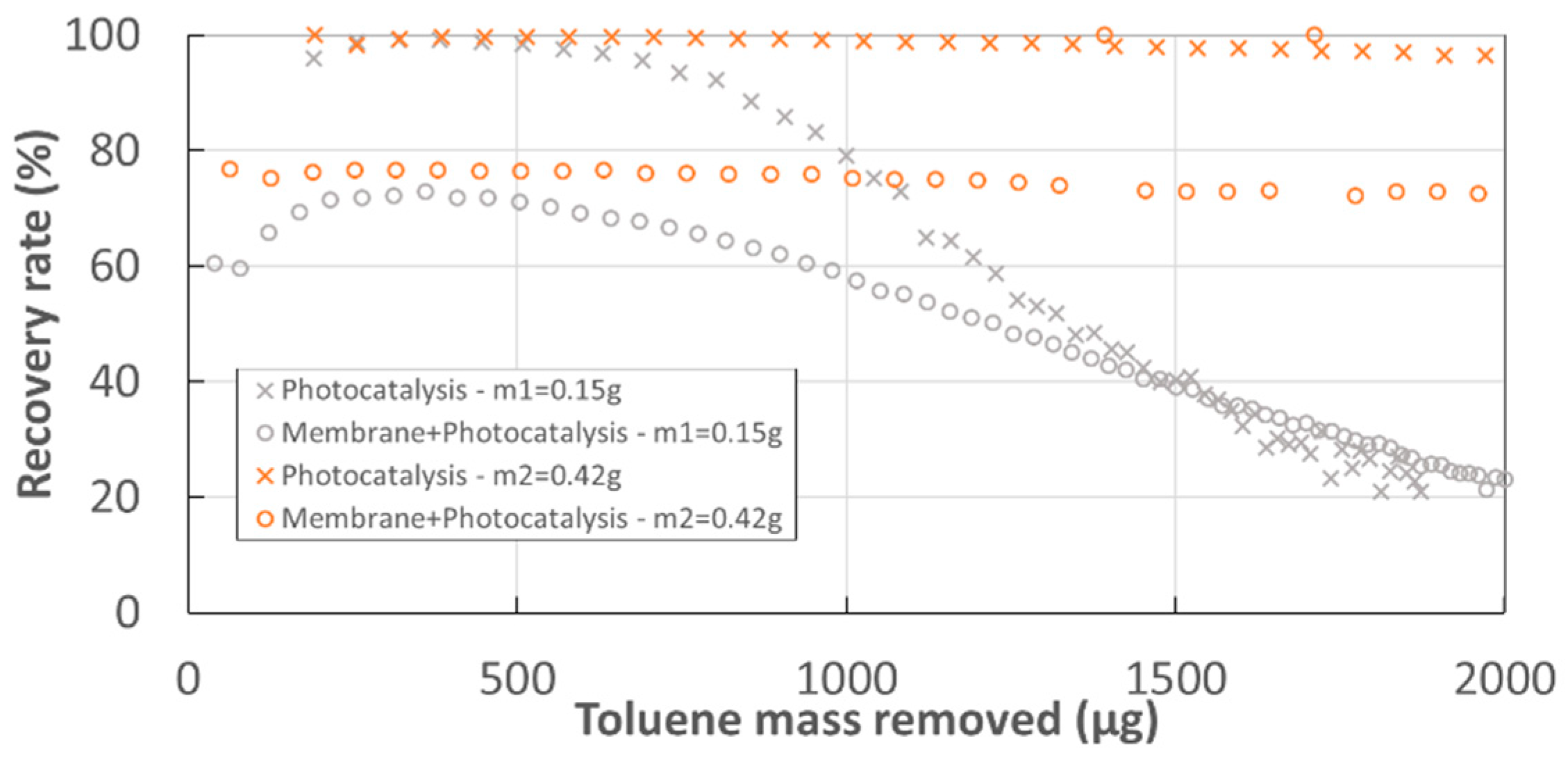
- The recovery ratio of toluene (%):
2.2. Modeling of Toluene Removal in the Experimental Module
3. Results and Discussion
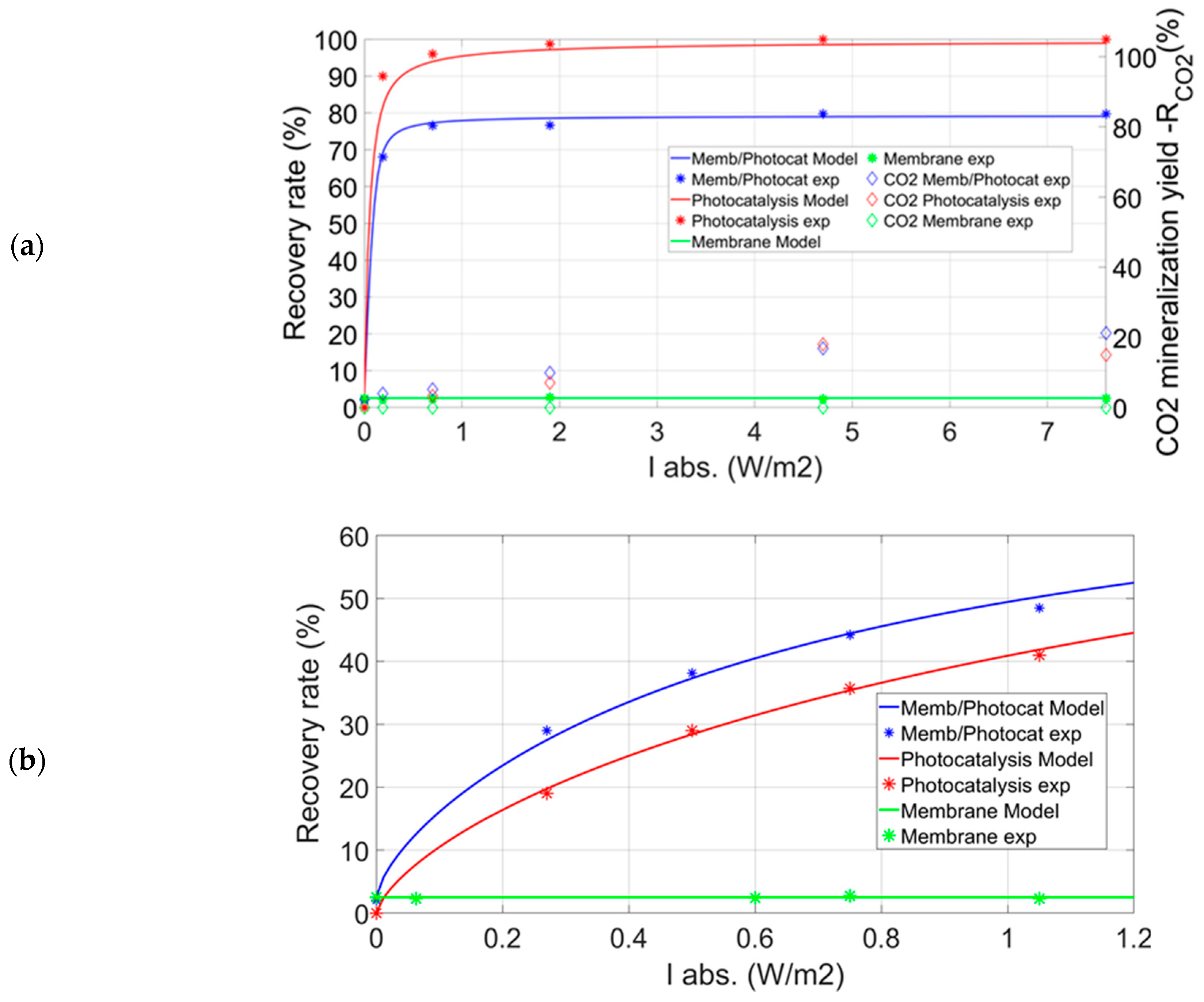
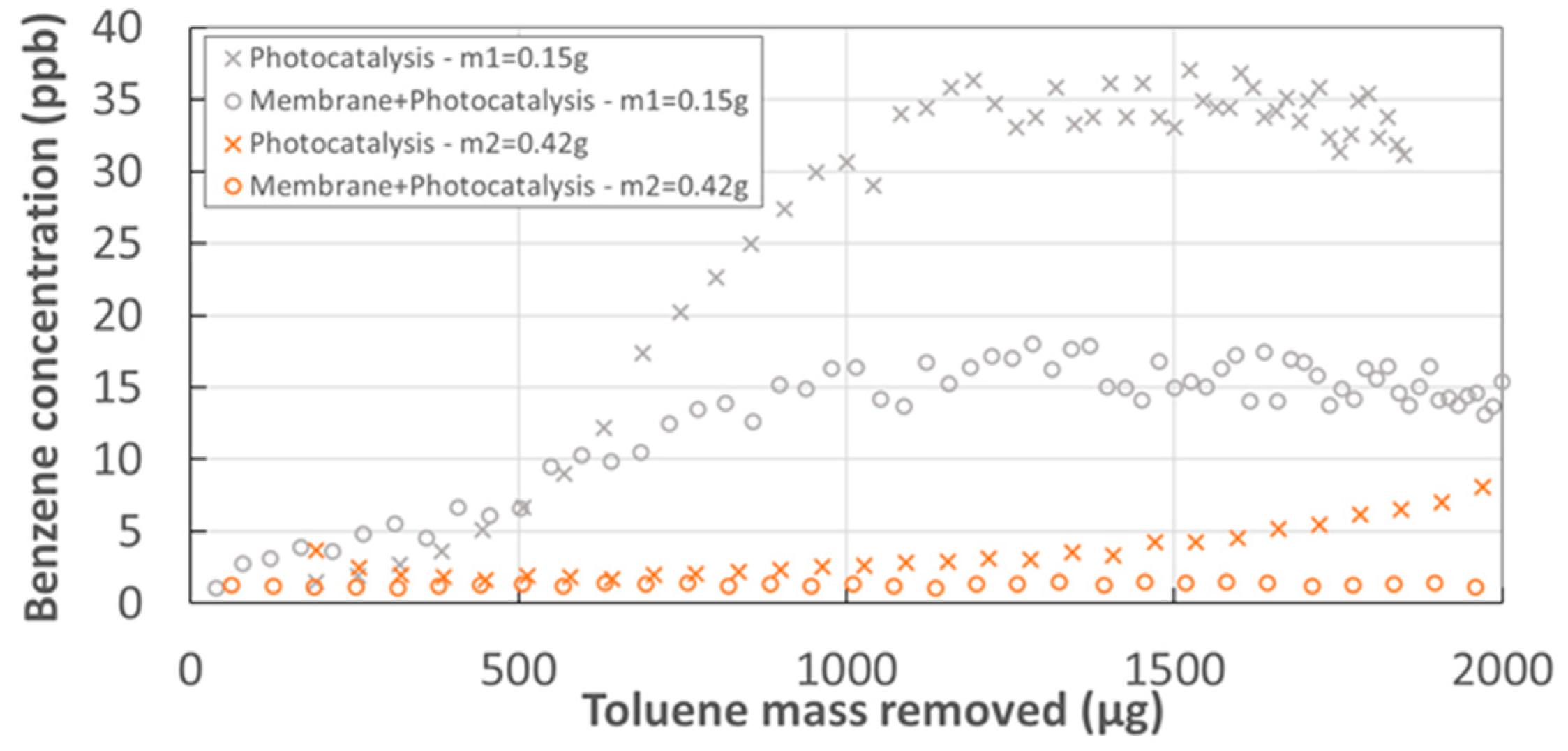
3.1. Toluene Removal with the Experimental Hybrid Module
- -
- Photocatalytic decomposition in the experimental module without membrane (CSTR), catalyst mass m2. The modeling of PCO reactor is detailed in Supplementary Material.
- -
- Membrane separation of toluene without PCO and with the same pressure ratio.
- -
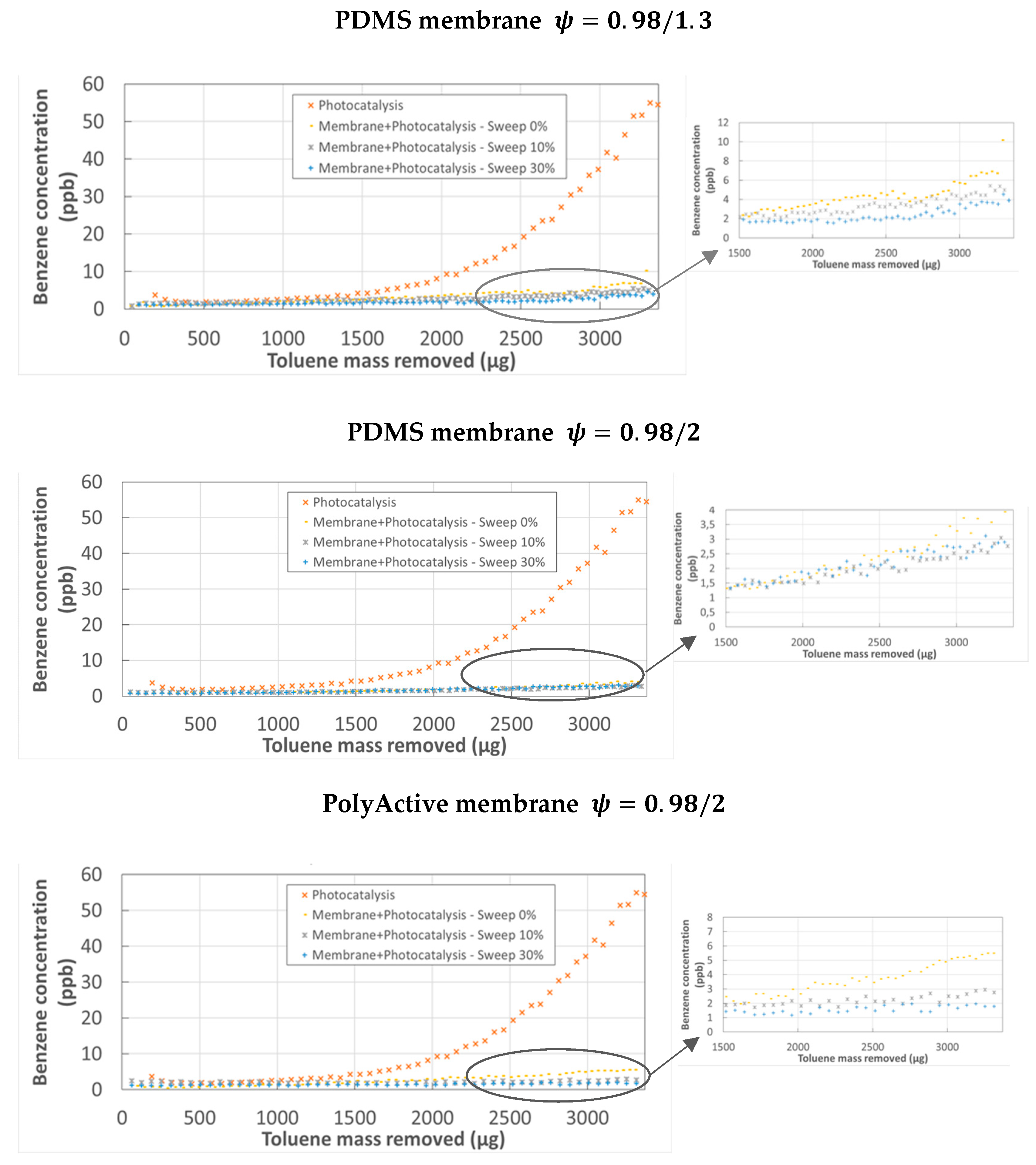
- Coupling membrane separation and PCO in the permeate compartment of the experimental module, catalyst mass m2, PDMS membrane, without sweep gas flow rate.
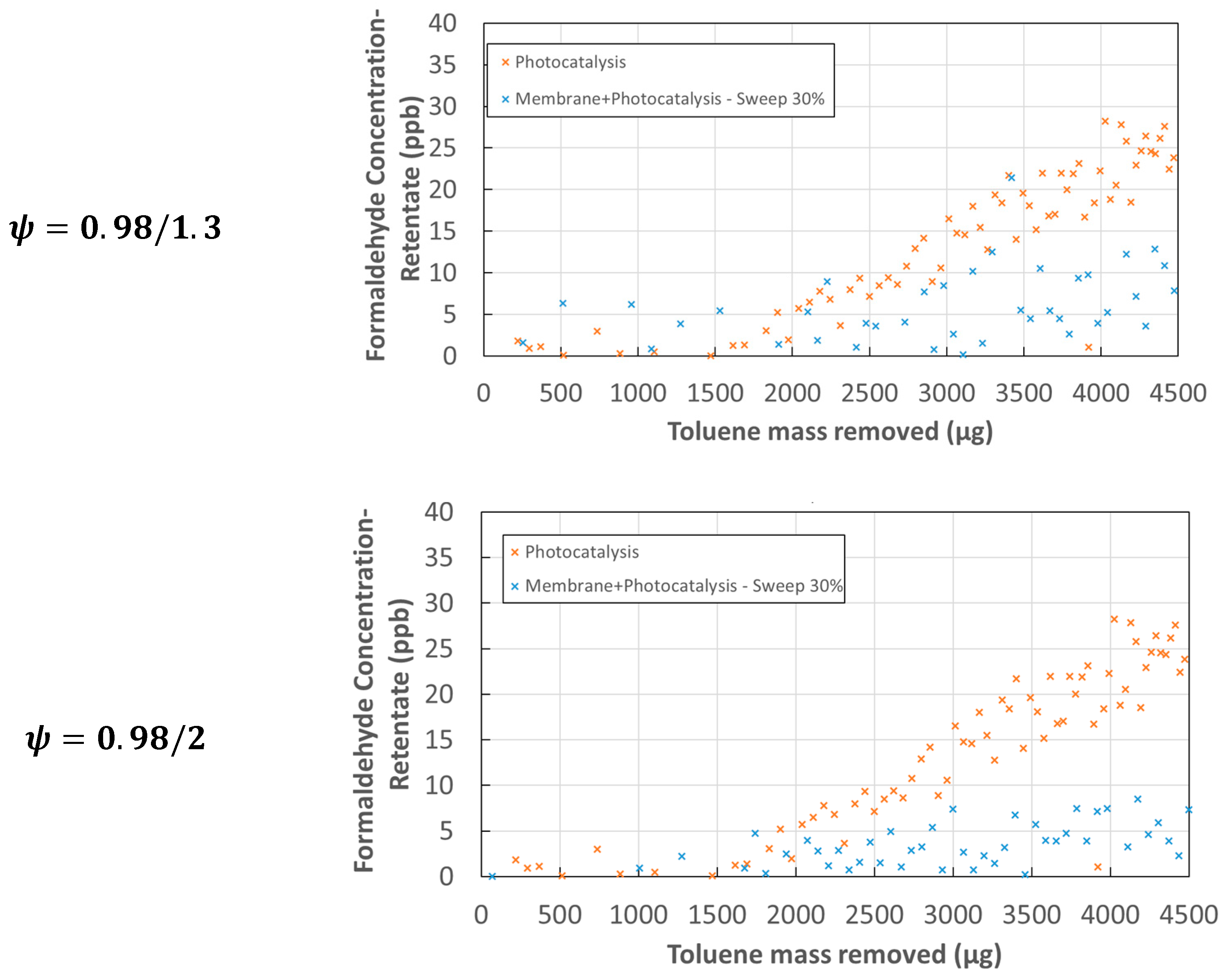
3.2. Identification and Monitoring of the Formation of Transformation Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Coombs, K.C.; Chew, G.L.; Schaffer, C.; Ryan, P.H.; Brokamp, C.; Grinshpun, S.A.; Adamkiewicz, G.; Chillrud, S.; Hedman, C.; Colton, M.; et al. Indoor Air Quality in Green-Renovated vs. Non-Green Low-Income Homes of Children Living in a Temperate Region of US (Ohio). Sci. Total Environ. 2016, 554, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Derbez, M.; Berthineau, B.; Cochet, V.; Pignon, C.; Ribéron, J.; Wyart, G.; Mandin, C.; Kirchner, S. A 3-Year Follow-up of Indoor Air Quality and Comfort in Two Energy-Efficient Houses. Build. Environ. 2014, 82, 288–299. [Google Scholar] [CrossRef]
- Stamatelopoulou, A.; Asimakopoulos, D.N.; Maggos, T. Effects of PM, TVOCs and Comfort Parameters on Indoor Air Quality of Residences with Young Children. Build. Environ. 2019, 150, 233–244. [Google Scholar] [CrossRef]
- Agents Classified by the IARC Monographs, Volumes 1–129. IARC Monographs on the Identification of Carcinogenic Hazards to Humans. Available online: https://monographs.iarc.who.int/agents-classified-by-the-iarc/ (accessed on 13 September 2021).
- Available online: https://www.epa.gov/indoor-air-quality-iaq/introduction-indoor-air-quality (accessed on 16 December 2021).
- WHO Guidelines for Indoor Air Quality: Selected Pollutants; WHO: Copenhagen, Denmark, 2010.
- González-Martín, J.; Kraakman, N.J.R.; Pérez, C.; Lebrero, R.; Muñoz, R. A State–of–the-Art Review on Indoor Air Pollution and Strategies for Indoor Air Pollution Control. Chemosphere 2021, 262, 128376. [Google Scholar] [CrossRef] [PubMed]
- Data Hub–ACGIH. Available online: https://www.acgih.org/data-hub-2022/ (accessed on 2 September 2022).
- Walser, T.; Juraske, R.; Demou, E.; Hellweg, S. Indoor Exposure to Toluene from Printed Matter Matters: Complementary Views from Life Cycle Assessment and Risk Assessment. Environ. Sci. Technol. 2014, 48, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Weisel, C.P. Benzene Exposure: An Overview of Monitoring Methods and Their Findings. Chem. Biol. Interact. 2010, 184, 58–66. [Google Scholar] [CrossRef]
- Arts, J.H.E.; Muijser, H.; Kuper, C.F.; Woutersen, R.A. Setting an Indoor Air Exposure Limit for Formaldehyde: Factors of Concern. Regul. Toxicol. Pharmacol. 2008, 52, 189–194. [Google Scholar] [CrossRef]
- Khan, F.I.; Ghoshal, A.K. Removal of Volatile Organic Compounds from Polluted Air. J. Loss Prev. Process Ind. 2000, 13, 527–545. [Google Scholar] [CrossRef]
- Le Bechec, M.; Kinadjian, N.; Ollis, D.; Backov, R.; Lacombe, S. Comparison of Kinetics of Acetone, Heptane and Toluene Photocatalytic Mineralization over TiO2 Microfibers and Quartzel® Mats. Appl. Catal. B Environ. 2015, 179, 78–87. [Google Scholar] [CrossRef]
- Farhanian, D. Impact of Design Parameters on the Performance of Ultraviolet Photocatalytic Oxidation Air Cleaner. Build. Environ. 2013, 66, 148–157. [Google Scholar] [CrossRef]
- Boyjoo, Y.; Sun, H.; Liu, J.; Pareek, V.K.; Wang, S. A Review on Photocatalysis for Air Treatment: From Catalyst Development to Reactor Design. Chem. Eng. J. 2017, 310, 537–559. [Google Scholar] [CrossRef]
- Vikrant, K.; Park, C.M.; Kim, K.-H.; Kumar, S.; Jeon, E.-C. Recent Advancements in Photocatalyst-Based Platforms for the Destruction of Gaseous Benzene: Performance Evaluation of Different Modes of Photocatalytic Operations and against Adsorption Techniques. J. Photochem. Photobiol. C Photochem. Rev. 2019, 41, 100316. [Google Scholar] [CrossRef]
- Wood, D.; Shaw, S.; Cawte, T.; Shanen, E.; Van Heyst, B. An Overview of Photocatalyst Immobilization Methods for Air Pollution Remediation. Chem. Eng. J. 2020, 391, 123490. [Google Scholar] [CrossRef]
- da Costa Filho, B.M.; Vilar, V.J.P. Strategies for the Intensification of Photocatalytic Oxidation Processes towards Air Streams Decontamination: A Review. Chem. Eng. J. 2020, 391, 123531. [Google Scholar] [CrossRef]
- Jędrzejczyk, M.; Zbudniewek, K.; Rynkowski, J.; Keller, V.; Grams, J.; Ruppert, A.M.; Keller, N. Wide Band Gap Ga2O3 as Efficient UV-C Photocatalyst for Gas-Phase Degradation Applications. Environ. Sci. Pollut. Res. 2017, 24, 26792–26805. [Google Scholar] [CrossRef]
- Stucchi, M.; Galli, F.; Bianchi, C.L.; Pirola, C.; Boffito, D.C.; Biasioli, F.; Capucci, V. Simultaneous Photodegradation of VOC Mixture by TiO2 Powders. Chemosphere 2018, 193, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Guo, Z.; Liu, H.; Zhang, S.; Wang, P.; Lu, J.; Tong, Y. Heterojunction Architecture of N-Doped WO3 Nanobundles with Ce2S3 Nanodots Hybridized on a Carbon Textile Enables a Highly Efficient Flexible Photocatalyst. Adv. Funct. Mater. 2019, 29, 1903490. [Google Scholar] [CrossRef]
- Moradi, Z.; Jahromi, S.Z.; Ghaedi, M. Chapter 9—Design of Active Photocatalysts and Visible Light Photocatalysis. In Interface Science and Technology; Ghaedi, M., Ed.; Photocatalysis: Fundamental Processes and Applications; Elsevier: Amsterdam, The Netherlands, 2021; Volume 32, pp. 557–623. [Google Scholar] [CrossRef]
- Debono, O.; Hequet, V.; Le Coq, L.; Locoge, N.; Thevenet, F. VOC Ternary Mixture Effect on Ppb Level Photocatalytic Oxidation: Removal Kinetic, Reaction Intermediates and Mineralization. Appl. Catal. B Environ. 2017, 218, 359–369. [Google Scholar] [CrossRef]
- Mamaghani, A.H.; Haghighat, F.; Lee, C.-S. Photocatalytic Degradation of VOCs on Various Commercial Titanium Dioxides: Impact of Operating Parameters on Removal Efficiency and by-Products Generation. Build. Environ. 2018, 138, 275–282. [Google Scholar] [CrossRef]
- Ardizzone, S.; Bianchi, C.L.; Cappelletti, G.; Naldoni, A.; Pirola, C. Photocatalytic Degradation of Toluene in the Gas Phase: Relationship between Surface Species and Catalyst Features. Environ. Sci. Technol. 2008, 42, 6671–6676. [Google Scholar] [CrossRef]
- Mo, J.; Zhang, Y.; Xu, Q.; Zhu, Y.; Lamson, J.J.; Zhao, R. Determination and Risk Assessment of By-Products Resulting from Photocatalytic Oxidation of Toluene. Appl. Catal. B Environ. 2009, 89, 570–576. [Google Scholar] [CrossRef]
- Vildozo, D.; Portela, R.; Ferronato, C.; Chovelon, J.-M. Photocatalytic Oxidation of 2-Propanol/Toluene Binary Mixtures at Indoor Air Concentration Levels. Appl. Catal. B Environ. 2011, 107, 347–354. [Google Scholar] [CrossRef]
- Korologos, C.A.; Philippopoulos, C.J.; Poulopoulos, S.G. The Effect of Water Presence on the Photocatalytic Oxidation of Benzene, Toluene, Ethylbenzene and m-Xylene in the Gas-Phase. Atmos. Environ. 2011, 45, 7089–7095. [Google Scholar] [CrossRef]
- Mo, J.; Zhang, Y.; Xu, Q. Effect of Water Vapor on the By-Products and Decomposition Rate of Ppb-Level Toluene by Photocatalytic Oxidation. Appl. Catal. B Environ. 2013, 132–133, 212–218. [Google Scholar] [CrossRef]
- Weon, S.; Choi, W. TiO 2 Nanotubes with Open Channels as Deactivation-Resistant Photocatalyst for the Degradation of Volatile Organic Compounds. Environ. Sci. Technol. 2016, 50, 2556–2563. [Google Scholar] [CrossRef]
- Chen, P.; Cui, W.; Wang, H.; Dong, X.; Li, J.; Sun, Y.; Zhou, Y.; Zhang, Y.; Dong, F. The Importance of Intermediates Ring-Opening in Preventing Photocatalyst Deactivation during Toluene Decomposition. Appl. Catal. B Environ. 2020, 272, 118977. [Google Scholar] [CrossRef]
- Gérardin, F.; Cloteaux, A.; Simard, J.; Favre, É. A Photodriven Energy Efficient Membrane Process for Trace VOC Removal from Air: First Step to a Smart Approach. Chem. Eng. J. 2021, 419, 129566. [Google Scholar] [CrossRef]
- Favre, E. Polymeric Membranes for Gas Separation. In Comprehensive Membrane Science and Engineering; Drioli, E., Giorno, L., Eds.; Elsevier: Oxford, UK, 2010; Volume 2, pp. 155–212. [Google Scholar]
- Yeom, C.K.; Kim, H.K.; Rhim, J.W. Removal of Trace VOCs from Water through PDMS Membranes and Analysis of Their Permeation Behaviors. J. Appl. Polym. Sci. 1999, 73, 601–611. [Google Scholar] [CrossRef]
- Yeom, C.K.; Lee, S.H.; Song, H.Y.; Lee, J.M. Vapor Permeations of a Series of VOCs/N2 Mixtures through PDMS Membrane. J. Membr. Sci. 2002, 198, 129–143. [Google Scholar] [CrossRef]
- Oh, H.-K.; Song, K.-H.; Lee, K.-R.; Rim, J.-M. Prediction of Sorption and ¯ux of Solvents through PDMS Membrane. Polymer 2001, 42, 6305–6312. [Google Scholar] [CrossRef]
- Schuldt, K.; Pohlmann, J.; Shishatskiy, S.; Brinkmann, T. Applicability of PolyActiveTM Thin Film Composite Membranes for CO2 Separation from C2H4 Containing Multi-Component Gas Mixtures at Pressures up to 30 Bar. Membranes 2018, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, P.; Drioli, E.; Golemme, G. Membrane Gas Separation: A Review/State of the Art. Ind. Eng. Chem. Res. 2009, 48, 4638–4663. [Google Scholar] [CrossRef]
- Gérardin, F.; Cloteaux, A.; Guillemot, M.; Faure, M.; André, J.C. Photocatalytic Conversion of Gaseous Nitrogen Trichloride into Available Chlorine—Experimental and Modeling Study. Environ. Sci. Technol. 2013, 47, 4628–4635. [Google Scholar] [CrossRef] [PubMed]
- Bounaceur, R.; Berger, E.; Pfister, M.; Ramirez Santos, A.A.; Favre, E. Rigorous Variable Permeability Modelling and Process Simulation for the Design of Polymeric Membrane Gas Separation Units: MEMSIC Simulation Tool. J. Membr. Sci. 2017, 523, 77–91. [Google Scholar] [CrossRef]

| Household (ppb) | Workplace (ppm) | |
|---|---|---|
| Toluene | 1–45 [7] | 0.001–1000 [7,9] |
| Benzene | 0.5–15 [10] | 0.002–1.1 [10] |
| Formaldehyde | 7–220 [7,11] | 0.04–0.8 [7,11] |
| Membrane | ψ | θexp (%) | θmod (%) |
|---|---|---|---|
| PDMS | 0.98/1.3 | 0.5 | 0.4 |
| 0.98/2 | 1.0 | 1.2 | |
| PolyActive | 0.98/1.3 | 3.0 | 2.4 |
| 0.98/2 | 7.8 | 8.0 |
| PCO | Membrane + PCO | Fresh Catalyst | |
|---|---|---|---|
| Formaldehyde | 7.9 | 6.1 | 5.1 |
| Acetaldehyde | 13 | 9.3 | 16.2 |
| Acrolein | 0.3 | 0.3 | 0 |
| Acetone | 46 | 103 | 32 |
| Propionaldehyde | 0.4 | 0.2 | 0.1 |
| Butanal | 1.9 | 1.1 | 0.1 |
| Benzaldehyde | 1460 | 964 | 1.7 |
| Pentanal | 1.4 | 1.1 | 0.2 |
| m-Tolualdehyde | 0.8 | 0.6 | 0.7 |
| Hexanal | 0.7 | 0.4 | 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gérardin, F.; Simard, J.; Favre, É. Experimental Proof of a Transformation Product Trap Effect with a Membrane Photocatalytic Process for VOC Removal. Membranes 2022, 12, 900. https://doi.org/10.3390/membranes12090900
Gérardin F, Simard J, Favre É. Experimental Proof of a Transformation Product Trap Effect with a Membrane Photocatalytic Process for VOC Removal. Membranes. 2022; 12(9):900. https://doi.org/10.3390/membranes12090900
Chicago/Turabian StyleGérardin, Fabien, Julien Simard, and Éric Favre. 2022. "Experimental Proof of a Transformation Product Trap Effect with a Membrane Photocatalytic Process for VOC Removal" Membranes 12, no. 9: 900. https://doi.org/10.3390/membranes12090900
APA StyleGérardin, F., Simard, J., & Favre, É. (2022). Experimental Proof of a Transformation Product Trap Effect with a Membrane Photocatalytic Process for VOC Removal. Membranes, 12(9), 900. https://doi.org/10.3390/membranes12090900







