3D Printed and Bioprinted Membranes and Scaffolds for the Periodontal Tissue Regeneration: A Narrative Review
Abstract
1. Introduction
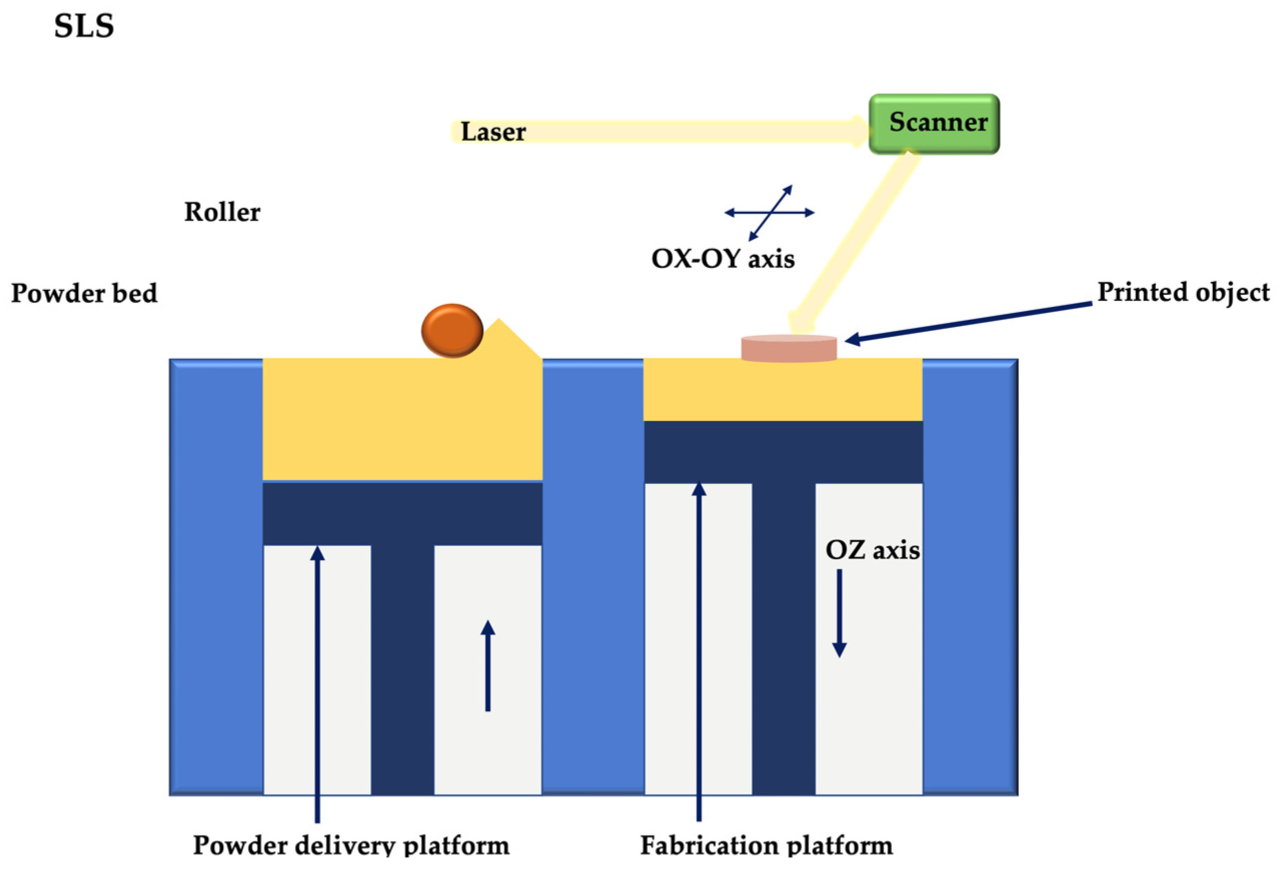
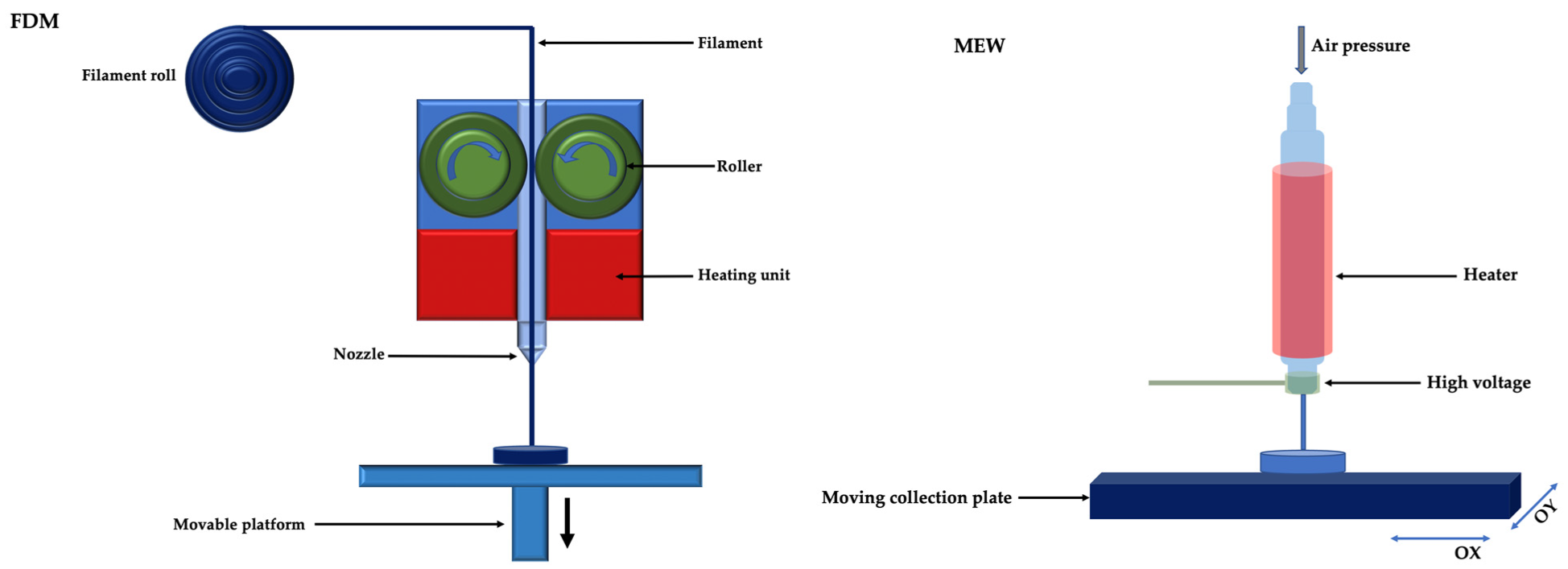
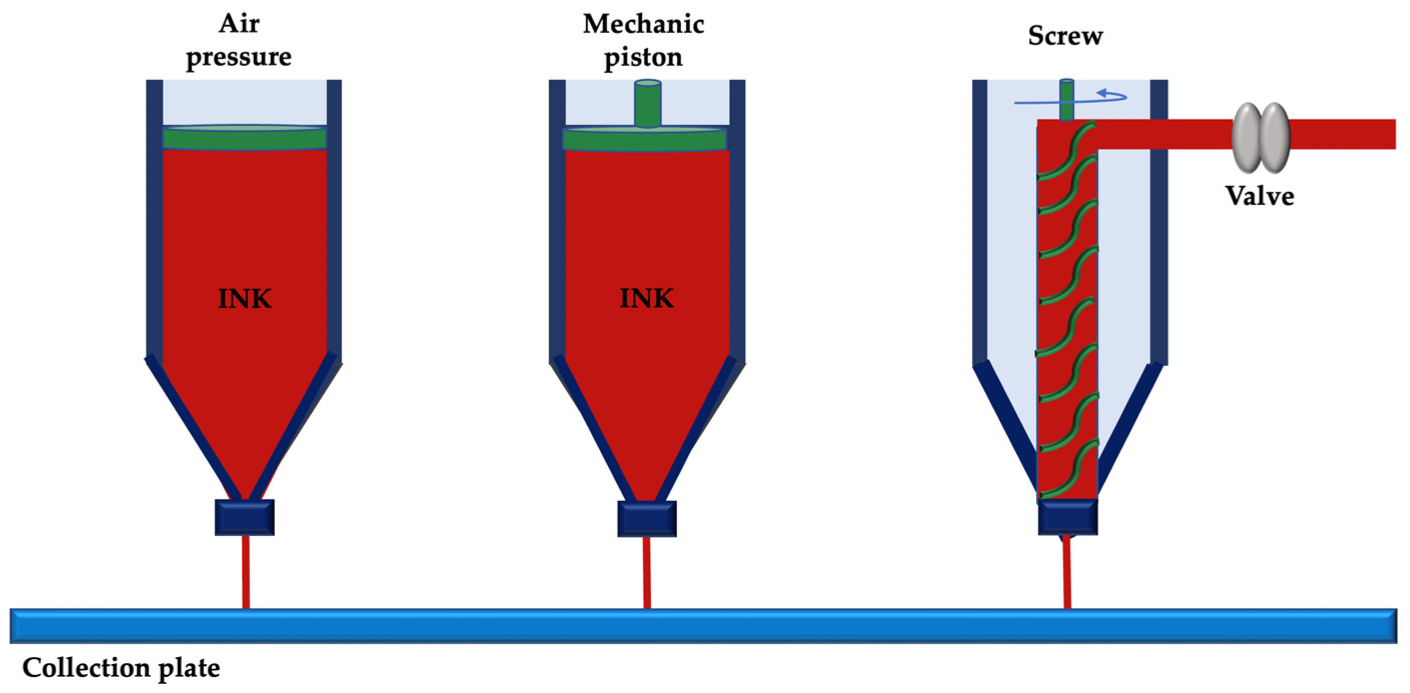
2. 3D Printing and Bioprinting Techniques
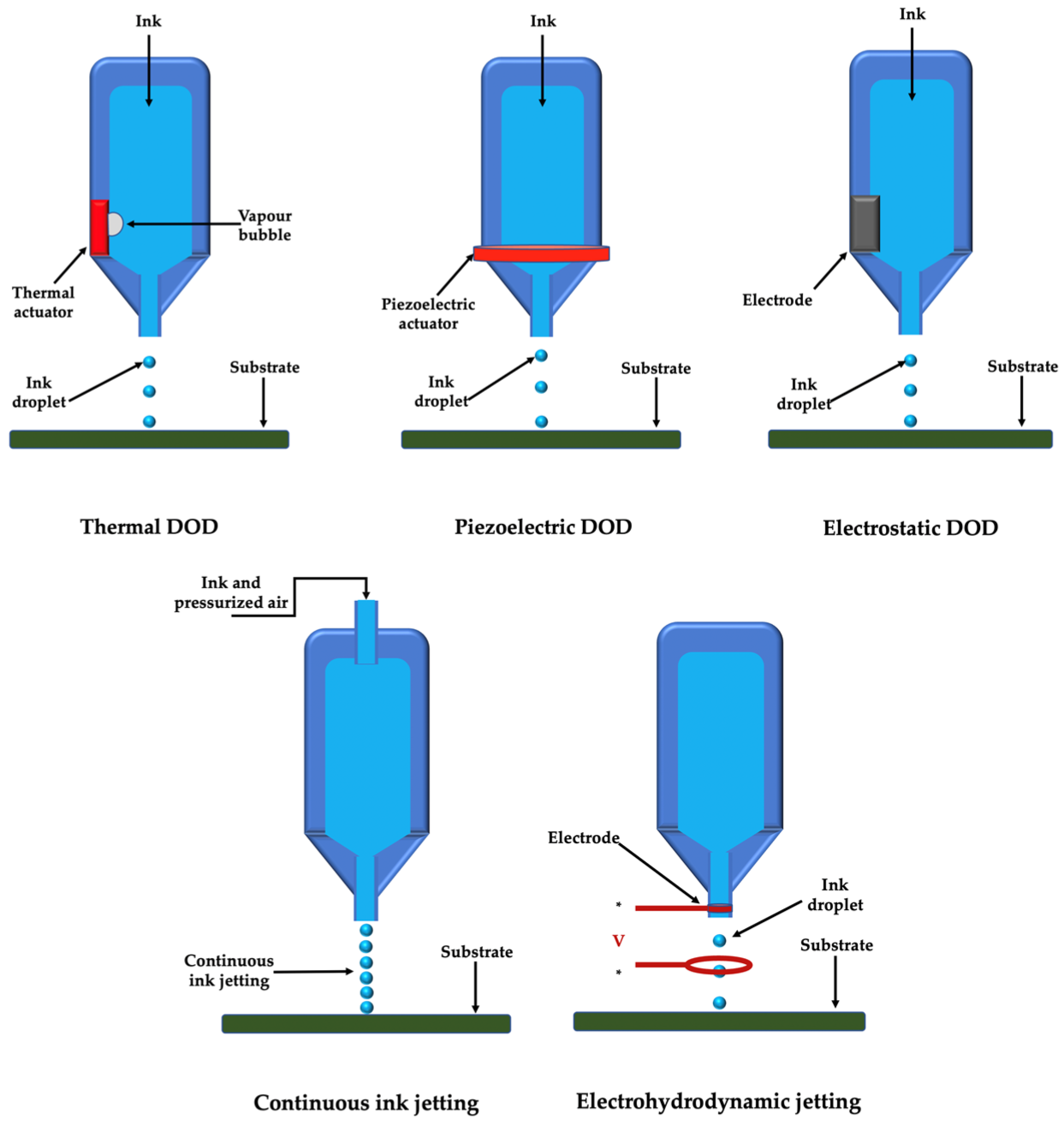
2.1. Droplet-Based Printing
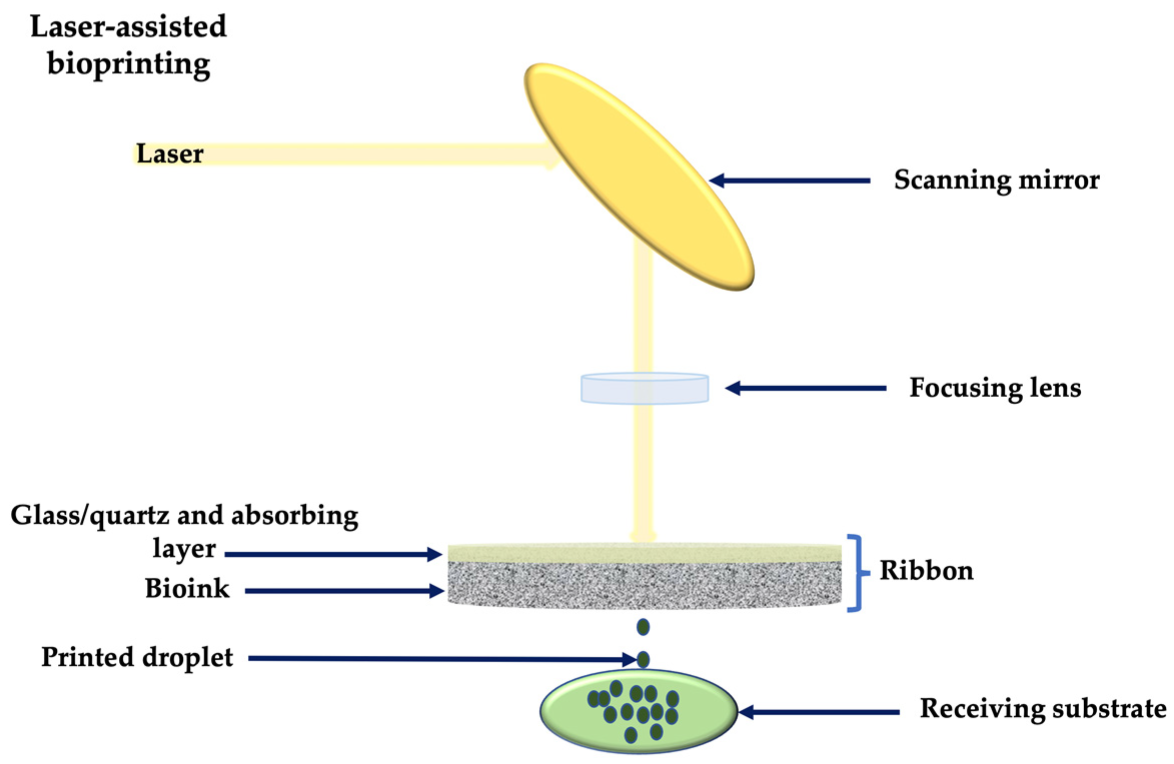
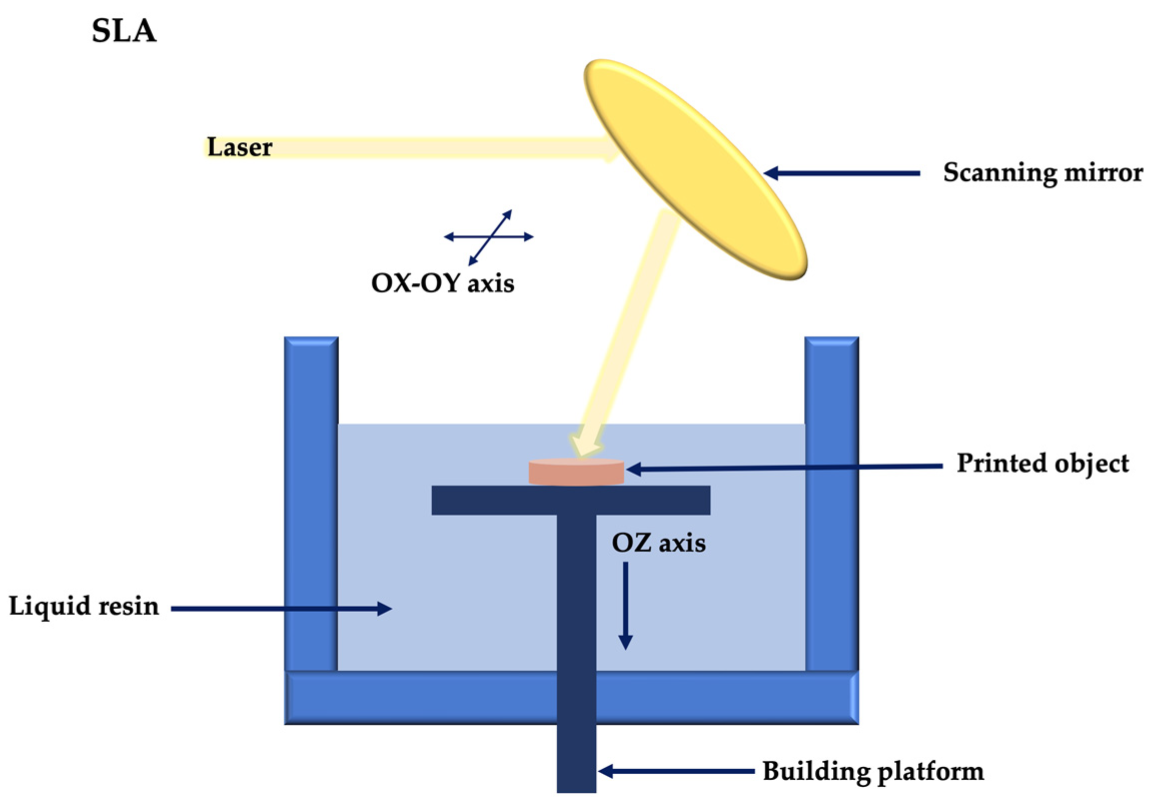
2.2. Light-Assisted 3D Printing
2.3. Extrusion 3D Printing
2.3.1. Thermal Extrusion 3D Printing
2.3.2. Non-Thermal Extrusion 3D Printing
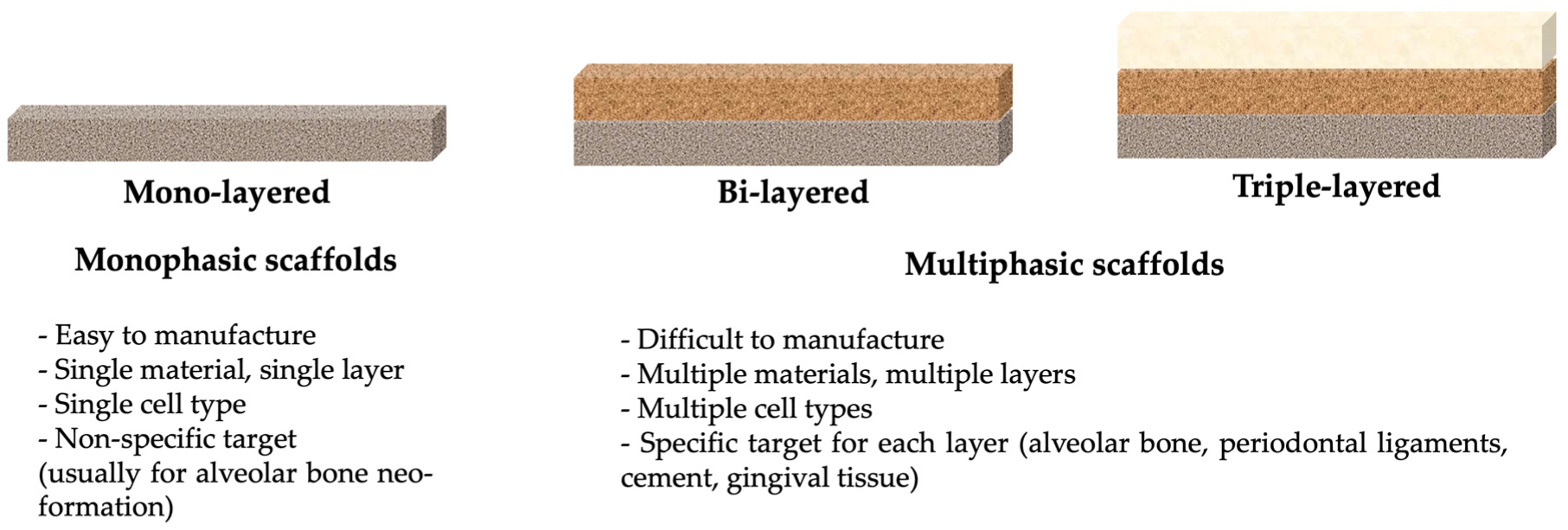
3. Applications of 3D Printing in Periodontology
3.1. 3D Printed Scaffolds in Periodontal Defects
3.2. Socket Preservation
3.3. Other Applications
4. Bioprinting
- Data acquisition, using X-ray scanning and reconstruction techniques, computed tomography (CT), magnetic resonance imaging (MRI), or directly using computer-aided design (CAD) software. These data will be processed with the help of specific software. The file is converted to a printer-readable file [119]. The data is then translated to allow estimation of the amount of material to be extruded, which depends on the desired height and width of the layer according to the shape of the bioink (droplet or filament) [31,120].
- The choice of bioink, which is made according to the printing technique and the requirements of the printed structures. Thus, the bioink must meet favorable mechanical properties, as well as biocompatibility and printability requirements. The bioink can contain isolated cells, growth factors and bioprinting materials. It is prepared according to the physiological temperature, pH and requirements of the printed structures [31].
- Setting the appropriate printing parameters, depending on the bioink and the desired structure of the printed product.
- The actual bioprinting, under close observation to make adjustments when necessary. Printing resolution is specific to the printer and the type of bioink. In cases of high resolution, the time to fabricate the object can be longer [121].
- Post-printing stage, which can include spinning and microscopical assessment of the printed object. The bioprinted object is kept in an incubator or bioreactor.
- Placement of the bioprinted product (in vivo or in vitro conditions).
5. Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- AlJehani, Y.A. Risk Factors of Periodontal Disease: Review of the Literature. Int. J. Dent. 2014, 2014, 182513. [Google Scholar] [CrossRef] [PubMed]
- Gibertoni, F.; Sommer, M.E.L.; Esquisatto, M.A.M.; Amaral, M.E.C.D.; Oliveira, C.A.; Andrade, T.A.M.; Mendonça, F.A.S.; Santamaria, M., Jr.; Felonato, M. Evolution of periodontal disease: Immune response and RANK/RANKL/OPG system. Braz. Dent. J. 2017, 28, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Van Dyke, T.E.; Bartold, P.M.; Reynolds, E.C. The nexus between periodontal inflammation and dysbiosis. Front. Immunol. 2020, 11, 511. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Liu, L.; Li, T.; Zhuang, D.; Dai, J.; Wang, B.; Bi, L. Exploring the imbalance of periodontitis immune system from the cellular to molecular level. Front. Genet. 2021, 12, 653209. [Google Scholar] [CrossRef]
- Song, J.; Zhao, H.; Pan, C.; Li, C.; Liu, J.; Pan, Y. Risk factors of chronic periodontitis on healing response: A multilevel modelling analysis. BMC Med. Inform. Decis. Mak. 2017, 17, 135. [Google Scholar] [CrossRef]
- Grzesik, W.J.; Narayanan, A.S. Cementum and periodontal wound healing and regeneration. Crit. Rev. Oral. Biol. Med. 2002, 13, 474–484. [Google Scholar] [CrossRef]
- Zhao, D.; Wu, M.Z.; Yu, S.Y.; Pelekos, G.; Yiu, K.H.; Jin, L. Periodontitis links to concurrent systemic comorbidities among ‘self-perceived health’ individuals. J. Periodontal. Res. 2022, 57, 632–643, Epub ahead of print. [Google Scholar] [CrossRef]
- Cirelli, J.A.; Fiorini, T.; Moreira, C.H.C.; de Molon, R.S.; Dutra, T.P.; Sallum, E.A. Periodontal regeneration: Is it still a goal in clinical periodontology? Braz. Oral Res. 2021, 35, 0097. [Google Scholar] [CrossRef]
- Cheng, X.; Yang, F. More than just a barrier-challenges in the development of guided bone regeneration membranes. Matter 2019, 1, 550–644. [Google Scholar] [CrossRef]
- Cho, Y.D.; Kim, K.H.; Lee, Y.M.; Ku, Y.; Seol, Y.J. Periodontal Wound Healing and Tissue Regeneration: A Narrative Review. Pharmaceuticals 2021, 14, 456. [Google Scholar] [CrossRef]
- Raveau, S.; Jordana, F. Tissue engineering and three-dimensional printing in periodontal regeneration: A literature review. J. Clin. Med. 2020, 9, 4008. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Menicanin, D.; Gronthos, S.; Bartold, P.M. Stem cells, tissue engineering and periodontal regeneration. Aust Dent. J. 2014, 59, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Dahlin, C.; Apatzidou, D.; Artzi, Z.; Bozic, D.; Calciolari, E.; De Bruyn, H.; Dommisch, H.; Donos, N.; Eickholz, P.; et al. Biomaterials and regenerative technologies used in bone regeneration in the craniomaxillofacial region: Consensus report of group 2 of the 15th European Workshop on Periodontology on Bone Regeneration. J. Clin. Periodontol. 2019, 46, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R.; Mataliotakis, G.I.; Calori, G.M.; Giannoudis, P.V. The role of barrier membranes for guided bone regeneration and restoration of large bone defects: Current experimental and clinical evidence. BMC Med. 2012, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Bottino, M.C.; Pankajakshan, D.; Nör, J.E. Advanced scaffolds for dental pulp and periodontal regeneration. Dent. Clin. 2017, 61, 689–711. [Google Scholar] [CrossRef] [PubMed]
- Aytac, Z.; Dubey, N.; Daghrery, A.; Ferreira, J.; de Souza Araujo, I.J.; Castilho, M.; Malda, J.; Bottino, M.C. Innovations in craniofacial bone and periodontal tissue engineering—From electrospinning to converged biofabrication. Int. Mat. Rev. 2021, 2021, 1946236. [Google Scholar] [CrossRef]
- Wang, H.L.; Boyapati, L. “PASS” principles for predictable bone regeneration. Implant Dent. 2006, 15, 8–17. [Google Scholar] [CrossRef]
- Tayebi, L.; Rasoulianboroujeni, M.; Moharamzadeh, K.; Almela, T.K.D.; Cui, Z.; Ye, H. 3D-printed membrane for guided tissue regeneration. Mat. Sci. Eng. C Mater. Biol. Appl. 2018, C84, 148–158. [Google Scholar] [CrossRef]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen–glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef]
- Takata, T.; Wang, H.L.; Miyauchi, M. Attachment, proliferation and differentiation of periodontal ligament cells on various guided tissue regeneration membranes. J. Periodontal. Res. 2001, 36, 322–327. [Google Scholar] [CrossRef]
- Rakhmatia, Y.D.; Ayukawa, Y.; Furuhashi, A.; Koyano, K. Current barrier membranes: Titanium mesh and other membranes for guided bone regeneration in dental applications. J. Prosthodont. Res. 2013, 57, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Jiang, H.B.; Ryu, J.H.; Kang, H.; Kim, K.M.; Kwon, J.S. Comparing properties of variable pore-sized 3D-printed PLA membrane with conventional PLA membrane for guided bone/tissue regeneration. Materials 2019, 12, 1718. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.-M.; Sufaru, I.-G.; Teslaru, S.; Ghiciuc, C.M.; Stafie, C.S. Finding the perfect membrane: Current knowledge on barrier membranes in regenerative procedures: A descriptive review. Appl. Sci. 2022, 12, 1042. [Google Scholar] [CrossRef]
- Sasaki, J.I.; Abe, G.L.; Aonan, L.; Thongthai, P.; Tsuboi, R.; Kohno, T.; Imazato, S. Barrier membranes for tissue regeneration in dentistry. Biomat. Investig. Dent. 2021, 8, 54–63. [Google Scholar] [CrossRef]
- Lee, H.S.; Byun, S.H.; Cho, S.W.; Yang, B.E. Past, present, and future of regeneration therapy in oral and periodontal tissue: A review. Appl. Sci. 2019, 9, 1046. [Google Scholar] [CrossRef]
- Rasperini, G.; Pilipchuk, S.; Flanagan, C.L.; Park, C.H.; Pagni, G.; Hollister, S.J.; Giannobile, W.V. 3D—Printed bioresorbable scaffold for periodontal repair. J. Dent. Res. 2015, 94, 153S–157S. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Witek, L.; Flores, R.L.; Tovar, N.; Torroni, A.; Coelho, P.G.; Kasper, F.K.; Wong, M.; Young, S. Three-dimensional printing for craniofacial bone tissue engineering. Tissue Eng. Part A 2020, 26, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Xie, L.; Yang, B.; Tian, W. Three-dimensional printing biotechnology for the regeneration of the tooth and tooth-supporting tissues. Biotech Bioeng 2019, 116, 452–468. [Google Scholar] [CrossRef]
- Groll, J.; Boland, T.; Blunk, T.; Burdick, J.A.; Cho, D.W.; Dalton, P.D.; Derby, B.; Forgacs, G.; Li, Q.; Mironov, V.A.; et al. Biofabrication: Reappraising the definition of an evolving field. Biofabrication 2016, 8, 013001. [Google Scholar] [CrossRef]
- Yamada, S.; Shanbhag, S.; Mustafa, K. Scaffolds in periodontal regenerative treatment. Dent. Clin. N. Am. 2022, 66, 111–130. [Google Scholar] [CrossRef]
- Fatimi, A.; Okoro, O.V.; Podstawczyk, D.; Siminska-Stanny, J.; Shavandi, A. Natural hydrogel-based bio-inks for 3D bioprinting in tissue engineering: A review. Gels 2022, 8, 179. [Google Scholar] [CrossRef] [PubMed]
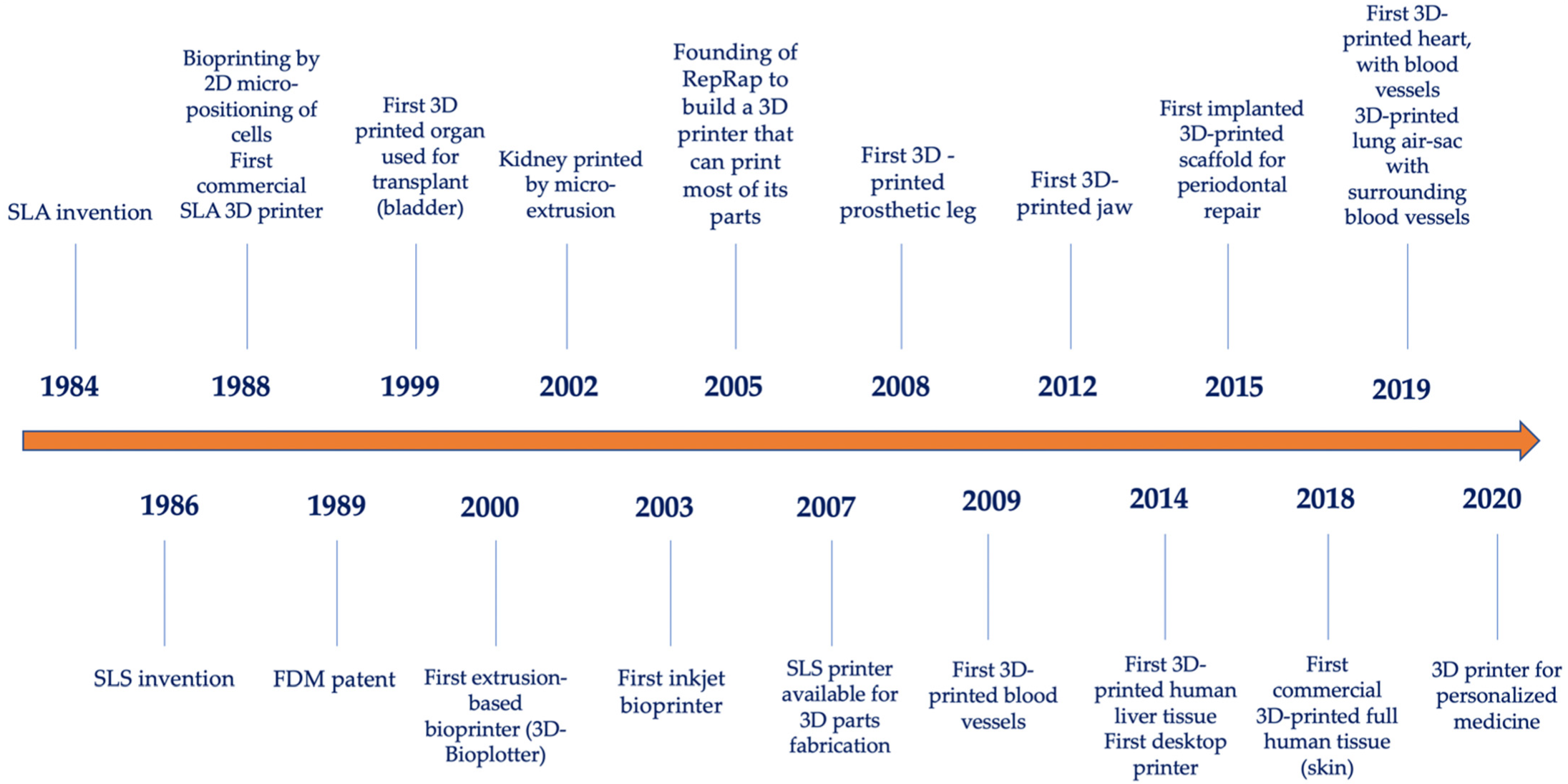
- US Patent for Apparatus for Production of Three-Dimensional Objects by Stereolithography Patent. U.S. Patent 4,575,330, 11 March 1986. Justia Patents Search. Available online: Patents.justia.com (accessed on 5 September 2022).
- Deckard, C. Method and apparatus for producing parts by selective sintering. U.S. Patent 4,863,538, 17 October 1986. published 5 September 1989. [Google Scholar]
- Klebe, R.J. Cytoscribing: A method for micropositioning cells and the construction of two- and three-dimensional synthetic tissues. Exp. Cell Res. 1988, 179, 362–373. [Google Scholar] [CrossRef]
- “Our Story”. 3D Systems. 3D Systems, Inc. Available online: https://www.3dsystems.com/our-story (accessed on 5 September 2022).
- Apparatus and Method for Creating Three-Dimensional Objects” (A System and a Method for Building Three-Dimensional Objects in a Layer-by-Layer Manner via Fused Deposition Modeling). U.S. Patent 5,121,329, 9 June 1989.
- Odde, D.J.; Renn, M.J. Laser-guided direct writing for applications in biotechnology. Trends Biotechnol. 1999, 17, 385–389. [Google Scholar] [CrossRef]
- Landers, R.; Hubner, U.; Schmelzeisen, R.; Mulhaupta, R. Rapid prototyping of scaffolds derived from thermoreversible hydrogels and tailored for applications in tissue engineering. Biomaterials 2002, 23, 4437–4447. [Google Scholar] [CrossRef]
- Wilson, W.C., Jr.; Boland, T. Cell and organ printing 1: Protein and cell printers. Anat. Rec. Part A 2003, 272, 491–496. [Google Scholar] [CrossRef]
- Jones, R.; Haufe, P.; Sells, E.; Iravani, P.; Olliver, V.; Palmer, C.; Bowyer, A. Reprap—The replicating rapid prototyper. Robotica 2011, 29, 177–191. [Google Scholar] [CrossRef]
- Charoo, N.A.; Ali, S.F.B.; Mohamed, E.M.; Kuttolamadom, M.A.; Ozkan, T.; Khan, M.A.; Rahman, Z. Selective laser sintering 3D printing—An overview of the technology and pharmaceutical applications. Drug Dev. Ind. Pharm. 2020, 46, 869–877. [Google Scholar] [CrossRef]
- The History of 3D Printing: 3D Printing Technologies from the 80s to Today. Available online: https://www.sculpteo.com/en/3d-learning-hub/basics-of-3d-printing/the-history-of-3d-printing/ (accessed on 5 September 2022).
- Gu, Z.; Fu, J.; Lin, H.; He, Y. Development of 3D bioprinting: From printing methods to biomedical applications. Asian J. Pharm. Sci. 2020, 15, 529–557. [Google Scholar] [CrossRef]
- Nickels, L. Worlds’s first patient-specific jaw implant. Metal Powder Rep. 2012, 67, 12–14. [Google Scholar] [CrossRef]
- Duan, B. State-of-the-art review of 3D bioprinting for cardiovascular tissue engineering. Ann. Biomed. Eng. 2017, 45, 195–209. [Google Scholar] [CrossRef]
- The Complete History of 3D Printing: From 1980 to 2022. Available online: https://www.3dsourced.com/guides/history-of-3d-printing/ (accessed on 4 September 2022).
- Beheshtizadeh, N.; Lotfibakhshaiesh, N.; Pazhouhnia, Z.; Hoseinpour, M.; Nafari, M. A review of 3D bio-printing for bone and skin tissue engineering: A commercial approach. J. Mat. Sci. 2020, 55, 3729–3749. [Google Scholar] [CrossRef]
- Noor, N.; Shapira, A.; Edri, R.; Gal, I.; Wertheim, L.; Dvir, T. 3D printing of personalized thick and perfusable cardiac patches and hearts. Adv. Sci. 2019, 6, 1900344. [Google Scholar] [CrossRef] [PubMed]
- Vaz, V.M.; Kumar, L. 3D printing as a promising tool in personalized medicine. AAPS PharmSciTech 2021, 22, 49. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, M.; Wei, X.; Hao, Y.; Wang, J. Evaluation of 3D-printed polycaprolactone scaffolds coated with freeze-dried platelet- rich plasma for bone regeneration. Materials 2017, 10, 831. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.S.; Shetty, S.R.; Shetty, R.M.; Vannala, V.; Sk, S.; Rajasekar, S. Focus on periodontal engineering by 3D printing technology—A systematic review. J. Oral. Res. 2020, 9, 522–531. [Google Scholar] [CrossRef]
- Kim, J.D.; Choi, J.S.; Kim, B.S.; Chan, C.Y.; Cho, Y.W. Piezoelectric inkjet printing of polymers: Stem cell patterning on polymer substrates. Polymer 2010, 51, 2147–2154. [Google Scholar] [CrossRef]
- Zhu, W.; Ma, X.Y.; Gou, M.L.; Mei, D.Q.; Zhang, K.; Chen, S.C. 3D printing of functional biomaterials for tissue engineering. Curr. Op. Biotechnol. 2016, 40, 103–112. [Google Scholar] [CrossRef]
- Xu, T.; Kincaid, H.; Atala, A.; Yoo, J.J. High-throughput production of single-cell microparticles using an inkjet printing technology. J. Manuf. Sci. Eng. 2008, 130, 021017. [Google Scholar] [CrossRef]
- Cui, X.F.; Boland, T.; D-Lima, D.D.; Lotz, M.K. Thermal inkjet printing in tissue engineering and regenerative medicine. Recent Pat. Drug Deliv. Formula 2012, 6, 149–155. [Google Scholar] [CrossRef]
- Saunders, R.E.; Gough, J.E.; Derby, B. Delivery of human fibroblast cells by piezoelectric drop-on-demand inkjet printing. Biomaterials 2008, 29, 193–203. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Onses, M.S.; Sutanto, E.; Ferreira, P.M.; Alleyne, A.G.; Rogers, J.A. Mechanisms, capabilities, and applications of high-resolution electrohydrodynamic jet printing. Small 2015, 11, 4237–4266. [Google Scholar] [CrossRef] [PubMed]
- Gasperini, L.; Maniglio, D.; Motta, A.; Migliaresi, C. An electrohydrodynamic bioprinter for alginate hydrogels containing living cells. Tissue Eng. Part C-Methods 2015, 21, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Workman, V.L.; Tezera, L.B.; Elkington, P.T.; Jayasinghe, S.N. Controlled generation of microspheres incorporating extracellular matrix fibrils for three-dimensional cell culture. Adv. Funct. Mater. 2014, 24, 2648–2657. [Google Scholar] [CrossRef]
- Hölzl, K.; Lin, S.; Tytgat, L.; Van Vlierberghe, S.; Gu, L.; Ovsianikov, A. Bioink properties before, during and after 3D bioprinting. Biofabrication 2016, 8, 032002. [Google Scholar] [CrossRef]
- Fan, D.; Li, Y.; Wang, X.; Zhu, T.; Wang, Q.; Cai, H.; Li, W.; Tian, Y.; Liu, Z. Progressive 3D printing technology and its application in medical materials. Front. Pharm. 2020, 11, 122. [Google Scholar] [CrossRef]
- Khorsandi, D.; Fahimipour, A.; Abasian, P.; Seyedig, M.; Ghanavatih, S.; Ahmadf, A.; Amoretti, A.; Fatemeh, S.; Makvandi, P.; Tay, F.R.; et al. 3D and 4D printing in dentistry and maxillofacial surgery: Printing techniques, materials, and applications. Acta Biomater. 2021, 122, 26–49. [Google Scholar] [CrossRef]
- Wang, Z.; Abdulla, R.; Parker, B.; Samanipour, R.; Ghosh, S.; Kim, K. A simple and high-resolution stereolithography-based 3D bioprinting system using visible light crosslinkable bioinks. Biofabrication 2015, 7, 045009. [Google Scholar] [CrossRef]
- Ge, L.; Dong, L.; Wang, D.; Ge, Q.; Gu, G. A digital light processing 3D printer for fast and high-precision fabrication of soft pneumatic actuators. Sens. Actuators A Phys. 2018, 273, 285–292. [Google Scholar] [CrossRef]
- Olakanmi, E.O.; Cochrane, R.F.; Dalgarno, K.W. A review on selective laser sintering/melting (SLS/SLM) of aluminium alloy powders: Processing, microstructure, and properties. Prog. Mater. Sci. 2015, 74, 401–477. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Park, C.H.; Rios, H.F.; Jin, Q.; Sugai, J.V.; Padial-Molina, M.; Taut, A.D.; Flanagan, C.L.; Hollister, S.J.; Giannobile, W.V. Tissue engineering bone-ligament complexes using fiber-guiding scaffolds. Biomaterials 2012, 33, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Vaquette, C.; Fan, W.; Xiao, Y.; Hamlet, S.; Hutmacher, D.W.; Ivanovski, S. A biphasic scaffold design combined with cell sheet technology for simultaneous regeneration of alveolar bone/periodontal ligament complex. Biomaterials 2012, 33, 5560–5573. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Hajibandeh, J.; Suzuki, T.; Fan, A.; Shang, P.; Mao, J.J. Three-dimensional printed multiphase scaffolds for regeneration of periodontium complex. Tissue Eng. Part A 2014, 20, 1342–1351. [Google Scholar] [CrossRef]
- Mwema, F.M.; Akinlabi, E.T. Basics of Fused Deposition modelling (FDM), in ‘Fused Deposition Modeling’; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–15. [Google Scholar]
- Kade, J.C.; Dalton, P.D. Polymers for melt electrowriting. Adv. Healthc. Mater. 2020, 10, 2001232. [Google Scholar] [CrossRef]
- Van Genderen, A.M.; Jansen, K.; Kristen, M.; van Duijn, J.; Li, Y.; Schuurmans, C.C.L.; Malda, J.; Vermonden, T.; Jansen, J.; Masereeuw, R.; et al. Topographic guidance in melt-electrowritten tubular scaffolds enhances engineered kidney tubule performance. BioRxiv 2020, 8, 617364. [Google Scholar] [CrossRef]
- Dalton, P.D. Melt electrowriting with additive manu- facturing principles. Curr. Opin. Biomed. Eng. 2017, 2, 49–57. [Google Scholar] [CrossRef]
- Chen, H.; de Malheiro, A.; van Blitterswijk, C.; Mota, C.; Wieringa, P.A.; Moroni, L. Direct writing electrospinning of scaffolds with multidimensional fiber architecture for hierarchical tissue engineering. ACS Appl. Mater. Interfaces 2017, 9, 38187–38200. [Google Scholar] [CrossRef]
- O’Connell, C.D.; Bridges, O.; Everett, C.; Antill-O’Brien, N.; Onofrillo, C.; Bella, C.D. Electrostatic distortion of melt-electrowritten patterns by 3D objects: Quantification, modeling, and toolpath correction. Adv. Mater. Tech. 2021, 6, 2100345. [Google Scholar] [CrossRef]
- Du, X.; Fu, S.; Zhu, Y. 3D printing of ceramic-based scaffolds for bone tissue engineering: An overview. J. Mater. Chem. B 2018, 6, 4397–4412. [Google Scholar] [CrossRef]
- Gillispie, G.; Prim, P.; Copus, J.; Fisher, J.; Mikos, A.G.; Yoo, J.J.; Atala, A.; Lee, S.J. Assessment methodologies for extrusion-based bioink printability. Biofabrication 2020, 12, 022003. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Huang, X. Elements of 3D bioprinting in periodontal regeneration: Frontiers and prospects. Processes 2021, 9, 1724. [Google Scholar] [CrossRef]
- Carrel, J.P.; Wiskott, A.; Moussa, M.; Rieder, P.; Scherrer, S.; Durual, S. A 3D printed TCP/HA structure as a new osteoconductive scaffold for vertical bone augmentation. Clin. Oral Implant Res. 2016, 27, 55–62. [Google Scholar] [CrossRef]
- Mangano, C.; Barboni, B.; Valbonetti, L.; Berardinelli, P.; Martelli, A.; Muttini, A.; Bedini, R.; Tetè, S.; Piattelli, A.; Mattioli, M. In Vivo Behavior of a custom-made 3D synthetic bone substitute in sinus augmentation procedures in sheep. J. Oral. Implantol. 2015, 41, 240–250. [Google Scholar] [CrossRef]
- Lakkaraju, R.; Guntakandla, V.; Gooty, J.; Palaparthy, R.; Vundela, R.; Bommireddy, V. Three-dimensional printing—A new vista for periodontal regeneration: A review. Int. J. Med. Rev. 2017, 4, 81–85. [Google Scholar] [CrossRef]
- Reçica, B.; Popovska, M.; Cana, A.; Bedxeti, L.Z.; Tefiku, U.; Spasovski, S.; Spasovska-Gjorgovska, A.; Kutllovci, T.; Ahmedi, J.F. Use of biomaterials for periodontal regeneration: A review. Open Access Maced J. Med. Sci. 2020, 8, 90–97. [Google Scholar] [CrossRef]
- Cho, H.; Tarafder, S.; Fogge, M.; Kao, K.; Lee, C.H. Periodontal ligament stem/progenitor cells with protein-releasing scaffolds for cementum formation and integration on dentin surface. Connect. Tissue Res. 2016, 57, 488–495. [Google Scholar] [CrossRef]
- Shim, J.-H.; Won, J.-Y.; Park, J.-H.; Bae, J.-H.; Ahn, G.; Kim, C.-H.; Lim, D.-H.; Cho, D.-W.; Yun, W.-S.; Bae, E.-B.; et al. Effects of 3D-printed polycaprolactone/β-tricalcium phosphate membranes on guided bone regeneration. Int. J. Mol. Sci. 2017, 18, 899. [Google Scholar] [CrossRef]
- Dubey, N.; Ferreira, J.A.; Daghrery, A.; Aytac, Z.; Malda, J.; Bhaduri, S.B.; Bottino, M.C. Highly tunable bioactive fiber-reinforced hydrogel for guided bone regeneration. Acta. Biomater. 2020, 113, 164–176. [Google Scholar] [CrossRef]
- Hsieh, H.Y.; Yao, C.C.; Hsu, L.F.; Tsai, L.H.; Jeng, J.H.; Young, T.H.; Chen, Y.J. Biological properties of human periodontal ligament cells spheroids cultivated on chitosan and polyvinyl alcohol membranes. J. Formosan. Med. Assoc. 2022. [Google Scholar] [CrossRef]
- Bai, L.; Ji, P.; Li, X.; Gao, H.; Li, L.; Wang, C. Mechanical characterization of 3D-printed individualized Ti-Mesh (membrane) for alveolar bone defects. J. Healthc Eng. 2019, 2019, 4231872. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Dong, H.; Niu, Y.; Fan, W.; Jiang, M.; Li, K.; Wei, Q.; Palin, W.; Zhang, Z. Electrophoretic deposition of novel semi- permeable coatings on 3D-printed Ti-Nb alloy meshes for guided alveolar bone regeneration. Dent. Mat. 2022, 38, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Rios, H.F.; Jin, Q.; Bland, M.E.; Flanagan, C.L.; Hollister, S.J.; Giannobile, W.V. Biomimetic hybrid scaffolds for engineering human tooth-ligament interfaces. Biomaterials 2010, 31, 5945–5952. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.F.; Vaquette, C.; Zhang, Q.; Reis, R.L.; Ivanovski, S.; Hutmacher, D.W. Advanced tissue engineering scaffold design for regeneration of the complex hierarchical periodontal structure. J. Clin. Periodontol. 2014, 41, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Chiu, Y.C.; Lee, A.K.; Lin, Y.A.; Lin, P.Y.; Shie, M.Y. Biofabrication of gingival fibroblast cell-laden collagen/strontium-doped calcium silicate 3D-printed bi-layered scaffold for osteoporotic periodontal regeneration. Biomedicines 2021, 9, 431. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.S.; Costa, P.F.; Vaquette, C.; Ivanovski, S.; Hutmacher, D.W.; Malda, J. Additive biomanufacturing: An advanced approach for periodontal tissue regeneration. Ann. Biomed. Eng. 2017, 45, 12–22. [Google Scholar] [CrossRef]
- Huang, R.Y.; Tai, W.C.; Ho, M.H.; Chang, P.C. Combination of a biomolecule-aided biphasic cryogel scaffold with a barrier membrane adhering PDGF-encapsulated nanofibers to promote periodontal regeneration. J. Periodontal. Re. 2020, 55, 529–538. [Google Scholar] [CrossRef]
- Daghrery, A.; de Souza, I.J.; Castilho, M.; Malda, J.; Bottino, M.C. Unveiling the potential of melt electrowritting in regenerative dental medicine. Acta Biomater. 2022, in press. [Google Scholar] [CrossRef]
- Park, S.A.; Lee, H.-J.; Kim, K.-S.; Lee, S.J.; Lee, J.-T.; Kim, S.-Y.; Chang, N.-H.; Park, S.-Y. In vivo evaluation of 3D-printed polycaprolactone scaffold implantation combined with β-TCP powder for alveolar bone augmentation in a beagle defect model. Materials 2018, 11, 238. [Google Scholar] [CrossRef]
- Goh, B.T.; The, L.Y.; Tan, D.B.; Zhang, Z.; Teoh, S.H. Novel 3D polycaprolactone scaffold for ridge preservation—A pilot randomised controlled clinical trial. Clin. Oral. Implants Res. 2014, 26, 271–277. [Google Scholar] [CrossRef]
- Kijartorn, P.; Thammarakcharoen, F.; Suwanprateeb, J.; Buranawat, B. The use of three dimensional printed hydroxyapatite granules in alveolar ridge preservation. Key Eng. Mater. 2017, 751, 663–667. [Google Scholar] [CrossRef]
- Gul, M.; Arif, A.; Ghafoor, R. Role of three-dimensional printing in periodontal regeneration and repair: Literature review. J. Indian Soc. Periodontol. 2019, 23, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Tamimi, F.; Alkhraisat, M.H.; Prados-Frutos, J.C.; Rastikerdar, E.; Gbureck, U.; Barralet, J.E.; López-Cabarcos, E. Vertical bone augmentation with 3D-synthetic monetite blocks in the rabbit calvaria. J. Clin. Periodontol. 2011, 38, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.H.; Stathopoulou, P.G. CAD/CAM and 3D-printing applications for alveolar ridge augmentation. Curr. Oral. Health Rep. 2018, 5, 127–132. [Google Scholar] [CrossRef]
- Reis, E.C.C.; Borges, A.P.; Araújo, M.V.; Mendes, V.C.; Guan, L.; Davies, J.E. Periodontal regeneration using a bilayered PLGA/calcium phosphate construct. Biomaterials 2011, 32, 9244–9253. [Google Scholar] [CrossRef]
- Obregon, F.; Vaquette, C.; Ivanovski, S.; Hutmacher, D.W.; Bertassoni, L.E. Three-dimensional bioprinting for regenerative dentistry and craniofacial tissue engineering. J. Dent. Res. 2015, 94, 143S–152S. [Google Scholar] [CrossRef]
- Park, C.H.; Kim, K.H.; Rios, H.F.; Lee, Y.M.; Giannobile, W.V.; Seol, Y.J. Spatiotemporally controlled microchannels of periodontal mimic scaffolds. J. Dent. Res. 2014, 93, 1304–1312. [Google Scholar] [CrossRef]
- Sumida, T.; Otawa, N.; Kamata, Y.U.; Kamakura, S.; Mtsushita, T.; Kitagaki, H.; Mori, S.; Sasaki, K.; Fujibayashi, S.; Takemoto, M.; et al. Custom-made titanium devices as membranes for bone augmentation in implant treatment: Clinical application and the comparison with conventional titanium mesh. J. Craniomaxillofac Surg. 2015, 43, 2183–2188. [Google Scholar] [CrossRef]
- Pilipchuk, S.P.; Monje, A.; Jiao, Y.; Hao, J.; Kruger, L.; Flanagan, C.L.; Hollister, S.J.; Giannobile, W.V. Integration of 3D printed and micropatterned polycaprolactone scaffolds for guidance of oriented collagenous tissue formation in vivo. Adv. Healthc Mater 2016, 5, 676–687. [Google Scholar] [CrossRef]
- Adel-Khattab, D.; Giacomini, F.; Gildenhaar, R.; Berger, G.; Gomes, C.; Linow, U.; Hardt, M.; Peleska, B.; Günster, J.; Stiller, M.; et al. Development of a synthetic tissue engineered three-dimensional printed bioceramic-based bone graft with homogenously distributed osteoblasts and mineralizing bone matrix in vitro. J. Tissue Eng. Regen. Med. 2018, 12, 44–58. [Google Scholar] [CrossRef]
- Lei, L.; Yu, Y.; Ke, T.; Sun, W.; Chen, L. The application of three-dimensional printing model and platelet-rich fibrin technology in guided tissue regeneration surgery for severe bone defects. J. Oral. Implantol. 2019, 45, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Bartnikowski, M.; Vaquette, C.; Ivanovski, S. Workflow for highly porous resorbable custom 3D printed scaffolds using medical grade polymer for large volume alveolar bone regeneration. Clin. Oral. Implants Res. 2020, 31, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Tamimi, F.; Torres, J.; Gbureck, U.; Lopez-Cabarcos, E.; Bassett, D.C.; Alkhraisat, M.H.; Barralet, J.E. Craniofacial vertical bone augmentation: A comparison between 3D printed monolithic monetite blocks and autologous onlay grafts in the rabbit. Biomaterials 2009, 30, 6318–6326. [Google Scholar] [CrossRef] [PubMed]
- Di Giacomo, G.A.; da Silva, J.V.; da Silva, A.M.; Paschoal, G.H.; Cury, P.R.; Szarf, G. Accuracy and complications of computer-designed selective laser sintering surgical guides for flapless dental implant placement and immediate definitive prosthesis installation. J. Periodontol. 2012, 83, 410–419. [Google Scholar] [CrossRef]
- Cassetta, M.; Di Mambro, A.; Giansanti, M.; Stefanelli, L.V.; Cavallini, C. The intrinsic error of a stereolithographic surgical template in implant guided surgery. Int. J. Oral. Maxillofac. Surg. 2013, 42, 264–275. [Google Scholar] [CrossRef]
- Pozzi, A.; Tallarico, M.; Marchetti, M.; Scarfò, B.; Esposito, M. Computer-guided versus free-hand placement of immediately loaded dental implants: 1-year post-loading results of a multicentre randomised controlled trial. Eur. J. Oral. Implantol. 2014, 7, 229–242. [Google Scholar]
- Stübinger, S.; Buitrago-Tellez, C.; Cantelmi, G. Deviations between placed and planned implant positions: An accuracy pilot study of skeletally supported stereolithographic surgical templates. Clin. Implant. Dent. Relat. Res. 2014, 16, 540–551. [Google Scholar] [CrossRef]
- Shen, P.; Zhao, J.; Fan, L.; Qiu, H.; Xu, W.; Wang, Y.; Zhang, S.; Kim, Y.-J. Accuracy evaluation of computer-designed surgical guide template in oral implantology. J. Craniomaxillofac Surg. 2015, 43, 2189–2194. [Google Scholar] [CrossRef]
- Verhamme, L.M.; Meijer, G.J.; Boumans, T.; de Haan, A.F.; Bergé, S.J.; Maal, T.J. A clinically relevant accuracy study of computer-planned implant placement in the edentulous maxilla using mucosa-supported surgical templates. Clin. Implant. Dent. Relat. Res. 2015, 17, 343–352. [Google Scholar] [CrossRef]
- Xu, L.W.; You, J.; Zhang, J.X.; Liu, Y.F.; Peng, W. Impact of surgical template on the accuracy of implant placement. J. Prosthodont. 2016, 25, 641–646. [Google Scholar] [CrossRef]
- Bernard, L.; Vercruyssen, M.; Duyck, J.; Jacobs, R.; Teughels, W.; Quirynen, M. A randomized controlled clinical trial comparing guided with nonguided implant placement: A 3-year follow-up of implant-centered outcomes. J. Prosthet. Dent. 2019, 121, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Kislitsyn, A.; Savinkov, R.; Novkovic, M.; Onder, L.; Bocharov, G. Computational approach to 3D Modeling of the lymph node geometry. Computation 2015, 3, 222–234. [Google Scholar] [CrossRef]
- Rodriguez, M.J.; Brown, J.; Giordano, J.; Lin, S.J.; Omenetto, F.G.; Kaplan, D.L. Silk based bioinks for soft tissue reconstruction using 3-dimensional (3D) printing with in vitro and in vivo assessments. Biomaterials 2017, 117, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Miri, A.K.; Mirzaee, I.; Hassan, S.; Mesbah Oskui, S.; Nieto, D.; Khademhosseini, A.; Zhang, Y.S. Effective bioprinting resolution in tissue model fabrication. Lab. Chip. 2019, 19, 2019–2037. [Google Scholar] [CrossRef] [PubMed]
- Tasoglu, S.; Demirci, U. Bioprinting for stem cell research. Trends Biotechnol. 2013, 31, 10–19. [Google Scholar] [CrossRef]
- Albritton, J.L.; Miller, J.S. 3D bioprinting: Improving in vitro models of metastasis with heterogeneous tumor microenvironments. Dis. Model. Mech. 2017, 10, 3–14. [Google Scholar] [CrossRef]
- Chang, R.; Nam, J.; Sun, W. Direct cell writing of 3D microorgan for in vitro pharmacokinetic model. Tissue Eng. Part C Methods 2008, 14, 157–166. [Google Scholar] [CrossRef]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.-H. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef]
- Melchels, F.P.; Domingos, M.A.; Klein, T.J.; Malda, J.; Bartolo, P.J.; Hutmacher, D.W. Additive manufacturing of tissues and organs. Prog. Polym. Sci. 2012, 37, 1079–1104. [Google Scholar] [CrossRef]
- Rodriguez-Salvador, M.; Ruiz-Cantu, L. Revealing emerging science and technology research for dentistry applications of 3D bioprinting. Int. J. Bioprint. 2018, 5, 170. [Google Scholar] [CrossRef]
- Ingber, D.E.; Mow, V.C.; Butler, D.; Niklason, L.; Huard, J.; Mao, J.; Yannas, I.; Kaplan, D.; Vunjak-Novakovic, G. Tissue engineering and developmental biology: Going biomimetic. Tissue Eng. 2006, 12, 3265–3283. [Google Scholar] [CrossRef] [PubMed]
- Shu, A.F. Bioprinting of human pluripotent stem cells and their directed differentiation into hepatocyte-like cells for the generation of mini-livers in 3D. Biofabrication 2015, 7, 44102. [Google Scholar]
- Li, J.P.; Chen, M.J.; Fan, X.Q.; Zhou, H.F. Recent advances in bioprinting techniques: Approaches, applications and future prospects. J. Transl. Med. 2016, 14, 271. [Google Scholar] [CrossRef]
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 2020, 226, 119536. [Google Scholar] [CrossRef]
- Kato, B.; Wisser, G.; Agrawal, D.K.; Wood, T.; Thankam, F.G. 3D bioprinting of cardiac tissue: Current challenges and perspectives. J. Mater. Sci. Mater. Med. 2021, 32, 54. [Google Scholar] [CrossRef]
- Thattaruparambil Raveendran, N.; Vaquette, C.; Meinert, C.; Ipe, D.S.; Ivanovski, S. Optimization of 3D bioprinting of periodontal ligament cells. Dent. Mater. 2019, 35, 1683–1694. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ji, Y.; Huang, G.; Ling, K.; Zhang, X.; Xu, F. Bioprinting 3D cell-laden hydrogel microarray for screening human periodontal ligament stem cell response to extracellular matrix. Biofabrication 2015, 7, 044105. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, W.; Chen, Y.; Wang, J. Alveolar bone repair of rhesus monkeys by using BMP-2 gene and mesenchymal stem cells loaded three-dimensional printed bioglass scaffold. Sci. Rep. 2019, 9, 18175. [Google Scholar] [CrossRef]
- Lin, H.-H.; Chao, P.-H.G.; Tai, W.-C.; Chang, P.-C. 3D-printed collagen-based waveform microfibrous scaffold for periodontal ligament reconstruction. Int. J. Mol. Sci. 2021, 22, 7725. [Google Scholar] [CrossRef]
- Vurat, M.T.; Şeker, Ş.; Lalegül-Ülker, Ö.; Parmaksiz, M.; Elçin, A.E.; Elçin, Y.M. Development of a multicellular 3D-bioprinted microtissue model of human periodontal ligament-alveolar bone biointerface: Towards a pre-clinical model of periodontal diseases and personalized periodontal tissue engineering. Genes Dis. 2020, 9, 1008–1023. [Google Scholar] [CrossRef]
- Li, Z.; Li, S.; Yang, J.; Ha, Y.; Zhang, Q.; Zhou, X.; He, C. 3D bioprinted gelatin/gellan gum-based scaffold with double-crosslinking network for vascularized bone regeneration. Carbohydr. Polym. 2022, 290, 119469. [Google Scholar] [CrossRef] [PubMed]
- Castilho, M.; de Ruijter, M.; Beirne, S.; Ito, K. Multitechnology biofabrication: A new approach for the manufacturing of functional tissue structures? Trends Biotechnol. 2020, 38, 1316–1328. [Google Scholar] [CrossRef] [PubMed]
- De Ruijter, M.; Ribeiro, A.; Dokter, I.; Castilho, M.; Malda, J. Simultaneous micropatterning of fibrous meshes and bioinks for the fabrication of living tissue constructs. Adv. Healthc. Mater. 2019, 8, 1800418. [Google Scholar] [CrossRef] [PubMed]
- Diloksumpan, P.; de Ruijter, M.; Castilho, M.; Gbureck, U.; Vermonden, T.; van Weeren, P.R.; Malda, J.; Levato, R. Combining multi-scale 3D printing technologies to engineer reinforced hydrogel-ceramic interfaces. Biofabrication 2020, 12, 025014. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, D.M.; de Annunzio, S.R.; Carmello, J.C.; Pavarina, A.C.; Fontana, C.R.; Correa, D.S. Combining coaxial electrospinning and 3D printing: Design of biodegradable bilayered membranes with dual drug delivery capability for periodontitis treatment. ACS Appl. Bio. Mater 2022, 5, 146–159. [Google Scholar] [CrossRef]
- Liu, J.; Zou, Q.; Wang, C.; Lin, M.; Li, Y.; Zhang, R.; Li, Y. Electrospinning and 3D printed hybrid bi-layer scaffold for guided bone regeneration. Mat. Design 2021, 210, 110047. [Google Scholar] [CrossRef]
- Gao, B.; Yang, Q.; Zhao, X.; Jin, G.; Ma, Y.; Xu, F. 4D Bioprinting for biomedical applications. Trends Biotechnol. 2016, 34, 746–756. [Google Scholar] [CrossRef]
- Betsch, M.; Cristian, C.; Lin, Y.Y.; Blaeser, A.; Schöneberg, J.; Vogt, M.; Buhl, E.M. Incorporating 4D into bioprinting: Real-time magnetically directed collagen fiber alignment for generating complex multi- layered tissues. Adv. Healthc. Mater. 2018, 7, 1800894. [Google Scholar] [CrossRef]
- Liu, F.; Wang, W.; Mirihanage, W.; Hinduja, S.; Bartolo, P. A plasma- assisted bioextrusion system for tissue engineering. CIRP Ann. 2018, 67, 229–232. [Google Scholar] [CrossRef]

| Material | Advantages | Disadvantages |
|---|---|---|
| Natural polymers Collagen Alginate Hyaluronic acid Chitosan | Biocompatible Good cell affinity Hydrophilicity Antibacterial effect | Low mechanical properties Fast degradation rate Lack of bioactivity |
| Synthetic polymers Polycaprolactone (PCL) Polylactic acid (PLA) Polyglycolic acid (PGA) Polyethylene glycol (PEG) Poly(lactic-co-glycolic) acid (PLGA) | Highly adjustable physiochemical and mechanical properties Wide range of degradation and resorption kinetics Good repeatability | Low bioactivity Slow degradation rate Acidic byproducts |
| Bio-ceramics Hydroxyapatite (HA) β-tricalcium phosphate (β-TCP) Bioactive glass | Bioactive Biocompatible Osteoconductive Potential osteoinductive Hydrophilicity | Not compatible with cell encapsulation Stiffness Brittleness Low ductility Low flexibility Inconsistent cell reactions (variations in surface quality) |
| Application | Authors | Type of Study | Method | Material | 3D Printer |
|---|---|---|---|---|---|
| GTR | Kim et al., 2010 [52] | In vivo | 3D-printed tooth scaffold | Poly-epsilon caprolactone and hydroxyapatite | Not mentioned |
| Park et al., 2010 [90] | In vivo | 3D-printed scaffold | PCL-PGA | 3D wax-printing system (ModelMaker II, Solidscape, Inc., Merrimack, NH, USA) | |
| Carlo Reis et al., 2011 [102] | In vivo | 3D-printed scaffold | PLGA/CaP bilayered biomaterial | Not mentioned | |
| Park et al., 2012 [68] | In vivo | 3D-printed scaffold | Poly-ε caprolactone solution (PCL) | 3-D rapid prototyping wax printer (ModelMaker II; Solidscape Inc., Merrimack, NH, USA) | |
| Obregon et al., 2015 [103] | In vivo | 3D-printed scaffold | Bilayered biomaterial | Not mentioned | |
| Vaquette et al., 2012 [69] | In vivo | FDM + solution electrospinning | PCL | FDM, Osteopore Inc. Singapore In-house solution spinning device | |
| Costa et al., 2014 [91] | In vivo | 3D-printed scaffold | Bilayered biomaterial | Not mentioned | |
| Park et al., 2014 [104] | In vivo | 3D-printed scaffold | Gelatin, chitosan | Not mentioned | |
| Lee et al., 2014 [70] | In vivo | Layer-by-layer deposition | PCL + hydroxyapatite | Bioplotter, EnvisionTEC | |
| Rasperini et al., 2015 [26] | Case report | 3D-printed Bioresorbable Scaffold | PCL | SLS (Formiga P100 System; EOS e-Manufacturing Solutions, Pflugerville, TX, USA)) | |
| Sumida et al., 2015 [105] | RCT | 3D-printed scaffold | Titanium | Not mentioned | |
| Pilipchuk et al., 2016 [106] | Preclinical study | 3D-printed scaffold | PCL | Not mentioned | |
| Adel-Khattab et al., 2018 [107] | In vitro | 3D-printed scaffold | Bioceramic | R1Series ExOne (PROMETAL, North Huntingdon, PA, USA) | |
| Lei et al. 2019 [108] | Case report | 3D-printed bone model | Not mentioned | Not mentioned | |
| Bartnikowski et al., 2020 [109] | RCT | Layer-by-layer deposition | PCL | Bioplotter, EnvisionTEC, Dearborn, MI, USA | |
| Socket preservation | Goh et al., 2015 [97] | Pilot RCT | 3D-printed bioresorbable scaffold | PCL | FDM techniques (FDM 3000; Stratasys, Eden Prairie, MN, USA) |
| Kijartorn et al., 2017 [98] | Prospective study | 3D-printed scaffold | Hydroxyapatite granules | Projet 160, 3D systems | |
| Park et al., 2018 [96] | In vivo | 3D-printed bioresorbable scaffold | PCL | 3D bioprinting system (laboratory -made system in Korea Institute of Machinery and Materials, Daejeon, Korea) | |
| Vertical bone augmentation | Tamimi et al., 2009 [110] | Case report | 3D-printed monolithic monetite blocks | Synthetic calcium phosphates | 3D-powder Printing system (Z-Corporation, Burlington, MA, USA) |
| Torres et al., 2011 [100] | In vivo | 3D-printed monolithic monetite blocks | A/b-tricalcium phosphate | 3D-powder Printing system (Z-Corporation, Burlington, MA, USA) | |
| Sinus augmentation | Mangano et al., 2015 [103] | In vivo | 3D synthetic bone substitute | Ceramic | Not mentioned |
| Guided implant placement | Di Giacomo et al., 2005 [111] | NRCT | SLA surgical guides | Polymer | Simplant CSI Materialise, Ann Arbor, MI, USA |
| Cassetta et al., 2013 [112] | Retrospective | 3D-printed surgical guide | Acrylic | SLA surgical guide (External Hex Safe1, Materialise Dental, Leuven, Belgium) | |
| Pozzi et al., 2014 [113] | Clinical trial | SLA surgical guides | Acrylic resin | Nobel Procera, Nobel Biocare, Zurich, Switzerland | |
| Stübinger et al., 2014 [114] | Prospective | 3D-printed surgical guide | Polymer | Astra Tech AB, Mölndal, Sweden | |
| Shen et al., 2015 [115] | RCT | SLA templates | Acrylic | Geomagic, version 10.0, Geomagic, Research triangle Park, NC, USA | |
| Verhamme et al., 2015 [116] | Prospective | 3D-printed surgical guide | Not mentioned | NobelGuide (Nobel Biocare, Gothenburg, Sweden | |
| Xu et al., 2016 [117] | In vitro | SLA surgical guides | Acrylic | Conne×350; Objet, Rehovot, Israel | |
| Bernard et al., 2019 [118] | RCT | SLA surgical guides | Acrylic | Simplant; Materialise Dental, Waltham, MA, USA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sufaru, I.-G.; Macovei, G.; Stoleriu, S.; Martu, M.-A.; Luchian, I.; Kappenberg-Nitescu, D.-C.; Solomon, S.M. 3D Printed and Bioprinted Membranes and Scaffolds for the Periodontal Tissue Regeneration: A Narrative Review. Membranes 2022, 12, 902. https://doi.org/10.3390/membranes12090902
Sufaru I-G, Macovei G, Stoleriu S, Martu M-A, Luchian I, Kappenberg-Nitescu D-C, Solomon SM. 3D Printed and Bioprinted Membranes and Scaffolds for the Periodontal Tissue Regeneration: A Narrative Review. Membranes. 2022; 12(9):902. https://doi.org/10.3390/membranes12090902
Chicago/Turabian StyleSufaru, Irina-Georgeta, Georgiana Macovei, Simona Stoleriu, Maria-Alexandra Martu, Ionut Luchian, Diana-Cristala Kappenberg-Nitescu, and Sorina Mihaela Solomon. 2022. "3D Printed and Bioprinted Membranes and Scaffolds for the Periodontal Tissue Regeneration: A Narrative Review" Membranes 12, no. 9: 902. https://doi.org/10.3390/membranes12090902
APA StyleSufaru, I.-G., Macovei, G., Stoleriu, S., Martu, M.-A., Luchian, I., Kappenberg-Nitescu, D.-C., & Solomon, S. M. (2022). 3D Printed and Bioprinted Membranes and Scaffolds for the Periodontal Tissue Regeneration: A Narrative Review. Membranes, 12(9), 902. https://doi.org/10.3390/membranes12090902










