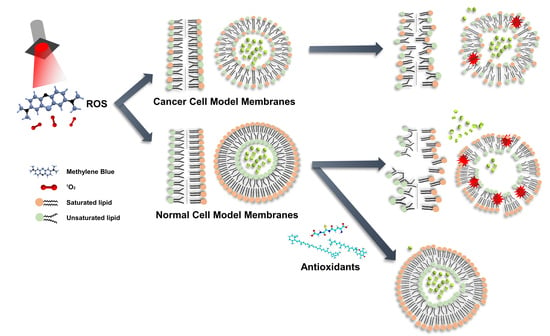
Elucidation of the Interactions of Reactive Oxygen Species and Antioxidants in Model Membranes Mimicking Cancer Cells and Normal Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bilayer Formation
2.2. Electrical Measurement
2.3. ROS Generation
2.4. Liposome Generation
2.5. Liposome Quenching Experiment
2.6. Antioxidant Test
3. Results and Discussion
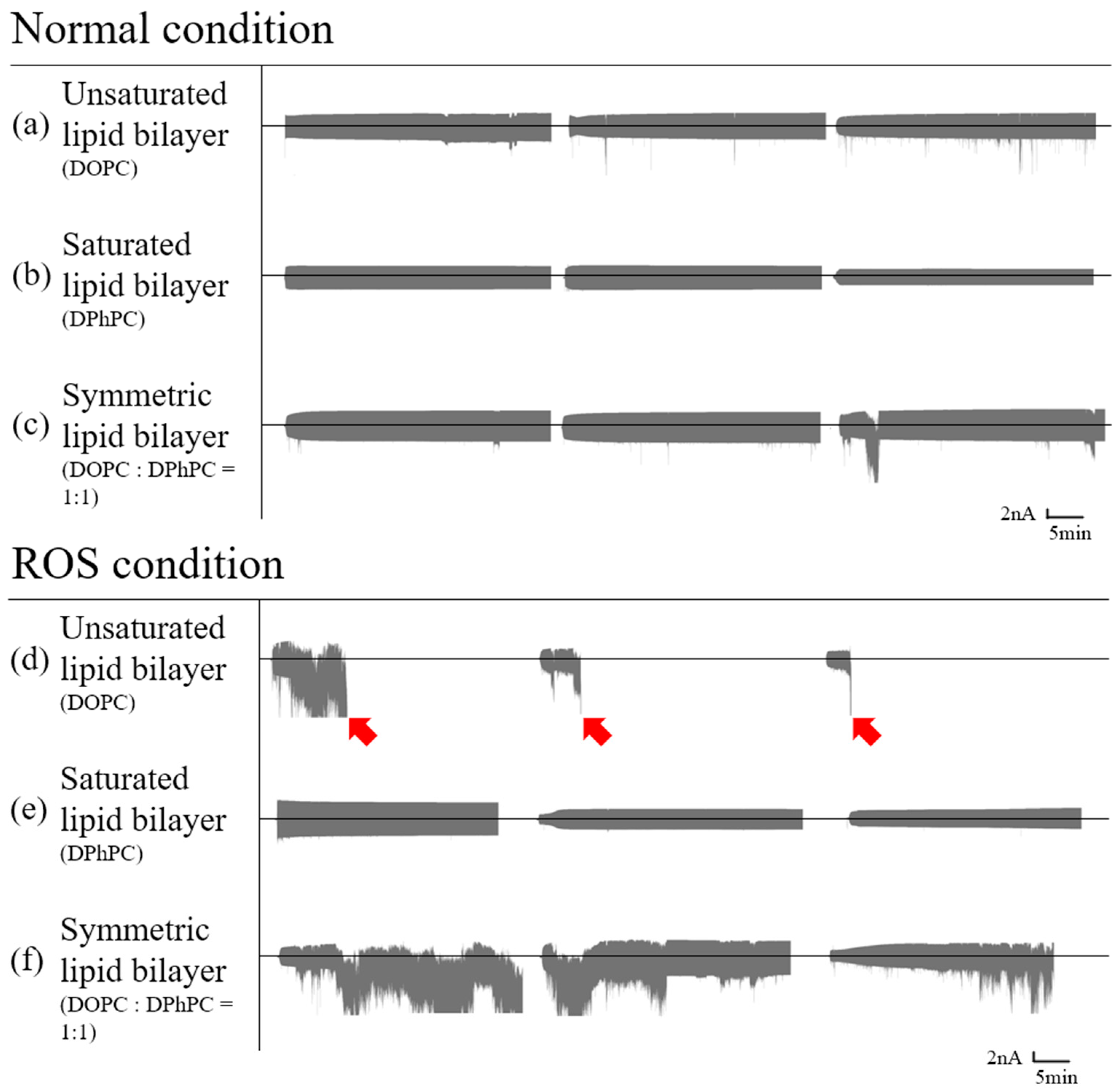
3.1. Planar Lipid Bilayer
3.1.1. Symmetric Bilayer
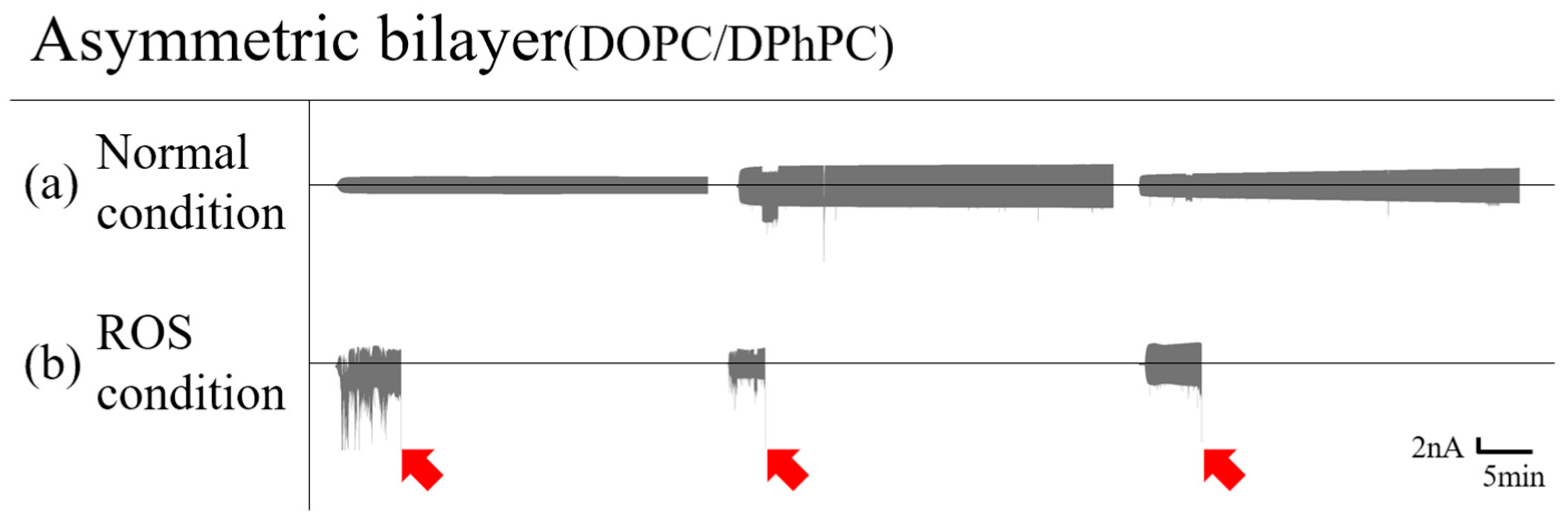
3.1.2. Asymmetric Bilayer
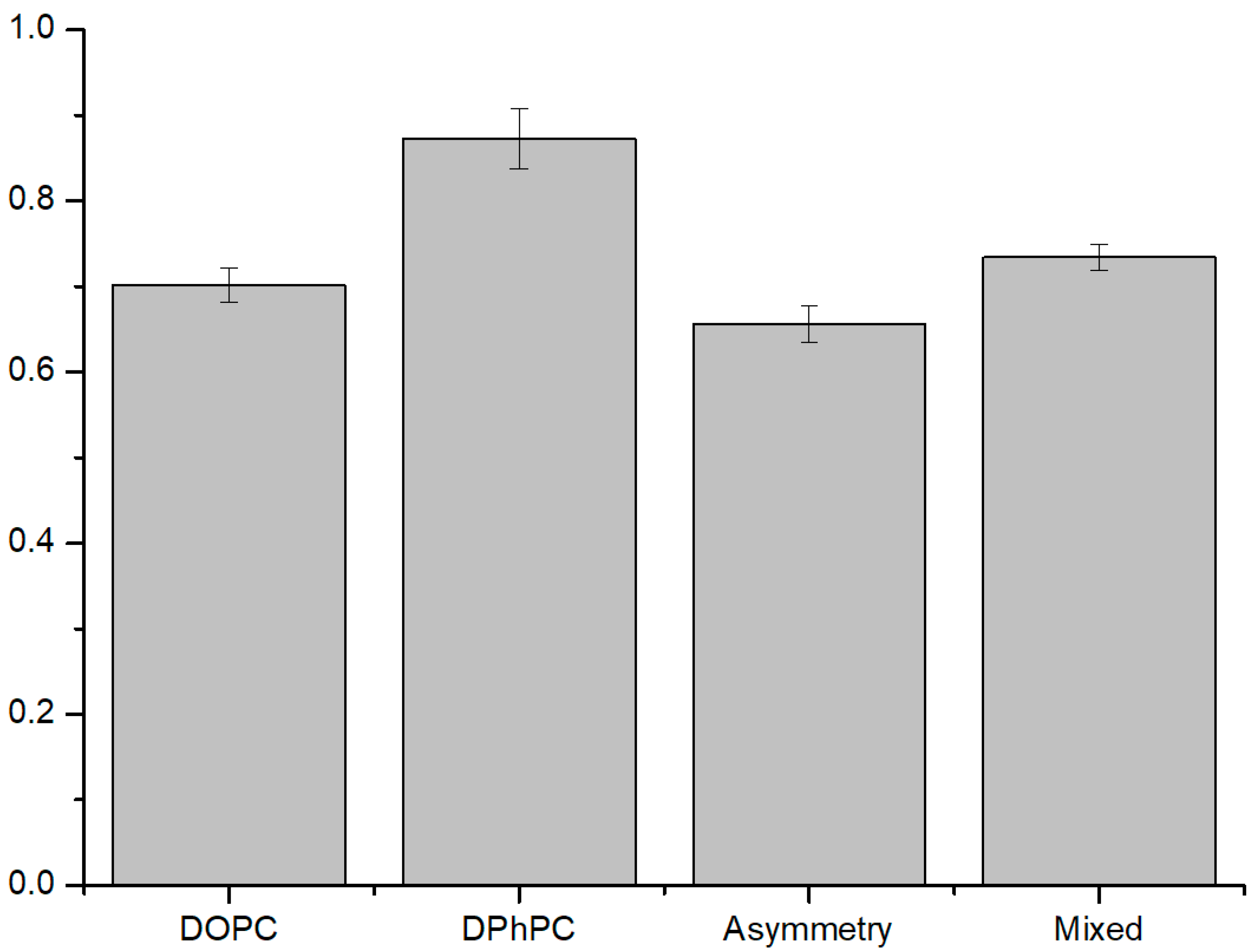
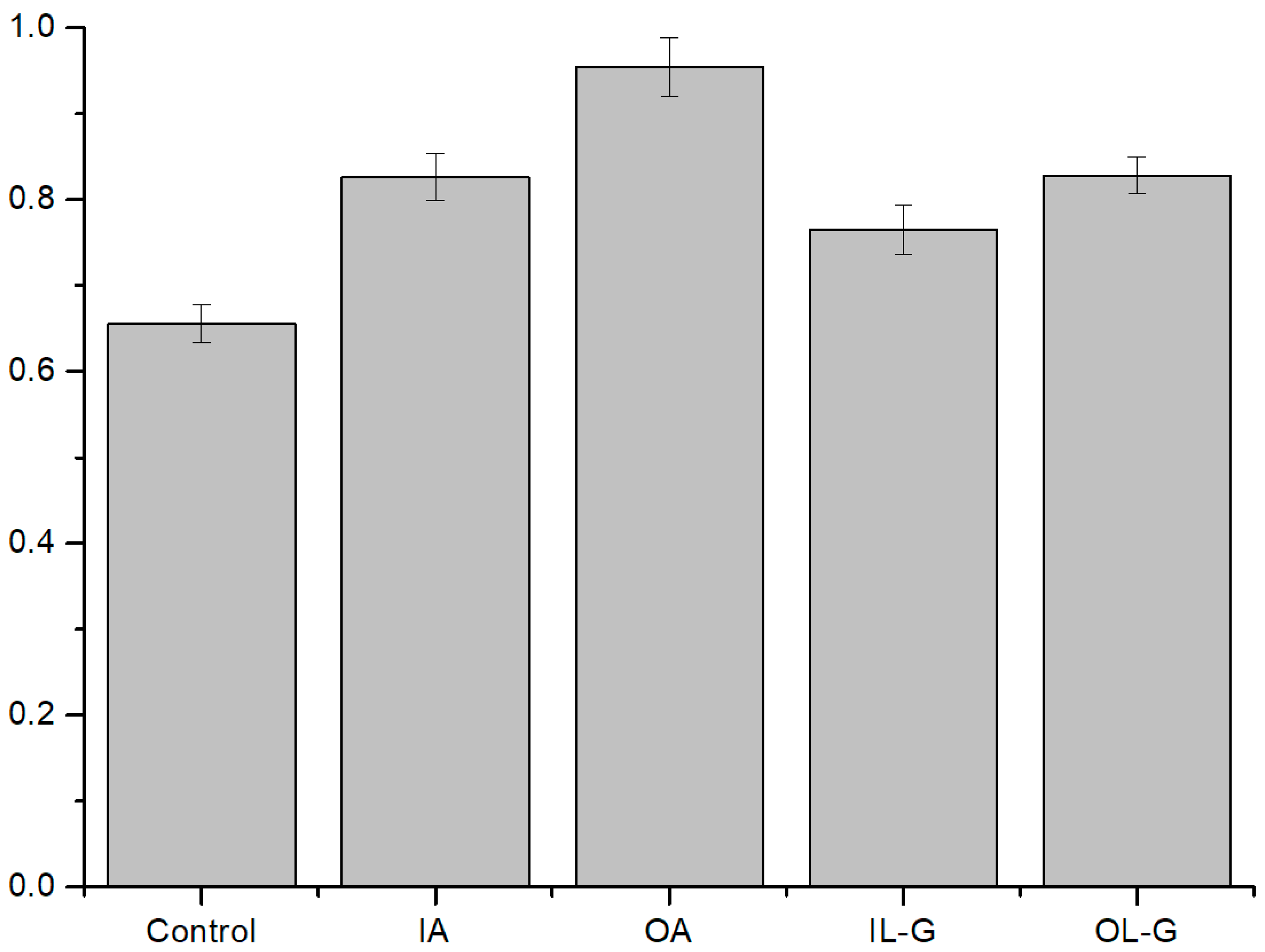
3.2. ROS Effect on Liposomes
3.2.1. ROS Effect on Several Compositions of Liposomes
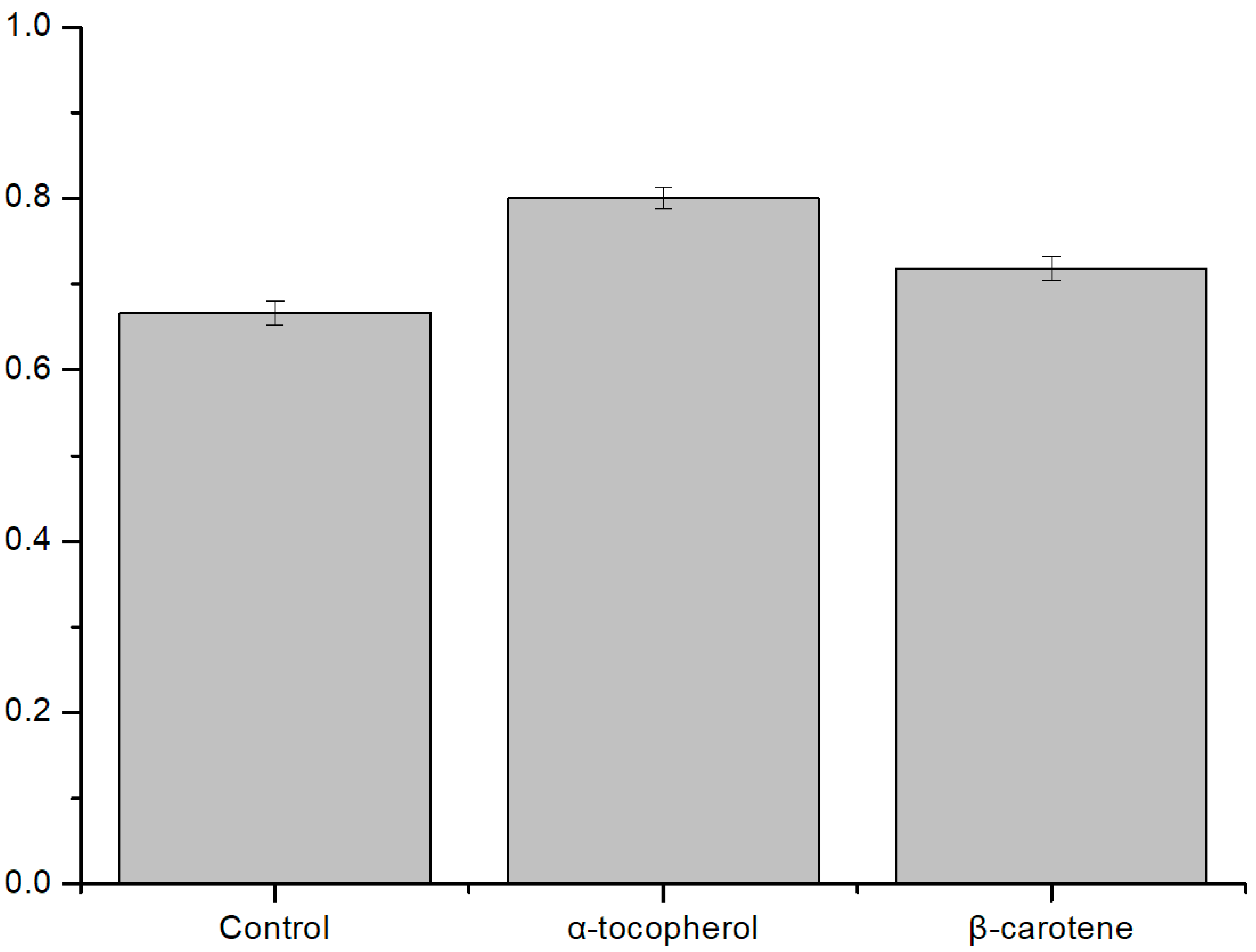
3.2.2. Antioxidant Effect on ROS Condition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Yi, J. Cancer cell killing via ROS: To increase or decrease, that is the question. Cancer Biol. Ther. 2008, 7, 1875–1884. [Google Scholar] [CrossRef] [PubMed]
- Van der Paal, J.; Neyts, E.C.; Verlackt, C.C.W.; Bogaerts, A. Effect of lipid peroxidation on membrane permeability of cancer and normal cells subjected to oxidative stress. Chem. Sci. 2015, 7, 489–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free Radicals and Antioxidants in Normal Physio-logical Functions and Human Disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Turrens, J.F. Mitochondrial Formation of Reactive Oxygen Species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef] [PubMed]
- de Jager, T.L.; Cockrell, A.E.; Du Plessis, S.S. Ultraviolet Light Induced Generation of Reactive Oxygen Species. Adv. Exp. Med. Biol. 2017, 996, 15–23. [Google Scholar] [PubMed]
- Caruso, E.; Malacarne, M.C.; Marras, E.; Papa, E.; Bertato, L.; Banfi, S.; Gariboldi, M.B. New BODIPYs for photodynamic therapy (PDT): Synthesis and activity on human cancer cell lines. Bioorg. Med. Chem. 2020, 28, 115737. [Google Scholar] [CrossRef] [PubMed]
- Szlasa, W.; Supplitt, S.; Drąg-Zalesińska, M.; Przystupski, D.; Kotowski, K.; Szewczyk, A.; Kasperkiewicz, P.; Saczko, J.; Kulbacka, J. Effects of curcumin based PDT on the viability and the organization of actin in melanotic (A375) and amelanotic melanoma (C32)—In vitro studies. Biomed. Pharmacother. 2020, 132, 110883. [Google Scholar] [CrossRef]
- Dougherty, T.J.; Kaufman, J.E.; Goldfarb, A.; Weishaupt, K.R.; Boyle, D.; Mittleman, A. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978, 38, 2628–2635. [Google Scholar]
- Singh, M.; Lee, K.E.; Vinayagam, R.; Kang, S.G. Antioxidant and Antibacterial Profiling of Pomegranate-pericarp Extract Functionalized-zinc Oxide Nanocomposite. Biotechnol. Bioprocess Eng. 2021, 26, 728–737. [Google Scholar] [CrossRef]
- Bae, C.-S.; Lee, C.-M.; Ahn, T. Encapsulation of Apoptotic Proteins in Lipid Nanoparticles to Induce Death of Cancer Cells. Biotechnol. Bioprocess Eng. 2020, 25, 264–271. [Google Scholar] [CrossRef]
- Jiang, L.; Zhou, S.; Zhang, X.; Li, C.; Ji, S.; Mao, H.; Jiang, X. Mitochondrion-specific dendritic lipopeptide liposomes for targeted sub-cellular delivery. Nat. Commun. 2021, 12, 2390. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Ding, L.; Xu, H.-J.; Shen, Z.; Ju, H.; Jia, L.; Bao, L.; Yu, J.-S. Cell-Specific and pH-Activatable Rubyrin-Loaded Nanoparticles for Highly Selective Near-Infrared Photodynamic Therapy against Cancer. J. Am. Chem. Soc. 2013, 135, 18850–18858. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cheng, L.; Liu, Y.; Wang, X.; Ma, X.; Deng, Z.; Li, Y.; Liu, Z. Imaging-Guided pH-Sensitive Photodynamic Therapy Using Charge Reversible Upconversion Nanoparticles under Near-Infrared Light. Adv. Funct. Mater. 2013, 23, 3077–3086. [Google Scholar] [CrossRef]
- Park, J.; Lee, Y.-K.; Park, I.-K.; Hwang, S.R. Current Limitations and Recent Progress in Nanomedicine for Clinically Available Photodynamic Therapy. Biomedicines 2021, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxid. Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef] [Green Version]
- Fujimoto, T.; Parmryd, I. Interleaflet Coupling, Pinning, and Leaflet Asymmetry—Major Players in Plasma Membrane Nanodomain Formation. Front. Cell Dev. Biol. 2017, 4, 155. [Google Scholar] [CrossRef] [Green Version]
- Fadok, V.; Bratton, D.L.; Frasch, S.C.; Warner, M.L.; Henson, P.M. The role of phosphatidylserine in recognition of apoptotic cells by phagocytes. Cell Death Differ. 1998, 5, 551–562. [Google Scholar] [CrossRef] [Green Version]
- Lorent, J.H.; Levental, K.R.; Ganesan, L.; Rivera-Longsworth, G.; Sezgin, E.; Doktorova, M.D.; Lyman, E.; Levental, I. Plasma membranes are asymmetric in lipid unsaturation, packing and protein shape. Nat. Chem. Biol. 2020, 16, 644–652. [Google Scholar] [CrossRef]
- Vallabhapurapu, S.D.; Blanco, V.M.; Sulaiman, M.K.; Lakshmi Vallabhapurapu, S.; Chu, Z.; Franco, R.S.; Qi, X. Variation in Human Cancer Cell External Phosphatidylserine Is Regulated by Flippase Activity and Intracellular Calcium. Oncotarget 2015, 6, 34375–34388. [Google Scholar] [CrossRef] [Green Version]
- Rivel, T.; Ramseyer, C.; Yesylevskyy, S. The asymmetry of plasma membranes and their cholesterol content influence the uptake of cisplatin. Sci. Rep. 2019, 9, 5627. [Google Scholar] [CrossRef] [Green Version]
- Choi, W.; Ryu, H.; Fuwad, A.; Goh, S.; Zhou, C.; Shim, J.; Takagi, M.; Kwon, S.; Kim, S.; Jeon, T.-J. Quantitative Analysis of the Membrane Affinity of Local Anesthetics Using a Model Cell Membrane. Membranes 2021, 11, 579. [Google Scholar] [CrossRef]
- Park, J.; Lim, M.C.; Ryu, H.; Shim, J.; Kim, S.M.; Kim, Y.R.; Jeon, T.J. Nanopore based detection of Bacillus thuringiensis HD-73 spores using aptamers and versatile DNA hairpins. Nanoscale 2018, 10, 11955–11961. [Google Scholar] [CrossRef]
- Chen, P.-C.; Chou, C.C.; Chiang, C.H. Systematically Studying Dissolution Process of 3D Printed Acrylonitrile Butadiene Styrene (ABS) Mold for Creation of Complex and Fully Transparent Polydimethylsiloxane (PDMS) Fluidic Devices. BioChip J. 2021, 15, 144–151. [Google Scholar] [CrossRef]
- Jung, S.H.; Choi, S.; Kim, Y.R.; Jeon, T.J. Storable Droplet Interface Lipid Bilayers for Cell-Free Ion Channel Studies. Bioprocess Biosyst. Eng. 2012, 35, 241–246. [Google Scholar] [CrossRef]
- Choi, S.; Yoon, S.; Ryu, H.; Kim, S.M.; Jeon, T.-J. Automated Lipid Bilayer Membrane Formation Using a Polydimethylsiloxane Thin Film. J. Vis. Exp. 2016, 113, e54258. [Google Scholar] [CrossRef]
- Smejtek, P.; Hsu, K.; Perman, W. Electrical conductivity in lipid bilayer membranes induced by pentachlorophenol. Biophys. J. 1976, 16, 319–336. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Kim, E.H.; Pham, T.H.Y.; Le, T.A.H.; Do, T.P.; Nguyen, T.N.; Trieu, H.P.; Kim, Y.-P. Colorimetric Determination of Singlet Oxygen Scavengers Using a Protein Photosensitizer. BioChip J. 2020, 14, 148–157. [Google Scholar] [CrossRef]
- DeRosa, M.; Crutchley, R.J. Photosensitized singlet oxygen and its applications. Coord. Chem. Rev. 2002, 233–234, 351–371. [Google Scholar] [CrossRef]
- Xu, B.; Ding, J.; Xu, J.; Yomo, T. Giant Vesicles Produced with Phosphatidylcholines (PCs) and Phosphatidylethanolamines (PEs) by Water-in-Oil Inverted Emulsions. Life 2021, 11, 223. [Google Scholar] [CrossRef]
- Breuer, W.; Epsztejn, S.; Millgram, P.; Cabantchik, I.Z. Transport of iron and other transition metals into cells as revealed by a fluorescent probe. Am. J. Physiol. Physiol. 1995, 268, C1354–C1361. [Google Scholar] [CrossRef]
- Catala, A. Lipid Peroxidation. Available online: https://www.intechopen.com/books/2553 (accessed on 16 January 2022).
- Yusupov, M.; Wende, K.; Kupsch, S.; Neyts, E.C.; Reuter, S.; Bogaerts, A. Effect of head group and lipid tail oxidation in the cell membrane revealed through integrated simulations and experiments. Sci. Rep. 2017, 7, 5761. [Google Scholar] [CrossRef]
- Weber, G.; Charitat, T.; Baptista, M.S.; Uchoa, A.F.; Pavani, C.; Junqueira, H.C.; Guo, Y.; Baulin, V.A.; Itri, R.; Marques, C.M.; et al. Lipid oxidation induces structural changes in biomimetic membranes. Soft Matter 2013, 10, 4241–4247. [Google Scholar] [CrossRef]
- Bour, A.; Kruglik, S.G.; Chabanon, M.; Rangamani, P.; Puff, N.; Bonneau, S. Lipid Unsaturation Properties Govern the Sensitivity of Membranes to Photoinduced Oxidative Stress. Biophys. J. 2019, 116, 910–920. [Google Scholar] [CrossRef] [Green Version]
- Skovsen, E.; Snyder, J.W.; Lambert, J.D.C.; Ogilby, P.R. Lifetime and Diffusion of Singlet Oxygen in a Cell. J. Phys. Chem. B 2005, 109, 8570–8573. [Google Scholar] [CrossRef]
- Montecinos, V.; Guzmán, P.; Barra, V.; Villagrán, M.; Munoz, C.; Sotomayor, K.; Escobar, E.; Godoy, A.; Mardones, L.; Sotomayor, P.; et al. Vitamin C Is an Essential Antioxidant That Enhances Survival of Oxidatively Stressed Human Vascular Endothelial Cells in the Presence of a Vast Molar Excess of Glutathione. J. Biol. Chem. 2007, 282, 15506–15515. [Google Scholar] [CrossRef] [Green Version]
- Moriwaki, S.-I.; Misawa, J.; Yoshinari, Y.; Yamada, I.; Takigawa, M.; Tokura, Y. Analysis of photosensitivity in Japanese cancer-bearing patients receiving photodynamic therapy with porfimer sodium (PhotofrinTM). Photodermatol. Photoimmunol. Photomed. 2008, 17, 241–243. [Google Scholar] [CrossRef]
- Berger, A.P.; Steiner, H.; Stenzl, A.; Akkad, T.; Bartsch, G.; Holtl, L. Photodynamic therapy with intravesical instillation of 5-aminolevulinic acid for patients with recurrent superficial bladder cancer: A single-center study. Urology 2003, 61, 338–341. [Google Scholar] [CrossRef]
- Kasche, A.; Luderschmidt, S.; Ring, J.; Hein, R. Photodynamic therapy induces less pain in patients treated with methyl aminolevulinate compared to aminolevulinic acid. J. Drugs Dermatol. 2006, 5, 353–356. [Google Scholar]
- Cowin, P.; Burket, B. Cytoskeleton-Membrane Interactions. Curr. Opin. Cell Biol. 1996, 8, 56–65. [Google Scholar] [CrossRef]
- Qiang, Y.; Liu, J.; Dao, M.; Du, E. In vitro assay for single-cell characterization of impaired deformability in red blood cells under recurrent episodes of hypoxia. Lab Chip 2021, 21, 3458–3470. [Google Scholar] [CrossRef] [PubMed]
- Kawano, A.; Yamasaki, R.; Sakakura, T.; Takatsuji, Y.; Haruyama, T.; Yoshioka, Y.; Ariyoshi, W. Reactive Oxygen Species Penetrate Persister Cell Membranes of Escherichia coli for Effective Cell Killing. Front. Cell. Infect. Microbiol. 2020, 10, 496. [Google Scholar] [CrossRef] [PubMed]
- Agmon, E.; Solon, J.; Bassereau, P.; Stockwell, B.R. Modeling the effects of lipid peroxidation during ferroptosis on membrane properties. Sci. Rep. 2018, 8, 5155. [Google Scholar] [CrossRef] [Green Version]
- Gu, L.; Wei, T.; Zhou, M.; Yang, H.; Zhou, Y. Impact of Lipid Peroxidation on the Response of Cell Membranes to High-Speed Equibiaxial Stretching: A Computational Study. J. Phys. Chem. B 2021, 125, 10736–10747. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, G.; Lee, D.; Kim, S.M.; Jeon, T.-J. Elucidation of the Interactions of Reactive Oxygen Species and Antioxidants in Model Membranes Mimicking Cancer Cells and Normal Cells. Membranes 2022, 12, 286. https://doi.org/10.3390/membranes12030286
Cho G, Lee D, Kim SM, Jeon T-J. Elucidation of the Interactions of Reactive Oxygen Species and Antioxidants in Model Membranes Mimicking Cancer Cells and Normal Cells. Membranes. 2022; 12(3):286. https://doi.org/10.3390/membranes12030286
Chicago/Turabian StyleCho, Geonho, Deborah Lee, Sun Min Kim, and Tae-Joon Jeon. 2022. "Elucidation of the Interactions of Reactive Oxygen Species and Antioxidants in Model Membranes Mimicking Cancer Cells and Normal Cells" Membranes 12, no. 3: 286. https://doi.org/10.3390/membranes12030286
APA StyleCho, G., Lee, D., Kim, S. M., & Jeon, T.-J. (2022). Elucidation of the Interactions of Reactive Oxygen Species and Antioxidants in Model Membranes Mimicking Cancer Cells and Normal Cells. Membranes, 12(3), 286. https://doi.org/10.3390/membranes12030286