A Study of the Mechanism and Separation of Structurally Similar Phenolic Acids by Commercial Polymeric Ultrafiltration Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Membrane Characterization
2.3. Experimental Procedure
2.3.1. Static Adsorption and Soaking Experiment
2.3.2. Membrane Filtration Experiments
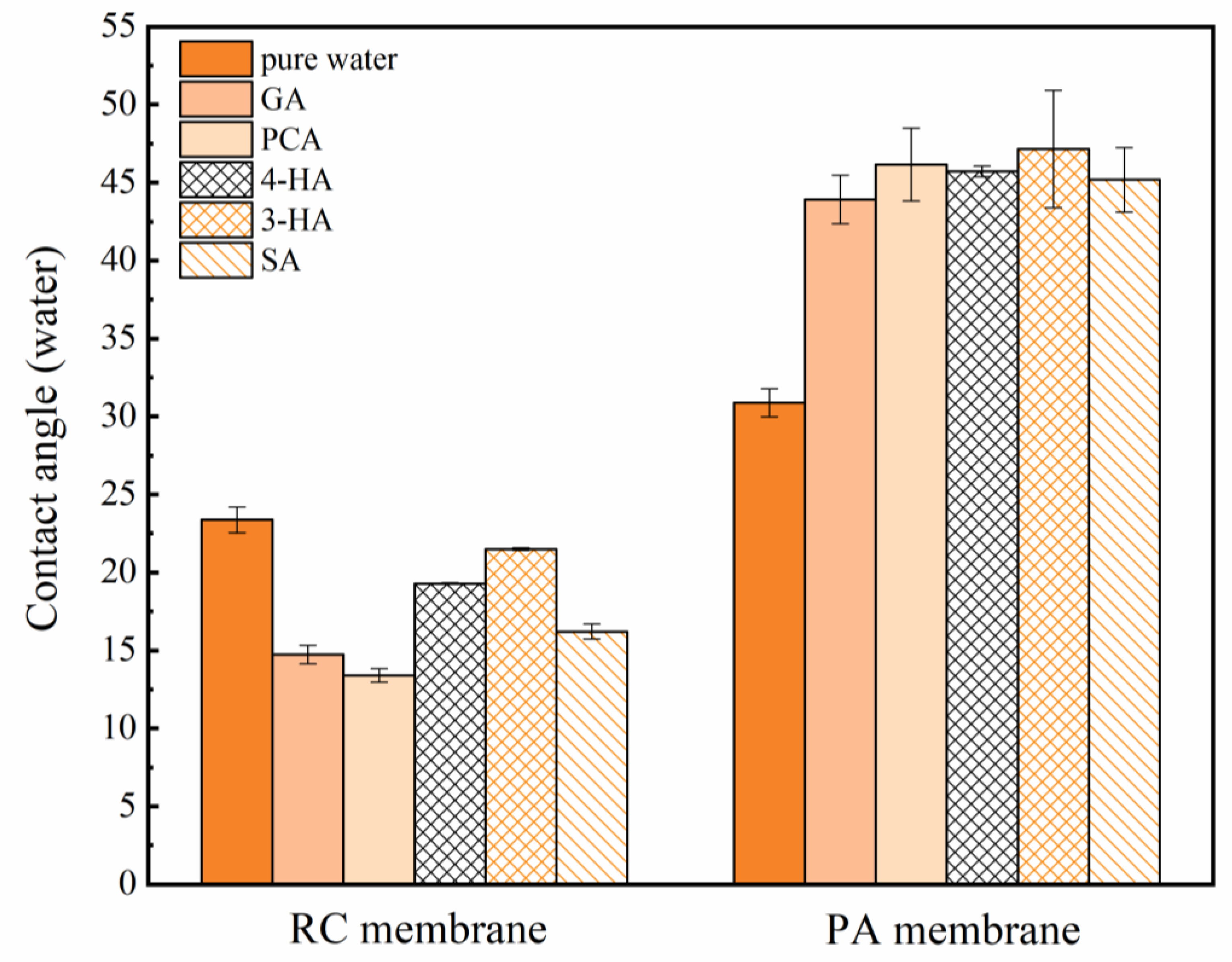
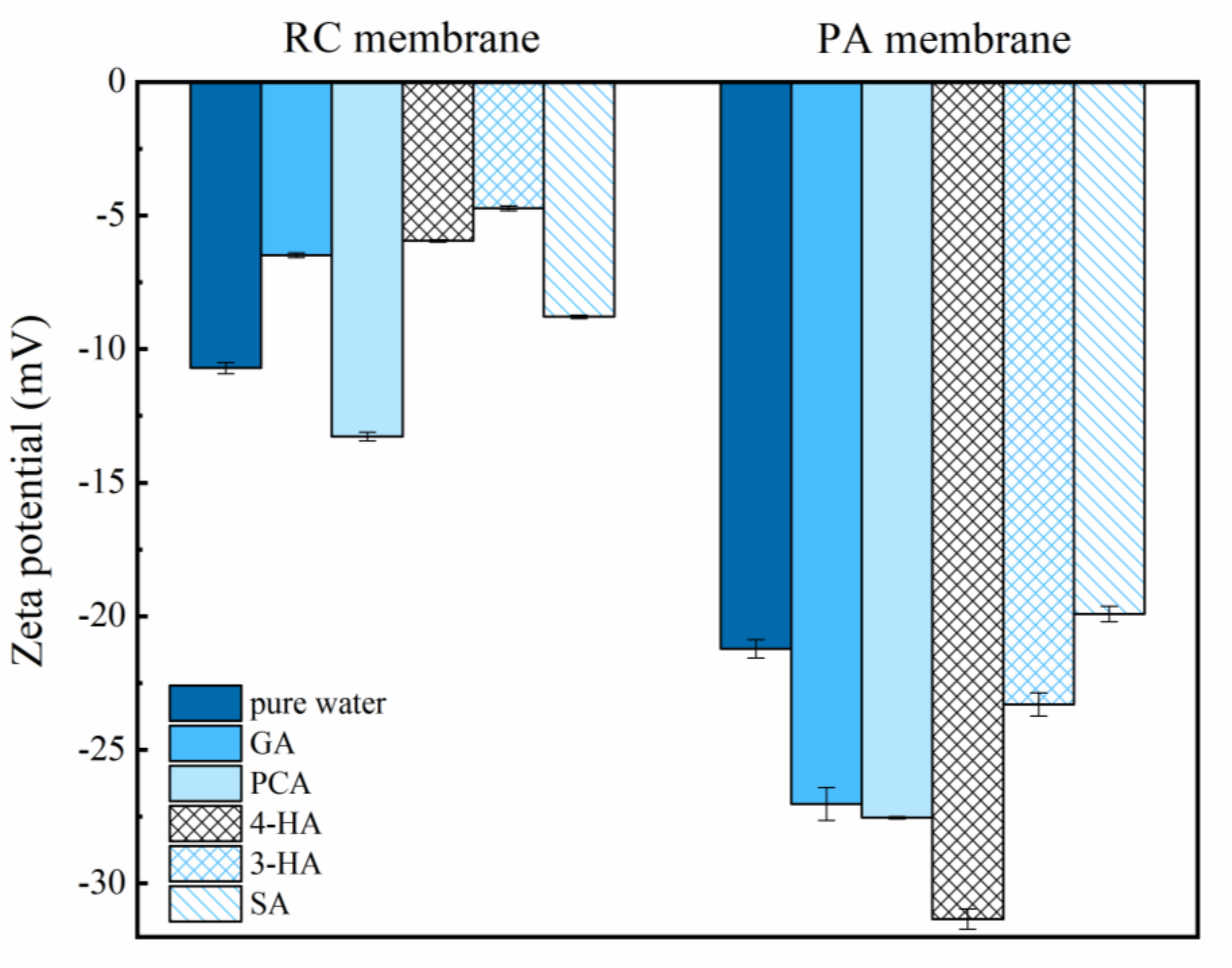
2.4. Membrane Characteristic Analysis
2.5. Analytical Methods
2.6. Statistical Analysis
3. Results
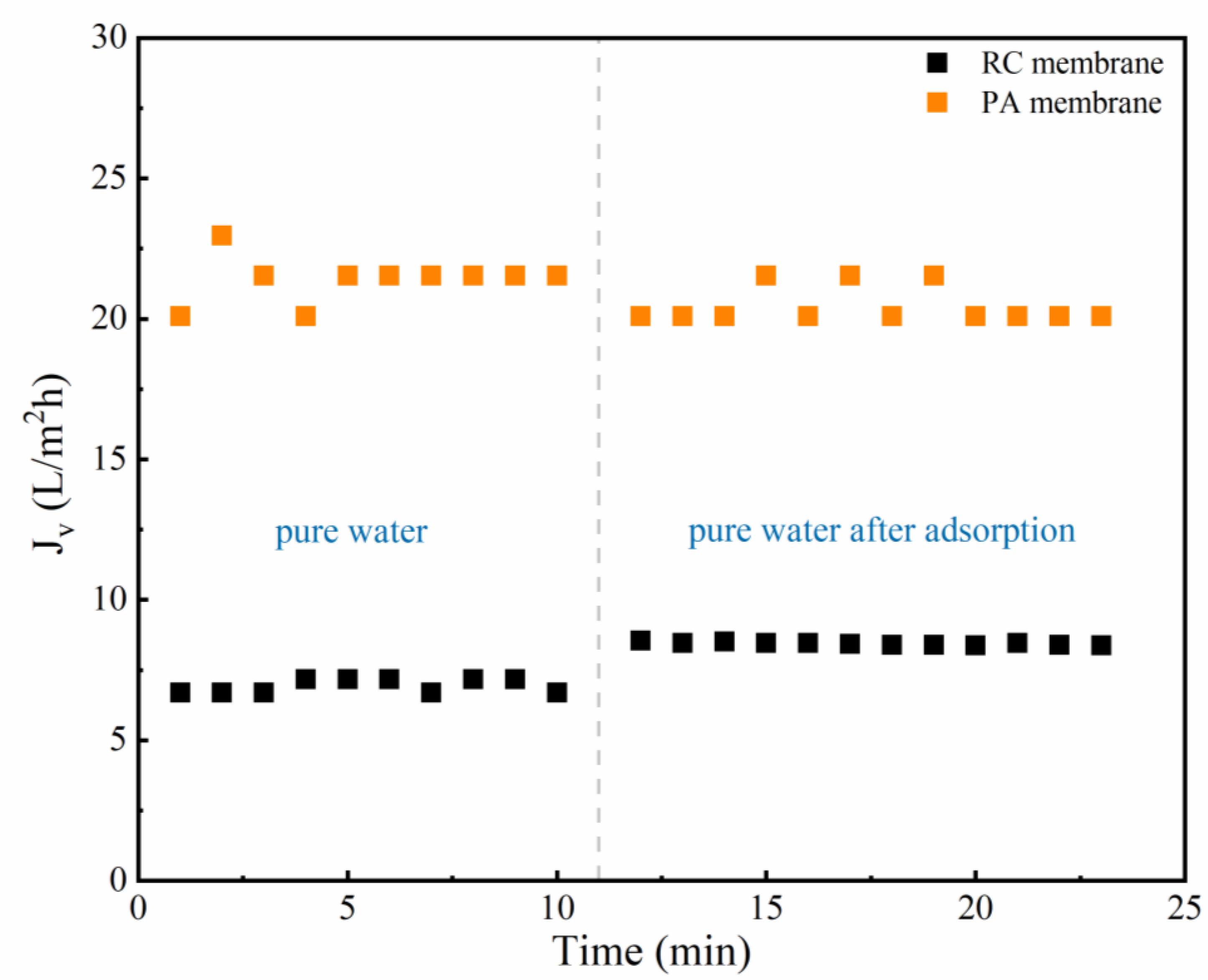
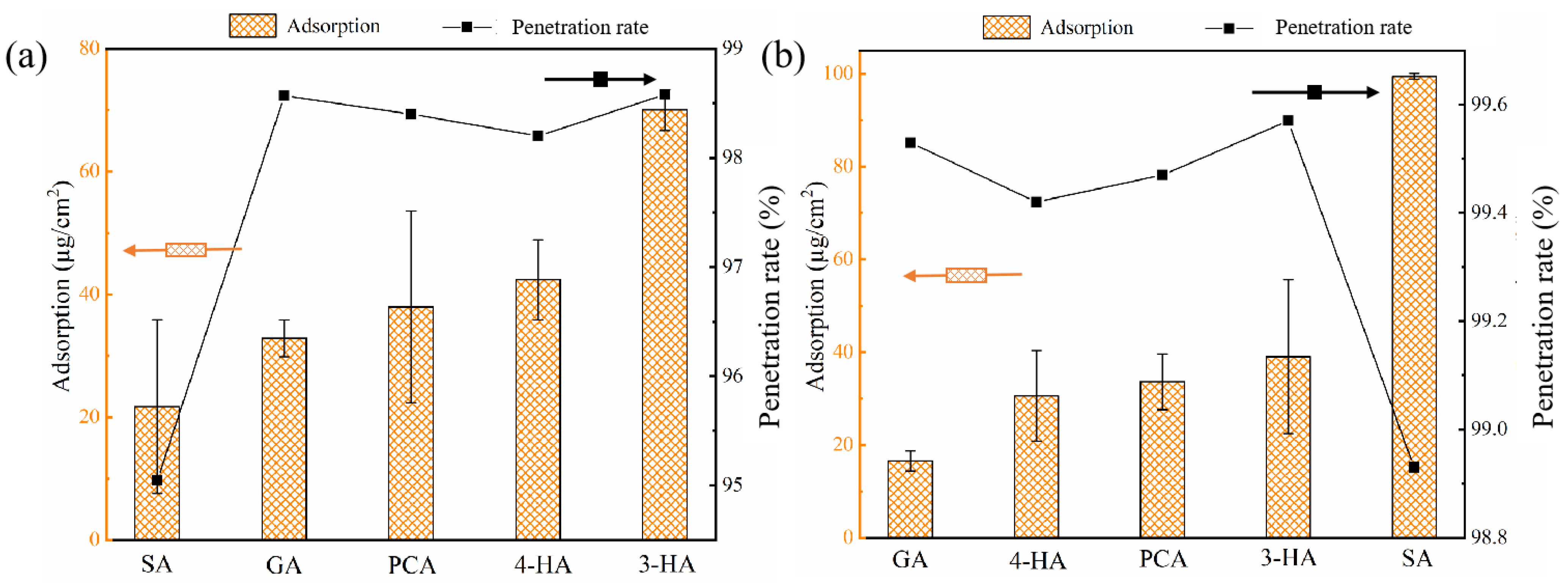
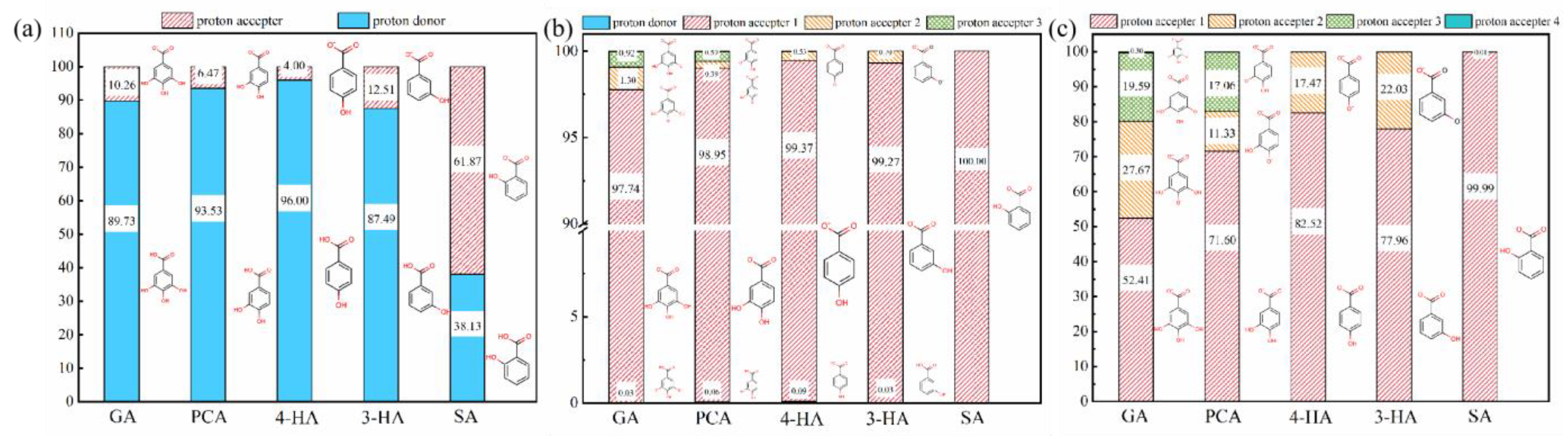
3.1. Effect of Phenolic Acids’ Adsorption on Membrane
3.2. Membrane Separation Capability
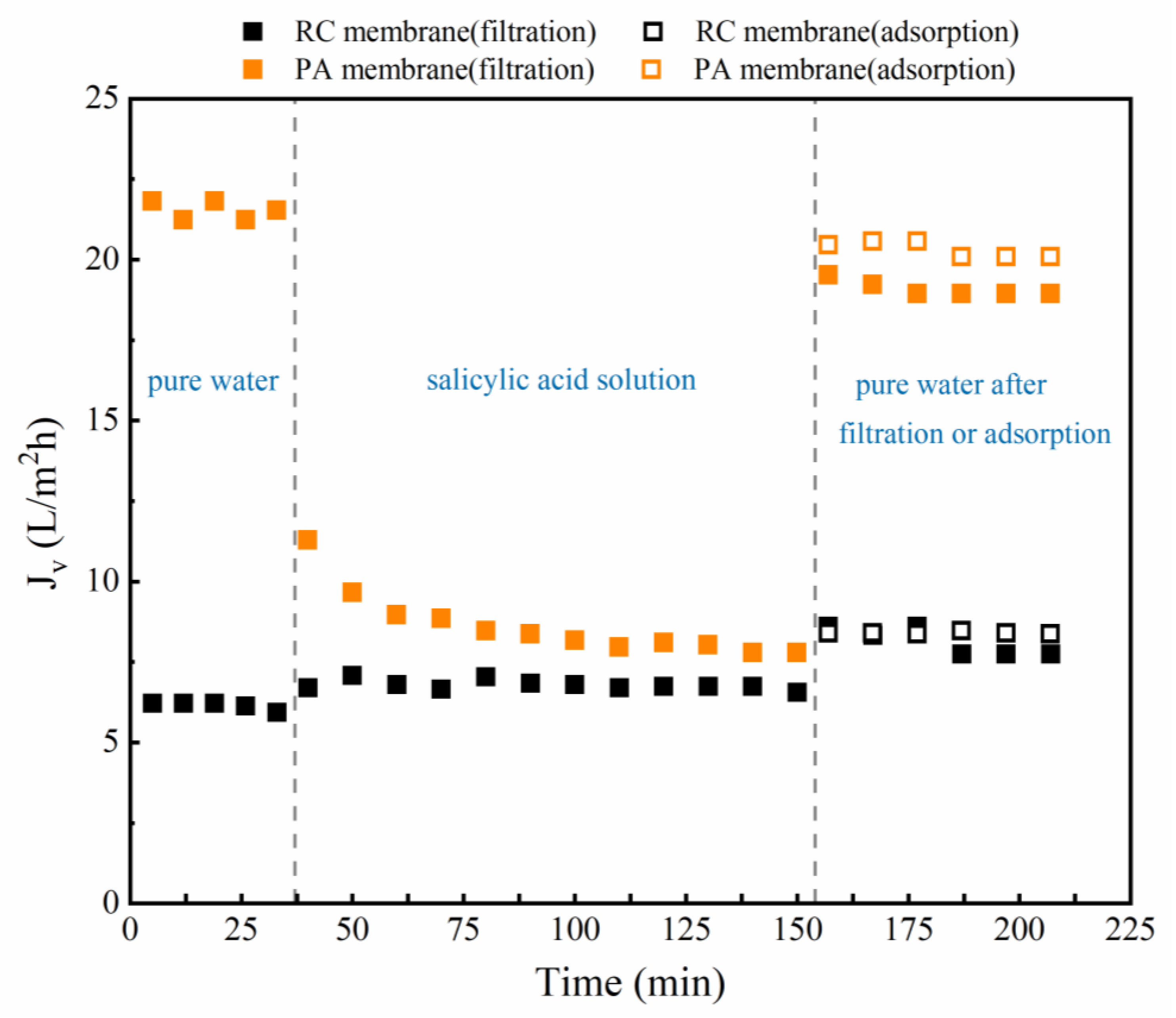
3.2.1. Effect of Phenolic Acids on Filtration Flux
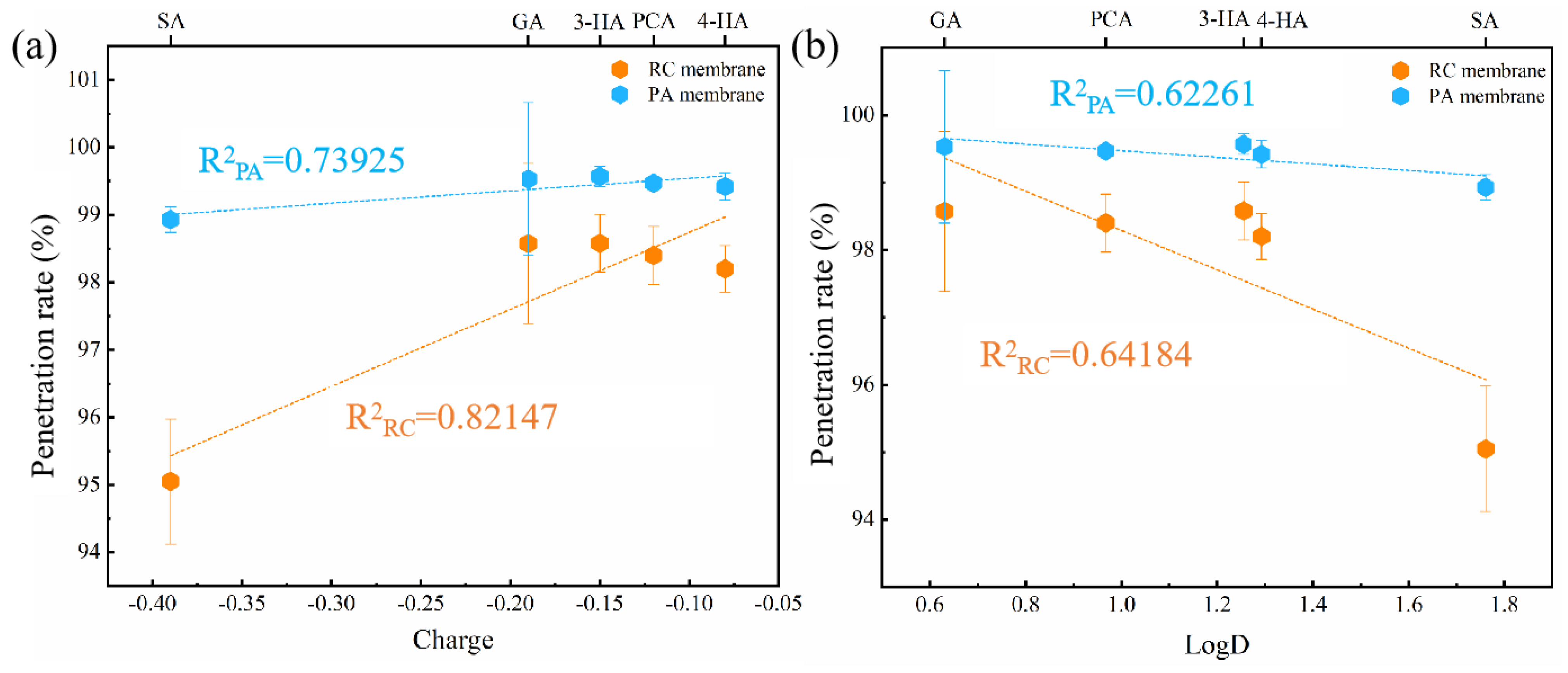
3.2.2. Different Phenolic Acids Penetration Rates
3.2.3. Effect of Phenolic Acids’ Concentration
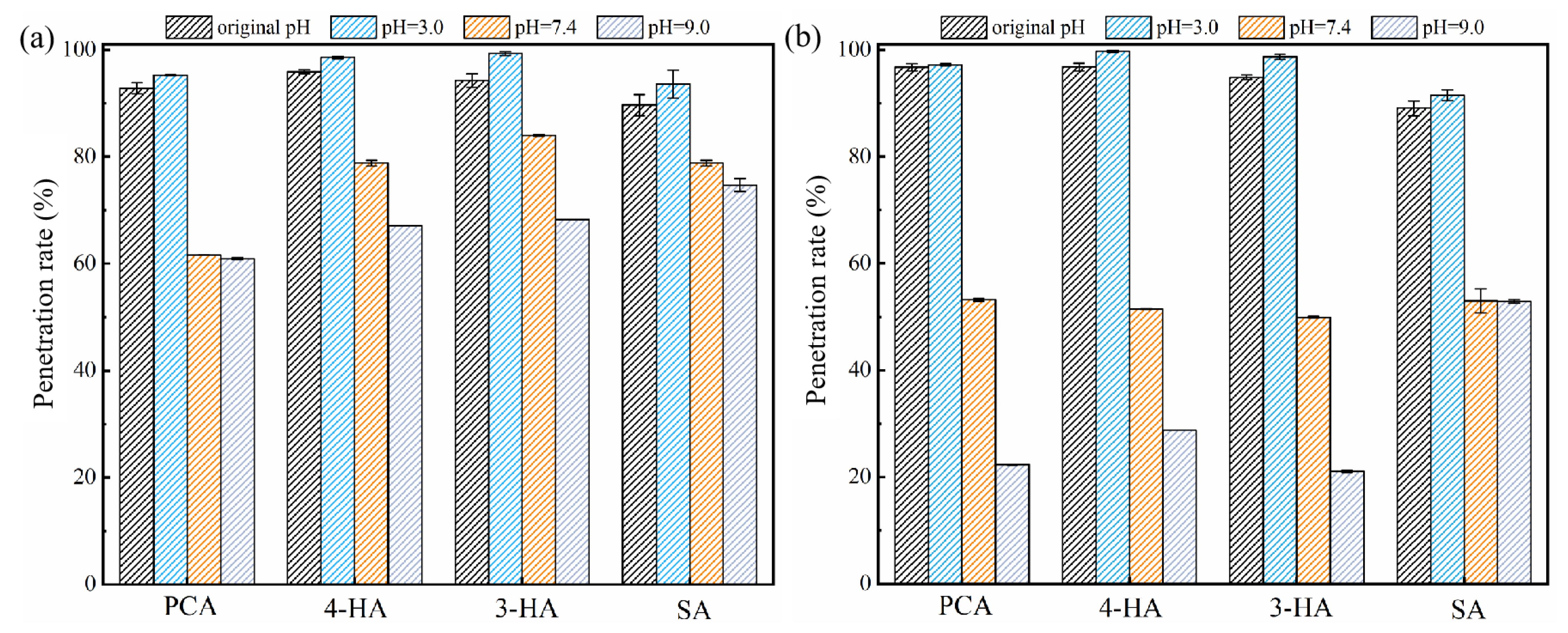
3.2.4. Effect of pH
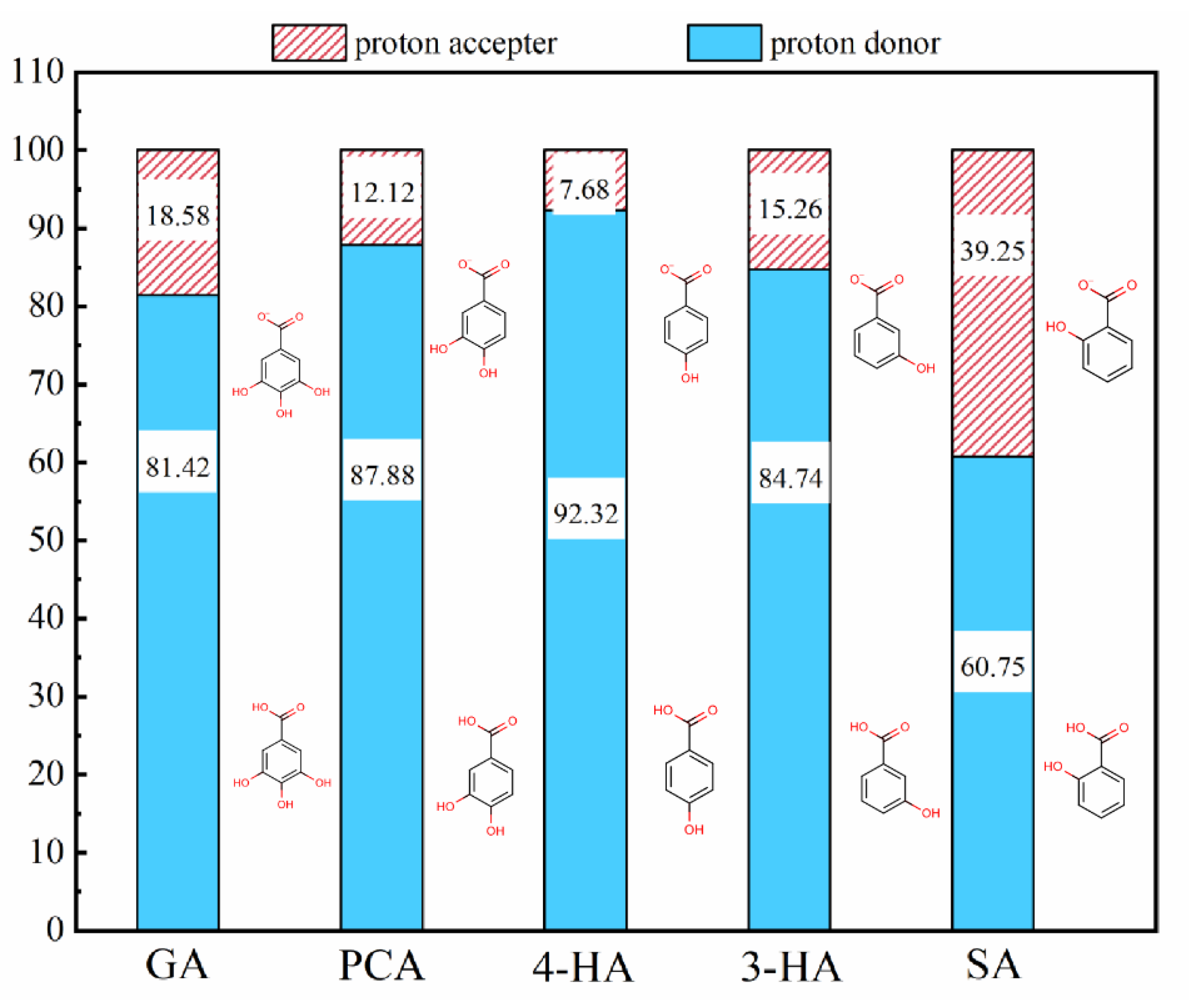
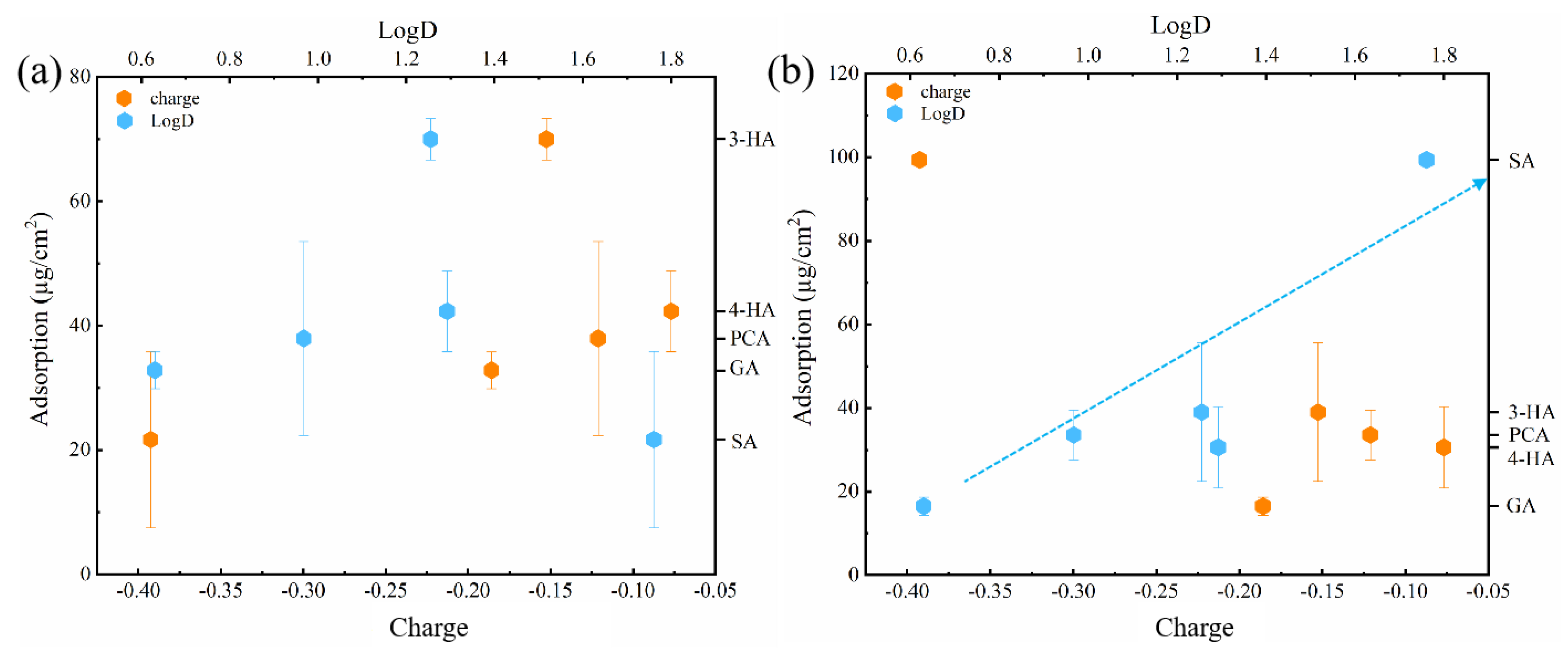
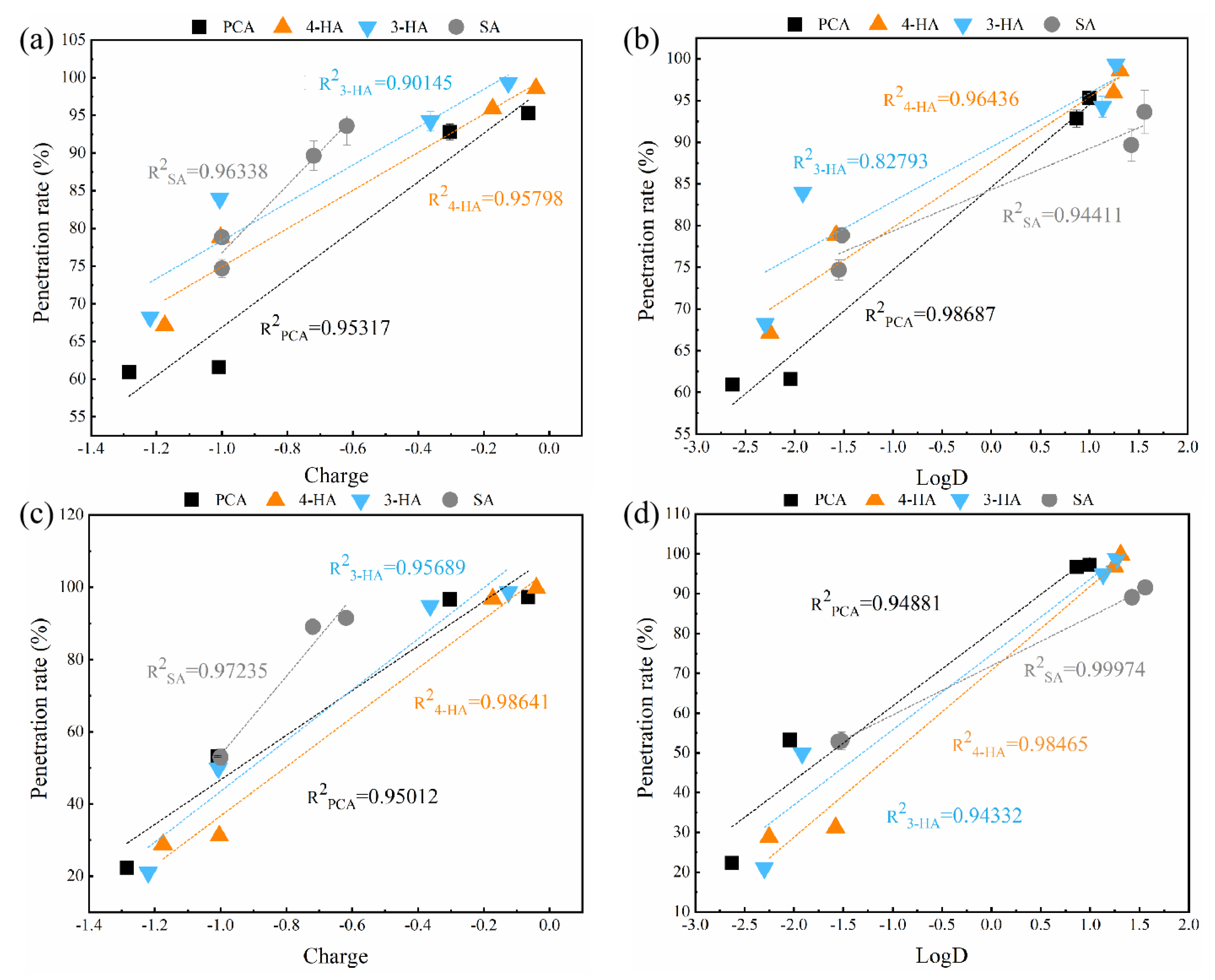
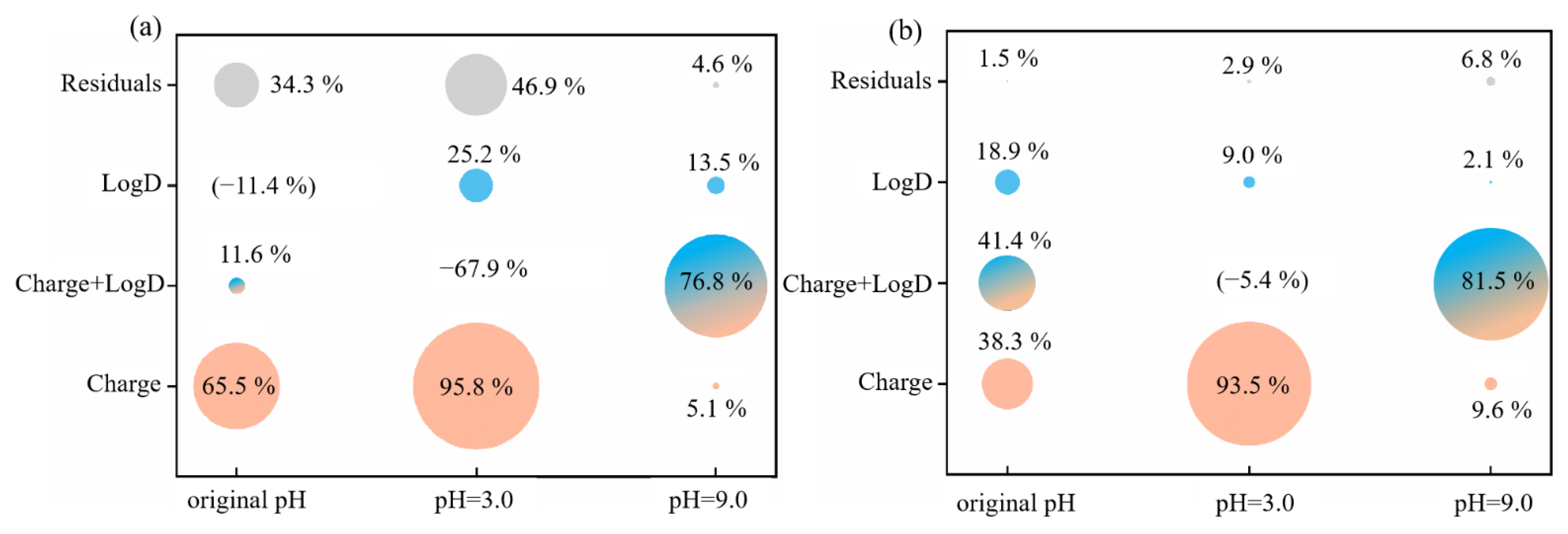
3.2.5. Relative Influence of Electrostatic and Hydrophobic Interactions on Phenolic Acid Membrane-Penetration
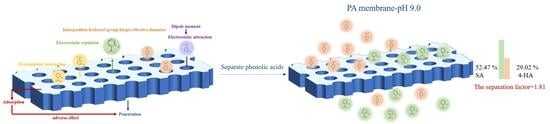
3.3. Practical Separation of Different Phenolic Acids in Mixtures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Fejzić, A.; Ćavar, S. Phenolic Compounds and Antioxidant Activity of Some Citruses. Bull. Chem. Technol. Bosnia Herzeg. 2014, 42, 1–4. [Google Scholar]
- Santos-Buelga, C.; Scalbert, A. Proanthocyanidins and tannin-like compounds—Nature, occurrence, dietary intake and effects on nutrition and health. J. Sci. Food Agric. 2000, 80, 1094–1117. [Google Scholar] [CrossRef]
- Jiang, H.; Yang, L.; Xing, X.; Yan, M.; Guo, X.; Yang, B.; Wang, Q.H.; Kuang, H.X. Development of an analytical method for separation of phenolic acids by ultra-performance convergence chromatography (UPC 2 ) using a column packed with a sub-2-μm particle. J. Pharm. Biomed. Anal. 2018, 153, 117–125. [Google Scholar] [CrossRef]
- Fernández, M.A.; Sáenz, M.T.; García, M.D. Natural Products: Anti-inflammatory Activity in Rats and Mice of Phenolic Acids Isolated from Scrophularia frutescens. J. Pharm. Pharmacol. 2011, 50, 1183–1186. [Google Scholar] [CrossRef]
- Bohm, B.A.; Tryon, R.M. Phenolic compounds in ferns. 1. A survey of some ferns for cinnamic acid and benzoic acid derivatives. Can. J. Bot. 1967, 45, 585–593. [Google Scholar] [CrossRef]
- Gomes, C.; Teresa, G.; Andrade, J.L.; Milhazes, N.; Borges, F.; Marques, M. Anticancer activity of phenolic acids of natural or synthetic origin: A structure-activity study. J. Med. Chem. 2003, 46, 5395–5401. [Google Scholar] [CrossRef] [Green Version]
- Fernández, M.A.; García, M.D.; Sáenz, M.T. Antibacterial activity of the phenolic acids fractions of Scrophularia frutescens and Scrophularia sambucifolia. J. Ethnopharmacol. 1996, 53, 11–14. [Google Scholar] [CrossRef]
- Sroka, Z.; Cisowski, W. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem. Toxicol. 2003, 41, 753–758. [Google Scholar] [CrossRef]
- Jamwal, S.; Dharela, R.; Gupta, R.; Ahn, J.-H.; Chauhan, G.S. Synthesis of crosslinked lipase aggregates and their use in the synthesis of aspirin. Chem. Eng. Res. 2015, 97, 159–164. [Google Scholar] [CrossRef]
- Filho, S.F.S.; Pereira, A.C.; Sarraguça, J.M.G.; Sarraguça, M.C.; Lopes, J.; Filho, P.F.F.; Santos, A.O.; Ribeiro, P.R.S. Synthesis of a glibenclamide cocrystal: Full spectroscopic and thermal characterization. J. Pharm. Sci. 2018, 107, 1597–1604. [Google Scholar] [CrossRef]
- Mark, R.; Lyu, X.; Lee, J.; Parra-Saldívar, R.; Chen, W.N. Sustainable production of natural phenolics for functional food applications. J. Funct. Foods 2019, 57, 233–254. [Google Scholar] [CrossRef]
- Boumya, W.; Laghrib, F.; Lahrich, S.; Farahi, A.; Achark, M.; Bakasse, M.; Mhammedi, M.A. Electrochemical behavior study of salicylic acid following azo dye formation with 2,4-dinitrophenylhydrazine: Analytical evaluation. South Afr. J. Chem. Eng. 2018, 25, 48–53. [Google Scholar] [CrossRef]
- Yüzbaşıoğlu, E.; Dalyan, E. Salicylic acid alleviates thiram toxicity by modulating antioxidant enzyme capacity and pesticide detoxification systems in the tomato (Solanum lycopersicum Mill.). Plant Physiol. Biochem. 2019, 135, 322–330. [Google Scholar] [CrossRef]
- D’Archivio, M.; Scazzocchio, B.; Giovannini, C.; Masella, R. Role of Protocatechuic Acid in Obesity-Related Pathologies. Polyphen. Hum. Health Dis. 2014, 1, 177–189. [Google Scholar]
- Elder, D.J.E.; Kelly, D.J. The bacterial degradation of benzoic acid and benzenoid compounds under anaerobic conditions: Unifying trends and new perspectives. FEMS Microbiol. Rev. 1994, 13, 441–468. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.M.; Zhou, T.; Carr, J.P. An update on salicylic acid biosynthesis, its induction and potential exploitation by plant viruses. Curr. Opin. Virol. 2020, 42, 8–17. [Google Scholar] [CrossRef]
- Bonnemain, J.L.; Chollet, J.F.; Rocher, F. Transport of salicylic acid and related compounds. In Salicylic Acid; Hayat, S., Ahmad, A., Alyemeni, M., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 43–59. [Google Scholar]
- U.S. Pharmacopeia. National Formulary; The United States Pharmacopeial Convention: Rockville, MD, USA, 2009. [Google Scholar]
- Jonsson, S.; Borén, H. Analysis of mono- and diesters of o-phthalic acid by solid-phase extractions with polystyrene-divinylbenzene-based polymers. J. Chromatogr. A 2002, 963, 393–400. [Google Scholar] [CrossRef]
- Kambia, K.; Dine, T.; Gressier, B.; Germe, A.F.; Luyckx, M.; Brunet, C.; Michaud, L.; Gottrand, F. High-performance liquid chromatographic method for the determination of di(2-ethylhexyl) phthalate in total parenteral nutrition and in plasma. J. Chromatogr. B Biomed. Sci. Appl. 2001, 755, 297–303. [Google Scholar] [CrossRef]
- Han, T.; Zhang, Q.Y.; Zhang, H.; Wen, J.; Wang, Y.; Huang, B.K.; Rahman, K.; Zheng, H.C.; Qin, L.P. Authentication and quantitative analysis on the chemical profile of Xanthium fruit (Cang-Er-Zi) by high-performance liquid chromatography-diode-array detection tandem mass spectrometry method. Anal. Chim. Acta 2009, 634, 272–278. [Google Scholar] [CrossRef]
- Wang, Z.; Hwang, S.H.; Huang, B.; Lim, S.S. Identification of tyrosinase specific inhibitors from Xanthium strumarium fruit extract using ultrafiltration-high performance liquid chromatography. J. Chromatogr. B 2015, 1002, 319–328. [Google Scholar] [CrossRef]
- Dong, G.; Xu, J.; Gu, Y.; Wei, Y. A general separation method of phenolic acids using pH-zone-refining counter-current chromatography and its application to oat bran. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 992, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Nevado, J.J.B.; PeñAlvo, G.C.; Robledo, V.R.; Martínez, G.V. New CE–ESI-MS analytical method for the separation, identification and quantification of seven phenolic acids including three isomer compounds in virgin olive oil. Talanta 2009, 79, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Cartoni, G.; Coccioli, F.; Jasionowska, R. Capillary electrophoretic separation of phenolic acids. J. Chromatogr. A 1995, 709, 209–214. [Google Scholar] [CrossRef]
- Nie, L.R.; Lu, J.; Zhang, W.; He, A.; Yao, S. Ionic liquid-modified silica gel as adsorbents for adsorption and separation of water-soluble phenolic acids from Salvia militiorrhiza Bunge. Sep. Purif. Technol. 2015, 155, 2–12. [Google Scholar] [CrossRef]
- Neoh, C.H.; Noor, Z.Z.; Mutamim, N.S.A.; Lim, C.K. Green technology in wastewater treatment technologies: Integration of membrane bioreactor with various wastewater treatment systems. Chem. Eng. J. 2016, 283, 582–594. [Google Scholar] [CrossRef] [Green Version]
- Anastas, P.; Eghbali, N. Green chemistry: Principles and practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- Meng, M.J.; Feng, Y.H.; Zhang, M.; Ji, Y.J.; Dai, J.D.; Liu, Y.; Yu, P.; Yan, Y.S. Optimization of surface imprinted layer attached poly(vinylidene fluoride) membrane for selective separation of salicylic acid from acetylsalicylic acid using central composite design. Chem. Eng. J. 2013, 231, 132–145. [Google Scholar] [CrossRef]
- Mao, Y.L.; Meng, M.J.; Yan, L.; Sun, F.Q.; Yan, Y.S.; Liu, S.J. Fabrication of highly selective molecularly imprinted membranes for the selective adsorption of methyl salicylate from salicylic acid. Rsc. Adv. 2016, 6, 91659–91668. [Google Scholar] [CrossRef]
- Liu, Y.; Meng, M.; Yao, J.; Da, Z.; Li, C. Selective separation of phenol from salicylic acid effluent over molecularly imprinted polystyrene nanospheres composite alumina membranes. Chem. Eng. J. 2016, 286, 622–631. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Tanioka, A.; Matsumoto, H. Molecularly imprinted nanofiber membranes. Curr. Opin. Chem. Eng. 2011, 1, 18–26. [Google Scholar] [CrossRef]
- Luo, J.Q.; Zeuner, B.; Morthensen, S.T.; Meyer, A.S.; Pinelo, M. Separation of phenolic acids from monosaccharides by low-pressure nanofiltration integrated with laccase pre-treatments. J. Membr. Sci. 2015, 482, 83–91. [Google Scholar] [CrossRef]
- Acero, J.L.; Benitez, F.J.; Leal, A.I.; Real, F.J. Removal of phenolic compounds in water by ultrafiltration membrane treatments. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2005, 40, 1585–1603. [Google Scholar] [CrossRef] [PubMed]
- Maiti, S.K.; Thuyavan, Y.L.; Singh, S.; Oberoi, H.S.; Agarwal, G.P. Modeling of the separation of inhibitory components from pretreated rice straw hydrolysate by nanofiltration membranes. Bioresour. Technol. 2012, 114, 419–427. [Google Scholar] [CrossRef]
- Ojajuni, O.; Saroj, D.; Cavalli, G. Removal of Organic Micropollutants Using Membrane-Assisted Processes: A Review of Recent Progress. Environ. Technol. Rev. 2015, 4, 17–37. [Google Scholar] [CrossRef]
- Bellona, C.; Drewes, J.E.; Xu, P.; Amy, G. Factors affecting the rejection of organic solutes during NF/RO treatment—A literature review. Water Res. 2004, 38, 2795–2809. [Google Scholar] [CrossRef]
- Einstein, A. Investigations on the Theory of Brownian Movement; Dover Publications, Inc.: Dover, UK, 1956. [Google Scholar]
- Geankoplis, C. Transport Processes and Unit Operations; Allyn Bacon: Boston, MA, USA, 1993. [Google Scholar]
- Shan, J.; Wang, C.; Wei, J.; Tang, C.Y. Rejection of pharmaceuticals by forward osmosis membranes. J. Hazard. Mater. 2012, 227–228, 55–61. [Google Scholar]
- Verliefde, A.R.; Heijman, S.G.; Cornelissen, E.R.; Amy, G.; Van der Bruggen, B.; van Dijk, J.C. Influence of electrostatic interactions on the rejection with NF and assessment of the removal efficiency during NF/GAC treatment of pharmaceutically active compounds in surface water. Water Res. 2007, 41, 3227–3240. [Google Scholar] [CrossRef]
- Worch, E. Eine neue Gleichung zur Berechnung von Diffusionskoeffizienten gelster Stoffe. Vom. Wasser. 1993, 81, 289–297. [Google Scholar]
- Aziz, M.; Ojumu, T. Exclusion of Estrogenic and Androgenic Steroid Hormones from Municipal Membrane Bioreactor Wastewater Using UF/NF/RO Membranes for Water Reuse Application. Membranes 2020, 10, 37. [Google Scholar] [CrossRef] [Green Version]
- Aziz, M.; Kasongo, G. The Removal of Selected Inorganics from Municipal Membrane Bioreactor Wastewater Using UF/NF/RO Membranes for Water Reuse Application: A Pilot-Scale Study. Membranes 2021, 11, 117. [Google Scholar] [CrossRef]
- Gai, W.X.; Zhao, D.L.; Chung, T.S. Novel thin film composite hollow fiber membranes incorporated with carbon quantum dots for osmotic power generation. J. Membr. Sci. 2018, 551, 94–102. [Google Scholar] [CrossRef]
- Rohani, M.M.; Zydney, A.L. Protein transport through zwitterionic ultrafiltration membranes. J. Membr. Sci. 2012, 397–398, 1–8. [Google Scholar] [CrossRef]
- Cheng, J.; Xie, S.; Wang, S.; Xue, Y.; Jiang, L.; Liu, L. Optimization of Protein Removal from Soybean Whey Wastewater Using Chitosan Ultrafiltration. J. Food Process Eng. 2017, 40, 12371–12379. [Google Scholar] [CrossRef]
- Lu, X.; Chen, Q.; Lu, J.; Xu, H.; Ji, J. Investigation of reinforced braided hollow fiber membrane containing silver-based butanediol for methyl linolenate separation:Better penetration rate, higher stability. J. Membr. Sci. 2021, 642, 119954. [Google Scholar] [CrossRef]
- Cohen, J.; Cohen, P.C.; West, S.G.; Aiken, L.S. Applied Multiple Regression/Correlation Analysis For The Behavioral Sciences; Lawrence Erlbaum Associates: Mahwah, NJ, USA, 2003. [Google Scholar]
- Persico, M.; Dhulster, P.; Bazinet, L. Redundancy analysis for determination of the main physicochemical characteristics of filtration membranes explaining their fouling by peptides. J. Membr. Sci. 2018, 563, 708–717. [Google Scholar] [CrossRef]
- Mu, S.; Wang, S.; Liang, S.; Xiao, K.; Huang, X. Effect of the Relative Degree of Foulant “Hydrophobicity” on Membrane Fouling. J. Membr. Sci. 2019, 570–571, 1–8. [Google Scholar] [CrossRef]
- Lin, W.; Li, M.C.; Xiao, K.; Huang, X. The role shifting of organic, inorganic and biological foulants along different positions of a two-stage nanofiltration process. J. Membr. Sci. 2020, 602, 117979. [Google Scholar] [CrossRef]
- Comerton, A.M.; Andrews, R.C.; Bagley, D.M.; Yang, P. Membrane adsorption of endocrine disrupting compounds and pharmaceutically active compounds. J. Membr. Sci. 2007, 303, 267–277. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Schafer, A.I.; Waite, T.D. Adsorptive interactions between membranes and trace contaminants. Desalination 2002, 147, 269–274. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Qiu, W.; Gao, W. Adsorption of estrone in microfiltration membrane filters. Chem. Eng. J. 2010, 165, 819–826. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Hullebusch, E.D.; Cretin, M.; Esposito, G.; Oturan, M.A. Coupling of membrane filtration and advanced oxidation processes for removal of pharmaceutical residues: A critical review. Sep. Purif. Technol. 2015, 156, 891–914. [Google Scholar] [CrossRef]
- Koo, C.H.; Mohammad, A.W.; Suja, F.; Talib, M.Z.M. Review of the effect of selected physicochemical factors on membrane fouling propensity based on fouling indices. Desalination 2012, 287, 167–177. [Google Scholar] [CrossRef]
- Licona, K.P.M.; Geaquinto, L.R.D.; Nicolini, J.V.; Figueiredo, N.G.; Chiapetta, S.C.; Habert, A.C.; Yokoyama, L. Assessing potential of nanofiltration and reverse osmosis for removal of toxic pharmaceuticals from water. J. Water Process Eng. 2018, 25, 195–204. [Google Scholar] [CrossRef]
- Wray, H.E.; Andrews, R.C.; Bérubé, P.R. Surface shear stress and retention of emerging contaminants during ultrafiltration for drinking water treatment. Sep. Purif. Technol. 2014, 122, 183–191. [Google Scholar] [CrossRef]
- Bruggen, B.V.D.; Schaep, J.; Wilms, D. Influence of molecular size, polarity and charge on the retention of organic molecules by nanofiltration. J. Membr. Sci. 1999, 156, 29–41. [Google Scholar] [CrossRef]
- Garcia-Ivars, J.; Durá-María, J.; Moscardó-Carreño, C.; Carbonell-Alcaina, C.; Alcaina-Miranda, M.I.; Iborra-Clar, M.I. Rejection of trace pharmaceutically active compounds present in municipal wastewaters using ceramic fine ultrafiltration membranes: Effect of feed solution pH and fouling phenomena. Sep. Purif. Technol. 2017, 175, 58–71. [Google Scholar] [CrossRef]
- Braeken, L.; Ramaekers, R.; Zhang, Y.; Maes, G.; Van der Bruggen, B.; Vandecasteele, C. Influence of hydrophobicity on retention in nanofiltration of aqueous solutions containing organic compounds. J. Membr. Sci. 2005, 252, 195–203. [Google Scholar] [CrossRef]
- Azais, A.; Mendret, J.; Petit, E.; Brosillon, S. Evidence of solute-solute interactions and cake enhanced concentration polarization during removal of pharmaceuticals from urban wastewater by nanofiltration. Water Res. 2016, 104, 156–167. [Google Scholar] [CrossRef]
- Friedman, M.; Jurgens, H.S. Effect of pH on the stability of plant phenolic compounds. J. Agric. Food Chem. 2000, 48, 2101–2110. [Google Scholar] [CrossRef]
- Wolters, J.; Tagliavini, M.; Schafer, A.I. Removal of steroid hormone micropollutants by UF-PBSAC composite in presence of organic matter. J. Membr. Sci. 2019, 592, 117315. [Google Scholar] [CrossRef]
- Bόdalo, A.; Gόmez, E.; Hidalgo, A.M.; Gόmez, M.; Murcia, M.D.; Lόpez, I. Nanofiltration membranes to reduce phenol concentration in wastewater. Desalination 2009, 245, 680–686. [Google Scholar] [CrossRef]
- Catalán, J.; Fernández-Alonso, J. A theoretical study of the stereochemistry of the intramolecular hydrogen bond of salicylic acid. J. Mol. Struct. 1975, 27, 59–65. [Google Scholar] [CrossRef]
- Paul, B.K.; Guchhait, N. Geometrical criteria versus quantum chemical criteria for assessment of intramolecular hydrogen bond (IMHB) interaction: A computational comparison into the effect of chlorine substitution on IMHB of salicylic acid in its lowest energy ground state conform. Chem. Phys. 2013, 412, 58–67. [Google Scholar] [CrossRef]
- Hu, J.Y.; Jin, X.; Ong, S.L. Rejection of estrone by nanofiltration: Influence of solution chemistry. J. Membr. Sci. 2007, 302, 188–196. [Google Scholar] [CrossRef]
- Moons, K.; Van der Bruggen, B. Removal of micropollutants during drinking water production from surface water with nanofiltration. Desalination 2006, 199, 245–247. [Google Scholar] [CrossRef]
- Taheran, M.; Brar, S.K.; Verma, M.; Surampalli, R.Y.; Zhang, T.C.; Valero, J.R. Membrane processes for removal of pharmaceutically active compounds (PhACs) from water and wastewaters. Sci. Total Environ. 2016, 547, 60–77. [Google Scholar] [CrossRef]
- Verliefde, A.; Cornelissen, E.; Amy, G.; Van der Bruggen, B.; van Dijk, H. Priority organic micropollutants in water sources in Flanders and the Netherlands and assessment of removal possibilities with nanofiltration. Environ. Pollut. 2007, 146, 281–289. [Google Scholar] [CrossRef]
- Yoon, Y.; Westerhoff, P.; Snyder, S.A.; Wert, E.C.; Yoon, J. Removal of endocrine disrupting compounds and pharmaceuticals by nanofiltration and ultrafiltration membranes. Desalination 2007, 202, 16–23. [Google Scholar] [CrossRef]
- Kiso, Y.; Sugiura, Y.; Kitao, T.; Nishimura, K. Effects of hydrophobicity and molecular size on rejection of aromatic pesticides with nanofiltration membranes. J. Membr. Sci. 2001, 192, 1–10. [Google Scholar] [CrossRef]
- Han, J.; Qiu, W.; Hu, J.Y.; Gao, W. Chemisorption of estrone in nylon microfiltration membranes: Adsorption mechanism and potential use for estrone removal from water. Water Res. 2012, 46, 873–881. [Google Scholar] [CrossRef]
- Liu, Y.L.; Wang, X.M.; Yang, H.W.; Xie, Y.F.F. Quantifying the influence of solute-membrane interactions on adsorption and rejection of pharmaceuticals by NF/RO membranes. J. Membr. Sci. 2018, 551, 37–46. [Google Scholar] [CrossRef]
- Ismail, N.H.; Salleh, W.; Ismail, A.F.; Hasbullah, H.; Jaafar, J. Hydrophilic polymer-based membrane for oily wastewater treatment: A review. Sep. Purif. Technol. 2019, 233, 116007. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, Y.; Sadam, H.; Ma, J.; Bai, Y.; Shen, X.; Kim, J.K.; Shao, L. Novel mussel-inspired zwitterionic hydrophilic polymer to boost membrane water-treatment performance. J. Membr. Sci. 2019, 582, 1–8. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, G.; Zhang, H.; Zhao, C.; Yang, F. Improvement of antifouling performances for modified PVDF ultrafiltration membrane with hydrophilic cellulose nanocrystal. Appl. Surf. Sci. 2018, 440, 1091–1100. [Google Scholar] [CrossRef]
- Cai, M.; Hou, W.Z.; Lv, Y.Q.; Sun, P.L. Behavior and rejection mechanisms of fruit juice phenolic compounds in model solution during nanofiltration. J. Food Eng. 2017, 195, 97–104. [Google Scholar] [CrossRef]
- Wang, J.; Mo, Y.; Mahendra, S.; Hoek, E.M.V. Effects of water chemistry on structure and performance of polyamide composite membranes. J Membr. Sci. 2014, 452, 415–425. [Google Scholar] [CrossRef]
- Oss, C.J. Development and applications of the interfacial tension between water and organic or biological surfaces. Colloids Surf. B Biointerfaces 2007, 54, 2–9. [Google Scholar]
- Liang, S.; Kang, Y.; Tiraferri, A.; Giannelis, E.P.; Huang, X.; Elimelech, M. Highly hydrophilic polyvinylidene fluoride (PVDF) ultrafiltration membranes via postfabrication grafting of surface-tailored silica nanoparticles. ACS Appl. Mater. Interfaces 2013, 5, 6694–6703. [Google Scholar] [CrossRef]
- Bellona, C.; Marts, M.; Drewes, J.E. The effect of organic membrane fouling on the properties and rejection characteristics of nanofiltration membranes. Sep. Purif. Technol. 2010, 74, 44–54. [Google Scholar] [CrossRef]
- Plakas, K.V.; Karabelas, A.J. A systematic study on triazine retention by fouled with humic substances NF/ULPRO membranes. Sep. Purif. Technol. 2011, 80, 246–261. [Google Scholar] [CrossRef]
- Subhi, N.; Verliefde, A.R.D.; Chen, V.; Le-Clech, P. Assessment of physicochemical interactions in hollow fibre ultrafiltration membrane by contact angle analysis. J. Membr. Sci. 2012, 403, 32–40. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Yasin, N.H.M.; Derek, C.J.C.; Lim, J.K. Harvesting of microalgal biomass using MF membrane: Kinetic model, CDE model and extended DLVO theory. J. Membr. Sci. 2013, 446, 341–349. [Google Scholar] [CrossRef]
- Gao, F.; Wang, J.; Zhang, H.W.; Hang, M.Q.A.; Cui, Z.; Yang, G. Interaction energy and competitive adsorption evaluation of different NOM fractions on aged membrane surfaces. J. Membr. Sci. 2017, 542, 195–207. [Google Scholar] [CrossRef]
- Oss, C.J.V. Interfacial Forces in Aqueous Media; Marcel Dekker Inc.: New York, NY, USA, 1994; pp. 209–210. [Google Scholar]
- Jin, X.; Huang, X.; Hoek, E.M. Role of specific ion interactions in seawater RO membrane fouling by alginic acid. Environ. Sci. Technol. 2009, 43, 3580–3587. [Google Scholar] [CrossRef]
- Hurwitz, G.; Guillen, G.R.; Hoek, E.M.V. Probing polyamide membrane surface charge, zeta potential, wettability, and hydrophilicity with contact angle measurements. J. Membr. Sci. 2010, 349, 349–357. [Google Scholar] [CrossRef]
- Luo, J.Q.; Ding, L.H.; Wan, Y.H.; Paullier, P.; Jaffrin, M.Y. Application of NF-RDM (nanofiltration rotating disk membrane) module under extreme hydraulic conditions for the treatment of dairy wastewater. Chem. Eng. J. 2010, 163, 307–316. [Google Scholar] [CrossRef]



| Compound | Molecular Formula | Molecular Structure | Mw (g/mol) | Stokes Radius a (nm) | PKa b | pH | LogD b,d | Charge b,d | Dipole moment c (Debye) | H-Bonding Donors b | H-Bonding Acceptors b |
|---|---|---|---|---|---|---|---|---|---|---|---|
| GA | C7H6O5 | 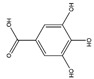 | 170.12 | 0.295 | 3.94 | 3.27 | 0.63 | −0.19 | −0.44 | 5 | 5 |
| PCA | C7H6O4 | 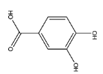 | 154.12 | 0.286 | 4.16 | 3.27 | 0.97 | −0.12 | 0.55 | 4 | 4 |
| 4-HA | C7H6O3 | 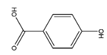 | 138.12 | 0.278 | 4.38 | 3.29 | 1.29 | −0.08 | 1.33 | 3 | 3 |
| 3-HA | C7H6O3 | 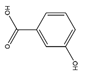 | 138.12 | 0.278 | 3.84 | 3.10 | 1.26 | −0.15 | 1.43 | 3 | 2 |
| SA | C7H6O3 | 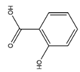 | 138.12 | 0.278 | 2.79 | 2.61 | 1.76 | −0.39 | 2.97 | 3 | 2 |
| Membrane | PLAC07610 | UA60 |
|---|---|---|
| Abbreviation | RC | PA |
| Structure | 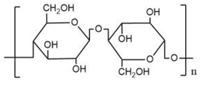 | 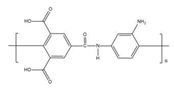 |
| Manufacturer | MILLIPORE | TRISEP |
| Material | Regenerated Cellulose | Poly (piperazine-amide) |
| MWCO(Da) | 1000 | 1000 |
| Pore Diameter d a (nm) | 1.59 | 1.59 |
| Max. pressure (bar) | 4.8 | 7.6 b |
| Max. temp. (°C) | 50 | 50 |
| pH range | 3–13 | 1–12 |
| NaCl rejection (%) | N.A. | 10 |
| Water permeability c (L/m2·h·bar) | 2.97 | 9.19 |
| Contact angle (water) c | 23.38 | 30.88 |
| Zeta-potential (mV) d | −10.71 | −21.22 |
| pH | Charge a | LogD a | ||||||
|---|---|---|---|---|---|---|---|---|
| PCA | 4-HA | 3-HA | SA | PCA | 4-HA | 3-HA | SA | |
| original b | −0.22 | −0.14 | −0.31 | −0.72 | 0.92 | 1.26 | 1.17 | 1.42 |
| 3.0 | −0.07 | −0.04 | −0.13 | −0.62 | 0.99 | 1.31 | 1.27 | 1.56 |
| 7.4 | −1.00 | −1.00 | −1.00 | −1.00 | −2.04 | −1.58 | −1.92 | −1.52 |
| 9.0 | −1.28 | −1.18 | −1.22 | −1.00 | −2.63 | −2.25 | −2.30 | −1.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Zhang, Y.; Zhang, X.; Li, Q.; Huang, M.; Huang, S.; Wu, Q.; Tang, Z.; Pan, L.; Zhang, Y.; et al. A Study of the Mechanism and Separation of Structurally Similar Phenolic Acids by Commercial Polymeric Ultrafiltration Membranes. Membranes 2022, 12, 285. https://doi.org/10.3390/membranes12030285
Wang Q, Zhang Y, Zhang X, Li Q, Huang M, Huang S, Wu Q, Tang Z, Pan L, Zhang Y, et al. A Study of the Mechanism and Separation of Structurally Similar Phenolic Acids by Commercial Polymeric Ultrafiltration Membranes. Membranes. 2022; 12(3):285. https://doi.org/10.3390/membranes12030285
Chicago/Turabian StyleWang, Qinshi, Yun Zhang, Xianli Zhang, Qi Li, Mingcong Huang, Shasha Huang, Qianlian Wu, Zhishu Tang, Linmei Pan, Yue Zhang, and et al. 2022. "A Study of the Mechanism and Separation of Structurally Similar Phenolic Acids by Commercial Polymeric Ultrafiltration Membranes" Membranes 12, no. 3: 285. https://doi.org/10.3390/membranes12030285
APA StyleWang, Q., Zhang, Y., Zhang, X., Li, Q., Huang, M., Huang, S., Wu, Q., Tang, Z., Pan, L., Zhang, Y., Liu, H., Li, B., & Zhu, H. (2022). A Study of the Mechanism and Separation of Structurally Similar Phenolic Acids by Commercial Polymeric Ultrafiltration Membranes. Membranes, 12(3), 285. https://doi.org/10.3390/membranes12030285