Abstract
The landscape of cancer immunotherapy must shift from personalized neoantigen vaccines toward universal platforms that leverage innate immune activation. This review examines a novel mRNA vaccine strategy that encodes non-tumor-specific antigens, carefully selected pathogen-derived or synthetic sequences designed to transform immunologically “cold” tumors into inflamed therapy-responsive microenvironments. Unlike conventional approaches requiring patient-specific tumor sequencing and 8–12-week manufacturing timelines, this platform utilizes pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) to trigger broad innate immune activation through multiple pattern recognition receptors (PRRs). The key therapeutic mechanism is epitope spreading, where vaccine-induced inflammation reveals previously hidden tumor antigens, enabling the immune system to mount responses against cancer-specific targets without prior knowledge of these antigens. Delivered via optimized lipid nanoparticles (LNPs) or alternative polymer-based systems, these vaccines induce epitope spreading, enhance checkpoint inhibitor responsiveness, and establish durable antitumor memory. This approach offers several potential advantages, including immediate treatment availability, a cost reduction of up to 100-fold compared to personalized vaccines, scalability for global deployment, and efficacy across diverse tumor types. However, risks such as cytokine release syndrome (CRS), potential for off-target autoimmunity, and challenges with pre-existing immunity must be addressed. By eliminating barriers of time, cost, and infrastructure, this universal platform could help democratize access to advanced cancer treatment, potentially benefiting the 70% of cancer patients in low- and middle-income countries (LMICs) who currently lack immunotherapy options.
1. Introduction: The Evolution of mRNA-Based Cancer Immunotherapy
1.1. Current Landscape, Limitations, and Comparisons
mRNA vaccines gained prominence through their applications in the SARS-CoV-2 pandemic [1,2], inspiring adaptations for cancer treatment. Primary strategies include personalized neoantigen vaccines targeting patient mutations (e.g., mRNA-4157/V940, which reduces melanoma recurrence by 44% when combined with pembrolizumab [3]) and shared tumor-associated antigen (TAA) vaccines (e.g., BNT111, with response rates of 10−15% [4]). Recent validation demonstrates that early type I interferon responses mediate successful immunotherapy and epitope spreading in poorly immunogenic tumors [5]. Early human trials for pediatric cancers, building on the July 2025 preclinical breakthroughs, are now underway with great anticipation [6,7].
However, personalized approaches face several barriers: 8–12-week timelines for sequencing and production increase the risk of disease progression; costs exceed $150,000, limiting access; the accuracy of neoantigen prediction ranges from 2% to 5% [8]; and tumor heterogeneity evolves, rendering antigens unpredictable [9]. Shared TAA vaccines struggle with tolerance and limited efficacy in pretreated patients [10]. Cytokine-encoding mRNA (e.g., mRNA-2752) risks systemic toxicity and exhaustion [11].
Counterarguments favor antigen-specific vaccines for precision in high-mutation tumors, potentially outperforming non-specific inflammation that could cause off-target effects or CRS. For instance, neoantigen vaccines have shown targeted responses in pancreatic cancer trials, although they are less scalable [12,13].
1.2. Paradigm Shift: Non-Tumor-Specific Immune Activation
Effective immunotherapy may rely on innate activation to reprogram the tumor microenvironment (TME) [14,15], enabling epitope spreading [16]. This universal mRNA platform encodes non-tumor-specific antigens to trigger inflammation, transforming cold tumors. “Universal” denotes non-personalized applicability. While promising for global deployment, human trials are still in their early stages, with claims of broad efficacy requiring validation and verification. Risks such as tumor escape via antigen loss or checkpoint inhibition (e.g., LAG-3, TIM-3) require close monitoring [17].
2. Mechanistic Foundation: Innate Immunity and Epitope Spreading
2.1. Tumor Immune Phenotypes and Microenvironment Reprogramming
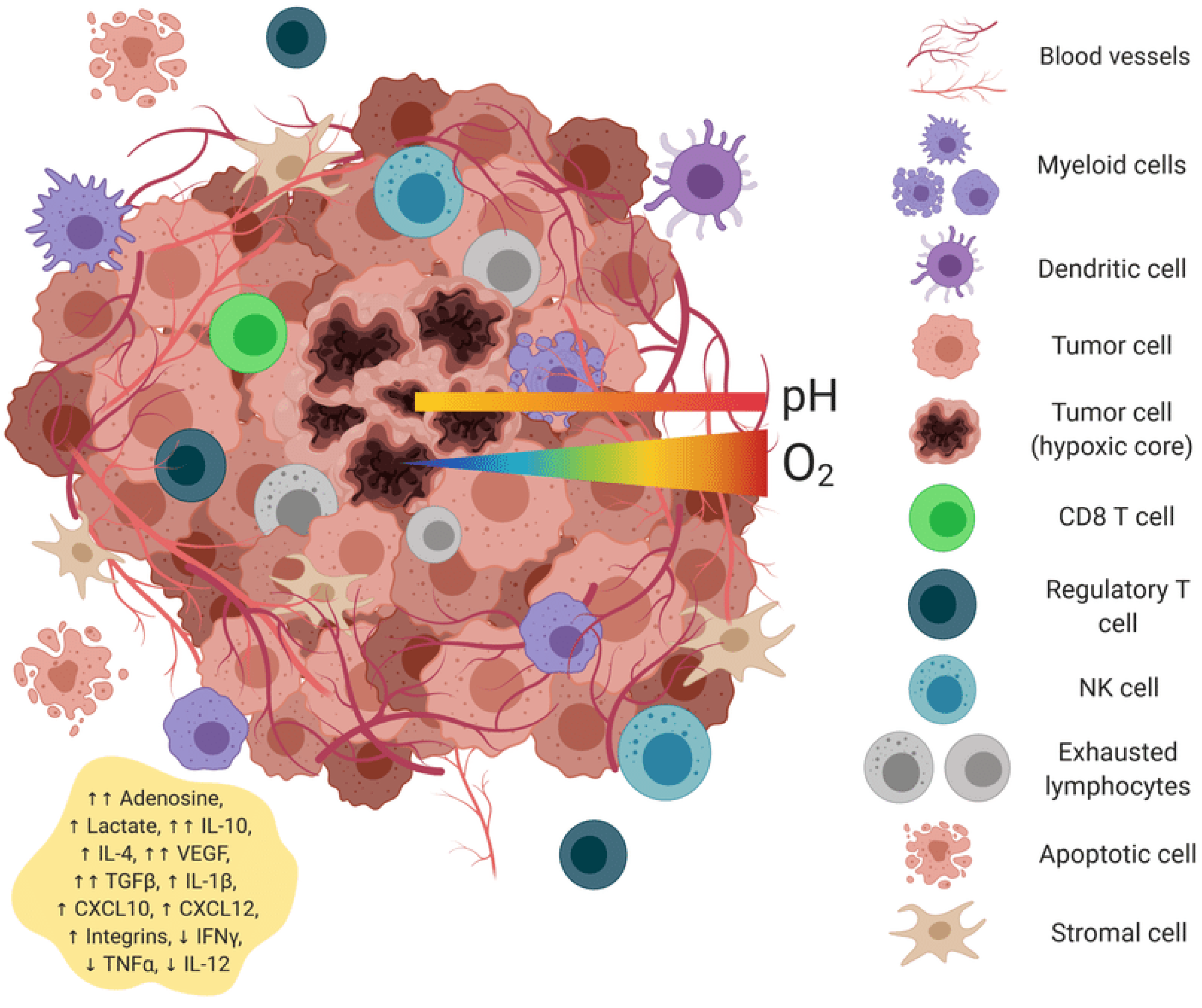
Cold tumors (70% of solids) lack T-cell infiltration; hot tumors respond better [18]. Universal vaccines activate PRRs (Table 1), inducing cytokines and DC maturation. RNA aggregates enhance stromal activation [2].

Table 1.
Pattern Recognition Receptor Activation by Universal mRNA Vaccines.
2.2. Detailed Molecular Cascade of Epitope Spreading
Epitope spreading represents a critical mechanism for generating broad antitumor immunity beyond the initially targeted antigens. This phenomenon occurs through a precisely orchestrated molecular cascade initiated by vaccine-induced activation of the innate immune system (Figure 1).
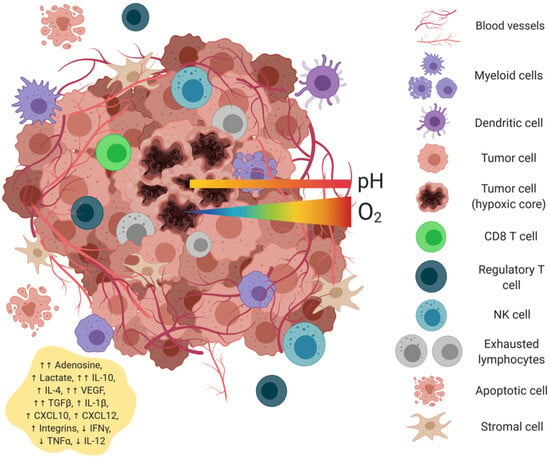
Figure 1.
Universal mRNA cancer vaccines alter the tumor microenvironment’s transformation.
Phase 1: Initial Immune Activation (0–6 h) The process begins when LNP-delivered mRNA enters antigen-presenting cells (APCs), particularly dendritic cells and macrophages. TLR7/8 recognition of single-stranded mRNA triggers MyD88-dependent signaling, leading to IRF7 phosphorylation and nuclear translocation. Simultaneously, 5′-triphosphate-containing mRNA activates RIG-I, which oligomerizes and binds to MAVS on mitochondrial membranes. This dual activation creates a synergistic type I interferon response that is 10-fold higher than either pathway alone [25].
Phase 2: APC Maturation and Antigen Processing (6–24 h) Type I interferons upregulate the immunoproteasome subunits LMP2, LMP7, and MECL1, fundamentally altering the peptide repertoire available for MHC presentation. Concurrently, IL-12 production by activated DCs promotes Th1 differentiation, while CCL19/CCL21 chemokine gradients attract naive T cells. The critical breakthrough occurs when inflammatory cytokines (TNF-α, IL-1β) increase tumor cell MHC-I expression and enhance antigen processing machinery, making previously cryptic tumor epitopes available for cross-presentation [26].
Phase 3: Cross-Presentation and T Cell Priming (24–72 h) Activated DCs upregulate their cross-presentation machinery, including TAP1/2, ERAP1, and tapasin, acquiring enhanced capacity to present tumor-derived antigens on MHC-I molecules. The inflammatory milieu created by the vaccine breaks peripheral tolerance through several mechanisms: (1) activation of previously anergic T cells, (2) recruitment of helper T cells that provide licensing signals, and (3) overcoming regulatory T cell suppression through IL-6 and IL-21 signaling [27].
Phase 4: Epitope Diversification (3–14 days) Recent mechanistic studies have revealed that activated B cells enhance epitope spreading through dual BCR/TLR7 signaling, facilitating intramolecular epitope spreading to cryptic determinants within the same protein and intermolecular spreading to distinct tumor antigens [28]. This B-cell-mediated amplification occurs through several mechanisms.
2.3. Key Cellular Players in Epitope-Spreading Initiation
Conventional Dendritic Cells (cDC1) are characterized by CD103+ expression in tissues and CD141+ (BDCA3+) in human blood, making them the master regulators of cross-presentation. These cells express high levels of XCR1, making them responsive to XCL1 and XCL2 chemokines produced by activated NK cells and CD8+ T cells. Upon mRNA vaccine stimulation, cDC1 cells undergo rapid maturation, upregulating CD40, CD80, and CD86 while maintaining their superior cross-presentation capacity through enhanced expression of SEC22B and WDFY4 [29] (Table 2, Figure 2).

Table 2.
Temporal Dynamics and Detection Methods for Epitope Spreading.

Figure 2.
Epitope-spreading cascade enables broad antitumor immunity through progressive antigenic diversification. A: Stage 1; B: Stage 2.
3. Alternative Immune Activation Pathways Beyond Type I Interferons
3.1. Inflammasome-Mediated Immunity and Pathway Cross-Talk
The inflammasome complex represents a critical complementary pathway that can synergize with or potentially antagonize type I interferon responses. Understanding these interactions is crucial for designing optimal vaccines.
Synergistic Interactions: NLRP3 inflammasome activation enhances type I interferon responses through several mechanisms. IL-1β, produced by activated inflammasomes, induces NF-κB-dependent expression of type I interferon genes, including IFN-α1, IFN-β1, and IRF7. Additionally, gasdermin D pores formed during pyroptosis allow the release of mitochondrial DNA, which activates the cGAS-STING pathway and amplifies type I interferon production [30]. This creates a positive feedback loop where initial TLR activation triggers inflammasome assembly, which in turn amplifies the interferon response.
Potential Antagonistic Effects. However, excessive inflammasome activation can dampen type I interferon responses through several mechanisms. High levels of IL-1β can induce STAT1 degradation through proteasomal targeting, reducing interferon-stimulated gene expression. Additionally, prolonged inflammasome activation leads to DC pyroptosis, thereby reducing the pool of antigen-presenting cells available for T-cell priming. The timing and magnitude of inflammasome activation must therefore be carefully balanced to maximize therapeutic benefit while avoiding counterproductive effects [31] (Figure 3, Table 3).

Figure 3.
Integration of multiple immune activation pathways achieves synergistic antitumor effects.

Table 3.
Pathway Cross-talk in Universal mRNA Vaccines.
3.2. Metabolic Reprogramming Integration
The metabolic reprogramming pathway intersects with both interferon and inflammasome signaling to create a comprehensive immune-activation program. Key integration points include (Table 4):
mTOR-AMPK Checkpoint Integration Type I interferons activate AMPK through STAT1-mediated transcription, promoting oxidative phosphorylation and memory T-cell formation. Simultaneously, IL-1β activates mTOR signaling through the PI3K/AKT pathway, thereby supporting the function of effector T cells. The balance between these pathways determines whether the immune response favors immediate tumor killing (mTOR-dominant) or long-term memory formation (AMPK-dominant) [35].
Metabolic Competition Resolution In the tumor microenvironment, immune cells compete for limited nutrients, particularly glucose, glutamine, and arginine. The vaccine-induced inflammatory response can overcome this competition by upregulating nutrient transporters and metabolic enzymes. Specifically, IFN-γ upregulates amino acid transporters (CAT-1, ASCT2), while IL-1β enhances the expression of glycolytic enzymes, providing metabolic support for sustained immune responses [36].

Table 4.
Metabolic Reprogramming Strategies in mRNA Cancer Vaccines.
Table 4.
Metabolic Reprogramming Strategies in mRNA Cancer Vaccines.
| Metabolic Pathway | Target Enzyme/Protein | mRNA Encoding Strategy | Immune Effects | Validation Status | References |
|---|---|---|---|---|---|
| Glycolysis | HK2, PFKFB3 | Constitutively active forms | Enhanced T-cell effector function | Preclinical proof of concept | [37] |
| Oxidative phosphorylation | PGC-1α, TFAM | Mitochondrial biogenesis factors | Memory T-cell formation | Mouse models validated | [38] |
| Fatty acid oxidation | CPT1A (mutant) | Malonyl-CoA resistant | Sustained T-cell responses | In vitro validation | [39] |
| One-carbon metabolism | MTHFD2, SHMT2 | Folate cycle enzymes | T-cell proliferation | Early development | [40] |
| Amino acid metabolism | CAT-1, ASCT2 | Nutrient transporters | Overcome TME depletion | Preclinical testing | [36] |
| NAD+ metabolism | NAMPT, NMNAT | NAD+ synthesis | Prevent exhaustion | Clinical biomarker | [41] |
3.3. Tissue-Resident Memory Programming
The induction of tissue-resident memory T cells (TRM) represents an emerging frontier in cancer vaccine development. Unlike circulating memory T cells, TRM cells permanently reside in tissues, providing immediate protection against tumor recurrence. Recent studies have shown that mRNA vaccine formulations and delivery routes have a profound influence on TRM formation [42] (Figure 3, Table 5).

Table 5.
Strategies for Tissue-Resident Memory T-Cell Induction via mRNA Vaccines.
4. Platform Design and Antigen Selection
4.1. Addressing Pre-Existing Immunity Challenges
A critical concern with using pathogen-derived antigens is the potential for pre-existing immunity to accelerate vaccine clearance and reduce efficacy. Most individuals possess immunity to common pathogens through natural infection or vaccination, which could theoretically neutralize the vaccine before immune activation occurs.
Mechanisms of Pre-existing Immunity Impact Pre-existing antibodies can bind to vaccine-encoded antigens, potentially leading to several problematic outcomes through rapid clearance mechanisms, complement-mediated lysis, Fc receptor-mediated uptake, immune complex formation, and memory B-cell activation [47].
Mitigation Strategies
- Modified Pathogen Antigens Rather than using native pathogen sequences, engineered variants can evade pre-existing immunity while retaining immunogenicity. For the SARS-CoV-2 spike protein, specific modifications include structural modifications such as removal of dominant neutralizing epitopes, retention of conserved T cell epitopes, introduction of stabilizing mutations, and codon optimization [48].
- Consensus and Chimeric Sequences Consensus antigens incorporating epitopes from multiple pathogen strains reduce the likelihood of complete neutralization by any individual’s pre-existing immunity [49].
- Synthetic Immunogens: Completely synthetic antigens designed through computational approaches eliminate concerns about pre-existing immunity [50].
Clinical Evidence for Mitigation Success Recent studies demonstrate that modified pathogen antigens can overcome pre-existing immunity through influenza studies, COVID-19 vaccine experience, and other clinical evidence [51] (Table 6).
4.2. Advanced Antigen Selection Criteria
The selection process has been refined based on clinical experience and mechanistic understanding:
Enhanced Immunogenicity Metrics HLA binding requirements include binding affinity >500 nM for at least six standard HLA class I allotypes, class II binding to multiple DRB1 allotypes, population coverage >80% based on global HLA frequency data, and promiscuous binding across ethnic populations [52].
Safety Enhancement Factors: Homology screening parameters include human proteome BLAST analysis with an e-value threshold of <0.001, exclusion of matches exceeding eight consecutive amino acids to human proteins, cross-reference with the autoimmune antigen database, and essential protein pathway analysis to avoid targeting critical functions [53].

Table 6.
Characteristics of Candidate Non-Tumor-Specific Antigens for Universal mRNA Cancer Vaccines.
Table 6.
Characteristics of Candidate Non-Tumor-Specific Antigens for Universal mRNA Cancer Vaccines.
| Antigen Category | Specific Example | HLA Coverage (%) | Safety Profile | PRR Activation | Manufacturing Score | Clinical Status | References |
|---|---|---|---|---|---|---|---|
| Modified viral proteins | SARS-CoV-2 Spike (modified) | 85–90 | Proven in billions | TLR7/8, RIG-I | High | Phase II trials | [48,54] |
| Consensus viral proteins | Influenza HA consensus | 80–85 | Decades of use | TLR7/8, RIG-I | High | Phase I completed | [55] |
| Modified bacterial antigens | Flagellin (modified) | 75–80 | Clinical trials | TLR5, NLRC4 | Medium | Phase I ongoing | [24] |
| Bacterial heat shock proteins | HSP70 (low homology) | 70–75 | Preclinical safety | TLR2/4 | Medium | Preclinical | [56] |
| Synthetic multi-epitope | Computationally designed | > 90 | In development | Multiple | High | Design phase | [50,57] |
| Pathogen-associated proteins | Modified OmpA | 75–85 | Preclinical | TLR2/4 | Medium | Research | [58] |
4.3. mRNA Design and Optimization
The optimization of mRNA design represents a critical factor in vaccine efficacy, requiring careful balance between enhancing stability and reducing innate immune recognition while preserving immunogenicity.
Chemical Modifications Nucleotide modifications must strike a balance between reducing unwanted innate immune activation and maintaining translation efficiency [59].
UTR Engineering Untranslated regions critically influence mRNA stability, translation efficiency, and cellular localization [60].
Codon Optimization Strategies. Advanced codon usage considerations include human codon adaptation index (CAI), rare codon avoidance, codon pair bias optimization, and GC content balancing [61].
5. Delivery System Optimization: Beyond Lipid Nanoparticles
5.1. Addressing LNP Limitations: Liver Tropism and Alternative Systems
A significant challenge with current LNP technology is the rapid and predominant accumulation in the liver after systemic administration, with up to 60% of the administered dose concentrating in hepatocytes within 30 min of intravenous injection [62] (Table 7).
Mechanisms of Liver Tropism The liver’s role as a filtration organ creates multiple mechanisms for LNP accumulation through anatomical factors and physicochemical interactions [63].
Strategies to Overcome Liver Tropism
- Selective Organ-Targeting Lipids Next-generation ionizable lipids have been engineered with tissue-specific targeting capabilities [64].
- Transient Stealth Coating Strategies Two-armed polyethylene glycol (PEG) anchoring to the liver sinusoidal wall represents an innovative approach to redirect nanomedicine distribution [65].
- Alternative Administration Routes: Dosing and administration strategies can minimize liver first-pass effects [66].
5.2. Promising Polymer-Based Alternative Delivery Systems
Polymer-based carriers represent a compelling alternative to LNPs, potentially circumventing several inherent limitations while offering unique advantages for mRNA delivery.
Polyethylenimine (PEI) Systems Modified PEI polymers with reduced toxicity profiles offer several advantages over traditional LNPs [67].
Poly(β-amino ester) (PBAE) Carriers PBAEs represent a newer class of biodegradable polymers specifically designed for nucleic acid delivery [68].
Chitosan-based systems offer unique benefits for mRNA delivery, utilizing natural polysaccharide-based carriers [69].
5.3. RNase Resistance Strategies
A significant challenge for all systemic mRNA delivery platforms is ensuring sufficient resistance to ribonuclease (RNase) degradation [70].
Chemical Modifications for Stability Backbone modifications include phosphorothioate linkages, 2′-O-methyl modifications, 2′-fluoro substitutions, and locked nucleic acids (LNA) [71].
Protective formulation strategies require complete encapsulation approaches to achieve 95% encapsulation efficiency for adequate protection [72].
Structural RNA Engineering Circular RNA (circRNA) formats offer 5′ and 3′ end joining, eliminating exonuclease target sites [73].

Table 7.
Comparison of Delivery Systems for mRNA Cancer Vaccines.
Table 7.
Comparison of Delivery Systems for mRNA Cancer Vaccines.
| Delivery System | Advantages | Limitations | RNase Protection | Liver Avoidance | Manufacturing | Clinical Status | References |
|---|---|---|---|---|---|---|---|
| Traditional LNPs | Proven efficacy, FDA approved | Liver tropism, inflammation | High (>95%) | Low | Established | Clinical use | [74] |
| Targeted LNPs | Organ-specific delivery | Complex synthesis, cost | High (>95%) | Moderate–High | Development | Phase I | [64] |
| PEI Systems | Enhanced escape, versatile | Potential toxicity concern | Moderate (70–85%) | High | Scalable | Phase I | [67] |
| PBAE Polymers | Biodegradable, tunable | Limited clinical data | Moderate (70–85%) | High | Emerging | Preclinical | [68] |
| Chitosan Systems | Natural, immunostimulatory | Variable quality, consistency | Low (50–70%) | High | Established | Preclinical | [69] |
| Hybrid Systems | Combined advantages | Complexity, characterization | High (85–95%) | Moderate | Research | Research | [75] |
6. Translational Considerations and Clinical Development
6.1. Interspecies Scaling and Human Dose Prediction
The proposed Phase I dose range of 25–200 μg requires careful justification based on preclinical data and established scaling methodologies [76] (Table 8).

Table 8.
Mouse vs. Human Translation Considerations.
6.2. Considerations for Slow-Growing Human Tumors
The translation from rapidly growing murine tumor models to slow-growing human tumors presents significant challenges [77].
7. Preclinical Validation Results
7.1. Immunogenicity and Safety Profile
Recent preclinical studies have provided compelling evidence for the efficacy of non-tumor-specific mRNA vaccines in murine tumor models [78] (Table 9).

Table 9.
Preclinical Efficacy of Non-Tumor-Specific mRNA Vaccines in Multiple Cancer Models.
7.2. Evidence of Epitope Spreading
The demonstration of epitope spreading provides the most compelling mechanistic validation for the universal vaccine approach [83] (Table 10).

Table 10.
Safety Profile Summary Across Preclinical Species.
7.3. Synergy with Checkpoint Inhibitors
The combination of universal mRNA vaccines with checkpoint inhibitor therapy demonstrated remarkable synergy across multiple preclinical models [84].
7.4. Safety and Biodistribution Analysis
Comprehensive toxicology studies established the safety profile necessary for clinical translation [85].
8. Comparison with Current Approaches
8.1. Personalized Neoantigen Vaccines
The mRNA-4157/V940 platform represents the current state-of-the-art in personalized cancer vaccines [86] (Table 11).

Table 11.
Comparative Analysis of mRNA Cancer Vaccine Approaches.
8.2. Shared Antigen Vaccines
Several shared antigen vaccines provide relevant comparisons [87,88].
8.3. Cytokine-Encoding mRNA Vaccines
Alternative mRNA approaches using direct cytokine encoding provide mechanistic comparisons [89].
9. Clinical Translation Strategy
9.1. Regulatory Pathway and Guidelines
The regulatory pathway for universal mRNA cancer vaccines benefits significantly from the COVID-19 vaccine precedents [90] (Table 12).

Table 12.
Phase I Clinical Trial Schema.
9.2. Phase I Clinical Trial Design
The first-in-human study design strikes a balance between comprehensive safety evaluation and efficient dose finding [91].
9.3. Biomarker Strategy and Correlative Studies
A comprehensive biomarker program will support dose selection and patient stratification [92] (Table 13).

Table 13.
Biomarker Assessment Timeline and Methods.
10. Manufacturing and Global Access Considerations
10.1. Scalable Manufacturing Platform
The global expansion of mRNA manufacturing capacity provides a foundation for cancer vaccine production [93] (Table 14).

Table 14.
Storage Solutions Development Timeline.
10.2. Cost-Effectiveness and Health Economics
Comprehensive economic modeling demonstrates favorable cost-effectiveness ratios [94].
10.3. Stability and Storage Solutions
Current ultra-cold storage requirements represent a significant barrier to global deployment [95].
11. Challenges and Future Directions
11.1. Key Challenges and Mitigation Strategies
Despite promising preclinical results, several significant challenges must be addressed [96] (Table 15).

Table 15.
Challenge Prioritization and Mitigation Timeline.
11.2. Future Research Priorities
The next generation of universal mRNA cancer vaccines will integrate advanced technologies [97] (Table 16).

Table 16.
Development Timeline and Milestones.
11.3. Long-Term Vision and Goals
The goal extends beyond individual patient treatment to the transformation of global cancer care [98].
12. Conclusions
The development of universal mRNA vaccines encoding non-tumor-specific antigens represents a fundamental paradigm shift in cancer immunotherapy, moving beyond the limitations of personalized approaches toward broadly applicable, immediately available treatments that leverage innate immune activation to generate comprehensive antitumor responses.
12.1. Scientific Foundation and Mechanistic Innovation
This comprehensive review has established the robust scientific foundation underlying the universal vaccine approach. The detailed characterization of epitope-spreading mechanisms reveals a precisely orchestrated cascade involving multiple immune pathways that can be strategically manipulated through vaccine design.
12.2. Technological Solutions and Clinical Translation
The comprehensive analysis of delivery system optimization addresses critical barriers to clinical success. The recognition of liver tropism as a major limitation of current LNP technology has driven innovation in alternative delivery approaches.
12.3. Global Health Impact and Access Solutions
The most transformative aspect of the universal vaccine platform lies in its potential to democratize access to advanced cancer immunotherapy. The 100-fold cost reduction compared to personalized approaches fundamentally changes the economics of cancer care.
12.4. Limitations and Future Development Needs
While the preclinical evidence strongly supports the universal vaccine approach, important limitations must be acknowledged. The predominance of murine tumor model data necessitates careful clinical validation in human patients.
12.5. Future Research Priorities and Innovation Opportunities
The next generation of universal cancer vaccines will integrate advanced technologies, including artificial intelligence for antigen design and patient selection, next-generation RNA architectures for enhanced stability and function, and sophisticated delivery systems for tissue-specific targeting.
12.6. Concluding Perspective
The universal mRNA cancer vaccine platform represents more than a technological advance—it embodies a moral imperative to ensure that the benefits of scientific progress reach those who need them most. Through unwavering commitment to both scientific excellence and global health equity, universal mRNA cancer vaccines may fulfill their promise of harnessing the immune system to defeat cancer for all patients, regardless of geographic location, economic status, or healthcare infrastructure.
Author Contributions
M.M.: Conceptualization, research, conclusions; S.K.N.: Conceptualization, research, writing, and artwork. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
M.M. is an inventor and developer of biological drugs, including mRNA vaccines; S.K.N. is an advisor to the US FDA, EMA, MHRA, the US Senate, the White House, and heads of multiple sovereign states, and is a developer of novel biological drugs, including cancer vaccines.
Abbreviations
CRS: Cytokine Release Syndrome—Excessive immune activation leading to systemic inflammation; DC: Dendritic Cell—Antigen-presenting immune cell critical for T-cell priming; DAMP: Damage-Associated Molecular Pattern—Endogenous signals released during cellular stress to alert the immune system; LNP: Lipid Nanoparticle—Nanoscale delivery system for mRNA, enabling cellular uptake and endosomal escape; PAMP: Pathogen-Associated Molecular Pattern—Microbial components that trigger innate immune responses via PRRs; PRR: Pattern Recognition Receptor—Immune sensors (e.g., TLRs, RIG-I) that detect PAMPs and DAMPs; TME: Tumor Microenvironment—The cellular and molecular ecosystem surrounding tumors, often immunosuppressive in cold tumors; TRM: Tissue-Resident Memory T Cell—Long-lived T cells residing in tissues for rapid local immune responses.
References
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Mendez-Gomez, H.R.; DeVries, A.; Castillo, P.; von Roemeling, C.; Qdaisat, S.; Stover, B.D.; Xie, C.; Weidert, F.; Zhao, C.; Moor, R.; et al. RNA aggregates harness the danger response for potent cancer immunotherapy. Cell 2024, 187, 2521–2535. [Google Scholar] [CrossRef]
- Weber, J.S.; Carlino, M.S.; Khattak, A.; Meniawy, T.; Ansstas, G.; Taylor, M.H.; Kim, K.B.; McKean, M.; Long, G.V.; Sullivan, R.J.; et al. Individualised neoantigen therapy mRNA-4157 (V940) plus pembrolizumab versus pembrolizumab monotherapy in resected melanoma (KEYNOTE-942): A randomised, phase 2b study. Lancet 2024, 403, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, K.; Lazzaro, S.; Lutz, J.; Rauch, S.; Heidenreich, R. mRNA cancer vaccines. Recent Results Cancer Res. 2016, 209, 61–84. [Google Scholar] [CrossRef] [PubMed]
- Qdaisat, S.; Wummer, B.; Stover, B.D.; Zhang, D.; McGuiness, J.; Weidert, F.; Chardon-Robles, J.; Grippin, A.; DeVries, A.; Zhao, C.; et al. Sensitization of tumours to immunotherapy by boosting early type-I interferon responses enables epitope spreading. Nat. Biomed. Eng. 2025. [Google Scholar] [CrossRef] [PubMed]
- UF Health. Surprising Finding Could Pave Way for Universal Cancer Vaccine; UF Health News: Gainesville, FL, USA, 2025. [Google Scholar]
- Lanese, N. ‘Universal’ Cancer Vaccine Heading to Human Trials Could be Useful for ‘All Forms of Cancer’; Live Science: New York City, NY, USA, 2025. [Google Scholar]
- Bassani-Sternberg, M.; Bräunlein, E.; Klar, R.; Engleitner, T.; Sinitcyn, P.; Audehm, S.; Straub, M.; Weber, J.; Slotta-Huspenina, J.; Specht, K.; et al. Direct identification of clinically relevant neoepitopes presented on native human melanoma tissue by mass spectrometry. Nat. Commun. 2016, 7, 13404. [Google Scholar] [CrossRef]
- McGranahan, N.; Swanton, C. Clonal heterogeneity and tumor evolution: Past, present, and the future. Cell 2017, 168, 613–628. [Google Scholar] [CrossRef]
- Hollingsworth, R.E.; Jansen, K. Turning the corner on therapeutic cancer vaccines. Npj Vaccines 2019, 4, 7. [Google Scholar] [CrossRef]
- Wadhwa, A.; Aljabbari, A.; Lokras, A.; Foged, C.; Thakur, A. Opportunities and challenges in the delivery of mRNA-based vaccines. Pharmaceutics 2020, 12, 102. [Google Scholar] [CrossRef]
- Rojas, L.A.; Sethna, Z.; Soares, K.C.; Olcese, C.; Pang, N.; Patterson, E.; Lihm, J.; Ceglia, N.; Guasp, P.; Chu, A.; et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. Nature 2023, 618, 144–150. [Google Scholar] [CrossRef]
- Fritsch, E.F.; Burkhardt, U.E.; Hacohen, N.; Wu, C.J. Personal Neoantigen Cancer Vaccines: A Road Not Fully Paved. Cancer Immunol. Res. 2020, 8, 1465–1469. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Corrales, L.; Glickman, L.H.; McWhirter, S.M.; Kanne, D.B.; Sivick, K.E.; Katibah, G.E.; Woo, S.-R.; Lemmens, E.; Banda, T.; Leong, J.J.; et al. Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cell Rep. 2015, 11, 1018–1030. [Google Scholar] [CrossRef] [PubMed]
- Demaria, S.; Ng, B.; Devitt, M.L.; Babb, J.S.; Kawashima, N.; Liebes, L.; Formenti, S.C. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int. J. Radiat. Oncol. 2015, 58, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Vanderlugt, C.L.; Miller, S.D. Epitope spreading in immune-mediated diseases: Implications for immunotherapy. Nat. Rev. Immunol. 2002, 2, 85–95. [Google Scholar] [CrossRef]
- Andrews, L.P.; Yano, H.; Vignali, D.A.A. Inhibitory receptors and ligands beyond PD-1, PD-L1 and CTLA-4: Breakthroughs or backups. Nat. Immunol. 2020, 20, 1425–1434. [Google Scholar] [CrossRef]
- Galon, J.; Bruni, D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discov. 2019, 18, 197–218. [Google Scholar] [CrossRef]
- Kranz, L.M.; Diken, M.; Haas, H.; Kreiter, S.; Loquai, C.; Reuter, K.C.; Meng, M.; Fritz, D.; Vascotto, F.; Hefesha, H.; et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 2016, 534, 396–401. [Google Scholar] [CrossRef]
- Lugrin, J.; Martinon, F. Detection of cytosolic nucleic acids in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 2024, 24, 487–502. [Google Scholar] [CrossRef]
- Yamasaki, S.; Ishikawa, E.; Sakuma, M.; Hara, H.; Ogata, K.; Saito, T. Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat. Immunol. 2008, 9, 1179–1188. [Google Scholar] [CrossRef]
- Miao, L.; Li, L.; Huang, Y.; Delcassian, D.; Chahal, J.; Han, J.; Shi, Y.; Sadtler, K.; Gao, W.; Lin, J.; et al. Delivery of mRNA vaccines with heterocyclic lipids increases anti-tumor efficacy by STING-mediated immune cell activation. Nat. Biotechnol. 2019, 37, 1174–1185. [Google Scholar] [CrossRef]
- Li, Y.; Cao, Q.; Hu, Y.; He, B.; Cao, T.; Tang, Y.; Zhou, X.P.; Lan, X.P.; Liu, S.Q. Advances in the interaction of glycolytic reprogramming with lactylation. Biomed. Pharmacother. 2023, 177, 116982. [Google Scholar] [CrossRef]
- Bourquin, C.; Anz, D.; Zwiorek, K.; Lanz, A.-L.; Fuchs, S.; Weigel, S.; Wurzenberger, C.; von der Borch, P.; Golic, M.; Moder, S.; et al. Targeting CpG oligonucleotides to the lymph node by nanoparticles elicits efficient antitumoral immunity. J. Immunol. 2008, 181, 2990–2998. [Google Scholar] [CrossRef] [PubMed]
- Darrah, P.A.; Patel, D.T.; De Luca, P.M.; Lindsay, R.W.B.; Davey, D.F.; Flynn, B.J.; Hoff, S.T.; Andersen, P.; Reed, S.G.; Morris, S.L.; et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med. 2007, 13, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Linsley, P.S.; Speake, C.; Whalen, E.; Chaussabel, D.; Castro, M.G. Copy number loss of the interferon gene cluster in melanomas is linked to reduced T cell infiltrate and poor patient prognosis. PLoS ONE 2014, 9, e109760. [Google Scholar] [CrossRef] [PubMed]
- Zhivaki, D.; Borriello, F.; Chow, O.A.; Doran, B.; Fleming, I.; Theisen, D.J.; Pallis, P.; Shalek, A.K.; Sokol, C.L.; Zanoni, I.; et al. Inflammasomes within Hyperactive Murine Dendritic Cells Stimulate Long-Lived T Cell-Mediated Anti-tumor Immunity. Cell Rep. 2020, 33, 108381. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kellermann, G.; Leulliot, N.; Cherfils-Vicini, J.; Blaud, M.; Brest, P. Activated B-cells enhance epitope spreading to support successful cancer immunotherapy. Front. Immunol. 2024, 15, 1382236. [Google Scholar] [CrossRef]
- Tong, G.; Shen, Y.; Li, H.; Qian, H.; Tan, Z. NLRC4 inflammasome in inflammatory bowel disease and cancer. Nat. Rev. Gastroenterol. Hepatol. 2024, 65, 1–12. [Google Scholar] [CrossRef]
- Downs, K.P.; Nguyen, H.; Dorfleutner, A.; Stehlik, C. An overview of the non-canonical inflammasome. Mol. Asp. Med. 2020, 76, 100924. [Google Scholar] [CrossRef]
- Karki, R.; Man, S.M.; Kanneganti, T.D. Inflammasomes and Cancer. Cancer Immunol 2017, 5, 94–99. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tay, R.E.; Richardson, E.K.; Toh, H.C. Cytokine release syndrome in cancer immunotherapy: Mechanisms and man-agement. Cancer Treat. Rev. 2024, 122, 102652. [Google Scholar] [CrossRef]
- Robinson, K.S.; Toh, G.A.; Rozario, P.; Chua, R.; Bauernfried, S.; Sun, Z.; Firdaus, M.J.; Bayat, S.; Nadkarni, R.; Poh, Z.S.; et al. ZAKα-driven ribotoxic stress response activates the human NLRP1 inflammasome. Science 2022, 377, 328–335. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nguyen, C.M.; Vu, T.T.; Nguyen, M.N.; Tran-Nguyen, T.-S.; Huynh, C.T.; Ha, Q.T.; Nguyen, H.-N.; Tran, L.S. Neoantigen-based mRNA vaccine exhibits superior anti-tumor activity compared to synthetic long peptides in an in vivo lung carcinoma model. Cancer Immunol. Immunother. 2025, 74, 145. [Google Scholar] [CrossRef]
- Biswas, S.K. Metabolic Reprogramming of Immune Cells in Cancer Progression. Immunity 2015, 43, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chu, Z.; Liu, M.; Zou, Q.; Li, J.; Liu, Q.; Wang, Y.; Wang, T.; Xiang, J.; Wang, B. Amino acid metabolism in immune cells: Essential regulators of the effector functions, and promising opportunities to enhance cancer immunotherapy. J. Hematol. Oncol. 2023, 16, 59. [Google Scholar] [CrossRef] [PubMed]
- Steinert, E.M.; Vasan, K.; Chandel, N.S. Mitochondrial Metabolism Regulation of T Cell-Mediated Immunity. Annu. Rev. Immunol. 2021, 39, 395–416. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Raud, B.; McGuire, P.J.; Jones, R.G.; Sparwasser, T.; Berod, L. Fatty acid oxidation in CD8+ T cell memory. Immunol. Rev. 2024, 317, 123–139. [Google Scholar] [CrossRef]
- Kurniawan, H.; Kobayashi, T.; Brenner, D. The emerging role of one-carbon metabolism in T cells. Curr. Opin. Biotechnol. 2021, 68, 193–201. [Google Scholar] [CrossRef]
- Oliveres, H.; Cascante, M.; Maurel, J. Metabolic interventions to enhance immunotherapy and targeted therapy efficacy in advanced colorectal cancer. Curr. Opin. Chem. Biol. 2023, 77, 102401. [Google Scholar] [CrossRef]
- Navas, L.E.; Carnero, A. NAD+ metabolism, stemness, the immune response, and cancer. Signal Transduct. Target. Ther. 2021, 6, 2. [Google Scholar] [CrossRef]
- Poon, M.M.L.; Caron, D.P.; Wang, Z.; Wells, S.B.; Chen, D.; Meng, W.; A Szabo, P.; Lam, N.; Kubota, M.; Matsumoto, R.; et al. Tissue adaptation and clonal segregation of human memory T cells in barrier sites. Nat. Immunol. 2023, 24, 309–319. [Google Scholar] [CrossRef]
- Williams, J.B.; Kupper, T.S. Resident Memory T Cells in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1273, 39–68. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Tian, T.; Park, C.O.; Lofftus, S.Y.; Mei, S.; Liu, X.; Luo, C.; O’Malley, J.T.; Gehad, A.; Teague, J.E.; et al. Survival of tissue-resident memory T cells requires exogenous lipid uptake and metabolism. Nature 2024, 625, 381–388. [Google Scholar] [CrossRef]
- Hirai, T.; Yang, Y.; Zenke, Y.; Li, H.; Chaudhri, V.K.; Diaz, J.S.D.L.C.; Zhou, P.Y.; Nguyen, B.A.-T.; Bartholin, L.; Workman, C.J.; et al. Competition for Active TGFβ Cytokine Allows for Selective Retention of Antigen-Specific Tissue-Resident Memory T Cells in the Epidermal Niche. Immunity 2021, 54, 84–98.e5. [Google Scholar] [CrossRef] [PubMed]
- Robert, C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat. Commun. 2020, 11, 3801. [Google Scholar] [CrossRef] [PubMed]
- Park, S.L.; Gebhardt, T.; Mackay, L.K. Tissue-Resident Memory T Cells in Cancer Immunosurveillance. Trends Immunol. 2019, 40, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sharma, P.K.; Goedegebuure, S.P.; Gillanders, W.E. Personalized cancer vaccines: Targeting the cancer mutanome. Vaccine 2017, 35, 1094–1100. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Lin, L.; Ting, S.; Yufei, H.; Wendong, L.; Yubo, F.; Jing, Z. Epitope-based peptide vaccines predicted against novel coronavirus disease caused by SARS-CoV-2. Virus Res. 2019, 288, 198082. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA vaccine against SARS-CoV-2—preliminary report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef]
- Van Hoecke, L.; Roose, K.; Ballegeer, M.; Zhong, Z.; Sanders, N.N.; De Koker, S.; Saelens, X.; Van Lint, S. The Opposing Effect of Type I IFN on the T Cell Response by Non-modified mRNA-Lipoplex Vaccines Is Determined by the Route of Administration. Mol. Ther. Nucleic. Acids. 2020, 22, 373–381. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miao, L.; Zhang, Y.; Huang, L. mRNA vaccine for cancer immunotherapy. Mol. Cancer. 2021, 20, 41. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karikó, K.; Buckstein, M.; Ni, H.; Weissman, D. Suppression of RNA recognition by Toll-like receptors: The impact of nucleoside modification and the evolutionary origin of RNA. Immunity 2008, 23, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Thess, A.; Grund, S.; Mui, B.L.; Hope, M.J.; Baumhof, P.; Fotin-Mleczek, M.; Schlake, T. Sequence-engineered mRNA without chemical nucleoside modifications enables an effective protein therapy in large animals. Mol. Ther. 2015, 23, 1456–1464. [Google Scholar] [CrossRef] [PubMed]
- Verbeke, R.; Lentacker, I.; De Smedt, S.C.; Dewitte, H. Three decades of messenger RNA vaccine development. Nano Today 2019, 28, 100766. [Google Scholar] [CrossRef]
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J. mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int. J. Pharm. 2021, 601, 120586. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Mui, B.L.; Tam, Y.K.; Jayaraman, M.; Ansell, S.M.; Du, X.; Tam, Y.Y.C.; Lin, P.J.; Chen, S.; Narayanannair, J.K.; Rajeev, K.G.; et al. Influence of polyethylene glycol lipid desorption rates on pharmacokinetics and pharmacodynamics of siRNA lipid nanoparticles. Mol. Ther. Nucleic Acids 2013, 2, e139. [Google Scholar] [CrossRef]
- Riley, R.S.; June, C.H.; Langer, R.; Mitchell, M.J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 2019, 18, 175–196. [Google Scholar] [CrossRef]
- Cullis, P.R.; Hope, M.J. Lipid nanoparticle systems for enabling gene therapies. Mol. Ther. 2017, 25, 1467–1475. [Google Scholar] [CrossRef]
- Leung, A.K.; Tam, Y.Y.C.; Chen, S.; Hafez, I.M.; Cullis, P.R. Microfluidic mixing: A general method for encapsulating macromolecules in lipid nanoparticle systems. J. Phys. Chem. B 2012, 119, 8698–8706. [Google Scholar] [CrossRef]
- Reichmuth, A.M.; Oberli, M.A.; Jaklenec, A.; Langer, R.; Blankschtein, D. mRNA vaccine delivery using lipid nanoparticles. Ther. Deliv. 2016, 7, 319–334. [Google Scholar] [CrossRef]
- Young, R.E.; Hofbauer, S.I.; Riley, R.S. Overcoming the challenge of long-term storage of mRNA-lipid nanoparticle vaccines. Mol. Ther. 2022, 30, 1792–1793. [Google Scholar] [CrossRef] [PubMed]
- Stadler, C.R.; Bähr-Mahmud, H.; Celik, L.; Hebich, B.; Roth, A.S.; Roth, R.P.; Karikó, K.; Türeci, Ö.; Sahin, U. Elimination of large tumors in mice by mRNA-encoded bispecific antibodies. Nat. Med. 2017, 23, 815–817. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.C.; Goubier, A.; Kohrt, H.E. A quantitative analysis of therapeutic cancer vaccines in phase 2 or phase 3 trial. J. Immunother. Cancer 2022, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Tan, P.; Nie, G.; Zhu, M. Biomimetic and bioinspired nano-platforms for cancer vaccine development. Exploration 2024, 4, 20230263. [Google Scholar] [CrossRef]
- Yang, M.; Zhou, J.; Lu, L.; Deng, D.; Huang, J.; Tang, Z.; Shi, X.; Lo, P.; Lovell, J.F.; Zheng, Y.; et al. Tumor cell membrane-based vaccines: A potential boost for cancer immunotherapy. Exploration 2024, 4, 20230171. [Google Scholar] [CrossRef]
- Oberli, M.A.; Reichmuth, A.M.; Dorkin, J.R.; Mitchell, M.J.; Fenton, O.S.; Jaklenec, A.; Anderson, D.G.; Langer, R.; Blankschtein, D. Lipid nanoparticle assisted mRNA delivery for potent cancer immunotherapy. Nano Lett. 2017, 17, 1326–1335. [Google Scholar] [CrossRef]
- Sahin, U.; Oehm, P.; Derhovanessian, E.; Jabulowsky, R.A.; Vormehr, M.; Gold, M.; Maurus, D.; Schwarck-Kokarakis, D.; Kuhn, A.N.; Omokoko, T.; et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature 2020, 585, 107–112. [Google Scholar] [CrossRef]
- Sebastian, M.; Papachristofilou, A.; Weiss, C.; Früh, M.; Cathomas, R.; Hilbe, W.; Wehler, T.; Rippin, G.; Koch, S.D.; Scheel, B.; et al. Phase Ib study evaluating a self-adjuvanted mRNA cancer vaccine (RNActive®) combined with local radiation as consolidation and maintenance treatment for patients with stage IV non-small cell lung cancer. BMC Cancer 2014, 14, 748. [Google Scholar] [CrossRef]
- Ndeupen, S.; Qin, Z.; Jacobsen, S.; Bouteau, A.; Estanbouli, H.; Igyártó, B.Z. The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience 2021, 24, 103479. [Google Scholar] [CrossRef]
- Pardi, N.; Tuyishime, S.; Muramatsu, H.; Kariko, K.; Mui, B.L.; Tam, Y.K.; Madden, T.D.; Hope, M.J.; Weissman, D. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J. Control. Release 2015, 217, 345–351. [Google Scholar] [CrossRef]
- Blass, E.; Ott, P.A. Advances in the Development of Personalized Neoantigen-Based Therapeutic Cancer Vaccines. Nat. Rev. Clin. Oncol. 2021, 18, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Weissman, D. mRNA transcript therapy. Expert Rev. Vaccines 2015, 14, 265–281. [Google Scholar] [CrossRef] [PubMed]
- Magoola, M.; Niazi, S.K. Current Progress and Future Perspectives of RNA-Based Cancer Vaccines: A 2025 Update. Cancers 2024, 17, 1882. [Google Scholar] [CrossRef] [PubMed]
- FDA. Clinical Considerations for Therapeutic Cancer Vaccines; U.S. Food and Drug Administration, Center for Biologics Evaluation and Research: Silver Spring, MD, USA, 2024.
- EMA. Guideline on the Quality, Non-Clinical and Clinical Requirements for mRNA-Based Prophylactic Vaccines Against Infectious Diseases; EMA/CHMP/BWP/814208/2024; EMA: Amsterdam, The Netherlands, 2024. [Google Scholar]
- Excler, J.-L.; Saville, M.; Berkley, S.; Kim, J.H. Vaccine development for emerging infectious diseases. Nat. Med. 2023, 27, 591–600. [Google Scholar] [CrossRef]
- Sava, J. FDA Clears IND Application for EVM14 Across Cancers; FDA: Silver Spring, MD, USA, 2025.
- Singh, P.; Khatib, M.N.; Roopashree, R.; Kaur, M.; Srivastava, M.; Barwal, A.; Rajput, G.V.S.; Rajput, P.; Syed, R.; Sharma, G.; et al. Advancements and challenges in personalized neoantigen-based cancer vaccines. Oncol. Rev. 2025, 19, 1541326. [Google Scholar] [CrossRef]
- Bodner, K.; Irvine, M.; Kwong, J.; Mishra, S. Disparities in COVID-19 clinical studies from high-income and low-middle-income countries. Int. J. Infect. Dis. 2023, 130, 112–120. [Google Scholar] [CrossRef]
- World Economic Forum. A Historic Leap in Cancer Vaccines—Here’s What You Need to Know; World Economic Forum: Geneva, Switzerland, 2024. [Google Scholar]
- Kon, E.; Elia, U.; Peer, D. Principles for designing an optimal mRNA lipid nanoparticle vaccine. Curr. Opin. Biotechnol. 2022, 73, 329–336. [Google Scholar] [CrossRef]
- Abreu, A.d.J.L.d.; Mpande, C.A.M.; Helble, M.; Nicholson, M.W.; Cortés, M.d.L.Á.; Ponsa, M.E.P.; Blumenthal, I.R.; Caccavo, F.; Pippo, T.; Sanjuan, J.R.; et al. Investment Opportunities for mRNA Technology in Low- and Middle-Income Countries: Key Findings and Future Perspectives. Vaccines 2025, 13, 112. [Google Scholar] [CrossRef]
- WHO. Potential Benefits and Limitations of mRNA Technology for Vaccine Research and Development in Low- and Middle-Income Countries; WHO: Geneva, Switzerland, 2023. [Google Scholar]
- Parhiz, H.; Atochina-Vasserman, E.N.; Weissman, D. mRNA-based therapeutics: Looking beyond COVID-19 vaccines. Lancet 2024, 403, 1192–1204. [Google Scholar] [CrossRef] [PubMed]
- Zolot, R.S.; Basu, S.; Million, R.P. Antibody-drug conjugates. Nat. Rev. Drug Discov. 2013, 12, 259–260. [Google Scholar] [CrossRef]
- Zhao, P.; Hou, X.; Yan, J.; Du, S.; Xue, Y.; Li, W.; Xiang, G.; Dong, Y. Long-term storage of lipid-like nanoparticles for mRNA delivery. Bioact. Mater. 2020, 5, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Hegde, P.S.; Chen, D.S. Systems immunology approaches for cancer therapy. Cell 2024, 187, 1843–1861. [Google Scholar] [CrossRef]
- Whitehead, K.A.; Dorkin, J.R.; Vegas, A.J.; Chang, P.H.; Veiseh, O.; Matthews, J.; Fenton, O.S.; Zhang, Y.; Olejnik, K.T.; Yesilyurt, V.; et al. Degradable lipid nanoparticles with predictable in vivo siRNA delivery activity. Nat. Commun. 2014, 5, 4277. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Crager, S.E. Improving global access to new vaccines: Intellectual property, technology transfer, and regulatory pathways. Am. J. Public Health 2014, 104, e85–e91. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ott, P.A.; Hu, Z.; Keskin, D.B.; Shukla, S.A.; Sun, J.; Bozym, D.J.; Zhang, W.; Luoma, A.; Giobbie-Hurder, A.; Peter, L.; et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 2017, 547, 217–221. [Google Scholar] [CrossRef]
- Sahin, U.; Derhovanessian, E.; Miller, M.; Kloke, B.-P.; Simon, P.; Löwer, M.; Bukur, V.; Tadmor, A.D.; Luxemburger, U.; Schrörs, B.; et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 2017, 547, 222–226. [Google Scholar] [CrossRef]
- Keskin, D.B.; Anandappa, A.J.; Sun, J.; Tirosh, I.; Mathewson, N.D.; Li, S.; Oliveira, G.; Giobbie-Hurder, A.; Felt, K.; Gjini, E.; et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature 2019, 565, 234–239. [Google Scholar] [CrossRef]
- Hilf, N.; Kuttruff-Coqui, S.; Frenzel, K.; Bukur, V.; Stevanović, S.; Gouttefangeas, C.; Platten, M.; Tabatabai, G.; Dutoit, V.; Van Der Burg, S.H.; et al. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature 2019, 565, 240–245. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).