Abstract
Background: Anti-HIV neutralizing antibodies (anti-HIV-nAbs) play a critical role in the immune defense against HIV by preventing viral entry and limiting replication. This study longitudinally evaluated the titers and variability of anti-HIV-nAbs in individuals coinfected with HIV and HCV. Samples were collected at three time points: before starting HCV treatment, one year after completion, and five years post-treatment. Methods: A retrospective analysis was conducted on 71 HIV/HCV-coinfected patients who achieved a sustained virologic response following antiviral therapy for HCV. A control group of 41 HIV-monoinfected individuals was also included. Anti-HIV-nAb titers were evaluated by HIV neutralization assays using a panel of six recombinant HIV viruses representing multiple genetic subtypes. Generalized Linear Mixed Models and Generalized Linear Models were used for statistical analysis. p-values were adjusted using the Benjamini–Hochberg procedure (q-value). Results: HIV-neutralizing antibody responses in HIV/HCV-coinfected individuals remained stable over five years following HCV therapy without significant changes (q-value > 0.05). The mean neutralization scores remained stable, with baseline scores of 6.1 (95% CI: 5.4–6.7), 6.2 (95% CI: 5.5–6.8) at one year post-HCV therapy, and 6.0 (95% CI: 5.3–6.7) at five years post-HCV therapy. HIV/HCV-coinfected individuals consistently showed lower neutralization scores compared to the control group throughout the follow-up (q-value < 0.05). Regression analyses adjusted for age, gender, nadir CD4+, and baseline CD4+ counts confirmed that the observed differences between HIV-monoinfected and HIV/HCV-coinfected individuals persisted (q-value < 0.05) at both the baseline and after HCV therapy completion. Conclusions: Successful HCV eradication in HIV/HCV-coinfected individuals did not normalize anti-HIV-nAb titers, which remained consistently lower than those in HIV-monoinfected controls over five years.
1. Introduction
Hepatitis C virus (HCV) and human immunodeficiency virus (HIV) coinfection is a significant global health issue due to their shared transmission routes and viral interactions [1]. An estimated 2.3 million people living with HIV (PLWH) worldwide are coinfected with HCV, representing approximately 6.2% of the global PLWH population. This coinfection is most prevalent among people who inject drugs (~80%) [2]. The introduction of direct-acting antivirals (DAAs) has transformed the treatment landscape for HCV, achieving cure rates >95% [3], even in populations with HIV coinfection [4].
Having both HCV and HIV infections is more detrimental to the immune system than having either infection alone. In PLWH, HCV coinfection is a major cause of non-AIDS-related illness and death because it impairs the recovery of CD4+ T cells after combination antiretroviral therapy (cART) [1]. Furthermore, in HIV/HCV-coinfected individuals, immune system dysfunction is worsened by chronic inflammation, altered B-cell and T-cell function, and increased immune exhaustion [1].
A DAA-mediated HCV cure in HIV/HCV-coinfected individuals rapidly lowers inflammatory markers and improves plasma cytokines, though not fully normalizing them. Post DAA therapy, the total CD4+ and CD8+ T-cell counts increase while activated T cells decrease [5]. While HCV-specific CD8+ T-cell proliferation may improve, a persistent “exhaustion scar” remains. Regulatory T-cells (Tregs) and myeloid-derived suppressor cells (MDSCs), which are associated with immune suppression, do not fully normalize after HCV cure, suggesting persistent immune dysregulation despite HCV eradication [1].
HIV/HCV-coinfection is characterized by severe B cell dysfunction, an exacerbation of the abnormalities already present in HIV-monoinfection. HIV alone causes altered B cell phenotypes, impaired antibody production, dysfunctional memory, and disrupted cytokine signaling, leading to weakened humoral immunity (e.g., HIV-neutralizing antibodies) and disease progression [6]. HCV coinfection amplifies this dysfunction through several mechanisms: increased B cell apoptosis and turnover [7]; an accumulation of exhausted/dysfunctional memory B cells that impair antibody production and long-term memory [6]; and persistent HCV-driven immune activation, which fuels B cell dysregulation and immune exhaustion [1]. Although HCV treatment and the subsequent sustained virologic response (SVR) in coinfected individuals can alleviate some B cell dysfunction, immune disruptions often persist. This residual dysregulation can compromise the generation of effective immune responses, including HIV-neutralizing antibodies, even after HCV clearance [1]. Thus, despite partial B cell recovery post-HCV treatment, lingering immune abnormalities may hinder effective antibody responses and long-term immunity.
Neutralizing antibodies (nAbs) play a critical role in the immune defense against HIV by preventing viral entry and limiting replication. These antibodies target key functional sites on the HIV-1 envelope glycoprotein (Env). The ongoing interaction between the host immune system and viral escape mechanisms can drive the emergence of broadly neutralizing antibodies (bNAbs). Unlike strain-specific nAbs, bNAbs can neutralize a diverse range of HIV-1 variants; however, they are often ineffective against autologous escape mutants [8].
In HIV/HCV-coinfected individuals, nAb dynamics are more complex. A recent study demonstrated reduced levels of anti-HCV-bNAbs in HIV/HCV-coinfected individuals compared to those with HCV monoinfection [9], illustrating the intricate relationship between coinfection and humoral immunity. However, the effects of a HCV cure on the dynamics of anti-HIV-nAbs remain poorly understood. HCV infection may influence the anti-HIV-nAb response, potentially affecting its efficacy. Furthermore, the long-term persistence and functionality of anti-HIV-nAbs after HCV cure are of particular importance, as they may provide insights into immune reconstitution and the potential need for adjunctive therapies in this population. Addressing this knowledge gap is crucial, especially given the high efficacy of DAAs and the increasing number of individuals achieving HCV clearance.
2. Objective
This longitudinal analysis focused on measuring the levels and variability of anti-HIV-nAbs in individuals coinfected with HIV and HCV. Samples were collected at three time points: before initiating HCV treatment and at one and five years following its successful completion.
3. Methods
3.1. Design Study
This retrospective study examined HIV/HCV-coinfected individuals selected from 15 healthcare centers in Spain (see Appendix A). Individuals were drawn from two prospective observational cohorts: the GeSIDA 3603b cohort [10] and the Escorial cohort [11]. All individuals underwent anti-HCV therapy between 2012 and 2016, receiving either IFN-based regimens (peg-IFN-α/ribavirin or peg-IFN-α/ribavirin/DAAs) or IFN-free DAAs. Following therapy, all individuals achieved SVR, defined as undetectable HCV-RNA levels 12–24 weeks post-treatment, depending on the therapeutic protocol. Participants were followed up for one year and five years after completing HCV treatment; the five-year follow-up occurred between January 2019 and May 2021. At baseline and both follow-up time points, all participants had been receiving stable cART for over six months, consistent with prevailing clinical guidelines at study inclusion. All participants maintained an undetectable HIV viral load (<50 RNA copies/mL) throughout this study. This study excluded individuals with acute hepatitis C, hepatic decompensation, hepatocellular carcinoma, or hepatitis B coinfection. Additionally, participants undergoing immunosuppressive therapy at any of the three time points were not included; as such, treatments could influence antibody responses. HCV RNA levels were not analyzed at the one- and five-year follow-ups; however, clinical confirmation ensured the absence of HCV reinfection.
A total of 71 HIV/HCV-coinfected individuals were included in this study at baseline, who were followed-up after one year (n = 71) and five years (n = 68) following HCV end-of-treatment (EOT). Thus, only 3 (4.2%) HIV/HCV-coinfected individuals were lost at the last visit, suggesting that there is no systematic bias due to loss to follow-up. As a control group, we utilized data from a previously published cohort of HIV-monoinfected participants with available neutralization data [12]. For appropriate comparison, we established a control group of 41 HCV-seronegative individuals who were receiving cART and had undetectable HIV viral loads. Crucially, this group was matched at baseline to the HIV/HCV-coinfected group in terms of age, sex distribution, current CD4+ T cell count (cells/mm3), and nadir CD4+ T cell count. This same control group was also used at the two subsequent follow-up points.
The study protocol was approved by the Ethics Committee of the Instituto de Salud Carlos III (#CEI PI 23_2011 and #CEI PI 41_2014) and conducted in accordance with the ethical standards of the 1975 Declaration of Helsinki. Prior to enrollment, all participants provided written informed consent.
3.2. Clinical Data and Sample Collection
Epidemiological and clinical data were extracted from the databases associated with the two prospective cohorts. This information was collected from medical records using an online form, ensuring compliance with all confidentiality requirements, and subsequently monitored for accuracy.
One and five years following HCV treatment, peripheral blood samples were obtained via venipuncture. These samples were collected in ethylenediaminetetraacetic acid (EDTA) tubes and promptly transported to the HIV HGM BioBank. Within 24 h of collection, the samples underwent processing and were stored at −80 °C until further analysis.
3.3. Laboratory Assays
3.3.1. Generation of a Panel of Recombinant Viruses
To generate full-length infectious molecular clones, the env region of HIVNL4–3 was replaced with env sequences from genetically diverse primary isolates, resulting in a panel of recombinant viruses, as previously described [12,13,14]. Virus strains were chosen based on the V3C3 region, spanning residues 300 to 392 of the gp120 glycoprotein (HxB2 numbering). This panel consists of six replication-competent recombinant viruses representing five distinct genetic subtypes: VI191 (clade A), AC10 (B), 92BR025 (C), 92UG024 (D), and CM244 (E). Additionally, the NL4–3 strain (B) was included as a neutralization-sensitive reference. To ensure specificity in immunoglobulin G (IgG) neutralization assays, an amphotropic vesicular stomatitis virus (VSV) env pseudotyped onto an HIV-1 core served as a control virus. Virus strains were selected to represent Tier 2 HIV-1 subtypes commonly circulating in the region, based on prior neutralization profiling studies and availability of well-characterized Env clones.
Virus stocks were produced by transfecting HEK293T cells with DNA constructs using the calcium phosphate transfection method, following the manufacturer’s protocol (Promega, Madison, WI, USA). Viral production was assessed by quantifying p24 capsid protein levels in the supernatant via an antigen capture assay (Innogenetics, Ghent, Belgium).
3.3.2. Purification of IgG
IgG was purified from plasma samples using protein A affinity chromatography (Pierce, Rockford, IL, USA). For IgG extraction, 100 μL of plasma was mixed with 500 μL of sterile phosphate-buffered saline, pH 7.4, and purified using a protein A spin column purification kit. To remove smaller molecules, the purified IgG was subjected to extensive dialysis with 50-kDa-cutoff membranes (Spectra/Por; Spectrum Medical Industries, Laguna Hills, CA, USA). As previously recommended, this molecular weight cutoff eliminates residual efavirenz bound to plasma proteins [15]. The flow-through fraction was further diluted to 0.2 µg/mL for neutralization assays.
3.3.3. HIV Neutralization Assays
The purified IgGs were evaluated in triplicate at 0.2 µg/mL concentration using the virus panel described above. Briefly, recombinant viruses (75 µL) were pre-incubated with 25 µL of plasma-purified IgGs at 0.2 µg/mL, corresponding to 1/200 plasma dilution, for 1 h at 37 °C in 96-well plates. Following this incubation, 5 × 103 TZM.bl cells in 100 µL were added to each well. Neutralization assays were conducted in triplicate. After 72 h of infection, luciferase activity in the cell lysates was measured using a 96-well plate luminometer (Orion; Berthold Technologies, Bad Wildbad, Germany). Luciferase activity from cell lysates infected with non-neutralized viruses was 100%. For each sample, a cumulative neutralization score was determined by averaging the neutralization percentages observed across the six viruses included in the panel, as previously described [12]. This cumulative score represents an integrative measure of the breadth and potency of the neutralizing antibody response. A higher score signifies greater breadth and/or stronger neutralizing activity. Employing a cumulative score addresses variability across different viral strains and facilitates standardized comparison among individuals and across timepoints.
Each sample was assayed in triplicate. To assess intra- and inter-assay variability, key samples underwent three independent repetitions in separate runs. HIV-negative plasma was included as a control to monitor specificity and assay performance. Additionally, negative controls and cell-only background wells were incorporated on each plate. Finally, two blinded investigators independently validated all results to ensure consistency and reproducibility.
4. Statistical Analysis
Statistical analyses were performed using SPSS v25.0 (IBM Corp., Chicago, IL, USA) and Stata v18.0 (STATA Corp., College Station, TX, USA). GraphPad Prism v10.0 was used to generate plots and graphs.
The statistical analysis involved the use of the Wilcoxon test for comparing paired groups and the Kruskal–Wallis (for multiple independent groups) and Mann–Whitney U (for two independent groups) tests. For the categorical variables, data are summarized as counts and percentages. Continuous variables, in contrast, are presented as medians along with their interquartile ranges (IQR), specifically the 25th to 75th percentiles.
To evaluate changes in anti-HIV-nAbs at three different time points, generalized linear mixed models (GLMMs) were applied, utilizing a gamma distribution and log link function. GLM with a family (gamma) and link (log) was used to evaluate differences in anti-HIV-nAbs between HIV-monoinfected and HIV/HCV-coinfected individuals at the baseline and following successful HCV therapy, adjusted by their age, gender, nadir CD4+, and baseline CD4+ counts, which were selected by a stepwise selection method (pin < 0.05 and pout < 0.10). GLMM and GLM analyses were performed using bootstrap replication (n = 500) and seed (1). Furthermore, the impact of the treatment regimen (IFN-based vs. IFN-free) was evaluated using GLMM and GLM analyses.
These analyses yielded arithmetic mean ratios (AMR), odds ratios (ORs), and 95% CI. To address the problem of multiple comparisons and reduce the risk of false positives, p-values were adjusted using the Benjamini–Hochberg procedure for the false discovery rate (FDR), yielding q-values. Statistical significance was defined as a two-tailed p-value below 0.05.
Statistical Power Analysis
Post hoc power analyses were conducted in R v4.4.2 (pwr package) for the main statistical models. For the repeated measures of GLMM assessing the anti-HIV-nAbs response over three time points (baseline, 1 and 5 years) in the HIV/HCV-coinfected group (N = 71), power was calculated to detect a medium-to-large overall effect of time (Cohen’s f = 0.30, ≈d = 0.6 for pairwise), assuming ρ = 0.5 and ε = 0.75 at alpha = 0.05. This analysis yielded 86.6% power, indicating adequate power for the main effect of time. For the GLM comparing the anti-HIV-nAbs response between the HIV/HCV (n = 71) and HIV-monoinfected (n = 41) groups (alpha = 0.05), a power analysis (alpha = 0.05, two-tailed) indicated approximately 84.7% power to detect a medium-to-large effect size (Cohen’s d = 0.6). Both analyses suggest this study was adequately powered to detect medium-to-large effects.
5. Results
5.1. Characteristics of the Study Participants
Table 1 presents the baseline epidemiological and clinical characteristics of the 71 HIV/HCV-coinfected individuals treated for hepatitis C. The median age was 51 years, and the majority were male (81.7%). Most individuals reported a history of intravenous drug use (77.5%) and current smoking (66.2%), while only 4.2% reported a current high-alcohol intake (>50 g/day).

Table 1.
Overview of the initial epidemiological and clinical characteristics of individuals coinfected with HIV and HCV who received hepatitis C treatment.
Regarding the HIV status, 30.4% had a nadir CD4+ count of <200 cells/mm3, and 52.1% had a baseline CD4+ count of >500 cells/mm3. The most common cART regimens consisted of nucleoside reverse transcriptase inhibitors plus integrase inhibitors (NRTI + II; 43.9%), followed by NRTI + non-NRTI (28.8%).
Concerning the HCV status, the median liver stiffness measurement (LSM) was 21.0 kPa, 45.1% of individuals presented an LSM of 25 kPa or higher, and 11.3% were diagnosed with decompensated cirrhosis. Over half of the cohort (57.7%) had received prior HCV therapy. Of those, 63.4% had undergone interferon (IFN)-based therapy, while 36.6% had received IFN-free DAA therapy. HCV genotype 1 (Gt1) was the most prevalent (76.1%), followed by Gt3 (11.3%) and Gt4 (9.9%). The median HCV-RNA viral load was 6.1 log10 IU/mL, with 62.9% of individuals exhibiting viral loads of 850,000 IU/mL or higher. Supplementary Table S1 provides further details on HCV genotypes and specific antiviral therapies.
5.2. Anti-HIV-nAbs Response
HIV-neutralizing antibody responses in HIV/HCV-coinfected individuals remained stable over five years following HCV therapy without significant changes (q-value > 0.05; Figure 1). Longitudinal trajectories for each participant (Supplementary Figure S1) further illustrate this overall stability across the cohort, with only a minority exhibiting noticeable fluctuations. Mean neutralization scores remained stable, with baseline values of 6.1 (95% confidence intervals [95% CI]: 5.4–6.7), 6.2 (95% CI: 5.5–6.8) at one-year post-HCV therapy, and 6.0 (95% CI: 5.3–6.7) at five years post-HCV therapy.
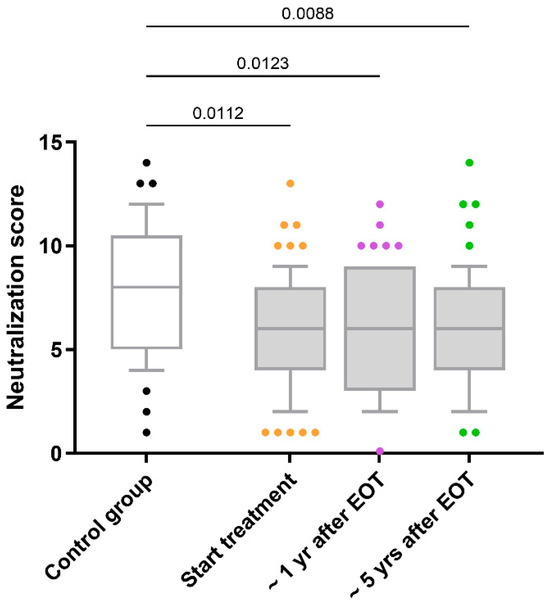
Figure 1.
Comparison of the neutralization scores for antibody responses against HIV in HIV/HCV-coinfected individuals. Abbreviations: EOT = end-of-treatment; Yr = year.
Additionally, HIV/HCV-coinfected individuals consistently exhibited lower neutralization scores than the control group throughout the follow-up period (q-value < 0.05; Figure 1). Both at the baseline and after the successful completion of HCV therapy, adjusted GLM analyses revealed that the differences between HIV-monoinfected and HIV/HCV-coinfected individuals persisted (AMR < 1; q-value < 0.05; Table 2).

Table 2.
Differences in neutralization scores between HIV-monoinfected and HIV/HCV-coinfected individuals at baseline and after successful DAA therapy.
5.3. Impact of Variables Related to HIV/HCV Coinfection on Anti-HIV-nAbs Response
We evaluated the impact of several variables related to HIV/HCV coinfection on the HIV neutralization score. These variables included the treatment regimen (IFN-based or IFN-free), baseline LSM (≤20 kPa or >20 kPa), baseline HCV RNA viral load (≤850,000 IU/mL or HCV-RNA >850,000 IU/mL), baseline HCV genotype (Non-HCV Gt1 or HCV Gt1), CD4 + T-cell categories (≤200 cells/mm3 or >200 cells/mm3), and baseline CD4+ T-cell categories (≤500 cells/mm3 or >500 cells/mm3).
Within the HIV/HCV-coinfected cohort, none of the evaluated variables significantly impacted the HIV neutralization score in the GLMM analyses (q-value > 0.05). However, when stratified by these variables, practically all resulting subgroups of HIV/HCV participants consistently showed systematically lower neutralization values compared to the control group (q-value < 0.05; Figure 2). This trend was confirmed by adjusted GLM analysis (Supplementary Table S2).
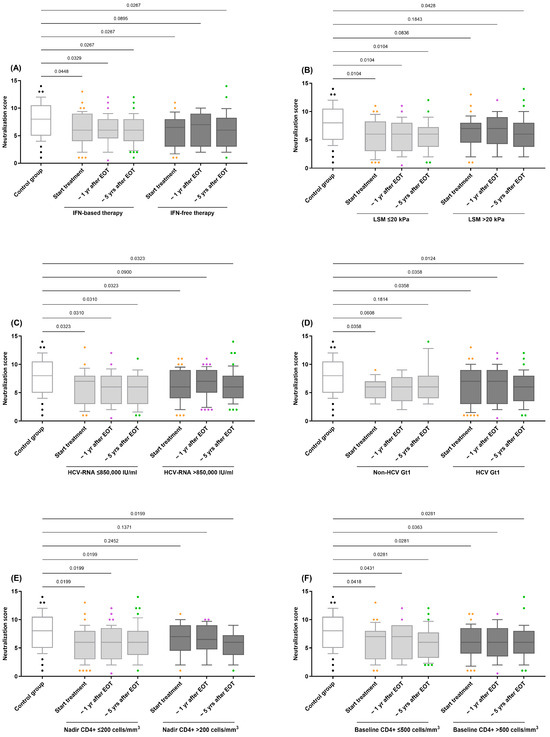
Figure 2.
Comparison of the neutralization scores for antibody responses against HIV in HIV/HCV-coinfected individuals stratified by variables related to HIV/HCV coinfection: (A) HCV therapy (IFN-based therapy vs. IFN-free therapy); (B) LSM (≤20 kPa vs. >20 kPa); (C) HCV-RNA (≤850,000 IU/mL vs. >850,000 IU/mL); (D) HCV Gt1 (Non-HCV Gt1 vs. HCV Gt1); (E) Nadir CD4+ (≤200 cells/mm3 vs. >200 cells/mm3); (F) Baseline CD4+ (≤500 cells/mm3 vs. >500 cells/mm3). Abbreviations: EOT = end-of-treatment; Yr = year; HIV = Human Immunodeficiency Virus; HCV = Hepatitis C Virus; IFN = Interferon; HCV-RNA = Hepatitis C Virus Ribonucleic Acid.
6. Discussion
The long-term dynamics of anti-HIV-nAb titers in HIV/HCV-coinfected individuals following successful HCV treatment are illuminated by this valuable longitudinal study. Our primary finding demonstrates that while HCV therapy effectively eliminates HCV, it does not lead to a significant improvement in anti-HIV-nAb titers in HIV/HCV-coinfected individuals. Notably, these anti-HIV-nAb titers remained consistently lower than those observed in HIV-monoinfected individuals throughout the five-year follow-up period. To the best of our knowledge, this is the first study to evaluate the long-term dynamics of anti-HIV-nAbs after a HCV cure in a population of HIV/HCV-coinfected individuals.
Our findings hold significant implications for HIV vaccine development and broader vaccinology regarding the anti-HIV-nAbs response. The persistent sub-optimal anti-HIV nAb titers in HIV/HCV-coinfected individuals, even post-HCV cure, suggest that chronic HCV coinfection induces immune dysregulation, leaving a lasting “immunological scar” on the B-cell compartment. This scar may lead to reduced responsiveness to standard nAb-eliciting HIV vaccine candidates, potentially requiring more potent or tailored strategies. More broadly, our results highlight that the HCV coinfection history and immune reconstitution profoundly modulate the nAb generation capacity, informing the vaccine design and evaluation in populations with complex immunological backgrounds.
The persistence of anti-HIV-nAbs, despite effective cART, is attributed to continuous antigenic stimulation from HIV reservoirs [16,17]. This is supported by studies showing that even individuals with undetectable viral loads maintain detectable anti-HIV-nAbs over extended periods [18,19]. The presence of these reservoirs likely ensures sustained antibody production, which could explain the observed stability over time. This suggests that in coinfected individuals, a HCV cure does not significantly alter this pre-existing immunological equilibrium. Given that HIV-specific neutralizing antibodies play a key role in limiting viral replication and represent a central focus in vaccine research [20,21], understanding the factors influencing their stability in coinfected populations is crucial for future therapeutic strategies.
Chronic HCV infection is known to induce systemic immune activation and dysregulation, which could theoretically affect humoral immunity [22,23]. Some studies have suggested that a HCV cure may restore immune homeostasis, leading to changes in antibody responses [24,25]. However, our findings align with research showing a minimal impact of HCV cure on immune parameters related to HIV [23,26]. Impaired HIV-specific neutralizing antibody responses can persist even after successful HCV eradication in individuals with HIV/HCV coinfection, suggesting that this may cause long-lasting changes in the humoral immune system, which are not fully reversed by clearing HCV. The persistence of these impairments is likely due to durable alterations in B cell function and antibody production.
The observation that anti-HIV-nAb levels remained stable, without a significant increase, for five years post-HCV cure is particularly noteworthy. This stability occurs despite the known association of chronic HIV/HCV coinfection with ongoing B cell dysfunction, including reduced memory B cell frequencies and an expansion of atypical or exhausted B-cell subsets [1]. Several factors may contribute to this persistent impairment of HIV-specific humoral immunity. Firstly, chronic immune activation and exhaustion, driven by both HIV and HCV, can lead to irreversible alterations in B-cell subsets and their functional capacity [27,28]. Secondly, prolonged exposure to HCV antigens might induce epigenetic modifications or alter B-cell receptor repertoires [29,30]. These changes could create a persistent bias towards HCV-specific responses, thereby diminishing HIV-specific immunity and limiting the production of new HIV-specific antibodies even after HCV eradication. Such immunological imprinting may not fully revert after HCV clearance, highlighting the need for targeted interventions to restore effective HIV-specific antibody responses. Alternatively, the absence of an increase in anti-HIV antibody levels could be due to feedback inhibition; pre-existing low levels of anti-HIV antibodies might bind to HIV antigens, thus preventing the stimulation required for further antibody production.
The persistence of diminished anti-HIV-nAb responses in HIV/HCV-coinfected individuals, even after achieving SVR, underscores the complex interplay between these two viral infections and their impact on humoral immunity. This observation is consistent with prior research that has highlighted the significant immunomodulatory effects of chronic HCV infection, which can result in persistent immune dysregulation even after viral clearance [31]. It is plausible, however, that the observed stability of the anti-HIV nAbs could reflect the effectiveness of cART in preserving the overall immune function and preventing the immunological decline in these HIV/HCV-coinfected individuals.
Our study also reinforces the notion that the immune dysregulation observed in HIV/HCV-coinfection is more profound than in HIV monoinfection. The consistent observation of lower anti-HIV-nAb titers in the HIV/HCV-coinfected cohort compared to the HIV-monoinfected control group, even after adjusting for potential confounders, highlights the additive impact of HCV on HIV-related immune dysfunction. This persistent difference underscores the importance of considering the unique immunological challenges faced by HIV/HCV-coinfected individuals.
Our study provides important clinical implications. Although the immediate benefits of HCV cure in HIV/HCV-coinfection are well established, including the reduced risk of liver complications and improved quality of life [32], our study suggests that the humoral immune compartment against HIV remains suboptimal in the long term. The persistent deficit in anti-HIV-nAb responses in HIV/HCV-coinfected individuals may have an impact on HIV disease progression and the potential need for adjunctive immunomodulatory therapies. For instance, strategies aimed at enhancing B-cell function or boosting HIV-specific antibody responses, such as therapeutic vaccination or broadly neutralizing antibody administration, might be particularly beneficial in this population. Furthermore, the long-term stability of nAb titers suggests that these individuals may not experience spontaneous improvements in HIV-specific humoral immunity following HCV cure, emphasizing the need for ongoing monitoring and targeted interventions.
7. Strengths and Limitations of This Study
This study has several strengths, including its long follow-up period (five years post-HCV treatment) and the use of a well-defined cohort of HIV/HCV-coinfected individuals. The methodology used to assess the neutralization activity across multiple HIV subtypes provides a robust measure of anti-HIV-nAb function.
However, several limitations must be considered when interpreting our findings. Firstly, the retrospective nature of the study design may introduce potential biases, although we employed strict selection criteria and advanced statistical methods to mitigate these effects. Secondly, the relatively small sample size may limit the study’s power to detect subtle differences in anti-HIV-nAb dynamics. Thirdly, data on the duration of antiretroviral treatment were unavailable for approximately half the patients. Consequently, this variable was excluded from the statistical analysis. While the ART duration might reflect the baseline immune status, we included the nadir CD4+ cell count and CD4+/mm3 values at the baseline as adjustment covariates in the GLM analysis. Moreover, given that all HIV/HCV-coinfected individuals have been on cART for over 6 months, and we have previously shown no correlation between the cART duration and neutralization score after this period [33], the time on cART is expected to have little impact on our study. Fourthly, HCV RNA data were not available during the one- and five-year follow-ups after HCV treatment to confirm the absence of HCV reinfection. However, the absence of HCV reinfection was clinically confirmed during the study period. Fifthly, while we evaluated the breadth and titers of anti-HIV-nAbs, key functional properties like affinity and avidity were not assessed. Therefore, future research involving detailed B-cell phenotyping and functional assays is crucial for a deeper understanding of these antibody responses.
8. Conclusions
In conclusion, this study demonstrates that achieving a HCV cure in HIV/HCV-coinfected individuals did not normalize anti-HIV-nAb responses, which remained consistently lower than those in HIV-monoinfected individuals over five years. This suggests that HCV eradication, while beneficial, does not fully restore humoral immunity against HIV. Further research is needed to explore mechanisms underlying this persistent deficit and potential adjunctive therapies to enhance HIV-specific immune responses in this population.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vaccines13050539/s1, Figure S1: Longitudinal trajectories of neutralization scores for individual participants; Table S1: HCV infecting genotype and antiviral therapies for HIV and HCV in study participants; Table S2: Comparison of Neutralization Scores by HIV Group and HCV Treatment (IFN-based vs. IFN-free) in HIV/HCV-Coinfected Individuals.
Author Contributions
Conceptualization: S.R. and E.Y.; Data curation: C.D., V.H., J.B., J.G.-G., D.S.-C., A.R.-P., F.G., and R.A.-S.; Formal analysis: D.S.-C., S.R., and V.S.-M.; Funding acquisition: S.R. and E.Y.; Investigation and methodology: D.S.-C., V.S.-M., R.A.-S., I.M., and E.Y.; Project Administration: S.R. and E.Y.; Resources: D.S.-C.: V.S.-M., A.R.-P., R.A.-S., and E.Y.; Supervision and validation: S.R. and E.Y.; Visualization: S.R. and E.Y.; Writing—original draft preparation: D.S.-C. and S.R.; Writing—Review and Editing: I.M. and E.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants from Instituto de Salud Carlos III (ISCII; grant numbers PI20/00474 to J.B., PI20/00507 to J.G.-G., PI19CIII/00009 to I.M., PI23CIII/00018 to D.S.-C. and I.M., PI20CIII/00039 to E.Y., and PI20CIII/00004 to S.R.). This study was also funded by the CIBER—Consorcio Centro de Investigación Biomédica en Red—(CB 2021), Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación and Unión Europea—NextGenerationEU (CB21/13/00044 and CB21/13/00039). D.S.-C. is a ’Miguel Servet’ researcher from ISCIII (grant number CP23CIII/00004).
Institutional Review Board Statement
This study was approved by the Ethics Committee of the Instituto de Salud Carlos III (CEI PI 23_2011 and CEI PI 41_2014). All individuals provided written informed consent before participating in this study and joining the cohorts.
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
We acknowledge the use of the AI tool ChatGPT4 by OpenAI for language editing, grammar correction, and enhancing readability. The authors supervised AI assistance and did not influence the manuscript’s intellectual content. We want to acknowledge the individuals participating in this study, collaborating Centers, and the Spanish HIV HGM BioBank for their help and collaboration. All the participants in this study (medical personnel, nursing staff, and data managers) who have participated in the project are listed in the Appendix A.
Conflicts of Interest
The authors declare no competing interests.
Abbreviations
| 95% CI | 95% confidence interval |
| AMR | arithmetic mean ratio |
| bNAbs | broadly neutralizing antibodies |
| cART | combination antiretroviral therapy |
| DAA | direct-acting antiviral |
| EDTA | ethylenediaminetetraacetic acid |
| Env | envelope glycoprotein |
| FDR | false discovery rate |
| GLM | generalized linear model |
| GLMM | generalized linear mixed model |
| Gt | HCV genotype |
| HCV | hepatitis C virus |
| HIV | human immunodeficiency virus |
| IFN | Interferon |
| IgG | immunoglobulin G |
| II | integrase inhibitors |
| LSM | liver stiffness measurement |
| MDSC | myeloid-derived suppressor cells |
| nAbs | neutralizing antibodies |
| NRTI | nucleoside reverse transcriptase inhibitor |
| OR | odds ratio |
| PEG-IFNα | pegylated interferon alpha |
| PLWH | people living with HIV |
| RNA | ribonucleic acid |
| SVR | sustained virologic response |
| SPSS | statistical package for social sciences |
| Tregs | regulatory T-cells |
| VSV | vesicular stomatitis virus |
Appendix A
Appendix A.1. The GESIDA 3603b Cohort Study Group
Hospital General Universitario Gregorio Marañón, Madrid: A Carrero, p Miralles, JC López, F Parras, B Padilla, T Aldamiz-Echevarría, F Tejerina, C Díez, L Pérez-Latorre, C Fanciulli, I Gutiérrez, M Ramírez, S Carretero, JM Bellón, J Bermejo, and J Berenguer.
Hospital Universitario La Paz, Madrid: V Hontañón, JR Arribas, ML Montes, I Bernardino, JF Pascual, F Zamora, JM Peña, F Arnalich, M Díaz, and J González-García.
Hospital de la Santa Creu i Sant Pau, Barcelona: p Domingo and JM Guardiola.
Hospital Universitari Vall d’Hebron, Barcelona: E Van den Eynde, M Pérez, E Ribera, and M Crespo.
Hospital Universitario Ramón y Cajal, Madrid: JL Casado, F Dronda, A Moreno, MJ Pérez-Elías, MA Sanfrutos, S Moreno, and C Quereda.
Hospital Universitario Príncipe de Asturias, Alcalá de Henares: A Arranz, E Casas, J de Miguel, S Schroeder, and J Sanz.
Hospital Universitario de La Princesa, Madrid: J Sanz and I Santos.
Hospital Donostia, San Sebastián: MJ Bustinduy, JA Iribarren, F Rodríguez-Arrondo, and MA Von-Wichmann.
Hospital Clínico San Carlos, Madrid: J Vergas and MJ Téllez.
Hospital Universitario San Cecilio, Granada: D. Vinuesa, L. Muñoz, and J. Hernández-Quero.
Hospital Clínico Universitario, Valencia: A Ferrer and MJ Galindo.
Hospital General Universitario, Valencia: L Ortiz and E Ortega.
Hospital Universitari La Fe, Valencia: M Montero, M Blanes, S Cuellar, J Lacruz, M Salavert, and J López-Aldeguer.
Hospital Universitario de Getafe, Getafe: G Pérez and G Gaspar.
Fundación SEIMC-GESIDA, Madrid: M Yllescas, p Crespo, E Aznar, and H Esteban
Appendix A.2. The ESCORIAL Study Group
Hospital General Universitario Gregorio Marañón (Madrid, Spain): Cristina Díez, Luis Ibáñez, Leire Pérez-Latorre, Diego Rincón, Teresa Aldámiz-Echevarría, Vega Catalina, Pilar Miralles, Teresa Aldámiz-Echevarría, Francisco Tejerina, María C Gómez-Rico, Esther Alonso, José M Bellón, Rafael Bañares, and Juan Berenguer.
Hospital Universitario La Paz/IdiPAZ (Madrid, Spain): José Arribas, José I Bernardino, Ana Delgado, Carmen Busca, Javier García-Samaniego, Víctor Hontañón, Luz Martín-Carbonero, Rafael Micán, María L Montes-Ramírez, Victoria Moreno, Antonio Olveira, Ignacio Pérez-Valero, Eulalia valencia, and Juan González-García.
Hospital Universitario Puerta de Hierro (Madrid, Spain): Elba Llop and José Luis Calleja.
Hospital Universitario Ramón y Cajal (Madrid, Spain): Javier Martínez and Agustín Albillos.
Fundación SEIMC/GeSIDA (Madrid, Spain): Marta de Miguel, María Yllescas, and Herminia Esteban.
References
- Gobran, S.T.; Ancuta, P.; Shoukry, N.H. A Tale of Two Viruses: Immunological Insights Into HCV/HIV Coinfection. Front. Immunol. 2021, 12, 726419. [Google Scholar] [CrossRef] [PubMed]
- Platt, L.; Easterbrook, P.; Gower, E.; McDonald, B.; Sabin, K.; McGowan, C.; Yanny, I.; Razavi, H.; Vickerman, P. Prevalence and burden of HCV co-infection in people living with HIV: A global systematic review and meta-analysis. Lancet Infect. Dis. 2016, 16, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Martinello, M.; Solomon, S.S.; Terrault, N.A.; Dore, G.J. Hepatitis C. Lancet 2023, 402, 1085–1096. [Google Scholar] [CrossRef]
- Lin, W.; Wang, X.; Zhang, J.; Wen, C.; Kang, W.; Mao, L.; Yang, J.; Dou, Y.; Shi, L.; Dang, B.; et al. A simple, feasible, efficient and safe treatment strategy of sofosbuvir/velpatasvir for chronic HCV/HIV-1 coinfected patients regardless of HCV genotypes: A multicenter, open-label study in China. Lancet Reg. Health West. Pac. 2023, 36, 100749. [Google Scholar] [CrossRef]
- Caraballo Cortes, K.; Osuch, S.; Perlejewski, K.; Radkowski, M.; Janiak, M.; Berak, H.; Rauch, A.; Fehr, J.S.; Hoffmann, M.; Gunthard, H.F.; et al. T-Cell Exhaustion in HIV-1/Hepatitis C Virus Coinfection Is Reduced After Successful Treatment of Chronic Hepatitis C. Open Forum Infect. Dis. 2023, 10, ofad514. [Google Scholar] [CrossRef]
- Chiodi, F.; Scarlatti, G. Editorial: HIV-Induced Damage of B Cells and Production of HIV Neutralizing Antibodies. Front. Immunol. 2018, 9, 297. [Google Scholar] [CrossRef]
- Laskus, T.; Kibler, K.V.; Chmielewski, M.; Wilkinson, J.; Adair, D.; Horban, A.; Stanczak, G.; Radkowski, M. Effect of hepatitis C infection on HIV-induced apoptosis. PLoS ONE 2013, 8, e75921. [Google Scholar] [CrossRef]
- Mouquet, H. Humoral immunity in HIV-1 post-treatment controllers. Curr. Opin. HIV AIDS 2025, 20, 80–85. [Google Scholar] [CrossRef]
- Sepulveda-Crespo, D.; Yelamos, M.B.; Diez, C.; Gomez, J.; Hontanon, V.; Torresano-Felipe, F.; Berenguer, J.; Gonzalez-Garcia, J.; Ibanez-Samaniego, L.; Llop, E.; et al. Negative impact of HIV infection on broad-spectrum anti-HCV neutralizing antibody titers in HCV-infected patients with advanced HCV-related cirrhosis. Biomed. Pharmacother. 2022, 150, 113024. [Google Scholar] [CrossRef]
- Garcia-Broncano, P.; Medrano, L.M.; Berenguer, J.; Brochado-Kith, O.; Gonzalez-Garcia, J.; Jimenez-Sousa, M.A.; Quereda, C.; Sanz, J.; Tellez, M.J.; Diaz, L.; et al. Mild profile improvement of immune biomarkers in HIV/HCV-coinfected patients who removed hepatitis C after HCV treatment: A prospective study. J. Infect. 2020, 80, 99–110. [Google Scholar] [CrossRef]
- Diez, C.; Berenguer, J.; Ibanez-Samaniego, L.; Llop, E.; Perez-Latorre, L.; Catalina, M.V.; Hontanon, V.; Jimenez-Sousa, M.A.; Aldamiz-Echevarria, T.; Martinez, J.; et al. Persistence of Clinically Significant Portal Hypertension After Eradication of Hepatitis C Virus in Patients With Advanced Cirrhosis. Clin. Infect. Dis. 2020, 71, 2726–2729. [Google Scholar] [CrossRef] [PubMed]
- Medina-Ramirez, M.; Sanchez-Merino, V.; Sanchez-Palomino, S.; Merino-Mansilla, A.; Ferreira, C.B.; Perez, I.; Gonzalez, N.; Alvarez, A.; Alcocer-Gonzalez, J.M.; Garcia, F.; et al. Broadly cross-neutralizing antibodies in HIV-1 patients with undetectable viremia. J. Virol. 2011, 85, 5804–5813. [Google Scholar] [CrossRef]
- Ferreira, C.B.; Merino-Mansilla, A.; Llano, A.; Perez, I.; Crespo, I.; Llinas, L.; Garcia, F.; Gatell, J.M.; Yuste, E.; Sanchez-Merino, V. Evolution of broadly cross-reactive HIV-1-neutralizing activity: Therapy-associated decline, positive association with detectable viremia, and partial restoration of B-cell subpopulations. J. Virol. 2013, 87, 12227–12236. [Google Scholar] [CrossRef]
- Sanchez-Merino, V.; Fabra-Garcia, A.; Gonzalez, N.; Nicolas, D.; Merino-Mansilla, A.; Manzardo, C.; Ambrosioni, J.; Schultz, A.; Meyerhans, A.; Mascola, J.R.; et al. Detection of Broadly Neutralizing Activity within the First Months of HIV-1 Infection. J. Virol. 2016, 90, 5231–5245. [Google Scholar] [CrossRef]
- Burrer, R.; Salmon-Ceron, D.; Richert, S.; Pancino, G.; Spiridon, G.; Haessig, S.; Roques, V.; Barre-Sinoussi, F.; Aubertin, A.M.; Moog, C. Immunoglobulin G (IgG) and IgA, but also nonantibody factors, account for in vitro neutralization of human immunodeficiency virus (HIV) type 1 primary isolates by serum and plasma of HIV-infected patients. J. Virol. 2001, 75, 5421–5424. [Google Scholar] [CrossRef]
- Leite, T.F.; Delatorre, E.; Cortes, F.H.; Ferreira, A.C.G.; Cardoso, S.W.; Grinsztejn, B.; de Andrade, M.M.; Veloso, V.G.; Morgado, M.G.; Guimaraes, M.L. Reduction of HIV-1 Reservoir Size and Diversity After 1 Year of cART Among Brazilian Individuals Starting Treatment During Early Stages of Acute Infection. Front. Microbiol. 2019, 10, 145. [Google Scholar] [CrossRef]
- Hong, F.F.; Mellors, J.W. Changes in HIV reservoirs during long-term antiretroviral therapy. Curr. Opin. HIV AIDS 2015, 10, 43–48. [Google Scholar] [CrossRef]
- Bitnun, A.; Ransy, D.G.; Brophy, J.; Kakkar, F.; Hawkes, M.; Samson, L.; Annabi, B.; Pagliuzza, A.; Morand, J.A.; Sauve, L.; et al. Clinical Correlates of Human Immunodeficiency Virus-1 (HIV-1) DNA and Inducible HIV-1 RNA Reservoirs in Peripheral Blood in Children With Perinatally Acquired HIV-1 Infection With Sustained Virologic Suppression for at Least 5 Years. Clin. Infect. Dis. 2020, 70, 859–866. [Google Scholar] [CrossRef]
- Sloan, D.D.; Lam, C.Y.; Irrinki, A.; Liu, L.; Tsai, A.; Pace, C.S.; Kaur, J.; Murry, J.P.; Balakrishnan, M.; Moore, P.A.; et al. Targeting HIV Reservoir in Infected CD4 T Cells by Dual-Affinity Re-targeting Molecules (DARTs) that Bind HIV Envelope and Recruit Cytotoxic T Cells. PLoS Pathog. 2015, 11, e1005233. [Google Scholar] [CrossRef]
- Schommers, P.; Kim, D.S.; Schlotz, M.; Kreer, C.; Eggeling, R.; Hake, A.; Stecher, M.; Park, J.; Radford, C.E.; Dingens, A.S.; et al. Dynamics and durability of HIV-1 neutralization are determined by viral replication. Nat. Med. 2023, 29, 2763–2774. [Google Scholar] [CrossRef]
- Lin, L.Y.; Gantner, P.; Li, S.; Su, B.; Moog, C. Unpredicted Protective Function of Fc-Mediated Inhibitory Antibodies for HIV and SARS-CoV-2 Vaccines. J. Infect. Dis. 2025, 231, e1–e9. [Google Scholar] [CrossRef] [PubMed]
- Bala, S.; Tilahun, Y.; Taha, O.; Alao, H.; Kodys, K.; Catalano, D.; Szabo, G. Increased microRNA-155 expression in the serum and peripheral monocytes in chronic HCV infection. J. Transl. Med. 2012, 10, 151. [Google Scholar] [CrossRef] [PubMed]
- Kuniholm, M.H.; O’Brien, T.R.; Prokunina-Olsson, L.; Augenbraun, M.; Plankey, M.; Karim, R.; Sarkar, M.; French, A.L.; Pierce, C.; Strickler, H.D.; et al. Association of Hepatitis C Virus Infection With CD4/CD8 Ratio in HIV-Positive Women. J. Acquir. Immune Defic. Syndr. 2016, 72, 162–170. [Google Scholar] [CrossRef]
- Shrivastava, S.; Bhatta, M.; Ward, H.; Romani, S.; Lee, R.; Rosenthal, E.; Osinusi, A.; Kohli, A.; Masur, H.; Kottilil, S.; et al. Multitarget Direct-Acting Antiviral Therapy Is Associated With Superior Immunologic Recovery in Patients Coinfected With Human Immunodeficiency Virus and Hepatitis C Virus. Hepatol. Commun. 2018, 2, 1451–1466. [Google Scholar] [CrossRef]
- Soares Correia, R.; Franca, M. An Immunological Non-responder Human Immunodeficiency Virus/Hepatitis C Virus Coinfected Patient: Considerations About a Clinical Case. Cureus 2023, 15, e37063. [Google Scholar] [CrossRef]
- Zhao, J.; Dang, X.; Zhang, P.; Nguyen, L.N.; Cao, D.; Wang, L.; Wu, X.; Morrison, Z.D.; Zhang, Y.; Jia, Z.; et al. Insufficiency of DNA repair enzyme ATM promotes naive CD4 T-cell loss in chronic hepatitis C virus infection. Cell Discov. 2018, 4, 16. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, S.; Sun, J.; Xu, J.; Zhang, X. Irreversible phenotypic perturbation and functional impairment of B cells during HIV-1 infection. Front. Med. 2017, 11, 536–547. [Google Scholar] [CrossRef]
- Moir, S.; Fauci, A.S. B cells in HIV infection and disease. Nat. Rev. Immunol. 2009, 9, 235–245. [Google Scholar] [CrossRef]
- Hamdane, N.; Jühling, F.; Crouchet, E.; El Saghire, H.; Thumann, C.; Oudot, M.A.; Bandiera, S.; Saviano, A.; Ponsolles, C.; Roca Suarez, A.A.; et al. HCV-Induced Epigenetic Changes Associated With Liver Cancer Risk Persist After Sustained Virologic Response. Gastroenterology 2019, 156, 2313–2329.e2317. [Google Scholar] [CrossRef]
- Underwood, A.P.; Gupta, M.; Wu, B.-R.; Eltahla, A.A.; Boo, I.; Wang, J.J.; Agapiou, D.; Abayasingam, A.; Reynaldi, A.; Keoshkerian, E.; et al. B-cell characteristics define HCV reinfection outcome. J. Hepatol. 2024, 81, 415–428. [Google Scholar] [CrossRef]
- Moretti, S.; Mancini, F.; Borsetti, A. Long term immunological perturbations post DAA therapy in chronic HCV/HIV co-infected patients. Biocell 2022, 46, 2695–2699. [Google Scholar] [CrossRef]
- Berenguer, J.; Alvarez-Pellicer, J.; Martin, P.M.; Lopez-Aldeguer, J.; Von-Wichmann, M.A.; Quereda, C.; Mallolas, J.; Sanz, J.; Tural, C.; Bellon, J.M.; et al. Sustained virological response to interferon plus ribavirin reduces liver-related complications and mortality in patients coinfected with human immunodeficiency virus and hepatitis C virus. Hepatology 2009, 50, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Merino, V.; Martin-Serrano, M.; Beltran, M.; Lazaro-Martin, B.; Cervantes, E.; Oltra, M.; Sainz, T.; Garcia, F.; Navarro, M.L.; Yuste, E. The Association of HIV-1 Neutralization in Aviremic Children and Adults with Time to ART Initiation and CD4+/CD8+ Ratios. Vaccines 2023, 12, 8. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).