Abstract
Campylobacter jejuni is the leading bacterial cause of human enteritis in developed countries. Chicken is the major animal reservoir of C. jejuni and a powerful infection model for human campylobacteriosis. No commercial vaccine against C. jejuni is available to date. The high affinity iron acquisition mediated through enterobactin (Ent), a small siderophore, plays a critical role in the colonization of C. jejuni in the intestine. Recently, an innovative Ent conjugate vaccine has been demonstrated to induce high-level of Ent-specific antibodies in rabbits; the Ent-specific antibodies displayed potent binding ability to Ent and inhibited Ent-dependent growth of C. jejuni. In this study, using specific-pathogen-free (SPF) chickens, we performed three trials to evaluate the immunogenicity of the Ent conjugate vaccine and its efficacy to control C. jejuni colonization in the intestine. The purified Ent was conjugated to the carrier keyhole limpet hemocyanin (KLH). Intramuscular immunization of chickens with the Ent–KLH conjugate for up to three times did not affect the body weight gain, the development of major immune organs and the gut microbiota. In the first two trials, immunizations of chickens with different regimens (two or three times of vaccination) consistently induced strong Ent-specific immune response when compared to control group. Consistent with the high-level of systemic anti-Ent IgG, C. jejuni colonization was significantly reduced by 3–4 log10 units in the cecum in two independent vaccination trials. The third trial demonstrated that single Ent–KLH vaccination is sufficient to elicit high level of systemic Ent-specific antibodies, which could persist for up to eight weeks in chickens. Taken together, the Ent–KLH conjugate vaccine could induce high-level of Ent-specific antibodies in chickens and confer host protection against C. jejuni colonization, which provides a novel strategy for Campylobacter control in poultry and humans.
1. Introduction
Campylobacter jejuni, one of the most important food-borne pathogens, is considered to be the most common bacterial cause of human gastroenteritis worldwide [1]. Additionally, the chronic sequelae of C. jejuni infection can lead to human autoimmune diseases such as Guillain–Barré syndrome and reactive arthritis [2]. Although these diseases are generally mild, they can be fatal among young children, elderly and immunosuppressed individuals [3]. Poultry is considered to be the major reservoir of C. jejuni. Consumption of raw or uncooked poultry is regarded as the major risk factor for human campylobacteriosis [4]. It was reported that poultry-related products cause about 60–80% of the global campylobacteriosis cases [3]. Colonization of chicken by C. jejuni can reach 106–1010 CFU/g in the intestine [5]. To effectively control the rate of incidence of human infections, it is important to reduce the colonization level of C. jejuni in poultry [6,7]. However, due to complex interaction between C. jejuni and its host, limited progress has been made in the development of effective intervention strategies against C. jejuni in both poultry and humans [5,8].
Antibiotics are required for treating Campylobacter infections in humans [3]. Antimicrobial use in the poultry sector has usually followed the approach of using broad spectrum antibiotics (e.g., the antibiotics against Gram-negative bacteria to treat infections caused by avian pathogenic Escherichia coli) [9]. This has led to alarming increase of antimicrobial resistance (AMR) in both pathogenic and commensal bacteria present in the gut [10,11]. An example of this is the zoonotic pathogen Campylobacter spp. [12,13]. Thus, due to worldwide trend to limit antibiotic use in animal production, the non-antibiotic approaches, such as vaccination, are extremely important to prevent the colonization of C. jejuni in poultry, consequently reducing the rate of incidence of human infections [14,15]. Multiple vaccine development strategies including whole-cell killed vaccines, subunit vaccines and delivery vector-based vaccines have been investigated in poultry [16]. However, to date, no vaccine has been approved globally to prevent C. jejuni infections [3].
Iron acquisition plays an important role in C. jejuni pathogenesis [17]. In particular, the enterobactin (Ent)-mediated high-affinity iron uptake system is considered to be essential for in vivo survival and colonization of C. jejuni, which has been demonstrated in chicken, an ideal infection model to study the colonization and immunogenicity of C. jejuni in the host [18,19,20]. Notably, inactivation of Fe-Ent receptors CfrA or CfrB significantly diminished and even abolished C. jejuni colonization in the intestine [18,19]. However, the vaccination strategies targeting the Fe-Ent receptor failed to confer protection against C. jejuni colonization in the host using chicken model [21,22]. Recently, an innovative Ent conjugate vaccine was developed; such vaccine could induce production of Ent-specific antibodies that showed inhibitory effect on C. jejuni growth in vitro [23]. Specifically, the Ent conjugate vaccine could trigger high level of Ent-specific antibodies in rabbits, which displayed similar bacteriostatic feature as lipocalins, the host innate immune effectors with potent Ent-binding ability [23]. Moreover, Wang et al. [23,24] demonstrated the Ent-specific antibodies significantly inhibited Ent-dependent growth of Campylobacter spp., E. coli and Salmonella enterica. However, to date, no information exists concerning protective efficacy of this novel Ent conjugate vaccine for Campylobacter control using appropriate animal model systems.
Here, we used chicken, a powerful infection model of C. jejuni, to examine the immune response and protective efficacy of the Ent conjugate vaccine against C. jejuni colonization. Specifically, Ent as a hapten was conjugated with keyhole limpet hemocyanin (KLH), a carrier protein, to prepare the Ent–KLH conjugate vaccine. Using specific-pathogen-free (SPF) chickens, we performed three trials to evaluate the immunogenicity of the Ent–KLH vaccine and its efficacy to control C. jejuni colonization in the intestine. The findings from the challenge trials with different vaccination regimens provided compelling evidence that the Ent–KLH conjugate vaccine could significantly reduce C. jejuni colonization in the chicken intestine, consistent with the drastically induced high level of Ent-specific antibodies in the chickens upon vaccination.
2. Materials and Methods
2.1. Ethics Statement
All chicken experiments were conducted in accordance with the guidelines approved by the Institutional Animal Care and Use Committee at Institute of Animal Husbandry and Veterinary Medicine, Beijing Academy of Agriculture and Forestry Sciences.
2.2. Bacterial Strain and Culture Conditions
Campylobacter jejuni NCTC 11168 was routinely grown in Mueller–Hinton (MH) broth (Difco, Sparks, MD, USA) or on agar at 42 °C in a microaerophilic incubator (5% O2, 10% CO2, 85% N2).
2.3. Enterobactin Purification and Conjugation
Enterobactin purification and conjugation procedures were performed as described previously [23]. Briefly, Ent was purified from the supernatant of an Ent transport mutant E.scherichia coli AN102. The purified Ent was dissolved in dimethylformamide (DMF) and coupled with KLH and bovine serum albumin (BSA) (Thermo Fisher Scientific, Waltham, MA, USA), respectively. Each batch of Ent–KLH and Ent–BSA conjugates was subjected to SDS-PAGE analysis to validate conjugation as described previously [23]. Finally, the purified Ent–KLH and Ent–BSA conjugates were adjusted to a concentration of 1 mg/mL in sodium phosphate buffer (PBS, 0.01 M, pH 7.2).
2.4. Enterobactin-Keyhole Limpet Hemocyanin (Ent–KLH) Conjugate Vaccine Preparation
The purified Ent–KLH conjugate (1 mg/mL) was emulsified with MONTANIDE™ RANGE (Seppic, Paris, France) in a volume ratio of 3:7 following the manufacturer’s instructions to prepare a water-in-oil emulsion for immunization in chicken.
2.5. Vaccination Procedures
All immunization experiments were performed using one-week-old SPF White Leghorn chickens (Beijing Boehringer Ingelheim Vital Biotechnology Co, Ltd., Beijing, China). All the chickens were determined to be negative for Campylobacter by culturing cloacal swabs prior to use, as described previously [18]. For each trial, the chickens were randomly divided into two groups in equal number. Each group was individually housed and fed ad libitum. Each chicken in the treatment group was immunized with 100 μg of Ent–KLH conjugate vaccine via intramuscular injection (inner thigh) while the chickens in the control group were immunized with PBS solution (0.01 M, pH 7.2) emulsified with the same adjuvant.
In Trial A, 50 SPF chickens (25 chickens per group) were used to evaluate the effects of immunization of chickens with the Ent–KLH vaccine for three times on growth performance, Ent-specific immune response and protection against C. jejuni colonization (Figure 1A). Chickens received three immunizations at age of 7, 21 and 35 days, respectively. Blood samples were collected 14 days after each immunization and the sera were separated for detection of Ent-specific antibody. Each chicken was weighted weekly. Nine chickens of each group were euthanized at 49 days old; major immune organs (bursa, spleen and thymus) and cecal contents were collected from each chicken as well. The development of each immune organ was determined according to the percentage of each immune organs (bursa, spleen or thymus) weight to its body weight at 49 days old. Cecal content from each euthanized chicken was immediately frozen in liquid nitrogen for microbiota analysis described below. The remaining 16 chickens in each group were orally challenged with C. jejuni (104 CFU per chicken) at age of 49 days old. Cloacal swabs were collected from each chicken on Days 1, 3, 5, 7 and 9 post inoculation. On Day 9 post inoculation, all chickens were euthanized and cecal contents were collected. Both cloacal swabs and cecal samples were subjected to 10-fold serial dilutions and plated on MH agar plates containing Campylobacter-specific selective supplements (SR117E, Oxoid, Bashingstoke, Hampshire, England) for enumeration of C. jejuni as described previously [18].
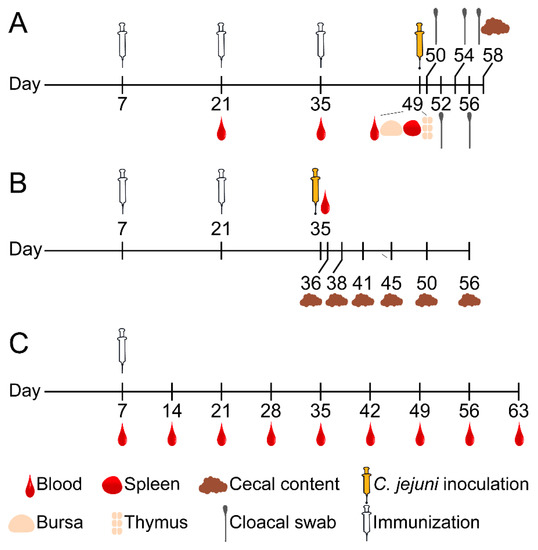
Figure 1.
Diagram of three chicken immunization trials. For each trial (20–30 SPF chickens per group), each chicken in the treatment group was immunized with 100 μg of Ent–KLH conjugate emulsified with MONTANIDE™ RANGE adjuvant (Seppic, Paris) via intramuscular injection (inner thigh) while the chicken in control group was immunized with PBS solution (0.01 M, pH 7.2) emulsified with the same adjuvant. (A) Chickens (25 birds per group) received three immunizations at age of 7, 21 and 35 days, respectively. Blood samples were collected 14 days after each of immunizations. Each chicken was weighed weekly. Nine chickens of each group were euthanized at 49 days old; major immune organs (bursa, spleen and thymus) and cecal contents were collected from each chicken as well. The remaining 16 chickens of each group were orally challenged with C. jejuni (104 CFU per chicken) two weeks after the last immunization. Cloacal swabs were collected from each chicken every 1–2 days post inoculation and cecal contents were also collected upon termination for CFU enumeration. (B) Chickens (30 bird per group) received two immunizations at age of 7 and 21 days, respectively. Blood samples were collected 14 days after the second immunization. All chickens were orally challenged with C. jejuni (104 CFU per chicken) two weeks after the second immunization. Five chickens in each group were euthanized at indicated age post challenge and cecal contents were collected from each chicken for CFU enumeration. (C) Chickens (20 birds per group) only received single immunization at age of one week old. Blood samples were collected immediately prior to immunization and weekly after the immunization until the chickens were euthanized at age of 63 days.
In Trial B, 60 SPF chickens (30 chickens per group) were used to determine immune response and protective efficacy of Ent–KLH conjugate vaccine for reducing C. jejuni colonization in chicken (Figure 1B). In this trial, chickens only received two immunizations at age of 7 and 21 days, respectively, followed by C. jejuni challenge. Blood samples were collected from all chickens 14 days after the second immunization and immediately prior to challenge for assessing Ent-specific antibody. All the chickens were orally challenged with C. jejuni (104 CFU per chicken) at age of 35 days old. Subsequently, five chickens in each group were euthanized on Days 1, 3, 6, 10, 15 and 21 post inoculation, respectively, for collecting cecal contents. The cecal contents were used for CFU enumeration of C. jejuni as described above.
In Trial C, 40 SPF chickens (20 chickens per group) were used to assess the persistence of Ent-specific antibodies upon single vaccination of chickens with the Ent–KLH conjugate vaccine (Figure 1C). Chickens only received single immunization at age of one week old. Blood samples were collected immediately prior to immunization and weekly after the immunization until the chickens were euthanized at age of 63 days. All the collected sera were subjected to ELISA analysis for detection of Ent-specific antibody.
2.6. Analysis of Gut Microbiota
Total DNA was extracted from each cecal sample using a Monarch® Genomic DNA Purification Kit (NEB, Ipswich, MA, USA) in accordance with the manufacturer’s protocols. The DNA extracts were subjected to microbial community analysis using multiplex tag-encoded 16S rRNA gene (rDNA) amplicon sequencing, which was performed by Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China) using Illumina Miseq platform. Raw data were demultiplexed and quality filtered by Trimmomatic. The 16S rDNA amplicon sequences were analyzed in terms of taxonomic classification as well as grouped in operational taxonomic units (OTUs) at different sequence similarity levels.
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
An indirect ELISA assay was used to examine Ent-specific antibody in serum samples as described previously with some modifications [23]. Briefly, given the lack of cross reactivity between KLH and BSA, the Ent–BSA was used as coating antigen for the detection of specific anti-Ent IgG level in the chickens immunized with Ent–KLH vaccine. The microplates were coated with 100 μL of Ent–BSA solution (2 ng/μL) in coating buffer (bicarbonate/carbonate coating buffer, pH 9.6) overnight at 4 °C. Each of the collected serum samples was diluted by 200-fold using blocking buffer (PBS containing 5% skim milk) and 100 μL of the diluted sample were added to each well. Each serum sample was performed in triplicate. After 1 h of incubation at 37 °C, the plates were washed three times with washing buffer (PBS containing 0.05% Tween 20). The HRP-labeled rabbit anti-chicken IgG (Millipore Sigma, St. Louis, MO, USA) was diluted 1:10,000 in blocking buffer, and 100 μL were added to each well as the secondary antibody. Following washing the plates three times with washing buffer, 100 μL of TMB (3, 3′, 5, 5′-tetramethylbenzidine) substrate (Beyotime Biotechnology, Shanghai, China) were added in each well and incubated at 37 °C for 30 min for color development. The reaction was stopped using 1 M H2SO4 solution (50 μL per well) and the absorbance was measured at OD450 using an ELISA reader (Synergy H1, BioTek, Winooski, VT, USA).
2.8. Statistical Analysis
Statistical analyses were conducted using an unpaired t test with two-tailed distribution. Data are presented as means ± the standard deviations. Results were considered significant if the p value was less than or equal to 0.05.
3. Results
3.1. Multiple Vaccinations of Chickens with the Ent–KLH Conjugate Vaccine Did Not Cause Phenotypic Changes in Physiology (Trial A)
One goal of Trial A was to determine if up to three vaccinations of chickens with the Ent–KLH conjugate influenced chicken physiology with respect to body weight gain, development of major immune organs (bursa, spleen and thymus) and composition of gut microbiota when compared to control group. As shown in Figure 2A, no significant difference was observed in body weight gain for chickens between the two groups (p = 0.65). The development of major immune organs, including bursa (p = 0.61), spleen (p = 0.14) and thymus (p = 0.13) also showed no significant difference between the two groups (Figure 2B). Microbiota analysis of the cecal contents from the chickens in two groups did not reveal significant differences regarding Shannon (p = 0.11) and Chao (p = 0.93) indices, which represent the bacterial diversity and abundance, respectively (Figure 2C). The diverse bacterial phyla were identified in the cecal contents from both groups. The dominant phyla identified in Ent–KLH and control groups were Firmicutes (81.17% vs. 84.15%), Bacteroidetes (12.71% vs. 7.90%), Actinobacteria (4.26% vs. 6.33%), Cyanobacteria (0.69% vs. 0.84%), Proteobacteria (0.90% vs. 0.46%), Desulfobacterota (0.27% vs. 0.28%), Verrucomicrobiota (0.0002% vs. 0.0003%) and Deinococcota (0.00001% vs. 0.00001%). No significant difference was observed among the eight phyla between the two groups (p > 0.99) (Figure 2D).
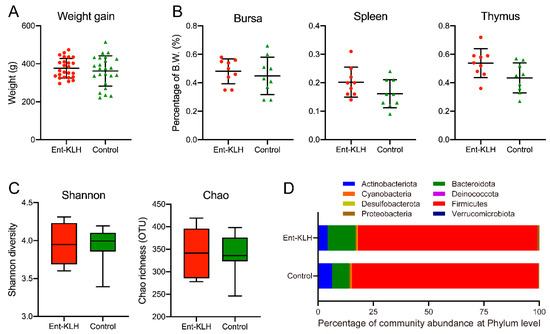
Figure 2.
Effects of vaccination of chickens with the Ent–KLH conjugate on growth performance, immune organ development, and gut microbiota. (A) Chicken body weight gain. Each symbol represents the body weight gain of each individual chicken between ages of 7 and 49 days. (B) The weight percentage of each immune organ (bursa, spleen or thymus) to its body weight at 49 days old. (C) The Shannon diversity and the Chao richness (OTU) based on 16S rDNA amplicon sequences of the cecal samples. (D) Relative abundance of the eight dominant phyla in the cecum between Ent–KLH and PBS-control groups.
3.2. The Ent–KLH Conjugate Vaccine Significantly Elicited Ent-Specific Antibody Response (Trial A)
To accurately determine the level of Ent-specific antibodies rather than those directed against whole conjugate vaccine in serum samples, the Ent–BSA conjugate was used as the coating antigen in the ELISA assay. Clearly, in Trial A, the chickens receiving three vaccinations displayed significantly (p < 0.01) higher level of anti-Ent antibodies than that the control group as early as two weeks after the first immunization (Figure 3A). The mean OD450 values of the serum samples from the Ent–KLH immunized chickens were 1.80, 3.71 and 3.80 at two weeks following the first, second and third immunization, respectively, while those from control group displayed significantly (p < 0.01) lower mean OD450 values (0.20–0.24). The Ent-specific antibody level at four and six weeks post immunization in treatment group did not show significant difference (p = 0.98) (Figure 3A).
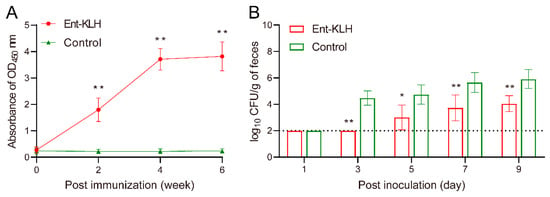
Figure 3.
Enterobactin-specific immune response and protective efficacy of the Ent–KLH conjugate vaccine to mitigate C. jejuni colonization in the chicken intestine (Trial A with a total of three immunizations). (A) The Ent-specific IgG in the chickens immunized with Ent–KLH or PBS. Each point represents the average of OD450 absorbance from 25 individual serum samples (200-fold dilution) with standard deviation indicated by error bar. (B) Comparison of colonization of C. jejuni NCTC 11168 in the chickens immunized with Ent–KLH or PBS. Each bar represents mean CFU/g feces ± the standard deviation in each group. The dotted line indicates detection limit. * p < 0.05, ** p < 0.01.
3.3. Multiple Vaccinations of Chickens with the Ent–KLH Conjugate Reduced Colonization of C. jejuni in Chicken Intestine (Trial A)
In Trial A, due to removal of nine chickens at age of 49 days old for sample collections (Figure 1A) and corresponding analyses (Figure 2), there were 16 birds left for C. jejuni challenge following three immunizations. To better monitor colonization dynamics and pattern of C. jejuni in the intestine, cloacal samples were collected from each bird for CFU enumeration at five different time points post inoculation. As shown in Figure 3B, the C. jejuni level in the feces from the chickens receiving Ent–KLH vaccinations, based on the assumption of 100 mg of feces per swab, were significantly lower than those from control chickens from three days post inoculation; the difference ranged from 1.75 to 2.73 log10 units. Notably, upon termination of the trial (nine days post inoculation), cecal contents were also collected from each bird in both groups for accurate enumeration of C. jejuni in the intestine, which showed that vaccination of chickens with Ent–KLH led to significant C. jejuni reduction (>4 log10 units) in cecum (4.61 log10 units) when compared to control group (8.65 log10 units) at nine days post C. jejuni challenge.
3.4. Different Vaccination Regimen Still Significantly Induced High Level of Ent-Specific Antibodies and Reduced Colonization of C. jejuni in the Chicken Intestine (Trial B)
To confirm the efficacy of the Ent–KLH vaccine observed in Trial A, a complementary chicken experiment (Trial B) was performed with following three modifications: (1) the chickens in treatment group only received two immunizations; (2) more chickens in each group (30 birds per group) were subjected to C. jejuni challenge so that five birds were euthanized at each of the six time points post inoculation for accurate assessment of C. jejuni level in the cecum, which overcame the limitation of semi-quantitative nature of using cloacal swabs in Trial A (Figure 3B); and (3) the time period for intestinal sample collection following C. jejuni challenge was greatly extended (up to 21 days post inoculation) when compared to Trial A (up to nine days post inoculation).
Despite different vaccination regimen used in Trial B (two immunizations), the mean OD450 value of the serum samples from the Ent–KLH immunized chickens (3.66) at two weeks following the second immunization was significantly higher (p < 0.01) than that from control group (0.16) (Figure 4A), indicating the reduced frequency of Ent–KLH vaccination still induced desired level of Ent-specific antibodies in the system.
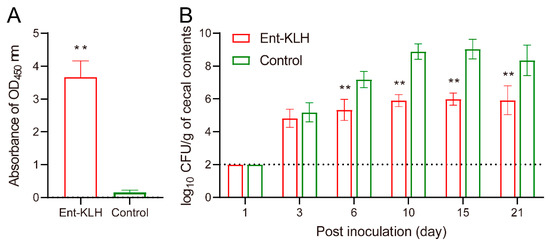
Figure 4.
Enterobactin-specific immune response and protective efficacy of the Ent–KLH conjugate vaccine to mitigate C. jejuni colonization in chickens (Trial B with two immunizations). (A) The Ent-specific IgG in the chickens immunized with Ent–KLH or PBS. Each bar represents the average of OD450 absorbance from 30 individual serum samples (200-fold dilution) with standard deviation indicated by error bar. (B) Comparison of colonization of C. jejuni NCTC 11168 in the chickens immunized with Ent–KLH or PBS. Each bar represents mean CFU/g cecal contents ± the standard deviation in each group. The dotted line indicates detection limit. ** p < 0.01.
Quantitative measurement of C. jejuni in cecal contents at different time points post inoculation (Figure 4B) confirmed the protective efficacy of the Ent–KLH vaccine observed in Trial A (Figure 3B): the C. jejuni level in the chickens immunized with the Ent–KLH was significantly (p < 0.01) lower than that in control group (up to 3.20 log10 units) from six days post inoculation (Figure 4B). Notably, at 21 days post inoculation, the C. jejuni colonization level in the cecum of the chickens receiving the conjugate vaccine was still more than 2 log10 units lower than that in control group (Figure 4B).
3.5. Persistence of the Ent-Specific Antibodies Upon Single Vaccination of Chickens with the Ent–KLH Conjugate Vaccine (Trial C)
The findings from Trial B strongly suggest that the chickens receiving reduced times of vaccination could still produce high level of Ent-specific antibodies, which could significantly mitigate C. jejuni colonization for a long time. To further optimize vaccination regimen for induction of desired immune response, in Trial C, chickens were vaccinated with the Ent–KLH conjugate one time at seven days old and the level and persistence of the Ent-specific antibodies in serum were monitored weekly for eight weeks post immunization. The mean OD450 values of sera from Ent–KLH group were significantly higher than those in the control group as early as one week post immunization (p < 0.01) (Figure 5). The Ent-specific antibody level in Ent–KLH group reached a peak at four weeks post immunization (mean OD450 = 3.08), sustained for at least two weeks and then dropped to mean OD450 of 2.24 and 2.38 at seven and eight weeks post immunization, respectively (Figure 5).
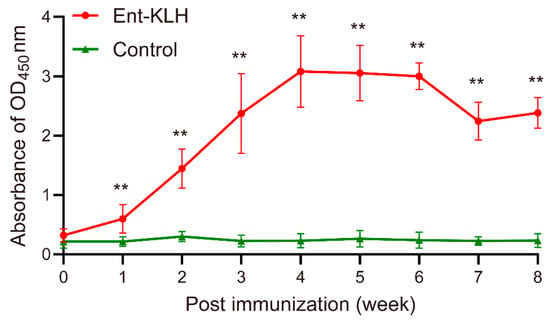
Figure 5.
Persistence of Ent-specific IgG upon single vaccination of chickens with the Ent–KLH conjugate vaccine (Trial C). Each point represents the average of OD450 absorbance from 20 individual serum samples (200-fold dilution) with standard deviation indicated by error bar. ** p < 0.01.
4. Discussion
Iron is an essential micronutrient for all organisms [25]. During the “tug-of-war” for iron between bacteria and host, the competition for iron is so intense that microbial iron acquisition systems are major determinants of virulence [25,26]. Siderophore-mediated iron uptake system plays an important role in modulation of bacteria colonization and growth in the host [27]. Currently, three strategies were developed to inhibit siderophore biosynthesis or utilization: (1) screening inhibitors of siderophore biosynthetic enzymes as novel antimicrobials [28]; (2) design of siderophore–antimicrobial conjugates against antimicrobial resistance [29,30]; and (3) development of siderophore-based conjugate vaccines [23,31,32].
Siderophore-based immunization strategy is promising to inhibit growth of different Gram-negative pathogens, such as E. coli and Salmonella infections in mice [31,32]. In the study by Mike et al. [32], the siderophore conjugate vaccine failed to elicit any detectable siderophore-specific antibodies in mice. Relevant to this work, Sassone-Corsi et al. [31] reported an Ent conjugate vaccine that was synthesized using a lengthy and complicated procedure; this Ent conjugate elicited weak mucosal response (i.e., <4-fold increase in Ent-specific antibodies) and no systemic response (IgG) in the immunized mice. In our recent study [23], we developed a new Ent conjugate vaccine using a simple and efficient protocol. This new vaccine could trigger strong systemic immune responses, resulting in up to 4,096-fold increase in the titer of Ent-specific IgG in serum. In addition, the Ent-specific antibodies induced by this novel Ent conjugate vaccine in rabbit could inhibit Ent-dependent growth of diverse Campylobacter, E. coli and S. enterica in vitro [23,24]. Therefore, the new Ent conjugate vaccine is promising for controlling Campylobacter and other Gram-negative pathogens in poultry. This study, for the first time, demonstrated that the Ent–KLH conjugate vaccine could elicit high level of systemic Ent-specific antibodies in chickens and significantly reduce C. jejuni colonization in the intestine, which was confirmed in two independent chicken trials (Figure 3 and Figure 4). In addition, immunization of the Ent–KLH conjugate vaccine had no effects on the body weight gain and the development of major immune organs (Figure 2A,B). These data indicate that the Ent–KLH conjugate vaccine has high potential for application in poultry industry to control infections caused by Campylobacter and even other Gram-negative pathogens.
As Ent is produced by most members of Enterobacteriaceae [27], the potential effects of an Ent conjugate vaccine on the composition and abundance of the gut microbiota should be investigated in animal experiments. Our results show that the diversity and abundance of the chicken gut microbiota were not affected by three times of vaccination of chickens with the Ent–KLH conjugate (Figure 2C,D). Previous study also showed that immunization of mice with the Ent-CTB conjugate vaccine did not significantly influence the composition of microbiota in mice without intestinal inflammation [31]. Moreover, reduction of Salmonella colonization was accompanied by significant expansion of Lactobacillus spp. in the inflamed gut of mice immunized with CTB-Ent conjugate vaccine, which provided additional benefits to the host by indirectly promoting the expansion of beneficial microbes in the inflamed gut [31]. Extensive microbiome studies have consistently shown that poor gut health is associated to the expansion of dysbiotic Proteobacteria [33], the most important bacterial population producing catecholate siderophores. Therefore, the Ent conjugate vaccine examined in this study may be beneficial for enhancing host gut health by controlling overpopulation of Proteobacteria species and by expanding beneficial microbes.
Stimulation of specific and sustained immune response is an important criteria for evaluating the protective efficacy of the vaccine [34]. As discussed above, the new Ent–KLH conjugate vaccine reported by Wang et al. [23] has shown significant advantage over other siderophore conjugate vaccines with respect to induction of high level of siderophore-specific antibodies [31,32]. The findings from three vaccination trials in this study further provided compelling evidence that intramuscular immunization of chickens with the Ent–KLH conjugate vaccine, regardless of vaccination regimens, significantly elicited Ent-specific antibodies (Figure 3A, Figure 4A and Figure 5). Another new and exciting finding in this study is that the high level of Ent-specific antibodies induced by single Ent–KLH vaccination could persist for up to eight weeks post immunization (Figure 5). This feature, which clearly makes this vaccine more economically feasible for application in poultry industry, may provide long-term protection of chickens against Campylobacter infections. This speculation needs to be examined in the future.
In addition to using the Ent–KLH conjugate vaccine to induce active immunity, passive immunization of food-producing animals and even humans with Ent-specific hyperimmune egg yolk antibodies is another promising approach to prevent and control Campylobacter. Laying hen has been recognized as an efficient “bioreactor” to produce a large quantity of specific egg yolk antibodies; therefore, passive immunization with specific egg yolk antibodies is regarded as a potential alternative to antibiotics for control of various infectious diseases [35]. For cost-efficient production of large quantities of specific egg yolk antibodies, it is essential to elicit high and steady immune response in laying hens. The findings from multiple vaccination trials in this study, particularly Trial C (Figure 5), demonstrated that single immunization of chickens with the Ent–KLH vaccine could trigger high-level of systemic Ent-specific antibodies sustaining for up to eight weeks. Thus, the finding from this study may provide a simple immunization procedure for efficient production of Ent-specific antibody in laying hens.
Consistent with previous in vitro finding that Ent-specific antibodies significantly inhibited the Ent-dependent growth of Campylobacter [23], the immunization-challenge trials in this study demonstrated that the Ent–KLH conjugate vaccine can reduce C. jejuni loads by up to 10,000-fold in chicken cecum, which exhibits a better protective efficacy than most reported vaccination strategies for C. jejuni control in chickens [8,36]. In addition to the novelty of the Ent–KLH conjugate vaccine and its potent protection against C. jejuni colonization in the chicken intestine, this study has some advantages in terms of experimental design when compared to previous chicken vaccination studies [8,36]. For example, the protective efficacy of the Ent–KLH was consistently observed in independent trials using different vaccination regimens. In addition, we showed long-term persistence of induced Ent-specific antibodies upon single immunization in this study, which is critically important for both active immunization and passive immunization approaches using the Ent–KLH conjugate vaccine in poultry. It is important to mention that high-quality SPF chickens were used for all trials in this study, which generates consistent and insightful findings for the immunogenicity and protective efficacy of the Ent–KLH vaccine in chickens. In the future, trials using commercial broilers are highly warranted to confirm the efficacy of the Ent-based immune intervention for Campylobacter control in poultry.
5. Conclusions
Chickens are a major source of human campylobacteriosis; however, there is no vaccine available for Campylobacter control in poultry. In this study, immunization of chickens with the innovative Ent–KLH conjugate vaccine using two different vaccination regimens consistently reduced C. jejuni colonization by 3–4 log10 units in the cecum. The Ent–KLH vaccine could significantly induce Ent-specific immune response in chickens but did not affect growth performance, development of immune organs and gut microbiota. Moreover, the high level of systemic Ent-specific antibodies due to single immunization could persist for eight weeks in chickens. In conclusion, the results of this study strongly support that the Ent–KLH conjugate vaccine is promising for Campylobacter control in poultry, consequently protecting food safety and public health.
Author Contributions
Conceptualization, J.L. and F.X.; methodology, Y.C., H.W., X.Z., J.L. and F.X.; validation, Y.C. and F.X.; formal analysis, Y.C., H.W., X.Z., J.L. and F.X.; investigation, Y.C., F.G., J.G., X.C., B.Y., H.Z., X.S. and F.X.; resources, J.L. and F.X.; data curation, Y.C. and F.X.; writing—original draft preparation, Y.C. and F.X.; writing—review and editing, Y.C., F.G., J.L. and F.X.; visualization, Y.C., J.L. and F.X.; project administration, J.L. and F.X.; and funding acquisition, J.L. and F.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (2018YFD0500506); the National Natural Science Foundation of China (31572527); and the Special Program on Science and Technology Innovation Capacity Building of BAAFS (KJCX201914). Support was also provided to the University of Tennessee by the USDA National Institute of Food and Agriculture through the Hatch-Multistate project NC1202, Enteric Diseases of Food Animals: Enhanced Prevention, Control, and Food Safety and by the United States Department of Agriculture National Institute of Food and Agriculture (NIFA) Award No. 2018-67015-28295.
Acknowledgments
We are grateful to Maojun Zhang (National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention) for providing the C. jejuni strain.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Kaakoush, N.O.; Castano-Rodriguez, N.; Mitchell, H.M.; Man, S.I.M. Global Epidemiology of Campylobacter Infection. Clin. Microbiol. Rev. 2015, 28, 687–720. [Google Scholar] [CrossRef]
- Facciola, A.; Riso, R.; Avventuroso, E.; Visalli, G.; Delia, S.A.; Lagana, P. Campylobacter: From microbiology to prevention. J. Prev. Med. Hyg. 2017, 58, E79–E92. [Google Scholar]
- Igwaran, A.; Okoh, A.I. Human campylobacteriosis: A public health concern of global importance. Heliyon 2019, 5, e02814. [Google Scholar] [CrossRef] [PubMed]
- Umaraw, P.; Prajapati, A.; Verma, A.K.; Pathak, V.; Singh, V.P. Control of Campylobacter in poultry industry from farm to poultry processing unit: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Sibanda, N.; McKenna, A.; Richmond, A.; Ricke, S.C.; Callaway, T.; Stratakos, A.C.; Gundogdu, O.; Corcionivoschi, N. A review of the effect of management practices on Campylobacter prevalence in poultry farms. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.J.; Shank, J.M.; Johnson, J.G. Current and potential treatments for reducing Campylobacter colonization in animal hosts and disease in humans. Front. Microbiol. 2017, 8, 487. [Google Scholar] [CrossRef]
- Hansson, I.; Sandberg, M.; Habib, I.; Lowman, R.; Engvall, E.O. Knowledge gaps in control of Campylobacter for prevention of campylobacteriosis. Transbound Emerg. Dis. 2018, 65 (Suppl. 1), 30–48. [Google Scholar] [CrossRef]
- Hermans, D.; Van Deun, K.; Messens, W.; Martel, A.; Van Immerseel, F.; Haesebrouck, F.; Rasschaert, G.; Heyndrickx, M.; Pasmans, F. Campylobacter control in poultry by current intervention measures ineffective: Urgent need for intensified fundamental research. Vet. Microbiol. 2011, 152, 219–228. [Google Scholar] [CrossRef]
- Fancher, C.A.; Zhang, L.; Kiess, A.S.; Adhikari, P.A.; Dinh, T.T.N.; Sukumaran, A.T. Avian pathogenic Escherichia coli and Clostridium perfringens: Challenges in no antibiotics ever broiler production and potential solutions. Microorganisms 2020, 8, 1533. [Google Scholar] [CrossRef]
- Bywater, R.J. Veterinary use of antimicrobials and emergence of resistance in zoonotic and sentinel bacteria in the EU. J. Vet. Med. B 2004, 51, 361–363. [Google Scholar] [CrossRef]
- Von Wintersdorff, C.J.H.; Penders, J.; van Niekerk, J.M.; Mills, N.D.; Majumder, S.; van Alphen, L.B.; Savelkoul, P.H.M.; Wolffs, P.F.G. Dissemination of antimicrobial resistance in microbial ecosystems through horizontal gene transfer. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Wang, Y.; Zhang, Q.; Shen, J. Antimicrobial resistance in Campylobacter spp. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef]
- Wieczorek, K.; Wolkowicz, T.; Osek, J. Antimicrobial resistance and virulence-associated traits of Campylobacter jejuni isolated from poultry food chain and humans with diarrhea. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Avci, F.Y. A chicken vaccine to protect humans from diarrheal disease. Glycobiology 2016, 26, 1137–1139. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Poly, F.; Noll, A.J.; Riddle, M.S.; Porter, C.K. Update on Campylobacter vaccine development. Hum. Vaccin. Immunother. 2019, 15, 1389–1400. [Google Scholar] [CrossRef] [PubMed]
- Jagusztyn-Krynicka, E.K.; Laniewski, P.; Wyszynska, A. Update on Campylobacter jejuni vaccine development for preventing human campylobacteriosis. Expert Rev. Vaccines 2009, 8, 625–645. [Google Scholar] [CrossRef]
- Miller, C.E.; Williams, P.H.; Ketley, J.M. Pumping iron: Mechanisms for iron uptake by Campylobacter. Microbiology 2009, 155, 3157–3165. [Google Scholar] [CrossRef][Green Version]
- Xu, F.; Zeng, X.; Haigh, R.D.; Ketley, J.M.; Lin, J. Identification and characterization of a new ferric enterobactin receptor, CfrB, in Campylobacter. J. Bacteriol. 2010, 192, 4425–4435. [Google Scholar] [CrossRef]
- Palyada, K.; Threadgill, D.; Stintzi, A. Iron acquisition and regulation in Campylobacter jejuni. J. Bacteriol. 2004, 186, 4714–4729. [Google Scholar] [CrossRef]
- Zeng, X.; Lin, J. Characterization of high affinity iron acquisition systems in Campylobacter jejuni. Methods Mol. Biol. 2017, 1512, 65–78. [Google Scholar] [CrossRef]
- Liu, X.; Adams, L.J.; Zeng, X.; Lin, J. Evaluation of in ovo vaccination of DNA vaccines for Campylobacter control in broiler chickens. Vaccine 2019, 37, 3785–3792. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.J.; Zeng, X.; Lin, J. Development and evaluation of two live Salmonella-vectored vaccines for Campylobacter control in broiler chickens. Foodborne Pathog. Dis. 2019, 16, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zeng, X.; Mo, Y.; He, B.; Lin, H.; Lin, J. Enterobactin-specific antibodies induced by a novel enterobactin conjugate vaccine. Appl. Environ. Microb. 2019, 85. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zeng, X.; Lin, J. Enterobactin-specific antibodies inhibit in vitro growth of different gram-negative bacterial pathogens. Vaccine 2020, 38, 7764–7773. [Google Scholar] [CrossRef] [PubMed]
- Carver, P.L. The battle for iron between humans and microbes. Curr. Med. Chem. 2018, 25, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Detweiler, C.S. A new way to beat intestinal pathogens. Trends Microbiol. 2017, 25, 169–170. [Google Scholar] [CrossRef][Green Version]
- Ellermann, M.; Arthur, J.C. Siderophore-mediated iron acquisition and modulation of host-bacterial interactions. Free Radic. Biol. Med. 2017, 105, 68–78. [Google Scholar] [CrossRef]
- Lamb, A.L. Breaking a pathogen’s iron will: Inhibiting siderophore production as an antimicrobial strategy. Biochim. Biophys. Acta 2015, 1854, 1054–1070. [Google Scholar] [CrossRef]
- Zheng, T.; Bullock, J.L.; Nolan, E.M. Siderophore-mediated cargo delivery to the cytoplasm of Escherichia coli and Pseudomonas aeruginosa: Syntheses of monofunctionalized enterobactin scaffolds and evaluation of enterobactin-cargo conjugate uptake. J. Am. Chem. Soc. 2012, 134, 18388–18400. [Google Scholar] [CrossRef]
- Negash, K.H.; Norris, J.K.S.; Hodgkinson, J.T. Siderophore-antibiotic conjugate design: New drugs for bad bugs? Molecules 2019, 24, 3314. [Google Scholar] [CrossRef]
- Sassone-Corsi, M.; Chairatana, P.; Zheng, T.; Perez-Lopez, A.; Edwards, R.A.; George, M.D.; Nolan, E.M.; Raffatellu, M. Siderophore-based immunization strategy to inhibit growth of enteric pathogens. Proc. Natl. Acad. Sci. USA 2016, 113, 13462–13467. [Google Scholar] [CrossRef] [PubMed]
- Mike, L.A.; Smith, S.N.; Sumner, C.A.; Eaton, K.A.; Mobley, H.L.T. Siderophore vaccine conjugates protect against uropathogenic Escherichia coli urinary tract infection. Proc. Natl. Acad. Sci. USA 2016, 113, 13468–13473. [Google Scholar] [CrossRef] [PubMed]
- Litvak, Y.; Byndloss, M.X.; Tsolis, R.M.; Baumler, A.J. Dysbiotic Proteobacteria expansion: A microbial signature of epithelial dysfunction. Curr. Opin. Microbiol. 2017, 39, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Slifka, M.K.; Amanna, I. How advances in immunology provide insight into improving vaccine efficacy. Vaccine 2014, 32, 2948–2957. [Google Scholar] [CrossRef]
- Diraviyam, T.; Zhao, B.; Wang, Y.; Schade, R.; Michael, A.; Zhang, X. Effect of chicken egg yolk antibodies (IgY) against diarrhea in domesticated animals: A systematic review and meta-analysis. PLoS ONE 2014, 9, e97716. [Google Scholar] [CrossRef]
- De Zoete, M.R.; van Putten, J.P.M.; Wagenaar, J.A. Vaccination of chickens against Campylobacter. Vaccine 2007, 25, 5548–5557. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).