The Roles of Osteopontin in the Pathogenesis of West Nile Encephalitis
Abstract
1. Osteopontin
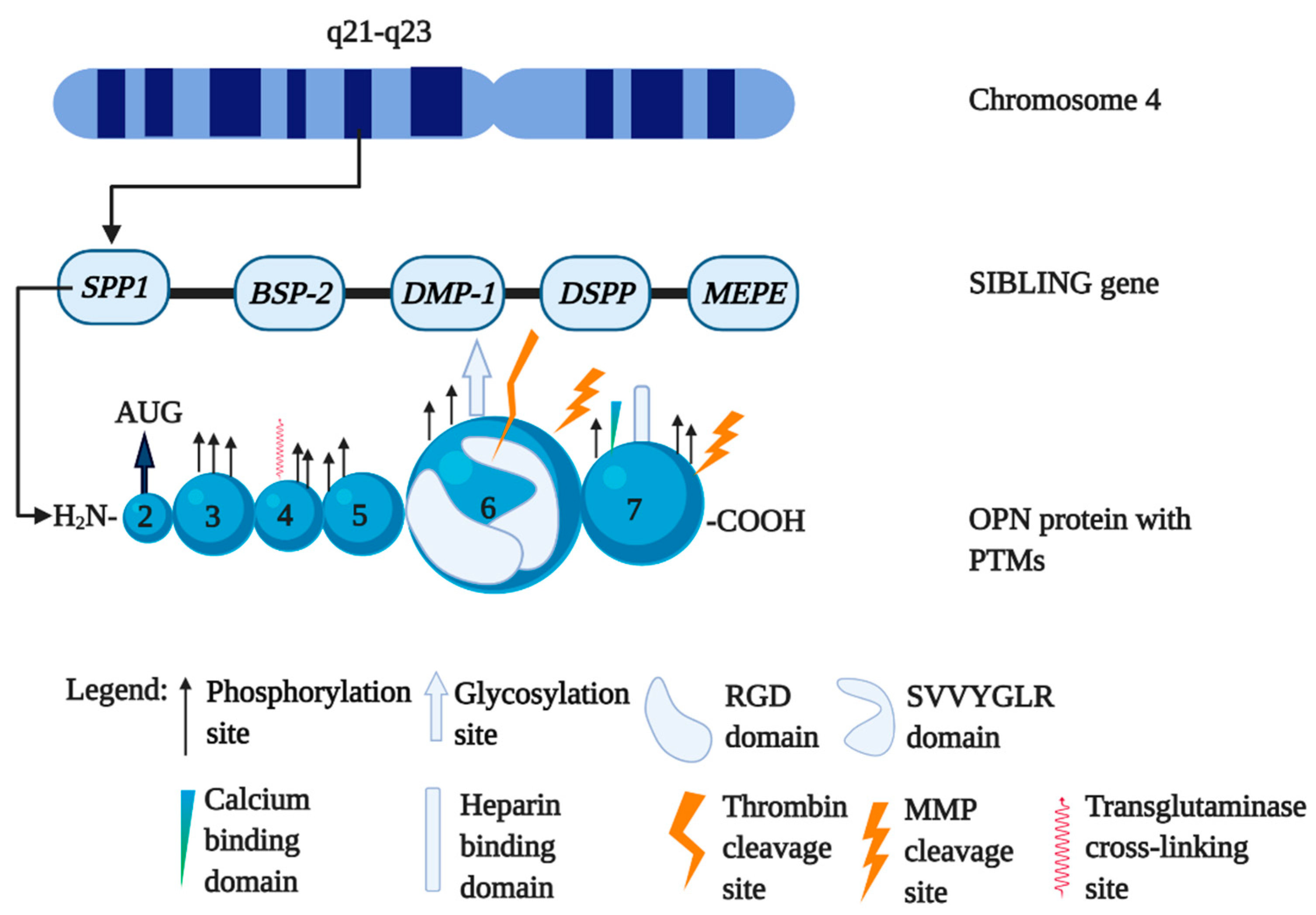
1.1. The Biology of Osteopontin
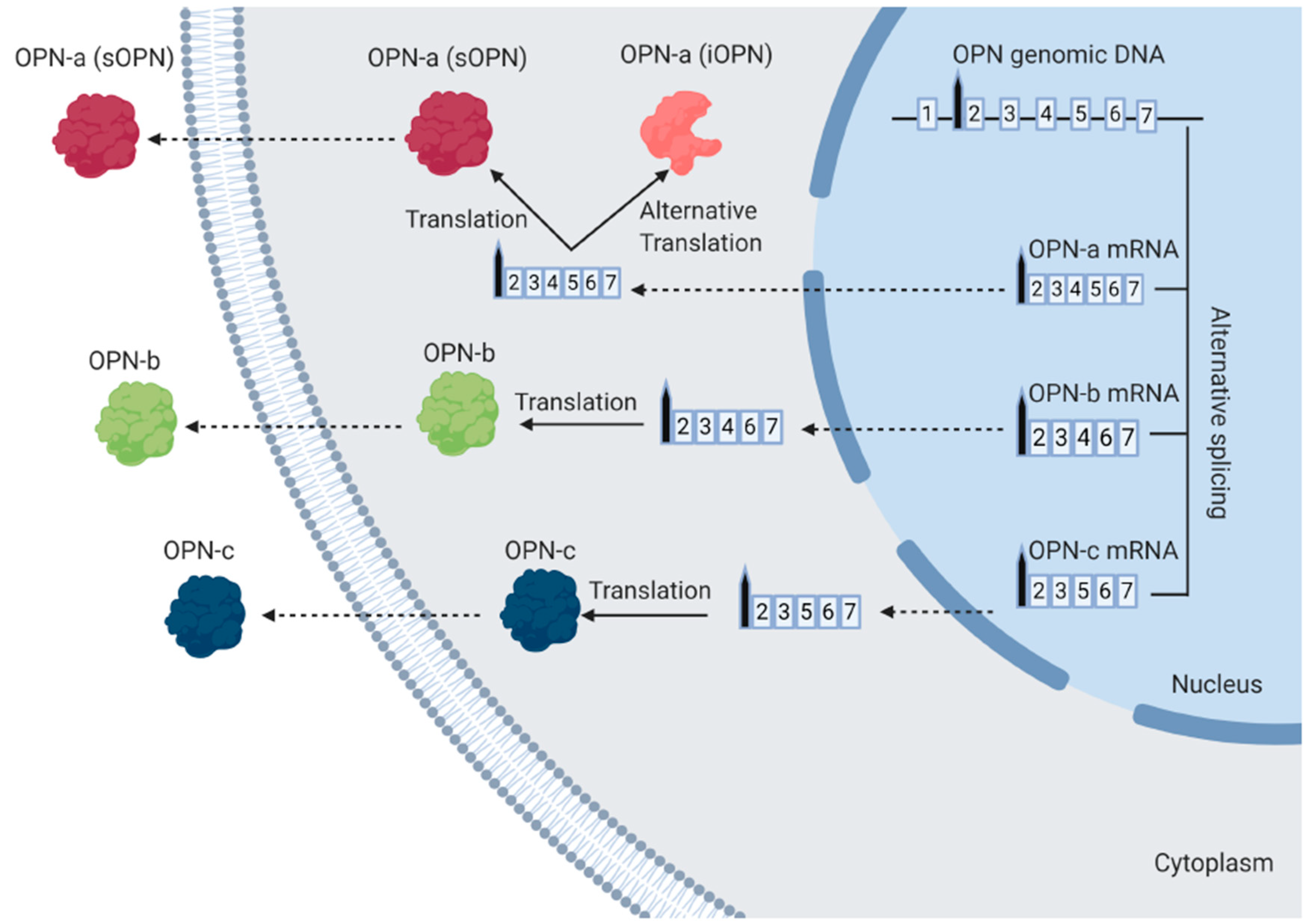
1.2. The Different Isoforms of OPN
1.3. The Function of Special Forms of OPN
1.3.1. Polymerized OPN
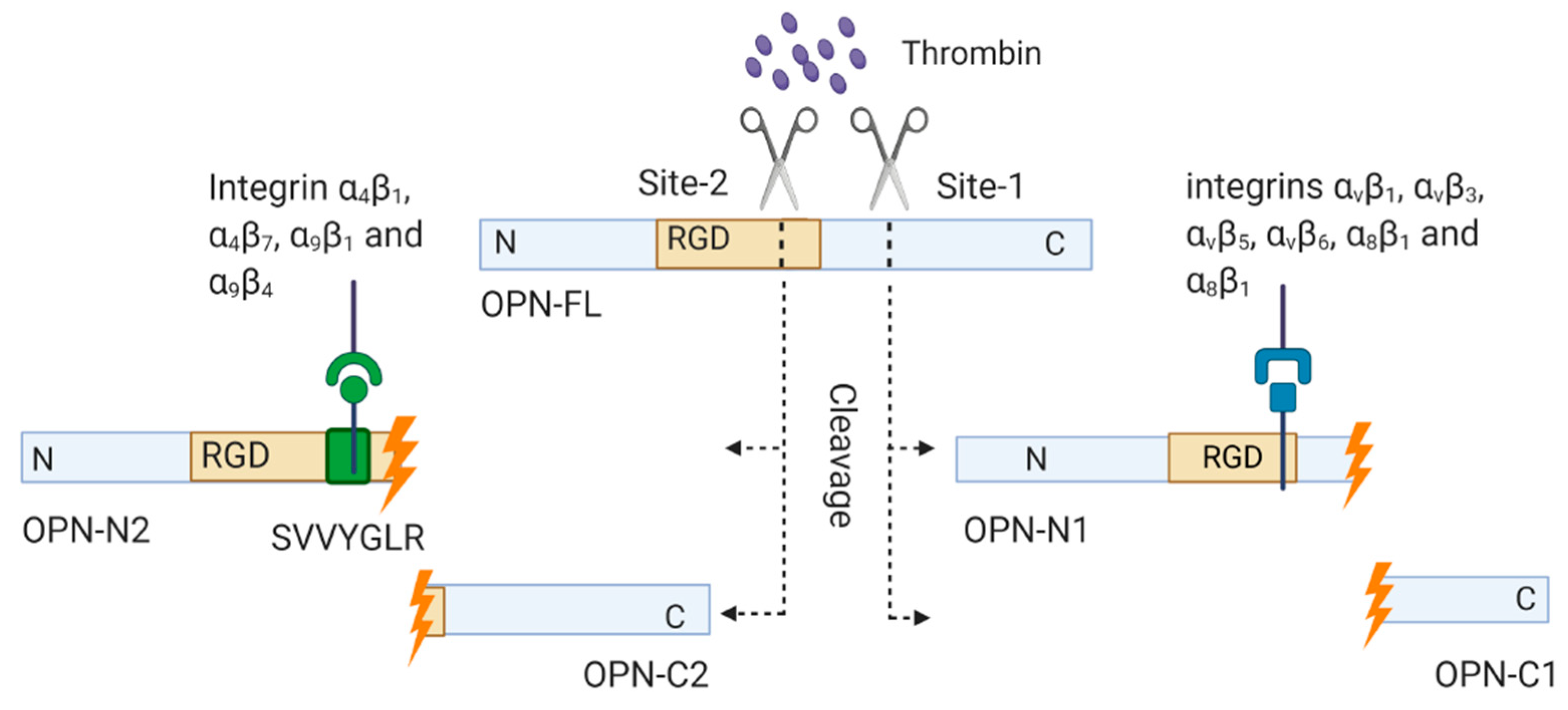
1.3.2. Thrombin-Cleaved OPN
2. Roles of OPN in the Pathogenesis of WNV
2.1. West Nile Virus
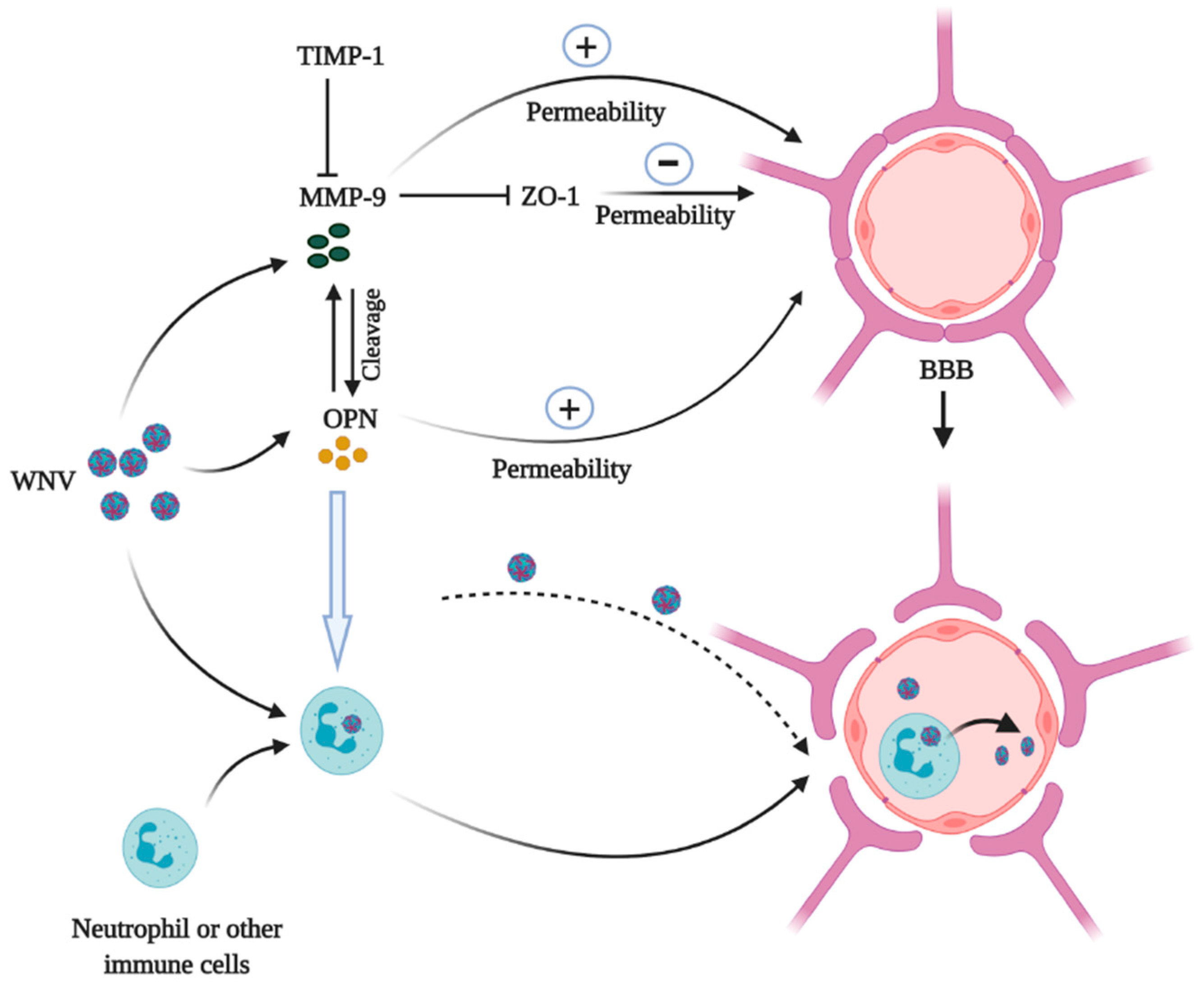
2.2. OPN and WNV
3. Concluding Remarks
Funding
Acknowledgments
Conflicts of Interest
References
- Hao, C.; Cui, Y.; Owen, S.; Li, W.; Cheng, S.; Jiang, W.G. Human osteopontin: Potential clinical applications in cancer (Review). Int. J. Mol. Med. 2017, 39, 1327–1337. [Google Scholar] [CrossRef]
- McKee, M.D.; Cole, W.G. Bone matrix and mineralization. In Pediatric Bone; Academic Press: San Diego, CA, USA, 2012; pp. 9–37. [Google Scholar]
- Qin, C.; Baba, O.; Butler, W.T. Post-translational modifications of sibling proteins and their roles in osteogenesis and dentinogenesis. Crit. Rev. Oral Biol. Med. 2004, 15, 126–136. [Google Scholar] [CrossRef]
- Chaplet, M.; De Leval, L.; Waltregny, D.; Detry, C.; Fornaciari, G.; Bevilacqua, G.; Fisher, L.W.; Castronovo, V.; Bellahcène, A. Dentin matrix protein 1 is expressed in human lung cancer. J. Bone Miner. Res. 2003, 18, 1506–1512. [Google Scholar] [CrossRef]
- Castello, L.M.; Raineri, D.; Salmi, L.; Clemente, N.; Vaschetto, R.; Quaglia, M.; Garzaro, M.; Gentilli, S.; Navalesi, P.; Cantaluppi, V.; et al. Osteopontin at the Crossroads of Inflammation and Tumor Progression. Mediat. Inflamm. 2017, 2017, 4049098. [Google Scholar] [CrossRef]
- Kaleta, B. Role of osteopontin in systemic lupus erythematosus. Arch. Immunol. Exp. 2014, 62, 475–482. [Google Scholar] [CrossRef]
- Denhardt, T.D.; Noda, M. Osteopontin expression and function: Role in bone remodeling. J. Cell. Biochem. 1998, 72, 92–102. [Google Scholar] [CrossRef]
- Ramaiah, K.S.; Rittling, S. Role of osteopontin in regulating hepatic inflammatory responses and toxic liver injury. Expert Opin. Drug Metab. Toxicol. 2007, 3, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Uede, T. Osteopontin, intrinsic tissue regulator of intractable inflammatory diseases. Pathol. Int. 2011, 61, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Denhardt, D.T.; Mistretta, D.; Chambers, A.F.; Krishna, S.; Porter, J.F.; Raghuram, S.; Rittling, S.R. Transcriptional regulation of osteopontin and the metastatic phenotype: evidence for a Ras-activated enhancer in the human OPN promoter. Clin. Exp. Metastasis. 2003, 20, 77–84. [Google Scholar] [CrossRef]
- Rittling, S.R.; Chen, Y.; Feng, F.; Wu, Y. Tumor-derived osteopontin is soluble, not matrix associated. J. Biol. Chem. 2002, 277, 9175–9182. [Google Scholar] [CrossRef]
- Boggio, E.; Dianzani, C.; Gigliotti, C.L. Thrombin Cleavage of Osteopontin Modulates Its Activities in Human Cells In Vitro and Mouse Experimental Autoimmune Encephalomyelitis In Vivo. J. Immunol. Res. 2016, 2016, 9345495. [Google Scholar] [CrossRef] [PubMed]
- Grassinger, J.; Haylock, D.N.; Storan, M.J. Thrombin-cleaved osteopontin regulates hemopoietic stem and progenitor cell functions through interactions with alpha9beta1 and alpha4beta1 integrins. Blood 2009, 114, 49–59. [Google Scholar] [CrossRef]
- Yokasaki, Y.; Sheppard, D. Mapping of the cryptic integrin-binding site in osteopontin suggests a new mechanism by which thrombin can regulate inflammation and tissue repair. Trends Cardiovasc. Med. 2000, 10, 155–159. [Google Scholar] [CrossRef]
- Katagiri, Y.U.; Sleeman, J.; Fujii, H.; Herrlich, P.; Hotta, H.; Tanaka, K.; Chikuma, S.; Yagita, H.; Okumura, K.; Murakami, M.; et al. CD44 variants but not CD44s cooperate with β1-containing integrins to permit cells to bind to osteopontin independently of arginine-glycine-aspartic acid, thereby stimulating cell motility and chemotaxis. Cancer Res. 1999, 59, 219–226. [Google Scholar]
- Weber, G.F. The metastasis gene osteopontin: A candidate target for cancer therapy. Biochim. Biophys. Acta 2001, 1552, 61–85. [Google Scholar] [CrossRef]
- Furger, K.A.; Menon, R.K.; Tuck, A.B.; Bramwell, V.H.C.; Chambers, A.F. The functional and clinical roles of osteopontin in cancer and metastasis. Curr. Mol. Med. 2001, 1, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Coombes, J.D.; Swiderska-Syn, M.; Dollé, L.; Reid, D.; Eksteen, B.; Claridge, L.; Briones-Orta, M.A.; Shetty, S.; Oo, Y.H.; Riva, A.; et al. Osteopontin neutralisation abrogates the liver progenitor cell response and fibrogenesis in mice. Gut. 2015, 64, 1120–1131. [Google Scholar] [CrossRef]
- Shimada, M.; Greer, P.A.; McMahon, A.P.; Bouxsein, M.L.; Schipani, E. In vivo targeted deletion of calpain small subunit, Capn4, in cells of the osteoblast lineage impairs cell proliferation, differentiation, and bone formation. J. Biol. Chem. 2008, 283, 21002–21010. [Google Scholar] [CrossRef]
- Shinohara, M.L.; Kim, J.H.; Garcia, V.A.; Cantor, H. Engagement of the type I interferon receptor on dendritic cells inhibits T helper 17 cell development: Role of intracellular osteopontin. Immunity 2008, 29, 68–78. [Google Scholar] [CrossRef]
- Young, M.F.; Kerr, J.M.; Termine, J.D.; Wewer, U.M.; Wang, M.G.; McBride, O.W.; Fisher, L.W. cDNA cloning, mRNA distribution and heterogeneity, chromosomal location, and RFLP analysis of human osteopontin (OPN). Genomics 1990, 7, 491–502. [Google Scholar] [CrossRef]
- Denhardt, D.T.; Noda, M.; O’Regan, A.W.; Pavlin, D.; Berman, J.S. Osteopontin as a means to cope with environmental insults: Regulation of inflammation, tissue remodeling, and cell survival. J. Clin. Investig. 2001, 107, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Junaid, A.; Moon, M.C.; Harding, G.E.; Zahradka, P. Osteopontin localizes to the nucleus of 293 cells and associates with polo-like kinase-1. Am. J. Physiol. Cell Physiol. 2007, 292, C919–C926. [Google Scholar] [CrossRef] [PubMed]
- O’Regan, A.; Berman, J.S. Osteopontin: A key cytokine in cell-mediated and granulomatous inflammation. Int. J. Exp. Pathol. 2000, 81, 373–390. [Google Scholar] [CrossRef]
- Lin, J.; Myers, A.L.; Wang, Z.; Nancarrow, D.J.; Ferrer-Torres, D.; Handlogten, A.; Leverenz, K.; Bao, J.; Thomas, D.G.; Wang, T.D.; et al. Osteopontin (OPN/SPP1) isoforms collectively enhance tumor cell invasion and dissemination in esophageal adenocarcinoma. Oncotarget 2015, 6, 22239. [Google Scholar] [CrossRef]
- Shinohara, M.L.; Kim, H.J.; Kim, J.H.; Garcia, V.A.; Cantor, H. Alternative translation of osteopontin generates intracellular and secreted isoforms that mediate distinct biological activities in dendritic cells. Proc. Natl. Acad. Sci. USA 2008, 105, 7235–7239. [Google Scholar] [CrossRef]
- Shinohara, M.L.; Lu, L.; Bu, J. Osteopontin expression is essential for interferon-α production by plasmacytoid dendritic cells. Nat. Immunol. 2006, 7, 498–506. [Google Scholar] [CrossRef]
- Suzuki, K.; Zhu, B.; Rittling, S.R. Colocalization of intracellular osteopontin with CD44 is associated with migration, cell fusion, and resorption in osteoclasts. J. Bone Min. Res. 2002, 17, 1486–1497. [Google Scholar] [CrossRef]
- Zohar, R.; Suzuki, N.; Suzuki, K. Intracellular osteopontin is an integral component of the CD44-ERM complex involved in cell migration. J. Cell Physiol. 2000, 184, 118–130. [Google Scholar] [CrossRef]
- Wung, J.K.; Perry, G.; Kowalski, A. Increased expression of the remodeling- and tumorigenic-associated factor osteopontin in pyramidal neurons of the Alzheimer’s disease brain. Curr. Alzheimer Res. 2007, 4, 67–72. [Google Scholar] [CrossRef]
- Christensen, B.; Kazanecki, C.C.; Petersen, T.E.; Rittling, S.R.; Denhardt, D.T.; Sørensen, E.S. Cell type-specific posttranslational modifications of mouse osteopontin are associated with different adhesive properties. J. Biol. Chem. 2007, 282, 19463–19472. [Google Scholar] [CrossRef]
- He, B.; Mirza, M.; Weber, G.F. An osteopontin splice variant induces anchorage independence in human breast cancer cells. Oncogene 2006, 25, 2192–2202. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, V.; Chackalaparampil, I.; Kundu, G.C.; Mukherjee, A.B.; Mukherjee, B.B. Altered sialylation of osteopontin prevents its receptor-mediated binding on the surface of oncogenically transformed tsB77 cells. Biochemistry 1997, 36, 5729–5738. [Google Scholar] [CrossRef] [PubMed]
- Christensen, B.; Zachariae, E.D.; Scavenius, C.; Thybo, M.; Callesen, M.M.; Kløverpris, S.; Oxvig, C.; Enghild, J.J.; Sorensen, E.S. Identification of transglutaminase reactive residues in human osteopontin and their role in polymerization. PLoS ONE 2014, 9, 113650. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.S.; Greenberg, C.S. TGM2 and implications for human disease: Role of alternative splicing. Front. Biosci. 2013, 18, 504–519. [Google Scholar]
- Lorand, L.; Graham, R.M. Transglutaminases: Crosslinking enzymes with pleiotropic functions. Nat. Rev. Mol. Cell Biol. 2003, 4, 140–156. [Google Scholar] [CrossRef]
- Kaartinen, M.T.; El-Maadawy, S.; Räsänen, N.H.; McKee, M.D. Tissue transglutaminase and its substrates in bone. J. Bone Miner. Res. 2002, 17, 2161–2173. [Google Scholar] [CrossRef]
- Kaartinen, M.T.; Murshed, M.; Karsenty, G.; McKee, M.D. Osteopontin upregulation and polymerization by transglutaminase 2 in calcified arteries of Matrix Gla protein-deficient mice. J. Histochem. Cytochem. 2007, 55, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Patarca, R.; Schwartz, J.; Singh, P.; Cantor, H. Definition of a specific interaction between the early T lymphocyte activation 1 (Eta-1) protein and murine macrophages in vitro and its effect upon macrophages in vivo. J. Exp. Med. 1990, 171, 1931–1942. [Google Scholar] [CrossRef]
- Nishimichi, N.; Hayashita-Kinoh, H.; Chen, C.; Matsuda, H.; Sheppard, D.; Yokosaki, Y. Osteopontin undergoes polymerization in vivo and gains chemotactic activity for neutrophils mediated by integrin α9β1. J. Biol. Chem. 2011, 286, 11170–11178. [Google Scholar] [CrossRef]
- Senger, D.R.; Perruzzi, C.A. Cell migration promoted by a potent GRGDS-containing thrombin-cleavage fragment of osteopontin. Biochim. Biophys. Acta 1996, 1314, 13–24. [Google Scholar] [CrossRef]
- Green, M.P.; Ludbrook, S.B.; Miller, D.D.; Horgan, C.M.T.; Barry, S.T. Structural elements of the osteopontin SVVYGLR motif important for the interaction with alpha(4) integrins. FEBS Lett. 2001, 503, 75–79. [Google Scholar] [CrossRef]
- Smith, L.L.; Cheung, H.K.; Ling, L.E. Osteopontin N-terminal domain contains a cryptic adhesive sequence recognized by alpha9beta1 integrin. J. Biol. Chem. 1996, 271, 28485–28491. [Google Scholar] [CrossRef]
- Yokosaki, Y.; Matsuura, N.; Sasaki, T.; Murakami, I.; Schneider, H.; Higashiyama, S.; Saitoh, Y.; Yamakido, M.; Taooka, Y.; Sheppard, D. The integrin α9β1 binds to a novel recognition sequence (SVVYGLR) in the thrombin-cleaved amino-terminal fragment of osteopontin. J. Biol. Chem. 1999, 274, 36328–36334. [Google Scholar] [CrossRef]
- Triantafilou, K.; Kar, S.; Vakakis, E.; Kotecha, S.; Triantafilou, M. Human respiratory syncytial virus viroporin SH: A viral recognition pathway used by the host to signal inflammasome activation. Thorax 2013, 68, 66–75. [Google Scholar] [CrossRef]
- Bai, F.; Thompson, E.A.; Vig, P.J.S.; Leis, A.A. Current Understanding of West Nile Virus Clinical Manifestations, Immune Responses, Neuroinvasion, and Immunotherapeutic Implications. Pathogens 2019, 8, 193. [Google Scholar] [CrossRef]
- Paul, A.M.; Acharya, D.; Duty, L. Osteopontin facilitates West Nile virus neuroinvasion via neutrophil “Trojan horse” transport. Sci. Rep. 2017, 7, 4722. [Google Scholar] [CrossRef]
- Bortell, N.; Flynn, C.; Conti, B.; Fox, H.S.; Marcondes, M.C.G. Osteopontin Impacts West Nile virus Pathogenesis and Resistance by Regulating Inflammasome Components and Cell Death in the Central Nervous System at Early Time Points. Mediat. Inflamm. 2017, 2017, 7582437. [Google Scholar] [CrossRef]
- Luissint, A.C.; Artus, C.; Glacial, F.; Ganeshamoorthy, K.; Couraud, P.O. Tight junctions at the blood brain barrier: Physiological architecture and disease-associated dysregulation. Fluids Barriers CNS 2012, 9, 23. [Google Scholar] [CrossRef]
- Zhao, Z.; Nelson, A.R.; Betsholtz, C.; Zlokovic, B.V. Establishment and Dysfunction of the Blood-Brain Barrier. Cell 2015, 163, 1064–1078. [Google Scholar] [CrossRef]
- Diamond, M.S.; Klein, R.S. West Nile virus: Crossing the blood-brain barrier. Nat. Med. 2004, 10, 1294–1295. [Google Scholar] [CrossRef]
- Samuel, M.A.; Diamond, M.S. Pathogenesis of West Nile Virus infection: A balance between virulence, innate and adaptive immunity, and viral evasion. J. Virol. 2006, 80, 9349–9360. [Google Scholar] [CrossRef] [PubMed]
- Samuel, M.A.; Wang, H.; Siddharthan, V.; Morrey, J.D.; Diamond, M.S. Axonal transport mediates West Nile virus entry into the central nervous system and induces acute flaccid paralysis. Proc. Natl. Acad. Sci. USA 2007, 104, 17140–17145. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Dai, J.; Bai, F. Matrix metalloproteinase 9 facilitates West Nile virus entry into the brain. J. Virol 2008, 82, 8978–8985. [Google Scholar] [CrossRef]
- Agrawal, S.; Anderson, P.; Durbeej, M. Dystroglycan is selectively cleaved at the parenchymal basement membrane at sites of leukocyte extravasation in experimental autoimmune encephalomyelitis. J. Exp. Med. 2006, 203, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Esparza, J.; Kruse, M.; Lee, J.; Michaud, M.; Madri, J.A. MMP-2 null mice exhibit an early onset and severe experimental autoimmune encephalomyelitis due to an increase in MMP-9 expression and activity. FASEB J. 2004, 18, 1682–1691. [Google Scholar] [CrossRef] [PubMed]
- Romanic, A.M.; Madri, J.A. Extracellular matrix-degrading proteinases in the nervous system. Brain Pathol. 1994, 4, 145–156. [Google Scholar] [CrossRef]
- Brilha, S.; Ong, C.W.M.; Weksler, B.; Romero, N.; Couraud, P.O.; Friedland, J.S. Matrix metalloproteinase-9 activity and a downregulated Hedgehog pathway impair blood-brain barrier function in an in vitro model of CNS tuberculosis. Sci. Rep. 2017, 7, 16031. [Google Scholar] [CrossRef]
- Tang, J.; Kang, Y.; Huang, L.; Wu, L.; Peng, Y. TIMP1 preserves the blood–brain barrier through interacting with CD63/integrin β1 complex and regulating downstream FAK/RhoA signaling. Acta Pharm. Sin. B 2020, 10, 987–1003. [Google Scholar] [CrossRef]
- Rangaswami, H.; Kundu, G.C. Osteopontin stimulates melanoma growth and lung metastasis through NIK/MEKK1-dependent MMP-9 activation pathways. Oncol. Rep. 2007, 18, 909–915. [Google Scholar] [CrossRef]
- Lindsey, M.L.; Zouein, F.A.; Tian, Y.; Padmanabhan, I.R.; de Castro Brás, L.E. Osteopontin is proteolytically processed by matrix metalloproteinase 9. Can. J. Physiol. Pharmacol. 2015, 93, 879–886. [Google Scholar] [CrossRef]
- Chan, J.L.; Reeves, T.M.; Phillips, L.L. Osteopontin expression in acute immune response mediates hippocampal synaptogenesis and adaptive outcome following cortical brain injury. Exp. Neurol. 2014, 261, 757–771. [Google Scholar] [CrossRef] [PubMed]
- Doyle, K.P.; Yang, T.; Lessov, N.S. Nasal administration of osteopontin peptide mimetics confers neuroprotection in stroke. J. Cereb. Blood Flow Metab. 2008, 28, 1235–1248. [Google Scholar] [CrossRef] [PubMed]
- Meller, R.; Stevens, S.L.; Minami, M. Neuroprotection by osteopontin in stroke. J. Cereb. Blood Flow Metab. 2005, 25, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Ayer, R.; Sugawara, T. Protective effects of recombinant osteopontin on early brain injury after subarachnoid hemorrhage in rats. Crit. Care Med. 2010, 38, 612–618. [Google Scholar] [CrossRef]
- Shin, T. Osteopontin as a two-sided mediator in acute neuroinflammation in rat models. Acta Histochem. 2012, 114, 749–754. [Google Scholar] [CrossRef]
- Bai, F.; Kong, K.F.; Dai, J. A paradoxical role for neutrophils in the pathogenesis of West Nile virus. J. Infect. Dis. 2010, 202, 1804–1812. [Google Scholar] [CrossRef]
- Beasley, D.W.; Li, L.; Suderman, M.T.; Barrett, A.D. Mouse neuroinvasive phenotype of West Nile virus strains varies depending upon virus genotype. Virology 2002, 296, 17–23. [Google Scholar] [CrossRef]
- Roulston, A.; Marcellus, R.C.; Branton, P.E. Viruses and apoptosis. Annu. Rev. Microbiol. 1999, 53, 577–628. [Google Scholar] [CrossRef]
- Song, G.; Ming, Y.; Mao, Y.; Bao, S.; Ouyang, G. Osteopontin Prevents Curcumin-Induced Apoptosis and Promotes Survival Through Akt Activation via αvβ3 Integrins in Human Gastric Cancer Cells. Exp. Biol. Med. 2008, 233, 1537–1545. [Google Scholar] [CrossRef]
- Iida, T.; Wagatsuma, K.; Hirayama, D.; Nakase, H. Is Osteopontin a Friend or Foe of Cell Apoptosis in Inflammatory Gastrointestinal and Liver Diseases? Int. J. Mol. Sci. 2017, 19, 7. [Google Scholar] [CrossRef]
- Rittling, R.S.; Chambers, A.F. Role of osteopontin in tumour progression. Br. J. Cancer 2004, 90, 1877–1881. [Google Scholar] [CrossRef] [PubMed]
- Saleh, S.; Thompson, D.E.; McConkey, J.; Murray, P.; Moorehead, R.A. Osteopontin regulates proliferation, apoptosis, and migration of murine claudin-low mammary tumor cells. BMC Cancer 2016, 16, 359. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, A.M.; Hocker, S.E.; Leis, A.A. West Nile virus induces a post-infectious pro-inflammatory state that explains transformation of stable ocular myasthenia gravis to myasthenic crises. J. Neurol. Sci. 2018, 395, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Leis, A.A.; Szatmary, G.; Ross, M.A.; Stokic, D.S. West nile virus infection and myasthenia gravis. Muscle Nerve 2014, 49, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Segovia, J.; Sabbah, A.; Mgbemena, V. TLR2/MyD88/NF-κB pathway, reactive oxygen species, potassium efflux activates NLRP3/ASC inflammasome during respiratory syncytial virus infection. PLoS ONE 2012, 7, e29695. [Google Scholar] [CrossRef] [PubMed]
- Han, R.K.; Cheng, Y.F.; Zhou, S.S. Increased circulating Th17 cell populations and elevated CSF osteopontin and IL-17 concentrations in patients with Guillain-Barré syndrome. J. Clin. Immunol. 2014, 34, 94–103. [Google Scholar] [CrossRef]
- Hassin-Baer, S.; Kirson, E.D.; Shulman, L. Stiff-person syndrome following West Nile fever. Arch. Neurol. 2004, 61, 938–941. [Google Scholar] [CrossRef]
- Leis, A.A.; Stokic, D.S. Neuromuscular manifestations of west nile virus infection. Front. Neurol. 2012, 3, 37. [Google Scholar] [CrossRef]
- Almhanna, K.; Palanichamy, N.; Sharma, M.; Hobbs, R.; Sil, A. Unilateral brachial plexopathy associated with West Nile virus meningoencephalitis. Clin. Infect. Dis. 2003, 36, 1629–1630. [Google Scholar] [CrossRef]
- Yu, H.; Liu, X.; Zhong, Y. The Effect of Osteopontin on Microglia. BioMed Res. Int. 2017, 2017, 1879437. [Google Scholar] [CrossRef]
- Clemente, N.; Raineri, D.; Cappellano, G. Osteopontin Bridging Innate and Adaptive Immunity in Autoimmune Diseases. J. Immunol. Res. 2016, 2016, 7675437. [Google Scholar] [CrossRef] [PubMed]
- Frenzel, D.F.; Borkner, L.; Scheurmann, J.; Singh, K.; Scharffetter-Kochanek, K.; Weiss, J.M. Osteopontin deficiency affects imiquimod-induced psoriasis-like murine skin inflammation and lymphocyte distribution in skin, draining lymph nodes and spleen. Exp. Derm. 2015, 24, 305–307. [Google Scholar] [CrossRef] [PubMed]
- Hur, E.M.; Youssef, S.; Haws, M.E.; Zhang, S.Y.; Sobel, R.A.; Steinman, L. Osteopontin-induced relapse and progression of autoimmune brain disease through enhanced survival of activated T cells. Nat. Immunol. 2007, 8, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Kon, S.; Nakayama, Y.; Matsumoto, N.; Ito, K.; Kanayama, M.; Kimura, C.; Kouro, H.; Ashitomi, D.; Matsuda, T.; Uede, T. A novel cryptic binding motif, LRSKSRSFQVSDEQY, in the C-terminal fragment of MMP-3/7-cleaved osteopontin as a novel ligand for α9β1 integrin is involved in the anti-type II collagen antibody-induced arthritis. PLoS ONE 2014, 9, e116210. [Google Scholar] [CrossRef] [PubMed]
- Rittling, R.S.; Singh, R. Osteopontin in Immune-mediated Diseases. J. Dent. Res. 2015, 94, 1638–1645. [Google Scholar] [CrossRef]
- Phillips, D.W.; Vincent, A. Pathogenesis of myasthenia gravis: Update on disease types, models, and mechanisms. F1000Research 2016, 5, 1513. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazneen, F.; Bai, F. The Roles of Osteopontin in the Pathogenesis of West Nile Encephalitis. Vaccines 2020, 8, 748. https://doi.org/10.3390/vaccines8040748
Nazneen F, Bai F. The Roles of Osteopontin in the Pathogenesis of West Nile Encephalitis. Vaccines. 2020; 8(4):748. https://doi.org/10.3390/vaccines8040748
Chicago/Turabian StyleNazneen, Farzana, and Fengwei Bai. 2020. "The Roles of Osteopontin in the Pathogenesis of West Nile Encephalitis" Vaccines 8, no. 4: 748. https://doi.org/10.3390/vaccines8040748
APA StyleNazneen, F., & Bai, F. (2020). The Roles of Osteopontin in the Pathogenesis of West Nile Encephalitis. Vaccines, 8(4), 748. https://doi.org/10.3390/vaccines8040748





