Abstract
Eimeria tenella (E. tenella) is a highly pathogenic and prevalent species of Eimeria that infects chickens, and it causes a considerable disease burden worldwide. The secreted proteins and surface antigens of E. tenella at the sporozoite stage play an essential role in the host–parasite interaction, which involves attachment and invasion, and these interactions are considered vaccine candidates based on the strategy of cutting off the invasion pathway to interrupt infection. We selected two highly expressed surface antigens (SAGs; Et-SAG13 and Et-SAG) and two highly expressed secreted antigens (rhoptry kinases Eten5-A, Et-ROPK-Eten5-A and dense granule 12, Et-GRA12) at the sporozoite stage. Et-ROPK-Eten5-A and Et-GRA12 were two unexplored proteins. Et-ROPK-Eten5-A was an E. tenella-specific rhoptry (ROP) protein and distributed in the apical pole of sporozoites and merozoites. Et-GRA12 was scattered in granular form at the sporozoite stage. To evaluate the potential of rEt-ROPK-Eten5-A, rEt-GRA12, rEt-SAG13 and rEt-SAG proteins as a coccidiosis vaccine, the protective efficacy was examined based on survival rate, lesion score, body weight gain, relative body weight gain and oocyst output. The survival rate was significantly improved in rEt-ROPK-Eten5-A (100%) and rEt-GRA12 (100%) immune chickens compared to the challenged control group (40%). The average body weight gains of rEt-ROPK-Eten5-A, rEt-GRA12, rEt-SAG13 and rEt-SAG immunized chickens were significantly higher than those of unimmunized chickens. The mean lesion score and oocyst output of the rEt-ROPK-Eten5-A immunized chickens were significantly reduced compared to unimmunized challenged chickens. These results suggest that the rEt-ROPK-Eten5-A protein effectively triggered protection against E. tenella in chickens and provides a useful foundation for future work developing anticoccidial vaccines.
1. Introduction
Coccidiosis is caused by the genus Eimeria, and it is one of the most widespread and economically detrimental diseases affecting the global poultry industry [1,2]. The annual loss due to coccidiosis exceeds $3 billion USD globally [3]. Eimeria tenella (E. tenella) is one of the most harmful species due to its wide prevalence and high pathogenicity [4]. E. tenella parasitizes chicken cecal epithelial cells and leads to reduced body weight gains, epithelial cell damage and death [5].
Conventional control strategies primarily rely on anticoccidial drugs [3,6]. However, alternative control strategies are urgently needed due to the rapid emergence of drug-resistant parasites, the high cost of new drug development and the increasing legislation restrictions on the use of anticoccidial drugs [7,8]. Although live oocyst vaccines are used commercially in some regions, virulence variation and the high cost of vaccine production restrict the application of live vaccines [9,10]. Only CoxAbic was successfully commercialized. However, CoxAbic is a subunit vaccine prepared from E. maxima affinity-purified gametocyte antigens (APGAs), and it is limited by a complicated purification process and high production cost [3,11]. These drawbacks have driven the development of new control strategies, especially the screening of effective immunoprotective proteins for the development of a subunit vaccine [7].
E. tenella belongs to the phylum Apicomplexa, and it has rhoptries (ROP), micronemes (MIC) and dense granule (GRA) secretory organelles. These secretory organelles produce a large number of secretory proteins that mediate parasite invasion and survival [12]. Proteomic and genomic sequence profiling of apicomplexans showed that many secreted proteins were ROP proteins [13,14,15]. The secreted proteins and surface antigens (SAGs) play a critical role in host–parasite interactions involving attachment and invasion, which are considered vaccine candidates [9]. The extracellular sporozoite stage of E. tenella is critical for invasion, and it is immunologically vulnerable and functionally important for the parasite [16]. Therefore, the screening of surface antigens and secreted antigens that are highly expressed at the sporozoite stage is significant for the development of anticoccidial vaccines. The strategy for these vaccines is to block parasite infection by cutting off the invasion process. Several sporozoite proteins of Eimeria were identified as anticoccidial vaccine candidates. A sporozoite antigen (micronemes 1, EtMIC1) improved the efficacy against E. tenella [17]. A rhomboid-like protease (ETRHO1) also showed value as a vaccine candidate because it imparted partial protection to chickens against E. tenella [18]. The sporozoite-specific SAG1 induced partial protective immunity as a recombinant protein vaccine [19,20,21]. Antigen refractile body protein (EtSO7) is located in sporozoite refractile bodies, and it provoked cellular and humoral immune responses to provide significant protection against cecal coccidiosis in immunized chickens [16]. Immune mapped protein 1 (IMP1) was recently identified as an anticoccidial vaccine candidate, and it is localized on the sporozoite cell membrane [22,23].
The present study (i) characterized four sporozoite antigens from E. tenella, (ii) expressed the four sporozoites antigens and located two unexplored proteins (Et-ROPK-Eten5-A and Et-GRA12) in different stages of parasites and (iii) evaluated the protective efficacy of these four antigens to develop an effective vaccine against coccidiosis.
2. Materials and Methods
2.1. Ethics Statement
All animal experiments were performed in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of the Ministry of Science and Technology of China. The Institutional Animal Care and Use Committee of China Agricultural University approved all experimental procedures (approval number: AW05(7)069102-2).
2.2. Parasites and Animals
E. tenella was maintained and propagated in two-week-old coccidia-free SPF chickens, as previously described [24]. The oocysts were collected and purified as previously described [25]. SPF chickens and female BALB/c mice were obtained from Merial Animal Health Co., Ltd. (Beijing, China). Sterilized food and clean water without anti-coccidia drugs were provided in sufficient supply.
2.3. Bioinformatic Analysis
The nucleotide sequences and amino acid sequences were downloaded from ToxoDB (https://toxodb.org/toxo/). The amino acid sequence alignment was performed using Basic Local Alignment Search Tool in NCBI (https://blast.ncbi.nlm.nih.gov/Blast.cgi) and Clustal X software version 1.83 (http://www.clustal.org/). A phylogenetic tree was constructed using the neighbor-joining method in MEGA software (version 5.05, State College, PA, USA) (http://www.megasoftware.net/). The signal peptide was analyzed using the SignalP 4.0 server (http://www.cbs.dtu.dk/services/SignalP/). Transcriptional level data were obtained from ToxoDB uploads from Walker et al. [26]. The kinase domain was predicted using SMART (http://smart.embl-heidelberg.de/).
2.4. Cloning, Expression and Purification of Four Sporozoite Antigens
Total RNA was isolated using Trizol® (Life Technologies, Carlsbad, MD, USA), and cDNA was synthesized using TransScript One-Step gDNA Removal and cDNA Synthesis SuperMix (Transgen Biotech, Beijing, China). The full coding sequence of Et-ROPK-Eten5-A without the signal peptide sequence was amplified from sporozoite cDNA using the primers in Supplementary Table S1 (Supplementary Materials). High-fidelity enzyme (ransgen Biotech, Beijing, China) was used for the PCR reaction, and the PCR protocol was used as recommended in the instructions. The expression plasmid was pET28a (+) (Novagen, Darmstadt, Germany), which was preserved in the Key Laboratory of Animal Parasitology (Beijing, China). The pET28a (+) plasmid was linearized using PCR and ligated with the Et-ROPK-Eten5-A sequence via seamless cloning (Vazyme Biotech, Co., Ltd., Nanjing, China). The recombinant plasmid was transformed into Transetta (DE3) cells (TransGen, Beijing, China). The expression and purification procedures for recombinant Et-ROPK-Eten5-A protein (rEt-ROPK-Eten5-A) were performed following the manufacturer’s protocol (Novagen, Darmstadt, Germany). Briefly, the cells were induced for 5 h at 37 °C with 1 mM isopropyl-D-1-thiogalactopyranoside (IPTG), harvested and broken via ultrasonication. The recombinant protein was purified using a Ni2+ affinity column. Protein concentration was measured using micro-BCA protein assay reagent (Pierce, Rockford, IL, USA). The amplification, expression and purification procedures of rEt-GRA12, rEt-SAG13 and rEt-SAG were the same as those for rEt-ROPK-Eten5-A.
2.5. Sera
Negative serum was obtained from coccidia-free SPF chickens. Positive serum against E. tenella was collected from SFP chickens artificially infected with E. tenella. Polyclonal antibodies against Et-rROPK-Eten5-A and rEt-GRA12 were produced as previously described [27]. Briefly, 100 μg rEt-ROPK-Eten5-A or rEt-GRA12 was emulsified with Complete Freund’s adjuvant, then injected subcutaneously into female BALB/c mice (4–6 weeks old), followed by two boosters at the same dose. The titers of polyclonal antibodies were examined using ELISA for rEt-ROPK-Eten5-A or rEt-GRA12 as the antigen.
2.6. Immunoblotting and Immunofluorescence Assay
Recombinant Et-ROPK-Eten5-A, Et-GRA12, Et-SAG13 and Et-SAG were analyzed using Western blot assays with anti-E. tenella chicken sera. The recombinant proteins were separated in 12% SDS-PAGE and transferred to nitrocellulose membranes (Millipore, Bedford, MA, USA). Membranes were treated with 5% skim milk and incubated with chicken serum against E. tenella (1:500 dilutions) overnight at 4 °C. The membrane was incubated with horseradish peroxidase (HRP)-conjugated goat anti-chicken IgY (Sigma, Saint Louis, MO, USA)) for 1 h at 37 °C, and proteins were visualized using chemiluminescence reagents (CoWin Biotech Co., Ltd., Beijing, China) and film exposures. Coccidia-free SPF chicken serum was used as a control. Sporozoite excystation and purification were consistent with a previous study [28]. The merozoites were purified from infected chicken ceca, as published previously [29]. For immunofluorescence, the purified sporozoites and merozoites were adhered to coverslips precoated with poly-lysine and fixed with 4% (w/v) paraformaldehyde. The coverslips were washed, and the parasites were permeabilized with 0.1% Triton and incubated with mouse anti-Et-ROPK-Eten5-A serum (1:200) for 1 h at 37 °C. FITC- or Cy3-conjugated antibodies were used for labeling (Sigma, Saint Louis, MO, USA). DNA was stained with Hoechst 33,258 (Sigma, Saint Louis, MO, USA). Images were obtained using a Leica confocal microscope system (Leica, TCS SP52, Heidelberg, Germany).
2.7. The Protective Efficacy Comparison of Four Recombinant Sporozoite Proteins
Fourteen-day-old chickens were weighed and randomly divided into six groups of 10 chickens per group. Animal experiments were performed as shown in Figure 1. Experimental groups were intramuscularly vaccinated with 100 µg corresponding recombinant protein per chicken in the thigh. The challenged control group and unchallenged control chickens were injected with Freund’s adjuvant. One week after primary immunization, a booster immunization was administered at the same dose. Seven days after the booster vaccination, all chickens were infected orally with 10,000 freshly sporulated E. tenella oocysts, and the unchallenged group received phosphate buffer saline (PBS). The protective efficacy was evaluated based on the survival rate (%), lesion score, body weight gain, relative body weight gain (%), oocyst output and oocyst decrease rate (%). Cecum lesion score was observed on the sixth day post-challenge and recorded consistent with a previous description [30]. Oocyst output was counted using a McMaster egg-counting chamber after each challenge infection. Total feces of each group was collected, mixed and weighed within five days. Three samples were randomly selected to calculate the oocysts per gram (OPG) per sample. The average amount of oocyst output per chicken was calculated. Decreases in oocyst rates were calculated as (oocyst output from positive control chickens—oocyst output from vaccinated chickens) × 100/oocyst output from positive control chickens. Body weight gain of chickens was calculated as weight at the time of slaughter—weight at the time of challenge. The relative body weight gain rate was calculated as weight gain of the experimental group × 100/weight gain of the unchallenged group.
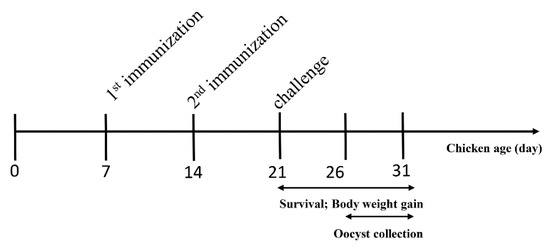
Figure 1.
Schematic outline of the experimental design.
2.8. Statistical Analysis
Graphs were created, and statistical analyses were performed, using Graph Pad Prism (San Diego, CA, USA). Graphs represent means, and error bars represent standard errors of means. All data were analyzed using t-tests. p-values are represented by asterisks in figures as follows: * p < 0.05, ** p < 0.01, and *** p < 0.001. All p < 0.05 were considered significant.
3. Results
3.1. Screening and Characterization of Four Sporozoite Antigens
Surface antigens and secreted proteins are considered vaccine candidates because the sporozoite stage is a critical stage for invasion. Therefore, screening for surface antigens or secreted antigens that are highly expressed at the sporozoite stage is significant for the development of anticoccidial vaccines. The present study analyzed the expression patterns of the SAG family proteins in different stages of E. tenella (Figure 2a) [26]. Based on the expression patterns, we selected two highly expressed SAG antigens at the sporozoite stage (ETH_00013178 and ETH_00034880). ETH_00013178 and ETH_00034880 were identified in a previous study and named Et-SAG13 and Et-SAG [31]. According to the homology analysis of the phylogenetic tree, Et-SAG13 and Et-SAG belonged to different branches (Figure 2b).
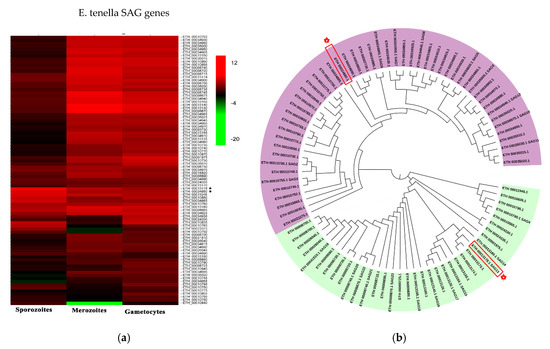
Figure 2.
Expression profile and evolutionary analysis of E. tenella SAG proteins. (a) Transcription levels of surface antigen (SAG) genes in sporozoites, merozoites and gametocytes were obtained from ToxoDB. The heatmap of the expression profile is drawn. Et-SAG and Et-SAG13 are indicated with asterisks (*). (b) The amino acid sequences were downloaded from ToxoDB. A phylogenetic tree was constructed using the neighbor-joining method in MEGA software (version 5.05). Et-SAG and Et-SAG13 are indicated with asterisks (*). The light purple and light green represent different branches of the SAG family.
Similarly, the expression patterns of ROP family and GAR family proteins in different stages of E. tenella were compared [26]. An ROP protein and a GRA protein (ETH_00005405 and ETH_00024035) were highly expressed at the sporozoite stage and selected (Figure 3a). Sequence alignment revealed that ETH_00024035 showed homology with GRA12 from Toxoplasma gondii, which was named Et-GRA12. A previous study revealed that ETH_00005405 was a specific ROP protein of E. tenella and belonged to the ROPK-Eten5 subfamily [32], named Et-ROPK-Eten5-A. Phylogenetic tree analysis showed that the Et-ROPK-Eten5 subfamily was a cluster alone, and Et-ROPK-Eten5-A belonged to this cluster (Figure 3b). Multiple sequence alignment revealed that Et-ROPK-Eten5-A shared high similarity with the Et-ROPK-Eten5-B (68.35%), but only 12.17%–26.30% with other clusters of ROP proteins (Figure 4). Comparison of the active site residues showed that Et-ROPK-Eten5-A contained a classical protein kinase domain at 265 to 499 amino acids and eight conserved motifs, including LYEDNESV, PYAMRL, ETT, SHNNLKLENF, GNFGT, AEMEL, SDMWG and DRLDA. However, Et-ROPK-Eten5-A lacked critical aspartates that participated in the kinase catalytic activity [32].
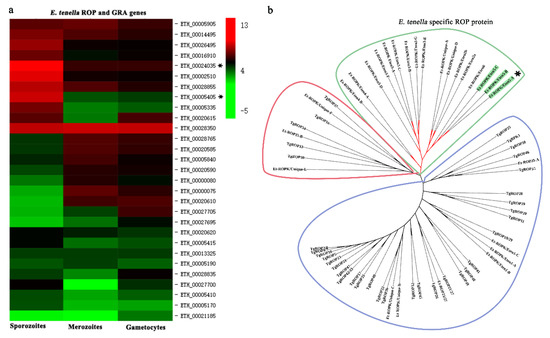
Figure 3.
Expression profile and evolutionary analysis of E. tenella ROP proteins. (a) Transcription levels of ROP genes in sporozoites, merozoites and gametocytes were obtained from ToxoDB. The heatmap of the expression profile is drawn. Et-ROPK-Eten5-A and Et-GRA 12 are indicated with asterisks (*). (b) A phylogenetic tree of E. tenella and T. gondii ROP proteins was constructed using the neighbor-joining method in MEGA software (version 5.05). Et-ROPK-Eten5-A is indicated with an asterisk. The Et-ROPK-Eten5 subfamily is indicated with asterisks (*) and green background. E. tenella specific proteins are circled in green. ROP proteins from different branches are circled in red and blue, respectively.
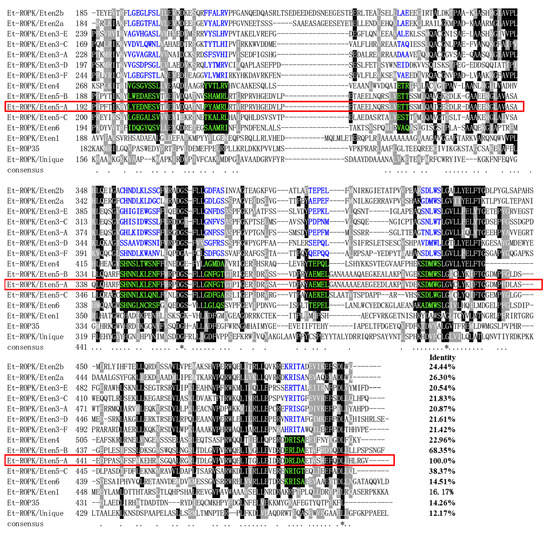
Figure 4.
Sequence alignment and conserved motif analysis of E. tenella rhoptries (ROP) proteins. Multiple sequence alignment was performed using Clustal X software version 1.83. The regions of high identity and similarity between ROP sequences are shown as black and gray columns, respectively. The identity of Et-ROPK-Eten5-A with each ROP is shown at the end of the alignment. The conserved motif regions of the kinase domain are highlighted. The conserved motifs of predicted noncanonical catalytic mechanisms are highlighted with blue letters. The conserved motifs of likely inactive rhoptry kinase subfamilies are highlighted with a black background and green letters. The catalytic activities of the conserved motifs were predicted in a previous report [32]. TheEt-ROPK-Eten5-A in this study is circled in red frame.
3.2. Cloning and Expression of the Four Sporozoite Proteins
The full-length coding sequences of Et-ROPK-Eten5-A, Et-GRA12, Et-SAG13 and Et-SAG were 1524, 1221, 789 and 762 bp nucleotides, respectively, which individually encoded 508, 407, 263 and 254 amino acids with predicted molecular weights of ~56, ~45, ~29 and ~29 kDa, respectively (Figure 5a). There was a signal peptide cleavage site in the Et-ROPK-Eten5-A and Et-GRA12 sequences, which indicated that both proteins were secretory proteins. The predicted molecular weights of Et-ROPK-Eten5-A and Et-GRA12 without the signal peptide were ~51 kDa and ~41 kDa, respectively. The expression of rEt-ROPK-Eten5-A, rEt-GRA12, rEt-SAG13 and rEt-SAG proteins in Escherichia coli transformed with the pET28a (+) plasmid were confirmed using SDS-PAGE and were consistent with the predicted sizes of ~57, ~47, ~35 and ~35 kDa, including a His-tag, respectively (Figure 5b).
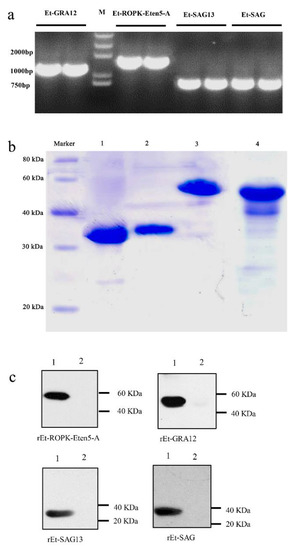
Figure 5.
Cloning, expression and immunogenicity identification of four sporozoite recombinant proteins. (a) The full coding sequences of Et-ROPK-Eten5-A, Et-GRA12, Et-SAG and Et-SAG13 without the signal peptide sequences were amplified from cDNA. (b) Purification of recombinant proteins. Lane 1, purified rEt-SAG13 protein; Lane 2, purified rEt-SAG protein; Lane 3, purified rEt-ROPK-Eten5-A protein; Lane 4, purified rEt-GRA12 protein. (c) Western blotting analysis of recombinant proteins. Lane 1, E. tenella positive serum; Lane 2, negative serum.
3.3. Immunoblot Analysis of the Four Recombinant Sporozoite Proteins
The rEt-ROPK-Eten5-A, rEt-GRA12, rEt-SAG13 and rEt-SAG proteins were tested using chicken E. tenella-positive serum, and a coccidia-free chicken serum was used as a control. The results showed that all four recombinant proteins reacted with positive serum, but no band was observed in the negative control (Figure 5c).
3.4. Immunolocalizations of Endogenous Et-ROPK-Eten5-A and Et-GRA12
Because Et-ROPK-Eten5-A and Et-GRA12 were not identified previously, localization studies were performed. The distributions of Et-ROPK-Eten5-A and Et-GRA12 in sporozoites and merozoites stages of E. tenella were assessed using immunofluorescence and a mouse anti-rEt-ROPK-Eten5-A antibody and mouse anti-Et-GRA12 antibody. Et-ROPK-Eten5-A was distributed in the apical area of sporozoites (Figure 6), and it distributed in the apical pole of merozoites as a slender form. Et-GRA12 was scattered in granular form in sporozoites (Figure 6), and it was not detected in the body of merozoites.
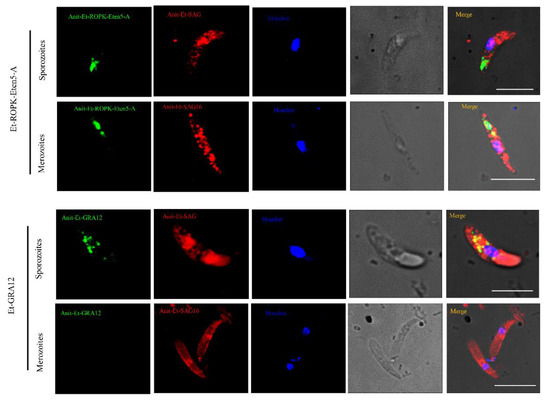
Figure 6.
Immunofluorescence localization of Et-ROPK-Eten5-A and Et-GRA12 in different stages of E. tenella. Immunofluorescence localization of Et-ROPK-Eten5-A and Et-GRA12 in sporozoites and merozoites using mouse anti-rEt-ROPK-Eten5-A and mouse anti-r Et-GRA12 serum. Scale bars: 10 μm. Et-SAG and Et-SAG16 (red) served as parasite surface markers. The morphology of parasites under a light microscopes is shown in the penultimate column.
3.5. Recombinant Et-ROPK-Eten5-A Induces Effective Protection against E. tenella
To evaluate the protective efficacy of rEt-ROPK-Eten5-A, rEt-GRA12, rEt-SAG13 and rEt-SAG proteins, groups of chickens were challenged with E. tenella. The survival rate was significantly improved in rEt-ROPK-Eten5-A- and rEt-GRA12-immunized chickens compared to the challenged control group (Figure 7a). There was a significant reduction in the mean lesion score of the rEt-ROPK-Eten5-A (p < 0.05) and rEt-GRA12 (p < 0.05) immunized chickens compared to unimmunized chickens (Figure 7b). No significant difference in rEt-SAG13 (p > 0.05) or rEt-SAG (p > 0.05) immunized birds was observed compared to unimmunized chickens. The average body weight gains of rEt-ROPK-Eten5-A (28.00 ± 3.50 g, p < 0.001), rEt-GRA12 (22.80 ± 5.53 g, p < 0.001), rEt-SAG13 (7.70 ± 7.80 g, p < 0.001) and rEt-SAG (12.00 ± 10.39, p < 0.001) immunized chickens were significantly higher than those of adjuvant-immunized chickens (−27 ± 14.24 g) and corresponded to relative body weight gain rates of 47% (rEt-ROPK-Eten5-A, p < 0.001), 38% (rEt-GRA12, p < 0.001), 12% (rEt-SAG13, p < 0.001) and 20% (rEt-SAG, p < 0.001) compared to control immunized chickens (−46%), respectively (Figure 7c,d). The decreases in oocyst output were 82.75% in the rEt-ROPK-Eten5-A-immunized group and 29.95%, 12.29% and 5.18% in the rEt-GRA12-, rEt-SAG13- and rEt-SAG-immunized groups compared to unimmunized challenged chickens (Figure 7e,f). These results demonstrated that the four recombinant sporozoite proteins triggered a heterologous immunoprotective effect in E. tenella-infected chickens, and the rEt-ROPK-Eten5-A protein had the best protection efficiency.
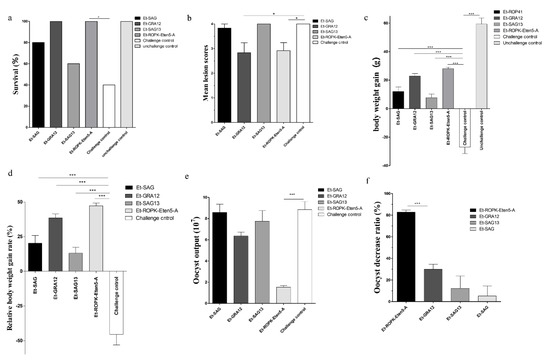
Figure 7.
Protective efficacy of the four sporozoite antigens against E. tenella. (a) Effects of vaccination with four sporozoite antigens on survival rate, (b) cecum lesion score, (c) body weight gain, (d) relative body weight gain, (e) oocyst output and (f) oocyst decrease rate. Data are the means ± SD (error bars) of three independent experiments. All data were analyzed using t-tests. p-values are represented by asterisks as follows: * p < 0.05, and *** p < 0.001.
4. Discussion
E. tenella belongs to the phylum Apicomplexa, and it has unique secretory organelles (micronemes, rhoptries and dense granules) that secrete proteins for the invasion process [12,33], which are considered vaccine candidates [9]. The present study focused on highly expressed proteins in sporozoites, including two secreted antigens and two surface antigens. E. tenella SAG genes are classified into four categories, each on a different chromosome. Family A is common to all species, but family B is restricted to E. tenella and E. necatrix [31]. Et-SAG13 belongs to family B, and Et-SAG belongs to family A. Phylogenetic tree analysis based on homology revealed that the SAG family may be divided into two clades, and the Et-SAG13 and Et-SAG proteins belonged to different branches. A previous study identified three ROPK subclades of particular interest, including a structurally conserved N-terminal extension to the kinase domain (NTE), E. tenella-specific expansion and a basal cluster [32]. Phylogenetic tree analysis showed that Et-ROPK-Eten5-A belonged to the Et-ROPK-Eten5 subfamily, which is the ROPK subclade of the E. tenella-specific expansion cluster. Our sequence alignment showed that Et-ROPK-Eten5-A contained a classical protein kinase domain and eight conserved motifs (LYEDNESV, PYAMRL, ETT, SHNNLKLENF, GNFGT, AEMEL, SDMWG and DRLDA) of the inactive rhoptry kinase subfamilies. However, Et-ROPK-Eten5-A lacked critical aspartates that participated in the kinase catalytic activity, and it is considered a pseudokinase [32].
Because Et-ROPK-Eten5-A and Et-GRA12 were not identified previously, localization studies were performed. The distribution of Et-ROPK-Eten5-A and Et-GRA12 in sporozoites and merozoites of E. tenella was assessed using immunofluorescence and a mouse anti-rEt-ROPK-Eten5-A antibody and mouse anti-Et-GRA12 antibody. Et-ROPK-Eten5-A was distributed in the apical area of sporozoites, which was similar to the localization of Et-ROP1 protein [34]. A previous report showed that free second-generation merozoites had a long, slender body with two rhoptries at the apical pole [35]. Et-ROPK-Eten5-A was distributed in the apical pole of merozoites with a slender form in our study, which is consistent with the distribution of identified ROP proteins. Et-GRA12 was scattered in a granular form in sporozoites, which is similar to the localization of dense granular proteins in the tachyzoites of Neospora caninum [36].
E. tenella is one of the most prevalent and pathogenic species of Eimeria infecting chickens [4]. E. tenella infection may result in severe lesions of the ceca, body weight loss, hemorrhagic diarrhea, hemorrhage and death [4,37]. Anticoccidial drugs are predominantly used to control Eimeria infection. However, the rise in drug resistance and public pressure for restrictions on foodborne animal chemicals continue to drive the development of anti-coccidiosis vaccines [3,38,39]. Therefore, it is urgent to develop safe and effective vaccines against avian coccidiosis [7,21,40]. The sporozoite stage plays a critical role in invasion. Therefore, the screening of sporozoites that highly expressed secreted antigens or surface antigens is crucial for the development of anticoccidial vaccines. The strategy for these vaccines is to block parasite infection by disturbing the invasion process. The present study successfully obtained recombinant Et-ROPK-Eten5-A, Et-GRA12, Et-SAG13 and Et-SAG proteins and assessed their immune protective efficiency against E. tenella. We detected the reaction of four recombinant proteins with positive serum of E. tenella infected chickens by Western blotting, respectively. The results implied that the chicken immune system would produce antibodies against these proteins under natural infection. Our results showed that vaccination with rEt-ROPK-Eten5-A significantly increased the survival rate and body weight gains, decreased the oocyst output and alleviated cecum lesions compared to the challenge control group. All of these results suggest that rEt-ROPK-Eten5-A provided effective protection for chickens against E. tenella. However, the protective immunities of rEt-GRA12, rEt-SAG13 and rEt-SAG proteins were not satisfactory compared to rEt-ROPK-Eten5-A. The groups immunized with rEt-GRA12 protein showed significantly increased body weight and reduced cecum lesion scores. However, vaccination with rEt-GRA12 was not effective in oocyst shedding, which suggests that rEt-GRA12 may not be a good vaccine candidate for controlling the oocyst output in infected chickens. For the rEt-SAG13 and rEt-SAG proteins, the index of survival rate, oocyst output reduction and weight gain were not as good as rEt-ROPK-Eten5-A, which suggest that they are not ideal antigens for vaccine.
A previous study investigated several sporozoite proteins of Eimeria as anticoccidial vaccine candidates. The efficacy of a recombinant sporozoite antigen (EtMIC1) protein in protecting against a homologous challenge was evaluated and only showed partial protection against homologous challenge in chickens [17]. The sporozoite-specific SAG1 also induced partial protective immunity as a subunit vaccine [19,20,21]. Antigens SO7 and ETRHO1 had been investigated to locate in the sporozoite refractile bodies and induce both cellular and humoral immune responses in immunized chickens. The oocyst reduction rate of rEt-ROPK-Eten5-A (82.75%) is better than that of SO7 (74.46%) [16,18]. The relative weight gain of the chickens immunized with Et-ROPK-Eten5-A was only 47%, but the weight gains of chickens immunized with SO7 and ETRHO1 were 86.77% and 87%, respectively. This seems to indicate that Et-ROPK-Eten5-A has less protective effect on intestinal injury in chickens than SO7 and ETRHO1 as previously reported. However, it is worth noting that different E. tenella strains have different levels of virulence; our challenge dose resulted in a weight loss of 46% in the unimmunized control group and a mortality rate of 60%, whereas the previous study using SO7 and ETRHO1 proteins reported weight gain rates of 69.79% and 47.80% in the respective unimmunized control groups. Therefore, rEt-ROPK-Eten5-A is considered an effective anticoccidial vaccine. The combination of rEt-ROPK-Eten5-A and other effective proteins (such as SO7 and ETRHO1) may be a new direction for the development of anticoccidial vaccines in the future. IMP1 was identified as an anticoccidial vaccine candidate, and it is localized in the sporozoite cell membrane [22]. However, the use of IMP1 in combination with a powerful immunological adjuvant (CD40L) did not improve the index of weight gain rate and oocyst decrease rate more than rEt-ROPK-Eten5-A [23].
5. Conclusions
In summary, we identified and characterized four sporozoite proteins from E. tenella, including two secreted antigens and two surface antigens. We presented the locations of Et-ROPK-Eten5-A and Et-GRA12 and provided novel insights into the biological functions of these proteins. Our study demonstrated that vaccination with rEt-ROPK-Eten5-A significantly increased the survival rate and body weight gains, decreased the oocyst output and alleviated cecum lesions, which indicate that Et-ROPK-Eten5-A may be an effective candidate for the development of vaccines against E. tenella.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-393X/8/3/452/s1, Table S1: Primers used in this study title.
Author Contributions
Conceived and designed the study, X.S., J.L. and Q.L.; Performed the experiments, X.S., X.Y. and T.Z.; Analyzed the data and drafted the manuscript, X.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Key Research and Development Program of China (2017YFD0501200).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Paul, G.C.; Friend, D.G. Clostridial enterotoxemia and coccidiosis in weanling cottontail rabbits (Sylvilagus audubonii, Sylvilagus floridanus, Sylvilagus nuttallii) from Colorado, USA. J. Wildl. Dis. 2019, 55, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Chapman, H.D.; Barta, J.R.; Blake, D.; Gruber, A.; Jenkins, M.; Smith, N.C.; Suo, X.; Tomley, F.M. A selective review of advances in coccidiosis research, in Advances in parasitology. Adv. Parasitol. 2013, 83, 93–171. [Google Scholar] [PubMed]
- Blake, D.P.; Tomley, F.M. Securing poultry production from the ever-present Eimeria challenge. Trends Parasitol. 2014, 30, 12–19. [Google Scholar] [CrossRef]
- Witcombe, D.M.; Smith, N.C. Strategies for anti-coccidial prophylaxis. Parasitology 2014, 141, 1379–1389. [Google Scholar] [CrossRef]
- Bussière, F.I.; Niepceron, A.; Sausset, A.; Esnault, E.; Silvestre, A.; Walker, R.A.; Smith, N.C.; Quéré, P.; Laurent, F. Establishment of an in vitro chicken epithelial cell line model to investigate Eimeria tenella gamete development. Parasit. Vectors 2018, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Odden, A.; Denwood, M.J.; Stuen, S.; Robertson, L.J.; Ruiz, A.; Hamnes, I.S.; Hektoen, L.; Enemark, H.L. Field evaluation of anticoccidial efficacy: A novel approach demonstrates reduced efficacy of toltrazuril against ovine Eimeria spp. in Norway. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 304–311. [Google Scholar] [CrossRef]
- Venkatas, J.; Adeleke, M. A review of Eimeria antigen identification for the development of novel anticoccidial vaccines. Parasitol. Res. 2019, 118, 1701–1710. [Google Scholar] [CrossRef]
- Song, X.; Ren, Z.; Yan, R.; Xu, L.; Li, X. Induction of protective immunity against Eimeria tenella, Eimeria necatrix, Eimeria maxima and Eimeria acervulina infections using multivalent epitope DNA vaccines. Vaccine 2015, 33, 2764–2770. [Google Scholar] [CrossRef]
- Zhao, P.; Li, Y.; Zhou, Y.; Zhao, J.; Fang, R. In vivo immunoprotective comparison between recombinant protein and DNA vaccine of Eimeria tenella surface antigen 4. Vet. Parasitol. 2020, 278, 109032. [Google Scholar] [CrossRef]
- Chapman, H.D.; Jeffers, T.K. Vaccination of chickens against coccidiosis ameliorates drug resistance in commercial poultry production. Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 214–217. [Google Scholar] [CrossRef]
- Ziomko, I.; Karamon, J.; Cencek, T.; Gornowicz, E.; Ashash, U. Prevention of broiler chick coccidiosis using the inactivated subunit vaccine CoxAbic®. Bull. Vet. Inst. Pulawy. 2005, 49, 299–302. [Google Scholar]
- Lebrun, M.; Carruthers, V.B.; Cesbron-Delauw, M.-F. Toxoplasma secretory proteins and their roles in cell invasion and intracellular survival. In Toxoplasma Gondii; Elsevier: Amsterdam, The Netherlands, 2014; pp. 389–453. [Google Scholar]
- Bradley, P.J.; Ward, C.; Cheng, S.J.; Alexander, D.L.; Coller, S.; Coombs, G.H.; Dunn, J.D.; Ferguson, D.J.; Sanderson, S.J.; Wastling, J.M.; et al. Proteomic Analysis of Rhoptry Organelles Reveals Many Novel Constituents for Host-Parasite Interactions in Toxoplasma gondii. J. Biol. Chem. 2005, 280, 34245–34258. [Google Scholar] [CrossRef] [PubMed]
- Hajj, H.E.; Demey, E.; Poncet, J.; Lebrun, M.; Wu, B.; Galéotti, N.; Fourmaux, M.N.; Mercereau-Puijalon, O.; Vial, H.; Labesse, G.; et al. The ROP2 family of Toxoplasma gondii rhoptry proteins: Proteomic and genomic characterization and molecular modeling. Proteomics 2006, 6, 5773–5784. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, L.; Chen, F.; Harb, O.S.; Davis, P.H.; Beiting, D.P.; Brownback, C.S.; Ouloguem, D.; Roos, D.S. Integrative Genomic Approaches Highlight a Family of Parasite-Specific Kinases that Regulate Host Responses. Cell Host Microbe 2010, 8, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Rafiqi, S.I.; Garg, R.; Reena, K.K.; Ram, H.; Singh, M.; Banerjee, P.S. Immune response and protective efficacy of Eimeria tenella recombinant refractile body protein, EtSO7, in chickens. Vet. Parasitol. 2018, 258, 108–113. [Google Scholar] [CrossRef]
- Subramanian, B.M.; Sriraman, R.; Rao, N.H.; Raghul, J.; Thiagarajan, D.; Srinivasan, V.A. Cloning, expression and evaluation of the efficacy of a recombinant Eimeria tenella sporozoite antigen in birds. Vaccine 2008, 26, 3489–3496. [Google Scholar] [CrossRef]
- Li, J.; Zheng, J.; Gong, P.; Zhang, X. Efficacy of Eimeria tenella rhomboid-like protein as a subunit vaccine in protective immunity against homologous challenge. Parasitol. Res. 2012, 110, 1139–1145. [Google Scholar] [CrossRef]
- Jahn, D.; Matros, A.; Bakulina, A.Y.; Tiedemann, J.; Schubert, U.; Giersberg, M.; Haehnel, S.; Zoufal, K.; Mock, H.P.; Kipriyanov, S.M. Model structure of the immunodominant surface antigen of Eimeria tenella identified as a target for sporozoite-neutralizing monoclonal antibody. Parasitol. Res. 2009, 105, 655. [Google Scholar] [CrossRef]
- Song, X.; Gao, Y.; Xu, L.; Yan, R.; Li, X. Partial protection against four species of chicken coccidia induced by multivalent subunit vaccine. Vet. Parasitol. 2015, 212, 80–85. [Google Scholar] [CrossRef]
- Blake, D.P.; Pastor-Fernández, I.; Nolan, M.J.; Tomley, F.M. Recombinant anticoccidial vaccines-a cup half full? Infect. Genet. Evol. 2017, 55, 358–365. [Google Scholar] [CrossRef]
- Jenkins, M.; Fetterer, R.; Miska, K.; Tuo, W.; Kwok, O.; Dubey, J.P. Characterization of the Eimeria maxima sporozoite surface protein IMP1. Vet. Parasitol. 2015, 211, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.; Lin, Q.; Qiu, J.; Qin, M.; Tang, X.; Suo, X.; Huang, Z.; Liu, X. Immunogenicity and protective efficacy of an Eimeria vaccine candidate based on Eimeria tenella immune mapped protein 1 and chicken CD40 ligand. Vet. Parasitol. 2015, 210, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Lin, J.; Han, H.; Zhao, Q.; Dong, H.; Zhu, S.; Huang, B. Identification and partial characterization of a serine protease inhibitor (serpin) of Eimeria tenella. Parasitol. Res. 2012, 110, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Long, P.L.; Millard, B.J.; Joyner, L.P.; Norton, C.C. A guide to laboratory techniques used in the study and diagnosis of avian coccidiosis. Folia Vet. Lat. 1976, 6, 201–217. [Google Scholar]
- Walker, R.A.; Sharman, P.A.; Miller, C.M.; Lippuner, C.; Okoniewski, M.; Eichenberger, R.M.; Ramakrishnan, C.; Brossier, F.; Deplazes, P.; Hehl, A.B. RNA Seq analysis of the Eimeria tenella gametocyte transcriptome reveals clues about the molecular basis for sexual reproduction and oocyst biogenesis. BMC Genom. 2015, 16, 94. [Google Scholar] [CrossRef]
- Li, M.; Wang, H.; Liu, J.; Hao, P.; Ma, L.; Liu, Q. The Apoptotic Role of Metacaspase in Toxoplasma gondii. Front. Microbiol. 2015, 6, 1560. [Google Scholar] [CrossRef]
- Schmatz, D.M.; Crane, M.S.; Murray, P.K. Purification of Eimeria sporozoites by DE-52 anion exchange chromatography. J. Eukaryot. Microbiol. 2010, 31, 181–183. [Google Scholar]
- Hu, D.; Wang, C.; Wang, S.; Tang, X.; Duan, C.; Zhang, S.; Suo, J.; Deng, M.; Lv, Y.; Suo, X. Comparative transcriptome analysis of Eimeria maxima (Apicomplexa: Eimeriidae) suggests DNA replication activities correlating with its fecundity. BMC Genom. 2018, 19, 1–10. [Google Scholar] [CrossRef]
- Johnson, J.; Reid, W.M. Anticoccidial drugs: Lesion scoring techniques in battery and floor-pen experiments with chickens. Exp. Parasitol. 1970, 28, 30–36. [Google Scholar] [CrossRef]
- Reid, A.J.; Blake, D.P.; Ansari, H.R.; Billington, K.; Browne, H.P.; Bryant, J.; Dunn, M.; Hung, S.S.; Kawahara, F.; Miranda-Saavedra, D. Genomic analysis of the causative agents of coccidiosis in domestic chickens. Genome Res. 2014, 24, 1676–1685. [Google Scholar] [CrossRef]
- Talevich, E.; Kannan, N. Structural and evolutionary adaptation of rhoptry kinases and pseudokinases, a family of coccidian virulence factors. BMC Evol. Biol. 2013, 13, 117. [Google Scholar] [CrossRef] [PubMed]
- Carruthers, V.; Boothroyd, J.C. Pulling together: An integrated model of Toxoplasma cell invasion. Curr. Opin. Microbiol. 2007, 10, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Diallo, M.A.; Sausset, A.; Gnahoui-David, A.; Silva, A.; Brionne, A.; Le Vern, Y.; Bussière, F.I.; Tottey, J.; Lacroix-Lamandé, S.; Laurent, F. Eimeria tenella ROP kinase EtROP1 induces G0/G1 cell cycle arrest and inhibits host cell apoptosis. Cell Microbiol. 2019, 21, e13027. [Google Scholar] [CrossRef]
- Rick, B.; Dubremetz, J.-F.; Entzeroth, R. A merozoite-specific 22-kDa rhoptry protein of the coccidium Eimeria nieschulzi (Sporozoa, Coccidia) is exocytosed in the parasitophorous vacuole upon host cell invasion. Parasitol. Res. 1998, 84, 291–296. [Google Scholar] [CrossRef]
- Yang, C.; Liu, J.; Ma, L.; Zhang, X.; Zhang, X.; Zhou, B.; Zhu, X.; Liu, Q. NcGRA17 is an important regulator of parasitophorous vacuole morphology and pathogenicity of Neospora caninum. Vet. Parasitol. 2018, 264, 26–34. [Google Scholar] [CrossRef]
- Song, X.; Zhao, X.; Xu, L.; Yan, R.; Li, X. Immune protection duration and efficacy stability of DNA vaccine encoding Eimeria tenella TA4 and chicken IL-2 against coccidiosis. Res. Vet. Sci. 2017, 111, 31–35. [Google Scholar] [CrossRef]
- Dong, H.; Yang, S.; Zhao, Q.; Han, H.; Zhu, S.; Zhu, X.; Li, C.; Wang, Z.; Xia, W.; Men, Q. Molecular characterization and protective efficacy of silent information regulator 2A from Eimeria tenella. Parasit. Vectors 2016, 9, 602. [Google Scholar] [CrossRef]
- Williams, R. Anticoccidial vaccines for broiler chickens: Pathways to success. Avian. Pathol. 2002, 31, 317–353. [Google Scholar] [CrossRef]
- Lin, R.-Q.; Lillehoj, H.S.; Lee, S.K.; Oh, S.; Panebra, A.; Lillehoj, E.P. Vaccination with Eimeria tenella elongation factor-1α recombinant protein induces protective immunity against E. tenella and E. maxima infections. Vet. Parasitol. 2017, 243, 79–84. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).