Visual Detection of Malaria Parasite-Parasitized Erythroblasts in Peripheral Blood via Immunization-Based Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Malaria Parasites and Blood-Stage Infection
2.3. Immunization (Live Vaccination) and Challenge Infection
2.4. Microscopic Examination of pEB
2.5. Evaluation of Blood–Brain Barrier
2.6. Staining of Parasitized Cells and Fluorescence Microscopy
2.7. Statistical Analysis
3. Results
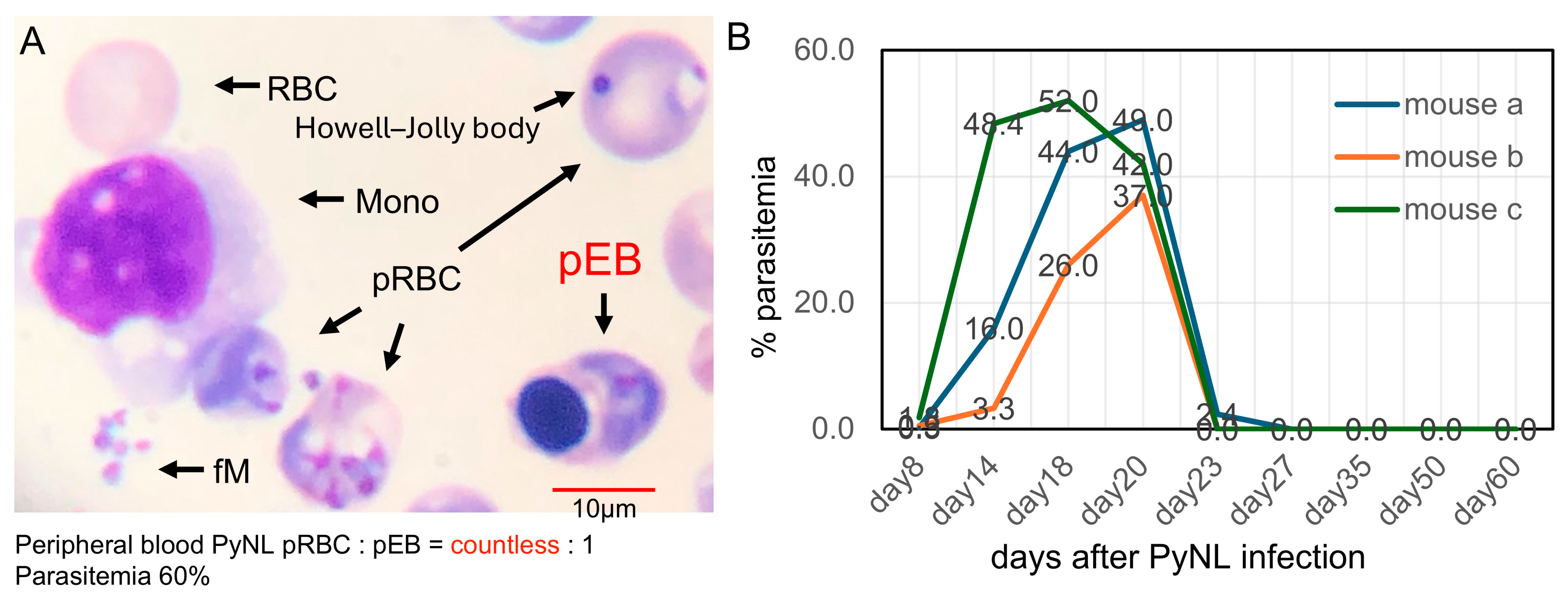
3.1. PyNL Infection and Rare Detection of Parasitized Erythroblasts in Peripheral Blood
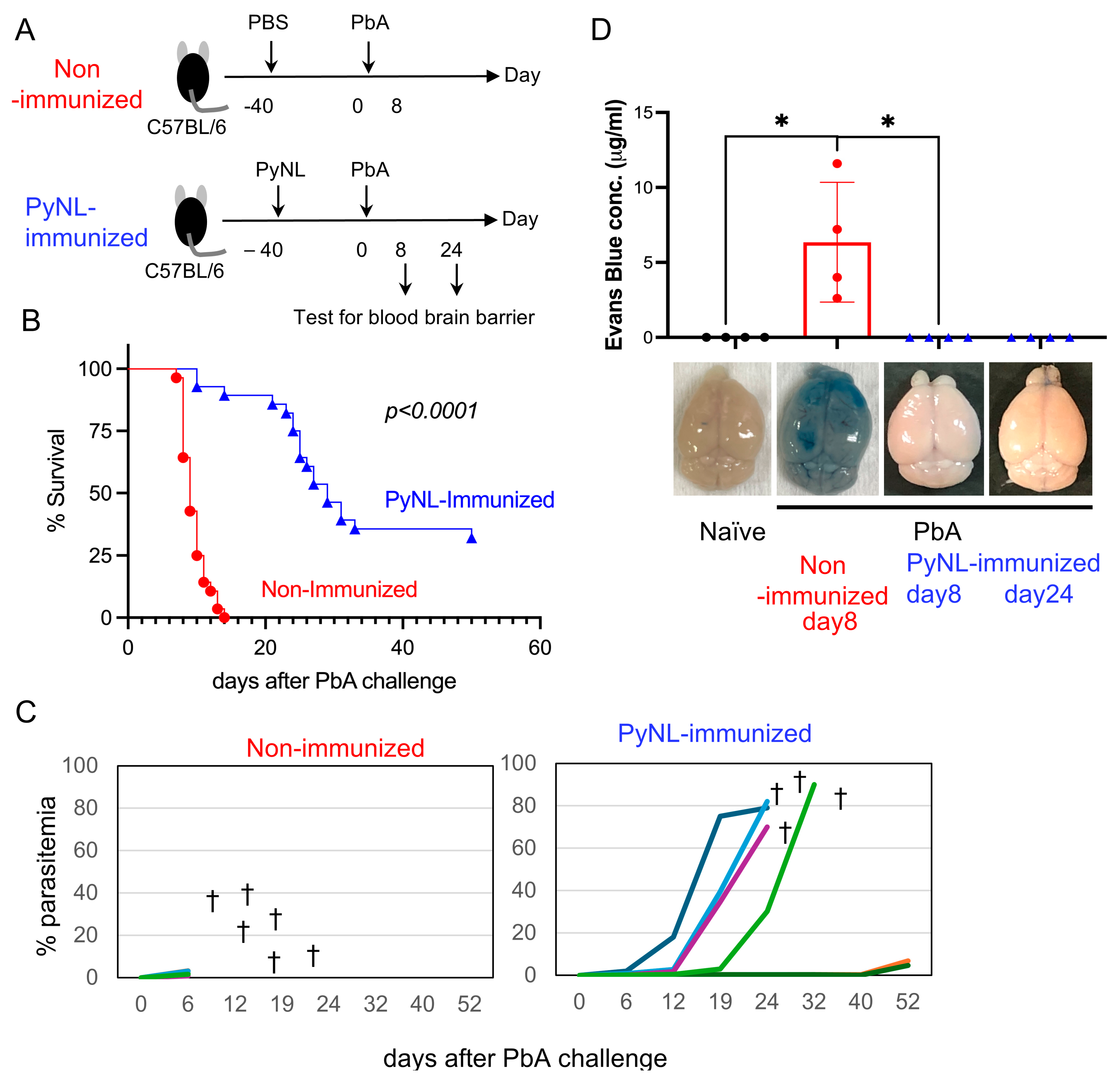
3.2. Live PyNL Immunization Partial Protection Against PbA Challenge Infection in Mice
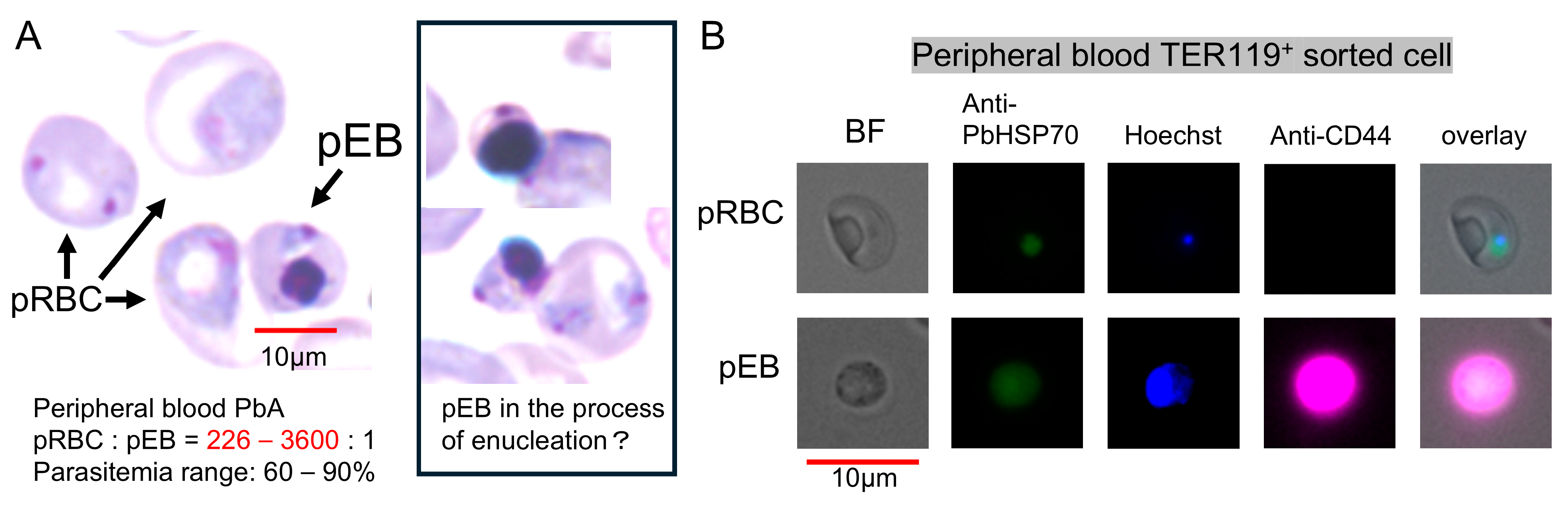
3.3. Visual Detection and Increased Proportion of pEBs in Peripheral Blood
3.4. Time-Course Analysis of Parasitemia and pEBs from Primary Infection with PyNL to Challenge Infection with PbA
3.5. Immunization Is Required for Increased Proportion of pEBs in Peripheral Blood
3.6. Catalog of PbA-Parasitized Erythroblasts Under Microscopic Examination

4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ECM | experimental cerebral malaria |
| fM | free merozoites |
| MHC | major histocompatibility complex |
| Mono | monocytes |
| pEB | parasitized erythroblast |
| PbA | Plasmodium berghei ANKA |
| Pf | Plasmodium falciparum |
| pRBC | parasitized red blood cell |
| PyL | Plasmodium yoelii yoelii 17XL |
| PyNL | Plasmodium yoelii yoelii 17XNL |
| RBC | red blood cell |
| SPF | specific pathogen-free |
References
- Franchini, J. Ch. Alphonse Laveran, 1845–1922: His Life and Works, Read on the Fiftieth Anniversary of the Malaria Parasite. Ann. Med. Hist. 1931, 3, 280–288. [Google Scholar] [PubMed]
- Ru, Y.X.; Mao, B.Y.; Zhang, F.K.; Pang, T.X.; Zhao, S.X.; Liu, J.H.; Wickramasinghe, S.N. Invasion of erythroblasts by Pasmodium vivax: A new mechanism contributing to malarial anemia. Ultrastruct. Pathol. 2009, 33, 236–242. [Google Scholar] [CrossRef]
- Tamez, P.A.; Liu, H.; Fernandez-Pol, S.; Haldar, K.; Wickrema, A. Stage-specific susceptibility of human erythroblasts to Plasmodium falciparum malaria infection. Blood 2009, 114, 3652–3655. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Ishida, H.; Suzue, K.; Hirai, M.; Taniguchi, T.; Okada, H.; Suzuki, T.; Shimokawa, C.; Hisaeda, H. CD8(+) T cell activation by murine erythroblasts infected with malaria parasites. Sci. Rep. 2013, 3, 1572. [Google Scholar] [CrossRef]
- Lee, R.S.; Waters, A.P.; Brewer, J.M. A cryptic cycle in haematopoietic niches promotes initiation of malaria transmission and evasion of chemotherapy. Nat. Commun. 2018, 9, 1689. [Google Scholar] [CrossRef]
- Joice, R.; Nilsson, S.K.; Montgomery, J.; Dankwa, S.; Egan, E.; Morahan, B.; Seydel, K.B.; Bertuccini, L.; Alano, P.; Williamson, K.C.; et al. Plasmodium falciparum transmission stages accumulate in the human bone marrow. Sci. Transl. Med. 2014, 6, 244re245. [Google Scholar] [CrossRef]
- De Niz, M.; Meibalan, E.; Mejia, P.; Ma, S.; Brancucci, N.M.B.; Agop-Nersesian, C.; Mandt, R.; Ngotho, P.; Hughes, K.R.; Waters, A.P.; et al. Plasmodium gametocytes display homing and vascular transmigration in the host bone marrow. Sci. Adv. 2018, 4, eaat3775. [Google Scholar] [CrossRef]
- Hentzschel, F.; Gibbins, M.P.; Attipa, C.; Beraldi, D.; Moxon, C.A.; Otto, T.D.; Marti, M. Host cell maturation modulates parasite invasion and sexual differentiation in Plasmodium berghei. Sci. Adv. 2022, 8, eabm7348. [Google Scholar] [CrossRef]
- Neveu, G.; Richard, C.; Dupuy, F.; Behera, P.; Volpe, F.; Subramani, P.A.; Marcel-Zerrougui, B.; Vallin, P.; Andrieu, M.; Minz, A.M.; et al. Plasmodium falciparum sexual parasites develop in human erythroblasts and affect erythropoiesis. Blood 2020, 136, 1381–1393. [Google Scholar] [CrossRef]
- Hu, J.; Liu, J.; Xue, F.; Halverson, G.; Reid, M.; Guo, A.; Chen, L.; Raza, A.; Galili, N.; Jaffray, J.; et al. Isolation and functional characterization of human erythroblasts at distinct stages: Implications for understanding of normal and disordered erythropoiesis in vivo. Blood 2013, 121, 3246–3253. [Google Scholar] [CrossRef] [PubMed]
- Pamer, E.; Cresswell, P. Mechanisms of MHC class I-restricted antigen processing. Annu. Rev. Immunol. 1998, 16, 323–358. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Ishida, H.; Suzue, K.; Taniguchi, T.; Okada, H.; Shimokawa, C.; Hisaeda, H. Cytotoxic activities of CD8(+) T cells collaborate with macrophages to protect against blood-stage murine malaria. eLife 2015, 4, e04232. [Google Scholar] [CrossRef] [PubMed]
- Sohawon, D.; Lau, K.K.; Lau, T.; Bowden, D.K. Extra-medullary haematopoiesis: A pictorial review of its typical and atypical locations. J. Med. Imaging Radiat. Oncol. 2012, 56, 538–544. [Google Scholar] [CrossRef]
- Kaechele, A.; Chawla, A.; Osher, M. Abnormal imaging presentations of extramedullary hematopoiesis in a 21-year old and 72-year old female. Radiol. Case Rep. 2022, 17, 2619–2625. [Google Scholar] [CrossRef]
- Gautier, E.F.; Ducamp, S.; Leduc, M.; Salnot, V.; Guillonneau, F.; Dussiot, M.; Hale, J.; Giarratana, M.C.; Raimbault, A.; Douay, L.; et al. Comprehensive Proteomic Analysis of Human Erythropoiesis. Cell Rep. 2016, 16, 1470–1484. [Google Scholar] [CrossRef]
- Trager, W.; Jensen, J.B. Human malaria parasites in continuous culture. Science 1976, 193, 673–675. [Google Scholar] [CrossRef]
- Landau, I.; Killick-Kendrick, R. Rodent plasmodia of the Republique Centrafricaine: The sporogony and tissue stages of Plasmodium chabaudi and P. berghei yoelii. Trans. R. Soc. Trop. Med. Hyg. 1966, 60, 633–649. [Google Scholar] [CrossRef]
- Cox, F.E. Protective immunity between malaria parasites and piroplasms in mice. Bull. World Health Organ. 1970, 43, 325–336. [Google Scholar] [PubMed]
- Playfair, J.H.; De Souza, J.B.; Cottrell, B.J. Reactivity and crossreactivity of mouse helper T cells to malaria parasites. Immunology 1977, 32, 681–687. [Google Scholar]
- Killick-Kendrick, R. Parasitic protozoa of the blood of rodents: A revision of Plasmodium berghei. Parasitology 1974, 69, 225–237. [Google Scholar] [CrossRef]
- Ortolan, L.S.; Bansal, P.; Primavera, V.I.; Freitas, R.; Wei, L.; Epiphanio, S.; Kaushansky, A.; Smith, J.D. Nilotinib attenuates vascular pathology in experimental cerebral malaria. Blood Adv. 2025, 9, 2473–2488. [Google Scholar] [CrossRef]
- Imai, T.; Ngo-Thanh, H.; Suzue, K.; Shimo, A.; Nakamura, A.; Horiuchi, Y.; Hisaeda, H.; Murakami, T. Live Vaccination with Blood-Stage Plasmodium yoelii 17XNL Prevents the Development of Experimental Cerebral Malaria. Vaccines 2022, 10, 762. [Google Scholar] [CrossRef]
- Imai, T.; Suzue, K.; Ngo-Thanh, H.; Shimokawa, C.; Hisaeda, H. Potential and Limitations of Cross-Protective Vaccine against Malaria by Blood-Stage Naturally Attenuated Parasite. Vaccines 2020, 8, 375. [Google Scholar] [CrossRef]
- Chen, K.; Liu, J.; Heck, S.; Chasis, J.A.; An, X.; Mohandas, N. Resolving the distinct stages in erythroid differentiation based on dynamic changes in membrane protein expression during erythropoiesis. Proc. Natl. Acad. Sci. USA 2009, 106, 17413–17418. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Ginzburg, Y.; Li, H.; Xue, F.; De Franceschi, L.; Chasis, J.A.; Mohandas, N.; An, X. Quantitative analysis of murine terminal erythroid differentiation in vivo: Novel method to study normal and disordered erythropoiesis. Blood 2013, 121, e43–e49. [Google Scholar] [CrossRef]
- Georgiadou, A.; Dunican, C.; Soro-Barrio, P.; Lee, H.J.; Kaforou, M.; Cunnington, A.J. Comparative transcriptomic analysis reveals translationally relevant processes in mouse models of malaria. eLife 2022, 11, e70763. [Google Scholar] [CrossRef] [PubMed]
- Chasis, J.A.; Mohandas, N. Erythroblastic islands: Niches for erythropoiesis. Blood 2008, 112, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, K.; Ghosh, K. Pathogenesis of anemia in malaria: A concise review. Parasitol. Res. 2007, 101, 1463–1469. [Google Scholar] [CrossRef]
- Dumarchey, A.; Lavazec, C.; Verdier, F. Erythropoiesis and Malaria, a Multifaceted Interplay. Int. J. Mol. Sci. 2022, 23, 12762. [Google Scholar] [CrossRef]
- Tumas, K.C.; Xu, F.; Wu, J.; Hernandez, M.; Pattaradilokrat, S.; Xia, L.; Peng, Y.C.; Lavali, A.M.; He, X.; Singh, B.K.; et al. Dysfunction of CD169(+) macrophages and blockage of erythrocyte maturation as a mechanism of anemia in Plasmodium yoelii infection. Proc. Natl. Acad. Sci. USA 2023, 120, e2311557120. [Google Scholar] [CrossRef] [PubMed]
- Yahata, K.; Treeck, M.; Culleton, R.; Gilberger, T.W.; Kaneko, O. Time-lapse imaging of red blood cell invasion by the rodent malaria parasite Plasmodium yoelii. PLoS ONE 2012, 7, e50780. [Google Scholar] [CrossRef] [PubMed]
- Janse, C.J.; Ramesar, J.; Waters, A.P. High-efficiency transfection and drug selection of genetically transformed blood stages of the rodent malaria parasite Plasmodium berghei. Nat. Protoc. 2006, 1, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Autengruber, A.; Gereke, M.; Hansen, G.; Hennig, C.; Bruder, D. Impact of enzymatic tissue disintegration on the level of surface molecule expression and immune cell function. Eur. J. Microbiol. Immunol. 2012, 2, 112–120. [Google Scholar] [CrossRef] [PubMed]
- DaMata, J.P.; Zelkoski, A.E.; Nhan, P.B.; Ennis, K.H.E.; Kim, J.S.; Lu, Z.; Malloy, A.M.W. Dissociation protocols influence the phenotypes of lymphocyte and myeloid cell populations isolated from the neonatal lymph node. Front. Immunol. 2024, 15, 1368118. [Google Scholar] [CrossRef]
- Lassoued, N.; Yero, A.; Jenabian, M.A.; Soret, R.; Pilon, N. Efficient enzyme-free method to assess the development and maturation of the innate and adaptive immune systems in the mouse colon. Sci. Rep. 2024, 14, 11063. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ito, K.; Tateishi, Y.S.; Imai, T.; Miyazaki, S.; Miyazaki, Y.; Kagaya, W.; Nakashima, M.; Sase, M.; Yoshioka-Takeda, M.; Shimokawa, C.; et al. Visual Detection of Malaria Parasite-Parasitized Erythroblasts in Peripheral Blood via Immunization-Based Model. Vaccines 2025, 13, 988. https://doi.org/10.3390/vaccines13090988
Ito K, Tateishi YS, Imai T, Miyazaki S, Miyazaki Y, Kagaya W, Nakashima M, Sase M, Yoshioka-Takeda M, Shimokawa C, et al. Visual Detection of Malaria Parasite-Parasitized Erythroblasts in Peripheral Blood via Immunization-Based Model. Vaccines. 2025; 13(9):988. https://doi.org/10.3390/vaccines13090988
Chicago/Turabian StyleIto, Kumpei, Yuki S. Tateishi, Takashi Imai, Shinya Miyazaki, Yukiko Miyazaki, Wataru Kagaya, Mai Nakashima, Miho Sase, Misato Yoshioka-Takeda, Chikako Shimokawa, and et al. 2025. "Visual Detection of Malaria Parasite-Parasitized Erythroblasts in Peripheral Blood via Immunization-Based Model" Vaccines 13, no. 9: 988. https://doi.org/10.3390/vaccines13090988
APA StyleIto, K., Tateishi, Y. S., Imai, T., Miyazaki, S., Miyazaki, Y., Kagaya, W., Nakashima, M., Sase, M., Yoshioka-Takeda, M., Shimokawa, C., Hayashi, K., Itokawa, K., Komagata, O., Ngo-Thanh, H., Shimo, A., Araki, T., Annoura, T., Murakami, T., & Hisaeda, H. (2025). Visual Detection of Malaria Parasite-Parasitized Erythroblasts in Peripheral Blood via Immunization-Based Model. Vaccines, 13(9), 988. https://doi.org/10.3390/vaccines13090988





