Individualized mRNA Vaccines in Melanoma—Where Do We Stand?
Abstract
1. Introduction
2. Opening a New Era in Melanoma
3. Mechanism of Action
4. Old Ideas Under a New Light: Targeting the Dendritic Cell
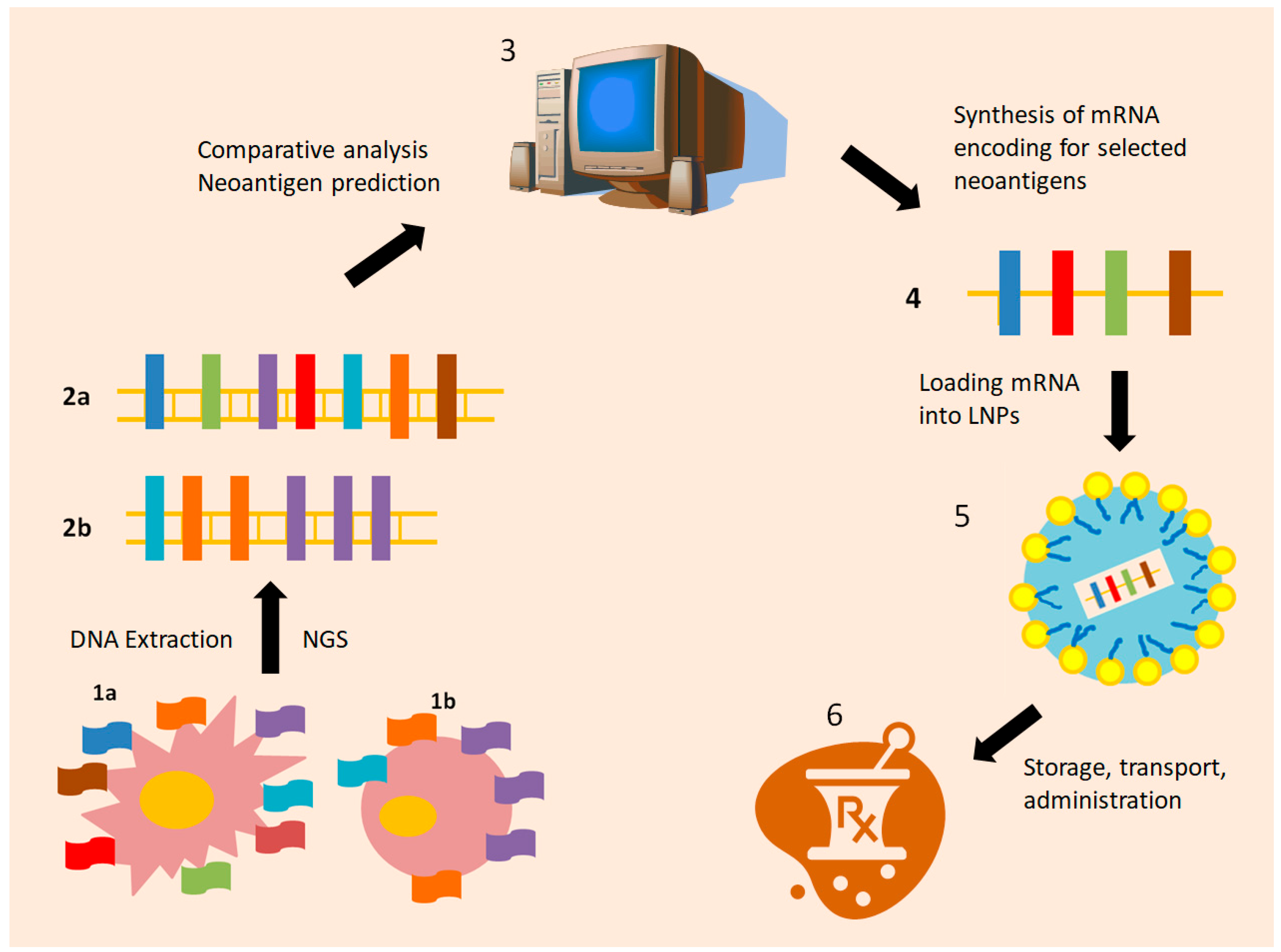
5. Dissecting the Footprints of Cancer: Individualized Neoantigen Treatment (INT)
6. Next Generation Packaging: The Nanoparticles
7. Proof of Concept: How mRNA Vaccination Affects Host Immunity
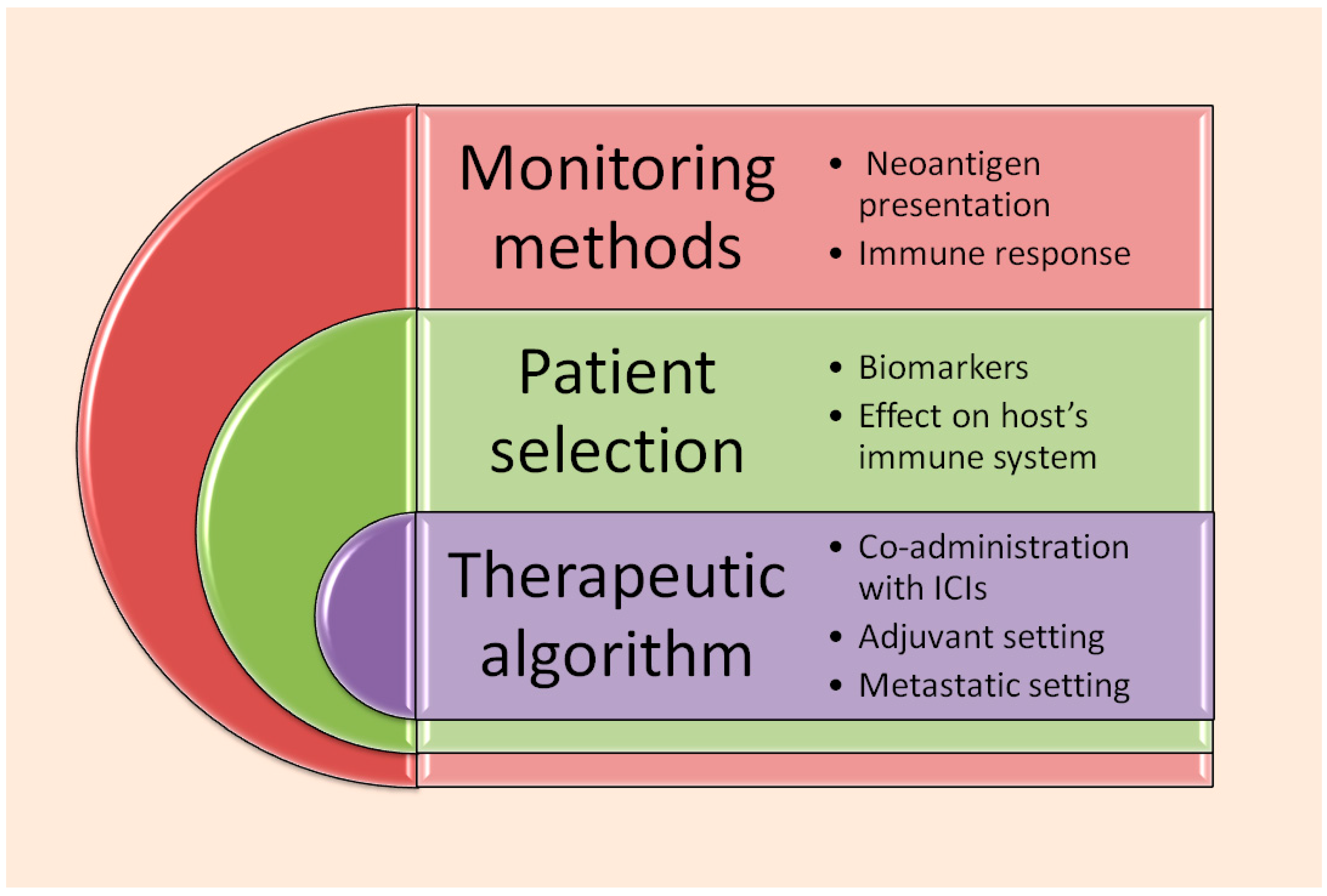
8. Future Prospects and Points Requiring Further Clarification
9. Critical Appraisal of Clinical Trials Involving mRNA Vaccines
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- SEER Cancer Statistics Network. Available online: https://seer.cancer.gov/statistics-network (accessed on 7 September 2025).
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef]
- Waseh, S.; Lee, J.B. Advances in Melanoma: Epidemiology, Diagnosis, and Prognosis. Front. Med. 2023, 10, 1268479. [Google Scholar] [CrossRef]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Barsouk, A. Epidemiology of Melanoma. Med. Sci. 2021, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Centeno, P.P.; Pavet, V.; Marais, R. The Journey from Melanocytes to Melanoma. Nat. Rev. Cancer 2023, 23, 372–390. [Google Scholar] [CrossRef]
- Vultur, A.; Herlyn, M. SnapShot: Melanoma. Cancer Cell. 2013, 23, 706–706.e1. [Google Scholar] [CrossRef]
- Castro-Pérez, E.; Singh, M.; Sadangi, S.; Mela-Sánchez, C.; Setaluri, V. Connecting the Dots: Melanoma Cell of Origin, Tumor Cell Plasticity, Trans-Differentiation, and Drug Resistance. Pigment Cell Melanoma Res. 2023, 36, 330–347. [Google Scholar] [CrossRef] [PubMed]
- Shain, A.H.; Bastian, B.C. From Melanocytes to Melanomas. Nat. Rev. Cancer 2016, 16, 345–358. [Google Scholar] [CrossRef]
- Tan, A.; Stein, J.A. Dermoscopic Patterns of Acral Melanocytic Lesions in Skin of Color. Cutis 2019, 103, 274–276. [Google Scholar]
- Cantwell-Dorris, E.R.; O’Leary, J.J.; Sheils, O.M. BRAFV600E: Implications for Carcinogenesis and Molecular Therapy. Mol. Cancer Ther. 2011, 10, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Darabi, S.; Stafford, P.; Braxton, D.R.; Zuazo, C.E.; Brodie, T.J.; Demeure, M.J. BRAF V600E Mutation Has Variable Tumor-Specific Effects on Expression of MAPK Pathway Genes That Could Affect Patient Outcome. Int. J. Mol. Sci. 2025, 26, 7910. [Google Scholar] [CrossRef]
- Robert, C.; Grob, J.J.; Stroyakovskiy, D.; Karaszewska, B.; Hauschild, A.; Levchenko, E.; Chiarion Sileni, V.; Schachter, J.; Garbe, C.; Bondarenko, I.; et al. Five-Year Outcomes with Dabrafenib plus Trametinib in Metastatic Melanoma. N. Engl. J. Med. 2019, 381, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Schadendorf, D.; Dummer, R.; Flaherty, K.T.; Robert, C.; Arance, A.; de Groot, J.W.B.; Garbe, C.; Gogas, H.J.; Gutzmer, R.; Krajsová, I.; et al. COLUMBUS 7-Year Update: A Randomized, Open-Label, Phase III Trial of Encorafenib plus Binimetinib versus Vemurafenib or Encorafenib in Patients with BRAF V600E/K-Mutant Melanoma. Eur. J. Cancer 2024, 204, 114073. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Dréno, B.; Larkin, J.; Liszkay, G.; Mihalcioiu, C.; Gogas, H.; Hoeller, C.; Grob, J.J.; Lao, C.D.; Mandalà, M. 5-Year Outcomes with Cobimetinib plus Vemurafenib in BRAFV600 Mutation-Positive Advanced Melanoma: Extended Follow-up of the coBRIM Study. Clin. Cancer Res. 2021, 27, 5225–5235. [Google Scholar] [CrossRef] [PubMed]
- Cameron, F.; Whiteside, G.; Perry, C. Ipilimumab: First Global Approval. Drugs 2011, 71, 1093–1104. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Chiarion-Sileni, V.; Rutkowski, P.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Queirolo, P.; Dummer, R.; Butler, M.O.; Hill, A.G.; et al. Final, 10-Year Outcomes with Nivolumab plus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2025, 392, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Carlino, M.S.; McNeil, C.; Ribas, A.; Gaudy-Marqueste, C.; Schachter, J.; Nyakas, M.; Kee, D.; Petrella, T.M.; Blaustein, A.; et al. Pembrolizumab versus Ipilimumab for Advanced Melanoma: 10-Year Follow-Up of the Phase III KEYNOTE-006 Study. Ann. Oncol. 2024, 35, 1191–1199. [Google Scholar] [CrossRef]
- Lipson, E.J.; Hodi, F.S.; Tawbi, H.; Schadendorf, D.; Ascierto, P.A.; Matamala, L.; Gutierrez, E.C.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; et al. Nivolumab plus Relatlimab in Advanced Melanoma: RELATIVITY-047 4-Year Update. Eur. J. Cancer 2025, 225, 115547. [Google Scholar] [CrossRef]
- Kirkwood, J.M.; Del Vecchio, M.; Weber, J.; Hoeller, C.; Grob, J.J.; Mohr, P.; Loquai, C.; Dutriaux, C.; Chiarion-Sileni, V.; Mackiewicz, J.; et al. Adjuvant Nivolumab in Resected Stage IIB/C Melanoma: Primary Results from the Randomized, Phase 3 CheckMate 76K Trial. Nat. Med. 2023, 29, 2835–2843. [Google Scholar] [CrossRef]
- Eggermont, A.M.; Kicinski, M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.; Dalle, S.; Haydon, A.; Meshcheryakov, A.; Khattak, A.; et al. Seven-Year Analysis of Adjuvant Pembrolizumab versus Placebo in Stage III Melanoma in the EORTC1325/KEYNOTE-054 Trial. Eur. J. Cancer 2024, 211, 114327. [Google Scholar] [CrossRef]
- Yoon, C.H.; Ross, M.I.; Gastman, B.R.; Luke, J.J.; Ascierto, P.A.; Long, G.V.; Rutkowski, P.; Khattak, M.; Del Vecchio, M.; de la Cruz Merino, L.; et al. Adjuvant Pembrolizumab in Stage II Melanoma: Outcomes by Primary Tumor Location in the Randomized, Double-Blind, Phase III KEYNOTE-716 Trial. Ann. Surg. Oncol. 2025, 32, 2756–2764. [Google Scholar] [CrossRef]
- Blank, C.U.; Lucas, M.W.; Scolyer, R.A.; van de Wiel, B.A.; Menzies, A.M.; Lopez-Yurda, M.; Hoeijmakers, L.L.; Saw, R.P.M.; Lijnsvelt, J.M.; Maher, N.G.; et al. Neoadjuvant Nivolumab and Ipilimumab in Resectable Stage III Melanoma. N. Engl. J. Med. 2024, 391, 1696–1708. [Google Scholar] [CrossRef]
- Reijers, I.L.M.; Menzies, A.M.; van Akkooi, A.C.J.; Versluis, J.M.; van den Heuvel, N.M.J.; Saw, R.P.M.; Pennington, T.E.; Kapiteijn, E.; van der Veldt, A.A.M.; Suijkerbuijk, K.P.M.; et al. Personalized Response-Directed Surgery and Adjuvant Therapy after Neoadjuvant Ipilimumab and Nivolumab in High-Risk Stage III Melanoma: The PRADO Trial. Nat. Med. 2022, 28, 1178–1188. [Google Scholar] [CrossRef]
- Lim, S.Y.; Shklovskaya, E.; Lee, J.H.; Pedersen, B.; Stewart, A.; Ming, Z.; Irvine, M.; Shivalingam, B.; Saw, R.P.M.; Menzies, A.M.; et al. The Molecular and Functional Landscape of Resistance to Immune Checkpoint Blockade in Melanoma. Nat. Commun. 2023, 14, 1516. [Google Scholar] [CrossRef]
- Patel, S.P.; Othus, M.; Chen, Y.; Wright, G.P., Jr.; Yost, K.J.; Hyngstrom, J.R.; Hu-Lieskovan, S.; Lao, C.D.; Fecher, L.A.; Truong, T.G.; et al. Neoadjuvant–Adjuvant or Adjuvant-Only Pembrolizumab in Advanced Melanoma. N. Engl. J. Med. 2023, 388, 813–823. [Google Scholar] [CrossRef]
- Lauss, M.; Phung, B.; Borch, T.H.; Harbst, K.; Kaminska, K.; Ebbesson, A.; Hedenfalk, I.; Yuan, J.; Nielsen, K.; Ingvar, C.; et al. Molecular Patterns of Resistance to Immune Checkpoint Blockade in Melanoma. Nat. Commun. 2024, 15, 3075. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Wu, H.; Wu, J.; Ding, P.; He, J.; Sang, M.; Liu, L. Mechanisms of Immune Checkpoint Inhibitors: Insights into the Regulation of Circular RNAs Involved in Cancer Hallmarks. Cell Death Dis. 2024, 15, 3. [Google Scholar] [CrossRef]
- Iranzo, P.; Callejo, A.; Assaf, J.D.; Molina, G.; Lopez, D.E.; Garcia-Illescas, D.; Pardo, N.; Navarro, A.; Martinez-Marti, A.; Cedres, S.; et al. Overview of Checkpoint Inhibitors Mechanism of Action: Role of Immune-Related Adverse Events and Their Treatment on Progression of Underlying Cancer. Front. Med. 2022, 9, 875974. [Google Scholar]
- Knight, A.; Karapetyan, L.; Kirkwood, J.M. Immunotherapy in Melanoma: Recent Advances and Future Directions. Cancers 2023, 15, 1106. [Google Scholar] [CrossRef] [PubMed]
- Yaremenko, A.V.; Khan, M.M.; Zhen, X.; Tang, Y.; Tao, W. Clinical Advances of mRNA Vaccines for Cancer Immunotherapy. Med. 2025, 6, 100562. [Google Scholar] [CrossRef] [PubMed]
- Al Fayez, N.; Nassar, M.S.; Alshehri, A.A.; Alnefaie, M.K.; Almughem, F.A.; Alshehri, B.Y.; Alawad, A.O.; Tawfik, E.A. Recent Advancement in mRNA Vaccine Development and Applications. Pharmaceutics 2023, 15, 1972. [Google Scholar] [CrossRef]
- Fan, T.; Xu, C.; Wu, J.; Cai, Y.; Cao, W.; Shen, H.; Zhang, M.; Zhu, H.; Yang, J.; Zhu, Z.; et al. Lipopolyplex-formulated mRNA cancer vaccine elicits strong neoantigen-specific T cell responses and antitumor activity. Sci. Adv. 2024, 10, eadn9961. [Google Scholar] [CrossRef] [PubMed]
- Khattack, A.; Jeffrey, S.W.; Tarek, M.; Matthew, H.T.; George, A.; Kevin, B.K.; Meredith, M.; Georgina, V.L.; Ryan, J.S.; Mark, B.F.; et al. Distant Metastasis-Free Survival Results from the Randomized, Phase 2 mRNA-4157-P201/KEYNOTE-942 Trial. J. Clin. Oncol. 2023, 41, LBA9503. [Google Scholar] [CrossRef]
- Weber, J.S.; Carlino, M.S.; Khattak, A.; Meniawy, T.; Ansstas, G.; Taylor, M.H.; Kim, K.B.; McKean, M.; Long, G.V.; Sullivan, R.J.; et al. Individualised Neoantigen Therapy mRNA-4157 (V940) Plus Pembrolizumab versus Pembrolizumab Monotherapy in Resected Melanoma (KEYNOTE-942): A Randomised, Phase 2b Study. Lancet 2024, 403, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Luke, J.J.; Carlino, M.S.; Khattak, A.; Meehan, R.S.; Brown, M.; Zhang, J.; Krepler, C.; Duic, J.P.; Long, G. INTerpath-001: Pembrolizumab with V940 (mRNA-4157) versus Pembrolizumab with Placebo for Adjuvant Treatment of High-Risk Stage II–IV Melanoma. J. Clin. Oncol. 2024, 42, TPS9616. [Google Scholar]
- ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov/study/NCT05933577 (accessed on 11 August 2025).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT06961006 (accessed on 11 August 2025).
- Burris, H.A.; Patel, M.R.; Cho, D.C.; Clarke, J.M.; Gutierrez, M.; Zaks, T.Z.; Frederick, J.; Hopson, K.; Mody, K.; Binanti, B. A phase I multicenter study to assess the safety, tolerability, and immunogenicity of mRNA-4157 alone in patients with resected solid tumors and in combination with pembrolizumab in patients with unresectable solid tumors. J. Clin. Oncol. 2019, 37, 2523. [Google Scholar] [CrossRef]
- Bauman, J.; Burris, H.; Clarke, J.; Patel, M.; Cho, D.; Gutierrez, M.; Julian, R.; Scott, A.; Cohen, P.; Frederick, J. Safety, tolerability, and immunogenicity of mRNA-4157 in combination with pembrolizumab in subjects with unresectable solid tumors (KEYNOTE-603): An update. J. Immunother. Cancer 2020, 8, A1–A559. [Google Scholar]
- Gainor, J.F.; Patel, M.R.; Weber, J.S.; Gutierrez, M.; Bauman, J.E.; Clarke, J.M.; Julian, R.; Scott, A.J.; Geiger, J.L.; Kirtane, K.; et al. T-cell Responses to Individualized Neoantigen Therapy mRNA-4157 (V940) Alone or in Combination with Pembrolizumab in the Phase 1 KEYNOTE-603 Study. Cancer Discov. 2024, 14, 2209–2223. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. ClinicalTrials.gov: Search Results for “mRNA-4157”. Available online: https://www.clinicaltrials.gov/search?intr=mRNA-4157 (accessed on 9 September 2025).
- Wang, B.; Pei, J.; Xu, S.; Liu, J.; Yu, J. Recent Advances in mRNA Cancer Vaccines: Meeting Challenges and Embracing Opportunities. Front. Immunol. 2023, 14, 1246682. [Google Scholar] [CrossRef]
- Available online: https://www.merck.com/news/moderna-and-merck-announce-mrna-4157-v940-an-investigational-personalized-mrna-cancer-vaccine-in-combination-with-keytruda-pembrolizumab-met-primary-efficacy-endpoint-in-phase-2b-keynote-94/ (accessed on 9 September 2025).
- Wang, Y.; Zhang, L.; Xu, Z.; Miao, L.; Huang, L. mRNA Vaccine with Antigen-Specific Checkpoint Blockade Induces an Enhanced Immune Response Against Established Melanoma. Mol. Ther. 2018, 26, 420–434. [Google Scholar] [CrossRef]
- Fan, T.; Zhang, M.; Yang, J.; Zhu, Z.; Cao, W.; Dong, C. Therapeutic Cancer Vaccines: Advancements, Challenges, and Prospects. Signal Transduct. Target Ther. 2023, 8, 450. [Google Scholar] [CrossRef]
- Patente, T.A.; Pinho, M.P.; Oliveira, A.A.; Evangelista, G.C.M.; Bergami-Santos, P.C.; Barbuto, J.A.M. Human Dendritic Cells: Their Heterogeneity and Clinical Application Potential in Cancer Immunotherapy. Front. Immunol. 2019, 9, 3176. [Google Scholar] [CrossRef]
- Zanna, M.Y.; Yasmin, A.R.; Omar, A.R.; Arshad, S.S.; Mariatulqabtiah, A.R.; Nur-Fazila, S.H.; Mahiza. Review of Dendritic Cells, Their Role in Clinical Immunology, and Distribution in Various Animal Species. Int. J. Mol. Sci. 2021, 22, 8044. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.Y.; Zhang, F.; Goedegebuure, S.P.; Gillanders, W.E. Dendritic cell subsets and implications for cancer immunotherapy. Front Immunol. 2024, 15, 1393451. [Google Scholar] [CrossRef] [PubMed]
- Giovanelli, P.; Sandoval, T.A.; Cubillos-Ruiz, J.R. Dendritic Cell Metabolism and Function in Tumors. Trends Immunol. 2019, 40, 699–718. [Google Scholar] [CrossRef]
- Knight, S.C.; Fryer, P.; Griffiths, S.; Harding, B. Class II Histocompatibility Antigens on Human Dendritic Cells. Immunology 1987, 61, 21–27. [Google Scholar]
- Willerslev-Olsen, A.; Krejsgaard, T.; Lindahl, L.M.; Bonefeld, C.M.; Wasik, M.A.; Koralov, S.B.; Geisler, C.; Kilian, M.; Iversen, L.; Woetmann, A.; et al. Bacterial Toxins Fuel Disease Progression in Cutaneous T-Cell Lymphoma. Toxins 2013, 5, 1402–1421. [Google Scholar] [CrossRef]
- Kambayashi, T.; Laufer, T. Atypical MHC Class II-Expressing Antigen-Presenting Cells: Can Anything Replace a Dendritic Cell? Nat. Rev. Immunol. 2014, 14, 719–730. [Google Scholar] [CrossRef]
- Abascal, J.; Oh, M.S.; Liclican, E.L.; Dubinett, S.M.; Salehi-Rad, R.; Liu, B. Dendritic Cell Vaccination in Non-Small Cell Lung Cancer: Remodeling the Tumor Immune Microenvironment. Cells 2023, 12, 2404. [Google Scholar] [CrossRef]
- Dubsky, P.; Ueno, H.; Piqueras, B.; Connolly, J.; Banchereau, J.; Palucka, A.K. Human dendritic cell subsets for vaccination. J. Clin. Immunol. 2025, 25, 551–572. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, X.; Cheng, Y.; Cao, X. Dendritic Cell Migration in Inflammation and Immunity. Cell Mol. Immunol. 2021, 18, 2461–2471. [Google Scholar] [CrossRef]
- Leithner, A.; Altenburger, L.M.; Hauschild, R.; Assen, F.P.; Rottner, K.; Stradal, T.E.B.; Diz-Muñoz, A.; Stein, J.V.; Sixt, M. Dendritic Cell Actin Dynamics Control Contact Duration and Priming Efficiency at the Immunological Synapse. J. Cell Biol. 2021, 220, e202006081. [Google Scholar] [CrossRef]
- de Vries, I.J.; Lesterhuis, W.J.; Barentsz, J.O.; Verdijk, P.; van Krieken, J.H.; Boerman, O.C.; Oyen, W.J.; Bonenkamp, J.J.; Boezeman, J.B.; Adema, G.J.; et al. Magnetic Resonance Tracking of Dendritic Cells in Melanoma Patients for Monitoring of Cellular Therapy. Nat. Biotechnol. 2005, 23, 1407–1413. [Google Scholar] [CrossRef]
- Cifuentes-Rius, A.; Desai, A.; Yuen, D.; Johnston, A.P.R.; Voelcker, N.H. Inducing Immune Tolerance with Dendritic Cell-Targeting Nanomedicines. Nat. Nanotechnol. 2021, 16, 37–46. [Google Scholar] [CrossRef]
- Schwartzentruber, D.J.; Lawson, D.H.; Richards, J.M.; Conry, R.M.; Miller, D.M.; Treisman, J.; Gailani, F.; Riley, L.; Conlon, K.; Pockaj, B.; et al. gp100 Peptide Vaccine and Interleukin-2 in Patients with Advanced Melanoma. N. Engl. J. Med. 2011, 364, 2119–2127. [Google Scholar] [CrossRef]
- Biri-Kovács, B.; Bánóczi, Z.; Tummalapally, A.; Szabó, I. Peptide Vaccines in Melanoma: Chemical Approaches Towards Improved Immunotherapeutic Efficacy. Pharmaceutics 2023, 15, 452. [Google Scholar] [CrossRef]
- Peterson, A.C.; Harlin, H.; Gajewski, T.F. Immunization with Melan-A Peptide-Pulsed Peripheral Blood Mononuclear Cells Plus Recombinant Human Interleukin-12 Induces Clinical Activity and T-Cell Responses in Advanced Melanoma. J. Clin. Oncol. 2003, 21, 2342–2348. [Google Scholar] [CrossRef]
- Dillman, R.O.; Cornforth, A.N.; Depriest, C.; McClay, E.F.; Amatruda, T.T.; de Leon, C.; Ellis, R.E.; Mayorga, C.; Carbonell, D.; Cubellis, J.M. Tumor Stem Cell Antigens as Consolidative Active Specific Immunotherapy: A Randomized Phase II Trial of Dendritic Cells Versus Tumor Cells in Patients with Metastatic Melanoma. J. Immunother. 2012, 35, 641–649. [Google Scholar] [CrossRef]
- Dörrie, J.; Schaft, N.; Schuler, G.; Schuler-Thurner, B. Therapeutic Cancer Vaccination with Ex Vivo RNA-Transfected Dendritic Cells—An Update. Pharmaceutics 2020, 12, 92. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. NCT04526899. Available online: https://clinicaltrials.gov/study/NCT04526899 (accessed on 14 August 2025).
- Terai, M.; Sato, T. Individualised Neoantigen Cancer Vaccine Therapy. Lancet 2024, 403, 590–591. [Google Scholar] [CrossRef]
- Duan, L.; Mukherjee, E. Janeway’s Immunobiology, Ninth Edition. Yale J. Biol. Med. 2016, 89, 424–425. [Google Scholar]
- Lancaster, E.M.; Jablons, D.; Kratz, J.R. Applications of Next-Generation Sequencing in Neoantigen Prediction and Cancer Vaccine Development. Genet. Test. Mol. Biomarkers 2020, 24, 59–66. [Google Scholar] [CrossRef]
- Peng, M.; Mo, Y.; Wang, Y.; Wu, P.; Zhang, Y.; Xiong, F.; Guo, C.; Wu, X.; Li, Y.; Li, X.; et al. Neoantigen Vaccine: An Emerging Tumor Immunotherapy. Mol. Cancer 2019, 18, 128. [Google Scholar] [CrossRef]
- Li, L.; Goedegebuure, P.; Mardis, E.R.; Ellis, M.J.; Zhang, X.; Herndon, J.M.; Fleming, T.P.; Carreno, B.M.; Hansen, T.H.; Gillanders, W.E. Cancer Genome Sequencing and Its Implications for Personalized Cancer Vaccines. Cancers 2011, 3, 4191–4211. [Google Scholar] [CrossRef]
- Imani, S.; Li, X.; Chen, K.; Maghsoudloo, M.; Jabbarzadeh, K.P.; Hashemi, M.; Khoushab, S.; Li, X. Computational Biology and Artificial Intelligence in mRNA Vaccine Design for Cancer Immunotherapy. Front. Cell Infect. Microbiol. 2025, 14, 1501010. [Google Scholar] [CrossRef]
- Kirchmair, A.; Finotello, F. In Silico Prediction of Tumor Neoantigens with TIminer. Methods Mol. Biol. 2020, 2120, 129–145. [Google Scholar]
- Wang, X.; Jiang, L.; Zhao, J.; Wu, M.; Xiong, J.; Wu, X.; Weng, X. In Silico Neoantigen Screening and HLA Multimer-Based Validation Identify Immunogenic Neopeptide in Multifocal Lung Adenocarcinoma. Front. Immunol. 2024, 15, 1456209. [Google Scholar] [CrossRef]
- Feola, S.; Chiaro, J.; Martins, B.; Cerullo, V. Uncovering the Tumor Antigen Landscape: What to Know About the Discovery Process. Cancers 2020, 12, 1660. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, C.; Li, Y.; Li, Z.; Zhu, Y.; Yang, L.; Hu, H.; Sun, Q.; Liu, M.; Cao, S. Anti-Cancer Immune Effect of Human Colorectal Cancer Neoantigen Peptide Based on MHC Class I Molecular Affinity Screening. Front. Immunol. 2024, 15, 1473145. [Google Scholar] [CrossRef]
- Shi, R.; Liu, X.; Wang, Y.; Pan, M.; Wang, S.; Shi, L.; Ni, B. Long-Term Stability and Immunogenicity of Lipid Nanoparticle COVID-19 mRNA Vaccine Is Affected by Particle Size. Hum. Vaccin. Immunother. 2024, 20, 2342592. [Google Scholar] [CrossRef]
- Liu, J.; Miao, L.; Sui, J.; Hao, Y.; Huang, G. Nanoparticle Cancer Vaccines: Design Considerations and Recent Advances. Asian, J. Pharm. Sci. 2020, 15, 576–590. [Google Scholar] [CrossRef]
- Chesney, J.A.; Ribas, A.; Long, G.V.; Kirkwood, J.M.; Dummer, R.; Puzanov, I.; Hoeller, C.; Gajewski, T.F.; Gutzmer, R.; Rutkowski, P.; et al. Randomized, Double-Blind, Placebo-Controlled, Global Phase III Trial of Talimogene Laherparepvec Combined With Pembrolizumab for Advanced Melanoma. J. Clin. Oncol. 2023, 41, 528–540. [Google Scholar] [CrossRef]
- Cheng, Z.F.; Deutscher, M.P. An Important Role for RNase R in mRNA Decay. Mol. Cell. 2005, 17, 313–318. [Google Scholar] [CrossRef]
- Shyu, A.B.; Wilkinson, M.F.; van Hoof, A. Messenger RNA Regulation: To Translate or To Degrade. EMBO J. 2008, 27, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Ben-Akiva, E.; Karlsson, J.; Hemmati, S.; Yu, H.; Tzeng, S.Y.; Pardoll, D.M.; Green, J.J. Biodegradable Lipophilic Polymeric mRNA Nanoparticles for Ligand-Free Targeting of Splenic Dendritic Cells for Cancer Vaccination. Proc. Natl. Acad. Sci. USA 2023, 120, e2301606120. [Google Scholar] [CrossRef]
- Han, H.D.; Byeon, Y.; Kang, T.H.; Jung, I.D.; Lee, J.W.; Shin, B.C.; Lee, Y.J.; Sood, A.K.; Park, Y.M. Toll-Like Receptor 3-Induced Immune Response by Poly (d,l-lactide-co-glycolide) Nanoparticles for Dendritic Cell-Based Cancer Immunotherapy. Int. J. Nanomed. 2016, 11, 5729–5742. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, E.; Ahmed, A.; Naguib, Y.W. Advances in mRNA LNP-Based Cancer Vaccines: Mechanisms, Formulation Aspects, Challenges, and Future Directions. J. Pers. Med. 2024, 14, 1092. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.; Rana, D.; Salave, S.; Benival, D.; Khunt, D.; Prajapati, B.G. Achieving Endo/Lysosomal Escape Using Smart Nanosystems for Efficient Cellular Delivery. Molecules 2024, 29, 3131. [Google Scholar] [CrossRef]
- Varkouhi, A.K.; Scholte, M.; Storm, G.; Haisma, H.J. Endosomal Escape Pathways for Delivery of Biologicals. J. Control Release 2011, 151, 220–228. [Google Scholar] [CrossRef]
- Chatterjee, S.; Kon, E.; Sharma, P.; Peer, D. Endosomal Escape: A Bottleneck for LNP-Mediated Therapeutics. Proc. Natl. Acad. Sci. USA 2024, 121, e2307800120. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Naradikian, M.S.; Parkhouse, K.; Cain, D.W.; Jones, L.; Moody, M.A.; Verkerke, H.P.; Myles, A.; Willis, E.; et al. Nucleoside-Modified mRNA Vaccines Induce Potent T Follicular Helper and Germinal Center B Cell Responses. J. Exp. Med. 2018, 215, 1571–1588. [Google Scholar] [CrossRef]
- Horejs, C. From Lipids to Lipid Nanoparticles to mRNA Vaccines. Nat. Rev. Mater. 2021, 6, 1075–1076. [Google Scholar] [CrossRef]
- Dolgin, E. The Tangled History of mRNA Vaccines. Nature 2021, 597, 318–324. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Simberg, D. Pro-Inflammatory Concerns with Lipid Nanoparticles. Mol. Ther. 2022, 30, 2109–2110. [Google Scholar] [CrossRef]
- Tahtinen, S.; Tong, A.J.; Himmels, P.; Oh, J.; Paler-Martinez, A.; Kim, L.; Wichner, S.; Oei, Y.; McCarron, M.J.; Freund, E.C.; et al. IL-1 and IL-1ra Are Key Regulators of the Inflammatory Response to RNA Vaccines. Nat. Immunol. 2022, 23, 532–542. [Google Scholar] [CrossRef]
- Omo-Lamai, S.; Wang, Y.; Patel, M.N.; Milosavljevic, A.; Zuschlag, D.; Poddar, S.; Wu, J.; Wang, L.; Dong, F.; Espy, C.; et al. Limiting Endosomal Damage Sensing Reduces Inflammation Triggered by Lipid Nanoparticle Endosomal Escape. Nat. Nanotechnol. 2025. [Google Scholar] [CrossRef]
- Prabhakar, P.K.; Upadhyay, T.K.; Sahu, S.K. mRNA-Based Cancer Vaccines: A Novel Approach to Melanoma Treatment. Adv. Immunol. 2025, 165, 117–162. [Google Scholar] [PubMed]
- Kostecki, K.L.; Iida, M.; Crossman, B.E.; Salgia, R.; Harari, P.M.; Bruce, J.Y.; Wheeler, D.L. Immune Escape Strategies in Head and Neck Cancer: Evade, Resist, Inhibit, Recruit. Cancers 2024, 16, 312. [Google Scholar] [CrossRef] [PubMed]
- Yamshchikov, G.V.; Mullins, D.W.; Chang, C.C.; Ogino, T.; Thompson, L.; Presley, J.; Galavotti, H.; Aquila, W.; Deacon, D.; Ross, W.; et al. Sequential Immune Escape and Shifting of T Cell Responses in a Long-Term Survivor of Melanoma. J. Immunol. 2005, 174, 6863–6871. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, K.; Schmidt, B.; Kastenmüller, W.; Busch, D.H.; Drexler, I.; Sutter, G.; Heike, M.; Peschel, C.; Bernhard, H. Melanoma-Reactive Class I-Restricted Cytotoxic T Cell Clones Are Stimulated by Dendritic Cells Loaded with Synthetic Peptides, but Fail to Respond to Dendritic Cells Pulsed with Melanoma-Derived Heat Shock Proteins In Vitro. J. Immunol. 2004, 172, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Ladak, R.J.; He, A.J.; Huang, Y.H.; Ding, Y. The Current Landscape of mRNA Vaccines Against Viruses and Cancer—A Mini Review. Front. Immunol. 2022, 13, 885371. [Google Scholar] [CrossRef]
- Leong, K.Y.; Tham, S.K.; Poh, C.L. Correction: Revolutionizing Immunization: A Comprehensive Review of mRNA Vaccine Technology and Applications. Virol. J. 2025, 22, 272. [Google Scholar] [CrossRef]
- Roudko, V.; Bozkus, C.C.; Orfanelli, T.; McClain, C.B.; Carr, C.; O’Donnell, T.; Chakraborty, L.; Samstein, R.; Huang, K.L.; Blank, S.V.; et al. Shared Immunogenic Poly-Epitope Frameshift Mutations in Microsatellite Unstable Tumors. Cell 2020, 183, 1634–1649. [Google Scholar] [CrossRef]
- Chakraborty, C.; Majumder, A.; Bhattacharya, M.; Chatterjee, S.; Lee, S.S. The Landscape of Neoantigens and Its Clinical Applications: From Immunobiology to Cancer Vaccines. Curr. Res. Biotechnol. 2024, 7, 100177. [Google Scholar] [CrossRef]
- Lu, T.; Zhang, Z.; Zhu, J.; Li, Y.; Zhao, Y.; Wang, G.; Xiong, Y.; Wang, H.; Xiong, D.; Li, S. Deep Learning-Based Prediction of the T Cell Receptor–Antigen Binding Specificity. Nat. Mach. Intell. 2021, 3, 864–875. [Google Scholar] [CrossRef]
- Li, X.; You, J.; Hong, L.; Liu, W.; Guo, P.; Hao, X. Neoantigen Cancer Vaccines: A New Star on the Horizon. Cancer Biol. Med. 2023, 21, 274–311. [Google Scholar] [CrossRef]
- Light, D.W.; Lexchin, J. The Costs of Coronavirus Vaccines and Their Pricing. J. R. Soc. Med. 2021, 114, 502–504. [Google Scholar] [CrossRef]
- Killeen, T.; Kermer, V.; Troxler Saxer, R. mRNA vaccine development during the COVID-19 pandemic: A retrospective review from the perspective of the Swiss affiliate of a global biopharmaceutical company. J. Pharm. Policy Pract. 2023, 16, 158. [Google Scholar] [PubMed]
- Magoola, M.; Niazi, S.K. Current Progress and Future Perspectives of RNA-Based Cancer Vaccines: A 2025 Update. Cancers 2025, 17, 1882. [Google Scholar] [CrossRef] [PubMed]
- The Lancet Oncology. Personalised Cancer Vaccines and New Regulatory Struggles. Lancet Oncol. 2025, 26, 265. [Google Scholar] [CrossRef] [PubMed]

| Study | Ref.No | Neoplasms | Phase | Setting | Study Regimen |
|---|---|---|---|---|---|
| KEYNOTE-603 | NCT03313778 | Miscellaneous | I | Systematic | Pembrolizumab plus mRNA-4157 |
| KEYNOTE-942 | NCT03897881 | Melanoma, resected | IIb | Adjuvant | Pembrolizumab with or without mRNA-4157 |
| V940-001 | NCT05933577 | Melanoma, resected | III | Adjuvant | Pembrolizumab with or without mRNA-4157 |
| V940-012/INTerpath-012 | NCT06961006 | Melanoma, unresectable | III | 1st line, systematic | Pembrolizumab with or without mRNA-4157 |
| V940-002/INTerpath-002 | NCT06077760 | Non-small Cell Lung Cancer, resected, st.II-IIIB | III | adjuvant | Pembrolizumab with or without mRNA-4157 |
| V940-009/INTerpath-009 | NCT06623422 | Non-small Cell Lung Cancer, resectable, not achieving pCR after preoperative platinum-based treatment | III | adjuvant | Pembrolizumab with or without mRNA-4157 |
| V940-004/INTerpath-004 | NCT06307431 | Renal Cell Carcinoma, Resected | II | adjuvant | Pembrolizumab with or without mRNA-4157 |
| V940-007 | NCT06295809 | Cutaneous Squamous Cell Carcinoma, resectable | II/III | (Neo)adjuvant | Pembrolizumab with or without mRNA-4157 |
| V940-011/INTerpath-011 | NCT06833073 | Bladder Cancer, high-risk, non-muscle invasive, endoscopically resected | II | adjuvant | Intravesical BCG with or without mRNA-4157 |
| Treatment | Mechanism of Action | Advantage | Disadvantage |
|---|---|---|---|
| Peptide vaccines | Peptides are injected into the host and activate APCs | Easy production | Modest clinical efficacy |
| Dendritic cell vaccines | Monocytes of the host are incubated ex vivo with tumor antigens and/or tumor tissue pieces, under the influence of growth factors and cytokines. The activated DCs produced are injected into the patient | Enhancement of the antigen-presenting capacity of the host Easy subcutaneous administration Have shown efficacy in the context of clinical trials | Modest clinical efficacy Difficult to produce The patient has to be treated in a center with the required facilities |
| mRNA-transfected DC vaccines | DCs are transfected ex vivo with mRNA encoding for tumor-associated antigens | DCs are able to produce tumor antigens Already established tumor-associated antigen sequences may serve as the template | Antigens encoded not unique to patient and tumor Challenging production chain Need for specialized hospital |
| mRNA vaccine encapsulated in lipid nanoparticle, not personalized (e.g., BNT111) | mRNA encoding for NY-ESO-1, MAGE-A3, tyrosinase, and TPTE in lipoplex formulation are injected into the host; DCs absorb mRNA and produce the four tumor-associated antigens and provoke immune reaction | Easy to transfer and administer Maybe more affordable Over 90% of patients with cutaneous melanomas express at least one of these four antigens | Not unique to every patient Lack of clinical data |
| mRNA vaccine encapsulated in lipid nanoparticle, individualized (e.g., mRNA-4157/V940) | Tumor and patient-specific, immunogenic neoantigen loci are identified, mRNA encoding for them is administered to the host, where it is absorbed by DCs, which then produce the targeted neoantigens, exposing them to immune cells. | Unique to every patient Makes use of tumor and host-specific neoantigens Immunogenicity of selected neoantigens may be predicted by in silico studies Easy to transfer, administer | State-of-the-art technology is required for production 1 drug per patient, cannot administer it to large groups High cost |
| mRNA Vaccine | mRNA-4157 | BNT111 |
|---|---|---|
| Encodes for | Up to thirty-four, individual, host, and tumor-specific neoantigens | Four melanoma associated peptides: NY-ESO-1, MAGE-A3, tyrosinase, TPTE |
| Vector | LNP | LNP |
| Combined with | pembrolizumab | cemiplimab |
| Significant trial | Keynote-942 (NCT03897881) | BNT111-01 (NCT04526899) |
| Available clinical data | Reduces recurrence of high-risk melanoma, combined with pembrolizumab in the adjuvant setting | Improves ORR in unresectable melanoma patients, compared to cemiplimab monotherapy |
| Potentially applicable to | Melanoma, NSCLC, bladder cancer, RCC | melanoma |
| advantage | Highly individualized treatment | May benefit larger groups of patients |
| Disadvantage | Not proven to have a benefit over universal mRNA vaccines Expensive to produce | Not patient-specific Not effective in tumors not expressing the specific encoded antigens |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gazouli, I.; Bafaloukos, D.; Koutserimpas, C.; Samonis, G. Individualized mRNA Vaccines in Melanoma—Where Do We Stand? Vaccines 2025, 13, 986. https://doi.org/10.3390/vaccines13090986
Gazouli I, Bafaloukos D, Koutserimpas C, Samonis G. Individualized mRNA Vaccines in Melanoma—Where Do We Stand? Vaccines. 2025; 13(9):986. https://doi.org/10.3390/vaccines13090986
Chicago/Turabian StyleGazouli, Ioanna, Dimitrios Bafaloukos, Christos Koutserimpas, and George Samonis. 2025. "Individualized mRNA Vaccines in Melanoma—Where Do We Stand?" Vaccines 13, no. 9: 986. https://doi.org/10.3390/vaccines13090986
APA StyleGazouli, I., Bafaloukos, D., Koutserimpas, C., & Samonis, G. (2025). Individualized mRNA Vaccines in Melanoma—Where Do We Stand? Vaccines, 13(9), 986. https://doi.org/10.3390/vaccines13090986






