Abstract
Background/Objectives: Mucosal vaccines, delivered intranasally or via inhalation, are being studied for respiratory infectious diseases like COVID-19 and influenza. These vaccines aim to provide non-invasive administration and strong immune responses at infection sites, making them a promising area of research. This systematic review and meta-analysis assessed their immunogenicity, safety, and protective efficacy. Methods: The study design was a systematic review and meta-analysis, searching PubMed and Cochrane databases up to 30 May 2025. Inclusion criteria followed the PICOS framework, focusing on mucosal vaccines for COVID-19, influenza, RSV, pertussis, and tuberculosis. Results: A total of 65 studies with 229,614 participants were included in the final analysis. Mucosal COVID-19 vaccines elicited higher neutralizing antibodies compared to intramuscular vaccines (SMD = 2.48, 95% CI: 2.17–2.78 for wild-type; SMD = 1.95, 95% CI: 1.32–2.58 for Omicron), with varying efficacy by route (inhaled VE = 47%, 95% CI: 22–74%; intranasal vaccine VE = 17%, 95% CI: 0–31%). Mucosal influenza vaccines protected children well (VE = 62%, 95% CI: 30–46%, I2 = 17.1%), but seroconversion rates were lower than those of intramuscular vaccines. RSV and pertussis vaccines had high seroconversion rates (73% and 52%, respectively). Tuberculosis vaccines were reviewed systemically, exhibiting robust cellular immunogenicity. Safety was comparable to intramuscular vaccines or placebo, with no publication bias detected. Conclusions: Current evidence suggests mucosal vaccines are immunogenic, safe, and protective, particularly for respiratory diseases. This review provides insights for future research and vaccination strategies, though limitations include varying efficacy by route and study heterogeneity.
1. Introduction
Respiratory infectious diseases, including COVID-19, influenza, respiratory syncytial virus (RSV), pertussis, and tuberculosis, pose a significant global health threat [1]. As of 25 February 2025, COVID-19 has affected over 770 million people, resulting in 7.09 million deaths [2]. Influenza impacts around 1 billion people annually, causing 3 to 5 million severe cases and 290,000 to 650,000 deaths each year [3]. RSV, a major cause of lower respiratory infections, affects 64 million globally, leading to 160,000 deaths annually [4]. There were 14,894 confirmed pertussis cases in England in 2024 [5], while in 2023, there were 8.2 million reported new tuberculosis diagnoses, leading to and 1.25 million deaths [6].
These diseases disproportionately affect vulnerable groups, such as infants, the elderly, and those with comorbidities, emphasizing the need for effective vaccination strategies. Traditional injectable vaccination induces systemic immunity but faces challenges like needle phobia, reducing acceptance, and limited effectiveness against transmission [7]. Mucosal vaccines, delivered intranasally or via inhalation, offer non-invasive options, enhancing public willingness and inducing site-specific immunity, including secretory IgA (sIgA) and tissue-resident memory cells, potentially preventing infection at entry points [7,8].
The COVID-19 pandemic has highlighted the need for innovative approaches, with mucosal vaccines like the inhaled adenovirus type 5 (Ad5) COVID-19 vaccine and intranasal FluMist showing promise [9]. However, comprehensive evaluations across pathogens are lacking, with previous studies focusing narrowly on specific aspects, such as humoral immunogenicity, leaving gaps in assessing overall efficacy and cellular responses [10].
Given the ongoing burden of respiratory diseases and rapid advancements in mucosal vaccine research, a systematic review is timely. Recent developments, spurred by COVID-19, include new clinical trials and the exploration of adenoviral vectors, yet gaps remain, particularly for RSV, pertussis, and tuberculosis, justifying a comprehensive synthesis to inform future strategies.
While current research on mucosal vaccines mainly consists of clinical trials and systematic reviews, there is a significant absence of meta-analysis evaluation [10]. Previous systematic reviews and meta-analyses have evaluated inhaled COVID-19 vaccines, demonstrating their favorable humoral immunogenicity and safety profile. However, comprehensive analyses concentrating on intranasal COVID-19 vaccines and assessments of cellular immune responses induced by COVID-19 mucosal vaccines remain insufficient [11]. Additionally, a meta-analysis published in 2021 investigated the humoral immunogenicity and safety of influenza intranasal vaccines [12]. Nevertheless, that study had notable limitations, as it failed to evaluate mucosal and cellular immune responses, as well as vaccine efficacy. To date, no meta-analyses are available for mucosal vaccines targeting respiratory syncytial virus (RSV), pertussis, or tuberculosis. The limitation impedes a holistic assessment of how mucosal vaccines compare to traditional injectable vaccines in terms of overall efficacy. Moreover, insufficient immunogenicity and the lack of suitable delivery vehicles remain limiting factors for the clinical use of mucosal vaccines.
This systematic review and meta-analysis aim to assess the immunogenicity, safety, and protective efficacy of mucosal vaccines, providing evidence to guide future research and vaccination strategies.
2. Methods
2.1. Study Design
This systematic review adhered to Systematic Reviews and Meta-Analyses (PRISMA) [13] and Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines (Supplementary Materials Table S1) [14]. A pre-registered protocol (PROSPERO ID: CRD420250649097) defined the research question, search strategy, inclusion/exclusion criteria, outcomes, data extraction, and synthesis.
2.2. Search Strategy and Selection Criteria
We searched Cochrane Central Register of Controlled Trials and PubMed up to 30 May 2025 for randomized controlled trials (RCTs), non-RCTs, and cohort studies evaluating the safety, immunogenicity, and protective efficacy of mucosal vaccines against respiratory infectious diseases. Search terms included “Vaccines”, “Inhalation”, “Intranasal”, their synonyms and MeSH terms, combined using Boolean operators (Supplementary Materials Table S2). Reference lists of included studies were screened, experts consulted, and corresponding authors contacted for missing or incomplete data.
Inclusion/exclusion criteria followed the Population, Intervention, Comparison, Outcome, and Study design (PICOS) design (Supplementary Materials Table S3) [15]. Studies were included if they (1) investigated mucosal vaccine safety, immunogenicity, or efficacy; (2) were RCTs, non-RCTs, or cohort studies; (3) focused on respiratory infectious diseases; and (4) were original English articles. Exclusion criteria included (1) non-inhaled/intranasal vaccines; (2) reviews, protocols, non-peer-reviewed, or non-full-text articles; (3) in vivo/in vitro studies; and (4) non-English articles. Two researchers independently screened titles/abstracts and full texts, resolving discrepancies with a third researcher.
2.3. Development of Meta-Analysis
Risk of bias was assessed as “low,” “high,” or “some concerns.” by two researchers using validated tools: the Risk of Bias-2 (RoB-2) for RCTs [16], the Newcastle–Ottawa Scale (NOS) for cohort studies [17], and the Risk of Bias in Non-Randomized Studies of Interventions (ROBINS-I) for non-RCTs [18]. RoB-2 evaluated randomization, deviations, missing data, outcome measurement, and result selection. ROBINS-I assessed confounding, selection, intervention classification, deviations, missing data, measurement, and reporting. NOS evaluated selection, comparability, and outcomes. Discrepancies were resolved through discussion with the corresponding author.
Data extraction used a pre-specified form, verified by a second researcher. Extracted data included study characteristics (author, year, country, design, period), participant characteristics (population, sample size, age, sex), intervention (comparison, dosage, vaccine composition), outcome (safety, efficacy, immunogenicity), funding, and conflicts of interest.
Immunogenicity, the primary outcome, was measured by seroconversion rates (four-fold antibody increase) and neutralizing/binding antibody geometric mean titers (nAb-GMT/bAb-GMT). Cellular immunity was assessed via T cell ELISpot IFN-γ responses and intracellular cytokine staining. Safety, the secondary outcome, was evaluated as risk ratio (RR) for adverse events. Protective efficacy, the third outcome, was calculated as vaccine efficacy (VE) using VE = (1 − RR) × 100 or 1-odds ratio (OR), with pooled VE derived from RR estimates. Dichotomous outcomes used RR with 95% CIs; continuous outcomes used standardized mean difference (SMDs) after logarithmic transformation.
2.4. Statistical Analysis
Meta-analysis was conducted using Stata (version 18.0) with p < 0.05 for significance. Pooled estimates used fixed or DerSimonian–Laird random-effects model based on heterogeneity [19], where the random-effects approach estimates between-study heterogeneity (τ2) using a moment-based approach with inverse-variance weighting. Heterogeneity was assessed via I2 (<25%: none; 25–50%: low; 50–75%: moderate; >75%: high) [20].
Subgroup analyses explored administration method, comparison group, and Omicron sub-lineages (Q-test, p < 0.05). Sensitivity analyses excluded individual studies to test robustness. Publication bias was evaluated using funnel plots and Egger’s regression asymmetry test, (p < 0.05) [21], with trim-and-fill applied if bias was detected [22].
3. Results
We identified 17,284 records through comprehensive database searches, including 15,356 from PubMed and 1928 records from Cochrane. After we excluded 914 duplicates, 16,370 records underwent title and abstract screening. During this phase, we excluded 12,378 records irrelevant to the topic, 3269 non-original articles, and 237 records in languages other than the target language. We then assessed 486 full-text articles for eligibility based on predefined inclusion criteria. Of these, 337 studies were excluded for not focusing on mucosal vaccines, 63 had outcomes not meeting our criteria, and 21 had data that could not be extracted. Ultimately, 65 studies were included in the systematic review, with 19 on COVID-19 [23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41], 32 on influenza [42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73], 5 on RSV [74,75,76,77,78], 5 on tuberculosis [79,80,81,82,83], and 4 on pertussis [84,85,86,87] (Figure 1). The same 65 studies were eligible for meta-analyses where data permitted.
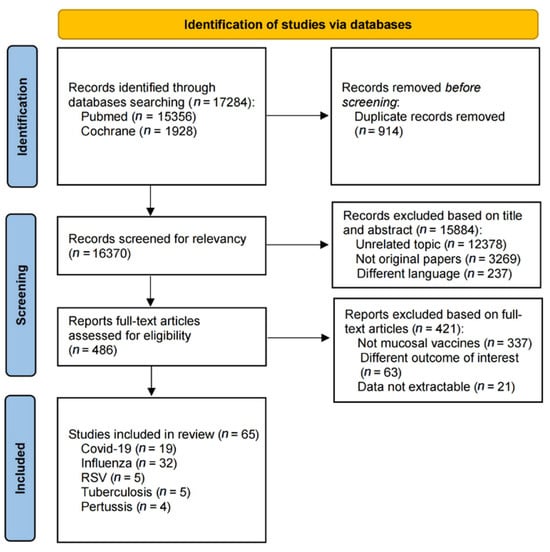
Figure 1.
Flow chart of the selection process.
4. Characteristics of the Included Studies
The included studies predominantly evaluated intranasal vaccines (51 studies) over inhaled vaccines (14 studies), with a focus on immunogenicity (54 studies) and safety (56 studies), while fewer assessed protective efficacy (11 studies). Randomized controlled trials were the most common design (49 studies), followed by non-randomized controlled trials (10 studies) and cohort studies (6 studies). Table 1 summarizes the main characteristics extracted, including vaccine type, target pathogen, study design, participant numbers, and outcomes measured.

Table 1.
Trials of vaccine immunogenicity, safety, and efficacy meeting inclusion criteria.
5. The Immunogenicity of Mucosal Vaccines
The primary assessment focused on the immunogenicity and safety of mucosal vaccines. For secretory IgA (sIgA), a meta-analysis of 10 studies (6 influenza, 4 COVID-19) showed that mucosal COVID-19 vaccines had a pooled standardized mean difference (SMD) for binding antibody geometric mean titer (bAb-GMT) of 0.67 (95% CI: 0.07–1.26, participants = 1220), with high heterogeneity (I2 = 93.7%) compared to controls (Figure 2). For mucosal influenza vaccines, pooled seroconversion rates were 65% (95% CI: 41–90%, participants = 229, I2 = 92.4%,) for A/H1N1, 45% (95% CI: 19–70%, participants = 252, I2 = 92.2%) for A/H3N2, and 44% (95% CI: 13–75%, participants = 151, I2 = 86.4%) for B strain (Supplementary Materials Figures S3–S5). For humoral immunity, a meta-analysis of 37 studies showed mucosal COVID-19 vaccines had a pooled SMD for neutralizing antibody geometric mean titer (nAb-GMT) of 2.48 (95% CI: 2.17–2.78, participants = 4406, I2 = 82.9%) for the wild-type strain and 1.95 (95% CI: 1.32–2.58, participants = 1359, I2 = 94.5%) for the Omicron strain, both higher than intramuscular inactivated vaccines (Figure 3). For mucosal influenza vaccines, the pooled relative risk (RR) of seroconversion rates for A/H1N1 was 0.89 (95% CI: 0.46–1.73, participants = 5191, I2 = 96.3%) compared to intramuscular vaccines, though higher than placebo (RR = 2.33, 95% CI: 1.39–3.91, participants = 1550, I2 = 41.3%). Similar trends were observed for A/H3N2 (RR = 0.65, 95% CI: 0.42–1.03, participants = 3761, I2 = 95.7%) compared to intramuscular vaccines, though higher than placebo (RR = 2.60, 95% CI: 1.18–5.75, participants = 1069, I2 = 30.5%), and for B strain (RR = 0.37, 95% CI: 0.16–0.84, participants = 4317, I2 = 98.5%) compared to intramuscular vaccines, though higher than placebo (RR = 7.70, 95% CI: 4.88–12.16, participants = 1060, I2 = 0.0%) (Figure 4). Pooled seroconversion rates for RSV and pertussis vaccines were 73% (95% CI: 51–94%, participants = 166, I2 = 88.1%, p < 0.0001) and 52% (95% CI: 23–82%, participants = 78, I2 = 85.6%), respectively (Supplementary Materials Figures S6 and S7). Cellular immunity showed mucosal vaccines for COVID-19 and influenza eliciting robust Th1 and CD8+ T cell responses, with tuberculosis vaccines (four studies) enhancing airway-resident memory T cells (Table 2).
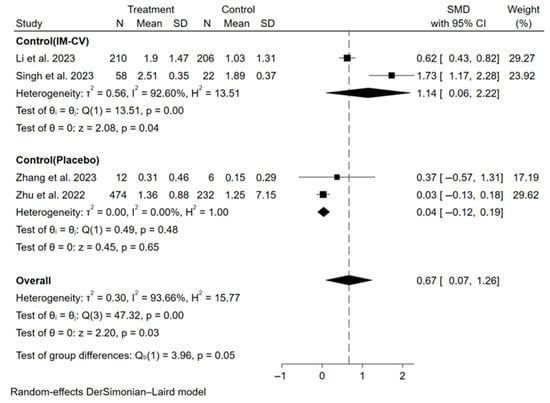
Figure 2.
Forest plot showing the SMD of bAb-GMT of sIgA for mucosal COVID-19 vaccines. IM-CV: intramuscular COVID-19 vaccines.
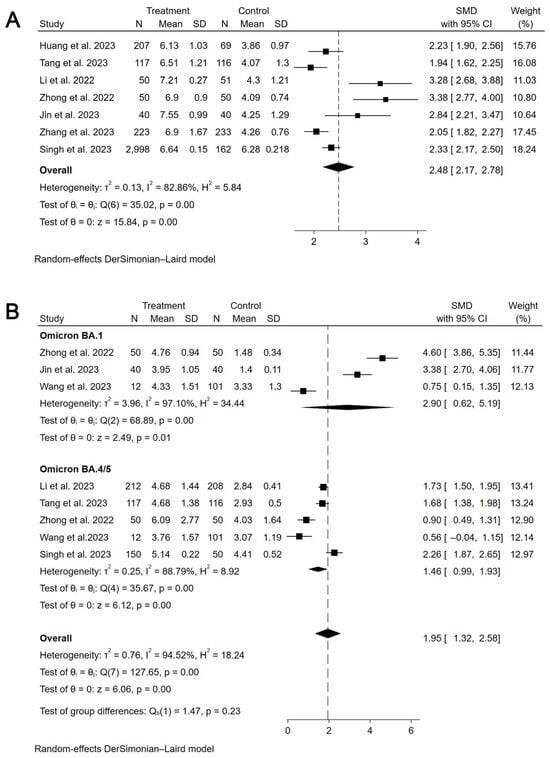
Figure 3.
Forest plot showing the SMD of nAb-GMT for mucosal COVID-19 vaccines (wide-type and Omicron). (A) the SMD of nAb-GMT for mucosal COVID-19 vaccines (wide-type). (B) The SMD of nAb-GMT for mucosal COVID-19 vaccines (Omicron).
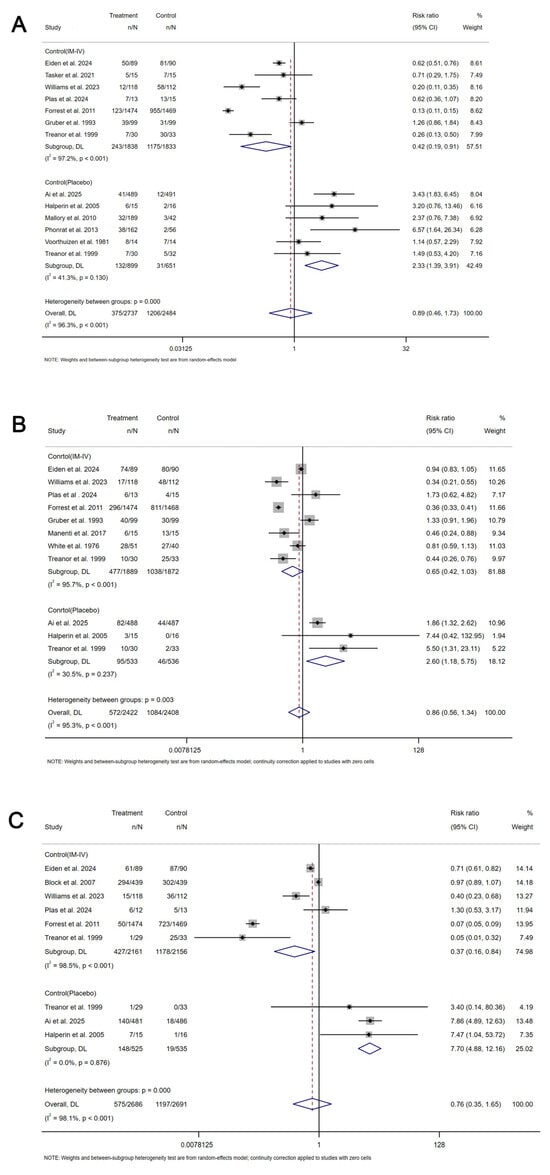
Figure 4.
Forest plot showing the risk ratio of seroconversion rate for mucosal influenza vaccines. (A) The risk ratio of seroconversion rate for mucosal influenza vaccines for A/H1N1 strain. (B) The risk ratio of seroconversion rate for mucosal influenza vaccines for A/H3N2 strain. (C) The risk ratio of seroconversion rate for mucosal influenza vaccines for B strain. IM-IV: intramuscular injection inactivated influenza vaccines. n: number of incidents. N: total number of participants.

Table 2.
The summary of the immunogenicity of tuberculosis mucosal vaccines.
6. The Safety of Mucosal Vaccines
In the secondary assessment, the safety analyses, based on 25 studies (Table 3), showed mucosal COVID-19 vaccines had a comparable risk of adverse events to intramuscular vaccines, including fever (RR = 2.24, 95% CI: 1.08–4.64, participants = 11,130, I2 = 0.0%), myalgia (RR = 1.12, 95% CI: 0.34–3.72, I2 = 0.0%), cough (RR = 2.86, 95% CI: 0.82–10.03, I2 = 0.0%), and sore throat (RR = 3.93, 95% CI: 1.08–14.27, I2 = 0.0%). For mucosal influenza vaccines, risks were higher than intramuscular vaccines for cough (RR = 2.27, 95% CI: 0.80–6.41, participants = 13,475, I2 = 85.7%) and nasal congestion (RR = 2.78, 95% CI: 0.79–9.77, I2 = 94.2%) but similar to placebo.

Table 3.
Safety profile of mucosal influenza vaccines compared to intramuscular vaccines and placebo.
7. The Vaccine Effectiveness of Mucosal Vaccines
In the third assessment, a meta-analysis of nine studies revealed a pooled vaccine effectiveness (VE) for mucosal COVID-19 vaccines of 35% (95% CI: 4–56%, I2 = 96.6%,), with inhalation at 47% (95% CI: 22–74%, I2 = 94.5%) and intranasal spray at 17% (95% CI: 0–31%, I2 = 34.1%). For influenza, pooled VE was 27% (95% CI: 13–41%, I2 = 86.4%), with 62% (95% CI: 30–46%, I2 = 17.1%) in children and 12% (95% CI: 5–19%, I2 = 0%) in adults (Figure 5).
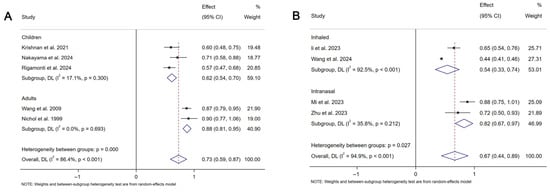
Figure 5.
Forest plot showing the vaccine efficacy for mucosal COVID-19 vaccines and mucosal influenza vaccines. (A) The vaccine efficacy for mucosal COVID-19 vaccines. (B) The vaccine efficacy for mucosal influenza vaccines.
8. Risk of Bias
Among randomized controlled trials, 17 of 49 had high risk due to selection and performance bias (Supplementary Materials Tables S4–S6). One of six cohort studies scored 4 on the NOS scale (high risk), with five at low risk (score ≥ 7). Eight of ten non-randomized trials had moderate risk due to confounding and selection bias, with two at low risk (Supplementary Materials Figures S1 and S2).
9. Sensitivity Analyses and Publication Bias
Heterogeneity was high in most analyses (I2 = 0–96.6%). Sensitivity analyses showed no single study significantly altered pooled estimates (Supplementary Materials Figures S8–S25). Egger’s test indicated no publication bias (Supplementary Materials Table S7).
10. Discussion
This meta-analysis and systematic review, the first to comprehensively assess mucosal vaccines against respiratory infectious diseases, analyzed 65 studies—19 on COVID-19, 32 on influenza, 5 on RSV, 5 on tuberculosis, and 4 on pertussis—offering critical insights into their immunogenicity, safety, and protective efficacy. Our findings reveal that mucosal vaccines induce robust mucosal immunity, particularly through secretory IgA, demonstrate a favorable safety profile with no increased risk of adverse events compared to intramuscular vaccines or placebo, and provide valid protective efficacy, with vaccine effectiveness (VE) of 35% (95% CI: 4–56%) for COVID-19 and 27% (95% CI: 13–41%) for influenza.
Our hypothesis posited that mucosal vaccines would elicit strong mucosal immunity and offer comparable or superior protection to intramuscular vaccines. The results support this: mucosal COVID-19 vaccines significantly increased sIgA levels (SMD = 0.67, 95% CI: 0.07–1.26) and neutralizing antibody titers against wild-type (SMD = 2.48, 95% CI: 2.17–2.78) and Omicron strains (SMD = 1.95, 95% CI: 1.32–2.58) compared to intramuscular vaccines. Mucosal influenza vaccines achieved high sIgA seroconversion rates (e.g., 65% for A/H1N1, 95% CI: 41–90%), though their neutralizing antibody levels were lower than intramuscular vaccines (RR = 0.42, 95% CI: 0.19–0.91) but exceeded placebo (RR = 2.33, 95% CI: 1.39–3.91). Limited data on RSV and pertussis showed moderate seroconversion (73% and 52%, respectively), while tuberculosis vaccines enhanced airway-resident T cells. These findings align with prior research, such as FluMist’s effectiveness in children [88], which our subgroup analysis confirmed with higher VE in pediatric populations (62% vs. 12% in adults).
The study’s strengths include its broad scope across multiple pathogens and outcomes, the novel use of sIgA as a mucosal immunity marker, and the inclusion of diverse study designs (49 RCTs, 10 non-RCTs, 6 observational), enhancing generalizability. However, limitations temper our conclusions. High heterogeneity (I2 up to 96.6%) arose from varied participant demographics, vaccine platforms, and administration routes, though sensitivity analyses confirmed robustness. Bias is a concern, with eight studies at high risk and ten with moderate risk, necessitating cautious interpretation. Publication bias was not evident, but sparse data on cellular immunity, long-term efficacy, and special populations (e.g., immunocompromised, pregnant) limit the findings’ scope. Most participants were healthy, reducing applicability to vulnerable groups.
No major controversies emerged, but discrepancies with other reviews exist. Some report lower systemic antibody responses for mucosal vaccines [89], consistent with our influenza findings, possibly due to differing study designs or analytical methods—our inclusion of observational data contrasts with RCT-focused reviews.
However, mucosal vaccination offers a strategic advantage for preventing infections but struggles with unique biological and technological constraints. Conventional live-attenuated vaccines pose safety risks, whereas modern alternatives often fail to elicit sufficient mucosal immune responses without tailored adjuvants or delivery vehicles. Overcoming these challenges requires innovative approaches to harmonize antigen presentation, adjuvant activity, and tissue-specific targeting at mucosal sites.
Future research should prioritize large-scale, high-quality trials to assess long-term efficacy, cellular immunity, and safety across diverse populations. Optimizing administration routes (e.g., inhaled vs. intranasal) and vaccine platforms also warrants investigation, given inhaled COVID-19 vaccines’ superior VE.
11. Conclusions
Collectively, the evidence suggests mucosal vaccines are a promising tool for respiratory disease prevention, leveraging mucosal immunity at pathogen entry points. Their non-invasive delivery could enhance uptake, particularly in children, and reduce healthcare burdens. Clinically, their significance lies in potentially controlling pandemics, though data gaps for RSV, tuberculosis, and cellular immunity require attention. For policy and practice, these findings advocate continued investment in mucosal vaccine development to bolster global health security, especially for pediatric and high-risk groups.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vaccines13080825/s1, Figure S1: Quality assessment of the included randomized controlled trials; Figure S2: Quality assessment of the included non-randomized controlled trials; Figure S3: The seroconversion rate of sIgA for mucosal influenza vaccines (A/H1N1); Figure S4: The seroconversion rate of sIgA for mucosal influenza vaccines (A/H3N2); Figure S5: The seroconversion rate of sIgA for mucosal influenza vaccines (B); Figure S6: The pooled seroconversion rate of mucosal RSV vaccines; Figure S7: The pooled seroconversion rate of mucosal pertussis vaccines; Figure S8: The sensitivity analysis for immunogenicity of mucosal COVID-19 vaccines (nAb-GMT, Wide-type); Figure S9: The sensitivity analysis for immunogenicity of mucosal COVID-19 vaccines (nAb-GMT, Omicron); Figure S10: The sensitivity analysis for immunogenicity of mucosal COVID-19 vaccines (bAb-GMT of sIgA); Figure S11. The sensitivity analysis for immunogenicity of mucosal influenza vaccines (RR of seroconversion rate, A/H1N1); Figure S12: The sensitivity analysis for immunogenicity of mucosal influenza vaccines (RR of seroconversion rate, A/H2N3); Figure S13: The sensitivity analysis for immunogenicity of mucosal influenza vaccines (RR of seroconversion rate, B); Figure S14: The sensitivity analysis for immunogenicity of mucosal influenza vaccines (RR for seroconversion rate of sIgA, A/H1N1); Figure S15: The sensitivity analysis for immunogenicity of mucosal influenza vaccines (RR for seroconversion rate of sIgA, A/H3N2). Figure S16: The sensitivity analysis for immunogenicity of mucosal influenza vaccines (RR for seroconversion rate of sIgA, B); Figure S17: The sensitivity analysis for immunogenicity of mucosal RSV vaccines (pooled seroconversion rate); Figure S18. The sensitivity analysis for immunogenicity of mucosal pertussis vaccines (pooled seroconversion rate); Figure S19: The sensitivity analysis for protective efficacy of mucosal COVID-19 vaccines (VE); Figure S20: The sensitivity analysis for protective efficacy of mucosal influenza vaccines (VE); Figure S21. The sensitivity analysis for safety of mucosal influenza vaccines (cough). Figure S22: The sensitivity analysis for safety of mucosal influenza vaccines (headache); Figure S23. The sensitivity analysis for safety of mucosal influenza vaccines (nasal congestion); Figure S24. The sensitivity analysis for safety of mucosal influenza vaccines (rhinorrhea); Figure S25: The sensitivity analysis for safety of mucosal influenza vaccines (sore throat). Table S1: PRISMA 2020 Checklist; Table S2: Search strategy by Pubmed and The Cochrane Central Register of Controlled Trials. Table S3: PICOS; Table S4: Quality assessment of randomized controlled clinical trials using the Cochrane risk of bias tool; Table S5: Quality assessment of non-randomized controlled clinical trials using the ROBINS-I tool; Table S6: Quality assessment of cohort studies using the Newcastle-Ottawa quality assessment scale tool; Table S7: The result data of Egger’s test (publication bias).
Author Contributions
J.Y. conceptualized and supervised the study. J.C. designed the study and, with W.L. (Weitong Lin), designed the search terms. X.C., H.H., X.L. and W.L. (Weitong Lin) screened studies for inclusion. X.C., H.H., X.L. and W.L. (Weitong Lin) collected data, with X.C., H.H., X.L. and C.Y. performing data extraction and quality assessment. J.C. and W.L. (Weitong Lin) analyzed and interpreted the data. J.C., W.L. (Weitong Lin) and C.Y. verified the underlying data. J.C. and W.L. (Weitong Lin) drafted the manuscript, with J.Y. and other authors, critically reviewed or revised the manuscript, and approved the final version for submission. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Major Project of Guangzhou National Laboratory (GZNL2023A01009 and GZNL2023A01005 to J.Y.), and the National Natural Science Foundation of China (NSFC 82371831 to J.Y.).
Data Availability Statement
All data supporting reported results are available within the manuscript and the Supplementary Materials.
Acknowledgments
We thank the authors of included studies who provided additional information upon request.
Conflicts of Interest
The authors declare no competing interests.
References
- Seo, J.; Polster, J.; Israelow, B.; Corbett-Helaire, K.S.; Martinez, D.R. Challenges for developing broad-based mucosal vaccines for respiratory viruses. Nat. Biotechnol. 2024, 42, 1765–1767. [Google Scholar] [CrossRef]
- COVID-19. Available online: https://data.who.int/dashboards/covid19 (accessed on 20 July 2025).
- Influenza (Seasonal). Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 20 July 2025).
- Respiratory Syncytial Virus (RSV). Available online: https://www.niaid.nih.gov/diseases-conditions/respiratory-syncytial-virus-rsv (accessed on 20 July 2025).
- Confirmed Cases of Pertussis in England by Month, to End December 2024. Available online: https://www.gov.uk/government/publications/pertussis-epidemiology-in-england-2024/confirmed-cases-of-pertussis-in-england-by-month (accessed on 20 July 2025).
- Global Tuberculosis Report 2024. Available online: https://www.who.int/teams/global-programme-on-tuberculosis-and-lung-health/tb-reports/global-tuberculosis-report-2024 (accessed on 20 July 2025).
- Tsai, C.J.Y.; Loh, J.M.S.; Fujihashi, K.; Kiyono, H. Mucosal vaccination: Onward and upward. Expert. Rev. Vaccines 2023, 22, 885–899. [Google Scholar] [CrossRef]
- Lavelle, E.C.; Ward, R.W. Mucosal vaccines—Fortifying the frontiers. Nat. Rev. Immunol. 2022, 22, 236–250. [Google Scholar] [CrossRef]
- Pilapitiya, D.; Wheatley, A.K.; Tan, H.X. Mucosal vaccines for SARS-CoV-2: Triumph of hope over experience. EBioMedicine 2023, 92, 104585. [Google Scholar] [CrossRef]
- Dotiwala, F.; Upadhyay, A.K. Next Generation Mucosal Vaccine Strategy for Respiratory Pathogens. Vaccines 2023, 11, 1585. [Google Scholar] [CrossRef]
- Song, G.; Li, R.; Cheng, M.Q. Safety, immunogenicity, and protective effective of inhaled COVID-19 vaccines: A systematic review and meta-analysis. J. Med. Virol. 2024, 96, e29625. [Google Scholar] [CrossRef]
- Perego, G.; Vigezzi, G.P.; Cocciolo, G.; Chiappa, F.; Salvati, S.; Balzarini, F.; Odone, A.; Signorelli, C.; Gianfredi, V. Safety and Efficacy of Spray Intranasal Live Attenuated Influenza Vaccine: Systematic Review and Meta-Analysis. Vaccines 2021, 9, 998. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ (Clin. Res. Ed.) 2021, 372, n71. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions; Wiley: Hoboken, NJ, USA, 2019. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Clin. Res. Ed.) 2011, 343, d5928. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, J. The Newcastle-Ottawa Scale (NOS) for Assessing The Quality of Nonrandomised Studies in Meta-analyses. Ott. Health Res. Inst. Web Site 2014, 7. [Google Scholar]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ (Clin. Res. Ed.) 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials revisited. Contemp. Clin. Trials 2015, 45, 139–145. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clin. Res. Ed.) 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. A Nonparametric “Trim and Fill” Method of Accounting for Publication Bias in Meta-Analysis. J. Am. Stat. Assoc. 2000, 95, 89–98. [Google Scholar] [CrossRef]
- Li, J.X.; Hou, L.H.; Gou, J.B.; Yin, Z.D.; Wu, S.P.; Wang, F.Z.; Zhang, Z.; Peng, Z.H.; Zhu, T.; Shen, H.B.; et al. Safety, immunogenicity and protection of heterologous boost with an aerosolised Ad5-nCoV after two-dose inactivated COVID-19 vaccines in adults: A multicentre, open-label phase 3 trial. Lancet Infect. Dis. 2023, 23, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Li, J.X.; Wu, S.P.; Guo, X.L.; Tang, R.; Huang, B.Y.; Chen, X.Q.; Chen, Y.; Hou, L.H.; Liu, J.X.; Zhong, J.; et al. Safety and immunogenicity of heterologous boost immunisation with an orally administered aerosolised Ad5-nCoV after two-dose priming with an inactivated SARS-CoV-2 vaccine in Chinese adults: A randomised, open-label, single-centre trial. Lancet Respir. Med. 2022, 10, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Huang, B.; Deng, Y.; Zhang, S.; Liu, X.; Wang, L.; Liu, Q.; Zhao, L.; Tang, L.; Wang, W.; et al. Neutralizing antibody levels associated with injectable and aerosolized Ad5-nCoV boosters and BA.2 infection. BMC Med. 2023, 21, 233. [Google Scholar] [CrossRef]
- Wu, S.; Huang, J.; Zhang, Z.; Wu, J.; Zhang, J.; Hu, H.; Zhu, T.; Zhang, J.; Luo, L.; Fan, P.; et al. Safety, tolerability, and immunogenicity of an aerosolised adenovirus type-5 vector-based COVID-19 vaccine (Ad5-nCoV) in adults: Preliminary report of an open-label and randomised phase 1 clinical trial. Lancet Infect. Dis. 2021, 21, 1654–1664. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, S.; Liu, Y.; Li, K.; Fan, P.; Song, X.; Wang, Y.; Zhao, Z.; Zhang, X.; Shang, J.; et al. Boosting with an aerosolized Ad5-nCoV elicited robust immune responses in inactivated COVID-19 vaccines recipients. Front. Immunol. 2023, 14, 1239179. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Zhang, S.; Dai, D.F.; Wang, B.S.; Zhuang, L.; Huang, H.T.; Wang, Z.F.; Zhao, J.S.; Li, Q.P.; Wu, S.P.; et al. Safety and immunogenicity of heterologous boosting with orally aerosolised or intramuscular Ad5-nCoV vaccine and homologous boosting with inactivated vaccines (BBIBP-CorV or CoronaVac) in children and adolescents: A randomised, open-label, parallel-controlled, non-inferiority, single-centre study. Lancet Respir. Med. 2023, 11, 698–708. [Google Scholar] [CrossRef]
- Tang, R.; Zheng, H.; Wang, B.S.; Gou, J.B.; Guo, X.L.; Chen, X.Q.; Chen, Y.; Wu, S.P.; Zhong, J.; Pan, H.X.; et al. Safety and immunogenicity of aerosolised Ad5-nCoV, intramuscular Ad5-nCoV, or inactivated COVID-19 vaccine CoronaVac given as the second booster following three doses of CoronaVac: A multicentre, open-label, phase 4, randomised trial. Lancet Respir. Med. 2023, 11, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Liu, S.; Cui, T.; Li, J.; Zhu, F.; Zhong, N.; Huang, W.; Zhao, Z.; Wang, Z. Heterologous booster with inhaled adenovirus vector COVID-19 vaccine generated more neutralizing antibodies against different SARS-CoV-2 variants. Emerg. Microbes Infect. 2022, 11, 2689–2697. [Google Scholar] [CrossRef]
- Jin, L.; Tang, R.; Wu, S.; Guo, X.; Huang, H.; Hou, L.; Chen, X.; Zhu, T.; Gou, J.; Zhong, J.; et al. Antibody persistence and safety after heterologous boosting with orally aerosolised Ad5-nCoV in individuals primed with two-dose CoronaVac previously: 12-month analyses of a randomized controlled trial. Emerg. Microbes Infect. 2023, 12, 2155251. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.Z.; Zhang, C.H.; Tang, L.; Rodewald, L.E.; Wang, W.; Liu, S.Y.; Wang, W.J.; Wu, D.; Liu, Q.Q.; Wang, X.Q.; et al. An Observational Prospective Cohort Study of Vaccine Effectiveness Against Severe Acute Respiratory Syndrome Coronavirus 2 Infection of an Aerosolized, Inhaled Adenovirus Type 5-Vectored Coronavirus Disease 2019 Vaccine Given as a Second Booster Dose in Guangzhou City, China. J. Infect. Dis. 2024, 229, 117–121. [Google Scholar] [CrossRef]
- Xu, J.W.; Wang, B.S.; Gao, P.; Huang, H.T.; Wang, F.Y.; Qiu, W.; Zhang, Y.Y.; Xu, Y.; Gou, J.B.; Yu, L.L.; et al. Safety and immunogenicity of heterologous boosting with orally administered aerosolized bivalent adenovirus type-5 vectored COVID-19 vaccine and B.1.1.529 variant adenovirus type-5 vectored COVID-19 vaccine in adults 18 years and older: A randomized, double blinded, parallel controlled trial. Emerg. Microbes Infect. 2024, 13, 2281355. [Google Scholar] [CrossRef]
- Sun, B.; Wang, Q.; Zheng, P.; Niu, X.; Feng, Y.; Guan, W.; Chen, S.; Li, J.; Cui, T.; Deng, Y.; et al. An intranasally administered adenovirus-vectored SARS-CoV-2 vaccine induces robust mucosal secretory IgA. JCI Insight 2024, 9. [Google Scholar] [CrossRef]
- Dodaran, M.S.; Banihashemi, S.R.; Es-Haghi, A.; Mehrabadi, M.H.F.; Nofeli, M.; Mokarram, A.R.; Mokhberalsafa, L.; Sadeghi, F.; Ranjbar, A.; Ansarifar, A.; et al. Immunogenicity and Safety of a Combined Intramuscular/Intranasal Recombinant Spike Protein COVID-19 Vaccine (RCP) in Healthy Adults Aged 18 to 55 Years Old: A Randomized, Double-Blind, Placebo-Controlled, Phase I Trial. Vaccines 2023, 11, 455. [Google Scholar] [CrossRef]
- Madhavan, M.; Ritchie, A.J.; Aboagye, J.; Jenkin, D.; Provstgaad-Morys, S.; Tarbet, I.; Woods, D.; Davies, S.; Baker, M.; Platt, A.; et al. Tolerability and immunogenicity of an intranasally-administered adenovirus-vectored COVID-19 vaccine: An open-label partially-randomised ascending dose phase I trial. EBioMedicine 2022, 85, 104298. [Google Scholar] [CrossRef]
- Mi, H.; Chen, Q.; Lin, H.; He, T.; Zhang, R.; Ren, S.; Liu, L.; Wang, J.; Huang, H.; Wang, M.; et al. Short-term effectiveness of single-dose intranasal spray COVID-19 vaccine against symptomatic SARS-CoV-2 Omicron infection in healthcare workers: A prospective cohort study. EClinicalMedicine 2024, 67, 102374. [Google Scholar] [CrossRef]
- Singh, C.; Verma, S.; Reddy, P.; Diamond, M.S.; Curiel, D.T.; Patel, C.; Jain, M.K.; Redkar, S.V.; Bhate, A.S.; Gundappa, V.; et al. Phase III Pivotal comparative clinical trial of intranasal (iNCOVACC) and intramuscular COVID-19 vaccine (Covaxin(®)). NPJ Vaccines 2023, 8, 125. [Google Scholar] [CrossRef]
- Zhang, R.; Chan, K.H.; Wang, P.; Zhou, R.; Yau, H.K.; Wong, C.K.; Au, M.W.; Tam, A.R.; Ng, C.T.; Lou, M.K.; et al. A Phase 1, Randomized, Double-Blinded, Placebo-Controlled and Dose-Escalation Study to Evaluate the Safety and Immunogenicity of the Intranasal DelNS1-nCoV-RBD LAIV for COVID-19 in Healthy Adults. Vaccines 2023, 11, 723. [Google Scholar] [CrossRef]
- Zhu, F.; Zhuang, C.; Chu, K.; Zhang, L.; Zhao, H.; Huang, S.; Su, Y.; Lin, H.; Yang, C.; Jiang, H.; et al. Safety and immunogenicity of a live-attenuated influenza virus vector-based intranasal SARS-CoV-2 vaccine in adults: Randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Respir. Med. 2022, 10, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Huang, S.; Liu, X.; Chen, Q.; Zhuang, C.; Zhao, H.; Han, J.; Jaen, A.M.; Do, T.H.; Peter, J.G.; et al. Safety and efficacy of the intranasal spray SARS-CoV-2 vaccine dNS1-RBD: A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir. Med. 2023, 11, 1075–1088. [Google Scholar] [CrossRef]
- Ambrose, C.S.; Wu, X. The safety and effectiveness of self-administration of intranasal live attenuated influenza vaccine in adults. Vaccine 2013, 31, 857–860. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.C.; Hsu, W.T.; Lee, W.S.; Wang, N.C.; Chen, T.J.; Liu, M.C.; Pai, H.C.; Hsu, Y.S.; Chang, M.; Hsieh, S.M. A double-blind, randomized controlled trial to evaluate the safety and immunogenicity of an intranasally administered trivalent inactivated influenza vaccine with the adjuvant LTh(αK): A phase II study. Vaccine 2020, 38, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Halperin, S.A.; Smith, B.; Clarke, K.; Treanor, J.; Mabrouk, T.; Germain, M. Phase I, randomized, controlled trial to study the reactogenicity and immunogenicity of a nasal, inactivated trivalent influenza virus vaccine in healthy adults. Hum. Vaccines 2005, 1, 37–42. [Google Scholar] [CrossRef]
- Kiseleva, I.; Isakova-Sivak, I.; Stukova, M.; Erofeeva, M.; Donina, S.; Larionova, N.; Krutikova, E.; Bazhenova, E.; Stepanova, E.; Vasilyev, K.; et al. A Phase 1 Randomized Placebo-Controlled Study to Assess the Safety, Immunogenicity and Genetic Stability of a New Potential Pandemic H7N9 Live Attenuated Influenza Vaccine in Healthy Adults. Vaccines 2020, 8, 296. [Google Scholar] [CrossRef]
- Forrest, B.D.; Steele, A.D.; Hiemstra, L.; Rappaport, R.; Ambrose, C.S.; Gruber, W.C. A prospective, randomized, open-label trial comparing the safety and efficacy of trivalent live attenuated and inactivated influenza vaccines in adults 60 years of age and older. Vaccine 2011, 29, 3633–3639. [Google Scholar] [CrossRef]
- Manenti, A.; Tete, S.M.; Mohn, K.G.; Jul-Larsen, Å.; Gianchecchi, E.; Montomoli, E.; Brokstad, K.A.; Cox, R.J. Comparative analysis of influenza A(H3N2) virus hemagglutinin specific IgG subclass and IgA responses in children and adults after influenza vaccination. Vaccine 2017, 35, 191–198. [Google Scholar] [CrossRef]
- Nichol, K.L.; Mendelman, P.M.; Mallon, K.P.; Jackson, L.A.; Gorse, G.J.; Belshe, R.B.; Glezen, W.P.; Wittes, J. Effectiveness of live, attenuated intranasal influenza virus vaccine in healthy, working adults: A randomized controlled trial. JAMA 1999, 282, 137–144. [Google Scholar] [CrossRef]
- Mallory, R.M.; Malkin, E.; Ambrose, C.S.; Bellamy, T.; Shi, L.; Yi, T.; Jones, T.; Kemble, G.; Dubovsky, F. Safety and immunogenicity following administration of a live, attenuated monovalent 2009 H1N1 influenza vaccine to children and adults in two randomized controlled trials. PLoS ONE 2010, 5, e13755. [Google Scholar] [CrossRef]
- Block, S.L.; Reisinger, K.S.; Hultquist, M.; Walker, R.E. Comparative immunogenicities of frozen and refrigerated formulations of live attenuated influenza vaccine in healthy subjects. Antimicrob. Agents Chemother. 2007, 51, 4001–4008. [Google Scholar] [CrossRef] [PubMed]
- Gruber, W.C.; Hinson, H.P.; Holland, K.L.; Thompson, J.M.; Reed, G.W.; Wright, P.F. Comparative trial of large-particle aerosol and nose drop administration of live attenuated influenza vaccines. J. Infect. Dis. 1993, 168, 1282–1285. [Google Scholar] [CrossRef]
- Hammitt, L.L.; Bartlett, J.P.; Li, S.; Rahkola, J.; Lang, N.; Janoff, E.N.; Levin, M.J.; Weinberg, A. Kinetics of viral shedding and immune responses in adults following administration of cold-adapted influenza vaccine. Vaccine 2009, 27, 7359–7366. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Deng, Y.; Cizmeci, D.; Fontana, L.; Carlock, M.A.; Hanley, H.B.; McNamara, R.P.; Lingwood, D.; Ross, T.M.; Alter, G. Distinct Functional Humoral Immune Responses Are Induced after Live Attenuated and Inactivated Seasonal Influenza Vaccination. J. Immunol. (Baltim. Md. 1950) 2024, 212, 24–34. [Google Scholar] [CrossRef]
- Krishnan, A.; Dar, L.; Saha, S.; Narayan, V.V.; Kumar, R.; Kumar, R.; Amarchand, R.; Dhakad, S.; Chokker, R.; Choudekar, A.; et al. Efficacy of live attenuated and inactivated influenza vaccines among children in rural India: A 2-year, randomized, triple-blind, placebo-controlled trial. PLoS Med. 2021, 18, e1003609. [Google Scholar] [CrossRef]
- Ai, L.; Gao, Z.; Lv, H.; Zhang, J.; Xu, N.; Zhao, H.; Lu, Q.; Zhu, H.; Shi, N.; Wei, W.; et al. Immunogenicity and safety of live attenuated influenza vaccine in children aged 3–17 years in China. Vaccine 2025, 46, 126653. [Google Scholar] [CrossRef] [PubMed]
- Phonrat, B.; Pitisuttithum, P.; Chamnanchanunt, S.; Puthavathana, P.; Ngaosuwankul, N.; Louisirirotchanakul, S.; Dhitavat, J.; Thirapakpoomanunt, S.; Chokevivat, V.; Wibulpolprasert, S. Safety and immune responses following administration of H1N1 live attenuated influenza vaccine in Thais. Vaccine 2013, 31, 1503–1509. [Google Scholar] [CrossRef]
- Williams, K.V.; Li, Z.N.; Zhai, B.; Alcorn, J.F.; Nowalk, M.P.; Levine, M.Z.; Kim, S.S.; Flannery, B.; Moehling Geffel, K.; Merranko, A.J.; et al. A Randomized Controlled Trial to Compare Immunogenicity to Cell-Based Versus Live-Attenuated Influenza Vaccines in Children. J. Pediatr. Infect. Dis. Soc. 2023, 12, 342–352. [Google Scholar] [CrossRef]
- Nakayama, T.; Hayashi, T.; Makino, K.; Oe, K. The efficacy and safety of a quadrivalent live attenuated influenza nasal vaccine in Japanese children: A phase 3, randomized, placebo-controlled study. J. Infect. Chemother. Off. J. Jpn. Soc. Chemother. 2025, 31, 102460. [Google Scholar] [CrossRef]
- Pitisuttithum, P.; Boonnak, K.; Chamnanchanunt, S.; Puthavathana, P.; Luvira, V.; Lerdsamran, H.; Kaewkungwal, J.; Lawpoolsri, S.; Thanachartwet, V.; Silachamroon, U.; et al. Safety and immunogenicity of a live attenuated influenza H5 candidate vaccine strain A/17/turkey/Turkey/05/133 H5N2 and its priming effects for potential pre-pandemic use: A randomised, double-blind, placebo-controlled trial. Lancet Infect. Dis. 2017, 17, 833–842. [Google Scholar] [CrossRef]
- Rudenko, L.; Kiseleva, I.; Naykhin, A.N.; Erofeeva, M.; Stukova, M.; Donina, S.; Petukhova, G.; Pisareva, M.; Krivitskaya, V.; Grudinin, M.; et al. Assessment of human immune responses to H7 avian influenza virus of pandemic potential: Results from a placebo-controlled, randomized double-blind phase I study of live attenuated H7N3 influenza vaccine. PLoS ONE 2014, 9, e87962. [Google Scholar] [CrossRef]
- Rudenko, L.; Kiseleva, I.; Stukova, M.; Erofeeva, M.; Naykhin, A.; Donina, S.; Larionova, N.; Pisareva, M.; Krivitskaya, V.; Flores, J. Clinical testing of pre-pandemic live attenuated A/H5N2 influenza candidate vaccine in adult volunteers: Results from a placebo-controlled, randomized double-blind phase I study. Vaccine 2015, 33, 5110–5117. [Google Scholar] [CrossRef] [PubMed]
- Rudenko, L.; Isakova-Sivak, I.; Naykhin, A.; Kiseleva, I.; Stukova, M.; Erofeeva, M.; Korenkov, D.; Matyushenko, V.; Sparrow, E.; Kieny, M.P. H7N9 live attenuated influenza vaccine in healthy adults: A randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Infect. Dis. 2016, 16, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Eiden, J.; Fierro, C.; White, A.; Davis, M.; Rhee, M.; Turner, M.; Murray, B.; Herber, R.; Aitchison, R.; Marshall, D.; et al. Safety and immunogenicity of the intranasal H3N2 M2-deficient single-replication influenza vaccine alone or coadministered with an inactivated influenza vaccine (Fluzone High-Dose Quadrivalent) in adults aged 65-85 years in the USA: A multicentre, randomised, double-blind, double-dummy, phase 1b trial. Lancet Infect. Dis. 2024, 24, 1118–1129. [Google Scholar] [CrossRef] [PubMed]
- Speroni, K.G.; Dawson, E.; Atherton, M.; Corriher, J. Influenza vaccination: Incidence of symptoms and resulting absenteeism in hospital employees. Aaohn J. 2005, 53, 477–483. [Google Scholar] [CrossRef]
- Tasker, S.; Wight O’Rourke, A.; Suyundikov, A.; Jackson Booth, P.G.; Bart, S.; Krishnan, V.; Zhang, J.; Anderson, K.J.; Georges, B.; Roberts, M.S. Safety and Immunogenicity of a Novel Intranasal Influenza Vaccine (NasoVAX): A Phase 2 Randomized, Controlled Trial. Vaccines 2021, 9, 224. [Google Scholar] [CrossRef]
- Treanor, J.J.; Kotloff, K.; Betts, R.F.; Belshe, R.; Newman, F.; Iacuzio, D.; Wittes, J.; Bryant, M. Evaluation of trivalent, live, cold-adapted (CAIV-T) and inactivated (TIV) influenza vaccines in prevention of virus infection and illness following challenge of adults with wild-type influenza A (H1N1), A (H3N2), and B viruses. Vaccine 1999, 18, 899–906. [Google Scholar] [CrossRef]
- Li, L.; Shi, N.; Xu, N.; Wang, H.; Zhao, H.; Xu, H.; Liu, D.; Zhang, Z.; Li, S.; Zhang, J.; et al. Safety and Viral Shedding of Live Attenuated Influenza Vaccine (LAIV) in Chinese Healthy Juveniles and Adults: A Phase|Randomized, Double-Blind, Placebo-Controlled Study. Vaccines 2022, 10, 1796. [Google Scholar] [CrossRef]
- van Voorthuizen, F.; Jens, D.; Saes, F. Characterization and clinical evaluation of live influenza A vaccine prepared from a recombinant of the A/USSR/92/77 (H1N1) and the cold-adapted A/Ann Arbor/6/60 (H2N2) strains. Antivir. Res. 1981, 1, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Vesikari, T.; Karvonen, A.; Smith, H.M.; Dunning, A.; Razmpour, A.; Saville, M.K.; Gruber, W.C.; Forrest, B.D. Safety and tolerability of cold-adapted influenza vaccine, trivalent, in infants younger than 6 months of age. Pediatrics 2008, 121, e568–e573. [Google Scholar] [CrossRef]
- van der Plas, J.L.; Haijema, B.J.; Leenhouts, K.; Paul Zoeteweij, J.; Burggraaf, J.; Kamerling, I.M.C. Safety, reactogenicity and immunogenicity of an intranasal seasonal influenza vaccine adjuvanted with gram-positive matrix (GEM) particles (FluGEM): A randomized, double-blind, controlled, ascending dose study in healthy adults and elderly. Vaccine 2024, 42, 125836. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Tobler, S.; Roayaei, J.; Eick, A. Live Attenuated or Inactivated Influenza Vaccines and Medical Encounters for Respiratory Illnesses Among US Military Personnel. JAMA 2009, 301, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Rigamonti, V.; Torri, V.; Morris, S.K.; Ieva, F.; Giaquinto, C.; Donà, D.; Di Chiara, C.; Cantarutti, A.; group, t.C.s. Real-World Effectiveness of Live Attenuated vs. Inactivated Influenza Vaccines in Children. medRxiv, 2024; 2024.2012.2004.24318492. [Google Scholar] [CrossRef]
- Gasparini, C.; Acunzo, M.; Biuso, A.; Roncaglia, S.; Migliavacca, F.; Borriello, C.R.; Bertolini, C.; Allen, M.R.; Orenti, A.; Boracchi, P.; et al. Nasal spray live attenuated influenza vaccine: The first experience in Italy in children and adolescents during the 2020-21 season. Ital. J. Pediatr. 2021, 47, 225. [Google Scholar] [CrossRef]
- Green, C.A.; Sande, C.J.; Scarselli, E.; Capone, S.; Vitelli, A.; Nicosia, A.; Silva-Reyes, L.; Thompson, A.J.; de Lara, C.M.; Taylor, K.S.; et al. Novel genetically-modified chimpanzee adenovirus and MVA-vectored respiratory syncytial virus vaccine safely boosts humoral and cellular immunity in healthy older adults. J. Infect. 2019, 78, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Verdijk, P.; van der Plas, J.L.; van Brummelen, E.M.J.; Jeeninga, R.E.; de Haan, C.A.M.; Roestenberg, M.; Burggraaf, J.; Kamerling, I.M.C. First-in-human administration of a live-attenuated RSV vaccine lacking the G-protein assessing safety, tolerability, shedding and immunogenicity: A randomized controlled trial. Vaccine 2020, 38, 6088–6095. [Google Scholar] [CrossRef]
- Cunningham, C.K.; Karron, R.A.; Muresan, P.; Kelly, M.S.; McFarland, E.J.; Perlowski, C.; Libous, J.; Oliva, J.; Jean-Philippe, P.; Moye, J.; et al. Evaluation of Recombinant Live-Attenuated Respiratory Syncytial Virus (RSV) Vaccines RSV/ΔNS2/Δ1313/I1314L and RSV/276 in RSV-Seronegative Children. J. Infect. Dis. 2022, 226, 2069–2078. [Google Scholar] [CrossRef]
- Karron, R.A.; Luongo, C.; Woods, S.; Oliva, J.; Collins, P.L.; Buchholz, U.J. Evaluation of the Live-Attenuated Intranasal Respiratory Syncytial Virus (RSV) Vaccine RSV/6120/ΔNS2/1030s in RSV-Seronegative Young Children. J. Infect. Dis. 2024, 229, 346–354. [Google Scholar] [CrossRef]
- Spearman, P.; Jin, H.; Knopp, K.; Xiao, P.; Gingerich, M.C.; Kidd, J.; Singh, K.; Tellier, M.; Radziewicz, H.; Wu, S.; et al. Intranasal parainfluenza virus type 5 (PIV5)-vectored RSV vaccine is safe and immunogenic in healthy adults in a phase 1 clinical study. Sci. Adv. 2023, 9, eadj7611. [Google Scholar] [CrossRef]
- Audran, R.; Karoui, O.; Donnet, L.; Soumas, V.; Fares, F.; Lovis, A.; Noirez, L.; Cavassini, M.; Fayet-Mello, A.; Satti, I.; et al. Randomised, double-blind, controlled phase 1 trial of the candidate tuberculosis vaccine ChAdOx1-85A delivered by aerosol versus intramuscular route. J. Infect. 2024, 89, 106205. [Google Scholar] [CrossRef]
- Manjaly Thomas, Z.R.; Satti, I.; Marshall, J.L.; Harris, S.A.; Lopez Ramon, R.; Hamidi, A.; Minhinnick, A.; Riste, M.; Stockdale, L.; Lawrie, A.M.; et al. Alternate aerosol and systemic immunisation with a recombinant viral vector for tuberculosis, MVA85A: A phase I randomised controlled trial. PLoS Med. 2019, 16, e1002790. [Google Scholar] [CrossRef]
- Jeyanathan, M.; Fritz, D.K.; Afkhami, S.; Aguirre, E.; Howie, K.J.; Zganiacz, A.; Dvorkin-Gheva, A.; Thompson, M.R.; Silver, R.F.; Cusack, R.P.; et al. Aerosol delivery, but not intramuscular injection, of adenovirus-vectored tuberculosis vaccine induces respiratory-mucosal immunity in humans. JCI Insight 2022, 7, e155655. [Google Scholar] [CrossRef]
- Satti, I.; Marshall, J.L.; Harris, S.A.; Wittenberg, R.; Tanner, R.; Lopez Ramon, R.; Wilkie, M.; Ramos Lopez, F.; Riste, M.; Wright, D.; et al. Safety of a controlled human infection model of tuberculosis with aerosolised, live-attenuated Mycobacterium bovis BCG versus intradermal BCG in BCG-naive adults in the UK: A dose-escalation, randomised, controlled, phase 1 trial. Lancet Infect. Dis. 2024, 24, 909–921. [Google Scholar] [CrossRef]
- Satti, I.; Meyer, J.; Harris, S.A.; Manjaly Thomas, Z.R.; Griffiths, K.; Antrobus, R.D.; Rowland, R.; Ramon, R.L.; Smith, M.; Sheehan, S.; et al. Safety and immunogenicity of a candidate tuberculosis vaccine MVA85A delivered by aerosol in BCG-vaccinated healthy adults: A phase 1, double-blind, randomised controlled trial. Lancet Infect. Dis. 2014, 14, 939–946. [Google Scholar] [CrossRef]
- Buddy Creech, C.; Jimenez-Truque, N.; Kown, N.; Sokolow, K.; Brady, E.J.; Yoder, S.; Solovay, K.; Rubin, K.; Noviello, S.; Hensel, E.; et al. Safety and immunogenicity of live, attenuated intranasal Bordetella pertussis vaccine (BPZE1) in healthy adults. Vaccine 2022, 40, 6740–6746. [Google Scholar] [CrossRef] [PubMed]
- Thorstensson, R.; Trollfors, B.; Al-Tawil, N.; Jahnmatz, M.; Bergström, J.; Ljungman, M.; Törner, A.; Wehlin, L.; Van Broekhoven, A.; Bosman, F.; et al. A phase I clinical study of a live attenuated Bordetella pertussis vaccine--BPZE1; a single centre, double-blind, placebo-controlled, dose-escalating study of BPZE1 given intranasally to healthy adult male volunteers. PLoS ONE 2014, 9, e83449. [Google Scholar] [CrossRef]
- Jahnmatz, M.; Richert, L.; Al-Tawil, N.; Storsaeter, J.; Colin, C.; Bauduin, C.; Thalen, M.; Solovay, K.; Rubin, K.; Mielcarek, N.; et al. Safety and immunogenicity of the live attenuated intranasal pertussis vaccine BPZE1: A phase 1b, double-blind, randomised, placebo-controlled dose-escalation study. Lancet Infect. Dis. 2020, 20, 1290–1301. [Google Scholar] [CrossRef] [PubMed]
- Keech, C.; Miller, V.E.; Rizzardi, B.; Hoyle, C.; Pryor, M.J.; Ferrand, J.; Solovay, K.; Thalen, M.; Noviello, S.; Goldstein, P.; et al. Immunogenicity and safety of BPZE1, an intranasal live attenuated pertussis vaccine, versus tetanus-diphtheria-acellular pertussis vaccine: A randomised, double-blind, phase 2b trial. Lancet (Lond. Engl.) 2023, 401, 843–855. [Google Scholar] [CrossRef]
- Carter, N.J.; Curran, M.P. Live attenuated influenza vaccine (FluMist®; Fluenz™): A review of its use in the prevention of seasonal influenza in children and adults. Drugs 2011, 71, 1591–1622. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, M.; Mirzaei, H.; Salemi, M.; Momeni, F.; Mousavi, M.J.; Sadeghalvad, M.; Arjeini, Y.; Solaymani-Mohammadi, F.; Sadri Nahand, J.; Namdari, H.; et al. Influenza vaccine: Where are we and where do we go? Rev. Med. Virol. 2019, 29, e2014. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).