Abstract
Background/Objectives: Pneumococcal conjugate vaccines (PCVs) were first introduced in the pediatric UK National Immunization Programme (NIP) in 2006 and subsequently led to a significant decline in invasive pneumococcal disease (IPD). In 2020, the UK NIP reduced the pediatric PCV dosing schedule from two infant doses and one toddler dose (2 + 1) to one infant dose and one toddler dose (1 + 1). This analysis evaluated the public health impact of pediatric vaccination with PCV15 versus PCV13 under a 1 + 1 schedule. Methods: A population-level compartmental model was previously adapted to the UK setting. The impact on the IPD incidence of vaccination with PCV15 versus PCV13 under a 1 + 1 schedule was evaluated over a 20-year time horizon. The uncertainty regarding the vaccine efficacy (VE) of PCV13 and PCV15 under a 1 + 1 schedule was investigated through a probabilistic sensitivity analysis, i.e., the PCV VE under a 1 + 1 schedule was assumed to be 0–24% lower than the PCV VE under a 2 + 1 schedule. Results: Relative to the initial IPD incidence, vaccination with PCV13 and PCV15 under a 1 + 1 schedule resulted in the IPD incidence in children <2 years old increasing by 11.1% (95% region: 8.4–14.5%) and 3.5% (0.2–7.7%), respectively, over the time horizon. At the end of the time horizon, in the overall population, PCV15 would lead to a 6.0% lower IPD incidence than PCV13 (10.70 IPD cases per 100,000 versus 11.38 per 100,000, respectively). Conclusions: Switching from PCV13 to PCV15 for routine pediatric vaccinations under the 1 + 1 dosing schedule in the UK led to a lower IPD incidence in both the pediatric and overall populations.
1. Introduction
Pneumococcal disease (PD), caused by Streptococcus pneumoniae, leads to significant morbidity and mortality, especially in young children [1]. Preventative pneumococcal conjugate vaccines (PCVs) targeting clinically relevant serotypes are recommended for routine administration in early childhood. PCV7 was initially licensed 25 years ago as a four-dose schedule, with three doses in infancy followed by a toddler dose (3 + 1) [1]. Based on immunogenicity data supporting reduced dosing schedules, coupled with economic pressures and crowded childhood immunization schedules, the United Kingdom (UK) became the first country to adopt PCV7 in a three-dose (2 + 1) schedule in their National Immunization Programme (NIP) in 2006 [2,3]. In the adult population, PPSV23 has been widely used for adults aged ≥65 years old since 2003 [4].
Before the introduction of PCVs in the UK, overall invasive pneumococcal disease (IPD) incidence was 14.8 per 100,000, with PCV7 serotypes accounting for approximately half of the IPD disease burden [4]. By 2010, the overall incidence of IPD had dropped to 10.1 cases per 100,000, with PCV7 serotypes comprising 14% of IPD [4]. However, a relative increase in the proportion of non-vaccine-type (NVT) PD led to the adoption of PCV13 in 2010, which covered 58% of the disease burden at that time [4]. By 2019–2020, the overall disease incidence reached a low of 9.4 per 100,000 and NVT IPD comprised about 81% of the disease burden [1]. The reduction in overall IPD, with NVT driving the residual burden of disease, motivated two changes for the pediatric PCV NIP—the move to a 1 + 1 dosing schedule, and interest in switching to a higher-valency PCV. These changes were further supported by several factors: (1) high vaccine coverage rates leading to good control of vaccine-type (VT) PD [3]; (2) a 2018 randomized control study by Goldblatt et al., which demonstrated similar immune responses to PCV13 for 2 + 1 and 1 + 1 dosing schedules [2]; and (3) a 2019 modeling study by Choi et al. projecting that the incidence of IPD and non-bacteremic pneumococcal pneumonia (NBPP) would not be affected by a reduction in dosing schedule [5].
The decision to move to a 1 + 1 dosing schedule was not without risk, as fewer doses in infancy, when the immune system is immature, may lead to subpar protection and breakthrough disease. A separate modeling study conducted by Wasserman et al. explored the projected potential effects of switching to a 1 + 1 PCV13 dosing schedule in the UK [6]. This study estimated that the reduction in the number of infant doses was projected to lead to an increase in IPD cases over a 10-year time horizon, with the greatest increases occurring in infants and older adults, also resulting in an increase in deaths [6]. This finding was consistent with the results of the randomized control study by Goldblatt et al., which, while supporting the switch to a 1 + 1 dosing schedule, also noted this potential risk [2].
In January of 2020, the 1 + 1 reduced dosing schedule was implemented in the UK, with the infant dose administered at 12 weeks of age and the toddler dose at 1 year [7]. Contagion control measures for the COVID-19 pandemic were implemented soon after this switch, rendering it difficult to estimate the effects of this policy change due to the overall reduction in PD. A real-world observational study, published in 2024, indicated that overall IPD incidence in 2022/23 decreased in the years since the implementation of the 1 + 1 dosing schedule (which included the period when COVID-19 pandemic measures were in effect) [1]. However, VT IPD incidence increased over this period, which may hint at the fact that the immune response for a 1 + 1 schedule is insufficient to maintain prior levels of control of VT IPD, though it is difficult to draw conclusions given the confounding effects of COVID-19 during this timeframe [1].
The percentages of PD due to VT and NVT in the UK are still substantial and warrant the consideration of higher-valency PCVs in the pediatric NIP. Due to the reduction in serotype-specific immunogenicity as more serotypes are added to a PCV (“immunogenicity-creep”), there is a risk of reduced effectiveness of higher-valency PCVs [8,9]. Nevertheless, the replacement of PCV7 with the higher-valency PCV13 in the UK did not lead to an increase in VT disease [4]. Two new expanded-valency PCVs were recently licensed in the UK—PCV15 (VaxneuvanceTM, Merck & Co., Inc., Rahway, NJ 07065, USA) and PCV20 (Prevnar 20TM, Wyeth Pharmaceuticals LLC, a subsidiary of Pfizer, Inc., New York, NY 10001-2192, USA)—that provide protection against additional NVT serotypes [1]. The clinical impact of these new PCVs and their potential for immunogenicity creep expected in 1 + 1 dosing remains to be seen.
The confluence of information from immunogenicity studies on reduced dosing schedules, epidemiological data, transmission models, and the impact of the COVID-19 pandemic on the epidemiology of infectious diseases paints a confusing picture of the potential effectiveness of a 1 + 1 dosing schedule for pediatric pneumococcal vaccination in the UK with PCV13, and potentially with higher-valency PCVs. This analysis employed a previously described and calibrated model to explore the potential impact of reduced vaccine effectiveness (VE) against disease resulting from a reduced dosing schedule, using pediatric vaccines PCV13 and PCV15.
2. Materials and Methods
2.1. Model Overview
The dynamic transmission model used in this analysis was a published deterministic, age-structured, population-level model accounting for (1) demographic components, including births, deaths, and aging, and (2) carriage transmission dynamics in the presence of historical vaccine introduction [10,11]. The demographic model assumed a constant population size and included age structure in the form of four age groups: <2-, 2–4-, 5–64-, and ≥65-year-olds [12,13]. Mixing among populations of different ages is particularly relevant for S. pneumoniae transmission and was accounted for with a previously published mixing matrix [14].
The epidemiological model was based on a system of ordinary differential equations and distinguished between pneumococcal carriage (single, double, and triple) and disease, as well as vaccination status. The progression from pneumococcal carriage to pneumococcal disease was modeled through an age-, serotype class- (STC), and vaccine-status-specific relationship, wherein disease occurred in some fraction of carriage episodes (Appendix A Figure A1). Given the relative infrequency of transmission from adults to children, the simplifying assumption was made that no transmission occurred from adults to children [15]. For the purpose of this model, the pediatric age strata comprised <2- and 2–4-year-olds and the adult age strata comprised 5–64- and ≥65-year-olds.
A complete description of the model, inputs, parameter estimates, and calibration results is provided by Oidtman et al. 2025 [11]. In brief, the model was calibrated using the Nelder–Mead simplex method implemented in the NMinimize function in Mathematica 13.3.1 (Wolfram Research, Champaign, IL, USA) to minimize a weighted sum of squared errors objective function over the annual age- and STC-specific IPD incidence data [16]. During the calibration, four sets of age- and STC-specific parameters were estimated: vaccine efficacy against carriage, carriage acquisition rate given contact, invasiveness (case-to-carrier ratio), and pairwise competition between STCs [11]. For the competition parameters, historical replacement dynamics were used to calibrate the specific STC-STC combinations. In the absence of observed data on replacement dynamics, as is the case between historical novel VTs in PCV13 and the novel VTs in PCV15, there was assumed to be no explicit competition.
2.2. Inputs and Data Sources
Annual age- and serotype-specific IPD incidence data for 2000–2019 were provided by the UK Health Security Agency (HSA) [4] and were used as a model calibration target for the pre-PCV NIP era steady state (2000–2005) and the vaccine period (2006–2019). As the model is driven by carriage dynamics, S. pneumoniae carriage data from 2006 were used as a baseline target for the pre-PCV NIP era [17,18]. Serotype-specific IPD and carriage data were aggregated into 11 STCs based on the inclusion of different serotypes in different vaccines to calibrate to historical data (Table 1) [19,20].

Table 1.
Serotype classes (STCs) with respective serotypes (STs) included in each class. Shading indicates inclusion in different vaccines. The vaccination group indicates how serotype classes were grouped for visualization purposes. Note that serotype 3 was included in a stand-alone STC due to evidence of a limited immunogenicity response from vaccines [21].
Serotype- and age-based data on VE against IPD were available for PCV13 [22] and PPSV23 [23]. Given the lack of real-world evidence for VE against IPD for novel PCV15 serotypes, VE was estimated as the weighted average (weighted by the number of STs in each PCV grouping) from prior vaccine-specific estimates (Appendix A Table A1 and Table A2). The base case values for VE against IPD (Table 2) were calculated by applying a 12.8% average reduction in the PCV13 VE under a 2 + 1 schedule, based on an observational study by Savulescu et al. [22]; additional details are provided in the Sensitivity Analysis subsection. Data on serotype-specific carriage clearance rates (i.e., the inverse of the duration of carriage) were available from studies from multiple countries and settings [24,25,26,27,28,29,30,31,32,33,34,35,36], which were averaged over serotype and age to obtain aggregate inputs into the model (more details are available in Appendix A Table A6). Data on vaccination coverage rates by age were available from the HSA (Appendix A Table A3, Table A4 and Table A5) [37].

Table 2.
Vaccine efficacy values used for the probabilistic sensitivity analysis. A 12.8% average reduction corresponds to the reduction from 0.897 (3 + 1) to 0.782 (2 + 1), as described by Savulescu et al. [22].
2.3. Projection Scenarios
The calibrated model was used to evaluate the impact of implementing a 1 + 1 dosing schedule among children <2 years old with either PCV13 or PCV15, beginning in 2020. Both scenarios assumed a continuation of PPSV23 in older-adult and risk-group populations. In both pediatric and adult populations, it was assumed that vaccine coverage rate (VCR) levels from 2019 remained constant into the projected 20-year time horizon (Appendix A Table A3, Table A4 and Table A5). Reductions in pneumococcal disease or contact patterns arising from protection measures enacted during the COVID-19 pandemic were not accounted for in these projections. The model projection scenarios are summarized in Table 3.

Table 3.
Projected vaccination scenarios for PCV13 and PCV15 in a 1 + 1 dosing schedule.
2.4. Sensitivity Analysis
To explore the uncertainty in the potential reductions in VE against IPD associated with a reduced pediatric dosing schedule, a probabilistic sensitivity analysis (PSA) was performed, which varied the vaccine effectiveness against IPD for both PCV13 and PCV15. This analysis did not consider potential reductions in vaccine efficacy against carriage.
Potential reductions in VE against IPD resulting from the adoption of a reduced dosing schedule were informed by the estimated 12.8% average reduction in PCV13 VE when shifting from a 3 + 1 schedule to a 2 + 1 schedule, based on an observational study by Savulescu et al. of PCV13 VE against IPD in pediatric populations in Europe [22]. The range in VE values for the PSA was defined as follows: the upper limit was set to equivalency, such that the VE would be equivalent in a 1 + 1 and 2 + 1 dosing schedule; the mean value was set to the average 12.8% reduction following Savulescu et al. [22]; and the lower limit was set to a maximum reduction informed by doubling the average reduction (Table 2).
The VEs were drawn from beta distributions with ranges approximately equal to those described in Table 2, and the parameters were varied in a Latin hypercube sampling method with a total of 100 random samples [38]. The PSA did not consider differences in VE by vaccine; for example, a reduced VE in PCV7 STs was applied equally to both PCV13 and PCV15.
3. Results
3.1. Model Calibration
The model was able to closely reproduce historically observed IPD dynamics, including the decline in incidence of VT IPD following the introduction of PCV7 and PCV13 and the subsequent increase in NVT IPD due to serotype replacement. In the <2- and 2–4-year-old populations, there were some observed dynamics that the model was not able to completely reproduce, such as the large increases in non-PCV15 serotypes beginning in 2014–2015 (Appendix A Figure A2).
The root mean squared error (RMSE) was used post hoc to compare model predictions to surveillance data by age, year, and STC, wherein an RMSE closer to zero indicates a better fit while a higher value indicates more error. The RMSE values were 1.47, 0.597, 0.312, and 1.29 for the <2-, 2–4-, 5–64-, and ≥65-year-old age groups (Appendix A Figure A3). Across all age groups, the RMSE was 1.03. A complete description of the model-fitting results and parameter estimates are available in the work by Oidtman et al. 2025 [11].
3.2. Projections of IPD in Children Less than Two Years Old
For both PCV13 and PCV15, the model projected an increase in overall IPD incidence in children less than two years old over a 20-year time horizon as a consequence of switching from a 2 + 1 to a 1 + 1 dosing schedule in 2020 (Figure 1). The model outcomes for PCV13 and PCV15 reflect an overlap in projected IPD incidence for shared STCs, with a 14.6–20.0% decline in the incidence of serotypes covered by both PCV13 and PCV15 over the 20-year time horizon following the switch to the reduced dosing schedule (Figure 1a,b, Table 4). IPD incidence due to PCV15-unique STCs declined by 93.1% over the time horizon with the implementation of PCV15, and increased by 6.3% with continued PCV13 vaccination (Figure 1c,d, Table 4). IPD incidence due to serotypes not included in PCV13 increased by 10.3% and 23.0% (Figure 1e,f), and IPD incidence due to serotypes not included in PCV15 increased by 30.7% and 26.4% for PCV15 and PCV13 scenarios, respectively (Figure 1g,h, Table 4). The absolute increase in overall IPD over the time horizon was smaller for PCV15 than for PCV13 (3.5% vs. 11.1%) (Figure 1i,j, Table 4). Sensitivity analyses reflect that results were robust to potential changes in the VE against IPD.
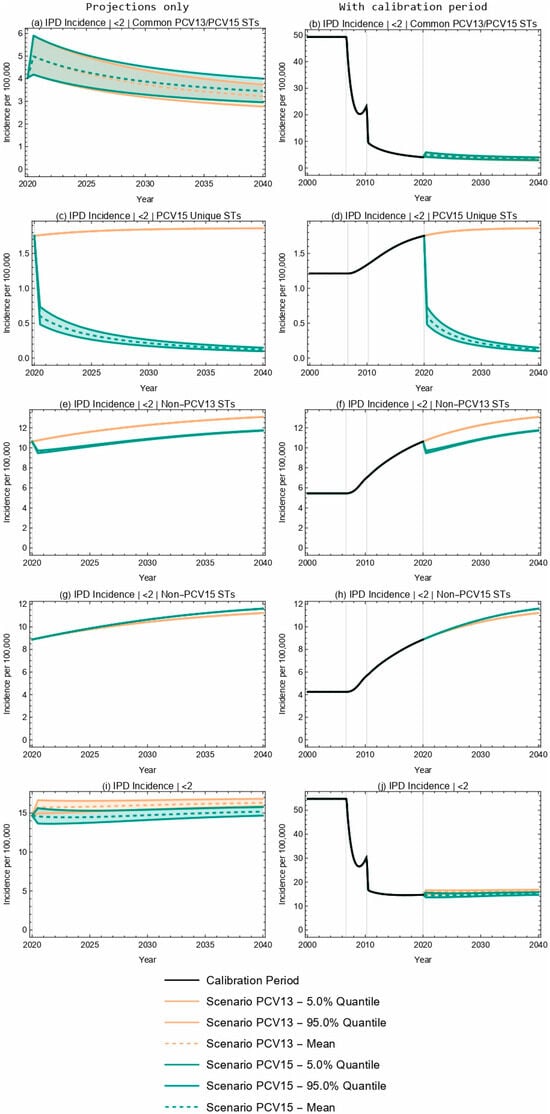
Figure 1.
Twenty-year projections of IPD incidence in <2-year-olds by serotype grouping. (a,b) serotypes common to both PCV13 and PCV15; (c,d) serotypes unique to PCV15 (i.e., covered by PCV15 but not PCV13); (e,f) serotypes not covered by PCV13; (g,h) serotypes not covered by PCV15; (i,j) all serotypes. The two columns showcase the same results; the left column focuses on the projection period only while the right column includes the calibration period. The first three rows illustrate IPD by ST grouping and the fourth row illustrates overall IPD incidence in <2-year-olds. ST—serotype.

Table 4.
Projected IPD incidence (per 100,000) by serotype grouping in <2-year-olds. IPD incidence at the start of the projection (0 years) and at the end of the 20-year time horizon, with the administration of either PCV13 or PCV15 in the <2-year-old population. The mean projections of IPD (over 100 Latin hypercube samples), the percent change from the start of projections, and the 90% intervals (5% and 95% quantiles) are included. NA values indicate the same value across all PSA realizations, and therefore, there is no informative interval. NA—not applicable.
3.3. Population-Level Projections of IPD
At the population level, projected overall IPD incidence outcomes for PCV13 and PCV15 overlapped for children aged 2–4 years old over the duration of the 20-year time horizon (Figure 2a,b). Further, the model projected a 4.2% decrease in IPD incidence in the 2–4-year-old age group following the introduction of PCV15, compared with a negligible change in IPD incidence for this same age group with the implementation of PCV13 (Table 5). The model projected a smaller increase in IPD incidence with the introduction of PCV15 when compared with PCV13 for the <2-year-old (3.5% increase vs. 11.2% increase, Table 4) and 5–64-year-old (5.1% increase vs. 7.8% increase) age groups, as well as for the overall population (0.6% increase vs. 7.1% increase, Figure 3 and Table 5). As seen with children <2 years of age, the sensitivity analyses reflect that projected IPD incidence results for all the modeled age groups were robust to potential changes in VE against IPD.
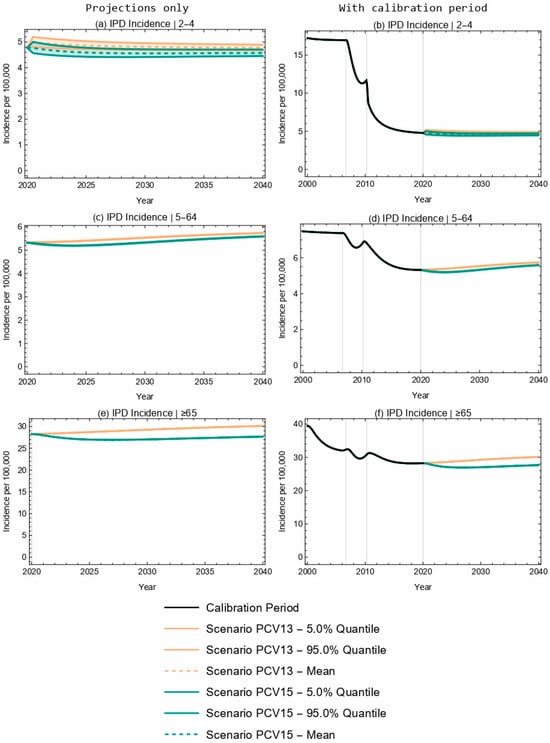
Figure 2.
Twenty-year projections of IPD in (a,b) 2–4-, (c,d) 5–64-, and (e,f) ≥65-year-olds. The two columns showcase the same results; the left column focuses on the projection period only, while the right column includes the calibration period. Each row represents a different age group.

Table 5.
Projected IPD incidence (per 100,000) by age. IPD incidence at the start of the projection (0 years) and at the end of the 20-year time horizon, with the administration of either PCV13 or PCV15 in the <2-year-old population. The mean projections of IPD, the percent change from the start of projections, and the 90% confidence intervals (5% and 95% quantiles) are included.
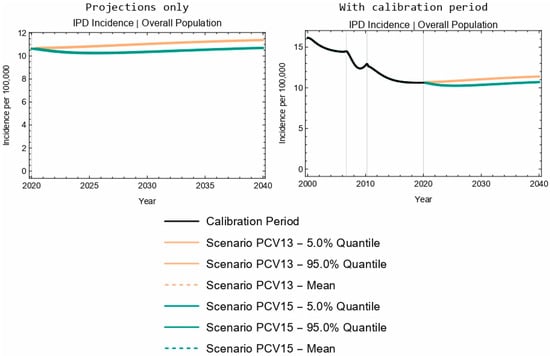
Figure 3.
Twenty-year projections of IPD in the entire population. The two columns showcase the same results; the left column focuses on the projection period only, while the right column includes the calibration period.
4. Discussion
Despite the inclusion of effective PCVs in pediatric vaccination programs, residual pneumococcal disease remains a public health matter in the UK and throughout the world. Here, a previously described and calibrated dynamic transmission model was used to investigate the clinical outcomes resulting from potential reductions in VE with two PCVs (PCV13 and PCV15) when routinely administered in a reduced pediatric dosing schedule (1 + 1) in the UK [10,11]. The model projections predicted that the use of PCV15 would significantly reduce IPD incidence, both in children <2 years of age, due to direct protection, and in older children and adults, due to indirect protection, with respect to the continued use of PCV13. These effects were consistent, regardless of potential changes in VE against disease.
Reductions in IPD in children <2 years old attributed to serotypes unique to PCV15 (22F and 33F) drove PCV15 to avert more disease than PCV13 in model projections. While both PCV13 and PCV15 led to projected decreases in IPD attributed to serotypes covered by both PCV13 and PCV15 in this age group, PCV15 led to a smaller projected decrease when compared with PCV13. Marginal increases in non-PCV15 serotypes were projected. Nevertheless, these differences were insufficient to counteract the overall estimated clinical benefits of pediatric PCV15 vaccination observed in the <2-year-old age group and in the population as a whole. With either vaccine, relative to the current 2019 estimates of IPD incidence, overall IPD incidence was predicted to increase over the 20-year time horizon, though the projected overall increase was predicted to be smaller with the implementation of PCV15 compared to PCV13 vaccination. These results were consistent with the findings of a 2015–2018 global surveillance analysis that found that, across all ages in over 40 countries, the proportion of IPD cases caused by PCV15 serotypes was approximately 4–10% greater than the proportion of IPD cases caused by PCV13 serotypes [39].
In contrast, a recent model by Choi et al. evaluated the implications of introducing higher-valency pediatric vaccines in the UK via the 1 + 1 schedule and concluded that the introduction of PCV15 could result in increased IPD relative to the standard of care of PCV13 [7]. These findings were, in part, a function of this study’s projection assumption on serotype replacement, wherein a decline in the carriage of a VT ST would be met with 100% replacement of carriage with an NVT ST, although this assumption is not substantiated by historical data to date. While it is necessary to make assumptions regarding the level of replacement expected upon the introduction of higher-valency vaccines, assuming 100% replacement may lead to unreasonably high predictions of the disease incidence of NVT STs. In contrast, the current analysis estimated competition between PCV13 and non-PCV13 STs through model calibration. However, there were insufficient data to estimate the competition parameters between PCV15 and non-PCV15 STs; thus, minimal competition between PCV15 and non-PCV15 STs was assumed. In addition, the Choi et al. analysis implemented two separate calibration models for model fitting to historical data, which has the potential to introduce bias and confounding effects due to incompatibilities between the two models [7]. The current analysis employed a single model for calibration and fitting, to ensure compatibility and consistency.
Several simplifying assumptions were made in the current analysis due to model complexity and limited availability of data. A potential reduction in VE against disease resulting from a change to a reduced pediatric vaccination schedule was considered, but there may also be reduced duration of protection and reduced VE against carriage [6]. Reductions in VE against carriage may lead to an increase in VT carriage, which could then increase VT-IPD in pediatric populations due to direct effects and in adult populations due to indirect effects. In addition, consistent with previous modeling studies [7], we did not consider the possibility of immunogenicity creep with the introduction of PCV15. This will be an important area of future research as real-world data become available for expanded-valency PCVs.
Limitations to this analysis are discussed below. First, the model did not account for pneumococcal dynamics during the COVID-19 pandemic. Post-pandemic surveillance calibration data have recently become available, and a recalibration of the model accounting for pneumococcal dynamics during the COVID-19 pandemic is planned as future research. Second, the model is very sensitive to estimates of the calibrated parameters, including VE against carriage. Although this model considered a range of potential reductions in VE against disease that could occur resulting from a change to a reduced dosing schedule, it could not consider potential changes in VE against carriage without conducting a more in-depth parameter identifiability analysis. Investigating the model sensitivity to calibrated parameters, including VE against carriage, is an ongoing area of research. Third, the PSA only considered the VE against disease after the toddler dose (i.e., at the completion of the 1 + 1 dosing schedule). VE in the first year of life (i.e., the VE after receiving the single infant dose in the 1 + 1 schedule) may be more critical to evaluate, given the vulnerability of infants to IPD [5]. However, since the model is not dose-dependent, it could not explicitly predict the effects of two infant doses (in a 2 + 1 schedule) compared to a single infant dose (in a 1 + 1 schedule). Modeling dose-dependent VEs and the potential for breakthrough IPD in infants with a reduced dosing schedule will be an important avenue for future research. Fourth, as there are limited data on real-world estimates of VEs against disease in a 1 + 1 dosing schedule, in part due to the COVID-19 pandemic coinciding with the start of the reduced dosing schedule in the UK, this PSA was instead informed by average changes when moving from a 3 + 1 to 2 + 1 dosing schedule [22] and by assuming parity between PCV13 and PCV15 among common serotypes. Although the assumption of VE parity between PCV13 and PCV15 is critical to this analysis, the assumption is justified by pharmacokinetic studies that predict a comparable efficacy between PCV13 and PCV15 among common serotypes [40,41]. As more estimates of VE against disease and carriage become available, it will be important to incorporate these into model-based predictions. Fifth, to reduce the computational load, this model stratified the population into four age groups, with the 5–64-year-old age group comprising the majority of the population. Since the model did not include restricted carriage transmission from adults to children, this age stratification may have resulted in an under-estimation of disease among all the age groups. While the results of this analysis may be relevant to other countries or regions in a qualitative sense, they are not directly transferable due to geographic variability in serotype distribution. Finally, future work should consider estimating the public health effects associated with other higher-valency pediatric vaccines, including a 20-valent PCV (PCV20).
5. Conclusions
Switching from PCV13 to PCV15 for routine pediatric vaccination via the 1 + 1 dosing schedule in the UK would not only further reduce IPD in the pediatric population, but would also lead to population-level reductions in IPD due to indirect protection. Although this model predicted short-term increases in VT-IPD associated with potential reductions in VE against disease in a reduced dosing schedule, the effects were not persistent into the future, and these transient increases were smaller in magnitude with the implementation of PCV15 than the continued use of PCV13. In countries with lower pediatric VCRs, nascent pneumococcal vaccination programs, or uncontrolled VT-IPD, a reduced dosing schedule may lead to more pronounced and persistent increases in VT-IPD.
Author Contributions
R.J.O., N.B., J.W., I.R.M., D.N., T.M.M. and O.S. led the study concept and design. R.J.O., O.S., T.M.M., G.M., D.N. and J.C.L. supported the model inputs, data analysis, and model adaptations. R.J.O., O.S., T.M.M., G.M. and J.C.L. supported the model calibration. All authors contributed to the interpretation of results and writing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. The funder provided support in the form of salaries or consulting fees for authors.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the relevant data are within the manuscript and Appendix A. This is a modeling study and, therefore, no primary data were collected in this study. All the inputs were from published literature and included only anonymized data.
Acknowledgments
We wish to thank UKHSA for providing seroprevalence data, and we wish to thank Kevin Bakker and Kenneth Klinker for their efforts in the development of the UK model adaptation. We also wish to thank Robert Nachbar for his useful comments and suggestions during model development and adaptation to the UK, and Colleen Burgess for her assistance in the preparation of this manuscript.
Conflicts of Interest
R.J.O., N.B., J.W., T.M.M., and O.S. are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. I.R.M. and D.N. are employees of MSD (UK) Ltd., London, United Kingdom. G.M. is an employee of Wolfram Research Inc. under contract to Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. J.C.L. is an employee of Merck Canada, Inc., Kirkland, QC, Canada. R.J.O., N.B., J.W., I.R.M., D.N., T.M.M., J.C.L., and O.S. may hold stock or stock options in Merck & Co., Inc., Rahway, NJ, USA.
Abbreviations
The following abbreviations are used in this manuscript:
| UK | United Kingdom |
| PCV | Pneumococcal conjugate vaccine |
| VE | Vaccine effectiveness |
| IPD | Invasive pneumococcal disease |
| NIP | National Immunization Programme |
| PD | Pneumococcal disease |
| NVT | Non-vaccine type |
| VT | Vaccine type |
| NBPP | Non-bacteremic pneumococcal pneumonia |
| ST | Serotype |
| STC | Serotype class |
| HSA | Health Security Agency |
| VCR | Vaccine coverage rate |
| PSA | Probabilistic sensitivity analysis |
| RMSE | Root mean squared error |
Appendix A
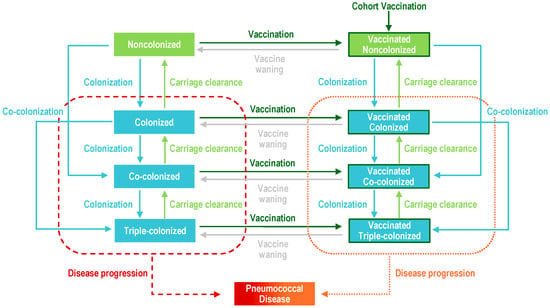
Figure A1.
Model diagram. Upon carriage acquisition, individuals moved to either the colonized, co-colonized, or triple-colonized classes, depending on whether one or two serotypes were acquired (denoted in blue). Some portion of individuals from these colonized classes would develop a pneumococcal disease (denoted in red and orange). Recovery then moved individuals back to the non-colonized class (denoted in light green), sequentially clearing one serotype at a time. Vaccination (dark green border) reduced the risk of carriage acquisition and disease development for vaccine serotypes.
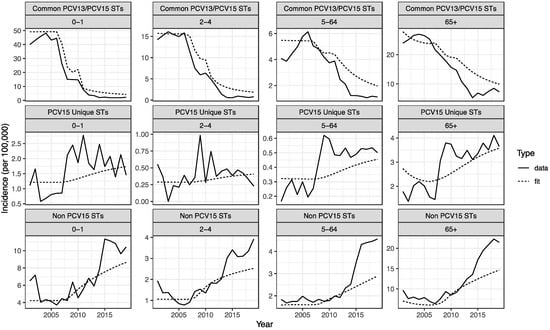
Figure A2.
Model calibration to historical observed IPD incidence per 100,000 people by ST grouping and age group. Comparison of data (solid line) to model fit (dotted line) during the calibration period, faceted by age group and vaccination grouping.

Figure A3.
Model validation of the calibrated model comparing the data to the model fit during the calibration period, by age group. Points denote annual and STC-specific incidence and are colored by vaccination grouping. The black line indicates a 1:1 line. The root mean squared error (RMSE) values were 1.47, 0.597, 0.312, and 1.29 for the <2-, 2–4-, 5–64-, and ≥65-year-old age groups. Across all age groups, the RMSE was 1.03.

Table A1.
Vaccine efficacy against disease for the <2-year-old age group [22] for a 2 + 1 schedule.
Table A1.
Vaccine efficacy against disease for the <2-year-old age group [22] for a 2 + 1 schedule.
| Serotype Class (ST) | VE Against Disease (PCV7) | VE Against Disease (PCV13) | VE Against Disease (PCV15) |
|---|---|---|---|
| 1 (4, 6B, 9V, 14, 18C, 19F, 23F) | 0.96 | 0.96 | 0.96 |
| 2 (1, 5) | 0 | 0.84 | 0.84 |
| 3 (3) | 0 | 0.41 | 0.41 |
| 4 (7F, 19A) | 0 | 0.93 | 0.93 |
| 5 (6A, 6C) | 0 | 0.96 | 0.96 |
| 6 (22F, 33F) | 0 | 0 | 0.94 |
| 7 (9N, 17F, 20) | 0 | 0 | 0 |
| 8 (8, 10A, 11A, 12F) | 0 | 0 | 0 |
| 9 (15B) | 0 | 0 | 0 |
| 10 (15A, 15C, 16F, 23A, 23B, 24F, 31, 35B) | 0 | 0 | 0 |
| 11 (NVTs) | 0 | 0 | 0 |
VE = vaccine efficacy; NVT = non-vaccine type; ST = serotype.

Table A2.
Vaccine efficacy against disease for the 2–4-year-old and 5–64-year-old risk groups and the ≥65-year-old population for PPSV23 [23]. Note that, given the lack of real-world evidence for vaccine effectiveness against disease for novel vaccine serotypes (STCs 6–10), we assumed equivalency with previous VTs in adult PCVs.
Table A2.
Vaccine efficacy against disease for the 2–4-year-old and 5–64-year-old risk groups and the ≥65-year-old population for PPSV23 [23]. Note that, given the lack of real-world evidence for vaccine effectiveness against disease for novel vaccine serotypes (STCs 6–10), we assumed equivalency with previous VTs in adult PCVs.
| Serotype Class (ST) | VE Against Disease (PPSV23) |
|---|---|
| 1 (4, 6B, 9V, 14, 18C, 19F, 23F) | 0.45 |
| 2 (1, 5) | 0.23 |
| 3 (3) | 0.22 |
| 4 (7F, 19A) | 0.59 |
| 5 (6A, 6C) | 0 |
| 6 (22F, 33F) | 0.54 |
| 7 (9N, 17F, 20) | 0.65 |
| 8 (8, 10A, 11A, 12F) | 0.57 |
| 9 (15B) | 0.53 |
| 10 (15A, 15C, 16F, 23A, 23B, 24F, 31, 35B) | 0 |
| 11 (NVTs) | 0 |
VE = vaccine efficacy; NVT = non-vaccine type.

Table A3.
PCV coverage for the <2-year-old age group [42]. NA—not applicable.
Table A3.
PCV coverage for the <2-year-old age group [42]. NA—not applicable.
| Year | VCR |
|---|---|
| 2005 | NA |
| 2006 | NA |
| 2007 | 89.1 |
| 2008 | 90.1 |
| 2009 | 92.9 |
| 2010 | 93.6 |
| 2011 | 94.2 |
| 2012 | 94.4 |
| 2013 | 94.1 |
| 2014 | 93.9 |
| 2015 | 93.5 |
| 2016 | 93.5 |
| 2017 | 93.3 |
| 2018 | 92.8 |
| 2019 | 93.2 |
VCR = vaccination coverage rate.

Table A4.
PPSV23 coverage for the ≥65-year-old age group. Data were from 2005–2018. For 2019, we assumed the same coverage as 2018 [43].
Table A4.
PPSV23 coverage for the ≥65-year-old age group. Data were from 2005–2018. For 2019, we assumed the same coverage as 2018 [43].
| Year | VCR |
|---|---|
| 2005 | 64.4 |
| 2006 | 66.6 |
| 2007 | 69.0 |
| 2008 | 68.2 |
| 2009 | 69.4 |
| 2010 | 70.5 |
| 2011 | 68.3 |
| 2012 | 69.1 |
| 2013 | 68.9 |
| 2014 | 69.8 |
| 2015 | 70.1 |
| 2016 | 69.8 |
| 2017 | 69.5 |
| 2018 | 69.2 |
| 2019 | 69.2 |
VCR = vaccination coverage rate.

Table A5.
PPSV23 coverage for the at-risk 2–4- and 5–64-year-old age groups. These calculations assumed that the VCR of clinical risk groups was 49.0% and that the proportion of the 2–64-year-old population that fell within a clinical risk group was 9.7% [43].
Table A5.
PPSV23 coverage for the at-risk 2–4- and 5–64-year-old age groups. These calculations assumed that the VCR of clinical risk groups was 49.0% and that the proportion of the 2–64-year-old population that fell within a clinical risk group was 9.7% [43].
| Year | VCR |
|---|---|
| 2005–2019 | 4.78 |
VCR = vaccination coverage rate.

Table A6.
Estimated duration of carriage (days) by age group and serotype class [11].
Table A6.
Estimated duration of carriage (days) by age group and serotype class [11].
| Serotype Class (ST) | 0–1 | 2–4 | 5–64 | 65+ |
|---|---|---|---|---|
| 1 (4, 6B, 9V, 14, 18C, 19F, 23F) | 70.24 | 30.60 | 24.94 | 26.83 |
| 2 (1, 5) | 13.38 | 9.58 | 7.04 | 6.56 |
| 3 (3) | 24.60 | 21.50 | 39.72 | 45.50 |
| 4 (7F, 19A) | 57.98 | 24.51 | 21.14 | 21.45 |
| 5 (6A, 6C) | 53.60 | 36.20 | 24.58 | 25.84 |
| 6 (22F, 33F) | 87.56 | 61.33 | 46.26 | 43.10 |
| 7 (9N, 17F, 20) | 34.73 | 25.07 | 18.03 | 16.58 |
| 8 (8, 10A, 11A, 12F) | 39.43 | 26.59 | 20.86 | 21.47 |
| 9 (15B) | 42.24 | 31.88 | 21.32 | 21.75 |
| 10 (15A, 15C, 16F, 23A, 23B, 24F, 31, 35B) | 36.62 | 26.04 | 17.22 | 17.48 |
| 11 (NVTs) | 35.26 | 30.70 | 29.32 | 31.21 |
References
- Bertran, M.; D’Aeth, J.C.; Abdullahi, F.; Eletu, S.; Andrews, N.J.; Ramsay, M.E.; Litt, D.J.; Ladhani, S.N. Invasive pneumococcal disease 3 years after introduction of a reduced 1+ 1 infant 13-valent pneumococcal conjugate vaccine immunisation schedule in England: A prospective national observational surveillance study. Lancet Infect. Dis. 2024, 24, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Goldblatt, D.; Southern, J.; Andrews, N.J.; Burbidge, P.; Partington, J.; Roalfe, L.; Pinto, M.V.; Thalasselis, V.; Plested, E.; Richardson, H. Pneumococcal conjugate vaccine 13 delivered as one primary and one booster dose (1+ 1) compared with two primary doses and a booster (2 + 1) in UK infants: A multicentre, parallel group randomised controlled trial. Lancet Infect. Dis. 2018, 18, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Goldblatt, D.; Southern, J.; Ashton, L.; Richmond, P.; Burbidge, P.; Tasevska, J.; Crowley-Luke, A.; Andrews, N.; Morris, R.; Borrow, R. Immunogenicity and boosting after a reduced number of doses of a pneumococcal conjugate vaccine in infants and toddlers. Pediatr. Infect. Dis. J. 2006, 25, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Ladhani, S.N.; Collins, S.; Djennad, A.; Sheppard, C.L.; Borrow, R.; Fry, N.K.; Andrews, N.J.; Miller, E.; Ramsay, M.E. Rapid increase in non-vaccine serotypes causing invasive pneumococcal disease in England and Wales, 2000–2017: A prospective national observational cohort study. Lancet Infect. Dis. 2018, 18, 441–451. [Google Scholar] [CrossRef]
- Choi, Y.H.; Andrews, N.; Miller, E. Estimated impact of revising the 13-valent pneumococcal conjugate vaccine schedule from 2 + 1 to 1 + 1 in England and Wales: A modelling study. PLoS Med. 2019, 16, e1002845. [Google Scholar] [CrossRef]
- Wasserman, M.; Lucas, A.; Jones, D.; Wilson, M.; Hilton, B.; Vyse, A.; Madhava, H.; Brogan, A.; Slack, M.; Farkouh, R. Dynamic transmission modelling to address infant pneumococcal conjugate vaccine schedule modifications in the UK. Epidemiol. Infect. 2018, 146, 1797–1806. [Google Scholar] [CrossRef]
- Choi, Y.H.; Bertran, M.; Litt, D.J.; Ladhani, S.N.; Miller, E. Potential impact of replacing the 13-valent pneumococcal conjugate vaccine with 15-valent or 20-valent pneumococcal conjugate vaccine in the 1+ 1 infant schedule in England: A modelling study. Lancet Public Health 2024, 9, e654–e663. [Google Scholar] [CrossRef]
- Hu, T.; Weiss, T.; Bencina, G.; Owusu-Edusei, K.; Petigara, T. Comprehensive value assessments for new pediatric pneumococcal conjugate vaccines. J. Med. Econ. 2021, 24, 1083–1086. [Google Scholar] [CrossRef]
- Yeh, S.H.; Gurtman, A.; Hurley, D.C.; Block, S.L.; Schwartz, R.H.; Patterson, S.; Jansen, K.U.; Love, J.; Gruber, W.C.; Emini, E.A. Immunogenicity and safety of 13-valent pneumococcal conjugate vaccine in infants and toddlers. Pediatrics 2010, 126, e493–e505. [Google Scholar] [CrossRef]
- Malik, T.M.; Bakker, K.M.; Oidtman, R.J.; Sharomi, O.; Meleleo, G.; Nachbar, R.B.; Elbasha, E.H. A dynamic transmission model for assessing the impact of pneumococcal vaccination in the United States. PLoS ONE 2025, 20, e0305892. [Google Scholar] [CrossRef]
- Oidtman, R.J.; Meleleo, G.J.; Sharomi, O.; Matthews, I.; Ntais, D.; Nachbar, R.; Malik, T.; Bakker, K.M. Modeling the epidemiological impact of different adult pneumococcal vaccination strategies in the United Kingdom. Infect. Dis. Ther. 2025, 14, 587–602. [Google Scholar] [CrossRef] [PubMed]
- Office for National Statistics. Office for National Statistics Conceptions in England and Wales. 2019. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/conceptionandfertilityrates/datasets/conceptionstatisticsenglandandwalesreferencetables (accessed on 17 December 2024).
- Office for National Statistics. Single Life Year Table—UK Edition. 2020. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/singleyearlifetablesuk1980to2018 (accessed on 17 December 2024).
- Prem, K.; Cook, A.R.; Jit, M. Projecting social contact matrices in 152 countries using contact surveys and demographic data. PLoS Comput. Biol. 2017, 13, e1005697. [Google Scholar] [CrossRef] [PubMed]
- Althouse, B.M.; Hammitt, L.L.; Grant, L.; Wagner, B.G.; Reid, R.; Larzelere-Hinton, F.; Weatherholtz, R.; Klugman, K.P.; Rodgers, G.L.; O’Brien, K.L. Identifying transmission routes of Streptococcus pneumoniae and sources of acquisitions in high transmission communities. Epidemiol. Infect. 2017, 145, 2750–2758. [Google Scholar] [CrossRef] [PubMed]
- Wolfram Research (2003), NMinimize, Wolfram Language function. (updated 2024). Available online: https://reference.wolfram.com/language/ref/NMinimize.html (accessed on 3 March 2025).
- Cleary, D.W.; Jones, J.; Gladstone, R.A.; Osman, K.L.; Devine, V.T.; Jefferies, J.M.; Bentley, S.D.; Faust, S.N.; Clarke, S.C. Changes in serotype prevalence of Streptococcus pneumoniae in Southampton, UK between 2006 and 2018. Sci. Rep. 2022, 12, 13332. [Google Scholar] [CrossRef]
- Hussain, M.; Melegaro, A.; Pebody, R.G.; George, R.; Edmunds, W.; Talukdar, R.; Martin, S.; Efstratiou, A.; Miller, E. A longitudinal household study of Streptococcus pneumoniae nasopharyngeal carriage in a UK setting. Epidemiol. Infect. 2005, 133, 891–898. [Google Scholar] [CrossRef]
- US Food and Drug Administration. CAPVAXIVE™ (Pneumococcal 21-Valent Conjugate Vaccine) Injection, for Intramuscular Use. Available online: https://www.fda.gov/media/179426/download (accessed on 3 March 2025).
- Løchen, A.; Anderson, R. Dynamic transmission models and economic evaluations of pneumococcal conjugate vaccines: A quality appraisal and limitations. Clin. Microbiol. Infect. 2020, 26, 60–70. [Google Scholar] [CrossRef]
- Moore, C.E.; Paul, J.; Foster, D.; Mahar, S.A.; Griffiths, D.; Knox, K.; Peto, T.E.; Walker, A.S.; Crook, D.W. Reduction of invasive pneumococcal disease 3 years after the introduction of the 13-valent conjugate vaccine in the Oxfordshire region of England. J. Infect. Dis. 2014, 210, 1001–1011. [Google Scholar] [CrossRef]
- Savulescu, C.; Krizova, P.; Valentiner-Branth, P.; Ladhani, S.; Rinta-Kokko, H.; Levy, C.; Mereckiene, J.; Knol, M.; Winje, B.A.; Ciruela, P.; et al. Effectiveness of 10 and 13-valent pneumococcal conjugate vaccines against invasive pneumococcal disease in European children: SpIDnet observational multicentre study. Vaccine 2022, 40, 3963–3974. [Google Scholar] [CrossRef]
- Andrews, N.J.; Waight, P.A.; George, R.C.; Slack, M.P.; Miller, E. Impact and effectiveness of 23-valent pneumococcal polysaccharide vaccine against invasive pneumococcal disease in the elderly in England and Wales. Vaccine 2012, 30, 6802–6808. [Google Scholar] [CrossRef]
- Hill, P.C.; Cheung, Y.B.; Akisanya, A.; Sankareh, K.; Lahai, G.; Greenwood, B.M.; Adegbola, R.A. Nasopharyngeal carriage of Streptococcus pneumoniae in Gambian infants: A longitudinal study. Clin. Infect. Dis. 2008, 46, 807–814. [Google Scholar] [CrossRef]
- Chaguza, C.; Senghore, M.; Bojang, E.; Lo, S.W.; Ebruke, C.; Gladstone, R.A.; Tientcheu, P.E.; Bancroft, R.E.; Worwui, A.; Foster-Nyarko, E.; et al. Carriage Dynamics of Pneumococcal Serotypes in Naturally Colonized Infants in a Rural African Setting During the First Year of Life. Front. Pediatr. 2021, 8, 587730. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, O.; Karani, A.; Tigoi, C.C.; Mugo, D.; Kungu, S.; Wanjiru, E.; Jomo, J.; Musyimi, R.; Lipsitch, M.; Scott, J.A. Rates of acquisition and clearance of pneumococcal serotypes in the nasopharynges of children in Kilifi District, Kenya. J. Infect. Dis. 2012, 206, 1020–1029. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, O.; Karani, A.; Tigoi, C.C.; Mugo, D.; Kungu, S.; Wanjiru, E.; Jomo, J.; Musyimi, R.; Lipsitch, M.; Scott, J.A. The prevalence and risk factors for pneumococcal colonization of the nasopharynx among children in Kilifi District, Kenya. PLoS ONE 2012, 7, e30787. [Google Scholar] [CrossRef] [PubMed]
- Dube, F.S.; Ramjith, J.; Gardner-Lubbe, S.; Nduru, P.; Robberts, F.J.L.; Wolter, N.; Zar, H.J.; Nicol, M.P. Longitudinal characterization of nasopharyngeal colonization with Streptococcus pneumoniae in a South African birth cohort post 13-valent pneumococcal conjugate vaccine implementation. Sci. Rep. 2018, 8, 12497. [Google Scholar] [CrossRef]
- Turner, P.; Turner, C.; Jankhot, A.; Helen, N.; Lee, S.J.; Day, N.P.; White, N.J.; Nosten, F.; Goldblatt, D. A longitudinal study of Streptococcus pneumoniae carriage in a cohort of infants and their mothers on the Thailand-Myanmar border. PLoS ONE 2012, 7, e38271. [Google Scholar] [CrossRef]
- Cauchemez, S.; Temime, L.; Valleron, A.J.; Varon, E.; Thomas, G.; Guillemot, D.; Boelle, P.Y.S. pneumoniae transmission according to inclusion in conjugate vaccines: Bayesian analysis of a longitudinal follow-up in schools. BMC Infect. Dis. 2006, 6, 14. [Google Scholar] [CrossRef]
- Grivea, I.N.; Priftis, K.N.; Giotas, A.; Kotzia, D.; Tsantouli, A.G.; Douros, K.; Michoula, A.N.; Syrogiannopoulos, G.A. Dynamics of pneumococcal carriage among day-care center attendees during the transition from the 7-valent to the higher-valent pneumococcal conjugate vaccines in Greece. Vaccine 2014, 32, 6513–6520. [Google Scholar] [CrossRef]
- Sleeman, K.L.; Griffiths, D.; Shackley, F.; Diggle, L.; Gupta, S.; Maiden, M.C.; Moxon, E.R.; Crook, D.W.; Peto, T.E. Capsular serotype-specific attack rates and duration of carriage of Streptococcus pneumoniae in a population of children. J. Infect. Dis. 2006, 194, 682–688. [Google Scholar] [CrossRef]
- Hogberg, L.; Ekdahl, K.; Sjostrom, K.; Olsson-Liljequist, B.; Walder, M.; Melander, E.; Ringberg, H.; Normark, B.H. Penicillin-resistant pneumococci in Sweden 1997–2003: Increased multiresistance despite stable prevalence and decreased antibiotic use. Microb. Drug Resist. 2006, 12, 16–22. [Google Scholar] [CrossRef]
- Hogberg, L.; Geli, P.; Ringberg, H.; Melander, E.; Lipsitch, M.; Ekdahl, K. Age- and serogroup-related differences in observed durations of nasopharyngeal carriage of penicillin-resistant pneumococci. J. Clin. Microbiol. 2007, 45, 948–952. [Google Scholar] [CrossRef]
- Auranen, K.; Mehtala, J.; Tanskanen, A.; Kaltoft, S.M. Between-strain competition in acquisition and clearance of pneumococcal carriage--epidemiologic evidence from a longitudinal study of day-care children. Am. J. Epidemiol. 2009, 171, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, D.; Hoti, F.; Syrjanen, R.; Sa-Leao, R.; Kaijalainen, T.; Gomes, M.G.; Auranen, K. Comparative analysis of Streptococcus pneumoniae transmission in Portuguese and Finnish day-care centres. BMC Infect. Dis. 2013, 13, 180. [Google Scholar] [CrossRef] [PubMed]
- Vaccine Uptake Guidance and the Latest Coverage Data. Published in 2023. Available online: https://www.gov.uk/government/collections/vaccine-uptake (accessed on 17 December 2024).
- Blower, S.M.; Dowlatabadi, H. Sensitivity and Uncertainty Analysis of Complex-Models of Disease Transmission—An Hiv Model, as an Example. Int. Stat. Rev. 1994, 62, 229–243. [Google Scholar] [CrossRef]
- Garcia Quesada, M.; Peterson, M.E.; Bennett, J.C.; Hayford, K.; Zeger, S.L.; Yang, Y.; Hetrich, M.K.; Feikin, D.R.; Cohen, A.L.; von Gottberg, A.; et al. Serotype distribution of remaining invasive pneumococcal disease after extensive use of ten-valent and 13-valent pneumococcal conjugate vaccines (the PSERENADE project): A global surveillance analysis. Lancet Infect. Dis. 2025, 25, 445–456. [Google Scholar] [CrossRef]
- Ryman, J.; Sachs, J.R.; Lai, Y.K.; Natalie, B.; Jessica, W.; and Weiss, T. Predicted serotype-specific effectiveness of pneumococcal conjugate vaccines V114 and PCV20 against invasive pneumococcal disease in children. Expert. Rev. Vaccines 2024, 23, 60–68. [Google Scholar] [CrossRef]
- Ryman, J.; Sachs, J.R.; Natalie, B.; Thomas, W.; Maurice, A.; Lai, Y.K.; and Weaver, J. Potential serotype-specific effectiveness against IPD of pneumococcal conjugate vaccines V114 and PCV20 in children given a 2 + 1 dosing regimen. Expert. Rev. Vaccines 2024, 23, 467–473. [Google Scholar] [CrossRef]
- NHS England. Childhood Vaccination Coverage Statistics—England: Pneumococcal Conjugate Vaccine (PCV). Available online: https://digital.nhs.uk/data-and-information/publications/statistical/nhs-immunisation-statistics/2021-22/6in-1-vaccine#pneumococcal-conjugate-vaccine-pcv- (accessed on 17 December 2024).
- UK Health Security Agency. Pneumococcal Polysaccharide Vaccine (PPV) Coverage Report, England, April 2021 to March 2022. Available online: https://www.gov.uk/government/publications/pneumococcal-polysaccharide-vaccine-ppv-vaccine-coverage-estimates/pneumococcal-polysaccharide-vaccine-ppv-coverage-report-england-april-2021-to-march-2022 (accessed on 6 March 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).