Therapeutic Vaccinations with p210 Peptides in Imatinib-Treated Chronic Myeloid Leukemia Patients: 10 Years Follow-Up of GIMEMA CML0206 and SI0207 Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Eligibility
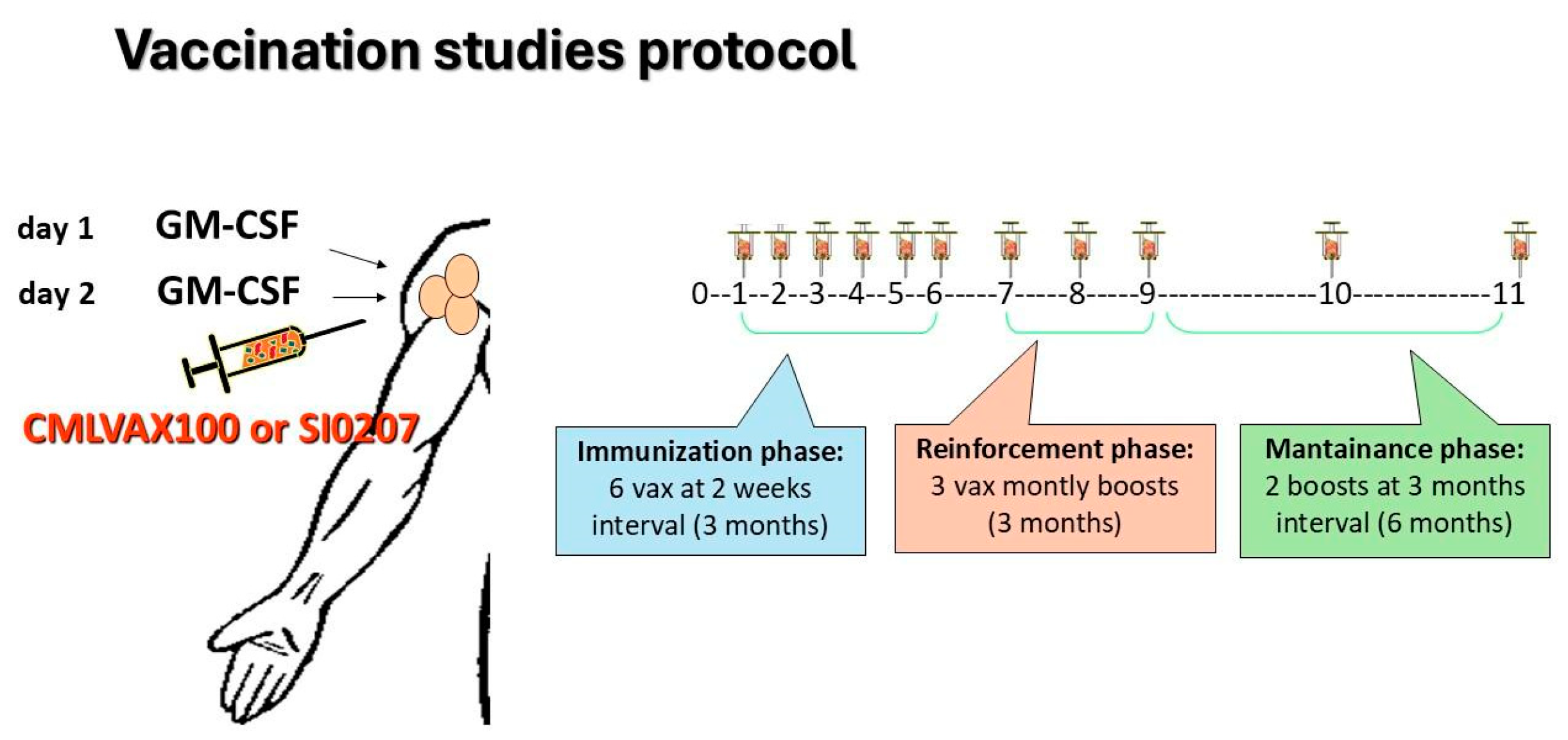
2.2. Study Design and Treatment
2.3. Endpoints
2.4. Peptide Vaccine Induced Immune Response Evaluation “In Vitro”
2.5. Molecular Response Evaluation
2.6. Statistical Methods
3. Results
3.1. Characteristics of Patients
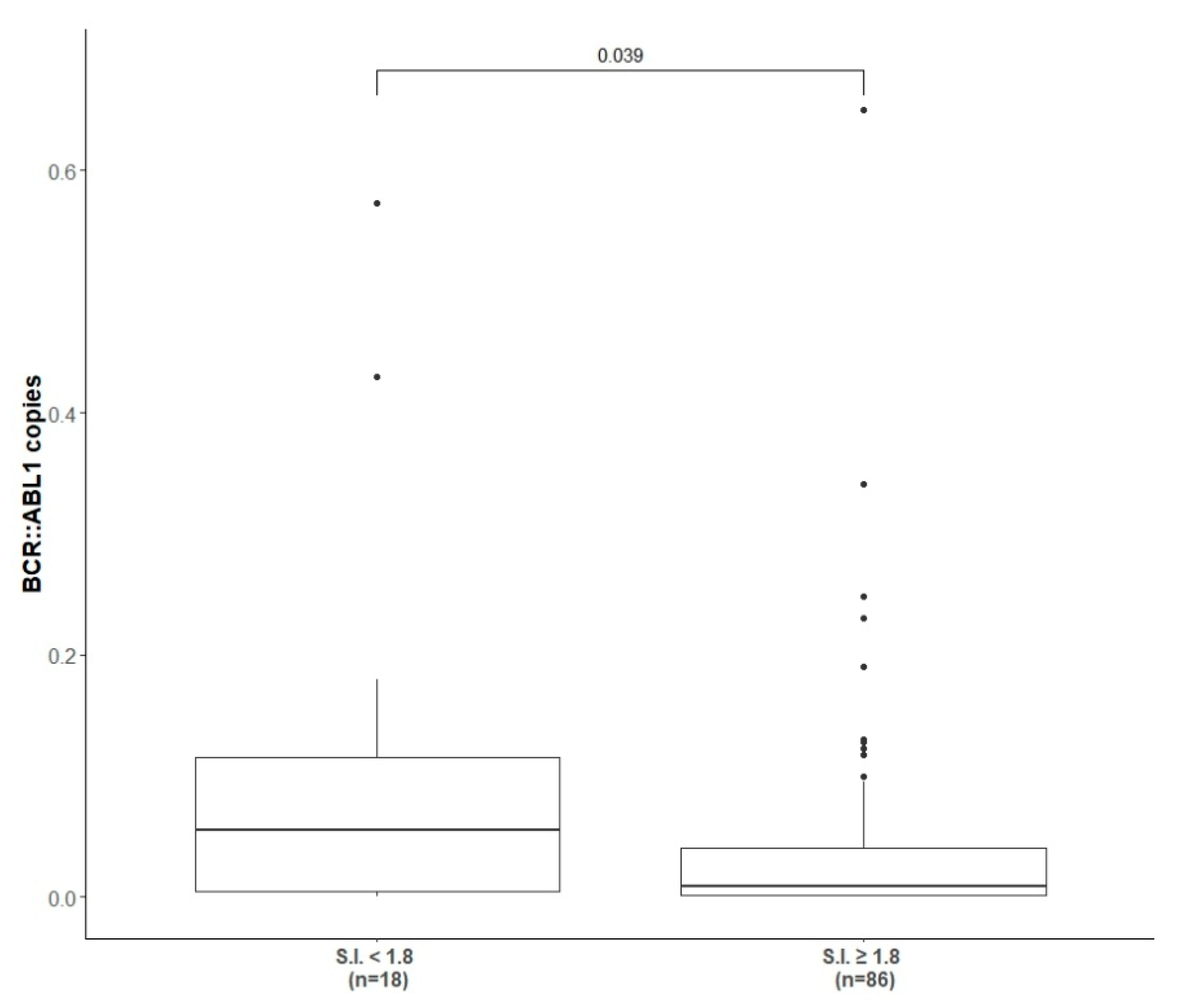
3.2. Stimulation Index Results
3.3. Molecular Responses After Vaccination
3.4. Vaccine Safety
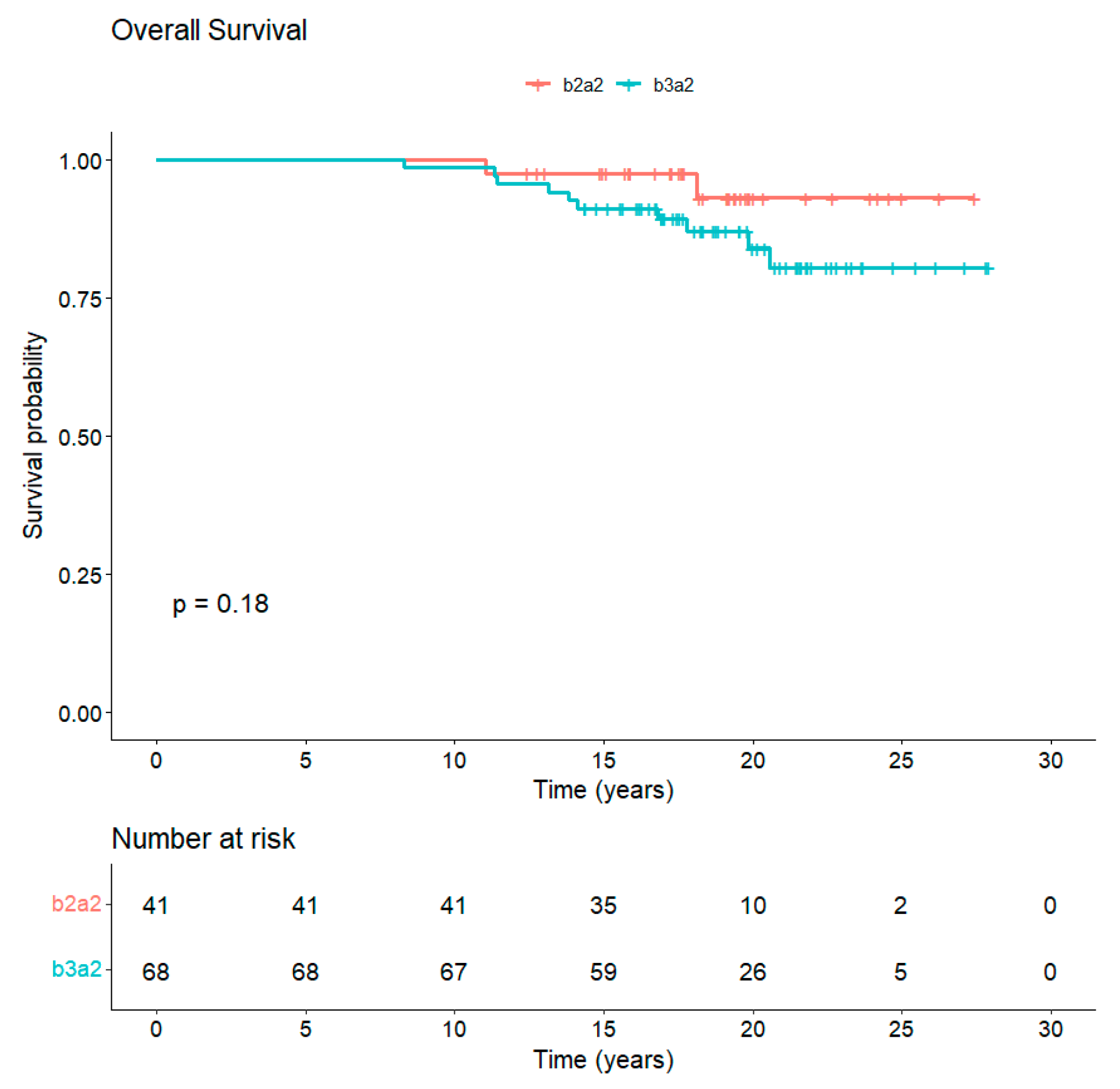
3.5. Long-Term Follow-Up
3.6. Treatment-Free Remission After Vaccination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kantarjian, H.; Sawyers, C.; Hochhaus, A.; Guilhot, F.; Schiffer, C.; Gambacorti-Passerini, C.; Niederwieser, D.; Resta, D.; Capdeville, R.; Zoellner, U.; et al. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N. Engl. J. Med. 2002, 346, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Baccarani, M.; Saglio, G.; Goldman, J.; Hochhaus, A.; Simonsson, B.; Appelbaum, F.; Apperley, J.; Cervantes, F.; Cortes, J.; Deininger, M.; et al. Evolving concepts in the management of chronic myeloid leukemia: Recommendations from an expert panel on behalf of the European LeukemiaNet. Blood 2006, 108, 1809–1820. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.P.; Kantarjian, H.; Quintás-Cardama, A.; Cortes, J. Evolution of therapies for chronic myelogenous leukemia. Cancer J. 2011, 17, 465–476. [Google Scholar] [CrossRef]
- Hughes, T.P.; Ross, D.M. Moving treatment-free remission into mainstream clinical practice in CML. Blood 2016, 128, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, A.; Helgason, G.V.; Schemionek, M.; Zhang, B.; Myssina, S.; Allan, E.K.; Nicolini, F.E.; Müller-Tidow, C.; Bhatia, R.; Brunton, V.G.; et al. Chronic myeloid leukemia stem cells are not dependent on Bcr-Abl kinase activity for their survival. Blood 2012, 119, 1501–1510. [Google Scholar] [CrossRef]
- Bhatia, R.; Holtz, M.; Niu, N.; Gray, R.; Snyder, D.S.; Sawyers, C.L.; Arber, D.A.; Slovak, M.L.; Forman, S.J. Persistence of malignant hematopoietic progenitors in chronic myelogenous leukemia patients in complete cytogenetic remission following imatinib mesylate treatment. Blood 2003, 101, 4701–4707. [Google Scholar] [CrossRef]
- Pacelli, P.; Santoni, A.; Sicuranza, A.; Abruzzese, E.; Giai, V.; Crugnola, M.; Annunziata, M.; Galimberti, S.; Iurlo, A.; Luciano, L.; et al. Prospective monitoring of chronic myeloid leukemia patients from the time of TKI discontinuation: The fate of peripheral blood CD26+ leukemia stem cells. Front. Pharmacol. 2023, 14, 1194712. [Google Scholar] [CrossRef]
- Hughes, A.; Yong, A.S.M. Immune Effector Recovery in Chronic Myeloid Leukemia and Treatment-Free Remission. Front. Immunol. 2017, 8, 469. [Google Scholar] [CrossRef]
- Bocchia, M.; Korontsvit, T.; Xu, Q.; Mackinnon, S.; Yang, S.; Sette, A.; Scheinberg, D. Specific human cellular immunity to bcr-abl oncogene derived peptides. Blood 1996, 87, 3587–3592. [Google Scholar] [CrossRef]
- Bosh, G.J.; Joosten, A.M.; Kessler, J.H.; Melief, C.J.; Leeksma, O.C. Recognition of BCR-ABL positive leukemia blast by human DC4+ T cells elicited by primary in vitro immunization with a BCR-ABL breakpoint peptide. Blood 1996, 88, 3522–3527. [Google Scholar] [CrossRef]
- Clark, R.E.; Dodi, I.A.; Hill, S.C.; Lill, J.R.; Aubert, G.; Macintyre, A.R.; Rojas, J.; Bourdon, A.; Bonner, P.L.R.; Wang, L.; et al. Direct evidence that leukemic cells present HLA-associated immunogenic peptides derived from the BCR-ABL b3a2 fusion protein. Blood 2001, 98, 2887–2893. [Google Scholar] [CrossRef] [PubMed]
- Bocchia, M.; Gentili, S.; Abruzzese, E.; Fanelli, A.; Iuliano, F.; Tabilio, A.; Amabile, M.; Forconi, F.; Gozzetti, A.; Raspadori, D.; et al. Effect of a p210 multipeptide vaccine associated with Imatinib or Interferon in patients with chronic myeloid leukemia and persistent residual disease: A multicentre observational trial. Lancet 2005, 365, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Baccarani, M.; Cortes, J.; Pane, F.; Niederwieser, D.; Saglio, G.; Apperley, J.; Cervantes, F.; Deininger, M.; Gratwohl, A.; Guilhot, F.; et al. Chronic myeloid leukemia: An update of concepts and management recommendations of European LeukemiaNet. J. Clin. Oncol. 2009, 27, 6041–6051. [Google Scholar] [CrossRef]
- Cortes, J.; Quintás-Cardama, A.; Kantarjian, H.M. Monitoring molecular response in chronic myeloid leukemia. Cancer 2011, 117, 1113–1122. [Google Scholar] [CrossRef]
- Cross, N.C.P.; E White, H.; Colomer, D.; Ehrencrona, H.; Foroni, L.; Gottardi, E.; Lange, T.; Lion, T.; Polakova, K.M.; Dulucq, S.; et al. Laboratory recommendations for scoring deep molecular responses following treatment for chronic myeloid leukemia. Leukemia 2015, 29, 999–1003. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap). A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Hochhaus, A.; Larson, R.A.; Guilhot, F.; Radich, J.P.; Branford, S.; Hughes, T.P.; Baccarani, M.; Deininger, M.W.; Cervantes, F.; Fujihara, S.; et al. IRIS Investigators. Long-Term Outcomes of Imatinib Treatment for Chronic Myeloid Leukemia. N. Engl. J. Med. 2017, 376, 917–927. [Google Scholar] [CrossRef]
- Hehlmann, R.; Lauseker, M.; Saußele, S.; Pfirrmann, M.; Krause, S.; Kolb, H.J.; Neubauer, A.; Hossfeld, D.K.; Nerl, C.; Gratwohl, A.; et al. Assessment of imatinib as first-line treatment of chronic myeloid leukemia: 10-year survival results of the randomized CML study IV and impact of non-CML determinants. Leukemia 2017, 31, 2398–2406. [Google Scholar] [CrossRef]
- Wolf, D.; Tilg, H.; Rumpold, H.; Gastl, G.; Wolf, A.M. The kinase inhibitor imatinib—An immunosuppressive drug? Curr. Cancer Drug Targets 2007, 7, 251–258. [Google Scholar] [CrossRef]
- Chan, O.; Talati, C.; Sweet, K.; Pinilla-Ibarz, J. Can increased immunogenicity in chronic myeloid leukemia improve outcomes? Expert Rev. Hematol. 2019, 12, 225–233. [Google Scholar] [CrossRef]
- Bocchia, M.; Defina, M.; Aprile, L.; Ippoliti, M.; Crupi, R.; Rondoni, M.; Gozzetti, A.; Lauria, F. Complete molecular response in CML after p210 BCR–ABL1-derived peptide vaccination. Nat. Rev. Clin. Oncol. 2010, 7, 600–603. [Google Scholar] [CrossRef]
- Cathcart, K.; Pinilla-Ibarz, J.; Korontsvit, T.; Schwartz, J.; Zakhaleva, V.; Papadopoulos, E.B.; Scheinberg, D.A. A multivalent bcr-abl fusion peptide vaccination trial in patients with chronic myeloid leukemia. Blood 2004, 103, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Reuben, J.M.; Kantarjian, H.; Li, C.; Gao, H.; Lee, B.; Cohen, E.N.; Ebarb, T.; Scheinberg, D.A.; Cortes, J. Synthetic tumor-specific breakpoint peptide vaccine in patients with chronic myeloid leukemia and minimal residual disease: A phase 2 trial. Cancer 2009, 115, 3924–3934. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.M.; Knight, K.; Wang, L.; Clark, R.E. Clinical evaluation of BCR-ABL peptide immunisation in chronic myeloid leukaemia: Results of the EPIC study. Leukemia 2007, 21, 2287–2295. [Google Scholar] [CrossRef] [PubMed]
- Mahon, F.-X.; Réa, D.; Guilhot, J.; Guilhot, F.; Huguet, F.; Nicolini, F.; Legros, L.; Charbonnier, A.; Guerci, A.; Varet, B.; et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: The prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010, 11, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Etienne, G.; Guilhot, J.; Rea, D.; Rigal-Huguet, F.; Nicolini, F.; Charbonnier, A.; Guerci-Bresler, A.; Legros, L.; Varet, B.; Gardembas, M.; et al. Long-Term Follow-Up of the French Stop Imatinib (STIM1) Study in Patients With Chronic Myeloid Leukemia. J. Clin. Oncol. 2017, 35, 298–305. [Google Scholar] [CrossRef]
- Saussele, S.; Richter, J.; Guilhot, J.; Gruber, F.X.; Hjorth-Hansen, H.; Almeida, A.; Janssen, J.J.W.M.; Mayer, J.; Koskenvesa, P.; Panayiotidis, P.; et al. Discontinuation of tyrosine kinase inhibitor therapy in chronic myeloid leukaemia (EURO-SKI): A prespecified interim analysis of a prospective, multicentre, non-randomised, trial. Lancet Oncol. 2018, 19, 747–757. [Google Scholar] [CrossRef]
- Haddad, F.G.; Sasaki, K.; Issa, G.C.; Garcia-Manero, G.; Ravandi, F.; Kadia, T.; Cortes, J.; Konopleva, M.; Pemmaraju, N.; Alvarado, Y.; et al. Treatment-free remission in patients with chronic myeloid leukemia following the discontinuation of tyrosine kinase inhibitors. Am. J. Hematol. 2022, 97, 856–864. [Google Scholar] [CrossRef]
- Chakraborty, C.; Sharma, A.R.; Bhattacharya, M.; Lee, S.S. From COVID-19 to Cancer mRNA Vaccines: Moving From Bench to Clinic in the Vaccine Landscape. Front. Immunol. 2021, 12, 679344. [Google Scholar] [CrossRef]
- Miao, L.; Zhang, Y.; Huang, L. mRNA vaccine for cancer immunotherapy. Mol. Cancer. 2021, 20, 41. [Google Scholar] [CrossRef]
| Total | b3a2 | b2a2 | ||
|---|---|---|---|---|
| Patients | 109 | 68 | 41 | |
| Median age (yr) at the diagnosis (range) | 43 (17–23 yr) | 43 (22–73 yr) | 50 (17–69 yr) | |
| Median age (yr) at the enrollment (range) | 50 (23–77 yr) | 51 (28–77 yr) | 46 (23–77 yr) | |
| Sex | male | 70 (64%) | 44 (64.7%) | 26 (63.4%) |
| female | 39 (36%) | 24 (35.3%) | 15 (36.6%) | |
| Sokal | high | 10 (9.2%) | 4 (5.9%) | 6 (14.6%) |
| intermediate | 31 (28.5%) | 21 (30.9%) | 10 (24.4%) | |
| low | 68 (62.3%) | 43 (63.2%) | 25 (61%) | |
| Disease status pre-vaccine | no MR3 | 13 (12%) | 10 (15%) | 3 (7%) |
| MR3 | 67 (61.4%) | 47 (69%) | 20 (49%) | |
| >MR3 | 29 (26.6%) | 11 (16%) | 18 (44%) | |
| Reduction >1 log BCR::ABL1 | Reduction <1 log BCR::ABL1 | Stable Disease with BCR::ABL1 fluctuation | Increase ≥1 log BCR::ABL1 | p Value | ||
|---|---|---|---|---|---|---|
| Whole cohort | 109 | 22 | 14 | 62 | 11 | |
| B3a2 | 68 | 16 (23.5%) | 9 (13.2%) | 38 (56%) | 5 (7.3%) | 0.494 |
| B2a2 | 41 | 6 (14.6%) | 5 (12.2%) | 24 (58.5%) | 6 (14.6%) | |
| INF-α yes | 42 (38.5%) | 9 (21.4%) | 7 (16.7%) | 22 (52.4%) | 4 (9.5%) | 0.655 |
| INF-α no | 67 (61.5%) | 11 (16.5%) | 7 (10.4%) | 42 (62.7%) | 7 (10.4%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sicuranza, A.; Breccia, M.; Iuliano, F.; Gugliotta, G.; Castagnetti, F.; Lunghi, M.; Patriarca, A.; Intermesoli, T.; Luciano, L.; Russo Rossi, A.; et al. Therapeutic Vaccinations with p210 Peptides in Imatinib-Treated Chronic Myeloid Leukemia Patients: 10 Years Follow-Up of GIMEMA CML0206 and SI0207 Studies. Vaccines 2025, 13, 419. https://doi.org/10.3390/vaccines13040419
Sicuranza A, Breccia M, Iuliano F, Gugliotta G, Castagnetti F, Lunghi M, Patriarca A, Intermesoli T, Luciano L, Russo Rossi A, et al. Therapeutic Vaccinations with p210 Peptides in Imatinib-Treated Chronic Myeloid Leukemia Patients: 10 Years Follow-Up of GIMEMA CML0206 and SI0207 Studies. Vaccines. 2025; 13(4):419. https://doi.org/10.3390/vaccines13040419
Chicago/Turabian StyleSicuranza, Anna, Massimo Breccia, Francesco Iuliano, Gabriele Gugliotta, Fausto Castagnetti, Monia Lunghi, Andrea Patriarca, Tamara Intermesoli, Luigiana Luciano, Antonella Russo Rossi, and et al. 2025. "Therapeutic Vaccinations with p210 Peptides in Imatinib-Treated Chronic Myeloid Leukemia Patients: 10 Years Follow-Up of GIMEMA CML0206 and SI0207 Studies" Vaccines 13, no. 4: 419. https://doi.org/10.3390/vaccines13040419
APA StyleSicuranza, A., Breccia, M., Iuliano, F., Gugliotta, G., Castagnetti, F., Lunghi, M., Patriarca, A., Intermesoli, T., Luciano, L., Russo Rossi, A., Rege Cambrin, G., Vucinic, V., Malagola, M., Malato, A., Abruzzese, E., D’Adda, M., Galimberti, S., Defina, M., Sammartano, V., ... Bocchia, M. (2025). Therapeutic Vaccinations with p210 Peptides in Imatinib-Treated Chronic Myeloid Leukemia Patients: 10 Years Follow-Up of GIMEMA CML0206 and SI0207 Studies. Vaccines, 13(4), 419. https://doi.org/10.3390/vaccines13040419








