In-Situ Self-Assembling Oligomeric Collagen Scaffold Enhances Vaccine Retention and Vaccine-Induced Humoral Immunity
Abstract
1. Introduction
2. Materials and Methods
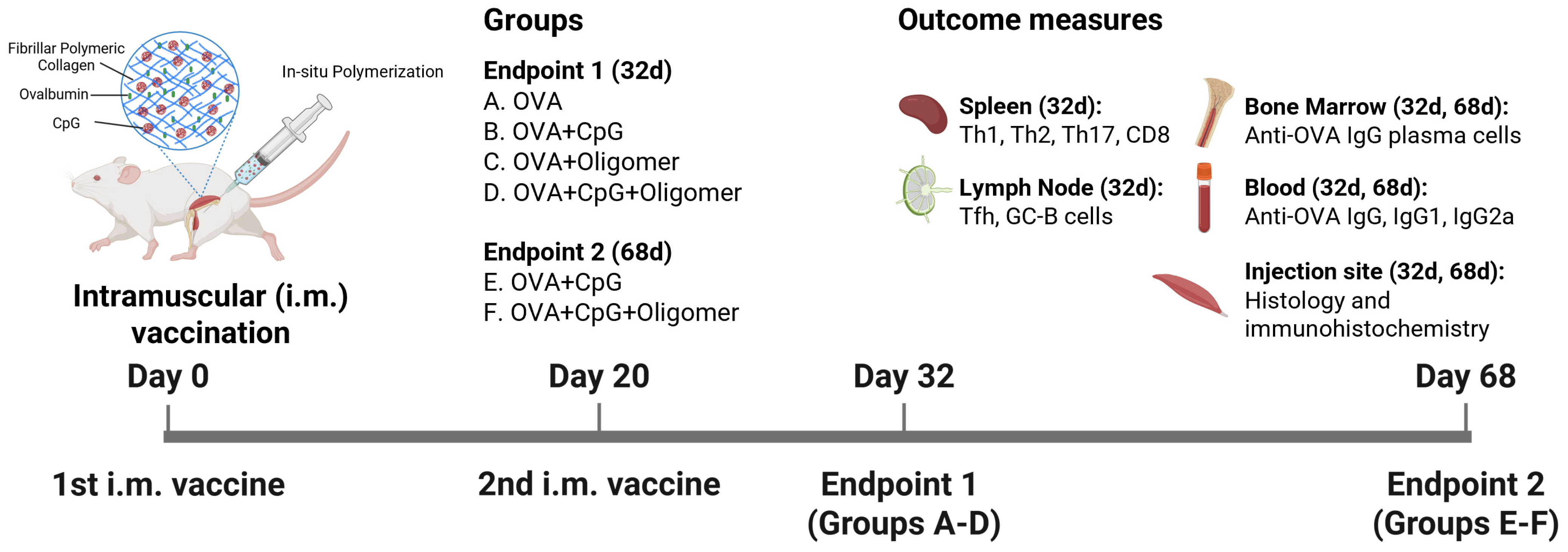
2.1. Animal Studies
2.2. Vaccine Preparations
2.3. Enzyme-Linked Immunosorbent Assay (ELISA)
2.4. Enzyme-Linked Immunospot (ELISpot) Assay
2.5. Flow Cytometry
2.6. Histology, Immunohistochemistry, and AF647-OVA Imaging
2.7. Statistical Analysis
3. Results
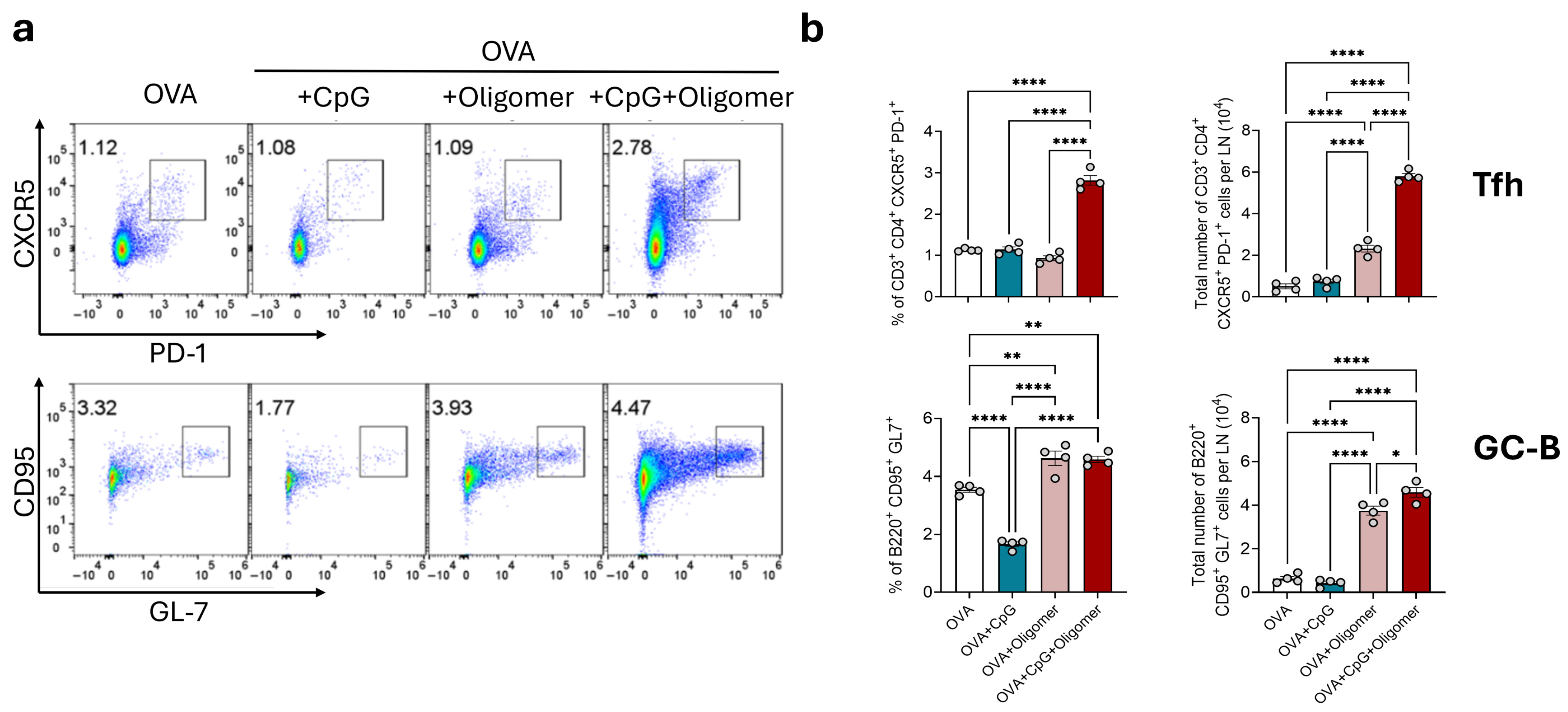
3.1. Oligomer Scaffold with CpG Enhances GC Responses Without Altering Baseline Lymph Node Composition
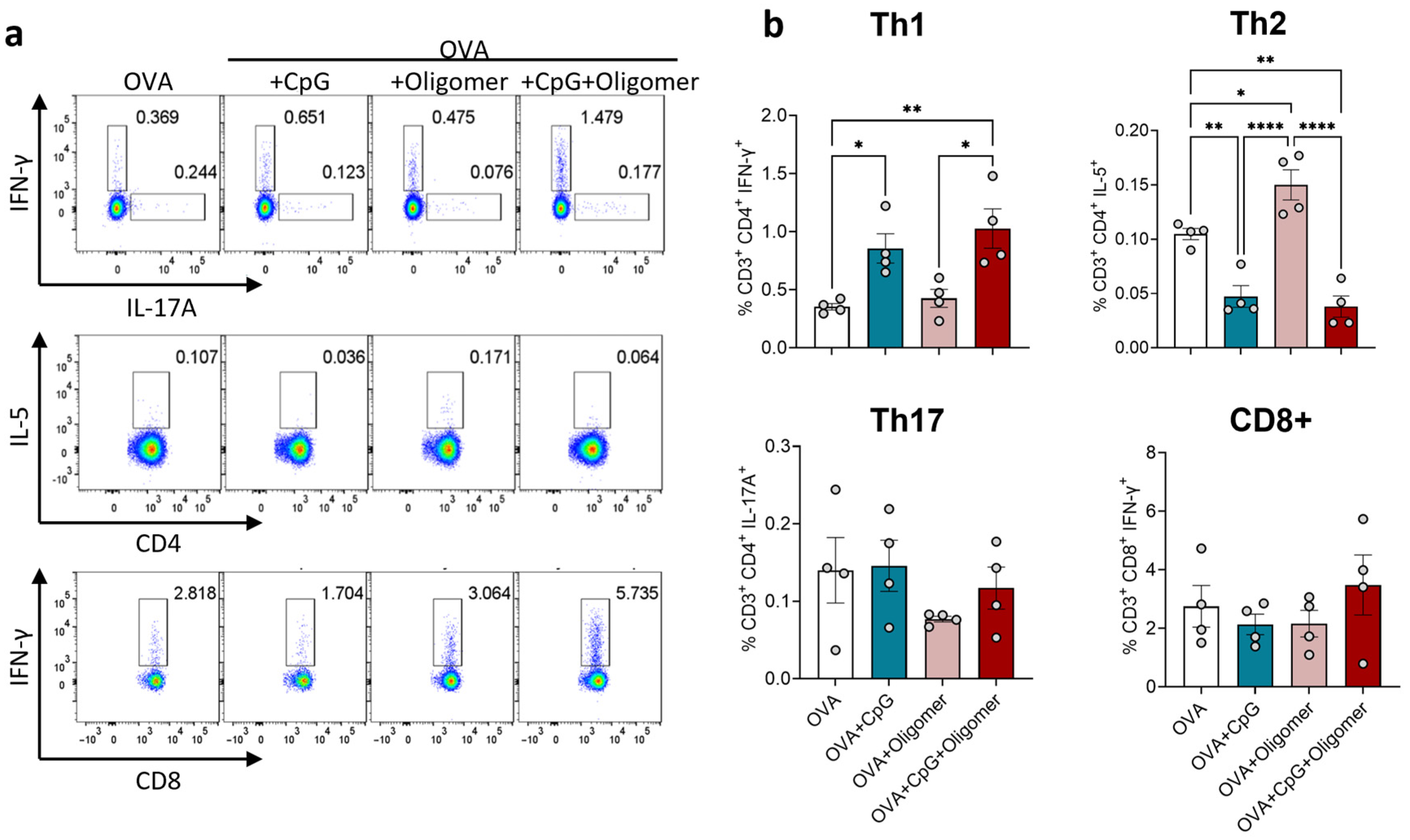
3.2. CpG Drives Th1 Polarization, with Oligomer Scaffold Delivery Maintaining Adjuvant-Induced T-Cell Bias
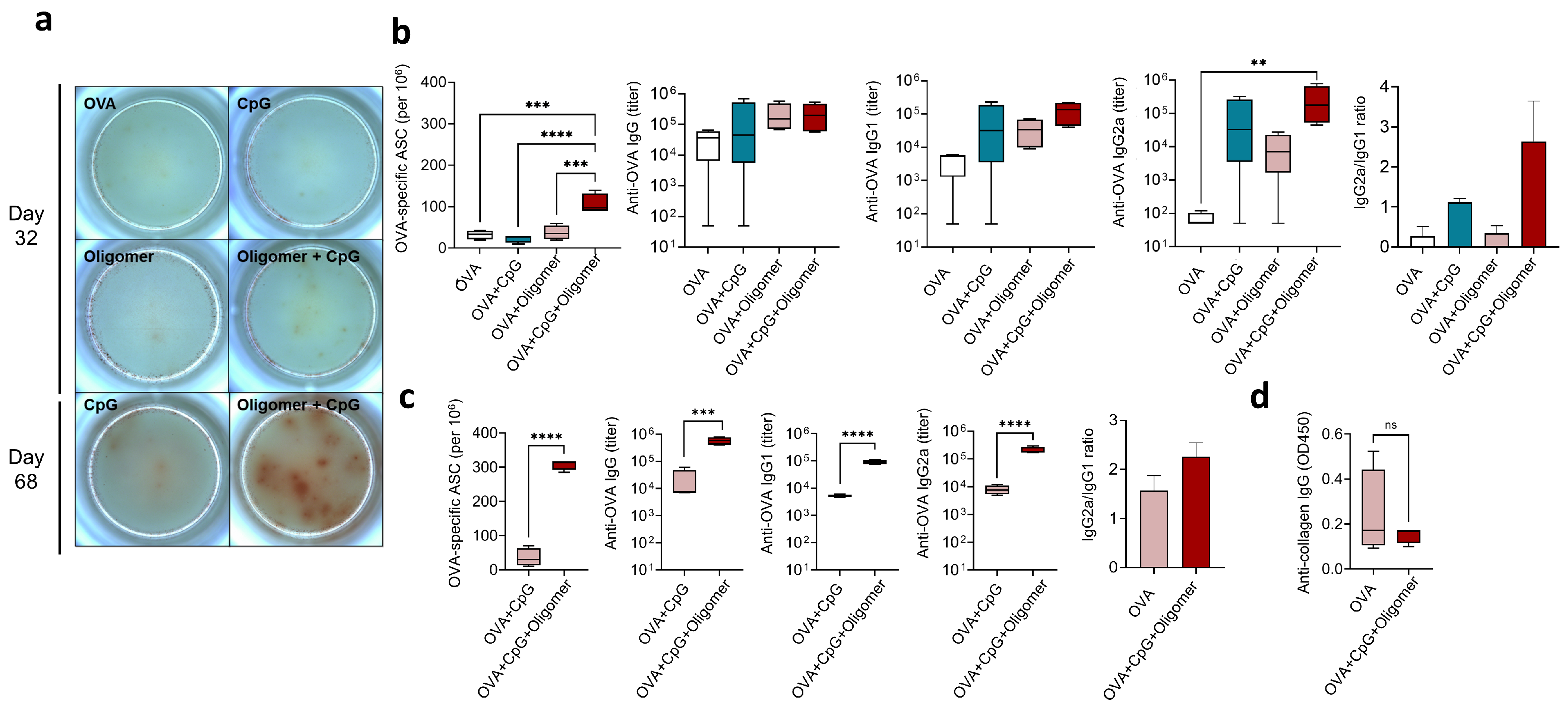
3.3. Oligomer Scaffold with CpG Enhances the Magnitude and Durability of Humoral Immunity Without Inducing Anti-Collagen Antibodies
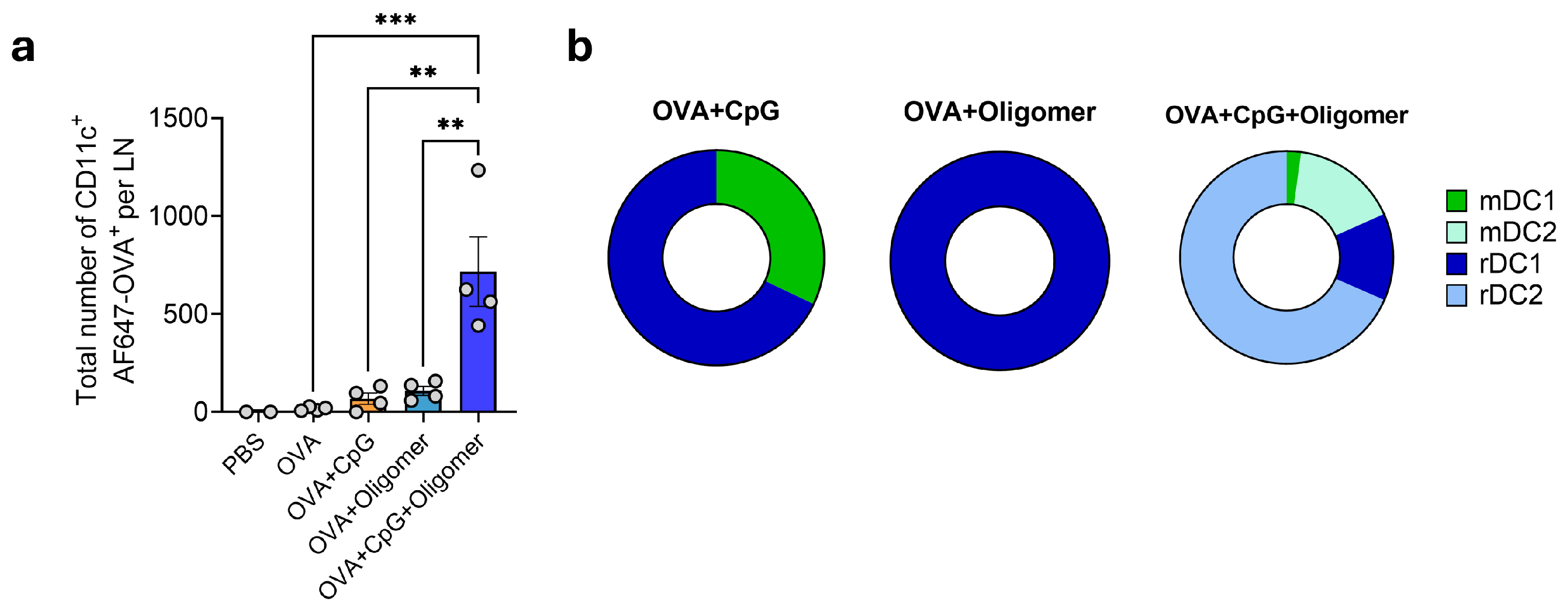
3.4. Oligomer Scaffold with CpG Enhances DC Uptake and Transport of Vaccine Antigen to Draining Lymph Nodes
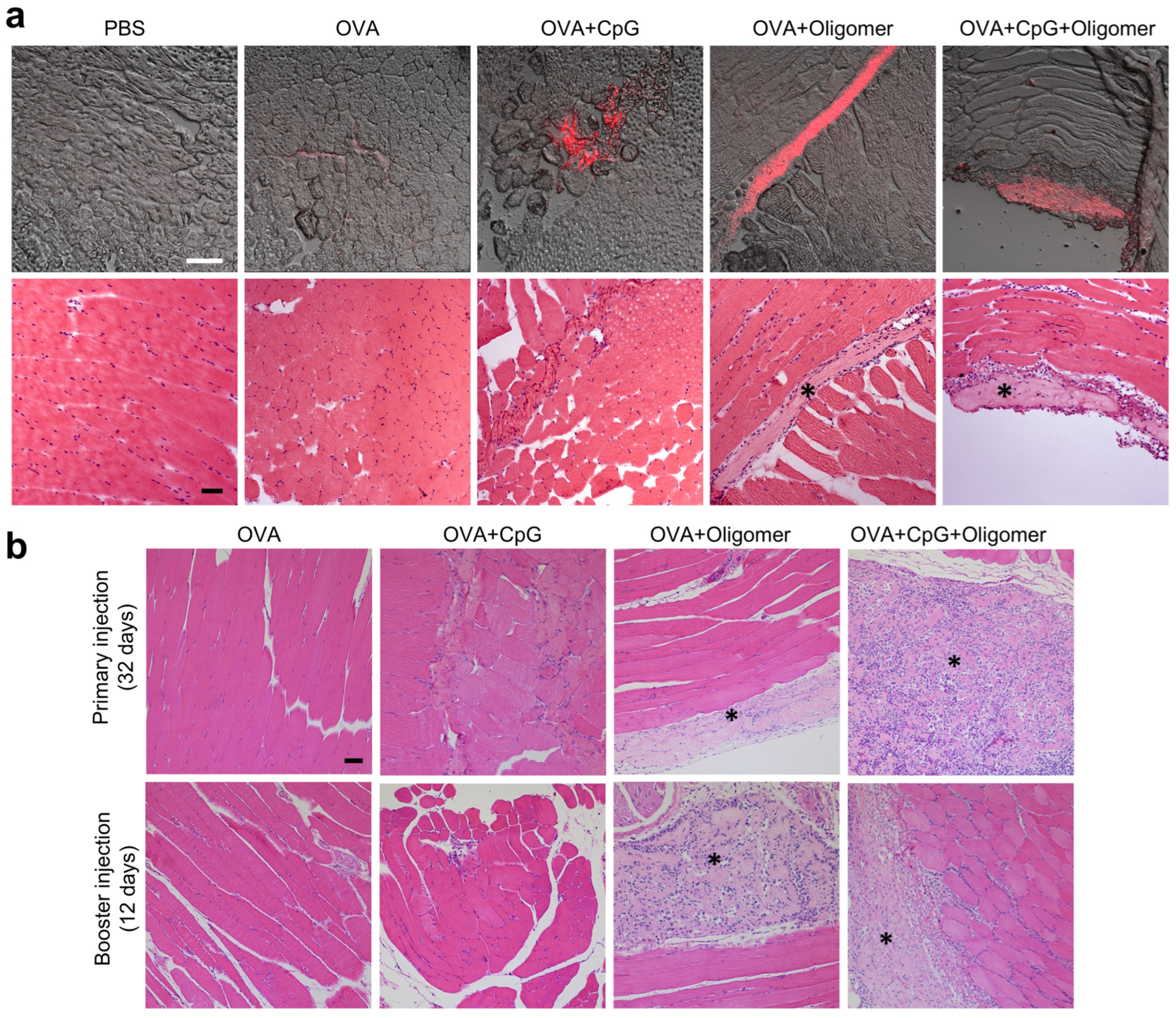
3.5. Oligomer Scaffold Enhances Antigen Retention and Supports Vaccine-Induced Immune Cell Recruitment at the Injection Site
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shattock, A.J.; Johnson, H.C.; Sim, S.Y.; Carter, A.; Lambach, P.; Hutubessy, R.C.W.; Thompson, K.M.; Badizadegan, K.; Lambert, B.; Ferrari, M.J.; et al. Contribution of vaccination to improved survival and health: Modelling 50 years of the Expanded Programme on Immunization. Lancet 2024, 403, 2307–2316. [Google Scholar] [CrossRef]
- Reed, S.G.; Orr, M.T.; Fox, C.B. Key roles of adjuvants in modern vaccines. Nat. Med. 2013, 19, 1597–1608. [Google Scholar] [CrossRef]
- HogenEsch, H.; Orr, M.T.; Fox, C.B. Vaccine adjuvants: Mechanisms of action. In Vaccine Development: From Concept to Clinic; Prasad, A.K., Ed.; The Royal Society of Chemistry: London, UK, 2023; pp. 214–234. [Google Scholar]
- Lavelle, E.C.; McEntee, C.P. Vaccine adjuvants: Tailoring innate recognition to send the right message. Immunity 2024, 57, 772–789. [Google Scholar] [CrossRef]
- Kasturi, S.P.; Skountzou, I.; Albrecht, R.A.; Koutsonanos, D.; Hua, T.; Nakaya, H.I.; Ravindran, R.; Stewart, S.; Alam, M.; Kwissa, M.; et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature 2011, 470, 543–547. [Google Scholar] [CrossRef]
- Ilyinskii, P.O.; Roy, C.J.; O’Neil, C.P.; Browning, E.A.; Pittet, L.A.; Altreuter, D.H.; Alexis, F.; Tonti, E.; Shi, J.; Basto, P.A.; et al. Adjuvant-carrying synthetic vaccine particles augment the immune response to encapsulated antigen and exhibit strong local immune activation without inducing systemic cytokine release. Vaccine 2014, 32, 2882–2895. [Google Scholar] [CrossRef]
- Lynn, G.M.; Laga, R.; Darrah, P.A.; Ishizuka, A.S.; Balaci, A.J.; Dulcey, A.E.; Pechar, M.; Pola, R.; Gerner, M.Y.; Yamamoto, A.; et al. In vivo characterization of the physicochemical properties of polymer-linked TLR agonists that enhance vaccine immunogenicity. Nat. Biotechnol. 2015, 33, 1201–1210. [Google Scholar] [CrossRef]
- Lu, F.; Mosley, Y.C.; Carmichael, B.; Brown, D.D.; HogenEsch, H. Formulation of aluminum hydroxide adjuvant with TLR agonists poly(I:C) and CpG enhances the magnitude and avidity of the humoral immune response. Vaccine 2019, 37, 1945–1953. [Google Scholar] [CrossRef]
- Ise, W.; Kurosaki, T. Plasma cell differentiation during the germinal center reaction. Immunol. Rev. 2019, 288, 64–74. [Google Scholar] [CrossRef]
- Cyster, J.G.; Allen, C.D.C. B Cell responses: Cell interaction dynamics and decisions. Cell 2019, 177, 524–540. [Google Scholar] [CrossRef]
- Cirelli, K.M.; Crotty, S. Germinal center enhancement by extended antigen availability. Curr. Opin. Immunol. 2017, 47, 64–69. [Google Scholar] [CrossRef]
- Wu, T.Y.; Singh, M.; Miller, A.T.; De Gregorio, E.; Doro, F.; D’Oro, U.; Skibinski, D.A.; Mbow, M.L.; Bufali, S.; Herman, A.E.; et al. Rational design of small molecules as vaccine adjuvants. Sci. Transl. Med. 2014, 6, 263ra160. [Google Scholar] [CrossRef]
- Tam, H.H.; Melo, M.B.; Kang, M.; Pelet, J.M.; Ruda, V.M.; Foley, M.H.; Hu, J.K.; Kumari, S.; Crampton, J.; Baldeon, A.D.; et al. Sustained antigen availability during germinal center initiation enhances antibody responses to vaccination. Proc. Natl. Acad. Sci. USA 2016, 113, E6639–E6648. [Google Scholar] [CrossRef]
- Cirelli, K.M.; Carnathan, D.G.; Nogal, B.; Martin, J.T.; Rodriguez, O.L.; Upadhyay, A.A.; Enemuo, C.A.; Gebru, E.H.; Choe, Y.; Viviano, F.; et al. Slow delivery immunization enhances HIV neutralizing antibody and germinal center responses via modulation of immunodominance. Cell 2019, 177, 1153–1171.e28. [Google Scholar] [CrossRef]
- Roth, G.A.; Picece, V.; Ou, B.S.; Luo, W.; Pulendran, B.; Appel, E.A. Designing spatial and temporal control of vaccine responses. Nat. Rev. Mater. 2022, 7, 174–195. [Google Scholar] [CrossRef]
- Irvine, D.J.; Aung, A.; Silva, M. Controlling timing and location in vaccines. Adv. Drug Deliv. Rev. 2020, 158, 91–115. [Google Scholar] [CrossRef]
- Lu, F.; Boutselis, I.; Borch, R.F.; HogenEsch, H. Control of antigen-binding to aluminum adjuvants and the immune response with a novel phosphonate linker. Vaccine 2013, 31, 4362–4367. [Google Scholar] [CrossRef]
- Moyer, T.J.; Kato, Y.; Abraham, W.; Chang, J.Y.H.; Kulp, D.W.; Watson, N.; Turner, H.L.; Menis, S.; Abbott, R.K.; Bhiman, J.N.; et al. Engineered immunogen binding to alum adjuvant enhances humoral immunity. Nat. Med. 2020, 26, 430–440. [Google Scholar] [CrossRef]
- Gregory, A.E.; Titball, R.; Williamson, D. Vaccine delivery using nanoparticles. Front. Cell. Infect. Microbiol. 2013, 3, 13. [Google Scholar] [CrossRef]
- Luzuriaga, M.A.; Shahrivarkevishahi, A.; Herbert, F.C.; Wijesundara, Y.H.; Gassensmith, J.J. Biomaterials and nanomaterials for sustained release vaccine delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1735. [Google Scholar] [CrossRef]
- Roth, G.A.; Gale, E.C.; Alcantara-Hernandez, M.; Luo, W.; Axpe, E.; Verma, R.; Yin, Q.; Yu, A.C.; Lopez Hernandez, H.; Maikawa, C.L.; et al. Injectable hydrogels for sustained codelivery of subunit vaccines enhance humoral immunity. ACS Cent. Sci. 2020, 6, 1800–1812. [Google Scholar] [CrossRef]
- Ou, B.S.; Saouaf, O.M.; Yan, J.; Bruun, T.U.J.; Baillet, J.; Zhou, X.; King, N.P.; Appel, E.A. Broad and durable humoral responses following single hydrogel immunization of SARS-CoV-2 subunit vaccine. Adv. Healthc. Mater 2023, 12, e2301495. [Google Scholar] [CrossRef]
- Rodgers, A.M.; Courtenay, A.J.; Donnelly, R.F. Dissolving microneedles for intradermal vaccination: Manufacture, formulation, and stakeholder considerations. Expert Opin. Drug Deliv. 2018, 15, 1039–1043. [Google Scholar] [CrossRef]
- Frey, S.; Castro, A.; Arsiwala, A.; Kane, R.S. Bionanotechnology for vaccine design. Curr. Opin. Biotechnol. 2018, 52, 80–88. [Google Scholar] [CrossRef]
- Kreger, S.T.; Bell, B.J.; Bailey, J.; Stites, E.; Kuske, J.; Waisner, B.; Voytik-Harbin, S.L. Polymerization and matrix physical properties as important design considerations for soluble collagen formulations. Biopolymers 2010, 93, 690–707. [Google Scholar]
- Puls, T.J.; Fisher, C.S.; Cox, A.; Plantenga, J.M.; McBride, E.L.; Anderson, J.L.; Goergen, C.J.; Bible, M.; Moller, T.; Voytik-Harbin, S.L. Regenerative tissue filler for breast conserving surgery and other soft tissue restoration and reconstruction needs. Sci. Rep. 2021, 11, 2711. [Google Scholar]
- Blum, K.M.; Novak, T.; Watkins, L.; Neu, C.P.; Wallace, J.M.; Bart, Z.R.; Voytik-Harbin, S.L. Acellular and cellular high-density, collagen-fibril constructs with suprafibrillar organization. Biomater. Sci. 2016, 4, 711–723. [Google Scholar] [CrossRef]
- Sohutskay, D.O.; Buganza Tepole, A.; Voytik-Harbin, S.L. Mechanobiological wound model for improved design and evaluation of collagen dermal replacement scaffolds. Acta Biomater. 2021, 135, 368–382. [Google Scholar]
- Stephens, C.H.; Morrison, R.A.; McLaughlin, M.; Orr, K.; Tersey, S.A.; Scott-Moncrieff, J.C.; Mirmira, R.G.; Considine, R.V.; Voytik-Harbin, S. Oligomeric collagen as an encapsulation material for islet/beta-cell replacement: Effect of islet source, dose, implant site, and administration format. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E388–E400. [Google Scholar] [CrossRef]
- Sohutskay, D.O.; Buno, K.P.; Tholpady, S.S.; Nier, S.J.; Voytik-Harbin, S.L. Design and biofabrication of dermal regeneration scaffolds: Role of oligomeric collagen fibril density and architecture. Regen. Med. 2020, 15, 1295–1312. [Google Scholar] [CrossRef]
- Morrison, R.A.; Brookes, S.; Puls, T.J.; Cox, A.; Gao, H.; Liu, Y.; Voytik-Harbin, S.L. Engineered collagen polymeric materials create noninflammatory regenerative microenvironments that avoid classical foreign body responses. Biomater. Sci. 2023, 11, 3278–3296. [Google Scholar] [CrossRef]
- Hernandez-Franco, J.F.; Mosley, Y.C.; Franco, J.; Ragland, D.; Yao, Y.; HogenEsch, H. Effective and safe stimulation of humoral and cell-mediated immunity by intradermal immunization with a cyclic dinucleotide/nanoparticle combination adjuvant. J. Immunol. 2021, 206, 700–711. [Google Scholar] [CrossRef]
- Bode, C.; Zhao, G.; Steinhagen, F.; Kinjo, T.; Klinman, D.M. CpG DNA as a vaccine adjuvant. Expert Rev. Vaccines 2011, 10, 499–511. [Google Scholar] [CrossRef]
- Chu, R.S.; Targoni, O.S.; Krieg, A.M.; Lehmann, P.V.; Harding, C.V. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J. Exp. Med. 1997, 186, 1623–1631. [Google Scholar] [CrossRef]
- Stevens, T.L.; Bossie, A.; Sanders, V.M.; Fernandez-Botran, R.; Coffman, R.L.; Mosmann, T.R.; Vitetta, E.S. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature 1988, 334, 255–258. [Google Scholar] [CrossRef]
- Huang, S.; Hendriks, W.; Althage, A.; Hemmi, S.; Bluethmann, H.; Kamijo, R.; Vilcek, J.; Zinkernagel, R.M.; Aguet, M. Immune response in mice that lack the interferon-gamma receptor. Science 1993, 259, 1742–1745. [Google Scholar] [CrossRef]
- Tidball, J.G. Regulation of muscle growth and regeneration by the immune system. Nat. Rev. Immunol. 2017, 17, 165–178. [Google Scholar] [CrossRef]
- Wang, X.; Sathe, A.A.; Smith, G.R.; Ruf-Zamojski, F.; Nair, V.; Lavine, K.J.; Xing, C.; Sealfon, S.C.; Zhou, L. Heterogeneous origins and functions of mouse skeletal muscle-resident macrophages. Proc. Natl. Acad. Sci. USA 2020, 117, 20729–20740. [Google Scholar] [CrossRef]
- Bachmann, M.F.; Jennings, G.T. Vaccine delivery: A matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 2010, 10, 787–796. [Google Scholar] [CrossRef]
- Sadeghi, I.; Byrne, J.; Shakur, R.; Langer, R. Engineered drug delivery devices to address Global Health challenges. J. Control. Release 2021, 331, 503–514. [Google Scholar] [CrossRef]
- Silva, A.L.; Soema, P.C.; Slutter, B.; Ossendorp, F.; Jiskoot, W. PLGA particulate delivery systems for subunit vaccines: Linking particle properties to immunogenicity. Hum. Vaccines Immunother. 2016, 12, 1056–1069. [Google Scholar] [CrossRef]
- Wang, Q.; Tan, M.T.; Keegan, B.P.; Barry, M.A.; Heffernan, M.J. Time course study of the antigen-specific immune response to a PLGA microparticle vaccine formulation. Biomaterials 2014, 35, 8385–8393. [Google Scholar] [CrossRef]
- Joshi, V.B.; Geary, S.M.; Salem, A.K. Biodegradable particles as vaccine antigen delivery systems for stimulating cellular immune responses. Hum. Vaccines Immunother. 2013, 9, 2584–2590. [Google Scholar] [CrossRef]
- Kim, J.; Li, W.A.; Choi, Y.; Lewin, S.A.; Verbeke, C.S.; Dranoff, G.; Mooney, D.J. Injectable, spontaneously assembling, inorganic scaffolds modulate immune cells in vivo and increase vaccine efficacy. Nat. Biotechnol. 2015, 33, 64–72. [Google Scholar] [CrossRef]
- Crawford, L.; Wyatt, M.; Bryers, J.; Ratner, B. Biocompatibility evolves: Phenomenology to toxicology to regeneration. Adv. Healthc. Mater. 2021, 10, e2002153. [Google Scholar] [CrossRef]
- Aachoui, Y.; Ghosh, S.K. Extracellular matrix from porcine small intestinal submucosa (SIS) as immune adjuvants. PLoS ONE 2011, 6, e27083. [Google Scholar] [CrossRef]
- Pal, S.; Chaudhari, R.; Baurceanu, I.; Hill, B.J.; Nagy, B.A.; Wolf, M.T. Extracellular matrix scaffold-assisted tumor vaccines induce tumor regression and long-term immune memory. Adv. Mater. 2024, 36, e2309843. [Google Scholar] [CrossRef]
- Lofthouse, S.; Nagahara, S.; Sedgmen, B.; Barcham, G.; Brandon, M.; Sano, A. The application of biodegradable collagen minipellets as vaccine delivery vehicles in mice and sheep. Vaccine 2001, 19, 4318–4327. [Google Scholar] [CrossRef]
| Feature | Benefit | Supporting Evidence |
|---|---|---|
| Regulatory clearance and safety | Demonstrates biocompatibility, safety, and translation readiness, with FDA clearance as a wound management device (510(k), product code KGN). | 510(k) Number: K250329 |
| Injectability | Supports minimally invasive delivery through small-gauge needles, enabled by in-situ polymerization. | [29]; current study |
| Tissue integration and homeostatic remodeling | Integrates with native tissue and undergoes homeostatic remodeling consistent with natural collagen turnover, without chronic inflammation, and fibrotic encapsulation. | [26,29,30]; current study |
| Immunologically neutral | Maintains local tissue health without triggering material-induced immune responses, supporting tunability of vaccine formulation for desired outcomes. Preserves self-tolerance to native collagen, with no autoimmunity detected following vaccination. | [29,31]; current study |
| Tunable properties with amenability to computational modeling | Enables tailoring of material properties, including fibrillar density and scaffold volume, to modulate depot effect and antigen/adjuvant bioavailability. | [28,29,30] |
| Formulation flexibility | Accommodates a broad range of antigens, adjuvants, and living cells, enabling diverse vaccine applications under physiological conditions. | [27,29,30]; current study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez-Franco, J.F.; Gude, S.; Morrison, R.A.; Castillo Perez, D.; Voytik-Harbin, S.L.; HogenEsch, H. In-Situ Self-Assembling Oligomeric Collagen Scaffold Enhances Vaccine Retention and Vaccine-Induced Humoral Immunity. Vaccines 2025, 13, 1146. https://doi.org/10.3390/vaccines13111146
Hernandez-Franco JF, Gude S, Morrison RA, Castillo Perez D, Voytik-Harbin SL, HogenEsch H. In-Situ Self-Assembling Oligomeric Collagen Scaffold Enhances Vaccine Retention and Vaccine-Induced Humoral Immunity. Vaccines. 2025; 13(11):1146. https://doi.org/10.3390/vaccines13111146
Chicago/Turabian StyleHernandez-Franco, Juan F., Sushma Gude, Rachel A. Morrison, Daniela Castillo Perez, Sherry L. Voytik-Harbin, and Harm HogenEsch. 2025. "In-Situ Self-Assembling Oligomeric Collagen Scaffold Enhances Vaccine Retention and Vaccine-Induced Humoral Immunity" Vaccines 13, no. 11: 1146. https://doi.org/10.3390/vaccines13111146
APA StyleHernandez-Franco, J. F., Gude, S., Morrison, R. A., Castillo Perez, D., Voytik-Harbin, S. L., & HogenEsch, H. (2025). In-Situ Self-Assembling Oligomeric Collagen Scaffold Enhances Vaccine Retention and Vaccine-Induced Humoral Immunity. Vaccines, 13(11), 1146. https://doi.org/10.3390/vaccines13111146






