Safety Profile of the 4CMenB (Bexsero®) Vaccine: A Systematic Review and Meta-Analysis of Adverse Events in Clinical Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Intervention
2.4. Comparators
2.5. Outcomes of Interest
2.6. Eligibility Criteria
2.7. Information Sources and Search Strategy
2.8. Study Selection
2.9. Data Extraction
2.10. Risk of Bias Assessment
2.11. Data Synthesis and Statistical Analysis
3. Results
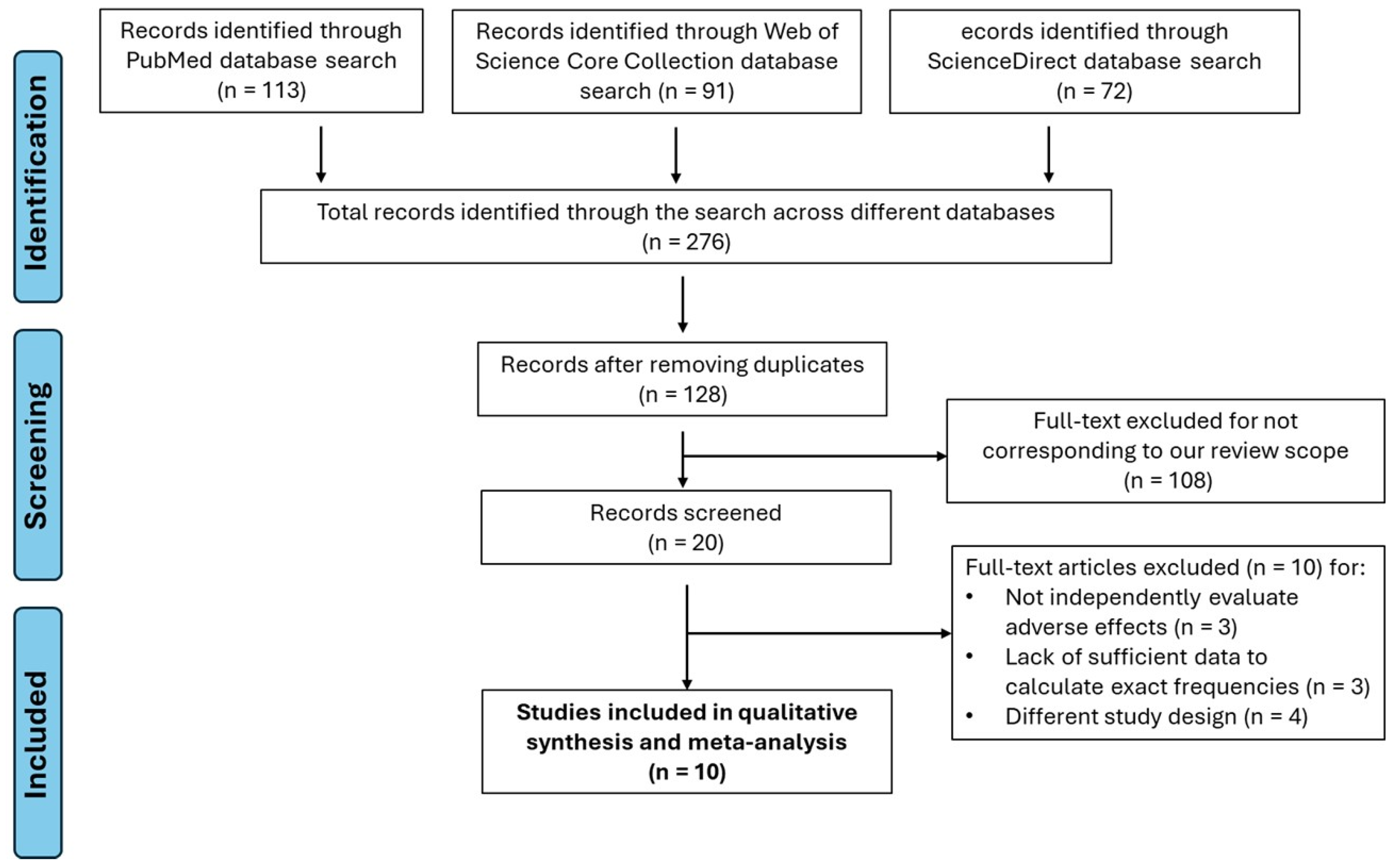
3.1. Study Selection
- Publication Bias
3.2. General Profile of Adverse Events
3.3. Local Adverse Events
3.4. Systemic Adverse Events
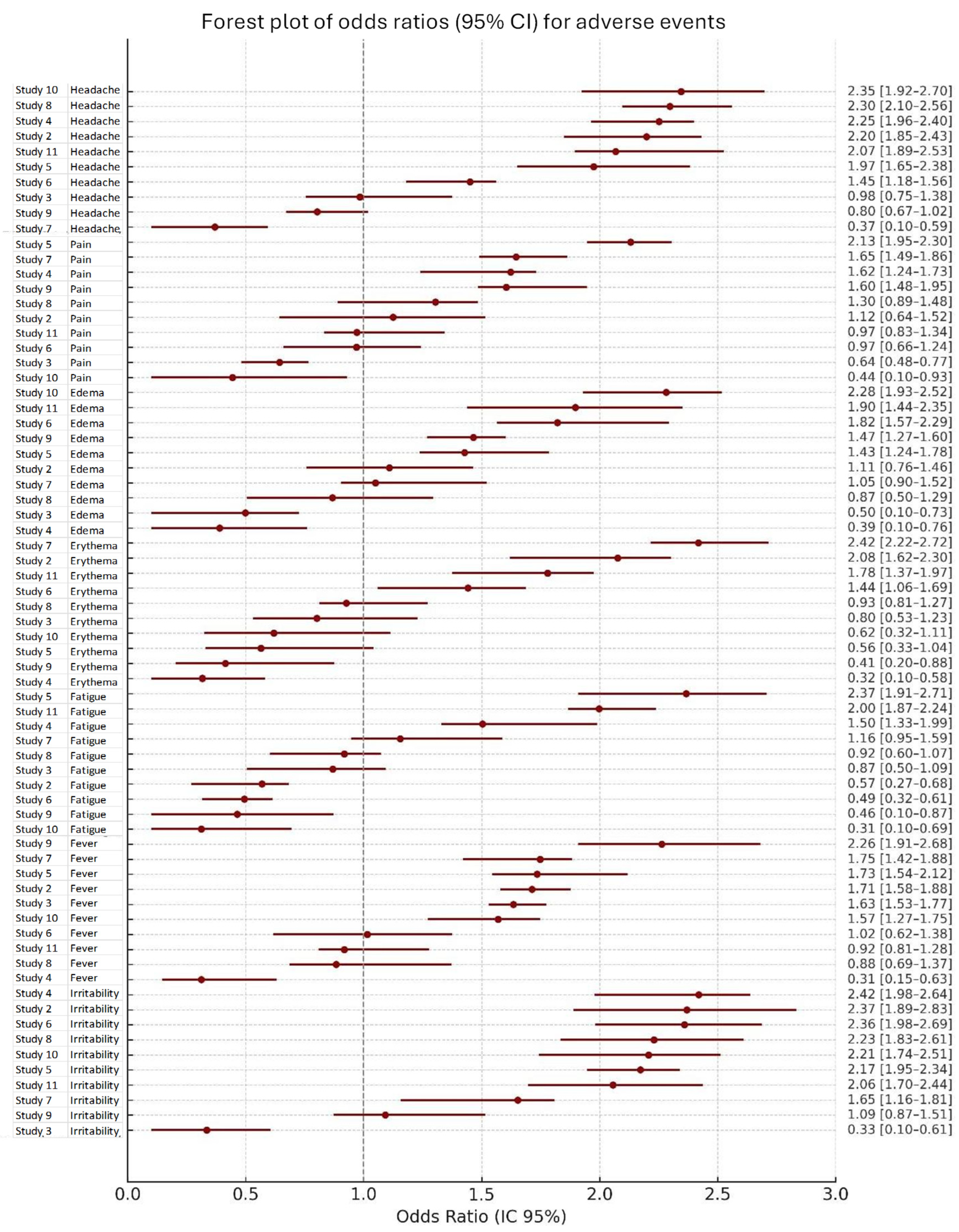
3.5. Heterogeneity and Statistical Model
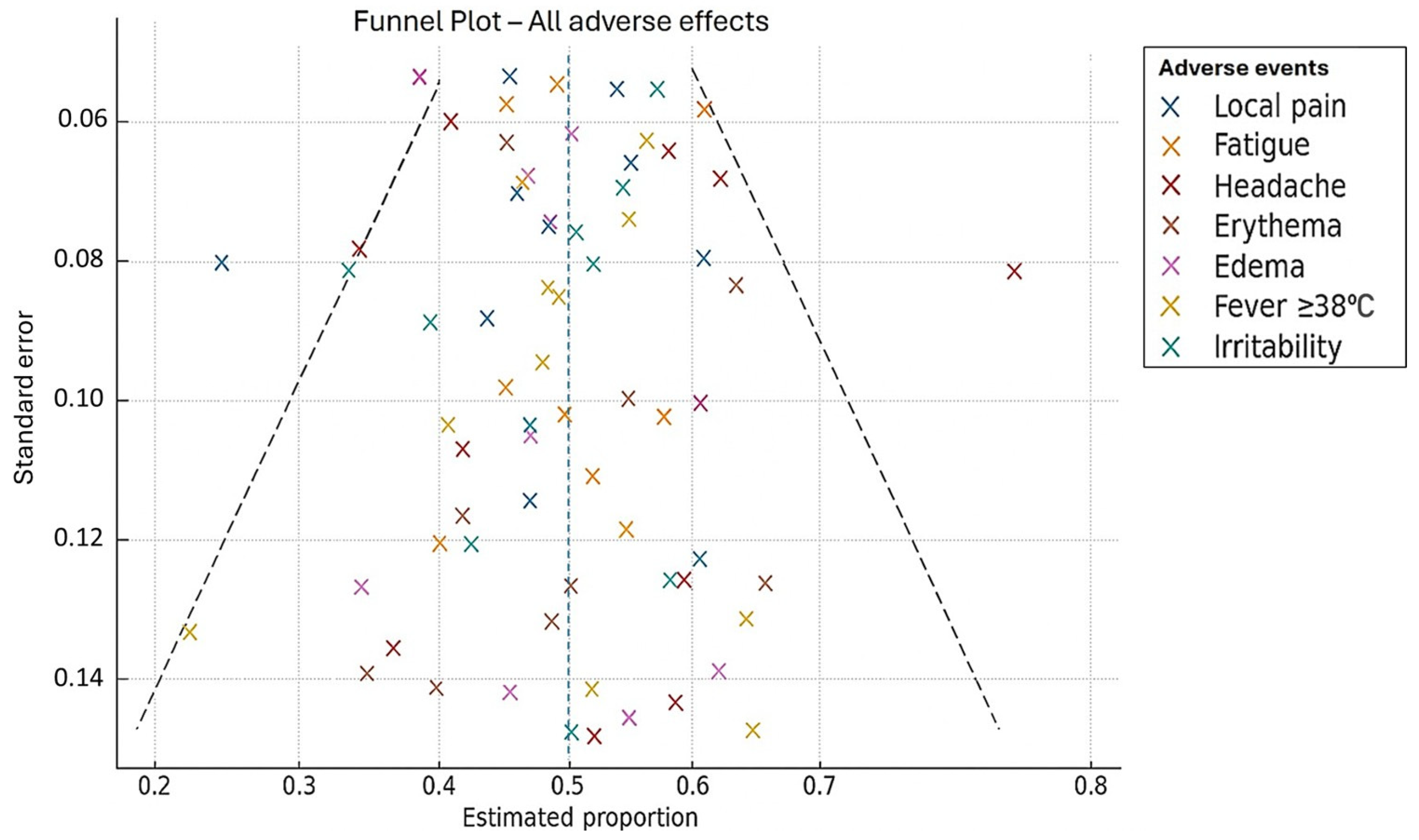
3.6. Publication Bias
4. Discussion
- Strengths and Limitations
- Implications for Public Health
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACWY | Meningococcal serogroups A, C, W, and Y |
| APC | Article Processing Charge |
| CDC | Centers for Disease Control and Prevention |
| CI | Confidence Interval |
| CT | Clinical Trial |
| EMA | European Medicines Agency |
| IMD | Invasive Meningococcal Disease |
| I2 I | I-squared statistics (measure of heterogeneity in meta-analyses) |
| MD | Meningococcal Disease |
| n | Number of participants with the event |
| N | Total number of participants evaluated |
| NR | Not Reported |
| OMV | Outer Membrane Vesicle |
| OR | Odds Ratio |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RCT | Randomized Controlled Trial |
| RoB 2 | Risk of Bias tool version 2 |
| SD | Standard Deviation |
References
- Shen, S.; Findlow, J.; Peyrani, P. Global Epidemiology of Meningococcal Disease-Causing Serogroups Before and After the COVID-19 Pandemic: A Narrative Review. Infect. Dis. Ther. 2024, 13, 2489–2507. [Google Scholar] [CrossRef] [PubMed]
- Olbrich, K.J.; Müller, D.; Schumacher, S.; Beck, E.; Meszaros, K.; Koerber, F. Systematic Review of Invasive Meningococcal Disease: Sequelae and Quality of Life Impact on Patients and Their Caregivers. Infect. Dis. Ther. 2018, 7, 421–438. [Google Scholar] [CrossRef]
- Duval, X.; Taha, M.K.; Lamaury, I.; Escaut, L.; Gueit, I.; Manchon, P.; Tubiana, S.; Hoen, B.; COMBAT Study Group. One-Year Sequelae and Quality of Life in Adults with Meningococcal Meningitis: Lessons from the COMBAT Multicentre Prospective Study. Adv. Ther. 2022, 39, 3031–3041. [Google Scholar] [CrossRef]
- Parra, M.M.; Gentile, A.; Narvaez, J.A.V.; Capdevila, A.; Minguez, A.; Carrascal, M.; Willemsen, A.; Bhusal, C.; Toneatto, D. Immunogenicity and safety of the 4CMenB and MenACWY-CRM meningococcal vaccines administered concomitantly in infants: A phase 3b, randomized controlled trial. Vaccine 2018, 36, 7609–7617. [Google Scholar] [CrossRef]
- Guedes, S.; Bertrand-Gerentes, I.; Evans, K.; Coste, F.; Oster, P. Invasive meningococcal disease in older adults in North America and Europe: Is this the time for action? A review of the literature. BMC Public Health 2022, 22, 380. [Google Scholar] [CrossRef]
- Villena, R.; Safadi, M.A.P.; Valenzuela, M.T.; Torres, J.P.; Finn, A.; O’Ryan, M. Global epidemiology of serogroup B meningococcal disease and opportunities for prevention with novel recombinant protein vaccines. Hum. Vaccin. Immunother. 2018, 14, 1042–1057. [Google Scholar] [CrossRef] [PubMed]
- Purmohamad, A.; Abasi, E.; Azimi, T.; Hosseini, S.; Safari, H.; Nasiri, M.J.; Imani Fooladi, A.A. Global estimate of Neisseria meningitidis serogroups proportion in invasive meningococcal disease: A systematic review and meta-analysis. Microb Pathog. 2019, 134, 103571. [Google Scholar] [CrossRef] [PubMed]
- Caugant, D.A.; Maiden, M.C.J. Meningococcal carriage and disease—Population biology and evolution. Vaccine 2009, 27 (Suppl. S2), B64–B70. [Google Scholar] [CrossRef]
- Presa, J.; Carrico, R.; Fergie, J.E.; Hanenberg, S.; Marshall, G.S.; Rivard, K.; Shaw, J.; Zimet, G.D.; Peyrani, P.; Cane, A. Preventing Meningococcal Disease in US Adolescents and Young Adults Through Vaccination. Infect. Dis. Ther. 2025, 14, 1381–1403. [Google Scholar] [CrossRef]
- Ministerio de Sanidad, Consumo y Bienestar Social. Vacunación Frente a la Meningitis: Preguntas y Respuestas [Internet]. Madrid: MSCBS. 2019. Available online: https://www.sanidad.gob.es/ (accessed on 8 March 2025).
- Ruiz-Montero, R.; Epstein, D.; Guzmán Herrador, B.; Espín Balbino, J. Evaluación económica de la inclusión de 4CMenB en el calendario vacunal en España. Gac. Sanit. 2020, 34, 318–325. [Google Scholar] [CrossRef]
- Castilla, J.; García Cenoz, M.; Abad, R.; Sánchez-Cambronero, L.; Lorusso, N.; Izquierdo, C.; Cañellas Llabrés, S.; Roig, J.; Malvar, A.; González Carril, F.; et al. Effectiveness of a meningococcal group B vaccine (4CMenB) in children. N. Engl. J. Med. 2023, 388, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Junta de Andalucía. Instrucción de Vacunación del Meningococo en Andalucía. Sevilla: Dirección Gral. Salud Pública. 2023. Available online: https://www.andavac.es/ (accessed on 8 March 2025).
- Asociación Española de Vacunología. Seguridad de la Vacuna Frente al Meningococo. Available online: http://www.vacunas.org/ (accessed on 8 March 2025).
- Rollier, C.S.; Dold, C.; Marsay, L.; Sadarangani, M.; Pollard, A.J. The capsular group B meningococcal vaccine, 4CMenB: Clinical experience and potential efficacy. Expert. Opin. Biol. Ther. 2015, 15, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Abitbol, V.; Sohn, W.Y.; Horn, M.; Safadi, M.A.P. Safety and immunogenicity of co-administered meningococcal serogroup B (4CMenB) vaccine: A literature review. Hum. Vaccin. Immunother. 2023, 19, 2245705. [Google Scholar] [CrossRef]
- Gossger, N.; Snape, M.D.; Yu, L.-M.; Finn, A.; Bona, G.; Esposito, S.; Principi, N.; Diez-Domingo, J.; Sokal, E.; Becker, B.; et al. Immunogenicity and tolerability of recombinant serogroup B meningococcal vaccine administered with routine infant vaccinations in the United Kingdom: A phase 2b randomized controlled trial. JAMA 2012, 307, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Vesikari, T.; Brzostek, J.; Ahonen, A.; Paassilta, M.; Majda-Stanislawska, E.; Szenborn, L.; Virta, M.; Clifford, R.; Jackowska, T.; Kimmel, M.; et al. Immunogenicity and safety of different schedules of the meningococcal ABCWY vaccine, with assessment of long-term antibody persistence and booster responses—Results from two phase 2b randomized trials in adolescents. Hum. Vaccin. Immunother. 2021, 17, 4689–4700. [Google Scholar] [CrossRef]
- O’Connor, D.; Pinto, M.V.; Sheerin, D.; Tomic, A.; Drury, R.E.; Channon-Wells, S.; Galal, U.; Dold, C.; Robinson, H.; Kerridge, S.; et al. Infant immune responses following 4CMenB vaccination: A systems biology analysis. Mol. Syst. Biol. 2020, 16, e9888. [Google Scholar] [CrossRef]
- Chiu, N.C.; Huang, L.M.; Willemsen, A.; Bhusal, C.; Arora, A.K.; Reynoso Mojares, Z.; Toneatto, D. Safety and immunogenicity of a meningococcal B recombinant vaccine when administered with routine vaccines to healthy infants in Taiwan: A phase 3, open-label, randomized study. Hum. Vaccin. Immunother. 2018, 14, 1075–1083. [Google Scholar] [CrossRef]
- Martinon-Torres, F.; Lamberth, E.; Natalini Martinez, S.; Salamanca de la Cueva, I.; Zolotas, L.; Oladipupo, I.; Maguire, J.D.; Trammel, J.; O’Neill, R.; Liberator, P.A.; et al. Safety, tolerability, and immunogenicity of pentavalent meningococcal MenABCWY vaccine in healthy infants: A phase 2b randomized clinical trial. Hum. Vaccin. Immunother. 2025, 21, 2463194. [Google Scholar] [CrossRef]
- Martinón-Torres, F.; Martínez, A.C.; Simkó, R.; Márquez, P.I.; Arimany, J.L.; Giménez-Sánchez, F.; Gianzo, J.A.C.; Kovács, É.; Rojo, P.; Wang, H.; et al. Antibody persistence and booster responses 24–36 months after different 4CMenB vaccination schedules in infants and children: A randomized trial. J. Infect. 2018, 76, 258–269. [Google Scholar] [CrossRef]
- Martinón-Torres, F.; Safadi, M.A.P.; Martinez, A.C.; Marquez, P.I.; Torres, J.C.T.; Weckx, L.Y.; Junior, E.D.M.; Mensi, I.; Calabresi, M.; Toneatto, D. Reduced schedules of 4CMenB vaccine in infants and catch-up series in children: Immunogenicity and safety results from a phase 3b randomized trial. Vaccine 2017, 35, 3548–3557. [Google Scholar] [CrossRef]
- Safadi, M.A.P.; Martinon-Torres, F.; Weckx, L.Y.; Junior, E.D.M.; da Fonseca Lima, E.J.; Mensi, I.; Calabresi, M.; Toneatto, D. Immunogenicity and safety of concomitant administration of 4CMenB and MenC-CRM vaccines in infants: A phase 3b randomized controlled trial. Vaccine 2017, 35, 2052–2059. [Google Scholar] [CrossRef]
- Findlow, J.; Bai, X.; Findlow, H.; Newton, E.; Kaczmarski, E.; Miller, E.; Borrow, R. Safety and immunogenicity of a four-component meningococcal group B vaccine (4CMenB) and a quadrivalent MenACWY conjugate vaccine administered concomitantly in healthy laboratory workers. Vaccine 2015, 33, 3322–3330. [Google Scholar] [CrossRef]
- Esposito, S.; Prymula, R.; Zuccotti, G.V.; Xie, F.; Barone, M.; Dull, P.M.; Toneatto, D. A phase 2 randomized controlled trial of the 4CMenB multicomponent meningococcal serogroup B vaccine in infants: Results of the second year of follow-up. Hum. Vaccin. Immunother. 2014, 10, 2005–2014. [Google Scholar] [CrossRef]
- Vesikari, T.; Esposito, S.; Prymula, R.; Ypma, E.; Kohl, I.; Toneatto, D.; Dull, P.; Kimura, A.; EU Meningococcal B Infant Vaccine Study Group. Immunogenicity and safety of the investigational multicomponent recombinant meningococcal B vaccine (4CMenB) administered concomitantly with routine infant and child vaccinations: Results of two randomized trials. Lancet 2013, 381, 825–835. [Google Scholar] [CrossRef]
- Riedmann, E.M. Human Vaccines and Immunotherapeutics: News. Hum. Vaccin. Immunother. 2013, 9, 7–10. [Google Scholar] [CrossRef]
- Santolaya, M.E.; O’Ryan, M.L.; Valenzuela, M.T.; Prado, V.; Vergara, R.; Muñoz, A.; Toneatto, D.; Graña, G.; Wang, H.; Clemens, R.; et al. Immunogenicity and tolerability of a multicomponent meningococcal B (4CMenB) vaccine in healthy adolescents in Chile: A phase 2b/3 randomized, observer-blind, placebo-controlled study. Lancet 2012, 379, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Thornton, V.; Lennon, D.; Rasanathan, K.; O’hallahan, J.; Oster, P.; Stewart, J.; Tilman, S.; Aaberge, I.; Feiring, B.; Nokleby, H.; et al. Safety and immunogenicity of the New Zealand strain meningococcal serogroup B OMV vaccine in healthy adults: Beginning of epidemic control. Vaccine 2006, 24, 1395–1400. [Google Scholar] [CrossRef] [PubMed]
- Parikh, S.R.; Andrews, N.J.; Beebeejaun, K.; Campbell, H.; Ribeiro, S.; Ward, C.; White, J.M.; Borrow, R.; Ramsay, M.E.; Ladhani, S.N. Effectiveness and impact of a reduced infant schedule of 4CMenB vaccine against group B meningococcal disease in England: A national observational cohort study. Lancet 2016, 388, 2775–2782. [Google Scholar] [CrossRef]
- Beran, J.; Dražan, D.; Enweonye, I.; Bhusal, C.; Toneatto, D. Immunogenicity and Safety of Investigational MenABCWY Vaccine and of 4CMenB and MenACWY Vaccines Administered Concomitantly or Alone: A Phase 2 Randomized Study of Adolescents and Young Adults. mSphere 2021, 6, e0055321. [Google Scholar] [CrossRef] [PubMed]
- Nolan, T.; Santolaya, M.E.; de Looze, F.; Marshall, H.; Richmond, P.; Henein, S.; Rheault, P.; Heaton, K.; Perrett, K.P.; Garfield, H.; et al. Antibody persistence and booster response in adolescents and young adults 4 and 7.5 years after immunization with 4CMenB vaccine. Vaccine 2019, 37, 1209–1218. [Google Scholar] [CrossRef]
- Perrett, K.P.; McVernon, J.; Richmond, P.C.; Marshall, H.; Nissen, M.; August, A.; Percell, S.; Toneatto, D.; Nolan, T. Immune responses to a recombinant, four-component, meningococcal serogroup B vaccine (4CMenB) in adolescents: A phase III, randomized, multicentre, lot-to-lot consistency study. Vaccine 2015, 33, 5217–5224. [Google Scholar] [CrossRef] [PubMed]
- McQuaid, F.; Snape, M.D.; John, T.M.; Kelly, S.; Robinson, H.; Yu, L.M.; Toneatto, D.; D’Agostino, D.; Dull, P.M.; Pollard, A.J. Persistence of specific bactericidal antibodies at 5 years of age after vaccination against serogroup B meningococcus in infancy and at 40 months. Cmaj 2015, 187, E215–E223. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Prymula, R.; Esposito, S.; Zuccotti, G.V.; Xie, F.; Toneatto, D.; Kohl, I.; Dull, P.M. A phase 2 randomized controlled trial of a multicomponent meningococcal serogroup B vaccine (I). Hum. Vaccines Immunother. 2014, 10, 1993–2004. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). Bexsero: Summary of Product Characteristics. 2023. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/bexsero (accessed on 3 September 2025).
- Centers for Disease Control and Prevention (CDC). Meningococcal Vaccination: What Everyone Should Know. 2024. Available online: https://www.cdc.gov/vaccines/vpd/mening/public/index.html (accessed on 3 September 2025).

| Study ID/ Reference | Design | N | Age | Sex (M/F) | Local Adverse Effects (n, %) | Systemic Adverse Effects (n) |
|---|---|---|---|---|---|---|
| #2 [32] | Phase 2 CT | 94 | 17.4 ± 4.64 years | 40/54 | Erythema: 10 (10.6%); Edema: 12 (12.8%); Induration: 12 (12.8%); Pain: 87 (92.6%) | Arthralgia (17), Fatigue (56), Nausea (17), Headache (39), Myalgia (38), Fever (1) |
| #3 [18] | Phase 2b CT | 353 | 14.5 ± 3.1/16.8 ± 3.1 years | 151/202 | Erythema: 45 (12.7%); Edema: 0 (0.0%); Induration: 44 (12.5%); Pain: 328 (92.9%) | Chills (72), Arthralgia (40), Fatigue (190), Nausea (66), Headache (164), Myalgia (84), Fever (11), Loss of appetite (61) |
| #4 [19] | NR | 92 | 8–12 weeks | NR | Erythema: 0 (0.0%); Edema: 0 (0.0%); Induration: 0 (0.0%); Pain: 92 (100.0%) | Fever (55) |
| #5 [33] | Phase 3b CT | 254 | 20.9 ± 2.69 years | 127/127 | Erythema: 29 (11.4%); Edema: 43 (16.9%); Induration: 43 (16.9%); Pain: 250 (98.4%) | Arthralgia (63), Fatigue (140), Nausea (51), Headache (125), Myalgia (98), Fever (9) |
| #6 [4] | Phase 3b CT | 228 | NR | NR | Erythema: 102 (44.7%); Edema: 86 (37.7%); Induration: 102 (44.7%); Pain: 159 (69.7%) | Fever (54), Loss of appetite (47), Sleep disturbance (78), Persistent crying (132), Vomiting (28), Diarrhea (41), Irritability (121), Skin rash (28) |
| #7 [20] | Phase 3 CT | 148 | NR | NR | Erythema: 54 (36.5%); Edema: 34 (23.0%); Induration: 63 (42.6%); Pain: 75 (50.7%) | Fever (71), Loss of appetite (92), Sleep disturbance (79), Persistent crying (96), Vomiting (21), Diarrhea (28), Irritability (111), Skin rash (18) |
| #11 [34] | Phase 3 | 342 | 11–17 | NR | Erythema: 156 (45.6%); Edema: 94 (27.5%); Induration: 93 (27.2%); Pain: 326 (95.3%) | Arthralgia (49), Fatigue (121), Nausea (64), Headache (117), Myalgia (192), Fever (9), Skin rash (14) |
| #13 [35] | Phase 2 CT | 50 | 5 years | NR | Erythema: 47 (94.0%); Edema: 19 (38.0%); Induration: 42 (84.0%); Pain: 23 (46.0%) | Arthralgia (10), Fever (5), Loss of appetite (17), Sleep disturbance (18), Vomiting (5), Diarrhea (4), Headache (5), Irritability (24), Skin rash (2) |
| #15 [36] | Phase 2 CT | 182 | NR | NR | Erythema: 59 (32.4%); Edema: 32 (17.6%); Induration: 55 (30.2%); Pain: 63 (34.6%) | Fever (99), Loss of appetite (42), Sleep disturbance (66), Persistent crying (52), Vomiting (13), Diarrhea (31), Irritability (70), Skin rash (3) |
| #19 [17] | Phase 2b/3 CT | 3330 | 11–17 | NR | Pain: 2863 (86.0%) | Fatigue (1703), Headache (1412), Fever (123) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García Flores, A.B.; Ruiz-Montero, R.; Onieva-García, M.Á.; Batista-Duharte, A.; López Cabrera, E.; Farouk Allam, M.; Salcedo, I. Safety Profile of the 4CMenB (Bexsero®) Vaccine: A Systematic Review and Meta-Analysis of Adverse Events in Clinical Trials. Vaccines 2025, 13, 1030. https://doi.org/10.3390/vaccines13101030
García Flores AB, Ruiz-Montero R, Onieva-García MÁ, Batista-Duharte A, López Cabrera E, Farouk Allam M, Salcedo I. Safety Profile of the 4CMenB (Bexsero®) Vaccine: A Systematic Review and Meta-Analysis of Adverse Events in Clinical Trials. Vaccines. 2025; 13(10):1030. https://doi.org/10.3390/vaccines13101030
Chicago/Turabian StyleGarcía Flores, Ana Belén, Rafael Ruiz-Montero, María Ángeles Onieva-García, Alexander Batista-Duharte, Estefanía López Cabrera, Mohamed Farouk Allam, and Inmaculada Salcedo. 2025. "Safety Profile of the 4CMenB (Bexsero®) Vaccine: A Systematic Review and Meta-Analysis of Adverse Events in Clinical Trials" Vaccines 13, no. 10: 1030. https://doi.org/10.3390/vaccines13101030
APA StyleGarcía Flores, A. B., Ruiz-Montero, R., Onieva-García, M. Á., Batista-Duharte, A., López Cabrera, E., Farouk Allam, M., & Salcedo, I. (2025). Safety Profile of the 4CMenB (Bexsero®) Vaccine: A Systematic Review and Meta-Analysis of Adverse Events in Clinical Trials. Vaccines, 13(10), 1030. https://doi.org/10.3390/vaccines13101030








