Longitudinal Evaluation of Humoral and Cellular Immunity After BNT162b2 COVID-19 Vaccination: Influence of Booster Type, Infection and Chronic Health Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Cohort Characteristics
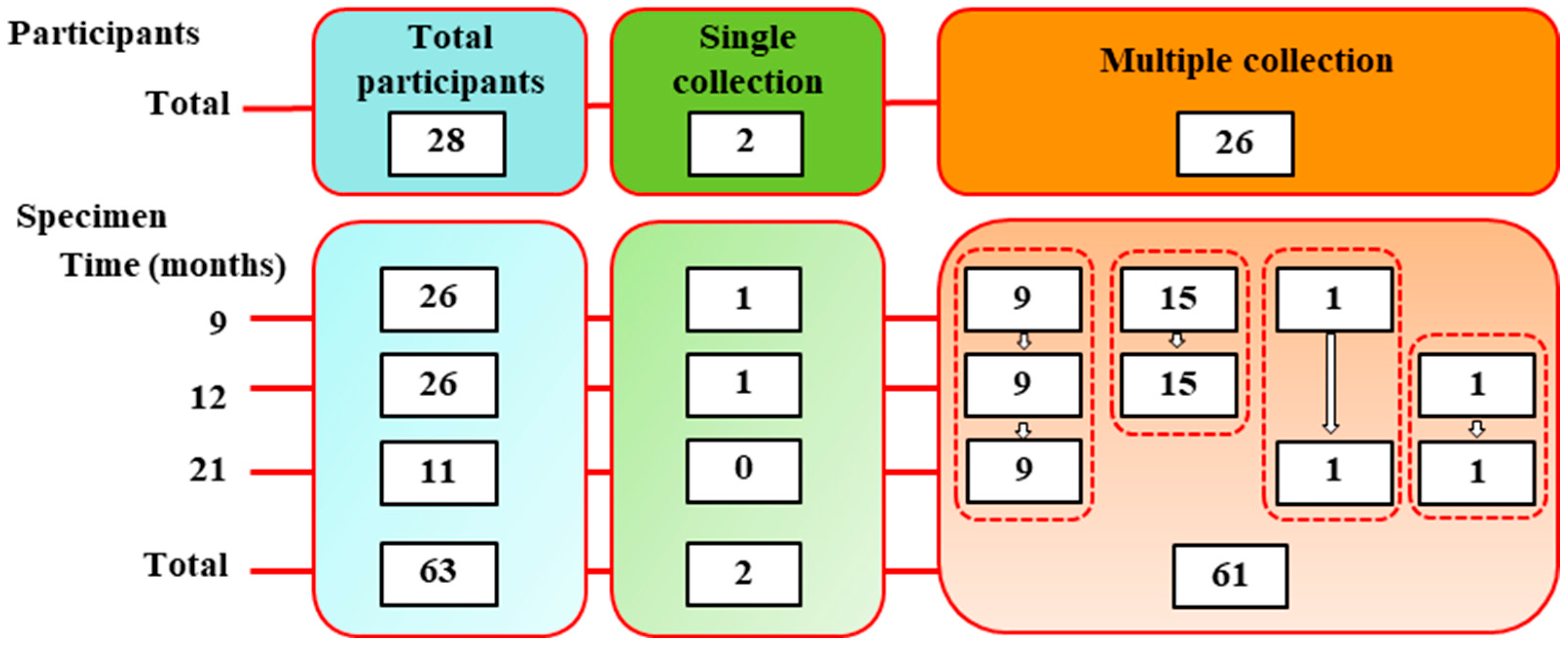
2.2. Blood Collection and Processing
2.3. Evaluation of Anti-SARS-CoV-2 Antibody Levels
2.4. T-Cell Activation and Intracellular Cytokine Assays
2.5. Analysis of SARS-CoV-2-Specific B Cells
2.6. Statistical Analysis
3. Results
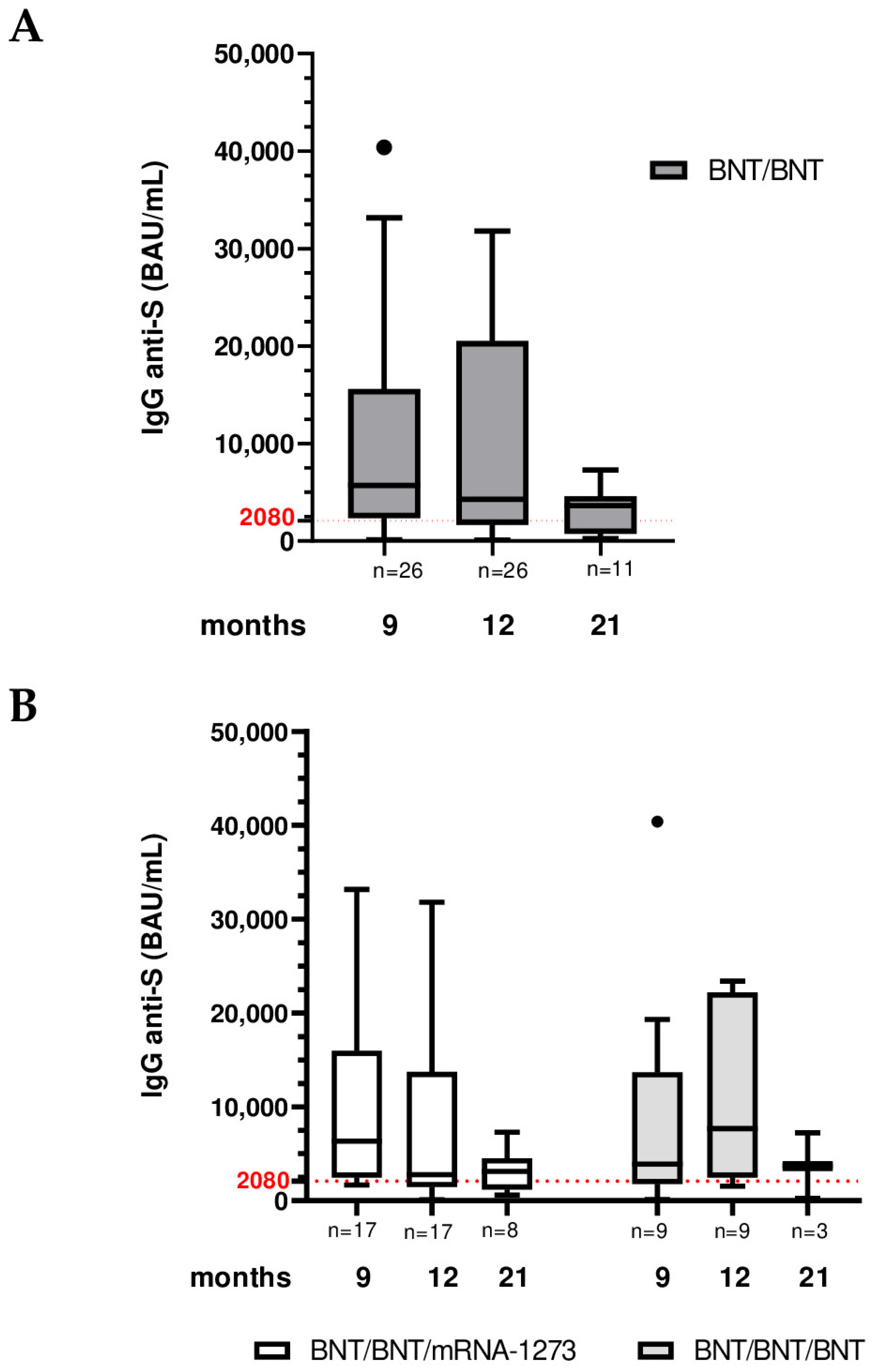
3.1. SARS-CoV-2 Longitudinal Analysis of Anti-Trimeric Spike IgG Levels at 9, 12 and 21 Months After Vaccination
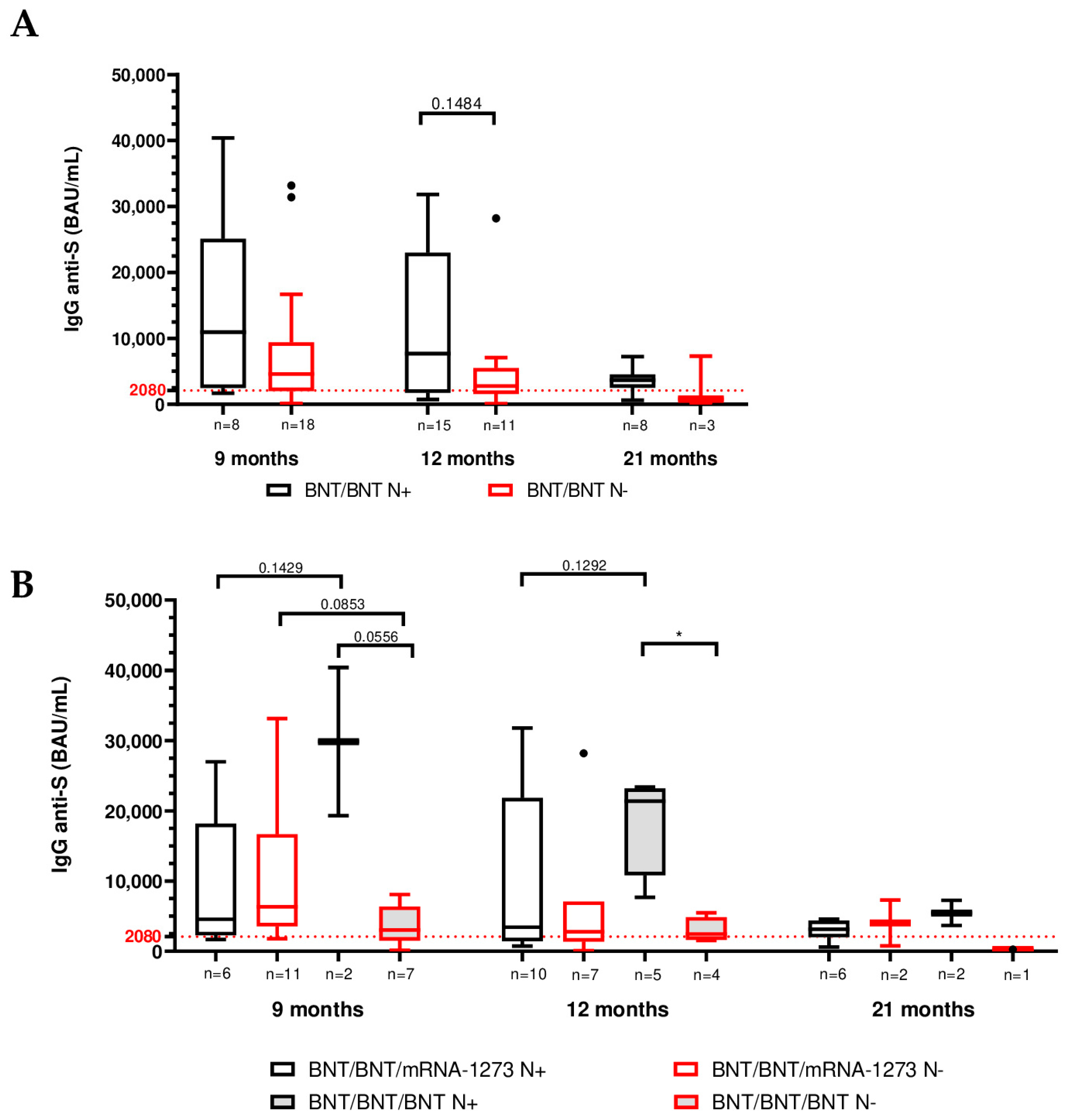
3.2. Role of SARS-CoV-2 Infection on Humoral Immunity
3.3. Clinical Characteristics of the Study Groups at 9, 12 and 21 Months Post-Vaccination
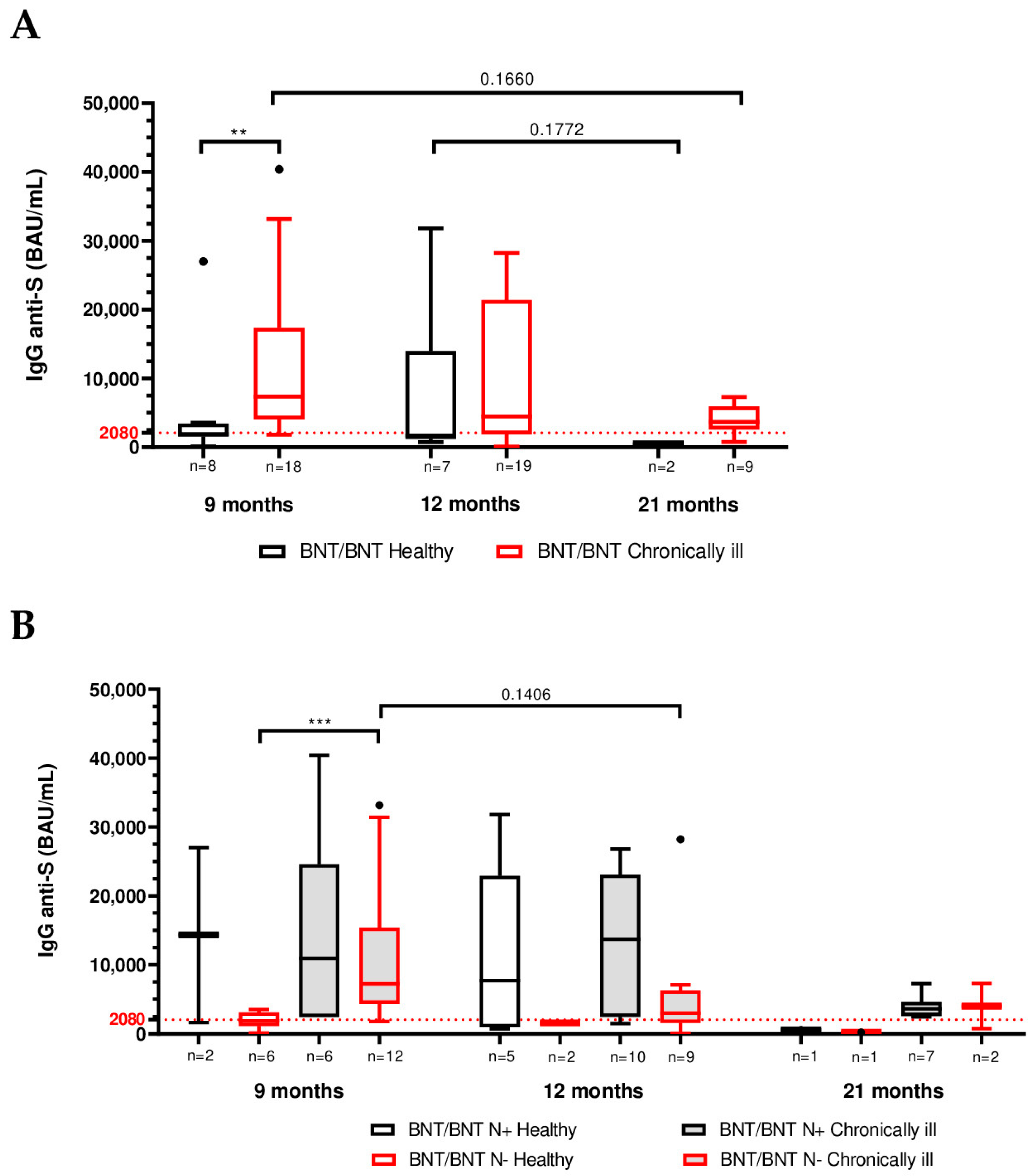
3.4. Impact of Chronic Health Status on Humoral Immunity
3.5. Spike-Specific Cellular Response at 21 Months Post-Vaccination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jamison, D.A.; Anand Narayanan, S.; Trovão, N.S.; Guarnieri, J.W.; Topper, M.J.; Moraes-Vieira, P.M.; Zaksas, V.; Singh, K.K.; Wurtele, E.S.; Beheshti, A. A Comprehensive SARS-CoV-2 and COVID-19 Review, Part 1: Intracellular Overdrive for SARS-CoV-2 Infection. Eur. J. Hum. Genet. 2022, 30, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Barros-Martins, J.; Hammerschmidt, S.I.; Cossmann, A.; Odak, I.; Stankov, M.V.; Morillas Ramos, G.; Dopfer-Jablonka, A.; Heidemann, A.; Ritter, C.; Friedrichsen, M.; et al. Immune Responses against SARS-CoV-2 Variants after Heterologous and Homologous ChAdOx1 nCoV-19/BNT162b2 Vaccination. Nat. Med. 2021, 27, 1525–1529. [Google Scholar] [CrossRef] [PubMed]
- Pawlowski, C.; Lenehan, P.; Puranik, A.; Agarwal, V.; Venkatakrishnan, A.J.; Niesen, M.J.M.; O’Horo, J.C.; Virk, A.; Swift, M.D.; Badley, A.D.; et al. FDA-Authorized mRNA COVID-19 Vaccines Are Effective per Real-World Evidence Synthesized across a Multi-State Health System. Med 2021, 2, 979–992.e8. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and Efficacy of the ChAdOx1 nCoV-19 Vaccine (AZD1222) against SARS-CoV-2: An Interim Analysis of Four Randomised Controlled Trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- CDC Staying up to Date with COVID-19 Vaccines. Available online: https://www.cdc.gov/covid/vaccines/stay-up-to-date.html (accessed on 2 July 2025).
- CDC Adult Immunization Schedule Notes. Available online: https://www.cdc.gov/vaccines/hcp/imz-schedules/adult-notes.html (accessed on 2 July 2025).
- Ndwandwe, D.; Wiysonge, C.S. COVID-19 Vaccines. Curr. Opin. Immunol. 2021, 71, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Khandker, S.S.; Godman, B.; Jawad, M.I.; Meghla, B.A.; Tisha, T.A.; Khondoker, M.U.; Haq, M.A.; Charan, J.; Talukder, A.A.; Azmuda, N.; et al. A Systematic Review on COVID-19 Vaccine Strategies, Their Effectiveness, and Issues. Vaccines 2021, 9, 1387. [Google Scholar] [CrossRef]
- Kim, D.; Lee, Y.J. Vaccination Strategies and Transmission of COVID-19: Evidence across Advanced Countries. J. Health Econ. 2022, 82, 102589. [Google Scholar] [CrossRef]
- Bansal, D.; Abdulmajeed, J.; Al-Shamali, M.H.M.A.; Albayat, S.S.A.; Himatt, S.M.; Cyprian, F.S.; Chivese, T.; Mundodan, J.M.A.; Khogali, H.S.; Baaboura, R.; et al. Duration of COVID-19 mRNA Vaccine Effectiveness against Severe Disease. Vaccines 2022, 10, 1036. [Google Scholar] [CrossRef]
- Figueroa, A.L.; Ali, K.; Berman, G.; Zhou, H.; Deng, W.; Xu, W.; Lussier, S.; Girard, B.; Dutko, F.J.; Slobod, K.; et al. Safety and Durability of mRNA-1273–Induced SARS-CoV-2 Immune Responses in Adolescents: Results from the Phase 2/3 TeenCOVE Trial. eClinicalMedicine 2024, 74, 102720. [Google Scholar] [CrossRef]
- Hartley, G.E.; Fryer, H.A.; Gill, P.A.; Boo, I.; Bornheimer, S.J.; Hogarth, P.M.; Drummer, H.E.; O’Hehir, R.E.; Edwards, E.S.J.; van Zelm, M.C. Homologous but Not Heterologous COVID-19 Vaccine Booster Elicits IgG4+ B-Cells and Enhanced Omicron Subvariant Binding. npj Vaccines 2024, 9, 129. [Google Scholar] [CrossRef]
- Asante, M.A.; Michelsen, M.E.; Balakumar, M.M.; Kumburegama, B.; Sharifan, A.; Thomsen, A.R.; Korang, S.K.; Gluud, C.; Menon, S. Heterologous versus Homologous COVID-19 Booster Vaccinations for Adults: Systematic Review with Meta-Analysis and Trial Sequential Analysis of Randomised Clinical Trials. BMC Med. 2024, 22, 263. [Google Scholar] [CrossRef]
- Stefan, N. Metabolic Disorders, COVID-19 and Vaccine-Breakthrough Infections. Nat. Rev. Endocrinol. 2022, 18, 75–76. [Google Scholar] [CrossRef]
- Morawska, M. Reasons and Consequences of COVID-19 Vaccine Failure in Patients with Chronic Lymphocytic Leukemia. Eur. J. Haematol. 2022, 108, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Torre, D.; Orlandi, C.; Conti, I.; Barocci, S.; Carlotti, E.; Magnani, M.; Casabianca, A.; Stefanetti, G. Analysis of Humoral and Cellular Immune Activation up to 21 Months after Heterologous and Homologous COVID-19 Vaccination. Front. Immunol. 2025, 16, 1579163. [Google Scholar] [CrossRef]
- Barocci, S.; Orlandi, C.; Diotallevi, A.; Buffi, G.; Ceccarelli, M.; Vandini, D.; Carlotti, E.; Galluzzi, L.; Rocchi, M.B.L.; Magnani, M.; et al. Evaluation of Two-Month Antibody Levels after Heterologous ChAdOx1-S/BNT162b2 Vaccination Compared to Homologous ChAdOx1-S or BNT162b2 Vaccination. Vaccines 2022, 10, 491. [Google Scholar] [CrossRef]
- Orlandi, C.; Stefanetti, G.; Barocci, S.; Buffi, G.; Diotallevi, A.; Rocchi, E.; Ceccarelli, M.; Peluso, S.; Vandini, D.; Carlotti, E.; et al. Comparing Heterologous and Homologous COVID-19 Vaccination: A Longitudinal Study of Antibody Decay. Viruses 2023, 15, 1162. [Google Scholar] [CrossRef] [PubMed]
- Bonelli, F.; Blocki, F.A.; Bunnell, T.; Chu, E.; O, A.D.L.; Grenache, D.G.; Marzucchi, G.; Montomoli, E.; Okoye, L.; Pallavicini, L.; et al. Evaluation of the Automated LIAISON® SARS-CoV-2 TrimericS IgG Assay for the Detection of Circulating Antibodies. Clin. Chem. Lab. Med. 2021, 59, 1463–1467. [Google Scholar] [CrossRef]
- Swadźba, J.; Anyszek, T.; Panek, A.; Martin, E. Anti-Spike SARS-CoV-2 IgG Assessment with a Commercial Assay during a 4-Month Course after COVID-19 Vaccination. Vaccines 2021, 9, 1367. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Jeyanathan, M.; Afkhami, S.; Smaill, F.; Miller, M.S.; Lichty, B.D.; Xing, Z. Immunological Considerations for COVID-19 Vaccine Strategies. Nat. Rev. Immunol. 2020, 20, 615–632. [Google Scholar] [CrossRef]
- Behrens, G.M.N.; Barros-Martins, J.; Cossmann, A.; Ramos, G.M.; Stankov, M.V.; Odak, I.; Dopfer-Jablonka, A.; Hetzel, L.; Köhler, M.; Patzer, G.; et al. BNT162b2-Boosted Immune Responses Six Months after Heterologous or Homologous ChAdOx1nCoV-19/BNT162b2 Vaccination against COVID-19. Nat. Commun. 2022, 13, 4872. [Google Scholar] [CrossRef]
- Yoo, J.; Kim, Y.; Cha, Y.m.; Lee, J.; Jeong, Y.J.; Kim, S.-H.; Maragakis, L.L.; Lee, S. Heterologous Vaccination (ChAdOx1 and BNT162b2) Induces a Better Immune Response against the Omicron Variant than Homologous Vaccination. J. Infect. Public Health 2023, 16, 1537–1543. [Google Scholar] [CrossRef]
- Atmar, R.L.; Lyke, K.E.; Deming, M.E.; Jackson, L.A.; Branche, A.R.; Sahly, H.M.E.; Rostad, C.A.; Martin, J.M.; Johnston, C.; Rupp, R.E.; et al. Homologous and Heterologous Covid-19 Booster Vaccinations. N. Engl. J. Med. 2022, 386, 1046–1057. [Google Scholar] [CrossRef]
- Yau, K.; Tam, P.; Chan, C.T.; Hu, Q.; Qi, F.; Abe, K.T.; Kurtesi, A.; Jiang, Y.; Estrada-Codecido, J.; Brown, T.; et al. BNT162b2 versus mRNA-1273 Third Dose COVID-19 Vaccine in Patients with CKD and Maintenance Dialysis Patients. Clin. J. Am. Soc. Nephrol. 2024, 19, 85–97. [Google Scholar] [CrossRef]
- Sezer, Z.; Pavel, S.T.I.; Inal, A.; Yetiskin, H.; Kaplan, B.; Uygut, M.A.; Aslan, A.F.; Bayram, A.; Mazicioglu, M.; Kalin Unuvar, G.; et al. Long-Term Immunogenicity and Safety of a Homologous Third Dose Booster Vaccination with TURKOVAC: Phase 2 Clinical Study Findings with 32-Week Post-Booster Follow-Up. Vaccines 2024, 12, 140. [Google Scholar] [CrossRef] [PubMed]
- Hellgren, F.; Rosdahl, A.; Arcoverde Cerveira, R.; Lenart, K.; Ols, S.; Gwon, Y.-D.; Kurt, S.; Delis, A.M.; Joas, G.; Evander, M.; et al. Modulation of Innate Immune Response to mRNA Vaccination after SARS-CoV-2 Infection or Sequential Vaccination in Humans. JCI Insight 2024, 9, e175401. [Google Scholar] [CrossRef] [PubMed]
- Peluso, M.J.; Donatelli, J.; Henrich, T.J. Long-Term Immunologic Effects of SARS-CoV-2 Infection: Leveraging Translational Research Methodology to Address Emerging Questions. Transl. Res. 2022, 241, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Vaibhav; Nishad, S.S.; Dongare, D.; Tripathi, A.C.P.; Tripathi, T.; Tripathi, P. Deciphering the Intricacies of Immune System Dysfunction and Its Impact on Diabetes Mellitus: Revisiting the Communication Strategies to Manage Diabetes Mellitus. Health Sci. Rev. 2024, 13, 100201. [Google Scholar] [CrossRef]
- Alexander, M.; Cho, E.; Gliozheni, E.; Salem, Y.; Cheung, J.; Ichii, H. Pathology of Diabetes-Induced Immune Dysfunction. Int. J. Mol. Sci. 2024, 25, 7105. [Google Scholar] [CrossRef]
- Daryabor, G.; Atashzar, M.R.; Kabelitz, D.; Meri, S.; Kalantar, K. The Effects of Type 2 Diabetes Mellitus on Organ Metabolism and the Immune System. Front. Immunol. 2020, 11, 1582. [Google Scholar] [CrossRef]
- Lampasona, V.; Secchi, M.; Scavini, M.; Bazzigaluppi, E.; Brigatti, C.; Marzinotto, I.; Davalli, A.; Caretto, A.; Laurenzi, A.; Martinenghi, S.; et al. Antibody Response to Multiple Antigens of SARS-CoV-2 in Patients with Diabetes: An Observational Cohort Study. Diabetologia 2020, 63, 2548–2558. [Google Scholar] [CrossRef]
- Warpechowski, J.; Leszczyńska, P.; Juchnicka, D.; Olichwier, A.; Szczerbiński, Ł.; Krętowski, A.J. Assessment of the Immune Response in Patients with Insulin Resistance, Obesity, and Diabetes to COVID-19 Vaccination. Vaccines 2023, 11, 1203. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Alterki, A.; Sindhu, S.; Alahmad, B.; Hammad, M.; Al-Sabah, S.; Alghounaim, M.; Jamal, M.H.; Aldei, A.; Mairza, M.J.; et al. Robust Antibody Levels in Both Diabetic and Non-Diabetic Individuals After BNT162b2 mRNA COVID-19 Vaccination. Front. Immunol. 2021, 12, 752233. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic Inflammation in the Etiology of Disease across the Life Span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Taeschler, P.; Adamo, S.; Deng, Y.; Cervia, C.; Zurbuchen, Y.; Chevrier, S.; Raeber, M.E.; Hasler, S.; Bächli, E.; Rudiger, A.; et al. T-cell Recovery and Evidence of Persistent Immune Activation 12 Months after Severe COVID-19. Allergy 2022, 77, 2468–2481. [Google Scholar] [CrossRef]
- Barnes, E.; Goodyear, C.S.; Willicombe, M.; Gaskell, C.; Siebert, S.; de Silva, T.I.; Murray, S.M.; Rea, D.; Snowden, J.A.; Carroll, M.; et al. SARS-CoV-2-Specific Immune Responses and Clinical Outcomes after COVID-19 Vaccination in Patients with Immune-Suppressive Disease. Nat. Med. 2023, 29, 1760–1774. [Google Scholar] [CrossRef]
- Blackwell, J.M.; Jamieson, S.E.; Burgner, D. HLA and Infectious Diseases. Clin. Microbiol. Rev. 2009, 22, 370–385. [Google Scholar] [CrossRef] [PubMed]
- Kalampokis, I.; Wong, C.S.; Ma, J.; Smith, L.M.; Masten, B.J.; Chabot-Richards, D.; Pisetsky, D.S. The Limitation of HLA Diversity as a Risk Factor for Pediatric-Onset Autoimmune Rheumatic Disease. J. Clin. Med. 2025, 14, 916. [Google Scholar] [CrossRef]
- Khan, T.; Rahman, M.; Ahmed, I.; Al Ali, F.; Jithesh, P.V.; Marr, N. Human Leukocyte Antigen Class II Gene Diversity Tunes Antibody Repertoires to Common Pathogens. Front. Immunol. 2022, 13, 856497. [Google Scholar] [CrossRef]
- Prugnolle, F.; Manica, A.; Charpentier, M.; Guégan, J.F.; Guernier, V.; Balloux, F. Pathogen-Driven Selection and Worldwide HLA Class I Diversity. Curr. Biol. 2005, 15, 1022–1027. [Google Scholar] [CrossRef]
| Vaccine Schedule | 9 Months | 12 Months | 21 Months | |||
|---|---|---|---|---|---|---|
| Primary | Booster | |||||
| BNT/BNT | Total | n = 26 | n = 26 | n = 11 | ||
| Gender | Male | 8/26 (30.8%) | 8/26 (30.8%) | 3/11 (27.3%) | ||
| Age | Years, median (IQR) | 54.5 (43.75–59.25) | 53 (43.75–59.25) | 53 (42–58) | ||
| BMI | Median (IQR) | 25.19 (22.8–28.74) | 24.84 (22.18–28.74) | 25.39 (22.41–28.69) | ||
| mRNA-1273 | n = 17 | n = 17 | n = 8 | |||
| Gender | Male | 7/17 (41.2%) | 7/17 (41.2%) | 3/8 (37.5%) | ||
| Age | Years, median (IQR) | 57 (43.5–59.5) | 56 (42.5–59.5) | 56.5 (38.25–58) | ||
| BMI | Median (IQR) | 26.99 (24.62–28.9) | 25.39 (24.03–28.9) | 27.62 (25.38–28.94) | ||
| BNT | n = 9 | n = 9 | n = 3 | |||
| Gender | Male | 1/9 (11.1%) | 1/9 (11.1%) | 0/3 (0.0%) | ||
| Age | Years, median (IQR) | 50 (42–60.5) | 53 (46.5–60.5) | 50 (47–53) | ||
| BMI | Median (IQR) | 23.24 (20.79–26.67) | 23.24 (20.88–26.67) | 22.41 (19.49–23.24) | ||
| Total (BNT/BNT) | BNT/BNT/ mRNA-1273 | BNT/BNT/ BNT | ||
|---|---|---|---|---|
| IgG titer (BAU/mL) | 9 months Median (IQR) | n = 26 5700 (2358–15,600) | n = 17 (65.4%) 6340 (2470–15,960) | n = 9 (34.6%) 3900 (1780–13,700) |
| 12 months Median (IQR) | n = 26 4280 (1660–20,500) | n = 17 (65.4%) 2780 (1455–13,710) | n = 9 (34.6%) 7680 (2462–22,200) | |
| 21 months Median (IQR) | n = 11 3640 (770–4600) | n = 8 (72.7%) 3130 (1198–4524) | n = 3 (27.3%) 3680 (256–7250) | |
| Total (BNT/BNT) | BNT/BNT/mRNA-1273 | BNT/BNT/BNT | |||||
|---|---|---|---|---|---|---|---|
| N+ | N− | N+ | N− | N+ | N− | ||
| IgG titer (BAU/mL) | 9 months | n = 8 (30.8%) | n = 18 (69.2%) | n = 6 (23.1%) | n = 11 (42.3%) | n = 2 (7.7%) | n = 7 (26.9%) |
| Median | 10,950 | 4580 | 4570 | 6340 | 29,860 | 3040 | |
| (IQR) | (2465–25,080) | (1991–9390) | (2265–18,180) | (3580–16,680) | (19,320–40,400) | (1510–6380) | |
| 12 months | n = 15 (57.7%) | n = 11 (42.3%) | n = 10 (38.5%) | n = 7 (26.9%) | n = 5 (19.2%) | n = 4 (15.4%) | |
| Median | 7680 | 2780 | 3420 | 2780 | 21400 | 2462 | |
| (IQR) | (1720–23,000) | (1540–5480) | (1449–21,850) | (1380–7100) | (10,830–23,200) | (1626–4870) | |
| 21 months | n = 8 (72.7%) | n = 3 (27.3%) | n = 6 (54.5%) | n = 2 (18.2%) | n = 2 (18.2%) | n = 1 (9.1%) | |
| Median | 3660 | 770 | 3130 | 4035 | 5465 | 256 | |
| (IQR) | (2515–4524) | (256–7300) | (2015–4371) | (770–7300) | (3680–7250) | ||
| Total (BNT/BNT) | BNT/BNT/mRNA-1273 | BNT/BNT/BNT | |||||
|---|---|---|---|---|---|---|---|
| Healthy | Chronically Ill | Healthy | Chronically Ill | Healthy | Chronically Ill | ||
| IgG titer (BAU/mL) | 9 months | n = 8 (30.8%) | n = 18 (69.2%) | n = 4 (23.5%) | n = 13 (76.5%) | n = 4 (44.4%) | n = 5 (55.6%) |
| Median | 1915 | 7370 | 2680 | 6660 | 1780 | 8080 | |
| (IQR) | (1553–3445) | (4050–17,340) | (1705–21,145) | (3290–15,960) | (481–2793) | (5140–29,860) | |
| 12 months | n = 7 (26.9%) | n = 19 (73.1%) | n = 4 (23.5%) | n = 13 (76.5%) | n = 3 (33.3%) | n = 6 (67.7%) | |
| Median | 1700 | 4460 | 1453 | 4100 | 7680 | 13440 | |
| (IQR) | (1206–13,980) | (1884–21,400) | (851–24,275) | (1625–13,710) | (1540–13,980) | (2751–23,100) | |
| 21 months | n = 2 (18.2%) | n = 9 (81.8%) | n = 1 (12.5%) | n = 7 (87.5%) | n = 1 (33.3%) | n = 2 (66.7%) | |
| Median | 437 | 3680 | 618 | 3640 | 256 | 5465 | |
| (IQR) | (256–618) | (2550–5925) | (2480–4600) | (3680–7250) | |||
| Healthy | Chronically Ill | ||||
|---|---|---|---|---|---|
| N+ | N− | N+ | N− | ||
| IgG titer (BAU/mL) | 9 months | n = 2 (7.7%) | n = 6 (23.1%) | n = 6 (23.1%) | n = 12 (46.2%) |
| Median | 14,340 | 1915 | 10,950 | 7230 | |
| (IQR) | (1680–27,000) | (1167–3175) | (2475–24,590) | (4340–15,375) | |
| 12 months | n = 5 (19.2%) | n = 2 (7.7%) | n = 10 (38.5%) | n = 9 (34.6%) | |
| Median | 7680 | 1620 | 13710 | 3040 | |
| (IQR) | (969.5–22,890) | (1540–1700) | (2485–23,100) | (1632–6290) | |
| 21 months | n = 1 (9.1%) | n = 1 (9.1%) | n = 7 (63.6%) | n = 2 (18.2%) | |
| Median | 618 | 256 | 3680 | 4035 | |
| (IQR) | (2620–4600) | (770–7300) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlandi, C.; Conti, I.; Torre, D.; Barocci, S.; Magnani, M.; Stefanetti, G.; Casabianca, A. Longitudinal Evaluation of Humoral and Cellular Immunity After BNT162b2 COVID-19 Vaccination: Influence of Booster Type, Infection and Chronic Health Conditions. Vaccines 2025, 13, 1031. https://doi.org/10.3390/vaccines13101031
Orlandi C, Conti I, Torre D, Barocci S, Magnani M, Stefanetti G, Casabianca A. Longitudinal Evaluation of Humoral and Cellular Immunity After BNT162b2 COVID-19 Vaccination: Influence of Booster Type, Infection and Chronic Health Conditions. Vaccines. 2025; 13(10):1031. https://doi.org/10.3390/vaccines13101031
Chicago/Turabian StyleOrlandi, Chiara, Ilaria Conti, Davide Torre, Simone Barocci, Mauro Magnani, Giuseppe Stefanetti, and Anna Casabianca. 2025. "Longitudinal Evaluation of Humoral and Cellular Immunity After BNT162b2 COVID-19 Vaccination: Influence of Booster Type, Infection and Chronic Health Conditions" Vaccines 13, no. 10: 1031. https://doi.org/10.3390/vaccines13101031
APA StyleOrlandi, C., Conti, I., Torre, D., Barocci, S., Magnani, M., Stefanetti, G., & Casabianca, A. (2025). Longitudinal Evaluation of Humoral and Cellular Immunity After BNT162b2 COVID-19 Vaccination: Influence of Booster Type, Infection and Chronic Health Conditions. Vaccines, 13(10), 1031. https://doi.org/10.3390/vaccines13101031








