Abstract
Preventive medicine has proven its long-term effectiveness and economic feasibility. Over the last century, vaccination has saved more lives than any other medical technology. At present, preventative measures against most infectious diseases are successfully used worldwide; in addition, vaccination platforms against oncological and even autoimmune diseases are being actively developed. At the same time, the development of medicine led to an increase in both life expectancy and the proportion of age-associated diseases, which pose a heavy socio-economic burden. In this context, the development of vaccine-based approaches for the prevention or treatment of age-related diseases opens up broad prospects for extending the period of active longevity and has high economic potential. It is well known that the development of age-related diseases is associated with the accumulation of senescent cells in various organs and tissues. It has been demonstrated that the elimination of such cells leads to the restoration of functions, rejuvenation, and extension of the lives of experimental animals. However, the development of vaccines against senescent cells is complicated by their antigenic heterogeneity and the lack of a unique marker. In addition, senescent cells are the body’s own cells, which may be the reason for their low immunogenicity. This mini-review discusses the mechanisms of central and peripheral tolerance that may influence the formation of an anti-senescent immune response and be responsible for the accumulation of senescent cells with age.
1. Introduction
The development of medicine led to a significant increase in life expectancy in most countries []. According to the World Health Organization (WHO), the number of people over 60 will double by 2050 and reach 2.1 billion people []. The global trend toward an aging population leads to an increase in age-associated diseases and places a burden on the world economies [,,]. However, as a result of scientific and technological progress, the arsenal of methods for treating age-related diseases is constantly expanding. In recent years, the possibility of using vaccines to prevent and treat the most common age-related diseases has been actively studied []. Thus, the first positive results were obtained from vaccines used to treat Alzheimer’s disease, type II diabetes mellitus, osteoarthritis, arterial hypertension, and some other cardiovascular diseases. Such vaccines can be based on different platforms and include peptide, protein, viral, cellular, and mRNA vaccines, as well as vaccines based on lipid particles (Table 1) [].

Table 1.
Vaccine approaches for combating age-related diseases.
A conceptually new approach is associated with the development of senolytic drugs. A number of studies have shown that aging of the body is accompanied by the accumulation of senescent cells in various tissues and organs [,]. This leads to tissue homeostasis and functional disorders typical of old age []. Senescent cells are cells with accumulated mutations, impaired autophagy, and defects in mitochondrial function, incapable of proliferating []. These cells are characterized by shortened telomeres, low heterochromatin orderliness, and defects in the nuclear membrane [,]. Morphologically, they are distinguished by an increased size and hypertrophy of the lysosomal apparatus [,,]. Senescent cells also typically express some age-related markers (such as p16INK4A, p21CIP1, etc.), have increased senescence-associated β-galactosidase (SA-β-Gal) activity, produce a number of cytokines and pro-inflammatory substances (Senescence-Associated Secretory Phenotype—SASP), and have various defects in the protein quality control machinery [,,,,]. The physiological features of senescent cells affect their antigen profiles []. Unique sets of mutations and abnormalities in post-translational modifications (PTMs) typical of senescent cells can alter the structure of various proteins [,]. In addition, the accumulating body of evidence points at the increased genomic instability, activation of retroelements, and awakening of some latent viruses in senescent cells, which also causes the emergence of new antigen determinants [,]. Thus, senescent cell antigens (SAs) can be divided into Senescent-Associated Antigens (SAAs) and Senescent-Specific Antigens (SSAs). The first case refers to unchanged self-antigens that have increased levels of expression on some types of senescent cells (e.g., uPAR) []. The second case refers to “neoantigens” that appeared de novo and are unique to senescent cells.
It is worth noting that senescent cells play an important role in some physiological processes. Thus, SASP factors are involved in tissue remodeling in early ontogenesis, as well as in repair and regeneration processes at later stages of development []. In addition, cell cycle arrest, which is the most important feature of senescence, prevents possible malignancy []. Senescent cells are normally present in tissues in limited numbers. However, the accumulation of these cells during the aging process is associated with the dysfunction of various tissues and organs, and the cumulative effect of SASP production contributes to chronic age-related inflammation, which increases the risk of developing age-related diseases []. At the same time, selective elimination of these cells during aging leads to the restoration of functions gradually lost with age and, in some cases, was demonstrated to extend the life of experimental animals [,].
Despite the promising results of using some drugs aimed at killing senescent cells (senolytics) or reducing the negative effects of SASP (senomorphics), the existing pharmacological approaches still do not possess the high specificity toward senescent cells, do not take into account their diversity, and are associated with the risk of side effects [,,,]. This significantly limits their translational potential. In this context, a promising alternative is the development of methods for targeted elimination of senescent cells with the help of adaptive immunity mechanisms. Various attempts to create senolytic vaccines that remove senescent cells from specific tissues have already been made. For example, vaccines against GPNMB (glycoprotein nonmetastatic melanoma protein B) and CD153 antigens showed positive results with minimal side effects [,]. In mice, targeting GPNMB with a peptide vaccine reduced senescence load, improved metabolic indices, and reduced atherosclerotic plaques []. Furthermore, the anti-CD153 peptide vaccine reduced the number of senescent T cells in adipose tissue, increased glucose tolerance, and improved the response to endogenous insulin []. In addition, CAR-T therapy against uPAR, a marker of senescent cells that can potentially be used in senolytic vaccines, showed a beneficial effect, increasing stamina and improving glucose tolerance in aged mice []. Targeting the senescent cell marker NKG2D with CAR-T selectively eliminates senescent cells in vitro with minimal impact on healthy cells, suggesting its therapeutic potential for senolytic vaccines []. However, the development of such vaccines is associated with certain problems. Unique SSAs absent in normal cells are still unknown, which hinders the development of safe senolytic vaccines due to the risk of damage to the healthy tissues [,]. The high antigenic diversity of senescent cells, both at individual and population levels, significantly complicates the search for target antigens for the creation of universal senolytic vaccines [,]. Obviously, tolerance to senescence-associated antigens is a serious problem [,]. Since senescent cells are the body’s own cells, the mechanisms of central and peripheral tolerance can act toward the antigens they express. They are carried out due to the elimination of autoreactive lymphocytes in the thymus and bone marrow, as well as through clonal inactivation and anergy of autoreactive clones in the periphery [,,]. Thus, overcoming tolerance to senescence-associated antigens is one of the key challenges in the development of senolytic vaccines.
2. Central Tolerance for Senescent Antigens
Mechanisms of central tolerance are crucial for preventing autoimmune diseases. The removal of potentially autoreactive immature T and B lymphocytes occurs during negative selection in the thymus and bone marrow, respectively [,]. The key role in this process is played by the diversity of self-antigens that immature lymphocytes encounter during maturation []. Unlike the thymus, the bone marrow does not have a specialized system for presenting self-antigens. Therefore, lymphocyte selection is less strict and limited to the default set of self-antigens present in the bone marrow. At the same time, the aging of bone marrow cells can lead to the emergence of senescence-associated antigens and the formation of tolerance of B cells to these antigens [,].
The situation is different in the thymus, where a complex system of presentation of self-antigens exists []. The multitude of antigens presented in the thymus forms the clonal diversity of effector (Teff) and regulatory (Treg) T lymphocytes and ensures equilibrium in the immune system and its ability to respond to antigen challenges, maintaining protection against oncological, autoimmune, and infectious diseases [,]. Apparently, the thymus is simultaneously involved in the formation of an anti-senescent immune response and the elimination of senescent cells, as well as in maintaining tolerance to a multitude of SA [,,]. The balance between these processes is determined by the repertoires of Teff and Treg cells that recognize SA []. In other words, the nature of the immune response to these antigens depends on their presentation in the thymus.
The action of the transcription factors AIRE, FezF2, and DEAF1 in cooperation with the helicase CHD4 in the thymus provides promiscuous gene expression (PGE) of the overwhelming majority of tissue-specific antigens []. Along with alternative splicing, the diversity of self-antigens in the thymus increases due to the expression of endogenous retroelements []. In addition, some dendritic cells migrate to the thymus and carry peripheral antigens with them (see Figure 1). The intensity of this process increases with age, which may be the reason for the formation of central tolerance to some SA [,,,]. Thus, a unique library of central tolerance antigens is formed in the thymus, which ensures the selection of thymocytes and participates in the formation of T-cell repertoires []. However, this library undergoes qualitative and quantitative changes during life. The age-related thymus involution is accompanied by a decrease in the number of medullary thymic epithelial cells (mTECs), a decrease in PGE, an increase in the number of dendritic cells with peripheral antigens, and an accumulation of cells with signs of senescence in Hassall’s corpuscles [,,]. This leads to a decrease in thymopoiesis (thymic output) and a decrease in the diversity of T-lymphocyte repertoires [,]. Apparently, age-related central tolerance to some SA is formed due to an increase in their share in the repertoire of antigens presented in the thymus. This is associated with the aging of the thymus itself and the appearance of senescent cells among mTEC and in Hassall’s corpuscles, as well as an increase in the influx of SA from the periphery due to the migration of dendritic cells [,,]. Accurate identification and determination of the number of these antigens is a complex task. Nevertheless, the described mechanism is a probable cause of the low efficiency of promising senolytic vaccines aimed at SA.
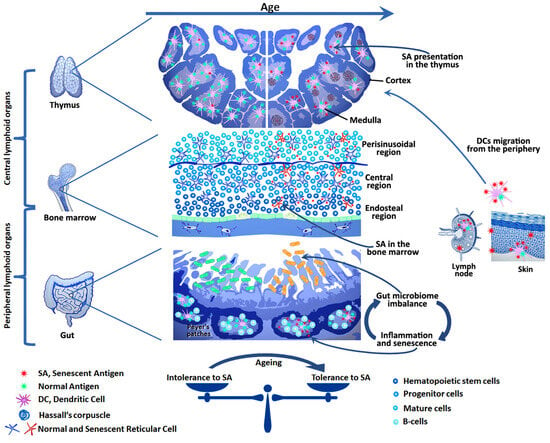
Figure 1.
In youth, aged and senescent cells are efficiently eliminated by the immune system. However, with age, the effectiveness of this process declines. This may be due to both the aging of the immune system itself and the development of tolerance to senescent cell antigens. Chronic inflammation caused by SASP factors, as well as the ever-increasing antigenic load, may play a key role in this by leading to exhaustion, anergy, or trans-differentiation of anti-senescent effector cells to Tregs. Senolytic vaccines aim to restore the immune system’s ability to selectively eliminate senescent cells without compromising the organism as a whole. To achieve this, it is necessary to consider age-related changes in the system of autotolerance: the appearance of SA in the bone marrow, thymus, and periphery, as well as the microbiome imbalance, as these changes affect the repertoires of effector and regulatory T and B cells specific for SA.
The solution to the problem can be found in a more detailed examination of the aging processes in the thymus and periphery. As noted earlier, PTM processes are disrupted in aging and senescent cells [,,]. This leads to changes in the immunopeptidome in these cells. On one hand, the appearance of a PTM peptide in the molecule of the major histocompatibility complex (MHC) can shield it from the T-cell receptor (TCR) []. On the other hand, such a peptide, on the contrary, is capable of causing a strong immune response []. The latter, for instance, can be observed in some autoimmune diseases, when a protein with the correct primary structure activates T cells due to the features of PTMs [,]. In the thymus, the intensity of PTMs is much lower than in the periphery, and PTM proteins are almost not involved in negative selection, which determines the absence of central tolerance towards many modified proteins []. Some authors consider this to be one of the main factors in the development of autoimmune diseases [,]. It is worth noting that due to the imbalance of pro- and anti-oxidant processes in senescent cells, non-enzymatic PTMs (such as cysteine redox PTMs, acyl transfers, glycation, formylation, etc.) are apparently most common, as are some enzymatic PTMs (such as mono- and poly-ADP-ribosylation) [,]. Thus, the appearance of specific modifications may be more likely in the senescent state and may not occur in normal cells. Therefore, further search for common patterns of PTMs characteristic of senescent cells, as well as identification of specific PTMs, may open prospects for the development of a relatively universal senolytic vaccine.
Another approach is related to the detection of the activity of transposable elements (TEs), DNA segments that are capable of moving within the genome and are genetic parasites []. This heterogeneous group includes more than 800 subfamilies and can be divided into the following three categories: LTR (Long Terminal Repeats), LINE, and SINE (Long and Short Interspersed Nuclear Elements, respectively). In total, TEs occupy approximately 45% of the human and mouse genomes []. Due to their mutagenic potential, TE activity is constantly suppressed in healthy cells. However, with age, their activity increases significantly and accompanies the transition of cells to a senescent state [,]. Interestingly, TEs play a physiological role in the thymus; their activity is increased in mTECs and in plasmacytoid dendritic cells, where TEs enrich MHC-I immunopeptide, thus participating in the negative selection of thymocytes []. Despite this, many TE protein products are highly immunogenic, which is associated with an increase in the risk of autoimmune diseases with age due to the activation of TEs in various tissues [,,,]. This suggests the formation of incomplete tolerance to the entire diversity of TE antigens. Currently, there is very limited data on the activity of specific TE subfamilies in the thymus, depending on age. In general, the highest activity of LINE and SINE is observed at a young age and decreases with thymus involution []. No such general pattern of decreased activity is observed for LTR subfamilies. Some of them are inactivated in early ontogenesis, while others demonstrate high activity in mTECs both before and after thymus involution []. Aging is generally associated with the activation of all TE categories. However, a common pattern characteristic of senescent cells is an increase in the activity of some LINE1 subfamilies and even the formation of retrovirus-like particles []. Thus, the search for the most characteristic senescent cell subfamilies of TEs with low activity in the thymus, as well as the identification of their protein products, opens up opportunities for the creation of effective senolytic vaccines directed against various types of senescent cells.
In addition, a recent study demonstrated that during skin aging, latent cytomegalovirus is awakened in senescent fibroblasts. This leads to the presentation of glycoprotein B epitopes in MHC-II molecules and the destruction of senescent cells by CD4+ lymphocytes with cytotoxic action [,]. It is plausible to speculate that cell aging is associated with the activation of other dormant pathogenic or commensal viruses, and the intensification of the immune response against them can serve to eliminate senescent cells [,]. Therefore, a detailed study of the genetic and antigen profiles of senescent cells, taking into account the features of the formation of central tolerance, opens up broad prospects for the development of universal, safe, and effective senolytic vaccines.
3. Peripheral Tolerance for Senescent Antigens
Negative selection in the thymus is not absolutely efficient, and a significant portion of potentially autoreactive T cells end up in the periphery. This requires the presence of peripheral mechanisms that suppress autoreactive cells and ensure irresponsiveness to self-antigens. The mechanisms of peripheral tolerance are very diverse and are well described by now [,]. In general, they are aimed at preventing or limiting the activation of autoreactive T and B cells. The tolerance of autoreactive lymphocytes to cognate antigens is achieved through various mechanisms: quiescence, ignorance, anergy, exhaustion, deletion of clones via the induction of apoptosis, and finally senescence []. It is worth noting that under conditions favorable for the induction of tolerance, lymphocytes of any specificity can be inactivated through these mechanisms. This can lead to the formation of tolerance to antigens that the immune system previously perceived as foreign []. Activation of a naive B cell is impossible without a T lymphocyte of the appropriate specificity, in particular due to the follicular exclusion mechanism [,]; therefore, further description of peripheral tolerance will be given from the T lymphocyte perspective. Naive T cells emerging from the thymus recognize various self-antigens in the peripheral area with low specificity. This provides them with a tonic TCR signal necessary for survival and homeostasis. At the same time, the intensity of this signal is below the activation threshold, which keeps lymphocytes in a quiescent state []. The mechanisms of ignorance are poorly understood; apparently, they are associated not only with low TCR specificity but also with low density and availability of self-antigens for recognition by naive T cells []. Deficiency of co-stimulation underlies T cell anergy and prevents activation of naive autoreactive T lymphocytes upon interaction with antigen-presenting cells (APCs) []. Exhaustion is typical of Teff cells that receive antigenic stimulation for a long time, for example, during persistent infections or autoimmune inflammation []. Exhausted cells react weakly to cognate antigens and produce cytokines poorly compared to normal Teff cells. These cells express inhibitory molecules (such as PD1, LAG3, TIGIT, CD38, CD39, TIM3, etc.) and also have special epigenetic, transcriptional, and metabolic features that do not allow them to enter a quiescent-like cell state, characteristic of memory T cells (Tmem) [,]. Like other cells, lymphocytes are also subject to aging. Repeated antigenic stimulation of TCR can lead to replicative exhaustion, shortening of telomeres, decreased reactivity, and other signs of senescence. Although lymphocyte senescence is poorly understood, there is evidence of both low reactivity and proliferative potential of these cells [,,]. One of the most important mechanisms of peripheral tolerance is the deletion of autoreactive cells [,,]. Death by apoptosis can occur in different situations. To form an immune response, activation of a threshold number of T cells or otherwise achieving a quorum of T cells is necessary []. Without the necessary quorum, the immune response does not develop, and previously activated T cells are likely to die by apoptosis []. Also, deletion of lymphocytes through apoptosis occurs with insufficient stimulation, and only a small part of these cells goes into anergy. Interaction with tolerogenic APCs or Tregs usually ends in the death of the lymphocyte. However, occasionally it leads to the induction of peripheral Tregs []. This is an essential mechanism of peripheral tolerance, which plays an important role in expanding the spectrum of antigens to which relatively stable tolerance is formed in the periphery.
Age-related changes in the repertoires of peripherally presented antigens can lead to activation or, conversely, suppression of the immune response due to the mechanisms described above [,]. Apparently, depletion and transition of T lymphocytes to a senescent state are important factors in suppression of the immune response during prolonged persistence of antigens. This is observed in various chronic infections or malignant processes []. At the same time, the relationship between persistent infections and the development of autoimmune diseases is well known, which implies non-specific activation of adaptive immunity and violation of auto-tolerance [,]. This relationship is largely explained by genetic factors, dysfunction of Treg cells, and antigen mimicry. However, there is another important mechanism associated with an increase in the probability of achieving a quorum by potentially autoreactive T cells. Thus, during acute or short-term infections, high-amplitude activation of a small number of T-lymphocyte clones specific to the most immunogenic antigens occurs [,]. In long-term persistent infections, the diversity of antigens that activate different clones of T cells is significantly higher. This is due to the duration of the process, tissue damage, and an increased availability of some autoantigens [,]. All this leads to an expansion of the repertoire of T lymphocytes involved in the immune response and increases the likelihood of the involvement of cross-reactive T cells capable of recognizing self-antigens. Moreover, the activation of such cells and their transition from naive cells to Teff and Tmem cells is accompanied by a decrease in the activation threshold due to increased expression of co-stimulation receptors, as well as oligomerization and formation of TCR nanoclusters [,]. A decrease in the activation threshold as well as a significant increase in the probability of achieving quorum by potentially autoreactive cells under inflammatory conditions are critical risk factors for the breakdown of auto-tolerance in persistent infections and oncological processes.
Senescent cells are constantly formed throughout life. With age, they accumulate in tissues. The constant expansion of the SA spectrum leads to an increase in the clonal diversity of anti-senescent T lymphocytes. Together with the production of inflammatory SASP factors, this increases the probability of activation of the threshold number of potentially autoreactive T lymphocytes []. This, apparently, is one of the risk factors for the development of autoimmune reactions associated with aging []. At the same time, senescent cells have developed certain mechanisms for evading immune surveillance. They increase the expression of HLA-E (H2-Qa-1), which protects them from NK and CD8+ lymphocytes, and also express the molecules CD24, CD47, and GD2, which transmit the “do not eat me” signal [,]. Apparently, the age-related decrease in the efficiency of elimination of senescent cells by the immune system is associated with the processes described earlier for persistent antigens. A gradual increase in the antigen load exhausts the reserves of the immune system; constant stimulation leads to the depletion of specific T cells or their transition to a senescent state.
Age-related changes in the repertoires of antigen-recognizing receptors of T and B lymphocytes are directly related to the dynamics of the diversity of commensal antigens. There is an increasing body of evidence pointing at the influence of the intestinal microbiome on the aging processes associated with the accumulation of senescent cells, an increase in systemic inflammation, and an increased predisposition to various age-related diseases []. Recent studies demonstrated that constant stimulation of the intestinal microbiome with antigens causes aging of B cells of the germinal centers in the lymph nodes of the small intestine. This leads to the accumulation of B cells with signs of senescence and a shift in the diversity of B cell-produced IgA molecules. In turn, this disrupts the balance of the microbiome and causes additional antigen stimulation, which closes the vicious circle (see Figure 1) []. Chronic local inflammation leads to the damage of tissue barriers and promotes bacterial translocation (the penetration of bacteria or their antigens into the systemic bloodstream) [,]. Chronic inflammation and stimulation with multiple commensal antigens lead to imbalance in the repertoires of antigen-recognizing receptors of Teff and Treg cells, accumulation of Tmem cells, and a decrease in the number of naive lymphocytes [,,]. Under these conditions, the activation threshold of a large number of T cells of various specificities decreases and the probability of achieving quorum by autoreactive lymphocytes increases. In addition, it has been shown that disturbances in the intestinal microbiome cause a decrease in the efficiency of hematopoiesis due to the suppression of multipotent progenitor cells []. Thus, constant antigen stimulation depletes the immune system and is one of the causes of immunoaging and decreased immunoreactivity in old age. This is reflected in an increased risk of autoimmune and oncological processes, as well as an increased susceptibility to infectious diseases and reduced vaccination efficiency in the elderly []. Therefore, the development of senotherapy methods aimed at reducing the number of senescent cells in the gastrointestinal tract along with restoring microbiome balance may be a promising strategy to mitigate the negative consequences of aging. In this context, the development of senolytic vaccines aimed at eliminating senescent B lymphocytes in the germinal centers of the small intestinal lymph nodes represents an example of a potential tissue-specific vaccination strategy. This is of interest from the point of view of the uniqueness of the pathogenetic mechanisms of aging inherent in each tissue or organ. Age-related changes in the microbiome adversely affect the bone marrow output of B cells, their differentiation, and antibody production []. The spontaneous development of germinal centers observed with aging, coupled with the accumulation of senescence-associated T and B cells, contributes to immunoaging and increases the risk of autoimmune reactions []. These changes, in turn, diminish the effectiveness of vaccination and elevate the risks of adverse reactions. This underscores the necessity for further research into the role of the microbiome in shaping the immune response to vaccination, as well as the development of effective strategies for microbiome correction in older individuals, particularly during vaccination periods. Recent studies have demonstrated that bifidobacterium can significantly enhance the vaccine response and represent an important component of the microbiome in youth []. It is evident that vaccination, when combined with microbiome correction, may enhance the efficacy and safety of the use of senolytic vaccines in older individuals.
Thus, the development of autoimmune reactions appears to be the greatest threat to the intensification of the immune response against SA, and both the formed tolerance to SA and the reduced immune reactivity typical of old age create additional difficulties in the development of senolytic vaccines []. Despite the attractiveness of such platforms and the demand for vaccine-based approaches for the prevention and therapy of age-related diseases, the development of such methods still presents a serious challenge for modern immunology and biotechnology []. However, alternative methods of combating the pathological accumulation of senescent cells, which rely on various senolytics and senomorphics, are currently being widely studied [,]. Perhaps the use of approaches combining specific stimulation of the immune response with the use of other methods of senotherapy will provide a safe and effective method for reducing the senescent load.
4. The Current State of the Senotherapeutic Approaches
In the last decade, encouraging results have been obtained in the study of senolytics, drugs that selectively destroy senescent cells by inducing apoptosis []. The demonstrated positive effect of dasatinib and quercetin on senescent cells led to widespread research into senolytics []. These drugs are able to induce apoptosis in senescent cells by suppressing ephrins (EFNB-1/3) and phosphatidylinositol 3-kinase (PI3K), respectively []. These targets play an important role in the molecular cascades that control senescent cell survival and apoptotic death, and their blockade causes the elimination of senescent cells of various localizations [,]. Although the precise mechanisms of action of senolytics are not yet fully understood, it is hypothesized that transcriptomic differences between healthy and senescent cells confer a degree of selectivity in the effects of these drugs [,]. The need to increase efficiency and specificity while reducing the risk of off-target effects led to the emergence of the second generation of senolytics. The substances in this group are diverse and can act through a wide range of senolytic mechanisms. These include inhibition of intracellular pathways essential for senescent cells, depletion of metabolites critical for these cells, disruption of proteostasis, electrolyte balance, aggravation of mitochondrial dysfunction, promotion of ROS accumulation, and many others. []. The main molecular targets of the second generation of senolytics include proteins of the Bcl-2 family, p16, p21, p53, Akt, HSP90, PI3K, FOXO4, SA-β-Gal, and others [,]. In addition, the action of some substances from this group, such as cardiac glycosides, is associated with suppression of Na+/K+-ATPase, which leads to destabilization of the membrane potential and a decrease in intracellular pH []. It is assumed that senescent cells are already at the limit of their adaptive reserve, and additional stress leads to their selective death.
Another strategy of senotherapy is aimed at minimizing the impact of senescent cells on the body. Usually, drugs from this group (senomorphics) inhibit the production of pro-inflammatory SASP factors in various ways without killing senescent cells []. The most well-known representatives of senomorphics are rapamycin, metformin, quercetin, resveratrol, aspirin, and statins []. The mechanisms of action of this group of substances are very diverse and have not been sufficiently studied. Most often, their action is directed at the molecular targets of the following signaling pathways: mTOR, NF-κB, AMPK, SIRT1, IGF-1, NRF2, p38MAPK, IRAK1, etc. [,]. Interestingly, more and more data are accumulating that senescent cells can perform useful physiological functions []. For example, it was demonstrated that in some cases, their elimination can negatively affect the structure of tissues, slow down regeneration and wound healing, and, at the early stages of development, even lead to disruption of the development of organs and tissues [,]. Therefore, in some situations, the use of senomorphics may be more preferable. The uniqueness of the aging mechanisms for each cell type is associated with the functional and structural features of various tissues and organs. Several SAAs have been identified. Some are common to various senescent cell types (e.g., NKG2DL and uPAR), while others exhibit tissue specificity (e.g., Cathepsin F in dermal fibroblasts, CD9 in endothelial cells, KLRG-1 and CD153 in T cells, and CD30 in B cells; see Table 2) [,,,,]. This suggests the potential for tissue-specific elimination of senescent cells, which is important in the context of the uneven aging of different tissues []. However, to minimize off-target effects, further investigation into both common and tissue-specific SSAs is required. This would broaden the therapeutic potential of targeted senotherapeutic approaches, including vaccine-based strategies.

Table 2.
Promising antigens for senolytic vaccine development.
The diversity of the nature of senescent cells explains the selectivity of the action of modern senotherapeutics in relation to certain cell types or subpopulations of senescent cells. Therefore, combinations of some senotherapeutic agents that demonstrated synergistic action and efficiency are currently being studied [,,]. The most studied senotherapeutics include various combinations of dasatinib, quercetin, fisetin, and navitoclax, which have been tested in clinical trials and shown positive results [,]. It should be emphasized that the causes and mechanisms of cellular aging differ for different physiological or pathological processes. This implies the uniqueness of the signs and markers of aging and senescent cells inherent in a specific cell type, tissue, or organ. Therefore, the development of senotherapeutic strategies should take into account not only the fundamental principles of aging but also the specific pathophysiological characteristics of the organism as well as the target tissue or organ. Therefore, the development of senotherapeutic strategies should take into account the specific pathophysiological characteristics of the organism as well as the target tissue or organ. Also promising are strategies targeting the fundamental causes of senescent cell accumulation. For example, selective activation of apoptosis in senescent cells while sparing healthy cells would allow the removal of harmful and dysfunctional cells with minimal side effects. Thus, a primary goal of senotherapy is the safe mitigation of aging’s effects and the prolongation of a healthy lifespan.
5. Discussion
The use of various senotherapy strategies opens up great opportunities for extending the period of active longevity. Positive effects from the use of various senolytics and senomorphics have been demonstrated in recent years [,]. However, their action is still insufficiently specific, is associated with the risk of adverse reactions, and requires higher efficiency []. Relatively high specificity and efficiency in experiments on mice were demonstrated by removing senescent cells using cytotoxic antibodies or CAR-T therapy [,,,,]. However, in the context of widespread use, these approaches are significantly inferior to senolytic vaccines due to their extremely high cost, difficulty of production, and transportation.
Vaccine development is, in principle, a complex and lengthy process that includes searching for and selecting a target antigen, choosing a platform and adjuvant, and assessing efficacy and safety. The latter is the most important point before widespread clinical use is approved. The development of senolytic vaccines opens a fundamentally new stage in the development of vaccine technology, which will require overcoming many pitfalls and hidden risks, primarily those related to safety. It is currently assumed that the restoration of the functions of old tissues and organs will be associated with the removal and replacement of senescent cells with new intact cells with normal vital activity. However, how timely and effective this process will be in various aged tissues remains unknown. For example, the depletion of cells positive for the senescence marker p16 in mouse models caused damage to the hemato-tissue barriers and led to fibrosis in various organs, while the removal of senescent endothelial cells did not lead to their replacement with new cells []. This demonstrates the possible negative effects of the elimination of senescent cells in various tissues, which must be taken into account when developing senolytic methods. Mass removal of senescent cells after vaccination can be associated with the development of a dangerous inflammatory response or the triggering of autoimmune reactions [,,,]. This must be taken into account in the context of the development of prophylactic or therapeutic vaccines. Apparently, preventing the accumulation of a large number of senescent cells in tissues by preventive vaccination carries fewer risks than using a therapeutic approach. In order to minimize the associated risks, senotherapeutic vaccines may be aimed at a narrower range of targets, act on specific target tissues or organs, and take into account their regenerative potential. Hypothetically, senolytic vaccines could be utilized for both therapeutic and preventive purposes. In the therapeutic context, it is proposed that they would eliminate accumulated senescent cells and restore tissue functions. In the preventive context, these vaccines may be administered at a younger age to prevent the age-related accumulation of senescent cells and reduce the likelihood of age-related changes. This is a promising prospect that necessitates further investigation.
Thus, in addition to identifying strictly specific antigens and obtaining an effective immune response that eliminates senescent cells, it is necessary to assess the potential risks associated with the removal of these cells. It is necessary to consider how the elimination of senescent cells will affect the function of the tissue or organ, as well as whether the regenerative potential is sufficient enough to replenish the lost elements in a timely manner []. It is impossible to “cancel” the formed immune response to the vaccine, so minimizing the likelihood of developing an excessive inflammatory response and triggering autoimmune processes is a critical aspect of the development of senolytic vaccines. Obviously, the creation of senolytic vaccines for various purposes will allow the use of personalized approaches for the prevention or treatment of certain age-associated diseases, as well as for the implementation of other anti-aging strategies.
6. Conclusions
In the last 10 years, the rapid development of genetic and omics technologies has opened up great opportunities for detailed research of various aspects of cell aging. High-resolution proteome assessment and single-cell transcriptome studies facilitate the search for new senescence-associated and senescence-specific antigens and allow for a more in-depth analysis of the heterogeneity of senescent cells within a tissue, organ, or between individuals. The novel coronavirus pandemic has accelerated the advancement of various platforms for the development of highly effective and safe vaccines. The new approaches rely on various adjuvants to selectively stimulate humoral or cellular immunity [,,]. In vitro and in silico models are being developed for personalized prediction of adverse effects from vaccination [,,]. In the long term, this will help overcome the main difficulties associated with the search for specific antigens absent in healthy tissues and develop a vaccine-based approach for safe and effective stimulation of the immune response to eliminate senescent cells of a specific type or a more universal method. Therefore, continued research in the field of senotherapy and the development of senolytic vaccines has great potential and opens up prospects for extending the period of active and healthy longevity.
Author Contributions
Conceptualization, D.V.S. and E.M.; writing—review and editing, D.V.S. and R.A.I.; writing—original draft preparation, M.I.V., D.V.S., K.S.M., V.V.S. and R.O.S.; project administration, D.V.S. and R.A.I.; funding acquisition, D.V.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Russian Scientific Foundation, project № 24-15-20003 https://rscf.ru/project/24-15-20003/ (accessed on 1 November 2024).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Vaupel, J.W.; Villavicencio, F.; Bergeron-Boucher, M.-P. Demographic Perspectives on the Rise of Longevity. Proc. Natl. Acad. Sci. USA 2021, 118, e2019536118. [Google Scholar] [CrossRef] [PubMed]
- Global Health Estimates: Life Expectancy and Leading Causes of Death and Disability. Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates (accessed on 30 November 2024).
- Crimmins, E.M. Lifespan and Healthspan: Past, Present, and Promise. Gerontologist 2015, 55, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.J. The Longevity Economy. Lancet Healthy Longev. 2021, 2, e828–e835. [Google Scholar] [CrossRef]
- Harinath, G.; Zalzala, S.; Nyquist, A.; Wouters, M.; Isman, A.; Moel, M.; Verdin, E.; Kaeberlein, M.; Kennedy, B.; Bischof, E. The Role of Quality of Life Data as an Endpoint for Collecting Real-World Evidence within Geroscience Clinical Trials. Ageing Res. Rev. 2024, 97, 102293. [Google Scholar] [CrossRef]
- Wu, R.; Sun, F.; Zhang, W.; Ren, J.; Liu, G.-H. Targeting Aging and Age-Related Diseases with Vaccines. Nat. Aging 2024, 4, 464–482. [Google Scholar] [CrossRef] [PubMed]
- Ghattas, M.; Dwivedi, G.; Lavertu, M.; Alameh, M.-G. Vaccine Technologies and Platforms for Infectious Diseases: Current Progress, Challenges, and Opportunities. Vaccines 2021, 9, 1490. [Google Scholar] [CrossRef]
- Muhs, A.; Hickman, D.T.; Pihlgren, M.; Chuard, N.; Giriens, V.; Meerschman, C.; van der Auwera, I.; van Leuven, F.; Sugawara, M.; Weingertner, M.-C.; et al. Liposomal Vaccines with Conformation-Specific Amyloid Peptide Antigens Define Immune Response and Efficacy in APP Transgenic Mice. Proc. Natl. Acad. Sci. USA 2007, 104, 9810–9815. [Google Scholar] [CrossRef]
- Hickman, D.T.; López-Deber, M.P.; Ndao, D.M.; Silva, A.B.; Nand, D.; Pihlgren, M.; Giriens, V.; Madani, R.; St-Pierre, A.; Karastaneva, H.; et al. Sequence-Independent Control of Peptide Conformation in Liposomal Vaccines for Targeting Protein Misfolding Diseases. J. Biol. Chem. 2011, 286, 13966–13976. [Google Scholar] [CrossRef]
- Rafii, M.S.; Sol, O.; Mobley, W.C.; Delpretti, S.; Skotko, B.G.; Burke, A.D.; Sabbagh, M.N.; Yuan, S.H.; Rissman, R.A.; Pulsifer, M.; et al. Safety, Tolerability, and Immunogenicity of the ACI-24 Vaccine in Adults with Down Syndrome: A Phase 1b Randomized Clinical Trial. JAMA Neurol. 2022, 79, 565–574. [Google Scholar] [CrossRef]
- Wang, C.Y.; Wang, P.-N.; Chiu, M.-J.; Finstad, C.L.; Lin, F.; Lynn, S.; Tai, Y.-H.; De Fang, X.; Zhao, K.; Hung, C.-H.; et al. UB-311, a Novel UBITh® Amyloid β Peptide Vaccine for Mild Alzheimer’s Disease. Alzheimer’s Dement. 2017, 3, 262–272. [Google Scholar] [CrossRef]
- Yu, H.J.; Dickson, S.P.; Wang, P.-N.; Chiu, M.-J.; Huang, C.-C.; Chang, C.-C.; Liu, H.; Hendrix, S.B.; Dodart, J.-C.; Verma, A.; et al. Safety, Tolerability, Immunogenicity, and Efficacy of UB-311 in Participants with Mild Alzheimer’s Disease: A Randomised, Double-Blind, Placebo-Controlled, Phase 2a Study. EBioMedicine 2023, 94, 104665. [Google Scholar] [CrossRef]
- Davtyan, H.; Ghochikyan, A.; Petrushina, I.; Hovakimyan, A.; Davtyan, A.; Cribbs, D.H.; Agadjanyan, M.G. The MultiTEP Platform-Based Alzheimer’s Disease Epitope Vaccine Activates a Broad Repertoire of T Helper Cells in Nonhuman Primates. Alzheimer’s Dement. 2014, 10, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Lacosta, A.-M.; Pascual-Lucas, M.; Pesini, P.; Casabona, D.; Pérez-Grijalba, V.; Marcos-Campos, I.; Sarasa, L.; Canudas, J.; Badi, H.; Monleón, I.; et al. Safety, Tolerability and Immunogenicity of an Active Anti-Aβ40 Vaccine (ABvac40) in Patients with Alzheimer’s Disease: A Randomised, Double-Blind, Placebo-Controlled, Phase I Trial. Alzheimer’s Res. Ther. 2018, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Theunis, C.; Crespo-Biel, N.; Gafner, V.; Pihlgren, M.; López-Deber, M.P.; Reis, P.; Hickman, D.T.; Adolfsson, O.; Chuard, N.; Ndao, D.M.; et al. Efficacy and Safety of a Liposome-Based Vaccine against Protein Tau, Assessed in Tau.P301L Mice That Model Tauopathy. PLoS ONE 2013, 8, e72301. [Google Scholar] [CrossRef] [PubMed]
- Novak, P.; Schmidt, R.; Kontsekova, E.; Kovacech, B.; Smolek, T.; Katina, S.; Fialova, L.; Prcina, M.; Parrak, V.; Dal-Bianco, P.; et al. FUNDAMANT: An Interventional 72-Week Phase 1 Follow-up Study of AADvac1, an Active Immunotherapy against Tau Protein Pathology in Alzheimer’s Disease. Alzheimer’s Res. Ther. 2018, 10, 108. [Google Scholar] [CrossRef] [PubMed]
- Novak, P.; Kovacech, B.; Katina, S.; Schmidt, R.; Scheltens, P.; Kontsekova, E.; Ropele, S.; Fialova, L.; Kramberger, M.; Paulenka-Ivanovova, N.; et al. ADAMANT: A Placebo-Controlled Randomized Phase 2 Study of AADvac1, an Active Immunotherapy against Pathological Tau in Alzheimer’s Disease. Nat. Aging 2021, 1, 521–534. [Google Scholar] [CrossRef]
- Cullen, N.C.; Novak, P.; Tosun, D.; Kovacech, B.; Hanes, J.; Kontsekova, E.; Fresser, M.; Ropele, S.; Feldman, H.H.; Schmidt, R.; et al. Efficacy Assessment of an Active Tau Immunotherapy in Alzheimer’s Disease Patients with Amyloid and Tau Pathology: A Post Hoc Analysis of the “ADAMANT” Randomised, Placebo-Controlled, Double-Blind, Multi-Centre, Phase 2 Clinical Trial. EBioMedicine 2024, 99, 104923. [Google Scholar] [CrossRef]
- Park, H.-H.; Lee, K.-Y.; Kim, S.; Lee, J.W.; Choi, N.-Y.; Lee, E.-H.; Lee, Y.J.; Lee, S.-H.; Koh, S.-H. Novel Vaccine Peptide GV1001 Effectively Blocks β-Amyloid Toxicity by Mimicking the Extra-Telomeric Functions of Human Telomerase Reverse Transcriptase. Neurobiol. Aging 2014, 35, 1255–1274. [Google Scholar] [CrossRef]
- Frenkel, D.; Maron, R.; Burt, D.S.; Weiner, H.L. Nasal Vaccination with a Proteosome-Based Adjuvant and Glatiramer Acetate Clears Beta-Amyloid in a Mouse Model of Alzheimer Disease. J. Clin. Investig. 2005, 115, 2423–2433. [Google Scholar] [CrossRef]
- Pang, Z.; Nakagami, H.; Osako, M.K.; Koriyama, H.; Nakagami, F.; Tomioka, H.; Shimamura, M.; Kurinami, H.; Takami, Y.; Morishita, R.; et al. Therapeutic Vaccine against DPP4 Improves Glucose Metabolism in Mice. Proc. Natl. Acad. Sci. USA 2014, 111, E1256-1263. [Google Scholar] [CrossRef]
- Cavelti-Weder, C.; Timper, K.; Seelig, E.; Keller, C.; Osranek, M.; Lässing, U.; Spohn, G.; Maurer, P.; Müller, P.; Jennings, G.T.; et al. Development of an Interleukin-1β Vaccine in Patients with Type 2 Diabetes. Mol. Ther. 2016, 24, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, X.-L.; Zha, J.; Mao, L.-Z.; Chai, J.-Q.; Liu, R.-T. Therapeutic Vaccine against IL-1β Improved Glucose Control in a Mouse Model of Type 2 Diabetes. Life Sci. 2018, 192, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Yokota, H.; Hayashi, H.; Hanaguri, J.; Yamagami, S.; Kushiyama, A.; Nakagami, H.; Nagaoka, T. Effect of Prorenin Peptide Vaccine on the Early Phase of Diabetic Retinopathy in a Murine Model of Type 2 Diabetes. PLoS ONE 2022, 17, e0262568. [Google Scholar] [CrossRef] [PubMed]
- Downham, M.R.; Auton, T.R.; Rosul, A.; Sharp, H.L.; Sjöström, L.; Rushton, A.; Richards, J.P.; Mant, T.G.K.; Gardiner, S.M.; Bennett, T.; et al. Evaluation of Two Carrier Protein-Angiotensin I Conjugate Vaccines to Assess Their Future Potential to Control High Blood Pressure (Hypertension) in Man. Br. J. Clin. Pharmacol. 2003, 56, 505–512. [Google Scholar] [CrossRef]
- Brown, M.J.; Coltart, J.; Gunewardena, K.; Ritter, J.M.; Auton, T.R.; Glover, J.F. Randomized Double-Blind Placebo-Controlled Study of an Angiotensin Immunotherapeutic Vaccine (PMD3117) in Hypertensive Subjects. Clin. Sci. 2004, 107, 167–173. [Google Scholar] [CrossRef]
- Ambühl, P.M.; Tissot, A.C.; Fulurija, A.; Maurer, P.; Nussberger, J.; Sabat, R.; Nief, V.; Schellekens, C.; Sladko, K.; Roubicek, K.; et al. A Vaccine for Hypertension Based on Virus-like Particles: Preclinical Efficacy and Phase I Safety and Immunogenicity. J. Hypertens. 2007, 25, 63–72. [Google Scholar] [CrossRef]
- Tissot, A.C.; Maurer, P.; Nussberger, J.; Sabat, R.; Pfister, T.; Ignatenko, S.; Volk, H.-D.; Stocker, H.; Müller, P.; Jennings, G.T.; et al. Effect of Immunisation against Angiotensin II with CYT006-AngQb on Ambulatory Blood Pressure: A Double-Blind, Randomised, Placebo-Controlled Phase IIa Study. Lancet 2008, 371, 821–827. [Google Scholar] [CrossRef]
- Chen, X.; Qiu, Z.; Yang, S.; Ding, D.; Chen, F.; Zhou, Y.; Wang, M.; Lin, J.; Yu, X.; Zhou, Z.; et al. Effectiveness and Safety of a Therapeutic Vaccine Against Angiotensin II Receptor Type 1 in Hypertensive Animals. Hypertension 2013, 61, 408–416. [Google Scholar] [CrossRef]
- Pan, Y.; Zhou, Z.; Zhang, H.; Zhou, Y.; Li, Y.; Li, C.; Chen, X.; Yang, S.; Liao, Y.; Qiu, Z. The ATRQβ-001 Vaccine Improves Cardiac Function and Prevents Postinfarction Cardiac Remodeling in Mice. Hypertens. Res. 2019, 42, 329–340. [Google Scholar] [CrossRef]
- Li, C.; Yan, X.; Wu, D.; Zhang, K.; Liang, X.; Pan, Y.; Zhou, Y.; Chen, F.; Chen, X.; Yang, S.; et al. Vaccine Targeted Alpha 1D-Adrenergic Receptor for Hypertension. Hypertension 2019, 74, 1551–1562. [Google Scholar] [CrossRef]
- Kurashiki, T.; Miyake, T.; Nakagami, H.; Nishimura, M.; Morishita, R. Prevention of Progression of Aortic Aneurysm by Peptide Vaccine Against Ang II (Angiotensin II) in a Rat Model. Hypertension 2020, 76, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liao, M.; Cao, M.; Qiu, Z.; Yan, X.; Zhou, Y.; Wu, H.; Wang, Y.; Zheng, J.; Ding, J.; et al. ATRQβ-001 Vaccine Prevents Experimental Abdominal Aortic Aneurysms. JAHA 2019, 8, e012341. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Kobiyama, K.; Winkels, H.; Tse, K.; Miller, J.; Vassallo, M.; Wolf, D.; Ryden, C.; Orecchioni, M.; Dileepan, T.; et al. Regulatory CD4+ T Cells Recognize Major Histocompatibility Complex Class II Molecule-Restricted Peptide Epitopes of Apolipoprotein B. Circulation 2018, 138, 1130–1143. [Google Scholar] [CrossRef] [PubMed]
- Momtazi-Borojeni, A.A.; Jaafari, M.R.; Badiee, A.; Sahebkar, A. Long-Term Generation of antiPCSK9 Antibody Using a Nanoliposome-Based Vaccine Delivery System. Atherosclerosis 2019, 283, 69–78. [Google Scholar] [CrossRef]
- Ma, Z.; Mao, C.; Chen, X.; Yang, S.; Qiu, Z.; Yu, B.; Jia, Y.; Wu, C.; Wang, Y.; Wang, Y.; et al. Peptide Vaccine Against ADAMTS-7 Ameliorates Atherosclerosis and Postinjury Neointima Hyperplasia. Circulation 2023, 147, 728–742. [Google Scholar] [CrossRef]
- Bourinbaiar, A.S.; Jirathitikal, V. Effect of Oral Immunization with Pooled Antigens Derived from Adipose Tissue on Atherosclerosis and Obesity Indices. Vaccine 2010, 28, 2763–2768. [Google Scholar] [CrossRef]
- Bourinbaiar, A.S.; Jirathitikal, V. Safety and Efficacy Trial of Adipose-Tissue Derived Oral Preparation V-6 Immunitor (V-6): Results of Open-Label, Two-Month, Follow-up Study. Lipids Health Dis. 2010, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- von Loga, I.S.; El-Turabi, A.; Jostins, L.; Miotla-Zarebska, J.; Mackay-Alderson, J.; Zeltins, A.; Parisi, I.; Bachmann, M.F.; Vincent, T.L. Active Immunisation Targeting Nerve Growth Factor Attenuates Chronic Pain Behaviour in Murine Osteoarthritis. Ann. Rheum. Dis. 2019, 78, 672–675. [Google Scholar] [CrossRef]
- Sobecki, M.; Chen, J.; Krzywinska, E.; Nagarajan, S.; Fan, Z.; Nelius, E.; Monné Rodriguez, J.M.; Seehusen, F.; Hussein, A.; Moschini, G.; et al. Vaccination-Based Immunotherapy to Target Profibrotic Cells in Liver and Lung. Cell Stem Cell 2022, 29, 1459–1474.e9. [Google Scholar] [CrossRef]
- Wu, D.; Zhou, Y.; Pan, Y.; Li, C.; Wang, Y.; Chen, F.; Chen, X.; Yang, S.; Zhou, Z.; Liao, Y.; et al. Vaccine Against PCSK9 Improved Renal Fibrosis by Regulating Fatty Acid β-Oxidation. J. Am. Heart Assoc. 2020, 9, e014358. [Google Scholar] [CrossRef]
- Meissner, W.G.; Traon, A.P.-L.; Foubert-Samier, A.; Galabova, G.; Galitzky, M.; Kutzelnigg, A.; Laurens, B.; Lührs, P.; Medori, R.; Péran, P.; et al. A Phase 1 Randomized Trial of Specific Active α-Synuclein Immunotherapies PD01A and PD03A in Multiple System Atrophy. Mov. Disord. 2020, 35, 1957–1965. [Google Scholar] [CrossRef] [PubMed]
- Nimmo, J.T.; Smith, H.; Wang, C.Y.; Teeling, J.L.; Nicoll, J.A.R.; Verma, A.; Dodart, J.-C.; Liu, Z.; Lin, F.; Carare, R.O. Immunisation with UB-312 in the Thy1SNCA Mouse Prevents Motor Performance Deficits and Oligomeric α-Synuclein Accumulation in the Brain and Gut. Acta Neuropathol. 2022, 143, 55–73. [Google Scholar] [CrossRef] [PubMed]
- Eijsvogel, P.; Misra, P.; Concha-Marambio, L.; Boyd, J.D.; Ding, S.; Fedor, L.; Hsieh, Y.-T.; Sun, Y.S.; Vroom, M.M.; Farris, C.M.; et al. Target Engagement and Immunogenicity of an Active Immunotherapeutic Targeting Pathological α-Synuclein: A Phase 1 Placebo-Controlled Trial. Nat. Med. 2024, 30, 2631–2640. [Google Scholar] [CrossRef] [PubMed]
- Haffer, K.N. Effects of Novel Vaccines on Weight Loss in Diet-Induced-Obese (DIO) Mice. J. Anim. Sci. Biotechnol. 2012, 3, 21. [Google Scholar] [CrossRef] [PubMed]
- Fulurija, A.; Lutz, T.A.; Sladko, K.; Osto, M.; Wielinga, P.Y.; Bachmann, M.F.; Saudan, P. Vaccination against GIP for the Treatment of Obesity. PLoS ONE 2008, 3, e3163. [Google Scholar] [CrossRef]
- Vizcarra, J.A.; Kirby, J.D.; Kim, S.K.; Galyean, M.L. Active Immunization against Ghrelin Decreases Weight Gain and Alters Plasma Concentrations of Growth Hormone in Growing Pigs. Domest. Anim. Endocrinol. 2007, 33, 176–189. [Google Scholar] [CrossRef]
- Zorrilla, E.P.; Iwasaki, S.; Moss, J.A.; Chang, J.; Otsuji, J.; Inoue, K.; Meijler, M.M.; Janda, K.D. Vaccination against Weight Gain. Proc. Natl. Acad. Sci. USA 2006, 103, 13226–13231. [Google Scholar] [CrossRef]
- Andrade, S.; Pinho, F.; Ribeiro, A.M.; Carreira, M.; Casanueva, F.F.; Roy, P.; Monteiro, M.P. Immunization against Active Ghrelin Using Virus-like Particles for Obesity Treatment. Curr. Pharm. Des. 2013, 19, 6551–6558. [Google Scholar] [CrossRef]
- Karin, O.; Agrawal, A.; Porat, Z.; Krizhanovsky, V.; Alon, U. Senescent Cell Turnover Slows with Age Providing an Explanation for the Gompertz Law. Nat. Commun. 2019, 10, 5495. [Google Scholar] [CrossRef]
- McHugh, D.; Gil, J. Senescence and Aging: Causes, Consequences, and Therapeutic Avenues. J. Cell Biol. 2018, 217, 65–77. [Google Scholar] [CrossRef]
- Tripathi, U.; Misra, A.; Tchkonia, T.; Kirkland, J.L. Impact of Senescent Cell Subtypes on Tissue Dysfunction and Repair: Importance and Research Questions. Mech. Ageing Dev. 2021, 198, 111548. [Google Scholar] [CrossRef]
- Matveeva, K.; Vasilieva, M.; Minskaia, E.; Rybtsov, S.; Shevyrev, D. T-Cell Immunity against Senescence: Potential Role and Perspectives. Front. Immunol. 2024, 15, 1360109. [Google Scholar] [CrossRef]
- Shin, D.-M.; Kucia, M.; Ratajczak, M.Z. Nuclear and Chromatin Reorganization during Cell Senescence and Aging—A Mini-Review. Gerontology 2010, 57, 76–84. [Google Scholar] [CrossRef]
- Sławińska, N.; Krupa, R. Molecular Aspects of Senescence and Organismal Ageing-DNA Damage Response, Telomeres, Inflammation and Chromatin. Int. J. Mol. Sci. 2021, 22, 590. [Google Scholar] [CrossRef]
- Wallis, R.; Milligan, D.; Hughes, B.; Mizen, H.; López-Domínguez, J.A.; Eduputa, U.; Tyler, E.J.; Serrano, M.; Bishop, C.L. Senescence-Associated Morphological Profiles (SAMPs): An Image-Based Phenotypic Profiling Method for Evaluating the Inter and Intra Model Heterogeneity of Senescence. Aging 2022, 14, 4220–4246. [Google Scholar] [CrossRef]
- Heckenbach, I.; Mkrtchyan, G.V.; Ezra, M.B.; Bakula, D.; Madsen, J.S.; Nielsen, M.H.; Oró, D.; Osborne, B.; Covarrubias, A.J.; Idda, M.L.; et al. Nuclear Morphology Is a Deep Learning Biomarker of Cellular Senescence. Nat. Aging 2022, 2, 742–755. [Google Scholar] [CrossRef]
- Park, J.T.; Lee, Y.-S.; Cho, K.A.; Park, S.C. Adjustment of the Lysosomal-Mitochondrial Axis for Control of Cellular Senescence. Ageing Res. Rev. 2018, 47, 176–182. [Google Scholar] [CrossRef]
- Ashraf, H.M.; Fernandez, B.; Spencer, S.L. The intensities of canonical senescence biomarkers integrate the duration of cell-cycle withdrawal. Nat. Commun. 2023, 14, 4527. [Google Scholar] [CrossRef]
- Hernandez-Segura, A.; Nehme, J.; Demaria, M. Hallmarks of Cellular Senescence. Trends Cell Biol. 2018, 28, 436–453. [Google Scholar] [CrossRef]
- Dodig, S.; Čepelak, I.; Pavić, I. Hallmarks of Senescence and Aging. Biochem. Med. 2019, 29, 030501. [Google Scholar] [CrossRef]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef]
- Coppé, J.-P.; Desprez, P.-Y.; Krtolica, A.; Campisi, J. The Senescence-Associated Secretory Phenotype: The Dark Side of Tumor Suppression. Annu. Rev. Pathol. 2010, 5, 99–118. [Google Scholar] [CrossRef]
- Marin, I.; Boix, O.; Garcia-Garijo, A.; Sirois, I.; Caballe, A.; Zarzuela, E.; Ruano, I.; Attolini, C.S.-O.; Prats, N.; López-Domínguez, J.A.; et al. Cellular Senescence Is Immunogenic and Promotes Antitumor Immunity. Cancer Discov. 2023, 13, 410–431. [Google Scholar] [CrossRef]
- Santos, A.L.; Lindner, A.B. Protein Posttranslational Modifications: Roles in Aging and Age-Related Disease. Oxid. Med. Cell Longev. 2017, 2017, 5716409. [Google Scholar] [CrossRef]
- Ebert, T.; Tran, N.; Schurgers, L.; Stenvinkel, P.; Shiels, P.G. Ageing—Oxidative Stress, PTMs and Disease. Mol. Aspects Med. 2022, 86, 101099. [Google Scholar] [CrossRef]
- Wu, Z.; Qu, J.; Liu, G.-H. Roles of Chromatin and Genome Instability in Cellular Senescence and Their Relevance to Ageing and Related Diseases. Nat. Rev. Mol. Cell Biol. 2024, 25, 979–1000. [Google Scholar] [CrossRef]
- Di Giorgio, E.; Ranzino, L.; Tolotto, V.; Dalla, E.; Burelli, M.; Gualandi, N.; Brancolini, C. Transcription of Endogenous Retroviruses in Senescent Cells Contributes to the Accumulation of Double-Stranded RNAs That Trigger an Anti-Viral Response That Reinforces Senescence. Cell Death Dis. 2024, 15, 157. [Google Scholar] [CrossRef]
- Amor, C.; Fernández-Maestre, I.; Chowdhury, S.; Ho, Y.-J.; Nadella, S.; Graham, C.; Carrasco, S.E.; Nnuji-John, E.; Feucht, J.; Hinterleitner, C.; et al. Prophylactic and Long-Lasting Efficacy of Senolytic CAR T Cells against Age-Related Metabolic Dysfunction. Nat. Aging 2024, 4, 336–349. [Google Scholar] [CrossRef]
- Bulbiankova, D.; Díaz-Puertas, R.; Álvarez-Martínez, F.J.; Herranz-López, M.; Barrajón-Catalán, E.; Micol, V. Hallmarks and Biomarkers of Skin Senescence: An Updated Review of Skin Senotherapeutics. Antioxidants 2023, 12, 444. [Google Scholar] [CrossRef]
- Schmitt, C.A.; Wang, B.; Demaria, M. Senescence and Cancer—Role and Therapeutic Opportunities. Nat. Rev. Clin. Oncol. 2022, 19, 619–636. [Google Scholar] [CrossRef]
- Fulop, T.; Larbi, A.; Pawelec, G.; Khalil, A.; Cohen, A.A.; Hirokawa, K.; Witkowski, J.M.; Franceschi, C. Immunology of Aging: The Birth of Inflammaging. Clin. Rev. Allergy Immunol. 2023, 64, 109–122. [Google Scholar] [CrossRef]
- Cai, Y.; Zhou, H.; Zhu, Y.; Sun, Q.; Ji, Y.; Xue, A.; Wang, Y.; Chen, W.; Yu, X.; Wang, L.; et al. Elimination of Senescent Cells by β-Galactosidase-Targeted Prodrug Attenuates Inflammation and Restores Physical Function in Aged Mice. Cell Res. 2020, 30, 574–589. [Google Scholar] [CrossRef]
- Chen, B.; Zhu, R.; Hu, H.; Zhan, M.; Wang, T.; Huang, F.; Wei, F.; Chai, Y.; Ling, Z.; Zou, X. Elimination of Senescent Cells by Senolytics Facilitates Bony Endplate Microvessel Formation and Mitigates Disc Degeneration in Aged Mice. Front. Cell Dev. Biol. 2022, 10, 853688. [Google Scholar] [CrossRef]
- Lorenzo, E.C.; Torrance, B.L.; Haynes, L. Impact of Senolytic Treatment on Immunity, Aging, and Disease. Front. Aging 2023, 4, 1161799. [Google Scholar] [CrossRef]
- Aguado, J.; Amarilla, A.A.; Taherian Fard, A.; Albornoz, E.A.; Tyshkovskiy, A.; Schwabenland, M.; Chaggar, H.K.; Modhiran, N.; Gómez-Inclán, C.; Javed, I.; et al. Senolytic Therapy Alleviates Physiological Human Brain Aging and COVID-19 Neuropathology. Nat. Aging 2023, 3, 1561–1575. [Google Scholar] [CrossRef]
- Martel, J.; Ojcius, D.M.; Wu, C.-Y.; Peng, H.-H.; Voisin, L.; Perfettini, J.-L.; Ko, Y.-F.; Young, J.D. Emerging Use of Senolytics and Senomorphics against Aging and Chronic Diseases. Med. Res. Rev. 2020, 40, 2114–2131. [Google Scholar] [CrossRef]
- Luís, C.; Maduro, A.T.; Pereira, P.; Mendes, J.J.; Soares, R.; Ramalho, R. Nutritional Senolytics and Senomorphics: Implications to Immune Cells Metabolism and Aging—From Theory to Practice. Front. Nutr. 2022, 9, 958563. [Google Scholar] [CrossRef]
- Suda, M.; Shimizu, I.; Katsuumi, G.; Hsiao, C.L.; Yoshida, Y.; Matsumoto, N.; Yoshida, Y.; Katayama, A.; Wada, J.; Seki, M.; et al. Glycoprotein Nonmetastatic Melanoma Protein B Regulates Lysosomal Integrity and Lifespan of Senescent Cells. Sci. Rep. 2022, 12, 6522. [Google Scholar] [CrossRef]
- Yoshida, S.; Nakagami, H.; Hayashi, H.; Ikeda, Y.; Sun, J.; Tenma, A.; Tomioka, H.; Kawano, T.; Shimamura, M.; Morishita, R.; et al. The CD153 Vaccine Is a Senotherapeutic Option for Preventing the Accumulation of Senescent T Cells in Mice. Nat. Commun. 2020, 11, 2482. [Google Scholar] [CrossRef]
- Lear, T.B.; Finkel, T. Senolytic Vaccination: A New Mandate for Cardiovascular Health? J. Cardiovasc. Aging 2022, 2, 17. [Google Scholar] [CrossRef]
- Deng, Y.; Kumar, A.; Xie, K.; Schaaf, K.; Scifo, E.; Morsy, S.; Li, T.; Ehninger, A.; Bano, D.; Ehninger, D. Targeting Senescent Cells with NKG2D-CAR T Cells. Cell Death Discov. 2024, 10, 217. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, A.R.; Larrick, J.W. Antiaging Vaccines Targeting Senescent Cells. Rejuvenation Res. 2022, 25, 39–45. [Google Scholar] [CrossRef]
- Prata, L.G.P.L.; Ovsyannikova, I.G.; Tchkonia, T.; Kirkland, J.L. Senescent Cell Clearance by the Immune System: Emerging Therapeutic Opportunities. Semin. Immunol. 2018, 40, 101275. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.I.; Devine, O.P.; Vukmanovic-Stejic, M.; Chambers, E.S.; Subramanian, P.; Patel, N.; Virasami, A.; Sebire, N.J.; Kinsler, V.; Valdovinos, A.; et al. Senescent Cells Evade Immune Clearance via HLA-E-Mediated NK and CD8+ T Cell Inhibition. Nat. Commun. 2019, 10, 2387. [Google Scholar] [CrossRef]
- Nemazee, D. Mechanisms of Central Tolerance for B Cells. Nat. Rev. Immunol. 2017, 17, 281–294. [Google Scholar] [CrossRef]
- Xing, Y.; Hogquist, K.A. T-Cell Tolerance: Central and Peripheral. Cold Spring Harb. Perspect. Biol. 2012, 4, a006957. [Google Scholar] [CrossRef]
- Getahun, A. Role of Inhibitory Signaling in Peripheral B Cell Tolerance. Immunol. Rev. 2022, 307, 27–42. [Google Scholar] [CrossRef]
- Kishimoto, H.; Sprent, J. Negative Selection in the Thymus Includes Semimature T Cells. J. Exp. Med. 1997, 185, 263–271. [Google Scholar] [CrossRef]
- Chen, J.W.; Schickel, J.-N.; Tsakiris, N.; Sng, J.; Arbogast, F.; Bouis, D.; Parisi, D.; Gera, R.; Boeckers, J.M.; Delmotte, F.R.; et al. Positive and Negative Selection Shape the Human Naive B Cell Repertoire. J. Clin. Investig. 2022, 132, e150985. [Google Scholar] [CrossRef] [PubMed]
- Shevyrev, D.; Tereshchenko, V.; Kozlov, V.; Sennikov, S. Phylogeny, Structure, Functions, and Role of AIRE in the Formation of T-Cell Subsets. Cells 2022, 11, 194. [Google Scholar] [CrossRef]
- Ding, P.; Gao, C.; Gao, Y.; Liu, D.; Li, H.; Xu, J.; Chen, X.; Huang, Y.; Zhang, C.; Zheng, M.; et al. Osteocytes Regulate Senescence of Bone and Bone Marrow. Elife 2022, 11, e81480. [Google Scholar] [CrossRef] [PubMed]
- Hellmich, C.; Moore, J.A.; Bowles, K.M.; Rushworth, S.A. Bone Marrow Senescence and the Microenvironment of Hematological Malignancies. Front. Oncol. 2020, 10, 230. [Google Scholar] [CrossRef] [PubMed]
- Chen, X. From Immune Equilibrium to Immunodynamics. Front. Microbiol. 2022, 13, 1018817. [Google Scholar] [CrossRef] [PubMed]
- Eberl, G. Immunity by Equilibrium. Nat. Rev. Immunol. 2016, 16, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Březina, J.; Vobořil, M.; Filipp, D. Mechanisms of Direct and Indirect Presentation of Self-Antigens in the Thymus. Front. Immunol. 2022, 13, 926625. [Google Scholar] [CrossRef]
- Barbouti, A.; Evangelou, K.; Pateras, I.S.; Papoudou-Bai, A.; Patereli, A.; Stefanaki, K.; Rontogianni, D.; Muñoz-Espín, D.; Kanavaros, P.; Gorgoulis, V.G. In Situ Evidence of Cellular Senescence in Thymic Epithelial Cells (TECs) during Human Thymic Involution. Mech. Ageing Dev. 2019, 177, 88–90. [Google Scholar] [CrossRef]
- Thomas, R.; Wang, W.; Su, D.-M. Contributions of Age-Related Thymic Involution to Immunosenescence and Inflammaging. Immun. Ageing 2020, 17, 2. [Google Scholar] [CrossRef]
- Carter, J.A.; Strömich, L.; Peacey, M.; Chapin, S.R.; Velten, L.; Steinmetz, L.M.; Brors, B.; Pinto, S.; Meyer, H.V. Transcriptomic Diversity in Human Medullary Thymic Epithelial Cells. Nat. Commun. 2022, 13, 4296. [Google Scholar] [CrossRef] [PubMed]
- Fergusson, J.R.; Morgan, M.D.; Bruchard, M.; Huitema, L.; Heesters, B.A.; van Unen, V.; van Hamburg, J.P.; van der Wel, N.N.; Picavet, D.; Koning, F.; et al. Maturing Human CD127+ CCR7+ PDL1+ Dendritic Cells Express AIRE in the Absence of Tissue Restricted Antigens. Front. Immunol. 2019, 9, 2902. [Google Scholar] [CrossRef]
- Baba, T.; Nakamoto, Y.; Mukaida, N. Crucial Contribution of Thymic Sirp Alpha+ Conventional Dendritic Cells to Central Tolerance against Blood-Borne Antigens in a CCR2-Dependent Manner. J. Immunol. 2009, 183, 3053–3063. [Google Scholar] [CrossRef]
- Agrawal, A.; Sridharan, A.; Prakash, S.; Agrawal, H. Dendritic Cells and Aging: Consequences for Autoimmunity. Expert. Rev. Clin. Immunol. 2012, 8, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, H.; Matsumoto, T. Mechanisms of Tolerance Induction by Dendritic Cells In Vivo. Front. Immunol. 2018, 9, 350. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sekai, M.; Matsui, T.; Fujii, Y.; Matsumoto, M.; Takeuchi, O.; Minato, N.; Hamazaki, Y. Hassall’s Corpuscles with Cellular-Senescence Features Maintain IFNα Production through Neutrophils and pDC Activation in the Thymus. Int. Immunol. 2019, 31, 127–139. [Google Scholar] [CrossRef]
- Liang, Z.; Dong, X.; Zhang, Z.; Zhang, Q.; Zhao, Y. Age-Related Thymic Involution: Mechanisms and Functional Impact. Aging Cell 2022, 21, e13671. [Google Scholar] [CrossRef]
- Li, Y.; Chen, P.; Huang, H.; Feng, H.; Ran, H.; Liu, W. Quantification of Dendritic Cell Subsets in Human Thymus Tissues of Various Ages. Immun. Ageing 2021, 18, 44. [Google Scholar] [CrossRef]
- Granadier, D.; Iovino, L.; Kinsella, S.; Dudakov, J.A. Dynamics of Thymus Function and T Cell Receptor Repertoire Breadth in Health and Disease. Semin. Immunopathol. 2021, 43, 119–134. [Google Scholar] [CrossRef]
- Britanova, O.V.; Putintseva, E.V.; Shugay, M.; Merzlyak, E.M.; Turchaninova, M.A.; Staroverov, D.B.; Bolotin, D.A.; Lukyanov, S.; Bogdanova, E.A.; Mamedov, I.Z.; et al. Age-Related Decrease in TCR Repertoire Diversity Measured with Deep and Normalized Sequence Profiling. J. Immunol. 2014, 192, 2689–2698. [Google Scholar] [CrossRef] [PubMed]
- Jennings, E.Q.; Fritz, K.S.; Galligan, J.J. Biochemical Genesis of Enzymatic and Non-Enzymatic Post-Translational Modifications. Mol. Aspects Med. 2022, 86, 101053. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.J.; Bloodworth, N.; Shao, Q.; Shabanowitz, J.; Hunt, D.; Meiler, J.; Pires, M.M. A Chemical Approach to Assess the Impact of Post-Translational Modification on MHC Peptide Binding and Effector Cell Engagement. ACS Chem. Biol. 2024, 19, 1991–2001. [Google Scholar] [CrossRef]
- Haro, I.; Sanmartí, R.; Gómara, M.J. Implications of Post-Translational Modifications in Autoimmunity with Emphasis on Citrullination, Homocitrullination and Acetylation for the Pathogenesis, Diagnosis and Prognosis of Rheumatoid Arthritis. Int. J. Mol. Sci. 2022, 23, 15803. [Google Scholar] [CrossRef]
- Doyle, H.A.; Mamula, M.J. Autoantigenesis: The Evolution of Protein Modifications in Autoimmune Disease. Curr. Opin. Immunol. 2012, 24, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Raposo, B.; Merky, P.; Lundqvist, C.; Yamada, H.; Urbonaviciute, V.; Niaudet, C.; Viljanen, J.; Kihlberg, J.; Kyewski, B.; Ekwall, O.; et al. T Cells Specific for Post-Translational Modifications Escape Intrathymic Tolerance Induction. Nat. Commun. 2018, 9, 353. [Google Scholar] [CrossRef] [PubMed]
- Hayward, A.; Gilbert, C. Transposable Elements. Curr. Biol. 2022, 32, R904–R909. [Google Scholar] [CrossRef] [PubMed]
- Larouche, J.-D.; Laumont, C.M.; Trofimov, A.; Vincent, K.; Hesnard, L.; Brochu, S.; Côté, C.; Humeau, J.F.; Bonneil, É.; Lanoix, J.; et al. Transposable Elements Regulate Thymus Development and Function. Elife 2024, 12, RP91037. [Google Scholar] [CrossRef]
- Pabis, K.; Barardo, D.; Sirbu, O.; Selvarajoo, K.; Gruber, J.; Kennedy, B.K. A Concerted Increase in Readthrough and Intron Retention Drives Transposon Expression during Aging and Senescence. Elife 2024, 12, RP87811. [Google Scholar] [CrossRef]
- Colombo, A.R.; Elias, H.K.; Ramsingh, G. Senescence Induction Universally Activates Transposable Element Expression. Cell Cycle 2018, 17, 1846–1857. [Google Scholar] [CrossRef]
- Kelly, M.; Lihua, S.; Zhe, Z.; Li, S.; Yoselin, P.; Michelle, P.; Sullivan Kathleen, E. Transposable Element Dysregulation in Systemic Lupus Erythematosus and Regulation by Histone Conformation and Hsp90. Clin. Immunol. 2018, 197, 6–18. [Google Scholar] [CrossRef]
- Stetson, D.B. Endogenous Retroelements and Autoimmune Disease. Curr. Opin. Immunol. 2012, 24, 692–697. [Google Scholar] [CrossRef]
- Volkman, H.E.; Stetson, D.B. The Enemy within: Endogenous Retroelements and Autoimmune Disease. Nat. Immunol. 2014, 15, 415–422. [Google Scholar] [CrossRef]
- De Cecco, M.; Ito, T.; Petrashen, A.P.; Elias, A.E.; Skvir, N.J.; Criscione, S.W.; Caligiana, A.; Brocculi, G.; Adney, E.M.; Boeke, J.D.; et al. L1 Drives IFN in Senescent Cells and Promotes Age-Associated Inflammation. Nature 2019, 566, 73–78. [Google Scholar] [CrossRef]
- Hasegawa, T.; Oka, T.; Son, H.G.; Oliver-García, V.S.; Azin, M.; Eisenhaure, T.M.; Lieb, D.J.; Hacohen, N.; Demehri, S. Cytotoxic CD4+ T Cells Eliminate Senescent Cells by Targeting Cytomegalovirus Antigen. Cell 2023, 186, 1417–1431.e20. [Google Scholar] [CrossRef] [PubMed]
- Bordon, Y. T Cells Target Viral Protein to Remove Senescent Fibroblasts. Nat. Rev. Immunol. 2023, 23, 271. [Google Scholar] [CrossRef] [PubMed]
- Klopack, E.T. Chronic Stress and Latent Virus Reactivation: Effects on Immune Aging, Chronic Disease Morbidity, and Mortality. J. Gerontol. B Psychol. Sci. Soc. Sci. 2023, 78, 1707–1716. [Google Scholar] [CrossRef] [PubMed]
- Stowe, R.P.; Kozlova, E.V.; Yetman, D.L.; Walling, D.M.; Goodwin, J.S.; Glaser, R. Chronic Herpesvirus Reactivation Occurs in Aging. Exp. Gerontol. 2007, 42, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Kyewski, B.; Klein, L. A Central Role for Central Tolerance. Annu. Rev. Immunol. 2006, 24, 571–606. [Google Scholar] [CrossRef]
- Kishimoto, H.; Sprent, J. The Thymus and Central Tolerance. Clin. Immunol. 2000, 95, S3-7. [Google Scholar] [CrossRef]
- ElTanbouly, M.A.; Noelle, R.J. Rethinking Peripheral T Cell Tolerance: Checkpoints across a T Cell’s Journey. Nat. Rev. Immunol. 2021, 21, 257–267. [Google Scholar] [CrossRef]
- Paul, E.; Nelde, A.; Verschoor, A.; Carroll, M.C. Follicular Exclusion of Autoreactive B Cells Requires FcgammaRIIb. Int. Immunol. 2007, 19, 365–373. [Google Scholar] [CrossRef]
- Chapman, N.M.; Boothby, M.R.; Chi, H. Metabolic Coordination of T Cell Quiescence and Activation. Nat. Rev. Immunol. 2020, 20, 55–70. [Google Scholar] [CrossRef]
- Schwartz, R.H. T Cell Anergy. Annu. Rev. Immunol. 2003, 21, 305–334. [Google Scholar] [CrossRef]
- Matveeva, K.S.; Shevyrev, D.V.; Rybtsov, S.A. Senescence-Associated β-Galactosidase Activity in Human Effector and Regulatory T Cells. Immunologiya 2024, 45, 290–299. [Google Scholar] [CrossRef]
- Matveeva, K.S.; Rybtsov, S.A.; Shevyrev, D.V. Investigating age-related dynamics and transcriptional signatures of CD8+HLA-DR+ regulatory T lymphocytes: Perspectives in understanding immune system aging. Med. Immunol. 2024, 26, 927–932. [Google Scholar] [CrossRef]
- Huang, M.; Wang, Y.; Fang, L.; Liu, C.; Feng, F.; Liu, L.; Sun, C. T Cell Senescence: A New Perspective on Immunotherapy in Lung Cancer. Front. Immunol. 2024, 15, 1338680. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Zamudio, R.I.; Dewald, H.K.; Vasilopoulos, T.; Gittens-Williams, L.; Fitzgerald-Bocarsly, P.; Herbig, U. Senescence-associated Β-galactosidase Reveals the Abundance of Senescent CD8+ T Cells in Aging Humans. Aging Cell 2021, 20, e13344. [Google Scholar] [CrossRef] [PubMed]
- Kurts, C.; Kosaka, H.; Carbone, F.R.; Miller, J.F.; Heath, W.R. Class I-Restricted Cross-Presentation of Exogenous Self-Antigens Leads to Deletion of Autoreactive CD8(+) T Cells. J. Exp. Med. 1997, 186, 239–245. [Google Scholar] [CrossRef]
- Davey, G.M.; Kurts, C.; Miller, J.F.A.P.; Bouillet, P.; Strasser, A.; Brooks, A.G.; Carbone, F.R.; Heath, W.R. Peripheral Deletion of Autoreactive CD8 T Cells by Cross Presentation of Self-Antigen Occurs by a Bcl-2-Inhibitable Pathway Mediated by Bim. J. Exp. Med. 2002, 196, 947–955. [Google Scholar] [CrossRef]
- Adler, A.J. Peripheral Tolerization of Effector and Memory T Cells: Implications for Autoimmunity and Tumor-Immunity. Curr. Immunol. Rev. 2005, 1, 21–28. [Google Scholar] [CrossRef][Green Version]
- Yin, R.; Melton, S.; Huseby, E.S.; Kardar, M.; Chakraborty, A.K. How Persistent Infection Overcomes Peripheral Tolerance Mechanisms to Cause T Cell-Mediated Autoimmune Disease. Proc. Natl. Acad. Sci. USA 2024, 121, e2318599121. [Google Scholar] [CrossRef]
- Wei, S.C.; Sharma, R.; Anang, N.-A.A.S.; Levine, J.H.; Zhao, Y.; Mancuso, J.J.; Setty, M.; Sharma, P.; Wang, J.; Pe’er, D.; et al. Negative Co-Stimulation Constrains T Cell Differentiation by Imposing Boundaries on Possible Cell States. Immunity 2019, 50, 1084–1098.e10. [Google Scholar] [CrossRef]
- Ness, S.; Lin, S.; Gordon, J.R. Regulatory Dendritic Cells, T Cell Tolerance, and Dendritic Cell Therapy for Immunologic Disease. Front. Immunol. 2021, 12, 633436. [Google Scholar] [CrossRef]
- Shevyrev, D.; Tereshchenko, V.; Blinova, E.; Knauer, N.; Pashkina, E.; Sizikov, A.; Kozlov, V. Regulatory T Cells Fail to Suppress Fast Homeostatic Proliferation In Vitro. Life 2021, 11, 245. [Google Scholar] [CrossRef] [PubMed]
- Paroli, M.; Schiaffella, E.; Di Rosa, F.; Barnaba, V. Persisting Viruses and Autoimmunity. J. Neuroimmunol. 2000, 107, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Sundaresan, B.; Shirafkan, F.; Ripperger, K.; Rattay, K. The Role of Viral Infections in the Onset of Autoimmune Diseases. Viruses 2023, 15, 782. [Google Scholar] [CrossRef]
- Bjornevik, K.; Cortese, M.; Healy, B.C.; Kuhle, J.; Mina, M.J.; Leng, Y.; Elledge, S.J.; Niebuhr, D.W.; Scher, A.I.; Munger, K.L.; et al. Longitudinal Analysis Reveals High Prevalence of Epstein-Barr Virus Associated with Multiple Sclerosis. Science 2022, 375, 296–301. [Google Scholar] [CrossRef]
- Lin, H.-C.; Wang, C.-H.; Tsai, F.-J.; Hwang, K.-P.; Chen, W.; Lin, C.-C.; Li, T.-C. Enterovirus Infection Is Associated with an Increased Risk of Childhood Type 1 Diabetes in Taiwan: A Nationwide Population-Based Cohort Study. Diabetologia 2015, 58, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Shevyrev, D.V.; Tereshchenko, V.P.; Sennikov, S.V. The Enigmatic Nature of the TCR-pMHC Interaction: Implications for CAR-T and TCR-T Engineering. Int. J. Mol. Sci. 2022, 23, 14728. [Google Scholar] [CrossRef] [PubMed]
- Pageon, S.V.; Tabarin, T.; Yamamoto, Y.; Ma, Y.; Nicovich, P.R.; Bridgeman, J.S.; Cohnen, A.; Benzing, C.; Gao, Y.; Crowther, M.D.; et al. Functional Role of T-Cell Receptor Nanoclusters in Signal Initiation and Antigen Discrimination. Proc. Natl. Acad. Sci. USA 2016, 113, E5454–E5463. [Google Scholar] [CrossRef]
- Saito, Y.; Yamamoto, S.; Chikenji, T.S. Role of Cellular Senescence in Inflammation and Regeneration. Inflamm. Regen. 2024, 44, 28. [Google Scholar] [CrossRef]
- Liu, Q.; Zheng, Y.; Goronzy, J.J.; Weyand, C.M. T Cell Aging as a Risk Factor for Autoimmunity. J. Autoimmun. 2023, 137, 102947. [Google Scholar] [CrossRef]
- Schloesser, D.; Lindenthal, L.; Sauer, J.; Chung, K.-J.; Chavakis, T.; Griesser, E.; Baskaran, P.; Maier-Habelsberger, U.; Fundel-Clemens, K.; Schlotthauer, I.; et al. Senescent Cells Suppress Macrophage-Mediated Corpse Removal via Upregulation of the CD47-QPCT/L Axis. J. Cell Biol. 2023, 222, e202207097. [Google Scholar] [CrossRef]
- Bosco, N.; Noti, M. The Aging Gut Microbiome and Its Impact on Host Immunity. Genes Immun. 2021, 22, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.-H.; Shin, J.-W.; Shim, E.; Ohtani, N.; Jeon, O.H. The Connection between Aging, Cellular Senescence and Gut Microbiome Alterations: A Comprehensive Review. Aging Cell 2024, 23, e14315. [Google Scholar] [CrossRef] [PubMed]
- Berg, R.D. Bacterial Translocation from the Gastrointestinal Tract. Adv. Exp. Med. Biol. 1999, 473, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Fine, R.L.; Manfredo Vieira, S.; Gilmore, M.S.; Kriegel, M.A. Mechanisms and Consequences of Gut Commensal Translocation in Chronic Diseases. Gut Microbes 2020, 11, 217–230. [Google Scholar] [CrossRef]
- Kawamoto, S.; Uemura, K.; Hori, N.; Takayasu, L.; Konishi, Y.; Katoh, K.; Matsumoto, T.; Suzuki, M.; Sakai, Y.; Matsudaira, T.; et al. Bacterial Induction of B Cell Senescence Promotes Age-Related Changes in the Gut Microbiota. Nat. Cell Biol. 2023, 25, 865–876. [Google Scholar] [CrossRef]
- Foth, S.; Völkel, S.; Bauersachs, D.; Zemlin, M.; Skevaki, C. T Cell Repertoire During Ontogeny and Characteristics in Inflammatory Disorders in Adults and Childhood. Front. Immunol. 2020, 11, 611573. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Zhang, W.; Wang, Y.; Qian, P.; Huang, H. Inflammation and Aging: Signaling Pathways and Intervention Therapies. Signal Transduct. Target. Ther. 2023, 8, 239. [Google Scholar] [CrossRef]
- Yan, H.; Baldridge, M.T.; King, K.Y. Hematopoiesis and the Bacterial Microbiome. Blood 2018, 132, 559–564. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; Ren, Y.; Wang, Y.; Fang, J.; Yue, H.; Ma, S.; Guan, F. Aging and Age-Related Diseases: From Mechanisms to Therapeutic Strategies. Biogerontology 2021, 22, 165–187. [Google Scholar] [CrossRef]
- Kumar, M.; James, M.M.; Kumawat, M.; Nabi, B.; Sharma, P.; Pal, N.; Shubham, S.; Tiwari, R.R.; Sarma, D.K.; Nagpal, R. Aging and Microbiome in the Modulation of Vaccine Efficacy. Biomedicines 2022, 10, 1545. [Google Scholar] [CrossRef]
- Mouat, I.C.; Goldberg, E.; Horwitz, M.S. Age-Associated B Cells in Autoimmune Diseases. Cell Mol. Life Sci. 2022, 79, 402. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.; Carding, S.R.; Hall, L.J. The Early-Life Gut Microbiome and Vaccine Efficacy. Lancet Microbe 2022, 3, e787–e794. [Google Scholar] [CrossRef]
- Weyand, C.M.; Goronzy, J.J. Aging of the Immune System. Mechanisms and Therapeutic Targets. Ann. Am. Thorac. Soc. 2016, 13 (Suppl. 5), S422–S428. [Google Scholar] [CrossRef]
- Rodrigues, C.M.C.; Plotkin, S.A. Impact of Vaccines; Health, Economic and Social Perspectives. Front. Microbiol. 2020, 11, 1526. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Pitcher, L.E.; Prahalad, V.; Niedernhofer, L.J.; Robbins, P.D. Targeting Cellular Senescence with Senotherapeutics: Senolytics and Senomorphics. FEBS J. 2023, 290, 1362–1383. [Google Scholar] [CrossRef] [PubMed]
- Lagoumtzi, S.M.; Chondrogianni, N. Senolytics and Senomorphics: Natural and Synthetic Therapeutics in the Treatment of Aging and Chronic Diseases. Free Radic. Biol. Med. 2021, 171, 169–190. [Google Scholar] [CrossRef]
- Deryabin, P.I.; Shatrova, A.N.; Borodkina, A.V. Apoptosis Resistance of Senescent Cells Is an Intrinsic Barrier for Senolysis Induced by Cardiac Glycosides. Cell Mol. Life Sci. 2021, 78, 7757–7776. [Google Scholar] [CrossRef]
- Zhu, Y.; Tchkonia, T.; Pirtskhalava, T.; Gower, A.C.; Ding, H.; Giorgadze, N.; Palmer, A.K.; Ikeno, Y.; Hubbard, G.B.; Lenburg, M.; et al. The Achilles’ Heel of Senescent Cells: From Transcriptome to Senolytic Drugs. Aging Cell 2015, 14, 644–658. [Google Scholar] [CrossRef] [PubMed]
- Chaib, S.; Tchkonia, T.; Kirkland, J.L. Cellular Senescence and Senolytics: The Path to the Clinic. Nat. Med. 2022, 28, 1556–1568. [Google Scholar] [CrossRef]
- Chan, K.T.; Blake, S.; Zhu, H.; Kang, J.; Trigos, A.S.; Madhamshettiwar, P.B.; Diesch, J.; Paavolainen, L.; Horvath, P.; Hannan, R.D.; et al. A Functional Genetic Screen Defines the AKT-Induced Senescence Signaling Network. Cell Death Differ. 2020, 27, 725–741. [Google Scholar] [CrossRef]
- L’Hôte, V.; Mann, C.; Thuret, J.-Y. From the Divergence of Senescent Cell Fates to Mechanisms and Selectivity of Senolytic Drugs. Open Biol. 2022, 12, 220171. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, D.E.; Miller, R.B.; Thiesfeldt, S.; Lakhani, H.V.; Shapiro, J.I.; Sodhi, K. The Role of Na/K-ATPase Signaling in Oxidative Stress Related to Aging: Implications in Obesity and Cardiovascular Disease. Int. J. Mol. Sci. 2018, 19, 2139. [Google Scholar] [CrossRef] [PubMed]
- von Kobbe, C. Targeting Senescent Cells: Approaches, Opportunities, Challenges. Aging 2019, 11, 12844–12861. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Hickson, L.J.; Eirin, A.; Kirkland, J.L.; Lerman, L.O. Cellular Senescence: The Good, the Bad and the Unknown. Nat. Rev. Nephrol. 2022, 18, 611–627. [Google Scholar] [CrossRef]
- Storer, M.; Mas, A.; Robert-Moreno, A.; Pecoraro, M.; Ortells, M.C.; Di Giacomo, V.; Yosef, R.; Pilpel, N.; Krizhanovsky, V.; Sharpe, J.; et al. Senescence Is a Developmental Mechanism That Contributes to Embryonic Growth and Patterning. Cell 2013, 155, 1119–1130. [Google Scholar] [CrossRef]
- Fukushima, Y.; Sakamoto, K.; Matsuda, M.; Yoshikai, Y.; Yagita, H.; Kitamura, D.; Chihara, M.; Minato, N.; Hattori, M. Cis Interaction of CD153 with TCR/CD3 Is Crucial for the Pathogenic Activation of Senescence-Associated T Cells. Cell Rep. 2022, 40, 111373. [Google Scholar] [CrossRef]
- Takaya, K.; Asou, T.; Kishi, K. Cathepsin F Is a Potential Marker for Senescent Human Skin Fibroblasts and Keratinocytes Associated with Skin Aging. GeroScience 2023, 45, 427–437. [Google Scholar] [CrossRef]
- Yang, M.; Harrison, B.R.; Promislow, D.E.L. Cellular Age Explains Variation in Age-Related Cell-to-Cell Transcriptome Variability. Genome Res. 2023, 33, 1906–1916. [Google Scholar] [CrossRef]
- Takaya, K.; Asou, T.; Kishi, K. New Senolysis Approach via Antibody-Drug Conjugate Targeting of the Senescent Cell Marker Apolipoprotein D for Skin Rejuvenation. Int. J. Mol. Sci. 2023, 24, 5857. [Google Scholar] [CrossRef]
- Takaya, K.; Asou, T.; Kishi, K. Identification of Apolipoprotein D as a Dermal Fibroblast Marker of Human Aging for Development of Skin Rejuvenation Therapy. Rejuvenation Res. 2023, 26, 42–50. [Google Scholar] [CrossRef]
- Amor, C.; Feucht, J.; Leibold, J.; Ho, Y.-J.; Zhu, C.; Alonso-Curbelo, D.; Mansilla-Soto, J.; Boyer, J.A.; Li, X.; Giavridis, T.; et al. Senolytic CAR T Cells Reverse Senescence-Associated Pathologies. Nature 2020, 583, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Kim, E.-C.; Son, Y.; Lee, D.-W.; Park, Y.S.; Choi, J.H.; Cho, K.-H.; Kwon, K.-S.; Kim, J.-R. CD9 Induces Cellular Senescence and Aggravates Atherosclerotic Plaque Formation. Cell Death Differ. 2020, 27, 2681–2696. [Google Scholar] [CrossRef] [PubMed]
- Shang, D.; Sun, D.; Shi, C.; Xu, J.; Shen, M.; Hu, X.; Liu, H.; Tu, Z. Activation of Epidermal Growth Factor Receptor Signaling Mediates Cellular Senescence Induced by Certain Pro-inflammatory Cytokines. Aging Cell 2020, 19, e13145. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Du, X.; Yu, J.; Wang, Y.; Jin, X.; Gu, B.; Yin, Q. Seno-Antigen-Pulsed Dendritic Cell Vaccine Induce Anti-Aging Immunity to Improve Adipose Tissue Senescence and Metabolic Abnormalities. Biomed. Pharmacother. 2024, 179, 117433. [Google Scholar] [CrossRef]
- Suda, M.; Shimizu, I.; Katsuumi, G.; Yoshida, Y.; Hayashi, Y.; Ikegami, R.; Matsumoto, N.; Yoshida, Y.; Mikawa, R.; Katayama, A.; et al. Senolytic Vaccination Improves Normal and Pathological Age-Related Phenotypes and Increases Lifespan in Progeroid Mice. Nat. Aging 2021, 1, 1117–1126. [Google Scholar] [CrossRef]
- Henson, S.M.; Akbar, A.N. KLRG1—More than a Marker for T Cell Senescence. Age 2009, 31, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, S.; Tang, X.; Peng, Y.; Jiang, T.; Zhang, X.; Li, J.; Liu, Y.; Yang, Z. The Role of KLRG1: A Novel Biomarker and New Therapeutic Target. Cell Commun. Signal. 2024, 22, 337. [Google Scholar] [CrossRef]
- Islam, M.T.; Tuday, E.; Allen, S.; Kim, J.; Trott, D.W.; Holland, W.L.; Donato, A.J.; Lesniewski, L.A. Senolytic Drugs, Dasatinib and Quercetin, Attenuate Adipose Tissue Inflammation, and Ameliorate Metabolic Function in Old Age. Aging Cell 2023, 22, e13767. [Google Scholar] [CrossRef]
- Raffaele, M.; Vinciguerra, M. The Costs and Benefits of Senotherapeutics for Human Health. Lancet Healthy Longev. 2022, 3, e67–e77. [Google Scholar] [CrossRef]
- Zonari, A.; Brace, L.E.; Al-Katib, K.; Porto, W.F.; Foyt, D.; Guiang, M.; Cruz, E.A.O.; Marshall, B.; Gentz, M.; Guimarães, G.R.; et al. Senotherapeutic Peptide Treatment Reduces Biological Age and Senescence Burden in Human Skin Models. NPJ Aging 2023, 9, 10. [Google Scholar] [CrossRef]
- Goronzy, J.J.; Weyand, C.M. Understanding Immunosenescence to Improve Responses to Vaccines. Nat. Immunol. 2013, 14, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Poblocka, M.; Bassey, A.L.; Smith, V.M.; Falcicchio, M.; Manso, A.S.; Althubiti, M.; Sheng, X.; Kyle, A.; Barber, R.; Frigerio, M.; et al. Targeted Clearance of Senescent Cells Using an Antibody-Drug Conjugate against a Specific Membrane Marker. Sci. Rep. 2021, 11, 20358. [Google Scholar] [CrossRef] [PubMed]
- Takaya, K.; Asou, T.; Kishi, K. Development of a Novel Senolysis Approach Targeting the Senescent Fibroblast Marker HTR2A via Antibody-Dependent Cellular Cytotoxicity. Rejuvenation Res. 2023, 26, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Rettko, N.J.; Campisi, J.; Wells, J.A. Engineering Antibodies Targeting P16 MHC-Peptide Complexes. ACS Chem. Biol. 2022, 17, 545–555. [Google Scholar] [CrossRef]
- Grosse, L.; Wagner, N.; Emelyanov, A.; Molina, C.; Lacas-Gervais, S.; Wagner, K.-D.; Bulavin, D.V. Defined p16High Senescent Cell Types Are Indispensable for Mouse Healthspan. Cell Metab. 2020, 32, 87–99.e6. [Google Scholar] [CrossRef]
- Rock, K.L.; Kono, H. The Inflammatory Response to Cell Death. Annu. Rev. Pathol. 2008, 3, 99–126. [Google Scholar] [CrossRef]
- Bock, F.J.; Riley, J.S. When Cell Death Goes Wrong: Inflammatory Outcomes of Failed Apoptosis and Mitotic Cell Death. Cell Death Differ. 2023, 30, 293–303. [Google Scholar] [CrossRef]
- Božič, B.; Rozman, B. Apoptosis and Autoimmunity. EJIFCC 2006, 17, 69–74. [Google Scholar]
- Brieske, C.; Lamprecht, P.; Kerstein-Staehle, A. Immunogenic Cell Death as Driver of Autoimmunity in Granulomatosis with Polyangiitis. Front. Immunol. 2022, 13, 1007092. [Google Scholar] [CrossRef]
- Mancinelli, L.; Intini, G. Age-Associated Declining of the Regeneration Potential of Skeletal Stem/Progenitor Cells. Front. Physiol. 2023, 14, 1087254. [Google Scholar] [CrossRef]
- Rapaka, R.R.; Cross, A.S.; McArthur, M.A. Using Adjuvants to Drive T Cell Responses for Next-Generation Infectious Disease Vaccines. Vaccines 2021, 9, 820. [Google Scholar] [CrossRef] [PubMed]
- Muslimov, A.; Tereshchenko, V.; Shevyrev, D.; Rogova, A.; Lepik, K.; Reshetnikov, V.; Ivanov, R. The Dual Role of the Innate Immune System in the Effectiveness of mRNA Therapeutics. Int. J. Mol. Sci. 2023, 24, 14820. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Cai, Y.; Jiang, Y.; He, X.; Wei, Y.; Yu, Y.; Tian, X. Vaccine Adjuvants: Mechanisms and Platforms. Sig Transduct. Target. Ther. 2023, 8, 283. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi, E.; Asgary, A.; Kharrazi, A.Y.; Tavakoli, N.; Zali, A.; Mehrazi, M.; Jamshidi, M.; Farrokhi, B.; Maher, A.; von Garnier, C.; et al. Personalized Predictions of Adverse Side Effects of the COVID-19 Vaccines. Heliyon 2023, 9, e12753. [Google Scholar] [CrossRef]
- Levi, Y.; Brandeau, M.L.; Shmueli, E.; Yamin, D. Prediction and Detection of Side Effects Severity Following COVID-19 and Influenza Vaccinations: Utilizing Smartwatches and Smartphones. Sci. Rep. 2024, 14, 6012. [Google Scholar] [CrossRef]
- Patrinos, G.P.; Sarhangi, N.; Sarrami, B.; Khodayari, N.; Larijani, B.; Hasanzad, M. Using ChatGPT to Predict the Future of Personalized Medicine. Pharmacogenom. J. 2023, 23, 178–184. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).