Abstract
Winter ulcer disease is a health issue in the Atlantic salmonid aquaculture industry, mainly caused by Moritella viscosa. Although vaccination is one of the effective ways to prevent bacterial outbreaks in the salmon farming industry, ulcer disease related to bacterial infections is being reported on Canada’s Atlantic coast. Here, we studied the immune response of farmed immunized Atlantic salmon to bath and intraperitoneal (ip) M. viscosa challenges and evaluated the immunogenicity of M. viscosa cell components. IgM titers were determined after infection, post boost immunization, and post challenge with M. viscosa. IgM+ (B cell) in the spleen and blood cell populations were also identified and quantified by 3,3 dihexyloxacarbocyanine (DiOC6) and IgM-Texas red using confocal microscopy and flow cytometry. At 14 days post challenge, IgM was detected in the serum and spleen. There was a significant increase in circulating neutrophils 3 days after ip and bath challenges in the M. viscosa outer membrane vesicles (OMVs) boosted group compared to non-boosted. Lymphocytes increased in the blood at 7 and 14 days after the ip and bath challenges, respectively, in OMVs boosted group. Furthermore, a rise in IgM titers was detected in the OMVs boosted group. We determined that a commercial vaccine is effective against M. viscosa strain, and OMVs are the most immunogenic component of M. viscosa cells.
1. Introduction
A recognized cause of winter ulcerative disease in Atlantic salmon is Moritella viscosa [1]. Two main clades (‘typical’ and ‘variant’) have been recognized in this pathogen. Typical M. viscosa has been isolated from Atlantic salmon cultured in European countries. Variant M. viscosa has been isolated from Atlantic salmon reared in Canada [2,3]. There are a few published studies on skin ulcerative diseases in Atlantic salmon, but less is known about it on Canada’s east coast [4,5]. The Atlantic salmon industry attempts to prevent or control bacterial outbreaks using vaccines [6]. Although vaccination is an effective management strategy that decreases disease outbreaks and minimizes the use of antibiotics in the aquaculture industry [7], ulcerative disease is frequently reported in vaccinated Atlantic salmon in eastern Canada [8]. To develop effective vaccines, an in-depth understanding of the immune response in the target species after immunization is required. However, there are many unknown aspects regarding fish immunology, and we are still far from understanding which immune mechanisms are responsible for protecting fish against some of these pathogens [9,10,11]. Most licensed vaccines for finfish are inactivated microbes mixed with adjuvant, which contain either single or combined heat or formalin-killed pathogenic microorganisms [12,13]. An efficient vaccine stimulates the development of long-lived plasma cells that produce high-affinity antibodies and memory B-cells. Antibodies play a significant role in limiting or preventing infection and can neutralize and remove pathogens before an infection becomes severe [14]. The pathogen’s capability to grow in an iron-restricted environment led to the synthesis of Iron-Regulated Outer-Membrane Proteins (IROMP), which have been proposed as important antigens that protect against bacteria [15]. Their interaction with the host immune system makes them suitable candidates for vaccine development [16]. Additionally, bacterial outer membrane vesicles (OMVs) contain protective antigens in their structures and can be used for vaccines as well [17,18,19,20,21].
Several studies have investigated aspects of the fish immune system [22,23,24,25,26,27,28,29], and examined hematological responses in vaccinated and infected fish species [30,31]. However, there are no studies assessing cell analysis and effective immunity in Atlantic salmon following M. viscosa infection. Hematological analyses are commonly utilized to assess a fish’s physiological status and health [32]. For example, leukocytes are polymorphic and multifunctional immune cells and carry out various physiological and immunological tasks. They help fish adjust to various biotic and abiotic factors and protect the body from foreign substances [33]. Changes in the number of leukocytes and their differential count (e.g., lymphocytes, neutrophils, eosinophils, and monocytes) are crucial clinical indicators, as they are symptomatic of several conditions, including acute and chronic stress and pathogen exposure/infection [34].
Here, we evaluated the hematological and immune response of vaccinated and boosted farmed Atlantic salmon after M. viscosa challenge. Farmed Atlantic salmon immunized with ALPHA JECT micro IV (Pharmaq, Norway) containing M. viscosa bacterin were bath and intraperitoneally challenged with M. viscosa. The vaccine efficacy and immune response were examined. Also, Atlantic salmon were boosted by M. viscosa antigens to evaluate the antigenicity of different bacterial components (bacterin and exudates’ (complete bacterin), bacterin cell, IROMP, OMVs). IgM titers, peripherical and spleen IgM+ cell populations were measured in vaccinated and challenged groups. White blood cells (WBCs) showed a significant increase after the challenge, with a higher cell percentage in the OMVs boosted group compared to the non-boosted one. IgM titers increased following the vaccination and challenge in OMVs boosted group. Our results showed M. viscosa OMVs are highly effective at stimulating an immune response in farmed Atlantic salmon.
2. Materials and Methods
2.1. Fish Holding
Vaccinated farmed Atlantic salmon (~350 ± 50 g) provided by Cook aquaculture industry were held at the Ocean Sciences Center (Memorial University of Newfoundland). Fish were kept and fed according to the standard protocols [8], and ethical procedures #18-01-JS, #18-03-JS, and biohazard license L-01 were implemented for the study.
2.2. Infection Trials
2.2.1. Inoculum Preparation
One colony of M. viscosa J311 (ATCC BAA-105) was cultivated in 3 mL of Trypticase Soy Broth (TSB, Difco) supplemented with 2% NaCl and set in a drum roller (TC7, New Brunswick, NJ, USA) at 15 °C for 24 h. Then, 300 µL of the culture was mixed with 30 mL of TSB 2% NaCl. Following, it was kept at 15 °C incubator for 24 h with aeration at 180 rpm. Bacterial proliferation was measured by Genesys 10 UV spectrophotometer (Thermo Spectronic, Thermo Fisher Scientific, Waltham, MA, USA) up to an OD ~0.7. Then, the bacterial culture was subjected to centrifugation at 4200× g for 10 min to isolate the sediment. The pellet was washed twice with filtered-sterilized seawater (0.22 μm) and resuspended in 300 µL of filtered-sterilized seawater, and its concentration was determined by plating method [35,36,37].
2.2.2. Challenge Assay in Vaccinated Atlantic Salmon
Atlantic salmon were vaccinated with ALPHA JECT micro IV when they reached a weight of approximately 60–80 g on 16 October 2020, six months before the challenge. This procedure was performed in the AQ3 biocontainment unit at the Cold-Ocean and Deep-Sea Research Facility (CDRF) at MUN. Fish were transferred to the AQ3-CDRF unit and acclimated for one week at 10 °C. All of the 240 immunized Atlantic salmon (~350 ± 50 g) were evenly distributed into six 500 L tanks containing 40 fish each. Fish were divided into three groups (Figure S1). Two tanks containing 80 fish were anesthetized with 50 mg/L MS-222 (Syndel Laboratories, Vancouver, BC, Canada) and intraperitoneally (ip) injected with 100 µL (1 × 106 CFU/dose) of M. viscosa, and two other tanks were bath challenged (1 × 106 CFU/mL) for 30 min. The final two tanks were not infected and were used as negative control groups. The susceptibility of Atlantic salmon was evaluated, and mortality rates were recorded daily up to 30 days post challenge (dpc).
2.3. Flow Cytometry
We performed flow cytometry, which is the most common technique used for single-cell analysis and isolation [38], to quantify the different blood cell populations in the blood and IgM+ cells in the spleen. Briefly, three fish from each group were netted at 3, 7, and 14 dpc and euthanized instantly with an overdose of MS222 (400 mg/L). Spleen and blood samples were aseptically collected. Spleens were homogenized using a 100 μm mesh strainer and resuspended in FACS media. Blood samples were heparinized (100 mg/mL. Pfizer Inc, NY, USA). All suspensions were preserved on ice until performing flow cytometry. The procedures and analyses were conducted according to previously described methods with modifications [39]. Briefly, fresh blood or spleen cell suspensions (20 μL) were diluted in FACS media (1:100 μL). Then, they were stained with 40 μL of 3,3 dihexyloxacarbocyanine (DiOC6) (1 μg/mL), 2 μL of Texas red-IgY anti-salmon IgM fresh solution. After staining, blood cell populations and IgM+ in the spleen were analyzed by a BD FACS Aria II flow cytometer (BD Biosciences, San Jose, CA, USA) and BD FACS Diva v7.0 software.
2.4. Immune Confocal Microscopy
Three healthy fish were euthanized with an overdose of MS222 (400 mg/L), and their spleens were aseptically collected, homogenized using a 100 µm mesh strainer, and resuspended in FACS media (PBS; 0.1% fetal bovine serum (FBS; Gibco, NY, USA)). Biotinylated chicken IgY anti-salmon IgM (1 mg/mL, Somru BioSciences, Charlottetown, PE, Canada) was labeled with Texas red-avidin (2.5 mg/mL, Thermo Fisher Scientific, MA, USA) by mixing in a 1:1 volume for 30 min at room temperature. Then 50 µL of the spleen cell suspensions were gently mixed with 3 µL of Texas red-IgY anti-salmon IgM, and 1 µL of 4,6-diamidino-2-phenylindole (DAPI, 5 mg/mL concentration) (Thermo Fisher Scientific, MA, USA) in a 1.5 mL centrifuge tube. A 3 µL aliquot of the stained cell suspension was added onto mountain media (Thermo Fisher Scientific, MA, USA) on a microscope slide, covered with a cover slide, and sealed. These samples were visualized by confocal microscope (Nikon AX/AX R, Long Island, NY, USA) for image acquisition. This study was conducted to verify the flow cytometry results.
2.5. IgM Titer Determination Using Indirect Enzyme-Linked Immunosorbent Assay (ELISA)
Six fish from each group were netted at 3, 7 and 14 dpc and anesthetized with 50 mg/L MS222. Blood samples were taken and centrifuged at 4200× g for 5 min at room temperature and preserved at −80 °C between 8 and 12 h. The samples were thawed in ice, and the complement system was inactivated by heating the samples to 56 °C for 30 min. Then, lipids were removed by adding 100 μL of chloroform (Sigma-Aldrich, St. Louis, MO, USA) and centrifuged at 4200× g for 10 min at room temperature. Next, the supernatant of each sample was collected and stored at −80 °C until analysis. Indirect ELISAs were conducted to quantify serum antibodies against M. viscosa according to published methods [40,41], with modifications. Briefly, the ideal antigen concentration (Formalin-killed M. viscosa cell antigen) to react with salmon serum was determined by a checkerboard titration method [42]. The anti-M. viscosa antibodies in the serum were measured at six distinct antigen concentrations (4, 2, 1, 0.5, 0.25, and 0.125 μg/mL) to interact with the pooled serum of Atlantic salmon (1:2 to 1:64). Antigen concentrations were coated into polystyrene 96-well microtiter plates (Thermo Scientific, MA, USA), and they were then incubated at 4 °C overnight. Following, the wells were blocked with 150 μL of ChonBlock™ (Chondrex Inc, Woodinville, WA, USA) for 1 h at 37 °C, washed three times with PBS-Tween, and then incubated with various dilutions of control and M. viscosa infected salmon. Based on the results of these assays, 4 μg/mL was chosen.
Finally, 96-well microtiter plates were coated with 100 μL (4 μg/mL) of M. viscosa in coating buffer (0.015 mM Na2CO3; 0.035 mM NaHCO3; pH 9.8) and were kept overnight at 4 °C. The antigen-coated plates were washed with 100 μL of PBS 0.1% Tween-20 (PBS-T) three times and blocked with 150 µL of Chondrex buffer for 1 h at 37 °C. Then, the 96-well plates were washed with 100 μL of PBS-T 0.1% three times, and 100 µL of Atlantic salmon serum from the infected or control group were added to the first well row, serially diluted 2-folds until the last row, and incubated at 37 °C for 1 h. Next, the wells were washed with 100 μL of PBS-T five times, and 100 µL of IgY anti-salmon IgM diluted with PBS-T (1:10,000) was added and the plates were incubated for 1 h at 37 °C. Afterward, the plates were washed five times with 100 μL of PBTS, and incubated with 100 µL of Streptavidin-HRP (Southern Biotech, Birmingham, AL, USA) diluted with PBST (1: 10,000) for 1 h at 37 °C. Following, the plates were washed three times with PBST. Finally, 50 µL of TBM buffer (3,3′, 5,5-tetramethylbenzidine; (Thermo Scientific, MA, USA) was added to each well and were incubated for 15 min. The chemical interaction was stopped by adding 50 µL of 2M H2SO4, and absorbance was determined at 450 nm using a microplate reader (SpectraMax M5 Multi-Mode Microplate Reader, Molecular Devices, Sunnyvale, CA, USA). To find the ideal cut-off, the specific antibody titer was determined by taking the maximum serum dilution, at which the O.D values began to rise as the dilution factor rose. This number was then normalized to a logarithmic base two scale [42].
2.6. M. visosa Antigenicity in Farmed Atlantic Salmon
2.6.1. Vaccine Preparations
Four different vaccines (bacterin cell, bacterin with exudates’ (complete bacterin), IROMP, OMVs) were used in this study. For preparing the bacterin cell, one colony of M. viscosa was cultivated in a tube containing 3 mL of TSB supplemented with 2% NaCl for 48 h at 15 °C with aeration (180 rpm). Then, 500 µL of the tube’s content was transferred to a 250 mL flask containing 50 mL of TSB 2% NaCl and grown at 15 °C up to an OD ~0.6–0.7 at 600 nm, and these bacteria were harvested by centrifugation (4200× g, 10 min, 4 °C) and washed two times with seawater and resuspended in 500 µL of seawater. Bacterial cell count was measured after 72 h incubation at 15 °C [37]. After counting, the bacterial cells were inactivated by adding 6% (v/v) of formalin (Sigma Chemical, St. Louis, MO, USA) and incubated at 4 °C for 48 h. Formaldehyde was removed by centrifugation, the cells were washed three times with PBS 1X, and then the cells were dialyzed (Spectrum™ Spectra/Por™ dialysis membrane; 12–14,000 Dalton molecular weight cut-off; Thermo Fisher, USA) in 1 L PBS 1X at 4 °C for 24 h with agitation in an orbital shaker [43]. M. viscosa inactivation was confirmed by plating onto TSA 2% NaCl for 24 h at 15 °C. The final concentration of bacterin (4.1 × 108 CFU/mL) was determined by a flow cytometer (Figure S2A) [44,45]. M. viscosa bacterin was kept at 4 °C until use. For vaccine preparation, 10% carbigen carbomer-based (Carbopol 934P) adjuvant were mixed with bacterin (4.1 × 108 CFU/mL) and buffered to pH 7 according to the manufacturer’s protocols (MVP Adjuvants®, Phibro Animal Health Corporation, Teaneck, NJ, USA).
For preparing bacterin with exudates (complete bacterin), M. viscosa was grown in a flask containing 50 mL TSB supplemented with 2% NaCl up to an OD ~0.6–0.7 at 600 nm, as previously described. Then, 5 mL of M. viscosa was grown in 500 mL of TSB 2% NaCl up to an OD ~0.9 at 600 nm. The bacteria were inactivated with 0.4% formaldehyde for 3 days with gentle shaking at room temperature. Then, the final concentration was calculated by flow cytometer as outlined previously (Figure S2B), and formalin-killed bacterin (4.2 × 107 CFU/mL) was mixed with 10% carbigen adjuvant and kept at 4 °C for immunization.
Laboratory preparation of M. viscosa outer-membrane proteins (OMPs), IROMP and OMVs, was carried out by established protocols [46,47,48,49]. Then, 100 µL of IROMP containing 50 µL of IRON (1504.4 µg/mL) and 50 µL M. viscosa OMP (1440 µg/mL) were added to 4450 µL of PBS and mixed with 450 µL of carbigen adjuvant, according to the manufacturer’s instructions, up to a concertation of 1 mg/mL (1:1 OMPs and IROMPs). For M. viscosa OMVs vaccines, 50 μL of OMVs (1546.3 µg/mL) were added to 4.5 mL of PBS and mixed with 450 μL of carbigen adjuvant.
2.6.2. Antigenicity of M. viscosa Components in Farmed Atlantic Salmon
This experiment was performed at the Dr. Joe Brown Aquatic Research Building (JBARB) in the Ocean Science Center, MUN. A total of 270 vaccinated Atlantic salmon with an average weight of ~380 ± 50 g were Passive Integrated Transponder (PIT)-tagged and divided equally into six 500 L tanks, each containing 45 fish. Atlantic salmon were anesthetized with 50 mg/L of MS222 and individually injected with 100 μL of the vaccine preparation (e.g., M. viscosa bacterin, M. vicosa cell, IROMP, OMVs). In each tank, 45 fish were intraperitoneally boosted with their respective vaccine, and one group were injected with PBS mixed with carbigen adjuvant and used as the control group (Figure S3). After immunization, blood samples from 6 fish were taken from each treatment every two weeks (2, 4, 6, and 8). The serum of each sample was collected and stored at −80 °C until IgM titer was determined by indirect ELISA.
2.6.3. M. viscosa Challenge in Atlantic Salmon
Atlantic salmon boosted with 4 vaccines and PBS mixed with carbigen adjuvant were transferred from JBARB to 6 tanks in the AQ3-CDRF and acclimated for 1 week at 10 °C under the described optimal conditions. Twelve weeks after being boosted, fish were challenged with M. viscosa. Briefly, two tanks containing 90 fish with 6 treatments were bath challenged with M. viscosa (1 × 106 CFU/mL) for 30 min without seawater flow through. The seawater flow through was reestablished after the challenge. The fish in two tanks containing different treatments were anesthetized with 50 mg/L MS222 (Syndel Laboratories, Vancouver, BC, Canada) and individually injected with 100 µL (1 × 106 CFU/dose) of M. viscosa. Two tanks were not challenged and remained as the control groups (Figure S4). Blood and spleen samples were aseptically collected at 3, 7, and 14 dpc to analyze the cell populations. IgM titers in the blood were detected using indirect ELISA. The mortality rates were recorded 30 days post challenge (Figure S5).
2.7. Statistical Analysis
The research data were analyzed using GraphPad Prism 10 (California, CA, USA). An arcsin (survival rate ratio) function was utilized to calculate fish survival rates. Shapiro–Wilk test was conducted to assess the normality of the data. All data are not normally distributed, and one-way ANOVA (non-parametric) followed by a Kruskal–Wallis’s test was utilized to determine differences (p ≤ 0.05) between cell populations. Two-way ANOVA (Dunnett’s multiple comparison tests) was performed to analyze the ELISA data. Asterisk (*) indicated considerable differences (p ≤ 0.0001) among the reciprocal titers.
3. Results
3.1. Susceptibility of Vaccinated Farmed Atlantic Salmon to M. viscosa Challenge
Mortality was not observed in the control (non-infected) group, in contrast, at 30 dpc, mortality in the bath and ip-challenged animals was 2.82% and 5.64%, respectively (Figure 1).
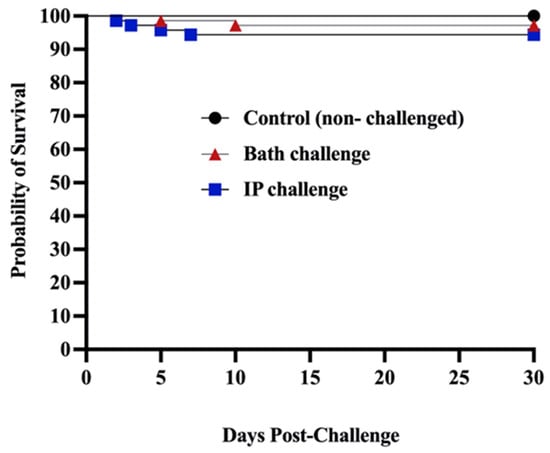
Figure 1.
Survival percentages of ALPHA JECT vaccinated farmed Atlantic salmon after being challenged with M. viscosa. The experimental fish were ip and bath challenged with 106 CFU/dose and 106 CFU/mL of M. viscosa, respectively. The control group was not infected.
3.2. Cell Populations in the Challenged Atlantic Salmon
Blood cell populations were analyzed by flow cytometry (Figure S6A,B). The IgM+ (B cell) population was defined in the spleen (Figure S7A–D).
The cell populations were divided into two parts. Red blood cells (RBCs) were shown in P1. WBCs were highlighted in P2 (Figure S6A). Then, P2 was divided into three distinct populations (Figure S6B). P3 exhibited IgM+ (B cells), P4 showed neutrophils and basophils, and P5 indicated monocytes (Figure S6B). Neutrophils and basophils increased significantly at 3 days post ip and bath challenge as compared to the non-infected salmon. Also, lymphocytes were considerably higher than the control group at 7 and 14 days post ip and bath challenges, respectively. Monocytes did not show meaningful differences between the groups (Figure 2).
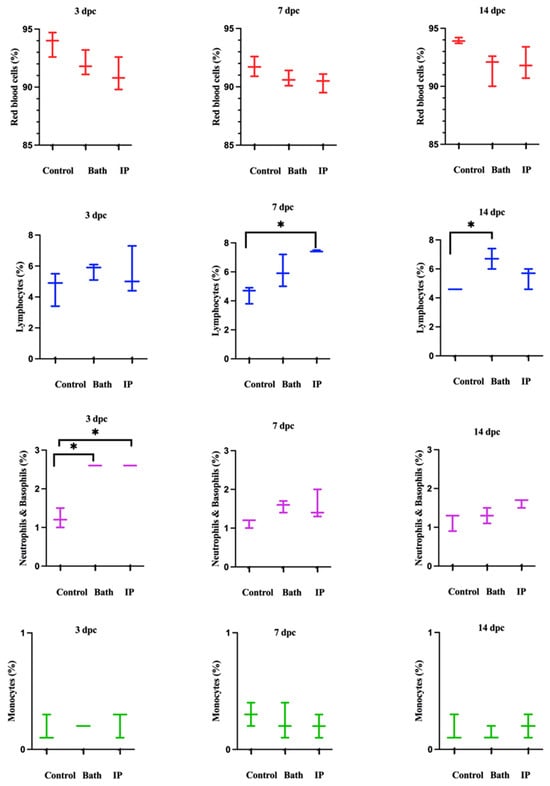
Figure 2.
Atlantic salmon blood cell populations identified by flow cytometry at 3, 7, and 14 dpc with M. viscosa. Differences were identified using one-way ANOVA (non-parametric) followed by Kruskal–Wallis post hoc tests at each sampling point. Asterisks (*) demonstrate a significant difference between groups (p ≤ 0.05).
We found no significant differences between the spleen IgM+ cell populations of control and challenged groups at 3 and 7 dpc. However, at 14 dpc, IgM+ cell population reached 15.4% in the ip challenged group and was significantly higher compared to the non-infected animals (Figure 3).
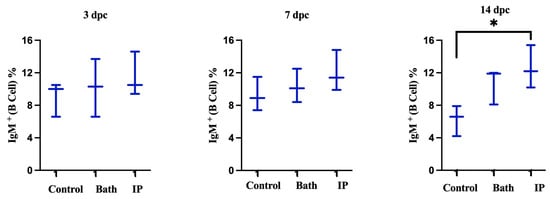
Figure 3.
Spleen IgM+ (B cells) detected by flow cytometry in ALPHA JECT-vaccinated Atlantic salmon at 3, 7, and 14 dpc with M. viscosa. Differences were identified by performing one-way ANOVA (non-parametric) followed by Kruskal–Wallis post hoc tests. At each time point, challenged groups are compared versus control (non-infected) fish. Asterisk (*) demonstrates a significant difference (p ≤ 0.05) between groups.
3.3. Confocal Microscopy Analysis
Immunostaining of white blood cells (WBCs) using DAPI, DiOC6, and Texas red IgY anti-salmon IgM revealed that peripheral IgM+ leukocytes were present in the spleen (Figure 4). This result validated the flow cytometry findings.
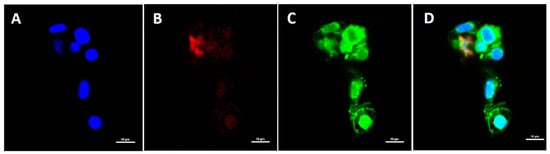
Figure 4.
Confocal microscopy of Atlantic salmon leukocytes from the spleen. (A) Cell nuclei were stained blue with DAPI; (B) IgM+ cells with red fluorescence were visible following staining with chicken anti-salmon-IgM/IgY and avidin-Texas Red (TX-R); (C) green fluorescence indicates cells that were stained with FITC; (D) overlay of all the used colors in confocal imaging.
3.4. IgM Titer Levels in the Challenged Atlantic Salmon
IgM titers did not change with time in the control group (non-infected) salmon. However, they were higher at 14 dpc in the ip-challenged group. Bath-challenged animals showed slightly higher IgM titers than the control group at all the examined time points. However, this difference was not significant (Figure 5).
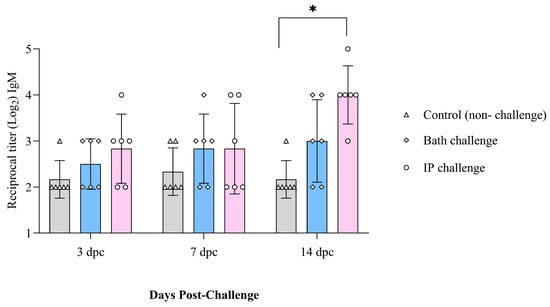
Figure 5.
IgM serum titers in Atlantic salmon at 3, 7, and 14 dpc with M. viscosa determined by indirect ELISA. Significant differences were identified using two-way ANOVA followed by Dunnett’s multiple comparisons test. The asterisk (*) indicates a significant difference (p ≤ 0.0001) between the treatment and the control group at a particular sampling point.
3.5. Detection of Antibody after Booster Dose by Prepared Vaccines
Immunized fish boosted with the OMVs vaccine had the highest IgM levels at all the examined time points, and this was significantly greater than in the control fish at all time points. In contrast, the IROMP-boosted group only had greater IgM titers at 4, 6, and 8 weeks post boost immunization compared to the non-boosted group, while that of the bacterin cell-boosted group was only higher than the control group at 6 weeks following the boost. Finally, the salmon boosted with the complete bacterin did not have IgM titers higher than those measured in the non-boosted (i.e., PBS adjuvant injected group) (Figure 6).
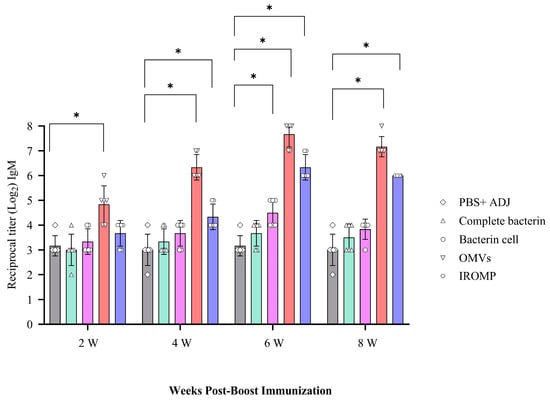
Figure 6.
IgM serum titers in Atlantic salmon at 2, 4, 6 and 8 weeks post boost immunization determined by indirect ELISA. Significant differences were identified using two-way ANOVA followed by Dunnett’s multiple comparisons post hoc test. Asterisks (*) demonstrate meaningful differences (p ≤ 0.0001) between the reciprocal titers.
3.6. Cell Population after Challenge in the Boosted Groups
Our findings showed that neutrophils and basophils increased in the OMVs boosted group at 3 dpc (ip and bath) compared to the non-boosted one (Figure 7 and Figure 8). Furthermore, lymphocytes were statistically higher in the OMVs boosted animals compared to the non-boosted group at 7 and 14 days post ip and bath challenge, respectively. A slight rise of leukocytes was observed in other boosted groups compared to the non-boosted; however, the non-parametric test revealed no significant differences between them (Figure 7 and Figure 8). IgM+ (B cell) increased in the spleen and peaked at 14 dpc in the OMVs-boosted group but was not statistically different from non-boosted animals (Figure 9).
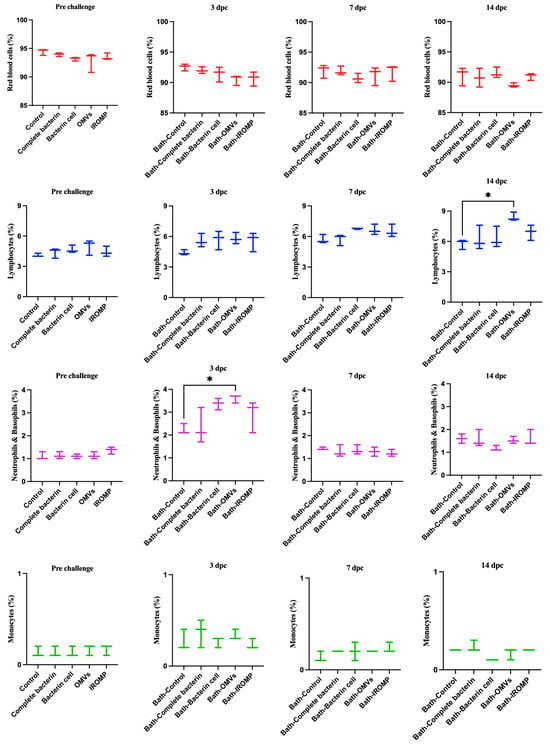
Figure 7.
Blood cell populations in boosted Atlantic salmon analyzed by flow cytometry at 3, 7 and 14 days post bath challenge with M. viscosa. Significant differences were identified by performing one-way ANOVA (non-parametric) followed by Kruskal–Wallis post hoc tests. Asterisks (*) reveal a meaningful difference (p ≤ 0.05) between experimental (i.e., boosted) and control fish (i.e., given a PBS injection with adjuvant).
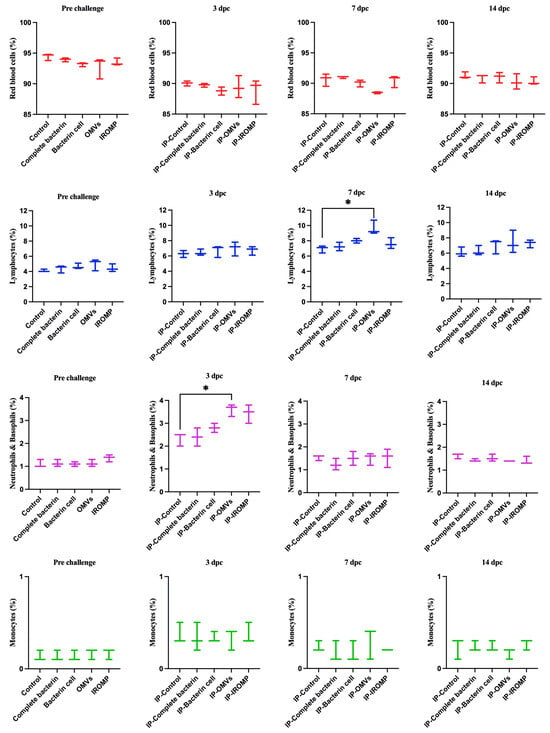
Figure 8.
Blood cell populations in boosted Atlantic salmon identified by flow cytometry at 3, 7, and 14 days post ip challenge with M. viscosa. Significant differences were identified using one-way ANOVA (non-parametric) followed by Kruskal–Wallis post hoc tests. Asterisks (*) indicate a significant difference (p ≤ 0.05) between experimental (i.e., boosted) and control fish (i.e., given a PBS injection with adjuvant).
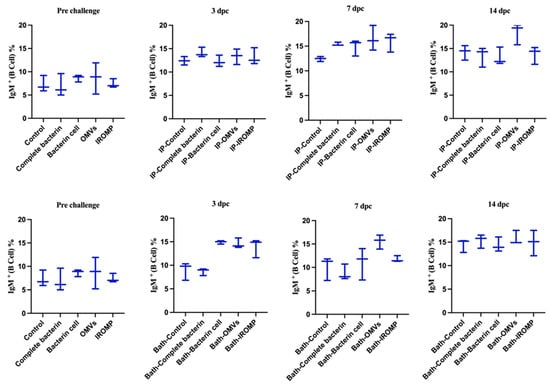
Figure 9.
IgM+ (B cells) in the spleen of boosted Atlantic salmon 3, 7 and 14 days after ip and bath challenges with M. viscosa. No significant differences (p ≤ 0.05) were identified between the non-boosted and boosted treatments after performing one-way ANOVA (non-parametric) followed by Kruskal–Wallis post hoc tests.
3.7. Detection of Antibody Levels after Challenge in the Boosted Groups
The antibody titer slightly increased from 3 to 14 days post challenge (dpc) (Figure 10A,B). The OMVs vaccine reached the highest antibody level at all the studied time points and was statistically higher at 14 dpc than the non-boosted animals (Figure 10B). The IROMP-boosted group displayed slightly higher antibody titer than the bacterin cell, complete bacterin, and control groups. However, statistical analysis indicated no significant difference between them. Antibody levels were slightly higher in the ip-challenged animals compared to the bath-challenged group. Also, no mortality was observed in the boosted animals until 30 dpc. This investigation indicated a positive correlation between survival proportions and antibody titers. All the vaccines induced the fish immune response, and the survival rate was 100% in all the treatments after the challenge (Figure S5).
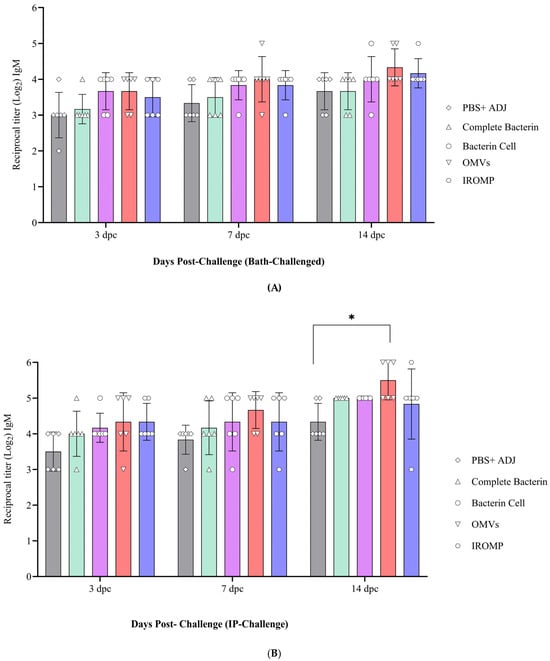
Figure 10.
Serum IgM titers in Atlantic salmon at 3, 7 and 14 days (A) post bath and (B) post ip challenges. Two-way ANOVA (Dunnett’s multiple comparisons tests) were used to detect significant differences. The asterisk (*) demonstrates a significant difference (p ≤ 0.05) between the boosted and non-boosted fish.
4. Discussion
Winter ulcer disease is mainly but not solely caused by M. viscosa [1,3,50]. Winter ulcers cause mortality, downgrading at slaughter, and animal welfare concerns [51]. Bacterin-based vaccines are usually used to control bacterial disease in the Atlantic salmon industry [52]. This study assessed farmed Atlantic salmon’s immunological response and susceptibility to M. viscosa challenge. ALPHA JECT-vaccinated Atlantic salmon were bath- and ip-challenged. Only 2.82% and 5.64% mortality were recorded in the bath and ip challenge, respectively. All the control (non-infected) groups survived, and the survival portions in the bath- and ip-challenged animals were 97.18% and 94.36%, respectively. The high survival percentage showed an appropriate immune response of infected fish and confirmed the effectiveness of the ALPHA JECT micro IV vaccine in protecting Atlantic salmon from M. viscosa challenge (Figure 1). The virulence of this M. viscosa strain has been previously verified in Atlantic salmon [8]. This result was expected since Norwegian strains are used as a vaccine component, such as the M. viscosa type strain used in this study. Efficacy against local strains of Atlantic Canada might be valuable for further study.
Other studies showed the efficacy of vaccines against M. viscosa. Vaccination against M. viscosa significantly reduced the clinical effects of this pathogen, including mortality and skin ulceration. Relative protection reached 91% compared to saline controls and 65% compared to the vaccine formulation lacking M. viscosa antigen. Exposure to M. viscosa antigens generally resulted in protective immunity. The survivors’ group did not exhibit the clinical symptoms of winter ulcer disease [3]. This result was consistent with our findings. In this study, no clinical signs of winter ulcer disease were observed after the challenge in the vaccinated Atlantic salmon.
Rozas-Serri et al. (2022) reviewed the circulating blood cells of farmed Atlantic salmon. They indicated lymphocytes had the largest proportion of white blood cells (WBCs) in all the age ranges in farmed Atlantic salmon [53], consistent with our current study results. Lymphocytes comprised most of the WBC populations, followed by neutrophils and monocytes (Figure 2, Figure 7 and Figure 8).
Hematological analysis showed an increase in the leukocyte population after M. viscosa challenge. Neutrophils and basophils increased significantly at 3 days post ip and bath challenges (Figure 2). This suggests a leukocyte adhesion cascade [54,55], crucial for early defense against infection [56].
Lymphocytes considerably increased at 7 and 14 days ip and bath challenge. Lymphocytes, including B cells and T cells, have essential roles in the adaptive immune response [57]. Our results showed that lymphocytes peaked earlier in the ip challenge compared to the bath route. This relatively early peak might indicate that adaptive immune response was triggered more rapidly in the ip challenge. The immune response in the ip route may have been accelerated, leading to the earlier recruitment and activation of lymphocytes. The delayed peak (at 14 days) in the bath challenge suggested that adaptive immune response took longer to develop in the bath route. An increase in leukocytes after bacterial infections was observed in other fish species [30,58,59,60], which is consistent with our finding after infection with M. viscosa (Figure 2).
IgM has been associated with mucosal and systemic immunity [29,61,62]. Teleost fish membrane-bound IgM+ (B lymphocytes) are a B cell subset implicated in innate and adaptive immune responses [63]. In this study, IgM+ (B cell) increased after the challenge and was statistically different from the non-infected animals at 14 days ip challenge in the spleen. B cells secrete antibodies against invading infections and are at the center of humoral immunity [64]. B lymphocytes that have undergone terminal differentiation and can secrete antibodies are plasma cells [64]. Lymphocytes were not statistically higher at 14 days ip challenge in the blood; however, a great volume of antibodies was observed in the serum (Figure 5). This finding suggested that B cells may differentiate into plasma cells and produce antibodies.
Rising the IgM after infection was reported in the spleen [65]. In our study, IgM increased at 2 weeks following the ip challenge (Figure 3). The early appearance of IgM+ in the spleen might suggest that vaccination quickly induced the immune response after infection. The pre and post infection serum IgM titers had been reported to be significantly greater in vaccinated fish compared to the unvaccinated. This result indicated an active immune response and phagocytic activity [31], which matched our results in this study (Figure 6 and Figure 10B).
Most licensed aquaculture vaccines are inactivated pathogenic microbes mixed with adjuvant, which contain either single or combined microorganisms [12,13]. These vaccine preparations contain not only immune protective antigens but also immune suppressive molecules and identify the most immunogenic bacterial components that can contribute to improving vaccine efficacy. This study used four different vaccines to boost the animals and reviewed the immunogenic response of M. viscosa antigen in vaccinated Atlantic salmon after the challenge of determining the immunogenicity of these vaccine preparations.
In this investigation, one group of salmon was boosted with the bacterin vaccine containing killed M. viscosa and exudates, such as the inactivated commercial vaccines that are commonly used in aquaculture. Also, one group of salmon was boosted with a bacterin cell vaccine containing only M. viscosa cells.
IROMP, a vaccine utilized in this investigation, is the combination of iron and the OMPs of M. viscosa. Gram-negative bacteria’s OMPs represent a significant portion of the cell surface. They are crucial structural elements of the membrane, acting in virulence-related processes like adhesion, invasion of host tissues, and reducing the host immune response. They are also highly antigenic [16,66]. Since they interact with the host immune system, they are important vaccine candidates [16].
OMVs, a vaccine preparation used in this study, are spherical lipid vesicles released from the outer membranes of Gram-negative bacteria to facilitate communication among the organisms as they grow in various environments and control the host immunological response [67,68]. OMVs are composed of OMPs, phospholipids, peptidoglycan, lipopolysaccharides (LPS), proteins, nucleic acids, ion metabolites, and signaling molecules [67,69]. OMVs have been classified and used in many ways. They contain adhesion, autolysins, cytotoxins, virulence factors, nutrition acquisition, stress responses, and toxin delivery that elude host defense mechanisms, among many more biomolecules [67]. In primary infection, OMVs are critical for delivering highly virulent factors to host cells, where they dramatically degrade the host cells’ enzymes and cause cell death. A crucial candidate for a new vaccine formulation is bacterial outer membrane vesicles carrying antigens. They efficiently phagocytize antigen-presenting cells due to the surface-associated antigens they transport. They also bring a variety of PAMPs (pathogen-associated molecular patterns) that trigger and support immune system responses [70]. It supported our findings in this study, which demonstrated a high capacity of OMVs to induce an immunological response in Atlantic salmon (Figure 6, Figure 7 and Figure 8).
OMVs have demonstrated the specific nature of the protective antibody and significant antigens that induce an adaptive memory immune response and possess self-adjuvant characteristics [71]. Our findings were in line with this. According to our research, adaptive immune response quickly triggered following the challenge, and OMVs-boosted group had higher lymphocytes and IgM titers than the other treatment (Figure 7, Figure 8 and Figure 10B).
It has been demonstrated that OMVs can cause immunological response and protect fish [72]. P. salmonis OMVs cause the generation of IgM in Atlantic salmon. IgM was produced against P. salmonis proteins in the serum taken from immunized fish after 14 days [73]. This was consistent with our findings in Atlantic salmon boosted with M. viscosa OMVs. IgM was detected at 14 days post boost immunization (Figure 6).
The OMVs vaccines have been evaluated in developing fish vaccines against Francisella noatunensis in zebrafish, P. salmonis in salmonids, and V. anguillarum in Japanese flounder [49,73,74]. These findings suggested that OMVs effectively induce stable immune responses, confer protection against bacteria, and are efficient antigens to produce vaccines [75,76]. In this study, OMVs were extracted from M. viscosa and used as the vaccine component. This is the first research that utilized the OMVs vaccine to boost the farmed Atlantic salmon against M. viscosa. The results of our experiment showed M. viscosa OMVs are the most immune-stimulating antigens compared to other vaccine elements in Atlantic salmon (Figure 6, Figure 7, Figure 8 and Figure 10B).
Our findings indicated that antibody titer in the boosted group with OMVs significantly differed from the control group at all the examined time points (2, 4, 6, and 8 weeks). Antibody titers peaked at 6 weeks in all the treatments and reached the highest in the OMVs boosted group. The IROMP and bacterin cell antibody levels were in second and third place, respectively, and they differed significantly from the control group at 6 weeks post boost immunization (Figure 6). Several studies were conducted on Atlantic salmon and they reviewed antibody titer by ELISA after vaccination. Romstad et al. (2012) indicated that Atlantic salmon immunized with A-layer positive vaccines demonstrated an increased mean antibody response with rising antigen dose [77]. According to Liu et al. (2020), all the immunized groups had noticeably higher antibody levels post vaccination in comparison to the control group [78]. Rising the antibody was consistent with what we observed in this study after boosting (Figure 6).
Also, in other fish species, significant rise of antibody was observed after vaccination. Japanese flounder was vaccinated against P. fluorescens and A. hydrophila. In all cases, specific antibodies were found at 5 weeks post vaccination and persisted for up to 8 weeks post vaccination [79]. Similar results were obtained in this study in Atlantic salmon. At 6 weeks post boost immunization, a significant difference in IgM titers was observed between the boosted groups (OMVs, IROMP, bacterin cell) and non-boosted animals. The IgM titers remained relatively high in OMVs and IROMP boosted animals compared to the non-boosted group to the end of the experiment at 8 weeks post boost immunization (Figure 6). A considerable rise in IgM titers after the challenge was reported in rainbow trout [80], similar to our results. High IgM titers were detected in the boosted animals compared to the non-boosted group (Figure 6). IgM titers were noticeably higher in the OMVs boosted animal at 14 days following ip challenge (Figure 10B). Villumsen et al. (2012) reported that the experimental vaccine produced noticeably greater IgM titers in each of the three intervals between immunization and challenge compared to the unvaccinated controls [81], which is similar to our results (Figure 6 and Figure 10B).
Hematological analysis revealed a considerable rise in the neutrophils and basophils in the OMVs boosted group compared to the non-boosted fish at 3 dpc (Figure 7 and Figure 8). Lymphocytes increased at 7 and 14 days post ip and bath challenge, respectively, in the OMVs boosted group (Figure 7 and Figure 8). A tendency of the rise was observed in the IgM+ cells in the spleen of OMVs-boosted salmon compared to the non-boosted group (Figure 9). M. viscosa OMVs might contain several immunogenic molecules and perhaps fewer immunosuppressors, thus, boosted salmon may have a faster activation of immune cells, including neutrophils, upon M. viscosa infection. Further studies on the M. viscosa OMVs protein profile might provide an antigenic profile for vaccine development against M. viscosa.
5. Conclusions
This is the first comprehensive study using flow cytometry to analyze the hematological cells of vaccinated and boosted farmed Atlantic salmon before and after M. viscosa challenge. This investigation evaluated the immune response of Atlantic salmon and reviewed the antibody titers after vaccination and challenge. Our research showed that the booster dose of OMVs vaccine had a strong capacity to trigger the farmed Atlantic salmon’s immune response following immunization and challenge with M. viscosa. Leukocytes were statistically higher in the OMVs boosted group, which indicated the appropriate haemato-immune response of the vaccine to control the infection. M. viscosa OMVs could be used as vaccines, heightened antibody response and potentially enhanced immune cell activity. These advantages contribute to the overall effectiveness of the vaccination strategy in conferring immunity against the specific pathogen.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vaccines12010070/s1. Figures S1–S7: Figure S1: Challenge assay with Moritella viscosa. Blood and spleen samples were collected at 3, 7 and 14 dpc. Figure S2: Vaccines concentration. The concentration of (A) bacterin cell and (B) complete bacterin vaccines were calculated based on the defined formula by flow cytometry (bacterial counting cell: (No of bacterial cell (p2))/(No of beads (p1)) ×106 × dilution (103) in flow cytometry. Figure S3: Post boost immunization design by in-house vaccines. Figure S4: Challenge assay with M. viscosa at 12 weeks post boost immunization. Figure S5: Survival of boosted Atlantic salmon after M. viscosa challenge. Figure S6: The flow cytometry algorithm for dividing blood populations (A) P1 shows red blood cells (RBCs) and P2 shows white blood cells (WBCs) in vaccinated farmed Atlantic salmon. (B) P3 shows lymphocytes. P4 indicates neutrophils and basophils and P5 displays monocytes. The value of the threshold operator was 5000 for FSC and SSC in flow cytometry. The voltages for FSC, SCC and FITC were 247, 231, and 313, respectively. Figure S7: The flow cytometry technique for defining IgM+ (B cells) in the spleen. (A) The red color displays IgM in the spleen. (B) Texas red is a marker to show IgM+ population. (C) Based on the DIOC6 the population of RBC and WBCs were separated. (D) The peak demonstrates the IgM+.
Author Contributions
Conceptualization: M.G. and J.S.; methodology: M.G., T.C., A.H. and J.S.; software: M.G., I.V. and J.S.; validation: M.G., I.V. and J.S.; formal analysis: M.G. and J.S.; investigation: M.G. and J.S.; resources, J.S. and M.G.; data curation: M.G. and J.S.; writing—original draft preparation: M.G.; writing—review and editing: M.G., I.V. and J.S.; visualization: M.G.; supervision: J.S.; funding acquisition: J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the Canadian Centre for Fisheries Innovation (CCFI; grant number A-2020-02), Innovate NL (contract number 5404-1209-106) and Cooke Aquaculture Inc., Canada First Excellence Research Fund—Ocean Frontier Institute (sub-module J3) and NSERC-Discovery grant (RGPIN-2018-05942).
Institutional Review Board Statement
Animal protocols #18-01-JS, #18-03-JS, and biohazard license L-01 were reviewed and approved by the Institutional Animal Care Committee (https://www.mun.ca/research/about/acs/acc/) following the Canadian Council of Animal Care guidelines (https://www.ccac.ca/).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article and supplementary materials.
Acknowledgments
We thank the staff at the Cold-Ocean and Deep-Sea Research Facility (CDRF) and the Joe Brown Aquaculture Research Building (JBARB) for technical assistance (fish holding). Also, the authors thank all the Santander’s lab members for providing technical assistance during the experiment.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Løvoll, M.; Wiik-Nielsen, C.; Tunsjø, H.S.; Colquhoun, D.; Lunder, T.; Sørum, H.; Grove, S. Atlantic salmon bath challenged with Moritella viscosa–pathogen invasion and host response. Fish Shellfish Immunol. 2009, 26, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Grove, S.; Wiik-Nielsen, C.; Lunder, T.; Tunsjø, H.; Tandstad, N.; Reitan, L.; Marthinussen, A.; Sørgaard, M.; Olsen, A.; Colquhoun, D. Previously unrecognised division within Moritella viscosa isolated from fish farmed in the North Atlantic. Dis. Aquat. Org. 2010, 93, 51–61. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Karlsen, C.; Thorarinsson, R.; Wallace, C.; Salonius, K.; Midtlyng, P.J. Atlantic salmon winter-ulcer disease: Combining mortality and skin ulcer development as clinical efficacy criteria against Moritella viscosa infection. Aquaculture 2017, 473, 538–544. [Google Scholar] [CrossRef]
- MacKinnon, B.; Groman, D.; Fast, M.D.; Manning, A.J.; Jones, P.; St-Hilaire, S. Atlantic salmon challenged with extracellular products from Moritella viscosa. Dis. Aquat. Org. 2019, 133, 119–125. [Google Scholar] [CrossRef]
- MacKinnon, B.; Jones, P.; Hawkins, L.; Dohoo, I.; Stryhn, H.; Vanderstichel, R.; St-Hilaire, S. The epidemiology of skin ulcers in saltwater reared Atlantic salmon (Salmo salar) in Atlantic Canada. Aquaculture 2019, 501, 230–238. [Google Scholar] [CrossRef]
- Mondal, H.; Thomas, J. A review on the recent advances and application of vaccines against fish pathogens in aquaculture. Aquac. Int. 2022, 30, 1971–2000. [Google Scholar] [CrossRef]
- Shoemaker, C.A.; Klesius, P.H.; Evans, J.J.; Arias, C.R. Use of modified live vaccines in aquaculture. J. World Aquac. Soc. 2009, 40, 573–585. [Google Scholar] [CrossRef]
- Ghasemieshkaftaki, M.; Vasquez, I.; Eshraghi, A.; Gamperl, A.K.; Santander, J. Comparative Genomic Analysis of a Novel Vibrio sp. Isolated from an Ulcer Disease Event in Atlantic Salmon (Salmo salar). Microorganisms 2023, 11, 1736. [Google Scholar] [CrossRef]
- Secombes, C. Will advances in fish immunology change vaccination strategies? Fish Shellfish Immunol. 2008, 25, 409–416. [Google Scholar] [CrossRef]
- Tafalla, C.; Bøgwald, J.; Dalmo, R.A. Adjuvants and immunostimulants in fish vaccines: Current knowledge and future perspectives. Fish Shellfish Immunol. 2013, 35, 1740–1750. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Quillet, E.; Boudinot, P.; Fischer, U. What could be the mechanisms of immunological memory in fish? Fish Shellfish Immunol. 2019, 85, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Kayansamruaj, P.; Areechon, N.; Unajak, S. Development of fish vaccine in Southeast Asia: A challenge for the sustainability of SE Asia aquaculture. Fish Shellfish Immunol. 2020, 103, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Bruce, T.J.; Jones, E.M.; Cain, K.D. A review of fish vaccine development strategies: Conventional methods and modern biotechnological approaches. Microorganisms 2019, 7, 569. [Google Scholar] [CrossRef] [PubMed]
- Evensen, Ø. Development of fish vaccines: Focusing on methods. In Fish Vaccines; Springer: Basel, Switzerland, 2016; pp. 53–74. [Google Scholar]
- Durbin, M.; McIntosh, D.; Smith, P.; Wardle, R.; Austin, B. Immunization against furunculosis in rainbow trout with iron-regulated outer membrane protein vaccines: Relative efficacy of immersion, oral, and injection delivery. J. Aquat. Anim. Health 1999, 11, 68–75. [Google Scholar] [CrossRef]
- Björnsson, H.; Marteinsson, V.; Friðjónsson, Ó.; Linke, D.; Benediktsdottir, E. Isolation and characterization of an antigen from the fish pathogen Moritella viscosa. J. Appl. Microbiol. 2011, 111, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Sartorio, M.G.; Pardue, E.J.; Feldman, M.F.; Haurat, M.F. Bacterial outer membrane vesicles: From discovery to applications. Annu. Rev. Microbiol. 2021, 75, 609–630. [Google Scholar] [CrossRef] [PubMed]
- Prior, J.T.; Davitt, C.; Kurtz, J.; Gellings, P.; McLachlan, J.B.; Morici, L.A. Bacterial-derived outer membrane vesicles are potent adjuvants that drive humoral and cellular immune responses. Pharmaceutics 2021, 13, 131. [Google Scholar] [CrossRef]
- Anand, D.; Chaudhuri, A. Bacterial outer membrane vesicles: New insights and applications. Mol. Membr. Biol. 2016, 33, 125–137. [Google Scholar] [CrossRef]
- Balhuizen, M.D.; Veldhuizen, E.J.; Haagsman, H.P. Outer membrane vesicle induction and isolation for vaccine development. Front. Microbiol. 2021, 12, 629090. [Google Scholar] [CrossRef]
- Mehanny, M.; Lehr, C.-M.; Fuhrmann, G. Extracellular vesicles as antigen carriers for novel vaccination avenues. Adv. Drug Deliv. Rev. 2021, 173, 164–180. [Google Scholar] [CrossRef]
- Gomez, D.; Sunyer, J.O.; Salinas, I. The mucosal immune system of fish: The evolution of tolerating commensals while fighting pathogens. Fish Shellfish Immunol. 2013, 35, 1729–1739. [Google Scholar] [CrossRef] [PubMed]
- Koshio, S. Immunotherapies targeting fish mucosal immunity–Current knowledge and future perspectives. Front. Immunol. 2016, 6, 643. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, Q.; Huang, Z.; Ding, L.; Xu, Z. Immunoglobulins, mucosal immunity and vaccination in teleost fish. Front. Immunol. 2020, 11, 567941. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, D.M.; Zaccone, G.; Alesci, A.; Kuciel, M.; Hussein, M.T.; Sayed, R.K. Main components of fish immunity: An overview of the fish immune system. Fishes 2023, 8, 93. [Google Scholar] [CrossRef]
- Smith, N.C.; Rise, M.L.; Christian, S.L. A comparison of the innate and adaptive immune systems in cartilaginous fish, ray-finned fish, and lobe-finned fish. Front. Immunol. 2019, 10, 2292. [Google Scholar] [CrossRef]
- Bengtén, E.; Clem, L.W.; Miller, N.W.; Warr, G.W.; Wilson, M. Channel catfish immunoglobulins: Repertoire and expression. Dev. Comp. Immunol. 2006, 30, 77–92. [Google Scholar] [CrossRef]
- Solem, S.T.; Stenvik, J. Antibody repertoire development in teleosts—A review with emphasis on salmonids and Gadus morhua L. Dev. Comp. Immunol. 2006, 30, 57–76. [Google Scholar] [CrossRef]
- Mu, Q.; Dong, Z.; Kong, W.; Wang, X.; Yu, J.; Ji, W.; Su, J.; Xu, Z. Response of immunoglobulin M in gut mucosal immunity of common carp (Cyprinus carpio) infected with Aeromonas hydrophila. Front. Immunol. 2022, 13, 1037517. [Google Scholar] [CrossRef]
- Isla, A.; Sánchez, P.; Ruiz, P.; Albornoz, R.; Pontigo, J.P.; Rauch, M.C.; Hawes, C.; Vargas-Chacoff, L.; Yáñez, A.J. Effect of low-dose Piscirickettsia salmonis infection on haematological-biochemical blood parameters in Atlantic salmon (Salmo salar). J. Fish Biol. 2022, 101, 1021–1032. [Google Scholar] [CrossRef]
- Monir, M.S.; Yusoff, S.b.M.; Zulperi, Z.b.M.; Hassim, H.b.A.; Mohamad, A.; Ngoo, M.S.b.M.H.; Ina-Salwany, M.Y. Haemato-immunological responses and effectiveness of feed-based bivalent vaccine against Streptococcus iniae and Aeromonas hydrophila infections in hybrid red tilapia (Oreochromis mossambicus × O. niloticus). BMC Vet. Res. 2020, 16, 226. [Google Scholar] [CrossRef]
- Fazio, F. Fish hematology analysis as an important tool of aquaculture: A review. Aquaculture 2019, 500, 237–242. [Google Scholar] [CrossRef]
- Gordeev, I.I.; Mikryakov, D.V.; Balabanova, L.V.; Mikryakov, V.R. Composition of leucocytes in peripheral blood of Patagonian toothfish (Dissostichus eleginoides, Smitt, 1898)(Nototheniidae). Polar Res. 2017, 36, 1374126. [Google Scholar] [CrossRef]
- Witeska, M.; Kondera, E.; Ługowska, K.; Bojarski, B. Hematological methods in fish–Not only for beginners. Aquaculture 2022, 547, 737498. [Google Scholar] [CrossRef]
- Soto-Dávila, M.; Hossain, A.; Chakraborty, S.; Rise, M.L.; Santander, J. Aeromonas salmonicida subsp. salmonicida early infection and immune response of Atlantic cod (Gadus morhua L.) primary macrophages. Front. Immunol. 2019, 10, 1237. [Google Scholar] [CrossRef] [PubMed]
- Connors, E.; Soto-Dávila, M.; Hossain, A.; Vasquez, I.; Gnanagobal, H.; Santander, J. Identification and validation of reliable Aeromonas salmonicida subspecies salmonicida reference genes for differential gene expression analyses. Infect. Genet. Evol. 2019, 73, 314–321. [Google Scholar] [CrossRef]
- Leboffe, M.J.; Pierce, B.E. Microbiology: Laboratory Theory and Application; Morton Publishing Company: Englewood, CO, USA, 2015. [Google Scholar]
- Gross, A.; Schoendube, J.; Zimmermann, S.; Steeb, M.; Zengerle, R.; Koltay, P. Technologies for single-cell isolation. Int. J. Mol. Sci. 2015, 16, 16897–16919. [Google Scholar] [CrossRef]
- Inoue, T.; Moritomo, T.; Tamura, Y.; Mamiya, S.; Fujino, H.; Nakanishi, T. A new method for fish leucocyte counting and partial differentiation by flow cytometry. Fish Shellfish Immunol. 2002, 13, 379–390. [Google Scholar] [CrossRef]
- Erkinharju, T.; Lundberg, M.R.; Isdal, E.; Hordvik, I.; Dalmo, R.A.; Seternes, T. Studies on the antibody response and side effects after intramuscular and intraperitoneal injection of Atlantic lumpfish (Cyclopterus lumpus L.) with different oil-based vaccines. J. Fish Dis. 2017, 40, 1805–1813. [Google Scholar]
- Rønneseth, A.; Haugland, G.T.; Colquhoun, D.J.; Brudal, E.; Wergeland, H.I. Protection and antibody reactivity following vaccination of lumpfish (Cyclopterus lumpus L.) against atypical Aeromonas salmonicida. Fish Shellfish Immunol. 2017, 64, 383–391. [Google Scholar] [CrossRef]
- Crowther, J.R. The ELISA Guidebook; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008; Volume 149. [Google Scholar]
- Dang, M.; Cao, T.; Vasquez, I.; Hossain, A.; Gnanagobal, H.; Kumar, S.; Hall, J.R.; Monk, J.; Boyce, D.; Westcott, J. Oral immunization of larvae and juvenile of lumpfish (Cyclopterus lumpus) against Vibrio anguillarum does not influence systemic immunity. Vaccines 2021, 9, 819. [Google Scholar] [CrossRef]
- Vasquez, I.; Cao, T.; Hossain, A.; Valderrama, K.; Gnanagobal, H.; Dang, M.; Leeuwis, R.H.; Ness, M.; Campbell, B.; Gendron, R. Aeromonas salmonicida infection kinetics and protective immune response to vaccination in sablefish (Anoplopoma fimbria). Fish Shellfish Immunol. 2020, 104, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Eslamloo, K.; Kumar, S.; Caballero-Solares, A.; Gnanagobal, H.; Santander, J.; Rise, M.L. Profiling the transcriptome response of Atlantic salmon head kidney to formalin-killed Renibacterium salmoninarum. Fish Shellfish Immunol. 2020, 98, 937–949. [Google Scholar] [CrossRef] [PubMed]
- Hirst, I.; Ellis, A.E. Iron-regulated outer membrane proteins of Aeromonas salmonicida are important protective antigens in Atlantic salmon against furunculosis. Fish Shellfish Immunol. 1994, 4, 29–45. [Google Scholar] [CrossRef]
- Santander, J.; Golden, G.; Wanda, S.-Y.; Curtiss III, R. Fur-regulated iron uptake system of Edwardsiella ictaluri and its influence on pathogenesis and immunogenicity in the catfish host. Infect. Immun. 2012, 80, 2689–2703. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Russell, D. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2001; Volume 413. [Google Scholar]
- Brudal, E.; Lampe, E.O.; Reubsaet, L.; Roos, N.; Hegna, I.K.; Thrane, I.M.; Koppang, E.O.; Winther-Larsen, H.C. Vaccination with outer membrane vesicles from Francisella noatunensis reduces development of francisellosis in a zebrafish model. Fish Shellfish Immunol. 2015, 42, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, C.; Vanberg, C.; Mikkelsen, H.; Sørum, H. Co-infection of Atlantic salmon (Salmo salar), by Moritella viscosa and Aliivibrio wodanis, development of disease and host colonization. Vet. Microbiol. 2014, 171, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Poppe, T.T.; Bæverfjord, G.; Hansen, T. Effects of intensive production with emphasis on on-growing production: Fast growth, deformities and production-related diseases. In Aquaculture Research: From Cage to Consumption; Norges Forskningsråd: Oslo, Norway, 2007; pp. 120–135. [Google Scholar]
- Adams, A. Progress, challenges and opportunities in fish vaccine development. Fish Shellfish Immunol. 2019, 90, 210–214. [Google Scholar] [CrossRef]
- Rozas-Serri, M.; Correa, R.; Walker-Vergara, R.; Coñuecar, D.; Barrientos, S.; Leiva, C.; Ildefonso, R.; Senn, C.; Peña, A. Reference Intervals for Blood Biomarkers in Farmed Atlantic Salmon, Coho Salmon and Rainbow Trout in Chile: Promoting a Preventive Approach in Aquamedicine. Biology 2022, 11, 1066. [Google Scholar] [CrossRef]
- Ley, K.; Laudanna, C.; Cybulsky, M.I.; Nourshargh, S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007, 7, 678–689. [Google Scholar] [CrossRef]
- Chavakis, E.; Choi, E.Y.; Chavakis, T. Novel aspects in the regulation of the leukocyte adhesion cascade. Thromb. Haemost. 2009, 102, 191–197. [Google Scholar] [CrossRef]
- Janeway Jr, C.A. Immunobiology the Immune System in Health and Disease; Garland Science: New York, NY, USA, 1997. [Google Scholar]
- Mutoloki, S.; Jørgensen, J.B.; Evensen, Ø. The adaptive immune response in fish. In Fish Vaccination; Wiley: Hoboken, NJ, USA, 2014; pp. 104–115. [Google Scholar]
- Harikrishnan, R.; Rani, M.N.; Balasundaram, C. Hematological and biochemical parameters in common carp, Cyprinus carpio, following herbal treatment for Aeromonas hydrophila infection. Aquaculture 2003, 221, 41–50. [Google Scholar] [CrossRef]
- Afiyanti, A.D.; Yuliani, M.G.A.; Handijatno, D. Leukocyte count and differential leukocyte count of carp (Cyprinus carpio Linn) after infected by Aeromonas salmonicida. Cell 2018, 2000, 3. [Google Scholar]
- Martins, M.; Mouriño, J.; Amaral, G.; Vieira, F.; Dotta, G.; Jatobá, A.; Pedrotti, F.; Jerônimo, G.; Buglione-Neto, C. Haematological changes in Nile tilapia experimentally infected with Enterococcus sp. Braz. J. Biol. 2008, 68, 657–661. [Google Scholar] [CrossRef]
- Sheng, X.-Z.; Xu, G.-J.; Tang, X.-Q.; Zhan, W.-B. Monoclonal antibodies recognizing mucus immunoglobulin and surface immunoglobulin-positive cells of flounder (Paralichthys olivaceus). Vet. Immunol. Immunopathol. 2012, 145, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Qian, X.; Tang, X.; Xing, J.; Zhan, W. Polymeric immunoglobulin receptor mediates immune excretion of mucosal IgM–antigen complexes across intestinal epithelium in flounder (Paralichthys olivaceus). Front. Immunol. 2018, 9, 1562. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Barreda, D.R.; Zhang, Y.-A.; Boshra, H.; Gelman, A.E.; LaPatra, S.; Tort, L.; Sunyer, J.O. B lymphocytes from early vertebrates have potent phagocytic and microbicidal abilities. Nat. Immunol. 2006, 7, 1116–1124. [Google Scholar] [CrossRef]
- Wu, L.; Yang, Y.; Gao, A.; Li, J.; Ye, J. Teleost fish IgM+ plasma-like cells possess IgM-secreting, phagocytic, and antigen-presenting capacities. Front. Immunol. 2022, 13, 1016974. [Google Scholar] [CrossRef]
- van der Wal, Y.A.; Jenberie, S.; Nordli, H.; Greiner-Tollersrud, L.; Kool, J.; Jensen, I.; Jørgensen, J.B. The importance of the Atlantic salmon peritoneal cavity B cell response: Local IgM secreting cells are predominant upon Piscirickettsia salmonis infection. Dev. Comp. Immunol. 2021, 123, 104125. [Google Scholar] [CrossRef]
- Dumetz, F.; Duchaud, E.; Claverol, S.; Orieux, N.; Papillon, S.; Lapaillerie, D.; Le Henaff, M. Analysis of the Flavobacterium psychrophilum outer-membrane subproteome and identification of new antigenic targets for vaccine by immunomics. Microbiology 2008, 154, 1793–1801. [Google Scholar] [CrossRef]
- Jan, A.T. Outer membrane vesicles (OMVs) of gram-negative bacteria: A perspective update. Front. Microbiol. 2017, 8, 1053. [Google Scholar] [CrossRef]
- Ellis, T.N.; Kuehn, M.J. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol. Mol. Biol. Rev. 2010, 74, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Koeppen, K.; Hampton, T.H.; Jarek, M.; Scharfe, M.; Gerber, S.A.; Mielcarz, D.W.; Demers, E.G.; Dolben, E.L.; Hammond, J.H.; Hogan, D.A. A novel mechanism of host-pathogen interaction through sRNA in bacterial outer membrane vesicles. PLoS Pathog. 2016, 12, e1005672. [Google Scholar] [CrossRef] [PubMed]
- Furuyama, N.; Sircili, M.P. Outer membrane vesicles (OMVs) produced by gram-negative bacteria: Structure, functions, biogenesis, and vaccine application. BioMed Res. Int. 2021, 2021, 1490732. [Google Scholar] [CrossRef] [PubMed]
- Schwechheimer, C.; Kuehn, M.J. Outer-membrane vesicles from Gram-negative bacteria: Biogenesis and functions. Nat. Rev. Microbiol. 2015, 13, 605–619. [Google Scholar] [CrossRef] [PubMed]
- Tandberg, J.; Oliver, C.; Lagos, L.; Gaarder, M.; Yáñez, A.J.; Ropstad, E.; Winther-Larsen, H.C. Membrane vesicles from Piscirickettsia salmonis induce protective immunity and reduce development of salmonid rickettsial septicemia in an adult zebrafish model. Fish Shellfish Immunol. 2017, 67, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Oliver, C.; Coronado, J.L.; Martínez, D.; Kashulin-Bekkelund, A.; Lagos, L.X.; Ciani, E.; Sanhueza-Oyarzún, C.; Mancilla-Nova, A.; Enríquez, R.; Winther-Larsen, H.C. Outer membrane vesicles from Piscirickettsia salmonis induce the expression of inflammatory genes and production of IgM in Atlantic salmon Salmo salar. Fish Shellfish Immunol. 2023, 139, 108887. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.-E.; Kim, D.-G.; Park, E.-M.; Nam, B.-H.; Kim, Y.-O.; Kong, I.-S. Identification of Vibrio anguillarum outer membrane vesicles related to immunostimulation in the Japanese flounder, Paralichthys olivaceus. Biosci. Biotechnol. Biochem. 2009, 73, 437–439. [Google Scholar] [CrossRef]
- Gerritzen, M.J.; Martens, D.E.; Wijffels, R.H.; van der Pol, L.; Stork, M. Bioengineering bacterial outer membrane vesicles as vaccine platform. Biotechnol. Adv. 2017, 35, 565–574. [Google Scholar] [CrossRef]
- Kashyap, D.; Panda, M.; Baral, B.; Varshney, N.; Bhandari, V.; Parmar, H.S.; Prasad, A.; Jha, H.C. Outer membrane vesicles: An emerging vaccine platform. Vaccines 2022, 10, 1578. [Google Scholar] [CrossRef]
- Romstad, A.B.; Reitan, L.J.; Midtlyng, P.; Gravningen, K.; Evensen, Ø. Development of an antibody ELISA for potency testing of furunculosis (Aeromonas salmonicida subsp salmonicida) vaccines in Atlantic salmon (Salmo salar L.). Biologicals 2012, 40, 67–71. [Google Scholar] [CrossRef]
- Liu, S.; Wang, D.; Cao, Y.; Lu, T.; Liu, H.; Li, S. Effects of propolis on the immune enhancement of the formalin-inactivated Aeromonas salmonicida vaccine. Aquac. Res. 2020, 51, 4759–4770. [Google Scholar] [CrossRef]
- Wang, H.-R.; Hu, Y.-H.; Zhang, W.-W.; Sun, L. Construction of an attenuated Pseudomonas fluorescens strain and evaluation of its potential as a cross-protective vaccine. Vaccine 2009, 27, 4047–4055. [Google Scholar] [CrossRef] [PubMed]
- Raida, M.K.; Nylén, J.; Holten-Andersen, L.; Buchmann, K. Association between plasma antibody response and protection in rainbow trout Oncorhynchus mykiss immersion vaccinated against Yersinia ruckeri. PLoS ONE 2011, 6, e18832. [Google Scholar] [CrossRef] [PubMed]
- Villumsen, K.R.; Dalsgaard, I.; Holten-Andersen, L.; Raida, M.K. Correction: Potential Role of Specific Antibodies as Important Vaccine Induced Protective Mechanism against Aeromonas salmonicida in Rainbow Trout. PLoS ONE 2012, 7, e46733. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).