Vaccinating Welders against Pneumococcus: Evidence from a Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Selection, Inclusion and Exclusion Criteria
- (a)
- (“Streptococcus pneumoniae” OR “pneumococcus” OR “pneumococcal infection”) AND (“meningitis” OR “pneumonia” OR “bacteremia” OR “invasive pneumococcal disease” OR “IPD”) AND (“occupation*” OR “work-related” OR “worker*” OR “job”)
- (b)
- (“Streptococcus pneumoniae”/exp OR “Streptococcus pneumoniae” OR “pneumococcal infection”) AND (“invasive pneumococcal infection” OR “meningitis” OR “infectious meningitis” OR “pneumonia” OR “IPD” OR “bacteremia”) AND (“occupation” OR “work” OR “workforce”)
- Reporting the crude number of assessed cases of PNX-related infections, IPD and/or PNX-related deaths: generic diagnoses such as “respiratory infections” or “pneumonia” not otherwise specified were removed from the analyses;
- Reporting the total number of exposed workers;
- Reporting the settings of occupational exposure, specifically focusing on the exposure to welding and metal fumes, assessed through job titles (e.g., welders) or job exposure matrix.
- Case reports, case series and outbreak reports were included but corresponding estimates were independently calculated.
2.2. Data Extraction
- Settings of the study (country, time, occupational settings);
- Number of included cases/deaths;
- Number of assessed workers;
- Number and characteristics of the reference groups (where available);
- Number of expected deaths for pneumococcal pneumonia in the reference group (where available);
- Pneumococcus serogroups, where available;
- Comorbidities, where available;
- Time elapsed between the first and the last case (where available).
2.3. Risk of Bias Assessment
2.4. Statistical Analysis
3. Results
3.1. Summary of Retrieved Studies
3.2. Summary of Case Series
3.3. Summary of Observational Studies
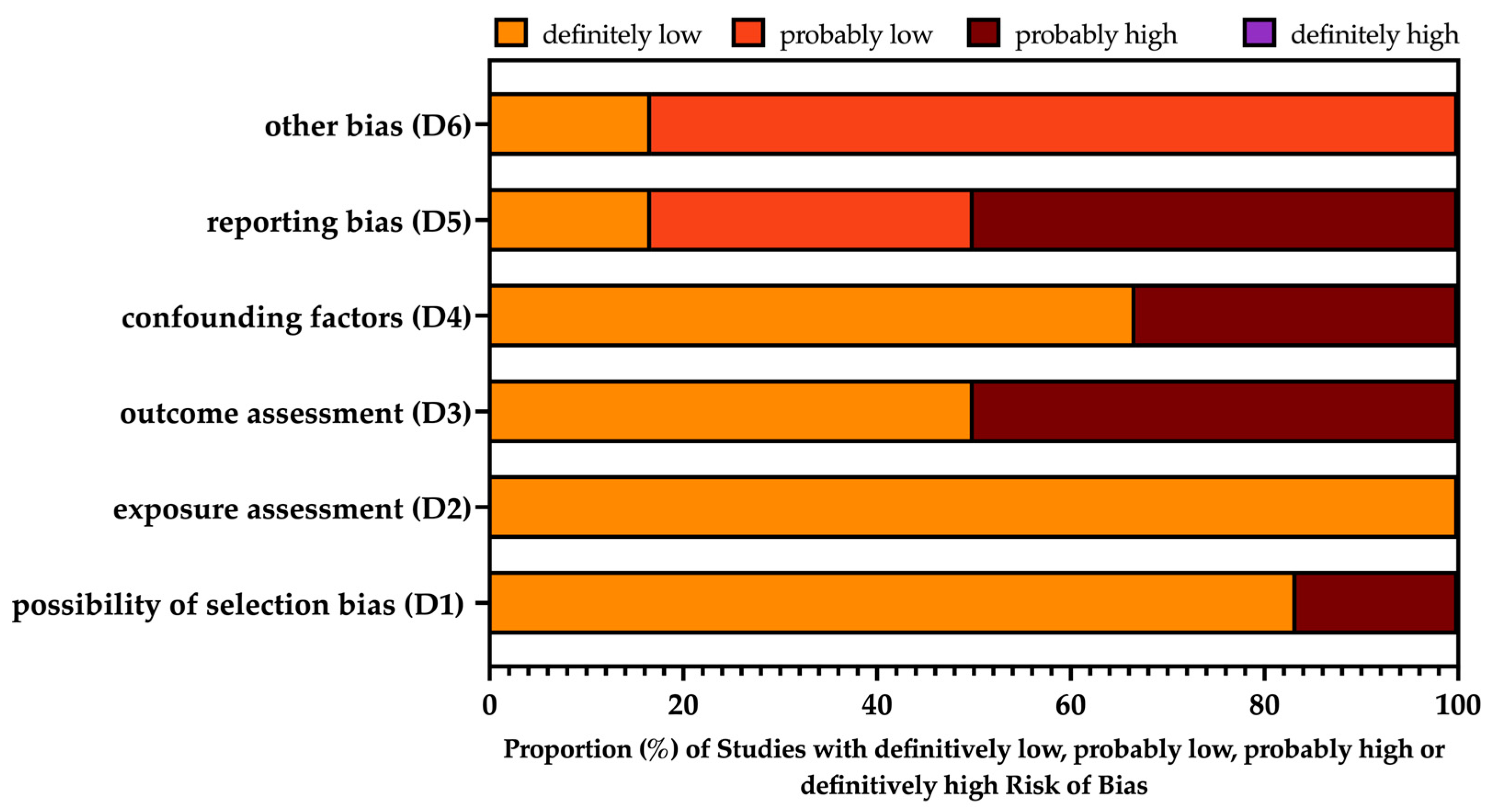
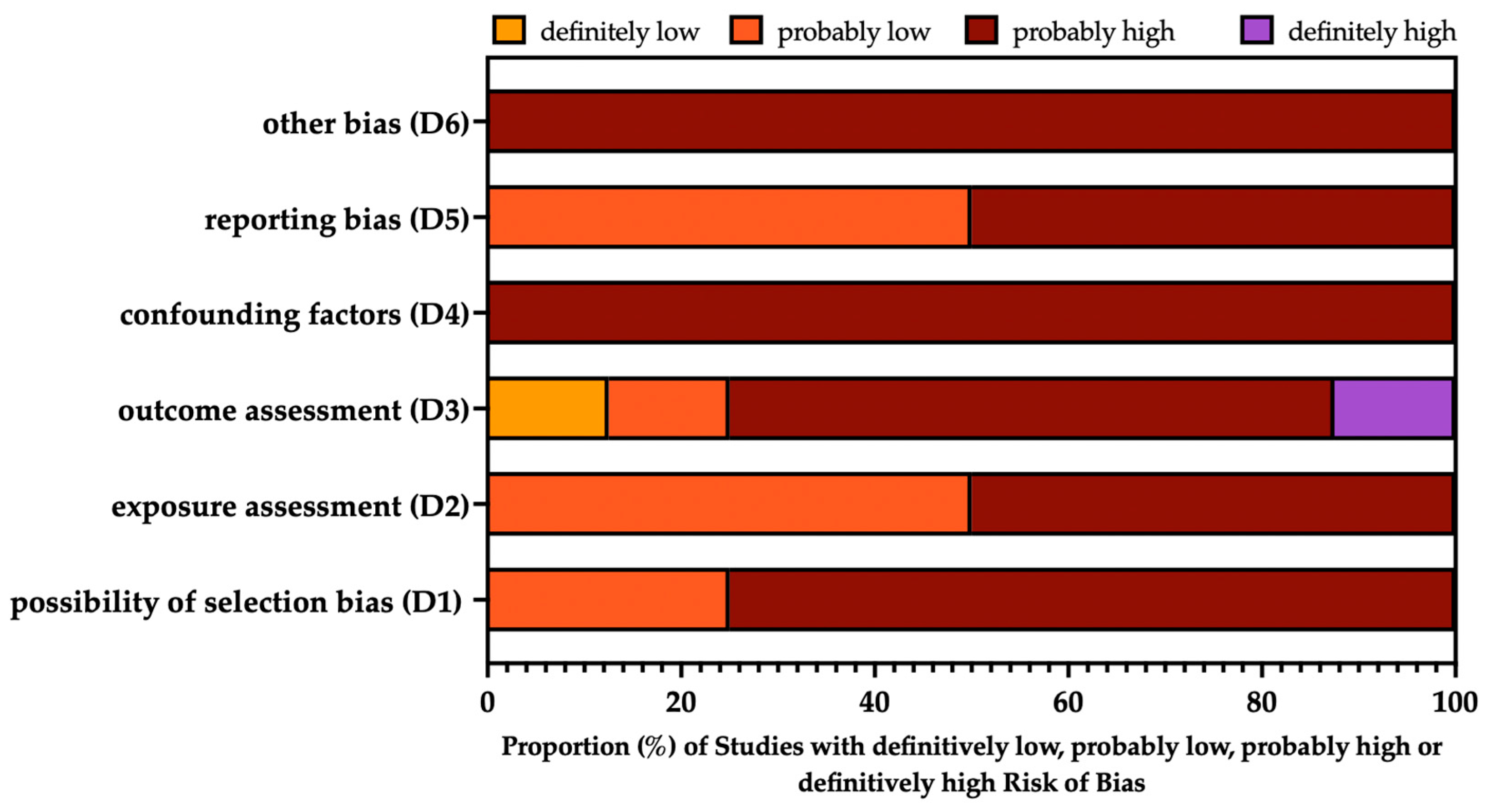
3.4. Risk of Bias Assessment
3.5. Meta-Analysis of Case Series Studies
3.6. Meta-Analysis of Observational Studies
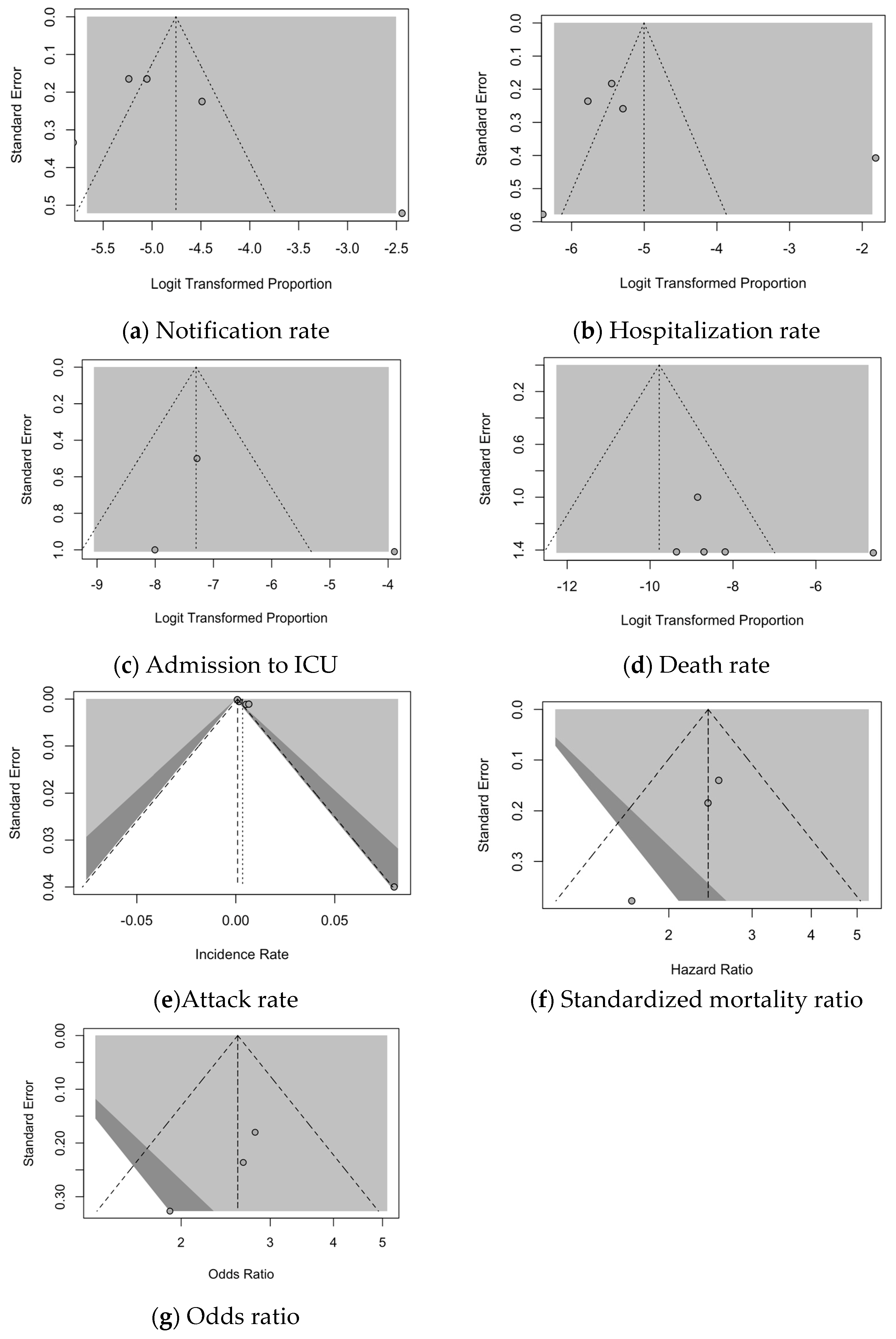
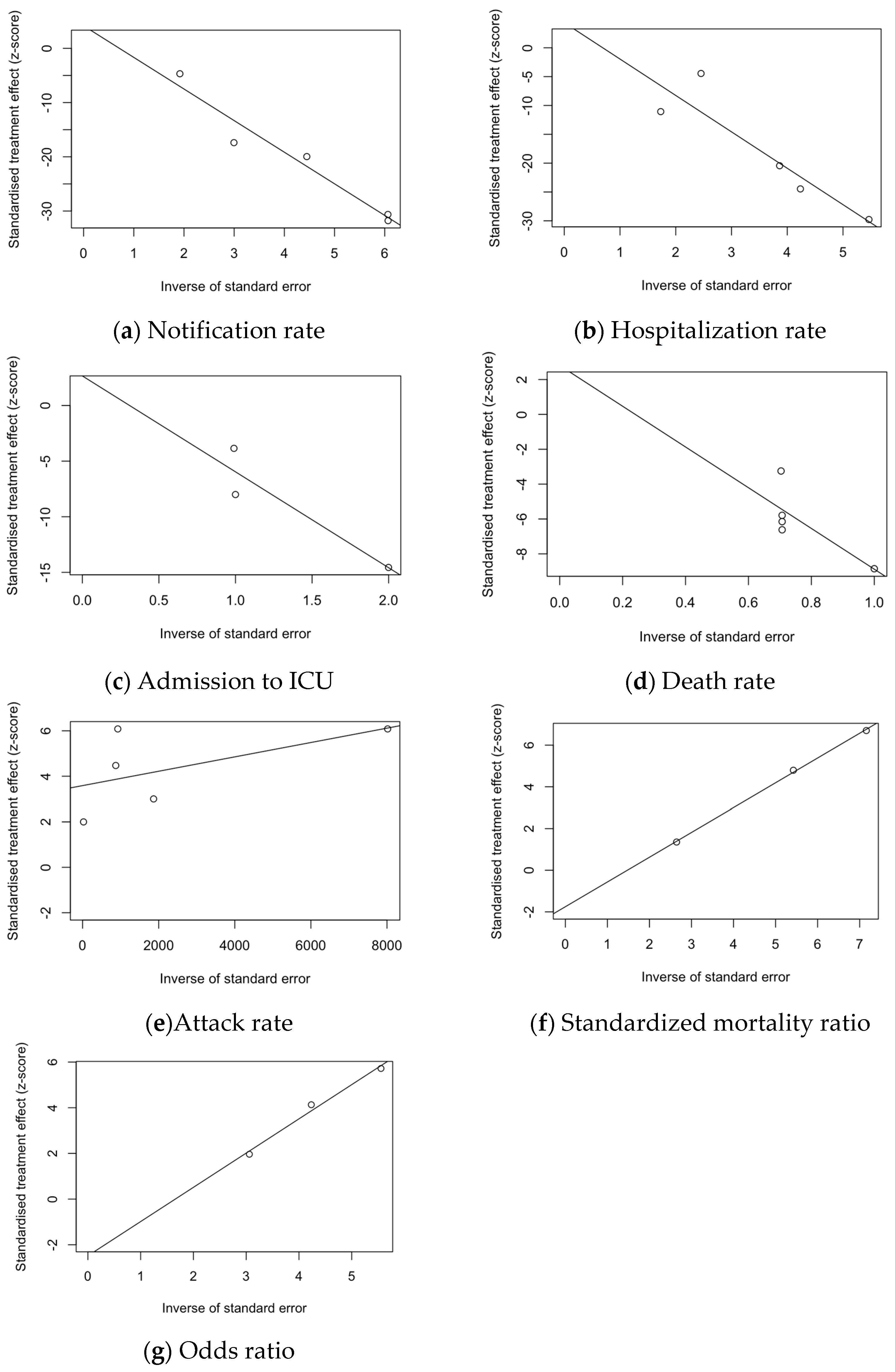
3.7. Analysis of Publication Bias
4. Discussion
Limits
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
 : definitively high;
: definitively high;  : probably high;
: probably high;  : probably low;
: probably low;  : definitively low.
: definitively low.
 : definitively high;
: definitively high;  : probably high;
: probably high;  : probably low;
: probably low;  : definitively low.
: definitively low.| Study | Risk of Bias | |||||
|---|---|---|---|---|---|---|
| D1 | D2 | D3 | D4 | D5 | D6 | |
| Cassir et al. [32] |  |  |  |  |  |  |
| Linkevivius et al. [42] |  |  |  |  |  |  |
| Kiang [41] |  |  |  |  |  |  |
| Berild et al. [30] |  |  |  |  |  |  |
| Ewing et al. [37] |  |  |  |  |  |  |
| Flodin et al. [38] |  |  |  |  |  |  |
 : definitively high;
: definitively high;  : probably high;
: probably high;  : probably low;
: probably low;  : definitively low.
: definitively low.
 : definitively high;
: definitively high;  : probably high;
: probably high;  : probably low;
: probably low;  : definitively low.
: definitively low.| Study | Risk of Bias | |||||
|---|---|---|---|---|---|---|
| D1 | D2 | D3 | D4 | D5 | D6 | |
| Beaumont and Weiss 1980 [29] |  |  |  |  |  |  |
| Coggon et al. 1994 [33] |  |  |  |  |  |  |
| Palmer et al. 2009 [35] |  |  |  |  |  |  |
| Palmer et al. 2003 [34] |  |  |  |  |  |  |
| Torén et al. 2011 [47] |  |  |  |  |  |  |
| Torén et al. 2022 [9] |  |  |  |  |  |  |
| Torén et al. 2020 [8] |  |  |  |  |  |  |
| Wong et al. 2010 [13] |  |  |  |  |  |  |
| Finding | t | df | Bias (SE) | Intercept (SE) | p Value |
|---|---|---|---|---|---|
| Notification rate | 1.13 | 3 | 4.166 (3.677) | −5.829 (0.799) | 0.340 |
| Hospitalization rate | 0.71 | 3 | 4.362 (6.164) | −6.312 (1.627) | 0.530 |
| Rate of ICU admission | 0.54 | 1 | 2.661 (4.949) | −8.623 (3.506) | 0.686 |
| Death rate | 0.64 | 3 | 2.807 (4.387) | −11.686 (5.668) | 0.568 |
| Attack rate | 3.57 | 3 | 3.586 (1.004) | 0.001 (0.001) | 0.038 |
| Notification rate | −1.37 | 8 | −1.865 (1.358) | 0.399 (0.652) | 0.207 |
| Standardized mortality ratio (SMR) | −8.79 | 1 | −1.753 (0.200) | 1.189 (0.037) | 0.072 |
| Odds ratio (OR) | −3.05 | 1 | −2.483 (0.814) | 1.499 (0.185) | 0.202 |
References
- Horn, E.K.; Wasserman, M.D.; Hall-Murray, C.; Sings, H.L.; Chapman, R.; Farkouh, R.A. Public Health Impact of Pneumococcal Conjugate Vaccination: A Review of Measurement Challenges. Expert Rev. Vaccines 2021, 20, 1291–1309. [Google Scholar] [CrossRef]
- Li, L.; Ma, J.; Yu, Z.; Li, M.; Zhang, W.; Sun, H. Epidemiological Characteristics and Antibiotic Resistance Mechanisms of Streptococcus Pneumoniae: An Updated Review. Microbiol. Res. 2023, 266, 127221. [Google Scholar] [CrossRef]
- Hanada, S.; Iwata, S.; Kishi, K.; Morozumi, M.; Chiba, N.; Wajima, T.; Takato, M.; Ubukata, K. Host Factors and Biomarkers Associated with Poor Outcomes in Adults with Invasive Pneumococcal Disease. PLoS ONE 2016, 11, e0147877. [Google Scholar] [CrossRef]
- Tsang, R.S.W. A Narrative Review of the Molecular Epidemiology and Laboratory Surveillance of Vaccine Preventable Bacterial Meningitis Agents: Streptococcus Pneumoniae, Neisseria meningitidis, Haemophilus influenzae and Streptococcus agalactiae. Microorganisms 2021, 9, 449. [Google Scholar] [CrossRef]
- Henriques-Normark, B.; Tuomanen, E.I. The Pneumococcus: Epidemiology, Microbiology, and Pathogenesis. Cold Spring Harb. Perspect. Med. 2013, 3, a010215. [Google Scholar] [CrossRef]
- Troeger, C.; Blacker, B.; Khalil, I.A.; Rao, P.C.; Cao, J.; Zimsen, S.R.M.; Albertson, S.B.; Deshpande, A.; Farag, T.; Abebe, Z.; et al. Estimates of the Global, Regional, and National Morbidity, Mortality, and Aetiologies of Lower Respiratory Infections in 195 Countries, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018, 18, 1191–1210. [Google Scholar] [CrossRef]
- Chen, H.; Matsumoto, H.; Horita, N.; Hara, Y.; Kobayashi, N.; Kaneko, T. Prognostic Factors for Mortality in Invasive Pneumococcal Disease in Adult: A System Review and Meta-Analysis. Sci. Rep. 2021, 11, 11865. [Google Scholar] [CrossRef]
- Torén, K.; Blanc, P.D.; Naidoo, R.N.; Murgia, N.; Qvarfordt, I.; Aspevall, O.; Dahlman-Hoglund, A.; Schioler, L. Occupational Exposure to Dust and to Fumes, Work as a Welder and Invasive Pneumococcal Disease Risk. Occup. Environ. Med. 2020, 77, 57–63. [Google Scholar] [CrossRef]
- Torén, K.; Blanc, P.D.; Naidoo, R.; Murgia, N.; Stockfelt, L.; Schiöler, L. Cumulative Occupational Exposure to Inorganic Dust and Fumes and Invasive Pneumococcal Disease with Pneumonia. Int. Arch. Occup. Environ. Health 2022, 95, 1797–1804. [Google Scholar] [CrossRef]
- Torén, K.; Naidoo, R.N.; Blanc, P.D. Pneumococcal Pneumonia on the Job: Unconvering the Past Story of Occupational Exposure to Metal Fumes and Dust. Am. J. Ind. Med. 2022, 65, 517–524. [Google Scholar] [CrossRef]
- Donoghue, A.M.; Wesdock, J.C. Pneumococcal Vaccination for Welders: Global Deployment within a Multi-National Corporation. Am. J. Ind. Med. 2019, 62, 69–73. [Google Scholar] [CrossRef]
- Palmer, K.T.; Cosgrove, M.P. Vaccinating Welders against Pneumonia. Occup. Med. 2012, 62, 325–330. [Google Scholar] [CrossRef]
- Wong, A.; Marrie, T.J.; Garg, S.; Kellner, J.D.; Tyrrell, G.J. Welders Are at Increased Risk for Invasive Pneumococcal Disease. Int. J. Infect. Dis. 2010, 14, e796–e799. [Google Scholar] [CrossRef]
- Steenland, K. Ten-Year Update on Mortality among Mild-Steel Welders. Scand. J. Work Environ. Health 2002, 28, 163–167. [Google Scholar] [CrossRef]
- Simonato, L.; Fletcher, A.C.; Andersen, A.; Anderson, K.; Becker, N.; Chang-Claude, J.; Ferro, G.; Gerin, M.; Gray, C.N.; Hansen, K.S.; et al. A Historical Prospective Study of European Stainless Steel, Mild Steel, and Shipyard Welders. Br. J. Ind. Med. 1991, 48, 145–154. [Google Scholar] [CrossRef]
- Blanc, P.D.; Redlich, C.A.; Annesi-Maesano, I.; Balmes, J.R.; Cummings, K.J.; Fishwick, D.; Miedinger, D.; Murgia, N.; Naidoo, R.N.; Reynolds, C.J.; et al. The Occupational Burden of Nonmalignant Respiratory Diseases an Official American Thoracic Society and European Respiratory Society Statement. Am. J. Respir. Crit. Care Med. 2019, 199, 1312–1334. [Google Scholar] [CrossRef]
- Health and Safety Executive. Pneumonia Vaccination for Employees Exposed to Welding and Metal Fume (EIS44); Health and Safety Executive (HSE): London, UK, 2014.
- Robert Koch-Institut Epidemiologisches Bulletin Empfehlungen Der Ständigen Impf-Kommission Beim Robert Koch-Institut 2023. Epidemiol. Bull. 2023, 2023, 133–152.
- Fischer, K.; Karnthaler, U.; Klein, J.-P.; Kohlfürst, D.; Kollaritsch, H.; Kundi, M.; Palmisano, G.; Paulke-Korinek, M.; Philadelphy, D.; Prieler, A.; et al. Impfplan Österreich 2023/2024; Bundesministeriums für Soziales, Gesundheit, Pflege und Konsumentenschutz: Vienna, Austria, 2023. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Morgan, R.L.; Whaley, P.; Thayer, K.A.; Schünemann, H.J. Identifying the PECO: A Framework for Formulating Good Questions to Explore the Association of Environmental and Other Exposures with Health Outcomes. Environ. Int. 2018, 121, 1027–1031. [Google Scholar] [CrossRef]
- Mintzker, Y.; Blum, D.; Adler, L. Replacing PICO in Non-Interventional Studies. BMJ Evid. Based Med. 2022, 28, 284. [Google Scholar] [CrossRef]
- Office of Health Assessment and Translation (OHAT). Handbook for Conducting a Literature-Based Health Assessment Using OHAT Approach for Systematic Review and Evidence Integration; Office of Health Assessment and Translation (OHAT): Research Triangle Park, NC, USA, 2019.
- Eick, S.M.; Goin, D.E.; Chartres, N.; Lam, J.; Woodruff, T.J. Assessing Risk of Bias in Human Environmental Epidemiology Studies Using Three Tools: Different Conclusions from Different Tools. Syst. Rev. 2020, 9, 249. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring Inconsistency in Meta-Analyses. Br. Med. J. 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Bell, A.; Fairbrother, M.; Jones, K. Fixed and Random Effects Models: Making an Informed Choice. Qual. Quant. 2019, 53, 1051–1074. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing: Reference Index; R Foundation for Statistical Computing: Vienna, Austria, 2010; ISBN 3900051070. [Google Scholar]
- Amin-Chowdhury, Z.; Iyanger, N.; Ramsay, M.E.; Ladhani, S.N. Outbreaks of Severe Pneumococcal Disease in Closed Settings in the Conjugate Vaccines Era, 2010–2018: A Systematic Review to Inform National Guidance in the UK. J. Infect. 2019, 79, 495–502. [Google Scholar] [CrossRef]
- Beaumont, J.J.; Weiss, N.S.; Beaumont, J.J. Mortality of Welders, Shipfitters, and Other Metal Trades Workers in Boilermakers Local No. 104, AFL-CIO. Am. J. Epidemiol. 1980, 112, 775–786. [Google Scholar]
- Berild, J.D.; Steens, A.; Askeland Winje, B.; Danielsen, T.E.; Fjeldheim, J.H.; Daae-Qvale Holmemo, H.; Frimann Vestrheim, D. Management and Control of an Outbreak of Vaccine-Preventable Severe Pneumococcal Disease at a Shipyard in Norway. J. Infect. 2020, 80, 590–592. [Google Scholar] [CrossRef]
- Moulin, J.J.; Wild, P.; Haguenoer, J.M.; Faucon, D.; de Gaudemaris, R.; Mur, J.M.; Mereau, M.; Gary, Y.; Toamain, J.P.; Birembaut, Y.; et al. A Mortality Study among Mild Steel and Stainless Steel Welders. Br. J. Ind. Med. 1993, 50, 234–243. [Google Scholar] [CrossRef]
- Cassir, N.; Pascal, L.; Ferrieux, D.; Bruel, C.; Guervilly, C.; Rebaudet, S.; Danis, K.; Kopec, L.; Fenollar, F.; Varon1, E.; et al. Outbreak of Pneumococcal Pneumonia among Shipyard Workers in Marseille, France, January to February 2020. Eurosurveillance 2020, 25, 2000162. [Google Scholar] [CrossRef]
- Coggon, D.; Inskip, H.; Winter, P.; Pannett, B. Lobar Pneumonia: An Occupational Disease in Welders. Lancet 1994, 344, 41–43. [Google Scholar] [CrossRef]
- Palmer, K.T.; Poole, J.; Ayres, J.G.; Mann, J.; Burge, P.S.; Coggon, D. Exposure to Metal Fume and Infectious Pneumonia. Am. J. Epidemiol. 2003, 157, 227–233. [Google Scholar] [CrossRef]
- Palmer, K.T.; Cullinan, P.; Rice, S.; Brown, T.; Coggon, D. Mortality from Infectious Pneumonia in Metal Workers: A Comparison with Deaths from Asthma in Occupations Exposed to Respiratory Sensitisers. Thorax 2009, 64, 983–986. [Google Scholar] [CrossRef] [PubMed]
- Danielsen, T.E. S-80 Outbreak of Invasive Pneumococcal Disease among Workers at a Norwegian Shipyard. Occup. Environ. Med. 2021, 78, A147. [Google Scholar] [CrossRef]
- Ewing, J.; Patterson, L.; Irvine, N.; Doherty, L.; Loughrey, A.; Kidney, J.; Sheppard, C.; Kapatai, G.; Fry, N.K.; Ramsay, M.; et al. Serious Pneumococcal Disease Outbreak in Men Exposed to Metal Fume—Detection, Response and Future Prevention through Pneumococcal Vaccination. Vaccine 2017, 35, 3945–3950. [Google Scholar] [CrossRef] [PubMed]
- Flodin, U.; Paues, J.; Åkerling, B.; Leanderson, P.; Sjögren, B. Svetsare—En Riskgrupp För Septisk Pneumoni. Lakartidningen 2017, 114, D6TW. [Google Scholar] [PubMed]
- Gladstone, R.A.; Siira, L.; Brynildsrud, O.B.; Vestrheim, D.F.; Turner, P.; Clarke, S.C.; Srifuengfung, S.; Ford, R.; Lehmann, D.; Egorova, E.; et al. International Links between Streptococcus Pneumoniae Vaccine Serotype 4 Sequence Type (ST) 801 in Northern European Shipyard Outbreaks of Invasive Pneumococcal Disease. Vaccine 2022, 40, 1054–1060. [Google Scholar] [CrossRef]
- Graham, W.G.B.; Costello, J.; Vacek, P.M. Vermont Granite Mortality Study: An Update with an Emphasis on Lung Cancer. J. Occup. Environ. Med. 2004, 46, 459–466. [Google Scholar] [CrossRef]
- Kiang, L.E. A Series of 3 Cases of Streptococcus Pneumoniae Pneumonia in 3 Foreign Shipyard Workers. Ann. Case Rep. 2018, 6, 1–9. [Google Scholar] [CrossRef]
- Linkevicius, M.; Cristea, V.; Siira, L.; Mäkela, H.; Toropainen, M.; Pitkäpaasi, M.; Dub, T.; Nohynek, H.; Puumalainen, T.; Rintala, E.; et al. Outbreak of Invasive Pneumococcal Disease among Shipyard Workers, Turku, Finland, May to November 2019. Euro. Surveill. 2019, 24, 1900681. [Google Scholar] [CrossRef]
- Newhouse, M.L.; Oakes, D.; Woolley, A.J. Mortality of Welders and Other Craftsmen at a Shipyard in NE England. Occup. Environ. Med. 1985, 42, 406–410. [Google Scholar] [CrossRef]
- Palmer, K. Community-Acquired Pneumonia and Welding. Clin. Med. 2013, 13, 214–2015. [Google Scholar] [CrossRef]
- Suri, R.; Periselneris, J.; Lanone, S.; Zeidler-Erdely, P.C.; Melton, G.; Palmer, K.T.; Andujar, P.; Antonini, J.M.; Cohignac, V.; Erdely, A.; et al. Exposure to Welding Fumes and Lower Airway Infection with Streptococcus Pneumoniae. J. Allergy Clin. Immunol. 2016, 137, 527–534.e7. [Google Scholar] [CrossRef] [PubMed]
- Sen, D.; Chen, Y. Vaccinating Welders against Pneumonia. Occup. Med. 2012, 62, 665–666. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Torén, K.; Qvarfordt, I.; Bergdahl, I.A.; Järvholm, B. Increased Mortality from Infectious Pneumonia after Occupational Exposure to Inorganic Dust, Metal Fumes and Chemicals. Thorax 2011, 66, 992–996. [Google Scholar] [CrossRef] [PubMed]
- Torén, K.; Albin, M.; Alderling, M.; Schiöler, L.; Åberg, M. Transmission Factors and Exposure to Infections at Work and Invasive Pneumococcal Disease. Am. J. Ind. Med. 2023, 66, 65–74. [Google Scholar] [CrossRef]
- Veiga, L.H.S.; Amaral, E.C.S.; Colin, D.; Koifman, S. A Retrospective Mortality Study of Workers Exposed to Radon in a Brazilian Underground Coal Mine. Radiat. Environ. Biophys. 2006, 45, 125–134. [Google Scholar] [CrossRef]
- Wergeland, E.; Iversen, B.G. Deaths from Pneumonia after Welding. Scand. J. Work Environ. Health 2001, 27, 353. [Google Scholar] [CrossRef]
- Koh, D.H.; Moon, K.T.; Kim, J.Y.; Choe, S.W. The Risk of Hospitalisation for Infectious Pneumonia in Mineral Dust Exposed Industries. Occup. Environ. Med. 2011, 68, 116–119. [Google Scholar] [CrossRef]
- Patterson, L.; Irvine, N.; Wilson, A.; Doherty, L.; Loughrey, A.; Jessop, L. Outbreak of Invasive Pneumococcal Disease at a Belfast Shipyard in Men Exposed to Welding Fumes, Northern Ireland, April–May 2015: Preliminary Report. Eurosurveillance 2015, 20, 21138. [Google Scholar] [CrossRef]
- Ochoa-Gondar, O.; Vila-Corcoles, A.; Rodriguez-Blanco, T.; Gomez-Bertomeu, F.; Figuerola-Massana, E.; Raga-Luria, X.; Hospital-Guardiola, I. Effectiveness of the 23-Valent Pneumococcal Polysaccharide Vaccine against Community-Acquired Pneumonia in the General Population Aged ≥60 Years: 3 Years of Follow-up in the CAPAMIS Study. Clin. Infect. Dis. 2014, 58, 909–917. [Google Scholar] [CrossRef]
- Palmborg, A.; Skovdal, M.; Molden, T.; Åhman, H.; Chen, L.; Banefelt, J. Invasive Pneumococcal Disease among the Elderly in the Later Era of Paediatric Pneumococcal Conjugate Vaccination—A Longitudinal Study over 10 Years Based on Public Surveillance Data in the Nordics. PLoS ONE 2023, 18, e0287378. [Google Scholar] [CrossRef]
- Ochoa-Gondar, O.; Torras-Vives, V.; de Diego-Cabanes, C.; Satué-Gracia, E.M.; Vila-Rovira, A.; Forcadell-Perisa, M.J.; Ribas-Seguí, D.; Rodríguez-Casado, C.; Vila-Córcoles, A. Incidence and Risk Factors of Pneumococcal Pneumonia in Adults: A Population-Based Study. BMC Pulm. Med. 2023, 23, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zivich, P.N.; Grabenstein, J.D.; Becker-Dreps, S.I.; Weber, D.J. Streptococcus Pneumoniae Outbreaks and Implications for Transmission and Control: A Systematic Review. Pneumonia 2018, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Aasen, T.B. Occupational Exposure and Respiratory Tract Infections—At Risk Workers in the International Contex. Curr. Respir. Med. Rev. 2016, 12, 5–9. [Google Scholar] [CrossRef]
- de Perio, M.A.; Kobayashi, M.; Wortham, J.M. Occupational Respiratory Infections. Clin. Chest. Med. 2020, 41, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Riccò, M.; Vezzosi, L.; Odone, A.; Signorelli, C. Invasive Meningococcal Disease on the Workplaces: A Systematic Review. Acta Biomed. 2017, 88, 337–351. [Google Scholar] [CrossRef]
- Riccò, M.; Vezzosi, L.; Marchesi, F. Vaccinating Front-Line Healthcare Workers: Results of a Pre-Pandemic Cross-Sectional Study from North-Eastern Italy on First Responders. Vaccines 2022, 10, 1492. [Google Scholar] [CrossRef]
- Reid, A.; Rhonda-Perez, E.; Schenker, M.B. Migrant Workers, Essential Work, and COVID-19. Am. J. Ind. Med. 2021, 64, 73–77. [Google Scholar] [CrossRef]
- Dyal, J.W.; Grant, M.P.; Broadwater, K.; Bjork, A.; Waltenburg, M.A.; Gibbins, J.D.; Hale, C.; Silver, M.; Fischer, M.; Steinberg, J.; et al. COVID-19 among Workers in Meat and Poultry Processing Facilities, April 2020. Morb. Mortal. Wkly. Rep. 2020, 69, e3. [Google Scholar] [CrossRef]
- Waltenburg, M.A.; Victoroff, T.; Rose, C.E.; Butterfield, M.; Jervis, R.H.; Fedak, K.M.; Gabel, J.A.; Feldpausch, A.; Dunne, E.M.; Austin, C.; et al. COVID-19 Among Workers in Meat and Poultry Processing Facilities—United States, April–May 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 888–892. [Google Scholar] [CrossRef]
- Ng, E.S.W.; Law, A. Keeping up! Older Workers’ Adaptation in the Workplace after Age 55. Can. J. Aging 2014, 33, 1–14. [Google Scholar] [CrossRef]
- Poscia, A.; Moscato, U.; La Milia, D.I.; Milovanovic, S.; Stojanovic, J.; Borghini, A.; Collamati, A.; Ricciardi, W.; Magnavita, N. Workplace Health Promotion for Older Workers: A Systematic Literature Review. BMC Health Serv Res. 2016, 16, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Magnavita, N.; Capitanelli, I.; Garbarino, S.; La Milia, D.I.; Moscato, U.; Pira, E.; Poscia, A.; Ricciardi, W. Workplace Health Promotion Programs for Older Workers in Italy. Med. Del. Lav. 2017, 108, 396–405. [Google Scholar] [CrossRef]
- Orsi, A.; Ansaldi, F.; Trucchi, C.; Rosselli, R.; Icardi, G. Pneumococcus and the Elderly in Italy: A Summary of Available Evidence Regarding Carriage, Clinical Burden of Lower Respiratory Tract Infections and on-Field Effectiveness of PCV13 Vaccination. Int. J. Mol. Sci. 2016, 17, 1140. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Considerations for Pneumococcal Vaccination in Older Adults. Wkly. Epidemiol. Rec. 2021, 96, 217–228. [Google Scholar]
- World Health Organization (WHO). Pneumococcal Conjugate Vaccines in Infants and Children under 5 Years of Age: WHO Position Paper—February 2019. Wkly. Epidemiol. Rec. 2019, 8, 85–104. [Google Scholar]
- World Health Organization (WHO) Pneumococcal Vaccines: WHO Position Paper on Their in Community Outbreak. Wkly. Epidemiol. Rec. 2021, 96, 105–110. [CrossRef]
- International Commission on Occupational Health (ICOH). International Code of Ethics for Occupational Health Professionals, 3rd ed.; International Commission on Occupational Health (ICOH): Rome, Italy, 2014; Available online: http://www.icohweb.org/site/multimedia/code_of_ethics/code-of-ethics-en.pdf (accessed on 14 July 2023).
- Esterhuizen, T.M.; Thabane, L. Con: Meta-Analysis: Some Key Limitations and Potential Solutions. Nephrol. Dial. Transplant. 2016, 31, 882–885. [Google Scholar] [CrossRef]
- Imrey, P.B. Limitations of Meta-Analyses of Studies with High Heterogeneity. JAMA Netw. Open 2020, 3, e1919325. [Google Scholar] [CrossRef]

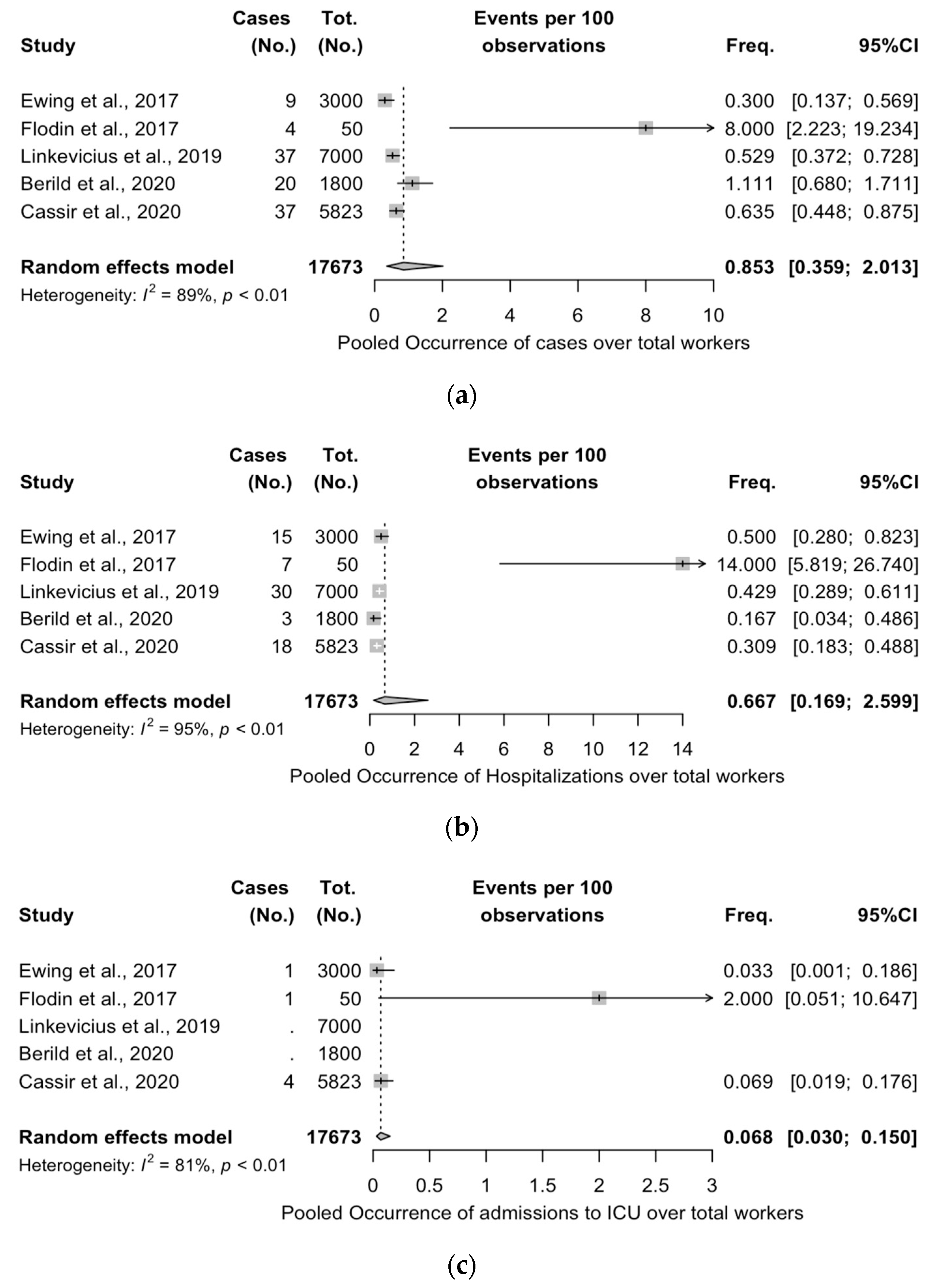
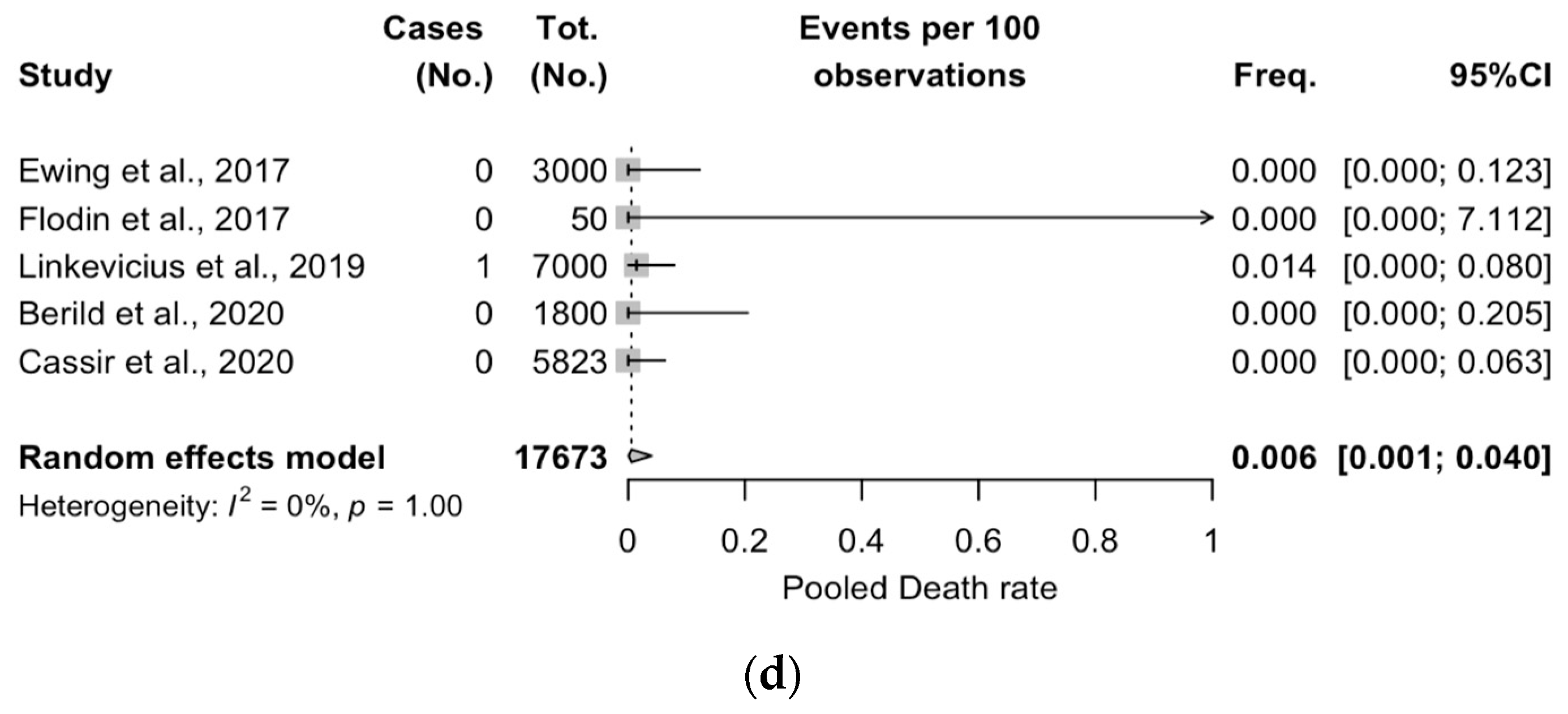
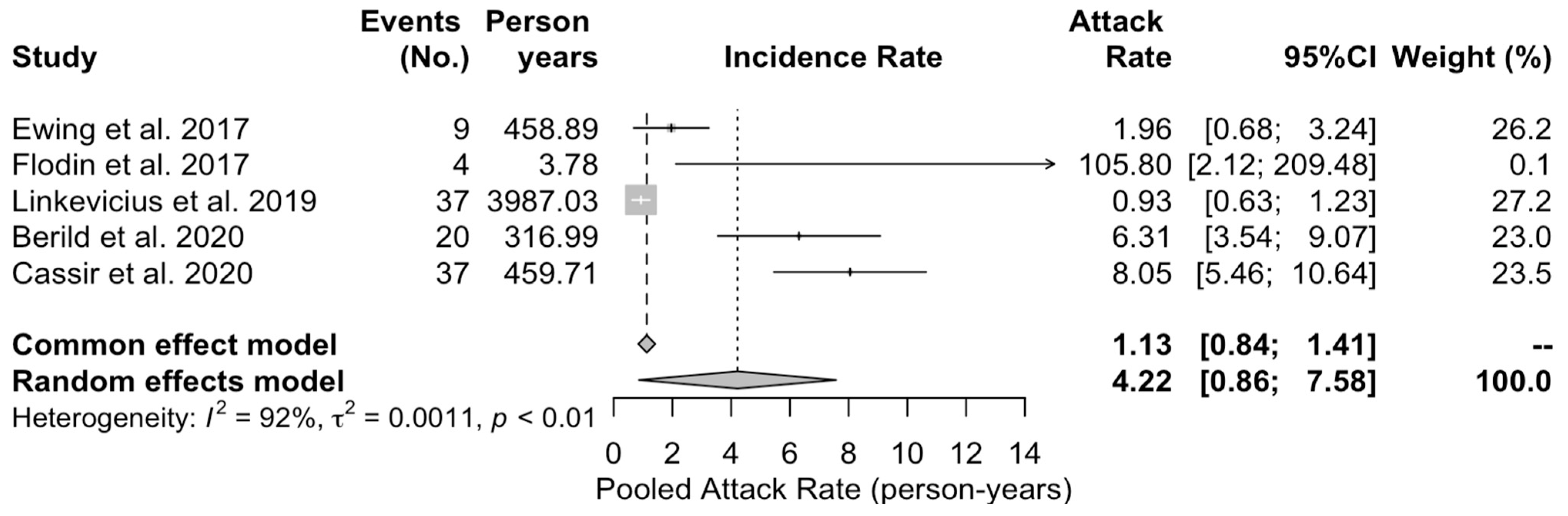

| Item | Definition |
|---|---|
| Population of interest | Among industrial workers, what is the effect of |
| Exposure | Occupational exposure to metal and welding fumes versus |
| Control/Comparator | General population and/or workers not occupationally exposed to metal and welding fumes in the |
| Outcome | Occurrence of PNX pneumonia, IPD, and PNX-related deaths |
| Authors | Year | Year of Cluster | Country | No. Cases (No., %) | No. Exposed Workers (No.) | Age (Years) | Hospitalizations (No., %) | ICU (No., %) | Deaths (No., %) | Serotypes (No., %) | Interval (Days) | Attack Rate (Cases per 100 Workers/Month) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Confirmed | Probable | Total * | Median | Range | |||||||||||
| Cassir et al. [32] | 2020 | 2020 | France | 18, 48.6% | 19, 51.4% | 37, 0.6% | 5823 | 39 | 22 to 66 | 18, 48.6% | 4, 10.8% | 0, - | 3 (1, 11.1%) 4 (5, 55.6%) 8 (2, 22.2%) 9N (1, 11.1%) | 29 | 0.66 |
| Linkevicius et al. [42] | 2019 | 2019 | Finland | 31, 83.8% | 6, 16.2% | 37, 0.5% | 7000 | 48 | 19 to 64 | 30, 81.1% | n.a. | 1, 2.7% | 12F (13, 52.0%) 4 (11, 44.0%) 8 (1, 4.0%) | 209 | 0.08 |
| Kiang [41] | 2018 | - | Singapore | 3, 100% | 0, - | 3 | n.a. | 24 | 20 to 41 | 3, 100% | 3, 100% | 0, - | n.a. | 90 | n.a. |
| Berild et al. [30] | 2020 | 2019 | Norway | 10, 50% | 10, 50% | 20, 1.1% | 1800 | 47 | 20 to 60 | 15, 75.0% | n.a. | 0, - | 4 (17, 100%) | 65 | 0.51 |
| Ewing et al. [37] | 2017 | 2015 | Northern Ireland | 4, 44.4% | 5, 55.6% | 9, 0.3% | 3000 | 43 | 20 to 60 | 7, 77.8% | 1, 11.1% | 0, - | 3 (1, 25.0%) 4 (3, 75.0%) | 56 | 0.16 |
| Flodin et al. [38] | 2017 | 2015 | Sweden | 4, 100% | 0, - | 4, 8.0% | 50 | 52 | 37 to 58 | 4, 100% | 1, 25.0% | 0, - | n.a. | 30 | 8.00 |
| Serogroup | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6A | 6B | 7F | 8 | 9N | 9V | 10A | 11A | 12F | 14 | 15B | 17F | 18C | 19A | 19F | 20 | 22F | 23F | 33F | |
| PPSV23 | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| PCV20 | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||
| PCV15 | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||
| PCV13 | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||||
| Cases (No, %) | 1, 1.4% | 2, 2.7% | 44, 60.3% | 6, 8.2% | 2, 2.7% | 1, 1.4% | 14, 19.2% | 1, 1.4% | 1, 1.4% | 1, 1.4% | ||||||||||||||
| Authors | Year | Timeframe | Country | Design | Settings | Exposure Assessment | Obs. (Welders) (No.) | Results | M.A. |
|---|---|---|---|---|---|---|---|---|---|
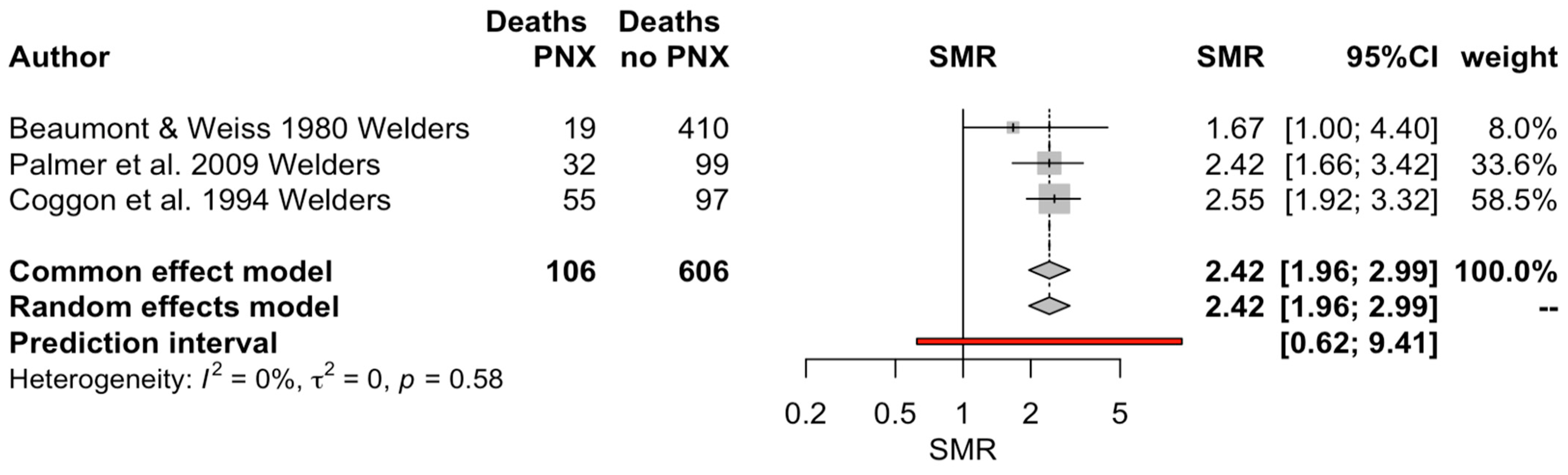
| Beaumont and Weiss [29] | 1980 | 1950 to 1973 | USA (Washington) | Analysis of death certificates | Metal trade workers in the area of Greater Seattle (≥3 years of seniority); original sample size of 3247 welders | Job title (Welders) | 19 | SMR 1.67, p < 0.001 for welders compared for pneumococcal pneumonia | Y |
| Coggon et al. [33] | 1994 | 1979 to 1980; 1982 to 1990 | United Kingdom | Analysis of death certificates | Nationwide data from official registries of metal-working occupations (occupational mortality data of Registrar General for England and Wales); original sample size of 729 death files. | Job title (Welders) | 55 | SMR 2.55, 95% CI 1.92 to 3.32 for pneumococcal pneumonia | Y |
| Palmer et al. [35] | 2009 | 1991 to 2000 | United Kingdom | Retrospective, based on death certificates | Nationwide data retrieved from the UK Office of Statistics, men aged 16 to 74 years from England and Wales. Original sample size of 794 death files. | Job title (Welders) | 32 | SMR 2.92, 95% CI 0.79 to 2.41 for pneumococcal pneumonia | Y |
| Palmer et al. [34] | 2003 | 1996 to 1999 | United Kingdom | Incident IPD cases, based on hospital records | Residents aged 20 to 64 years (650,000 individuals) from five metropolitan districts of the West Midlands. Admission to any of the acute medical services of the area. Original sample size, 525 cases of pneumonia. | Occupational exposure to welding fumes by detailed job titles | 22 | OR 1.8, 95% CI 0.6 to 5.2 for exposure to welding fumes in cases compared to control | Y |
| Torén et al. [47] | 2011 | 1971 to 2003 | Sweden | Mortality from infectious bacterial pneumonia, including pneumococcal pneumonia | Swedish Construction Workers from Swedish Construction Industry’s Organization for Working Environment; causes of death retrieved from national Cause of Death Register. Original sample of 773 cases of deaths from IPD. | Occupational exposure to metal fumes derived from the analysis of detailed job titles | 8 | RR 5.77, 95% CI 1.53 to 21.73 (calculated by means of Poisson’s regression analysis) for exposure to metal fumes compared to reference group. | N |
| Torén et al. [9] | 2022 | 2006 to 2019 | Sweden | Incident IPD cases, based on official Swedish registry | Swedish national Hospital Discharge Registry, identification of IPD. Controls retrieved from Swedish National Population registry. Original sample size of 3184 cases with pneumonia. | Occupational exposure calculated through a job exposure matrix based on the job title. | 836 | OR 2.24, 95% CI 1.41 to 3.35, calculated by means of logistic regression analysis for IPD in any exposure from metal fumes. | N |
| Torén et al. [8] | 2020 | 2006 to 2014 | Sweden | Incident IPD cases, based on official Swedish registry | Swedish National Hospital Discharge Registry, identification of IPD. Controls retrieved from Swedish national population registry. Original sample size of 4438 cases of IPD. | Occupational exposure calculated through the detailed job title. | 27 | OR 2.99, 95% CI 2.09 to 4.30 for welders. | Y |
| Wong et al. [13] | 2010 | 2000 to 2004 | Canada (Alberta) | Incident IPD cases, based on official registries | Official notification data on laboratory-confirmed IPD from the Canadian state of Alberta. Original sample size of 1768 IPD cases from a working population of around 3,000,000 inhabitants. | Job title (Welders) | 18 | OR 2.653, 95% CI 1.670 to 4.215 for working as welder compared to the general population | Y |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riccò, M.; Ferraro, P.; Zaffina, S.; Camisa, V.; Marchesi, F.; Gori, D. Vaccinating Welders against Pneumococcus: Evidence from a Systematic Review and Meta-Analysis. Vaccines 2023, 11, 1495. https://doi.org/10.3390/vaccines11091495
Riccò M, Ferraro P, Zaffina S, Camisa V, Marchesi F, Gori D. Vaccinating Welders against Pneumococcus: Evidence from a Systematic Review and Meta-Analysis. Vaccines. 2023; 11(9):1495. https://doi.org/10.3390/vaccines11091495
Chicago/Turabian StyleRiccò, Matteo, Pietro Ferraro, Salvatore Zaffina, Vincenzo Camisa, Federico Marchesi, and Davide Gori. 2023. "Vaccinating Welders against Pneumococcus: Evidence from a Systematic Review and Meta-Analysis" Vaccines 11, no. 9: 1495. https://doi.org/10.3390/vaccines11091495
APA StyleRiccò, M., Ferraro, P., Zaffina, S., Camisa, V., Marchesi, F., & Gori, D. (2023). Vaccinating Welders against Pneumococcus: Evidence from a Systematic Review and Meta-Analysis. Vaccines, 11(9), 1495. https://doi.org/10.3390/vaccines11091495











