Abstract
One of the main mechanisms of inducing an antiviral response depends on 2′-5′-oligoadenylate synthetases (OAS), which sense double-stranded RNA in the cytoplasm and activate RNase L. Mutations leading to the loss of functional OAS1 and OAS2 genes have been identified as important modifiers of the human immune response against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Here, we performed comparative genomics to search for inactivating mutations of OAS genes in other species of mammals and to establish a model for the diversifying evolution of the OAS gene family. We found that a recombination of the OAS and OAS-like (OASL) loci has led to the loss of OAS2 in camelids, which also lack OAS3. Both paralogs of OASL and OAS3 are absent in Asian pangolins. An evolutionarily ancient OAS paralog, which we tentatively name OAS4, has been lost in pangolins, bats and humans. A previously unknown OAS gene, tentatively named OAS5, is present in Yangochiroptera, a suborder of bats. These differences in the OAS gene repertoire may affect innate immune responses to coronaviruses and other RNA viruses.
Keywords:
oligoadenylate synthetase; innate immunity; evolution; zoonoses; SARS-CoV-2; camel; bat; gene loss; gene duplication; gene family 1. Introduction
Mammalian cells have different mechanisms to sense viral infections and initiate an innate immune response. One of the main approaches is the binding of specific sensor proteins to nucleic acids with infection-associated features, such as cytoplasmic localization of DNA, presence of double-stranded RNA or presence of Z-nucleic acid structures [1,2,3]. These sensors trigger signaling cascades that lead to reactions of the infected cell, the tissue and the immune system aimed at stopping the replication and spread of viruses. 2′-5′ oligoadenylate synthetases (OASs) bind double-stranded (ds) RNA in the cytoplasm and subsequently catalyze the oligomerization of ATP to 2′-5′-oligoadenylate. This oligomer activates RNase L, which subsequently degrades viral RNA to suppress the replication of the virus [1]. RNA degradation also generates small self-RNA, which amplifies antiviral innate immunity by binding to other receptors [4]. Humans have three OAS proteins comprising either one, two or three repeats of a nucleotidyl-transferase (NT) and an OAS1 C-terminal (OAS1C) domain. Besides catalytically active OAS enzymes, an OAS-like (OASL) protein contributes to the control of signaling in response to dsRNA in the cytoplasm. OASL activates retinoic acid inducible gene I (RIG-I), a sensor of cytoplasmic dsRNA [5,6], and suppresses cyclic GMP-AMP synthase (cGAS), a sensor of cytoplasmic DNA, leading to reduced replication of RNA viruses and enhanced replication of DNA viruses [7] (Figure 1).
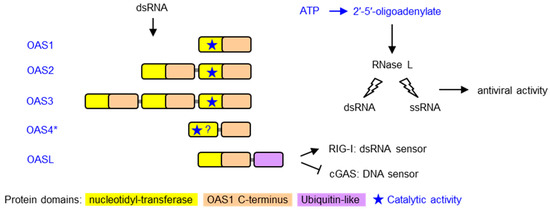
Figure 1.
Domain organization and mechanism of antiviral activity of OAS family proteins. Proteins of the 2′-5′-oligoadenylate synthetase (OAS) family are depicted schematically. Domains are color-coded as described at the bottom of the figure. OAS proteins are characterized by the presence of one or more nucleotidyl-transferase (cd05400: NT_2-5OAS_ClassI-CCAase) and OAS1 C-terminal (pfam10421: OAS1_C) [8] domains. The location of the catalytically active site is indicated by a star. OASL proteins contain a ubiquitin-like domain at the carboxy-terminus and lack catalytic activity. Upon binding to double-stranded RNA (dsRNA), OAS proteins catalyze the oligomerization of ATP to 2′-5′-oligoadenylate, which activates RNase L and thereby induces the degradation of dsRNA and single-stranded RNA (ssRNA) to suppress virus replication. OASL activates RIG-I, a sensor of cytoplasmic dsRNA, and suppresses cGAS, a sensor of cytoplasmic DNA *, OAS4 is the tentative name of an OAS paralog that was identified by Wang and colleagues who described the corresponding gene as “OAS1 in the ERAP2 (endoplasmic reticulum amino peptidase 2)-RIOK2 (RIO kinase 2) region (E-R region)” [9].
The OAS-RNase L pathway is involved in the defense against many viruses, some of which have evolved counteractive strategies [10,11,12,13]. Recent research has demonstrated that this pathway is also involved in the human immune response against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [14]. Specifically, mutations inactivating OAS1, OAS2 or RNase L impair the normal immune response and lead to SARS-CoV-2–related multisystem inflammatory syndrome in children [14]. OAS1 was reported to inhibit SARS-CoV-2 through its prenylated isoform [15]. Interestingly, the antiviral activity of OAS1 differs among primates due to mutations of its amino acid sequence, suggesting that a reduction in or loss of OAS1 catalytic activity may have had advantages in evolution [16]. Another report linked the decay of OAS1 mRNAs with the risk of COVID-19 hospitalization [17].
OAS genes are evolutionarily ancient [9,18]. Species from the major phylogenetic metazoan clades contain different sets of OAS genes, and even within tetrapods, significant variation has been reported [9,19]. For instance, OAS1 has undergone duplications in rodents and cattle [15,20]. In contrast to humans, some other placental mammals and marsupials have two copies of OASL [18], indicating that two OASL genes were also present in the genome of evolutionary ancestors of humans, and one of these copies was lost in the lineage leading to humans. Recently, a new OAS paralog, hereafter referred to as OAS4, was reported to be present in some amphibian, sauropsid and mammalian species [9]. Phylogenetic analysis suggested that this paralog emerged earlier in evolution than OAS1, OAS2 and OAS3 and that it was lost in rodents and primates [9]. Likewise, OAS3 has been lost in cetartiodactyls and in the tree shrew [20,21].
Comparative genomic studies have revealed a high diversity of innate immune genes in mammals with striking cases of gene degeneration in bats and pangolins [22,23,24,25,26]. Genes involved in the sensing of cytoplasmic DNA, such as CGAS and STING1, and cytoplasmic RNA, such as IFIH1/MDA5 and ZBP1, have been lost during the evolution of pangolins [23,24]. As bats are considered a likely source of SARS-CoV-2 and pangolins possibly were intermediate hosts of this virus [27,28], we put forward the hypothesis that alterations in antiviral innate immunity may contribute to the differential persistence of viruses in populations of such species with the risk of virus spillover potentially causing pandemics.
Here, we extended the concept of gene loss as a driver of inter-species variation in innate immunity and screened a selected group of mammalian species for cases of gene loss in the OAS gene family. The results of this study have implications for comparative immunology, and the selection of animal models for studying host–virus interactions.
2. Materials and Methods
Comparative genomics was performed according to an approach reported previously [23,29]. Nucleotide and amino acid sequences were downloaded from GenBank. Accession numbers are indicated in the text. Genes were identified in the genome sequences of Homo sapiens, assembly: GRCh38.p14 (GCF_000001405.40); Camelus bactrianus, assembly: Ca_bactrianus_MBC_1.0 (GCF_000767855.1); Camelus dromedarius, assembly: CamDro3 (GCF_000803125.2); Vicugna pacos, assembly: VicPac3.1 (GCF_000164845.3); Canis familiaris, assembly: ROS_Cfam_1.0 (GCF_014441545.1); Manis javanica, assembly: YNU_ManJav_2.0 (GCF_014570535.1); Manis pentadactyla, assembly: YNU_ManPten_2.0 (GCF_014570555.1); Rhinolophus sinicus, assembly: ASM188883v1 (GCF_001888835.1); Molossus molossus, assembly: mMolMol1.p (GCF_014108415.1); Myotis myotis, assembly: mMyoMyo1.p (GCF_014108235.1); and Artibeus jamaicensis, assembly: WHU_Ajam_v2 (GCF_014825515.1).
The Basic Local Alignment Search Tool (BLAST) [30] was used to determine sequence similarities. Sequence alignments were made with Multalin (http://multalin.toulouse.inra.fr/multalin/, accessed on 30 December 2022) and MUSCLE (https://www.ebi.ac.uk/Tools/msa/muscle/, accessed on 30 December 2022). Protein domains were identified with the NCBI Conserved Domain search tool [31]. Phylogenetic relationships and divergence times of phylogenetic lineages were obtained from the Timetree website (www.timetree.org, accessed on 30 December 2022) [32].
3. Results
3.1. Comparative Genomics Reveals Loss of OAS Genes during the Evolution of Humans and Camelids
We performed comparative genomics to determine the presence or absence of OAS genes in a selected subset of mammalian species. This study was focused on humans and clades of mammals (camelids, pangolins and bats), which were suspected or confirmed as reservoirs of coronaviruses with zoonotic potential [27,33,34,35]. An overview of the distribution of OAS family genes in the various species is provided in Table 1. The GenBank accession numbers of proteins encoded by these genes are listed in Table S1, and the corresponding amino acid sequences are documented in Figure S1. Together with the knowledge of phylogenetic relationships of mammals [32], the distribution of OAS paralogs in the various species allowed us to infer which genes were lost or gained during the evolutionary history of particular species or clades.

Table 1.
Conservation of OAS genes in mammalian species investigated in this study.
The gene loci of the OAS family share the same neighboring genes (synteny) in many but not all species. Comparative analysis indicated that chromosomal positions 12q24.13, 12q24.31 and 5q15 in the human genome correspond to the evolutionarily ancestral loci of OAS gene paralogs. The conservation of OAS4 in cetartiodactyls (Figure 2), African elephant and mouse lemur (Figure S1), and the absence of an OAS4 gene at human chromosome 5q15 indicated that, in agreement with a recent report [9], OAS4 was lost in the human lineage (Figure 2). Likewise, one of two ancestral OASL genes was lost during the evolution of humans (Figure 2).
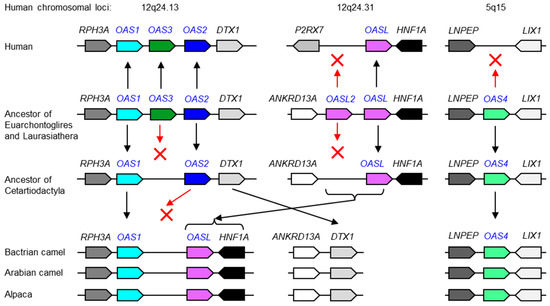
Figure 2.
Evolution of OAS gene loci in camelids in comparison to humans based on comparative genomics. Gene loci are schematically depicted. Genes are represented by rightwards and leftwards finger-post arrow symbols pointing in the direction of transcription. Note that, for simplification, genes that are not conserved among species have been omitted. The arrangement of genes in ancestors is inferred from shared patterns of gene arrangement (synteny) in extant species. Inheritance of genes is indicated by upwards and downwards arrows. Red Xs indicate gene loss. The scientific names of the species and accession numbers of proteins encoded by OAS family genes are provided in Table S1. Chromosomal loci of the human OAS gene family are shown at the top of the figure.
Camelids, which are a subclade of cetartiodactyls, have lost OASL2 but retained OAS4 (Figure 2). In line with a previous report on artiodactyls [20], OAS3 is absent in camels and alpaca (Figure 2). Camelids are unique within terrestrial cetartiodactyls because they lack OAS2 (Figure 2). The unusual tandem arrangement of OAS1 and OASL in camels and alpacas indicates that a recombination event occurred in a common ancestor of camelids, and OAS2 was probably lost in the course of this chromosomal rearrangement.
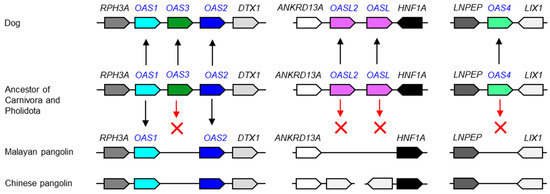
3.2. Pangolins have Lost Multiple OAS Genes
Next, we investigated pangolins, which are carriers of SARS-CoV-2-like viruses [28] and have a degenerated set of innate immune genes [23,24,25]. Pangolins constitute the clade Pholidota, which is most closely related to Carnivora (dog-like and cat-like mammals). Therefore, we compared the genomes of the Malayan pangolin (Manis javanica), Chinese pangolin (Manis pentadactyla) and the dog. In contrast to the dog, which has the full set of ancestral OAS genes, the Asian pangolins lack OAS3, OAS4, and both OASL1 and OASL2 (Figure 3 and Figure S2). At present, gene annotations are not available for African pangolins. Due to the loss of four ancestral genes, the OAS gene family is massively degenerated in Malayan and Chinese pangolins (Figure 3).
3.3. The Evolution of Bats Was Associated with the Diversification of OAS Paralogs
Bats, comprising the phylogenetic clade Chiroptera, have special adaptations of the immune system that allow them to act as reservoirs of many viruses [36,37,38,39], most likely including the virus from which SARS-CoV-2 evolved [27]. We performed an exploratory analysis of OAS genes in a subset of bats and found that OAS2, OAS3 and at least one OASL gene are conserved in bats (Figure 4). OAS1 is also present in all species investigated; however, the OAS1 ortholog of Molossus molossus contains inactivating mutations. OAS3 of the same species is predicted to contain an extended number of domains (Figure S3). The sequence modifications and their impact on the gene function remain to be investigated because sequence confirmations and analyses of gene transcripts were not within the scope of the present study. OAS4 was absent in all bats investigated, suggesting that this gene has been lost (Figure 4). Unexpectedly, we identified an as-yet-uncharacterized paralog, tentatively named OAS5, by sequence similarity searches in the genomes of species of the suborder Yangochiroptera [40]. OAS5 is located between the TBCK and NPNT genes, a locus that does not contain an OAS paralog in any species investigated except those of the clade Yangochiroptera. OAS5 genes encode proteins with high sequence similarity to OAS1 (Figure 5). In contrast to all other known OAS paralogs, OAS5 does not have introns, so the open reading frame is entirely contained in a single exon, suggesting that OAS5 has arisen by reverse transcription of an OAS1 mRNA followed by insertion of the complementary DNA into the genome of a germ cell in an ancestor of Yangochiroptera (Figure 4).
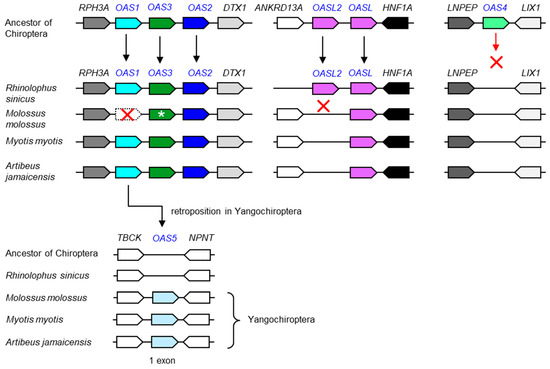
Figure 4.
Evolution of OAS gene loci in bats. Gene loci of the OAS family are schematically depicted with symbols described in the legend of Figure 2. For simplification, genes that are not conserved among species have been omitted. The arrangement of genes in ancestors is inferred from shared patterns of gene arrangement (synteny) in extant species. The open reading frame OAS1 of Molossus molossus is disrupted by point mutations. OAS3 of Molossus molossus (green symbol with a white asterisk) contains more exons than its orthologs in other species and is predicted to encode a protein with 6 repeats of the NT and OAS1C domains (Figure S3). The gene tentatively named OAS5 consists of only one exon, suggesting that it originated by retroposition [41]. GenBank accession numbers of OAS5 genes: LOC118635736 (Molossus molossus), LOC118673356 (Myotis myotis), LOC119056133 (Artibeus jamaicensis). Species: Chinese rufous horseshoe bat (Rhinolophus sinicus), Pallas’s mastiff bat (Molossus molossus), greater mouse-eared bat (Myotis myotis), Jamaican fruit bat (Artibeus jamaicensis). Accession numbers of proteins encoded by OAS family genes are provided in Table S1.
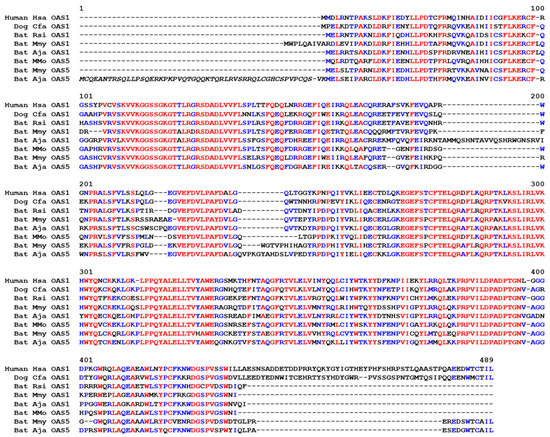
Figure 5.
Amino acid sequence alignment of OAS1 and OAS5 proteins. Amino acid sequences were aligned with the Multalin program. Red fonts indicate residues conserved in all sequences; blue fonts indicate residues conserved in at least 50% of sequences. The italicized segment of the sequence Bat-Aj_OAS5 is likely to correspond to an erroneous extension of the coding sequence in the protein prediction of GenBank. The accession numbers of the sequences are shown in Figure S1. Numbers above the sequences indicate amino acid sequence positions. Species: Hsa, Homo sapiens; Cfa, Canis familiaris; Rsi, Rhinolophus sinicus; Mmo, Molossus molossus; Mmy, Myotis myotis; Aja, Artibeus jamaicensis.
Taken together, these data show that the OAS gene family underwent lineage-specific changes leading to significant differences in the repertoire of OAS paralogs in major clades of extant mammals (Figure 6).
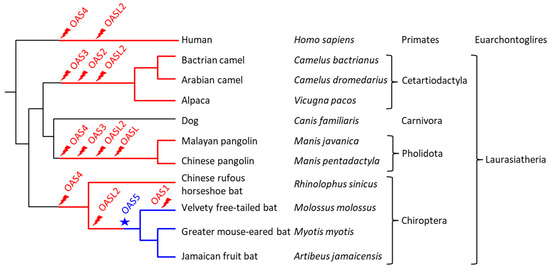
Figure 6.
Gene loss and gain events mapped onto a phylogenetic tree of the species investigated in this study. The tree on the left shows the relationship between species. Higher taxonomic ranks are shown on the right. Gene loss (flash symbol, red) and gain (star, blue) are mapped onto this tree on the basis of gene absence or presence in the extant species. Red lines indicate lineages in which at least one member of the OAS gene family was missing. Blue lines indicate the presence of OAS5 in Yangochiroptera. The chronological order of gene loss events between nodes of the tree is not known.
4. Discussion
The OAS–RNase L pathway is one of the central mechanisms of antiviral defense. A large body of literature exists on the roles of OAS genes in human cells and cells of model organisms [2,42,43,44]. It was noted early on that the OAS gene complement differs between humans and rodents as the latter have an amplification of OAS1 genes [45]. Studies of other species, including many domestic and wild mammals, revealed further inter-species differences [18,20,21,46]. The present study extends the comparative analysis of the OAS family to a set of species implicated in the spread of coronaviruses and reveals previously unknown taxon-specific compositions of the OAS gene family.
Our results show that the OAS gene family is larger than the set of OAS1, OAS2, OAS3 and OASL genes in humans. Two copies of OASL genes have previously been identified in other species, and an as-yet-uncharacterized OAS paralog, which we name OAS4, was reported previously [9]. The present study identified another paralog, tentatively named OAS5, in a subgroup of bats. It will be important to investigate OAS4 and OAS5 proteins with regard to RNA-binding properties, catalytic activities and functions in antiviral defense and other processes.
Importantly, peculiar features of the OAS gene sets were found in camelids, pangolins and bats, which have been implicated in the origin of viral zoonoses [27,33,34]. These findings provide a basis for studying the impact of particular OAS gene combinations on the induction of anti-viral defense in follow-up studies. For a comprehensive evaluation of the significance of OAS genes in zoonoses, reports on other hosts of coronaviruses [27,47,48] and zoonoses involving other viruses should also be considered [35,49,50,51,52].
Vaccines play key roles in the fight against viral pathogens in human and veterinary medicine. Therefore, it is of special clinical interest to transfer basic immunological knowledge into the development of highly effective vaccines. OAS proteins are involved in early immune responses against RNA viruses and perhaps also against modified-live RNA virus vaccines [10,53]. Yellow fever vaccination induced upregulation of OAS1 among other innate antiviral molecules and also a strong acquired serological and cellular immune response [54]. A drawback in the development of vaccines directed against RNA viruses is their generally high rate of sequence mutations, as exemplified by one of the economically most important RNA viruses in veterinary medicine, the porcine reproductive and respiratory syndrome virus (PRRSV) [55,56]. Despite its high mutation rate, it is possible to design effective vaccines [57]. The PRRSV is an ssRNA virus, but dsRNA intermediates are formed during intracellular replication. Thus, veterinary species such as pigs could serve as models for investigating the role of OAS gene family members and other components of innate immunity in modulating the efficacy of vaccines.
This study has limitations that need to be considered in the interpretation of the data. First, most genes were predicted by automated computational analysis, which is used by GenBank to annotate genes of non-model species [58]. Although these predictions are useful for compiling orthologous genes, structural details of the predicted genes need to be corrected in some cases [59,60]. Second, the structure of the predicted mRNAs and proteins has not been experimentally confirmed yet. However, the mapping of RNA-seq reads onto the GenBank genome sequences confirms that the predicted exons are indeed present in mature mRNAs of many species investigated (Figure S3A). Finally, the proteins encoded by the predicted OAS family genes remain to be characterized with regard to their biochemical features and their roles in intracellular signaling in response to viral infections.
The differences in the conservation of established OAS family members, OAS1, OAS2, OAS3, OASL and OASL2, and possibly also the presence or absence of OAS4 and OAS5, may contribute to differences in responses to viral dsRNA in mammalian species. Studies are warranted to determine the impact of specific OAS gene sets on the control of viruses in phylogenetically diverse mammals [39,49,61,62].
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/vaccines11020419/s1, Table S1. Proteins encoded by genes of the OAS family in mammalian species investigated in this study. Figure S1. Amino acid sequences of OAS family proteins encoded by genes that have been investigated in this study. Figure S2. Phylogenetic tree of species investigated in this study and mapping of gene loss and gain events. Figure S3. Prediction of exons for OAS1 and OAS2 genes of the Chinese pangolin (Manis pentadactyla). Figure S4. Predicted structure of OAS3 in Pallas’s mastiff bat (Molossus molossus). Figure S5. Amino acid sequence alignment of OAS1 and OAS5 proteins.
Author Contributions
Conceptualization, L.E.; investigation, L.E.; data analysis, L.E. and W.S.; writing—original draft preparation, L.E.; writing—review and editing, L.E. and W.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data are contained within the article and the Supplementary Materials.
Acknowledgments
We thank Heinz Fischer and Erwin Tschachler for the helpful discussions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hornung, V.; Hartmann, R.; Ablasser, A.; Hopfner, K.P. OAS proteins and cGAS: Unifying concepts in sensing and responding to cytosolic nucleic acids. Nat. Rev. Immunol. 2014, 14, 521–528. [Google Scholar] [CrossRef]
- Hur, S. Double-stranded RNA sensors and modulators in innate immunity. Annu. Rev. Immunol. 2019, 37, 349–375. [Google Scholar] [CrossRef] [PubMed]
- Ablasser, A.; Hur, S. Regulation of cGAS- and RLR-mediated immunity to nucleic acids. Nat. Immunol. 2020, 21, 17–29. [Google Scholar] [CrossRef]
- Malathi, K.; Dong, B.; Gale MJr Silverman, R.H. Small self-RNA generated by RNase L amplifies antiviral innate immunity. Nature 2007, 448, 816–819. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhang, Y.; Ghosh, A.; Cuevas, R.A.; Forero, A.; Dhar, J.; Ibsen, M.S.; Schmid-Burgk, J.L.; Schmidt, T.; Ganapathiraju, M.K.; et al. Antiviral activity of human OASL protein is mediated by enhancing signaling of the RIG-I RNA sensor. Immunity 2014, 40, 936–948. [Google Scholar] [CrossRef]
- Rong, E.; Wang, X.; Chen, H.; Yang, C.; Hu, J.; Liu, W.; Wang, Z.; Chen, X.; Zheng, H.; Pu, J.; et al. Molecular mechanisms for the adaptive switching between the OAS/RNase L and OASL/RIG-I pathways in birds and mammals. Front. Immunol. 2018, 9, 1398. [Google Scholar] [CrossRef]
- Ghosh, A.; Shao, L.; Sampath, P.; Zhao, B.; Patel, N.V.; Zhu, J.; Behl, B.; Parise, R.A.; Beumer, J.H.; O’Sullivan, R.J.; et al. Oligoadenylate-synthetase-family protein OASL inhibits activity of the DNA sensor cGAS during DNA virus infection to limit interferon production. Immunity 2019, 50, 51–63.e5. [Google Scholar] [CrossRef]
- Lu, S.; Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S.; et al. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020, 48, D265–D268. [Google Scholar] [CrossRef]
- Wang, X.; Hu, J.; Song, L.; Rong, E.; Yang, C.; Chen, X.; Pu, J.; Sun, H.; Gao, C.; Burt, D.W.; et al. Functional divergence of oligoadenylate synthetase 1 (OAS1) proteins in tetrapods. Sci. China Life Sci. 2022, 65, 1395–1412. [Google Scholar] [CrossRef] [PubMed]
- Drappier, M.; Michiels, T. Inhibition of the OAS/RNase L pathway by viruses. Curr. Opin. Virol. 2015, 15, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Bignon, E.; Miclot, T.; Terenzi, A.; Barone, G.; Monari, A. Structure of the 5′ untranslated region in SARS-CoV-2 genome and its specific recognition by innate immune system via the human oligoadenylate synthase 1. Chem. Commun. 2022, 58, 2176–2179. [Google Scholar] [CrossRef] [PubMed]
- Hancks, D.C.; Hartley, M.K.; Hagan, C.; Clark, N.L.; Elde, N.C. Overlapping patterns of rapid evolution in the nucleic acid sensors cGAS and OAS1 suggest a common mechanism of pathogen antagonism and escape. PLoS Genet. 2015, 11, e1005203. [Google Scholar] [CrossRef] [PubMed]
- Mozzi, A.; Pontremoli, C.; Forni, D.; Clerici, M.; Pozzoli, U.; Bresolin, N.; Cagliani, R.; Sironi, M. OASes and STING: Adaptive evolution in concert. Genome Biol. Evol. 2015, 7, 1016–1032. [Google Scholar] [CrossRef]
- Lee, D.; Le Pen, J.; Yatim, A.; Dong, B.; Aquino, Y.; Ogishi, M.; Pescarmona, R.; Talouarn, E.; Rinchai, D.; Zhang, P.; et al. Inborn errors of OAS-RNase L in SARS-CoV-2-related multisystem inflammatory syndrome in children. Science 2023, 379, eabo3627. [Google Scholar] [CrossRef]
- Wickenhagen, A.; Sugrue, E.; Lytras, S.; Kuchi, S.; Noerenberg, M.; Turnbull, M.L.; Loney, C.; Herder, V.; Allan, J.; Jarmson, I.; et al. A prenylated dsRNA sensor protects against severe COVID-19. Science 2021, 374, eabj3624. [Google Scholar] [CrossRef] [PubMed]
- Carey, C.M.; Govande, A.A.; Cooper, J.M.; Hartley, M.K.; Kranzusch, P.J.; Elde, N.C. Recurrent loss-of-function mutations reveal costs to OAS1 antiviral activity in primates. Cell Host Microbe 2019, 25, 336–343. [Google Scholar] [CrossRef]
- Banday, A.R.; Stanifer, M.L.; Florez-Vargas, O.; Onabajo, O.O.; Papenberg, B.W.; Zahoor, M.A.; Mirabello, L.; Ring, T.J.; Lee, C.H.; Albert, P.S.; et al. Genetic regulation of OAS1 nonsense-mediated decay underlies association with COVID-19 hospitalization in patients of European and African ancestries. Nat. Genet. 2022, 54, 1103–1116. [Google Scholar] [CrossRef]
- Kjaer, K.H.; Poulsen, J.B.; Reintamm, T.; Saby, E.; Martensen, P.M.; Kelve, M.; Justesen, J. Evolution of the 2′-5′-oligoadenylate synthetase family in eukaryotes and bacteria. J. Mol. Evol. 2009, 69, 612–624. [Google Scholar] [CrossRef]
- Kumar, S.; Mitnik, C.; Valente, G.; Floyd-Smith, G. Expansion and molecular evolution of the interferon-induced 2′-5′ oligoadenylate synthetase gene family. Mol. Biol. Evol. 2000, 17, 738–750. [Google Scholar] [CrossRef]
- Perelygin, A.A.; Zharkikh, A.A.; Scherbik, S.V.; Brinton, M.A. The mammalian 2′-5′ oligoadenylate synthetase gene family: Evidence for concerted evolution of paralogous Oas1 genes in Rodentia and Artiodactyla. J. Mol. Evol. 2006, 63, 562–576. [Google Scholar] [CrossRef]
- Yao, Y.L.; Yu, D.; Xu, L.; Fan, Y.; Wu, Y.; Gu, T.; Chen, J.; Lv, L.B.; Yao, Y.G. Molecular characterization of the 2′,5′-oligoadenylate synthetase family in the Chinese tree shrew (Tupaia belangeri chinensis). Cytokine 2019, 114, 106–114. [Google Scholar] [CrossRef]
- Banerjee, A.; Baker, M.L.; Kulcsar, K.; Misra, V.; Plowright, R.; Mossman, K. Novel insights into immune systems of bats. Front. Immunol. 2020, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Fischer, H.; Tschachler, E.; Eckhart, L. Pangolins lack IFIH1/MDA5, a cytoplasmic RNA sensor that initiates innate immune defense upon coronavirus infection. Front. Immunol. 2020, 11, 939. [Google Scholar] [CrossRef]
- Fischer, H.; Tschachler, E.; Eckhart, L. Cytosolic DNA sensing through cGAS and STING is inactivated by gene mutations in pangolins. Apoptosis 2020, 25, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Salova, M.; Sipos, W.; Tschachler, E.; Eckhart, L. NOD2 and reproduction-associated NOD-like receptors have been lost during the evolution of pangolins. Immunogenetics 2022, 74, 261–268. [Google Scholar] [CrossRef]
- Haley, P.J. From bats to pangolins: New insights into species differences in the structure and function of the immune system. Innate Immun. 2022, 28, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Holmes, E.C.; Goldstein, S.A.; Rasmussen, A.L.; Robertson, D.L.; Crits-Christoph, A.; Wertheim, J.O.; Anthony, S.J.; Barclay, W.S.; Boni, M.F.; Doherty, P.C.; et al. The origins of SARS-CoV-2: A critical review. Cell 2021, 184, 4848–4856. [Google Scholar] [CrossRef]
- Lam, T.T.; Jia, N.; Zhang, Y.W.; Shum, M.H.; Jiang, J.F.; Zhu, H.C.; Tong, Y.G.; Shi, Y.X.; Ni, X.B.; Liao, Y.S.; et al. Identifying SARS-CoV-2-related coronaviruses in Malayan pangolins. Nature 2020, 583, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Eckhart, L.; Uthman, A.; Sipos, W.; Tschachler, E. Genome sequence comparison reveals independent inactivation of the caspase-15 gene in different evolutionary lineages of mammals. Mol. Biol. Evol. 2006, 23, 2081–2089. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Marchler-Bauer, A.; Bo, Y.; Han, L.; He, J.; Lanczycki, C.J.; Lu, S.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; et al. CDD/SPARCLE: Functional classification of proteins via subfamily domain architectures. Nucleic Acids Res. 2017, 45, D200–D203. [Google Scholar] [CrossRef]
- Hedges, S.B.; Marin, J.; Suleski, M.; Paymer, M.; Kumar, S. Tree of life reveals clock-like speciation and diversification. Mol. Biol. Evol. 2015, 32, 835–845. [Google Scholar] [CrossRef]
- Azhar, E.I.; El-Kafrawy, S.A.; Farraj, S.A.; Hassan, A.M.; Al-Saeed, M.S.; Hashem, A.M.; Madani, T.A. Evidence for camel-to-human transmission of MERS coronavirus. N. Engl. J. Med. 2014, 370, 2499–2505. [Google Scholar] [CrossRef]
- Li, W.; Shi, Z.; Yu, M.; Ren, W.; Smith, C.; Epstein, J.H.; Wang, H.; Crameri, G.; Hu, Z.; Zhang, H.; et al. Bats are natural reservoirs of SARS-like coronaviruses. Science 2005, 310, 676–679. [Google Scholar] [CrossRef]
- Tian, J.; Sun, J.; Li, D.; Wang, N.; Wang, L.; Zhang, C.; Meng, X.; Ji, X.; Suchard, M.A.; Zhang, X.; et al. Emerging viruses: Cross-species transmission of coronaviruses, filoviruses, henipaviruses, and rotaviruses from bats. Cell Rep. 2022, 39, 110969. [Google Scholar] [CrossRef]
- Xie, J.; Li, Y.; Shen, X.; Goh, G.; Zhu, Y.; Cui, J.; Wang, L.F.; Shi, Z.L.; Zhou, P. Dampened STING-dependent interferon activation in bats. Cell Host Microbe 2018, 23, 297–301.e4. [Google Scholar] [CrossRef]
- Gonzalez, V.; Banerjee, A. Molecular, ecological, and behavioral drivers of the bat-virus relationship. iScience 2022, 25, 104779. [Google Scholar] [CrossRef]
- Irving, A.T.; Ahn, M.; Goh, G.; Anderson, D.E.; Wang, L.F. Lessons from the host defences of bats, a unique viral reservoir. Nature 2021, 589, 363–370. [Google Scholar] [CrossRef]
- Jacquet, S.; Culbertson, M.; Zhang, C.; El Filali, A.; De La Myre Mory, C.; Pons, J.B.; Filippi-Codaccioni, O.; Lauterbur, M.E.; Ngoubangoye, B.; Duhayer, J.; et al. Adaptive duplication and genetic diversification of protein kinase R contribute to the specificity of bat-virus interactions. Sci. Adv. 2022, 8, eadd7540. [Google Scholar] [CrossRef]
- Tsagkogeorga, G.; Parker, J.; Stupka, E.; Cotton, J.A.; Rossiter, S.J. Phylogenomic analyses elucidate the evolutionary relationships of bats. Curr. Biol. 2013, 23, 2262–2267. [Google Scholar] [CrossRef]
- Carelli, F.N.; Hayakawa, T.; Go, Y.; Imai, H.; Warnefors, M.; Kaessmann, H. The life history of retrocopies illuminates the evolution of new mammalian genes. Genome Res. 2016, 26, 301–314. [Google Scholar] [CrossRef]
- Rebouillat, D.; Hovanessian, A.G. The human 2′,5′-oligoadenylate synthetase family: Interferon-induced proteins with unique enzymatic properties. J. Interferon Cytokine Res. 1999, 19, 295–308. [Google Scholar] [CrossRef]
- Sadler, A.J.; Williams, B.R. Interferon-inducible antiviral effectors. Nat. Rev. Immunol. 2008, 8, 559–568. [Google Scholar] [CrossRef]
- Hu, J.; Wang, X.; Xing, Y.; Rong, E.; Ning, M.; Smith, J.; Huang, Y. Origin and development of oligoadenylate synthetase immune system. BMC Evol. Biol. 2018, 18, 201. [Google Scholar] [CrossRef] [PubMed]
- Mashimo, T.; Glaser, P.; Lucas, M.; Simon-Chazottes, D.; Ceccaldi, P.E.; Montagutelli, X.; Desprès, P.; Guénet, J.L. Structural and functional genomics and evolutionary relationships in the cluster of genes encoding murine 2′,5′-oligoadenylate synthetases. Genomics 2003, 82, 537–552. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wu, X.; Shang, Y.; Wang, X.; Zhou, S.; Zhang, H. Adaptive evolution of the OAS gene family provides new insights into the antiviral ability of laurasiatherian mammals. Animals 2023, 13, 209. [Google Scholar] [CrossRef]
- Liu, H.L.; Yeh, I.J.; Phan, N.N.; Wu, Y.H.; Yen, M.C.; Hung, J.H.; Chiao, C.C.; Chen, C.F.; Sun, Z.; Jiang, J.Z.; et al. Gene signatures of SARS-CoV/SARS-CoV-2-infected ferret lungs in short- and long-term models. Infect Genet. Evol. 2020, 85, 104438. [Google Scholar] [CrossRef] [PubMed]
- Han, B.A.; Castellanos, A.A.; Schmidt, J.P.; Fischhoff, I.R.; Drake, J.M. The ecology of zoonotic parasites in the Carnivora. Trends Parasitol. 2021, 37, 1096–1110. [Google Scholar] [CrossRef] [PubMed]
- Sipos, W.; Lutonsky, C. Amendments suggested for zoo medical research strategies with focus on the D-A-CH region. Tierarztl. Prax. Ausg. G. Grosstiere Nutztiere 2021, 49, 256–260. [Google Scholar] [CrossRef]
- Mollentze, N.; Streicker, D.G. Viral zoonotic risk is homogenous among taxonomic orders of mammalian and avian reservoir hosts. Proc. Natl. Acad. Sci. USA. 2020, 117, 9423–9430. [Google Scholar] [CrossRef] [PubMed]
- MacNeill, A.L. Comparative pathology of zoonotic orthopoxviruses. Pathogens 2022, 11, 892. [Google Scholar] [CrossRef] [PubMed]
- Medina, R.A.; García-Sastre, A. Influenza A viruses: New research developments. Nat. Rev. Microbiol. 2011, 9, 590–603. [Google Scholar] [CrossRef] [PubMed]
- Short, E.C., Jr.; Fulton, R.W. Induction and measurement of 2′,5′-oligoadenylate synthetase in Madin-Darby bovine kidney cells and in cattle. J. Clin. Microbiol. 1987, 25, 1735–1740. [Google Scholar] [CrossRef]
- Azamor, T.; da Silva, A.M.V.; Melgaço, J.G.; Dos Santos, A.P.; Xavier-Carvalho, C.; Alvarado-Arnez, L.E.; Batista-Silva, L.R.; de Souza Matos, D.C.; Bayma, C.; Missailidis, S.; et al. Activation of an effective immune response after yellow fever vaccination is associated with the genetic background and early response of IFN-γ and CLEC5A. Viruses 2021, 13, 96. [Google Scholar] [CrossRef]
- Du, T.; Nan, Y.; Xiao, S.; Zhao, Q.; Zhou, E.M. Antiviral strategies against PRRSV infection. Trends Microbiol. 2017, 25, 968–979. [Google Scholar] [CrossRef] [PubMed]
- Indik, S.; Schmoll, F.; Sipos, W.; Klein, D. Genetic variability of PRRS virus in Austria: Consequences for molecular diagnostics and viral quantification. Vet. Microbiol. 2005, 107, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Sipos, W.; Duvigneau, C.; Pietschmann, P.; Höller, K.; Hartl, R.; Wahl, K.; Steinborn, R.; Gemeiner, M.; Willheim, M.; Schmoll, F. Parameters of humoral and cellular immunity following vaccination of pigs with a European modified-live strain of porcine reproductive and respiratory syndrome virus (PRRSV). Viral. Immunol. 2003, 16, 335–346. [Google Scholar] [CrossRef]
- Souvorov, A.; Kapustin, Y.; Kiryutin, B.; Chetvernin, V.; Tatusova, T.; Lipman, D. Gnomon—NCBI Eukaryotic Gene Prediction Tool. 2010. Available online: http://www.ncbi.nlm.nih.gov/RefSeq/Gnomon-description.pdf (accessed on 29 December 2022).
- Ehrlich, F.; Lachner, J.; Hermann, M.; Tschachler, E.; Eckhart, L. Convergent evolution of cysteine-rich keratins in hard skin appendages of terrestrial vertebrates. Mol. Biol. Evol. 2020, 37, 982–993. [Google Scholar] [CrossRef]
- Holthaus, K.B.; Lachner, J.; Ebner, B.; Tschachler, E.; Eckhart, L. Gene duplications and gene loss in the epidermal differentiation complex during the evolutionary land-to-water transition of cetaceans. Sci. Rep. 2021, 11, 12334. [Google Scholar] [CrossRef]
- McDougal, M.B.; Boys, I.N.; De La Cruz-Rivera, P.; Schoggins, J.W. Evolution of the interferon response: Lessons from ISGs of diverse mammals. Curr. Opin. Virol. 2022, 53, 101202. [Google Scholar] [CrossRef] [PubMed]
- Glidden, C.K.; Nova, N.; Kain, M.P.; Lagerstrom, K.M.; Skinner, E.B.; Mandle, L.; Sokolow, S.H.; Plowright, R.K.; Dirzo, R.; De Leo, G.A.; et al. Human-mediated impacts on biodiversity and the consequences for zoonotic disease spillover. Curr. Biol. 2021, 31, R1342–R1361. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).