Abstract
Background: This study aimed to assess the safety and immunogenicity of MVC-COV1901, a recombinant COVID-19 protein vaccine, containing S-2P protein adjuvanted with CpG 1018 and aluminum hydroxide, for people living with HIV (PWH). Methods: A total of 57 PWH of ≥20 years of age who are on stable antiretroviral therapy were compared with 882 HIV-negative participants. Participants received two doses of MVC-COV1901 28 days apart. Results: No vaccine-related serious adverse events (SAEs) were recorded. Seroconversion rates (SCRs) of 100% and 99.8% were achieved in PWH and comparators, respectively, 28 days after the second dose. After adjusting for sex, age, BMI category, and comorbidity, the adjusted GMT ratio of comparator/PWH was 3.2 (95% CI 2.5–4). A higher CD4/CD8 ratio was associated with a higher GMT (R = 0.27, p = 0.039). MVC-COV1901 has shown robust safety but elicited weaker immune responses in PWH. Conclusions: Further investigations may be needed to determine whether PWH require distinct immunization strategies with improved immunogenicity. The main study is registered at ClinicalTrials.gov (NCT04695652).
1. Introduction
The Coronavirus Disease 2019 (COVID-19), caused by the novel coronavirus SARS-CoV-2, was first reported in Wuhan, Hubei Province, People’s Republic of China (PRC) in late December 2019. The disease spread rapidly around the globe and was declared a pandemic by the WHO on 11 March 2020 [1,2]. As of November 2022, nearly 643 million infections and over 6 million deaths have occurred worldwide [3]. Unfortunately, this disease has severely impacted people with poor socioeconomic status, and that includes populations vulnerable to HIV infection [3,4].
People who are living with HIV (PWH) are subject to many risk factors that predispose them to severe COVID-19. These factors include high rates of smoking, drug use, comorbidities including cardiovascular diseases, diabetes mellitus, chronic renal diseases, chronic liver diseases, and lung diseases [5,6,7,8]. Furthermore, many PWH are overweight or obese, which correlates with the widespread use of second-generation integrase inhibitors [9,10] and is an additional risk factor for severe COVID-19 [11,12]. Health disparities due to socioeconomic status and stigmatization may also hinder the early diagnosis of SARS-CoV-2 infection and the timely provision of healthcare [4]. Several case series and meta-analyses also pointed towards the association between PWH and severe COVID-19 and higher mortality rates. The U.S. RedCap data showed that COVID-19 affects PWH that are predominantly of older age (mean 51.4 years), African-American (47.5%), and having high rates of comorbidity (80%), with 57.3% greater risk for hospitalization, 16.5% for ICU admission, and 9.4% for mortality [13]. Data captured from the ISARIC WHO CCP study showed that after adjusting for age, PWH have 47% higher mortality rates (adjusted hazard ratio 1.47, and 95% confidence interval [CI] 1.04–2.25) in England [14]. The astonishingly rapid spread of COVID-19 and expansion of several variants of concern (VOCs), such as Alpha, Beta, Delta, Gamma, and Omicron variants, spurred the desperate need of creating an effective COVID-19 vaccination strategy for PWH.
MVC-COV1901 is a subunit vaccine based on the stabilized prefusion SARS-CoV-2 spike protein S-2P (15 mcg) with furin cleavage site mutation and T4 fibritin for trimerization, and it formulated in adjuvant composed of a toll-like receptor 9 (TLR9) agonist CpG 1018 and aluminium hydroxide. It is based on the ancestral strain of the SARS-CoV-2 virus [15,16,17]. Previous phase 1 and 2 clinical trials have shown that the MVC-COV1901 vaccine was well-tolerated and elicited robust immune responses [18,19]. In this study, PWH, matched with HIV-negative subjects, were inoculated with MVC-COV1901 and compared in terms of safety and immunogenicity.
2. Materials and Methods
2.1. Study Design and Participants
This is a substudy within a Phase 2, prospective, double-blinded, and multi-centre study to evaluate the SARS-CoV-2 vaccine MVC-COV1901. During enrolment, individuals underwent clinical laboratory tests and physical examinations including evaluation of medical history. Existing HIV infection was noted. The main Phase 2 study started on 30 December 2020, for which the interim report was completed in June 2021 [19]. Retrospective data for PWH of the substudy was collected between 24 September and 15 November 2021. The PWH included were on stable antiretroviral therapy with CD4+ T cell count greater than 350 cells/mm3 and HIV viral load less than 103 copies/mL. We compared PWH from a per-protocol immunogenicity (PPI) subset of the main study with HIV-negative participants of the main study (Figure 1). There were 57 individuals belonging to the HIV-positive group while 882 were included in the HIV-negative group. A total of 326 HIV-negative individuals were included in the comparator post-propensity score matching. Participants’ ages ranged from 20–87, and all received two standard doses of 15 mcg MVC-COV1901, administered 28 days apart via IM injection.
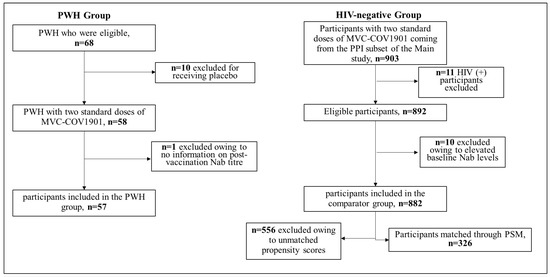
Figure 1.
The algorithm of the PWH group and the HIV-negative group receiving the MVC-COV1901 protein vaccine. Abbreviations: PWH = People Living with HIV; Nab = neutralizing antibodies; HIV = Human Immunodeficiency Virus; PSM = Propensity Score Matching.
Immediate adverse events (AEs), both solicited local and systemic, unsolicited AEs, and adverse events of special interest (AESIs) were recorded throughout the study period. For the safety data, the full safety set of participants from the main study was included in the analysis and compared to the HIV-positive group. The safety set involved participants in the main study who had at least one dose of the MVC-COV1901. A comprehensive evaluation of the safety profile of the two groups includes the recording of adverse events of people who received at least one dose of the study intervention, thus adverse events that occurred in the HIV-positive group were compared to the adverse events that occurred in the full safety set of the main study. For the safety profile comparison, we wanted to have as much data as possible to detect any unusual safety signals. Since in the process of propensity score matching, the unmatched individuals were discarded, to avoid the full safety picture being compromised, we chose to show the full scope of comparison.
Immunogenicity was assessed by measuring GMTs and seroconversion rates (SCRs) of neutralizing antibody. The detection and characterization of neutralizing antibodies were performed with central laboratories using validated pseudovirus and/or live virus neutralization assays [19]. To measure neutralizing antibody titers, wildtype SARS-CoV-2, Taiwan CDC strain number 4 (hCoV-19/Taiwan/4/2020; Global Initiative on Sharing All Influenza Data accession ID EPI_ISL_411927), was titrated to calculate the 50% tissue culture infective dose (TCID50). The hCoV-19/Taiwan/4/2020 is identical to the prototype virus strain. Vero E6 cells were seeded in 96-well plates (at 1.2 × 10⁴ cells per well) and incubated. Starting from a 1:8 dilution, the serum samples were diluted two-fold eight times to a final dilution of 1:1024. The diluted serum samples were then mixed with an equal volume of 100 TCID50 per 50 μL of virus. After incubating the serum–virus mixture at 37 °C for 1 h, it was added to the wells containing Vero E6 cells. The cells were then incubated at 37 °C in a 5% CO2 incubator for 4–5 days. The neutralizing titer (NT50) was defined as the reciprocal of the highest dilution capable of inhibiting 50% of the cytopathic effect. The NT50 results were derived from quadruplicates and calculated with the Reed-Muench method. Neutralizing antibody titers were converted to the WHO Standardized Unit, IU/mL. The conversion is based on the WHO validated NIBSC reference panel. The conversion formula was established based on the NT50 data of measuring the standards with defined IU/mL, including WHO SARS-CoV-2 international standard 20/136, WHO reference panel 20/268, and also the first international standard 20/130 before WHO IS 20/136 was launched. Each of the standards was measured 3 times (as 3 independent samples).
Written informed consent was obtained from all participants. The trial protocol and informed consent form were approved by the Taiwan Food and Drug Administration (TFDA) and the ethics committees at the conducting sites: National Taiwan University Hospital, Tri-Service General Hospital, Taipei Veterans General Hospital, Taipei Medical University Hospital, Taipei Municipal Wan Fang Hospital, Linkou Chang Gung Memorial Hospital, Taoyuan General Hospital Ministry of Health and Welfare, China Medical University Hospital, Changhua Christian Hospital, National Cheng Kung University Hospital, and Kaohsiung Medical University Chung-Ho Memorial Hospital. This trial was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice (GCP) guidelines.
2.2. Statistical Analysis
For the statistical analyses, descriptive statistics were first obtained and used to present sociodemographic and other characteristics. SCRs and 95% confidence interval (CI) were computed for individuals with at least a fourfold increase from the baseline. Fisher’s exact test was used to compare seroconversion between the HIV group and the main group. GMTs were estimated from neutralizing antibody titers measured at 28 days after the second dose of the study intervention. GMT ratios, computed as the ratio between the GMT of neutralizing antibodies in the HIV subgroup versus the control group, were also estimated. To assess the magnitude of the difference in immune response between the two groups, an analysis of the covariance (ANCOVA) model was used. The model included the log-transformed antibody titers at Day 57 as the dependent variable and the group (HIV subgroup and main group) as an explanatory variable, and they were adjusted for age, BMI, gender, and comorbidity profile. The 95% CI for the adjusted neutralizing antibody titers of each vaccine group was obtained. Then, the adjusted GMT was back-transformed to the original scale. The adjusted GMT ratio of the two groups and the corresponding 95% CI were also estimated. The correlation between GMT and CD4/CD8 ratio, and GMT and absolute CD4+ T cell count were analyzed using Spearman’s test. A one-way ANOVA was applied using log-transformed Nab titers for calculating the association between GMT and HIV classification stages. Out of the 57 HIV-positive individuals, three were excluded from this analysis due to a lack of information on the HIV classification stage. Lastly, using age and gender as covariates and a digit-based greedy and nearest neighbor approach with a 1:5 ratio, propensity score matching was employed for robust comparison between the two groups. Propensity scores were first estimated based on a multivariable logit regression. Propensity scores for the HIV-positive (case) group were then matched to that of the HIV-negative (control) individuals in a ratio of 1:5 without replacement. Matching without replacement meant that a patient from the control group who was already matched to a patient from the case group was not eligible for matching to another individual from the case group. Only subjects with a propensity score within 25% of the standard deviation of a case’s propensity score were matched. Any unmatched control was discarded. Differences between treatment groups for each covariate were assessed before and after matching to determine if there was sufficient balance. Standardized mean differences were evaluated to determine imbalances. The demographic characteristics of the excluded individuals are presented in Table S1.
3. Results
3.1. Study Population
In the main study, a total of 3854 participants were randomized to treatment groups. Among them, 58 PWH were randomized to the MVC-COV1901 group (Figure 1). One PWH was excluded from the analytic sample due to a lack of information on an outcome indicator (i.e., neutralizing antibody titer). There were 882 participants who were HIV-negative and aged 20 to 87 in the PPI subset from the main study (Table 1). Propensity matching resulted in a total of 57 participants in the HIV-positive group and 326 individuals in the HIV-negative group (Figure 1).

Table 1.
Demographic characteristics of the matched participants.
The demographic characteristics of the 57 PWH and the 326 matched comparators from the main study are summarized in Table 1. The mean age of the HIV group and the main group was 38.6 (Interquartile range [IQR] 19.0) and 42.8 (IQR 23.0) years, respectively (p = 0.058); 94.7% and 95.1% were male, respectively (p = 1); 19.3% and 15.0% had BMI ≥ 30 kg/m2, respectively (p = 0.413); and 10.5% and 18.1% had comorbidities at baseline, respectively (p = 0.16) (Table 1).
3.2. Safety
Overall, the percentages of participants in each category (solicited adverse events, unsolicited adverse events, and other adverse events after vaccination) were comparable between the HIV group and the main group in all age groups (Table 2a,b). The percentages of participants that reported solicited local adverse events were 58.6% and 72.3% for the PWH and main group, respectively. For solicited systemic adverse events, 63.8% and 53.8% were reported for the PWH and main group, respectively. In both PWH and HIV-negative participants, pain or tenderness at the injection site was the most common reported local reaction (67.2% and 71.2%, respectively). This event was slightly less common among PWH than HIV-negative individuals. Among systemic reactions, malaise and headache were the most common reaction in both groups but were predominantly mild to moderate in intensity.

Table 2.
(a) Solicited adverse events after any dosing. (b) Summary of unsolicited adverse events and other adverse events.
3.3. Immunogenicity
An assessment of immunogenicity among matched samples suggests that after two doses of study intervention, the wild-type SARS-CoV-2 neutralizing GMT’s ratio (95% CI) of HIV-negative/HIV-positive was 3.0 (95% CI 2.4–3.8) on Day 57 (i.e., 28 days after the 2nd dose), with 412.0 IU/mL (95% CI 378.7–448.3) in the HIV-negative group and 137.7 IU/mL (95% CI 110.7–171.3) in the HIV group, respectively (Table 3). After adjusting for sex, age, BMI category, and comorbidity profile, the adjusted GMT ratio (95% CI) of HIV-negative/HIV-positive was 3.2 (95% CI 2.5–4.0).

Table 3.
Comparison of wild-type SARS-CoV-2 neutralizing antibody (Nab) in IU/mL among matched participants.
The seroconversion rates on Day 57 based on the wild-type SARS-CoV-2 GMT were 100% in the PWH group and 99.8% in the main group with only two participants in the group failing to seroconvert.
3.4. Correlation of GMT and CD4/CD8 Ratio
CD4/CD8 ratio was positively associated with GMT (R = 0.27, p = 0.039) (Figure 2). No correlations were found between the GMTs and CD4+ T cell count (p = 0.3) and the GMT and HIV classification stages (p = 0. 35) (Figure 3 and Table 4).
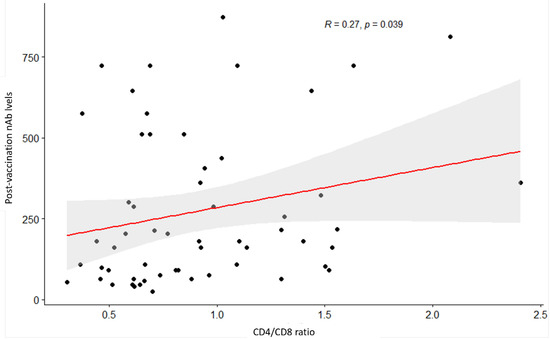
Figure 2.
Scatterplot between CD4/CD8 ratio and post-vaccination neutralization antibody titers. p value (i.e., 0.039) for the Spearman’s test indicates a significant relationship at a 5% significance level.
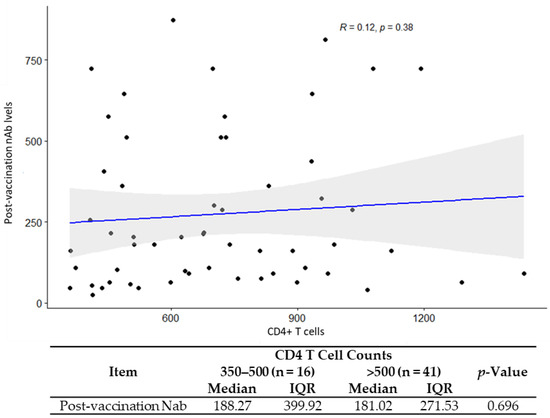
Figure 3.
Correlation analysis for CD4+ T cell counts with post vaccination neutralization antibody titers.

Table 4.
Correlation analysis for HIV classification stage with post vaccination neutralization antibody titers.
4. Discussion
In this study, the safety and immunogenicity of recombinant S-2P protein vaccine against COVID-19 were assessed in PWH. The interim analysis demonstrated that in PWH participants aged 20 years and older, two doses of the MVC-COV1901 vaccine were safe and well-tolerated but less immunogenic than in HIV-negative controls. By the time the report was written, all participants had been followed up with for up to six months after the second booster dose.
Based on the resulting GMTs, the immunogenicity of MVC-COV1901 may be compared to that of two doses of AZD1222 among healthy individuals. In an immunobridging study, the GMT ratio of Nab’s between MVC-COV1901 and AZD1222 was 3.8 times with a 95% confidence interval of 3.4–4.3 [20]. This is similar to the adjusted Nab GMT ratio between HIV-negative and HIV-positive individuals inoculated with MVC-COV1901 (i.e., 3.2 [95% CI: 2.5–4.0]). Although no correlates of protection have been established yet, neutralizing antibody levels might indicate similar efficacy to AZD1222 [21]. Previous research raised the possibility that the immune status of PWH negatively modulates the immune responses to COVID-19 vaccines. Specifically, PWH have diminished or less durable responses to hepatitis B and yellow fever vaccination [22,23,24], and people with low CD4 cell counts have diminished antibody titers to pneumococcus, influenza, diphtheria, tetanus, and poliomyelitis [25,26,27]. Despite these observations, most trials for COVID-19 vaccines have not addressed the PWH subpopulation with a subgroup analysis or comparison of PWH with HIV-negative control groups [28,29,30,31,32]. Several studies exhibited the same strength of immune response and safety profile, refs. [33,34,35,36] compared to HIV-negative comparators; the others showed weaker immune responses [37,38,39,40]. (Table 4). Despite the abovementioned studies, the numbers of PWH participating in clinical trials that evaluated COVID-19 vaccines is still very limited [41]. Weaker neutralization antibody responses to the Spike protein were demonstrated in this study, similar to the protein-based vaccine, NVX-CoV2373, studied in South Africa, ref. [38] and the inactivated SARS-CoV-2 vaccine studied in China [39].
CD4+ T cells orchestrate the response to acute and chronic viral infections by coordinating the immune system. These cells activate B cells to generate the efficient neutralisation antibodies, cytotoxic CD8+ T cells to kill infected cells, and multiple cells of the innate immune system and non-immune cells. Thus, CD4+ T cells play a key role for the establishment of long-term cellular and humoral antigen-specific immunity, which is the basis of life-long protection for many viral infections and vaccines [42,43]. In addition, CD4+ and CD8+ T cells produce interferon-gamma (commonly referred to as a “type 1” immune response), which is believed to be protective for the host [44]. It is therefore a legitimate concern that the immune response could be impeded in PWH with abnormal T cell counts as measured by depleted memory T cells and inversed CD4+/CD8+ ratios that may be indicative of the response of exhausted cytotoxic T cells toward HIV and persistent immune activation and inflammation even during stable antiretroviral therapy (ART) [45,46,47]. Nevertheless, the generation of neutralizing antibodies was a key endpoint in this vaccine study.
Notably, in the study with MVC-COV1901 presented here, CD4+ T cell counts did not significantly correlate with GMT in the vaccinated PWH while increasing CD4/CD8 ratios did correlate, unlike other studies [37,40]. Despite this, however, a “weaker” correlation was seen between GMT and CD4/CD8 ratios, which may partly be explained by the small size of the HIV-positive group and other factors of HIV disease not taken into account. Nevertheless, the adjuvant of MCV-COV1901, CpG 1018 (a toll-like receptor 9 agonist), may explain this correlation because it binds to the DNA receptor on plasmacytoid dendritic cells and enhances immunogenicity by stimulating CD4+ helper and CD8+ cytotoxic T cells simultaneously [48,49]. Consistent with these observations, an independent HBV vaccine study in PWH demonstrated that CD4/CD8 ratios > 0.4 were associated with a high rate (86%) of HBV seroconversion [50].
Despite these indications that cellular immunity may be important for durable protective immunity, there are arguments that long-lived plasma cells may have the potential to produce antibodies for decades in the absence of a re-encounter with the antigen or specific T cells [51]. Add to this the 100% of seroconversion in PWH with MVC-COV1901, and it is clear that the role of T cell memory in durable protective immunity against SARS-CoV-2 deserves further study.
5. Limitation
Despite the insights generated by our study, some limitations to interpretation may exist. First, the sample size in the PWH group was relatively small. Furthermore, all PWH were on stable ART and had CD4+ T cell counts greater than 350 cells/mm3 and HIV viral load less than 1000 genome copies/mL. Thus, extrapolation to people with HIV with lower CD4 counts or without suppressed HIV viral loads is not suggested. Recent studies have reported PWH with CD4+ T cell counts less than 200 cells/mm3 presented weaker immunologic responses [37,38,39,40,41]. Second, our study was initiated when SARS-CoV-2 was not endemic in Taiwan, and the low viral transmission rate made it difficult to ascertain the efficacy of the vaccine as an exploratory endpoint. Specifically, low levels of 1% of neutralizing antibody titer were detected both at baseline and on Day 57 in the placebo group, suggesting that COVID-19 was rare, and natural infection had not boosted the neutralizing antibody titers [19]. Third, the short duration of the follow-up period in this study did not allow for assessing the durability of immune responses after Day 57. Fourth, although Th1-skewed immune responses had been demonstrated in the phase I MVC-COV1901 study 18, the T-cell responses to the vaccine among PWH were not assessed in this study. Finally, neutralization activities for emerging VOCs were not tested, and the cross-reactivity remains unknown.
6. Conclusions
This report describes a good safety profile but weaker immunogenicity of MCV-COV1901 in PWH, especially in those PWH with low CD4/CD8 ratios. MCV-COV1901 has emergency authorization use in Taiwan as of 19 July 2021 and has since advanced to larger clinical trials, including a trial initiated by the WHO [52]. Additional information accumulates from these trials, but further studies are needed to see if PWH require distinct immunization strategies with improved immunogenicity, such as boosters or additional doses [53,54], heterologous revaccination [55], or higher doses as with hepatitis B [56] and that for the influenza vaccine [57]. These studies may include, at this point, effectiveness studies focusing on PWH, which may justify the use of strategies such as additional doses of the vaccine or heterologous boosting. Future research should also investigate the effectiveness of vaccines among PWH who have higher levels of immunodeficiency and PWH against Omicron or future variants [58].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vaccines11010018/s1, Figure S1: Propensity scores for people living with HIV, comparing unmatched participants from the main study, and comparing controls with matched propensity scores; Table S1: Demographic characteristics for the group from the main study and controls with matched propensity scores.
Author Contributions
S.-H.C., C.E.L. and W.-S.L. conceived and designed the study. W.-S.L., C.-Y.C., W.-D.L., S.-M.H., C.-L.L., W.-C.K., Y.-H.C., H.-T.C., C.-T.H., S.-J.H., N.-C.W., M.-C.L. and Y.-L.L. acquired and interpreted the data. C.E.L., I.-C.T., and J.A.G.E. analyzed the data. S.-H.C., W.-S.L. and C.E.L. drafted and prepared the manuscript. C.E.L. and I.-C.T. had full access to and verified all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. S.-H.C., C.E.L. and W.-S.L. reviewed and edited the final version, and S.-H.C., C.E.L., W.-S.L. and T.-Y.L. had final responsibility for the decision to submit for publication. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by Medigen Vaccine Biologics (study sponsor), the Taiwan Centers for Disease Control and Ministry of Health and Welfare. The sponsor co-designed the trial and coordinated interactions with contract Clinical Research Organization (CRO) staff and regulatory authorities. The CRO operated trial operation to meet the standards of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use and good clinical practice guidelines. The IDMC oversaw the safety data and communicated recommendations to the sponsor. The interim analysis was done by the CRO.
Institutional Review Board Statement
Institutional review board of Linkou Chang Gung Memorial Hospital had approved the version 2.0, 29-DEC-2020 protocol of the main study (202002080A0C601), and then the other study sites could follow the approval in fast track. So as the approval of the substudy of HIV approved by IRB of Taoyuan General Hospital Ministry of Health and Welfare (TYGH110043). The study and all associated methods were performed in accordance with the approved protocol, the principles of the Declaration of Helsinki, and Good Clinical Practice guidelines.
Informed Consent Statement
Written informed consent was obtained from all participants.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available as it is an interim analysis of data from an ongoing study.
Acknowledgments
We thank Stanley Chang, and Charles Chen at Medigen Vaccine Biologics; the investigational staff at National Taiwan University Hospital, Taiwan Taipei Veterans General Hospital, Tri-Service General Hospital, Taipei Veterans General Hospital, Taipei Medical University Hospital, Taipei Municipal Wan Fang Hospital, Linkou Chang Gung Memorial Hospital, Taoyuan General Hospital Ministry of Health and Welfare, China Medical University Hospital, Changhua Christian Hospital, National Cheng Kung University Hospital, and Kaohsiung Medical University Chung-Ho Memorial Hospital, for their dedication to this trial; the Clinipace Clinical Research team (Taipei, Taiwan), for their involvement in conducting the trial; Barney S Graham at the Vaccine Research Centre, US National Institute of Allergy and Infectious Diseases, for the development of S-2P pre-fusion protein; Robert Janssen at Dynavax Technologies for providing the CpG 1018 adjuvant and related important intellectual content; members at Protech Pharmaservices (Taipei, Taiwan) for conducting the spike-specific IgG ELISA assay; members at the Department of Laboratory Medicine, Linkou Chang Gung Memorial Hospital (Taoyuan, Taiwan), and members at Institute of Biomedical Sciences, Academia Sinica (Taipei, Taiwan) for conducting the neutralisation assay.
Conflicts of Interest
C.E.L., J.A.G.E. and I.-C.T. are employees of Medigen Vaccine Biologics (Taipei, Taiwan) and they received grants from Taiwan Centers for Disease Control, Ministry of Health and Welfare, during the conduct of the study. S.-H.C., S.-M.H., C.-Y.C., W.-D.L., C.-L.L., W.-C.K., Y.-H.C., C.-T.H., H.-T.C., S.-J.H., N.-C.W., M.-C.L., Y.-L.L., T.-Y.L. and W.-S.L. had nothing to declare.
References
- World Health Organization. WHO Director-General’s Opening Remarks at the Mission Briefing on COVID-19–11 March 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 5 June 2021).
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Done, E.; Du, I.; Garder, L. COVID-19 Dashboard by the Centrefor Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). John Hopkins University, 2020. Available online: https://coronavirus.jhu.edu/map.html (accessed on 30 November 2022).
- Yancy, C.W. COVID-19 and African Americans. JAMA 2020, 323, 1891–1892. [Google Scholar] [CrossRef] [PubMed]
- Mdodo, R.; Frazier, E.L.; Dube, S.R.; Mattson, C.L.; Sutton, M.Y.; Brooks, J.T.; Skarbinski, J. Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: Cross-sectional surveys. Ann. Intern. Med. 2015, 162, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Shiau, S.; Arpadi, S.M.; Yin, M.T.; Martins, S.S. Patterns of drug use and HIV infection among adults in a nationally representative sample. Addict. Behav. 2017, 68, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Hasse, B.; Ledergerber, B.; Furrer, H.; Battegay, M.; Hirschel, B.; Cavassini, M.; Bertisch, B.; Bernasconi, E.; Weber, R. Morbidity and aging in HIV-infected persons: The Swiss HIV cohort study. Clin. Infect. Dis. 2011, 53, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.; Gange, S.J.; Moore, R.D.; Justice, A.C.; Buchacz, K.; Abraham, A.G.; Rebeiro, P.; Koethe, J.R.; Martin, J.N.; Horberg, M.A.; et al. Multimorbidity Among Persons Living with Human Immunodeficiency Virus in the United States. Clin. Infect. Dis. 2017, 66, 1230–1238. [Google Scholar] [CrossRef]
- Eckard, A.R.; McComsey, G.A. Weight gain and integrase inhibitors. Curr. Opin. Infect. Dis. 2020, 33, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Sax, P.E.; Erlandson, K.M.; Lake, J.E.; McComsey, G.A.; Orkin, C.; Esser, S.; Brown, T.T.; Rockstroh, J.K.; Wei, X.; Carter, C.C.; et al. Weight Gain Following Initiation of Antiretroviral Therapy: Risk Factors in Randomized Comparative Clinical Trials. Clin. Infect. Dis. 2020, 71, 1379–1389. [Google Scholar] [CrossRef]
- Liu, B.; Spokes, P.; He, W.; Kaldor, J. High risk groups for severe COVID-19 in a whole of population cohort in Australia. BMC Infect. Dis. 2021, 21, 685. [Google Scholar] [CrossRef]
- Kang, I.S.; Kong, K.A. Body mass index and severity/fatality from coronavirus disease 2019: A nationwide epidemiological study in Korea. PLoS ONE 2021, 16, e0253640. [Google Scholar] [CrossRef]
- Dandachi, D.; Geiger, G.; Montgomery, M.W.; Karmen-Tuohy, S.; Golzy, M.; Antar, A.A.; Llibre, J.M.; Camazine, M.; Díaz-De Santiago, A.; Carlucci, P.M.; et al. Characteristics, Comorbidities, and Outcomes in a Multicenter Registry of Patients with HIV and Coronavirus Disease-19. Clin. Infect. Dis. 2021, 73, e1964–e1972. [Google Scholar] [CrossRef] [PubMed]
- Geretti, A.M.; Stockdale, A.J.; Kelly, S.H.; Cevik, M.; Collins, S.; Waters, L.; Villa, G.; Docherty, A.; Harrison, E.M.; Turtle, L.; et al. Outcomes of COVID-19 related hospitalization among people with HIV in the ISARIC WHO Clinical Characterization Protocol (UK): A prospective observational study. Clin. Infect. Dis. 2021, 73, e2095–e2106. [Google Scholar] [CrossRef] [PubMed]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.-L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.Y.; Lin, M.Y.; Coffman, R.L.; Campbell, J.D.; Traquina, P.; Lin, Y.J.; Liu, L.T.; Cheng, J.; Wu, Y.; Wu, C.; et al. Development of CpG-adjuvanted stable prefusion SARS-CoV-2 spike antigen as a subunit vaccine against COVID-19. Sci. Rep. 2020, 10, 20085. [Google Scholar] [CrossRef]
- Lien, C.E.; Lin, Y.J.; Chen, C.; Lian, W.C.; Kuo, T.Y.; Campbell, J.D.; Traquina, P.; Lin, M.Y.; Liu, L.T.; Chuang, Y.S.; et al. CpG-adjuvanted stable prefusion SARS-CoV-2 spike protein protected hamsters from SARS-CoV-2 challenge. Sci. Rep. 2021, 11, 8761. [Google Scholar] [CrossRef]
- Hsieh, S.-M.; Liu, W.-D.; Huang, Y.-S.; Lin, Y.-J.; Hsieh, E.-F.; Lian, W.-C.; Chen, C.; Janssen, R.; Shih, S.-R.; Huang, C.-G.; et al. Safety and immunogenicity of a Recombinant Stabilized Prefusion SARS-CoV-2 Spike Protein Vaccine (MVC-COV1901) Adjuvanted with CpG 1018 and Aluminum Hydroxide in healthy adults: A Phase 1, dose-escalation study. Eclinicalmedicine 2021, 38, 100989. [Google Scholar] [CrossRef]
- Hsieh, S.-M.; Liu, M.-C.; Chen, Y.-H.; Lee, W.-S.; Hwang, S.-J.; Cheng, S.-H.; Ko, W.-C.; Hwang, K.-P.; Wang, N.-C.; Lee, Y.-L.; et al. Safety and immunogenicity of CpG 1018 and aluminium hydroxide-adjuvanted SARS-CoV-2 S-2P protein vaccine MVC-COV1901: Interim results of a large-scale, double-blind, randomised, placebo-controlled phase 2 trial in Taiwan. Lancet Respir. Med. 2021, 9, 1396–1406. [Google Scholar] [CrossRef]
- Estrada, J.A.; Cheng, C.-Y.; Ku, S.-Y.; Hu, H.-C.; Yeh, H.-W.; Lin, Y.-C.; Chen, C.-P.; Cheng, S.-H.; Janssen, R.; Lin, I.-F. An Immunobridging Study to Evaluate the Neutralizing Antibody Titer in Adults Immunized with Two Doses of Either ChAdOx1-nCov-19 (AstraZeneca) or MVC-COV1901. Vaccines 2022, 10, 655. [Google Scholar] [CrossRef]
- Feng, S.; Phillips, D.J.; White, T.; Sayal, H.; Aley, P.K.; Bibi, S.; Dold, C.; Fuskova, M.; Gilbert, S.C.; Hirsch, I.; et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 2032–2040. [Google Scholar] [CrossRef]
- Van den Berg, R.; van Hoogstraten, I.; van Agtmael, M. Non-responsiveness to hepatitis B vaccination in HIV seropositive patients; possible causes and solutions. AIDS Rev. 2009, 11, 157–164. [Google Scholar]
- Avelino-Silva, V.I.; Miyaji, K.T.; Hunt, P.W.; Huang, Y.; Simoes, M.; Lima, S.B.; Freire, M.S.; Caiaffa-Filho, H.H.; Hong, M.A.; Costa, D.A.; et al. CD4/CD8 Ratio and KT Ratio Predict Yellow Fever Vaccine Immunogenicity in HIV-Infected Patients. PLoS Negl. Trop. Dis. 2016, 10, e0005219. [Google Scholar] [CrossRef] [PubMed]
- O’Bryan, T.; Rini, E.; Okulicz, J.; Messner, O.; Ganesan, A.; Lalani, T.; Bavaro, M.; O’Connell, R.; Agan, B.; Landrum, M. HIV viraemia during hepatitis B vaccination shortens the duration of protective antibody levels. HIV Med. 2015, 16, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Kroon, F.P.; Van Dissel, J.T.; Labadie, J.; Van Loon, A.M.; Van Furth, R. Antibody response to diphtheria, tetanus, and poliomyelitis vaccines in relation to the number of CD4+ T lymphocytes in adults infected with human immunodeficiency virus. Clin. Infect. Dis. 1995, 21, 1197–1203. [Google Scholar] [CrossRef]
- Kroon, F.P.; van Dissel, J.T.; de Jong, J.C.; van Furth, R. Antibody response to influenza, tetanus and pneumococcal vaccines in HIV-seropositive individuals in relation to the number of CD4+ lymphocytes. AIDS 1994, 8, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.-C.; Chang, S.-Y.; Su, C.-T.; Chen, Y.-Y.; Yang, C.-Y.; Liu, W.-C.; Wu, C.-H. A 5-year longitudinal follow-up study of serological responses to 23-valent pneumococcal polysaccharide vaccination among patients with HIV infection who received highly active antiretroviral therapy. HIV Med. 2009, 11, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Peebody, R. Have COVID-19 Vaccines Been Tested in People with HIV? 2021. Available online: https://www.aidsmap.com/about-hiv/have-covid-19-vaccines-been-tested-people-hiv (accessed on 30 July 2021).
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Janssen Biotech, Inc. FDA Briefing Document: Janssen Ad26.COV2.S Vaccine for the Prevention of COVID-19. 2021. Available online: https://www.fda.gov/media/146217/download (accessed on 27 November 2021).
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef]
- Frater, J.; Ewer, K.J.; Ogbe, A.; Pace, M.; Adele, S.; Adland, E.; Alagaratnam, J.; Aley, P.K.; Ali, M.; Ansari, M.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in HIV infection: A single-arm substudy of a phase 2/3 clinical trial. Lancet HIV 2021, 8, e474–e485. [Google Scholar] [CrossRef]
- Madhi, S.A.; Koen, A.L.; Izu, A.; Fairlie, L.; Cutland, C.L.; Baillie, V.; Padayachee, S.D.; Dheda, K.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in people living with and without HIV in South Africa: An interim analysis of a randomised, double-blind, placebo-controlled, phase 1B/2A trial. Lancet HIV 2021, 8, e568–e580. [Google Scholar] [CrossRef]
- Woldemeskel, B.A.; Karaba, A.H.; Garliss, C.C.; Beck, E.J.; Wang, K.H.; Laeyendecker, O.; Cox, A.L.; Blankson, J.N. The BNT162b2 mRNA Vaccine Elicits Robust Humoral and Cellular Immune Responses in People Living with HIV. Clin. Infect. Dis. 2021, 74, 1268–1270. [Google Scholar] [CrossRef] [PubMed]
- Levy, I.; Finesod, A.W.; Litchevsky, V.; Biber, A.; Indenbaum, V.; Olmer, L.; Huppert, A.; Mor, O.; Goldstein, M.; Levin, E.G.; et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in people living with HIV-1. Clin. Microbiol. Infect. 2021, 27, 1851–1855. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, M.A.; Peluso, M.J.; Lynch, K.L.; Yun, C.; Glidden, D.V.; Henrich, T.J.; Deeks, S.G.; Gandhi, M. Differences in Post-mRNA Vaccination SARS-CoV-2 IgG Concentrations and Surrogate Virus Neutralization Test Response by HIV Status and Type of Vaccine: A Matched Case-Control Observational Study. Clin. Infect. Dis. 2022, 75, e916–e919. [Google Scholar] [CrossRef] [PubMed]
- Shinde, V.; Bhikha, S.; Hoosain, Z.; Archary, M.; Bhorat, Q.; Fairlie, L.; Lalloo, U.; Masilela, M.S.L.; Moodley, D.; Hanley, S.; et al. Efficacy of NVX-CoV2373 Covid-19 Vaccine against the B.1.351 Variant. N. Engl. J. Med. 2021, 384, 1899–1909. [Google Scholar] [CrossRef]
- Huang, X.; Yan, Y.; Su, B.; Xiao, D.; Yu, M.; Jin, X.; Duan, J.; Zhang, X.; Zheng, S.; Fang, Y.; et al. Comparing Immune Responses to Inactivated Vaccines against SARS-CoV-2 between People Living with HIV and HIV-Negative Individuals: A Cross-Sectional Study in China. Viruses 2022, 14, 277. [Google Scholar] [CrossRef] [PubMed]
- Hassold, N.; Brichler, S.; Ouedraogo, E.; Leclerc, D.; Carroue, S.; Gater, Y.; Alloui, C.; Carbonnelle, E.; Bouchaud, O.; Mechai, F.; et al. Impaired antibody response to COVID-19 vaccination in advanced HIV infection. AIDS 2022, 36, F1–F5. [Google Scholar] [CrossRef]
- Díaz, N.A.; de Miguel, R.; Agüero, F.; Sued, O.; Arribas, J.R.; Ambrosioni, J.; Hospital Clinic COVID-19 in HIV Investigators. Prevention and Treatment of SARS-CoV2 Infection in People Living with HIV: The Need for Specific Data. Infect. Dis. Ther. 2021, 28, 1–13. [Google Scholar] [CrossRef]
- Kaech, S.M.; Wherry, E.J.; Ahmed, R. Effector and memory T-cell differentiation: Implications for vaccine development. Nat. Rev. Immunol. 2002, 2, 251–262. [Google Scholar] [CrossRef]
- Peng, X.; Ouyang, J.; Isnard, S.; Lin, J.; Fombuena, B.; Zhu, B.; Routy, J.-P. Sharing CD4+ T Cell Loss: When COVID-19 and HIV Collide on Immune System. Front. Immunol. 2020, 11, 596631. [Google Scholar] [CrossRef]
- Chen, Z.; John, W.E. T cell responses in patients with COVID-19. Nat. Rev. Immunol. 2020, 20, 529–536. [Google Scholar] [CrossRef]
- Papagno, L.; Spina, C.A.; Marchant, A.; Salio, M.; Rufer, N.; Little, S.; Dong, T.; Chesney, G.; Waters, A.; Easterbrook, P.; et al. Immune Activation and CD8+ T-Cell Differentiation towards Senescence in HIV-1 Infection. PLoS Biol. 2004, 2, E20. [Google Scholar] [CrossRef] [PubMed]
- McCune, J.M. The dynamics of CD4+ T-cell depletion in HIV disease. Nature 2001, 410, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, M.; Van Lunzen, J.; Soghoian, D.Z.; Kuhl, B.D.; Ranasinghe, S.; Kranias, G.; Flanders, M.D.; Cutler, S.; Yudanin, N.; Muller, M.I.; et al. Expansion of HIV-specific T follicular helper cells in chronic HIV infection. J. Clin. Investig. 2012, 122, 3271–3280. [Google Scholar] [CrossRef] [PubMed]
- Hornung, V.; Rothenfusser, S.; Britsch, S.; Krug, A.; Jahrsdörfer, B.; Giese, T.; Endres, S.; Hartmann, G. Quantitative Expression of Toll-Like Receptor 1–10 mRNA in Cellular Subsets of Human Peripheral Blood Mononuclear Cells and Sensitivity to CpG Oligodeoxynucleotides. J. Immunol. 2002, 168, 4531–4537. [Google Scholar] [CrossRef] [PubMed]
- Hyer, R.; McGuire, D.K.; Xing, B.; Jackson, S.; Janssen, R. Safety of a two-dose investigational hepatitis B vaccine, HBsAg-1018, using a toll-like receptor 9 agonist adjuvant in adults. Vaccine 2018, 36, 2604–2611. [Google Scholar] [CrossRef]
- Fuster, F.; Vargas, J.I.; Jensen, D.; Sarmiento, V.; Acuna, P.; Peirano, F.; Fuste, F.; Arab, J.P.; Martínez, F. CD4/CD8 ratio as a predictor of the response to HBV vaccination in HIV-positive patients: A prospective cohort study. Vaccine 2016, 34, 1889–1895. [Google Scholar] [CrossRef]
- Hammarlund, E.; Thomas, A.; Amanna, I.J.; Holden, L.A.; Slayden, O.D.; Park, B.; Gao, L.; Slifka, M.K. Plasma cell survival in the absence of B cell memory. Nat. Commun. 2017, 8, 1781. [Google Scholar] [CrossRef]
- World Health Orgnization. WHO Statement on Solidarity Trial Vaccines. Available online: https://www.who.int/news/item/26-10-2021-who-statement-on-solidarity-trial-vaccines (accessed on 26 October 2021).
- Krause, P.R.; Fleming, T.R.; Peto, R.; Longini, I.M.; Figueroa, J.P.; Sterne, J.A.C.; Cravioto, A.; Rees, H.; Higgins, J.P.T.; Boutron, I.; et al. Considerations in boosting COVID-19 vaccine immune responses. Lancet 2021, 398, 1377–1380. [Google Scholar] [CrossRef]
- Winthrop, K.L.; Whitley, R.J.; Aletaha, D. SARS-CoV-2 and the rheumatology patient: The last 12 months and a boost in the future. Ann. Rheum. Dis. 2021, 80, 1249–1251. [Google Scholar] [CrossRef]
- Zhang, J.; He, Q.; An, C.; Mao, Q.; Gao, F.; Bian, L.; Wu, X.; Wang, Q.; Liu, P.; Song, L.; et al. Boosting with heterologous vaccines effectively improves protective immune responses of the inactivated SARS-CoV-2 vaccine. Emerg. Microbes Infect. 2021, 10, 1598–1608. [Google Scholar] [CrossRef]
- Cruciani, M.; Mengoli, C.; Serpelloni, G.; Lanza, A.; Gomma, M.; Nardi, S.; Rimondo, C.; Bricolo, F.; Consolaro, S.; Trevisan, M.; et al. Serologic response to hepatitis B vaccine with high dose and increasing number of injections in HIV infected adult patients. Vaccine 2009, 27, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Caldera, F.; Mercer, M.; Samson, S.I.; Pitt, J.M.; Hayney, M.S. Influenza vaccination in immunocompromised populations: Strategies to improve immunogenicity. Vaccine 2021, 39, A15–A23. [Google Scholar] [CrossRef] [PubMed]
- Chambers, C.; Samji, H.; Cooper, C.L.; Costiniuk, C.T.; Janjua, N.Z.; Kroch, A.E.; Arbess, G.; Benoit, A.C.; Buchan, S.A.; Chung, H.; et al. Coronavirus disease 2019 vaccine effectiveness among a population-based cohort of people living with HIV. AIDS 2022, 36, F17–F26. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).