Cellular and Humoral Immunity against Different SARS-CoV-2 Variants Is Detectable but Reduced in Vaccinated Kidney Transplant Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Volunteers
2.2. CoV-iSpot for Interferon-γ and Interleukin-2
2.3. In-House ELISpot Assay
2.4. Cells and Viruses
2.5. Assessment of Neutralizing Antibodies by Cell Culture-Based Neutralization Assay
2.6. Assessment of Neutralizing Antibodies by Competitive Immunofluorescence
2.7. Antibody ELISA
2.8. Statistical Analysis
3. Results
3.1. T Cell Responses in Kidney Transplant Patients and Healthy Volunteers
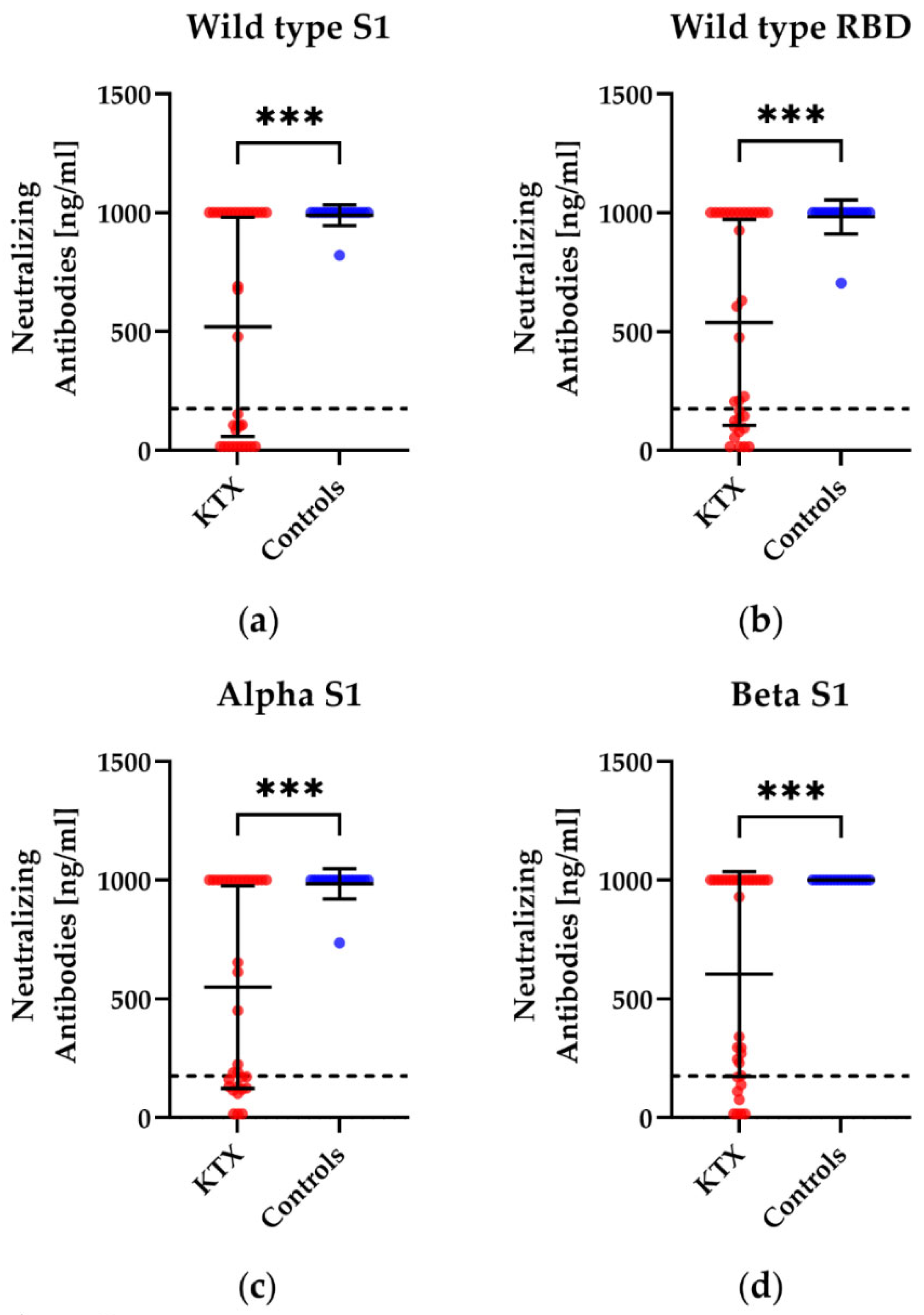
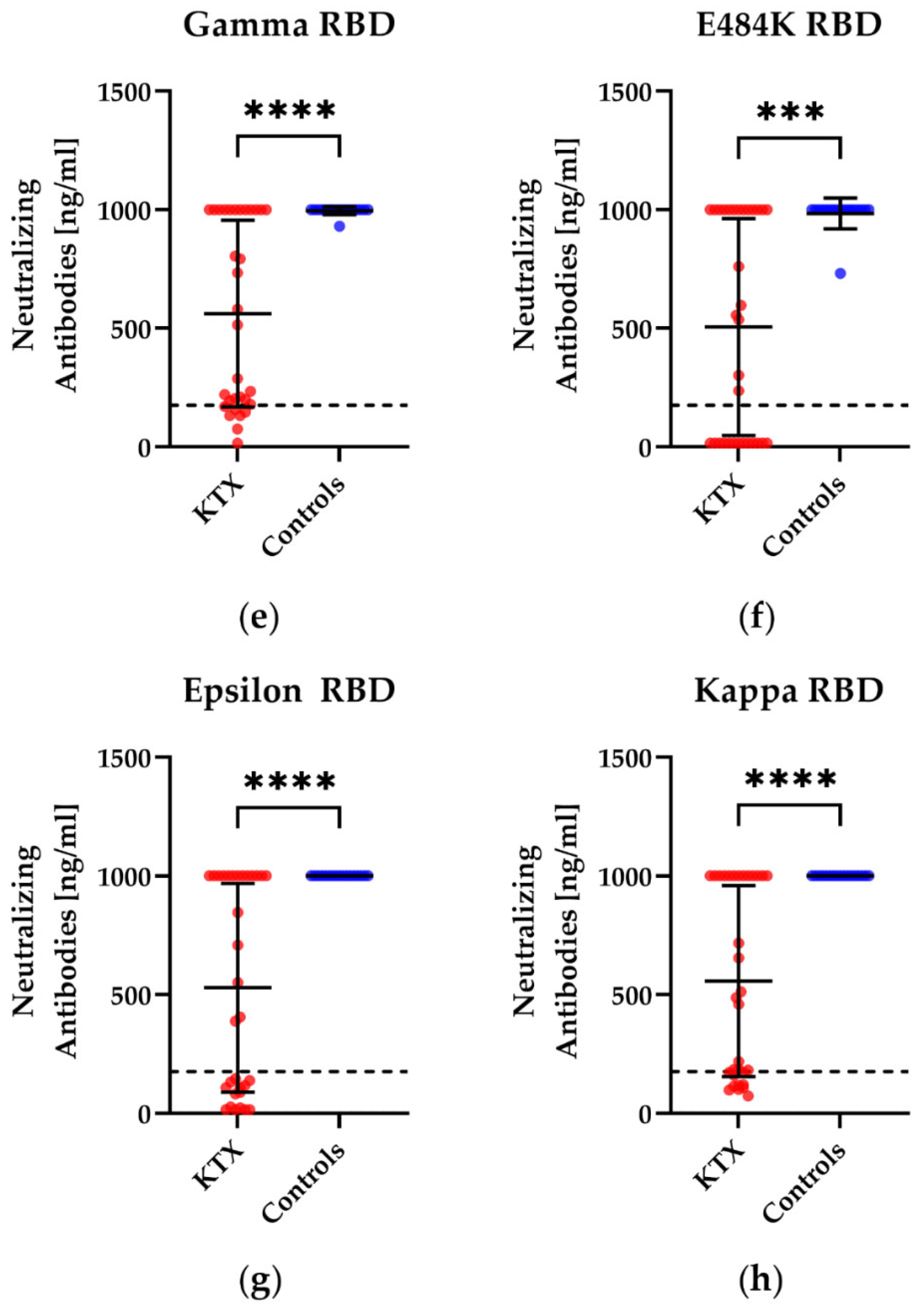
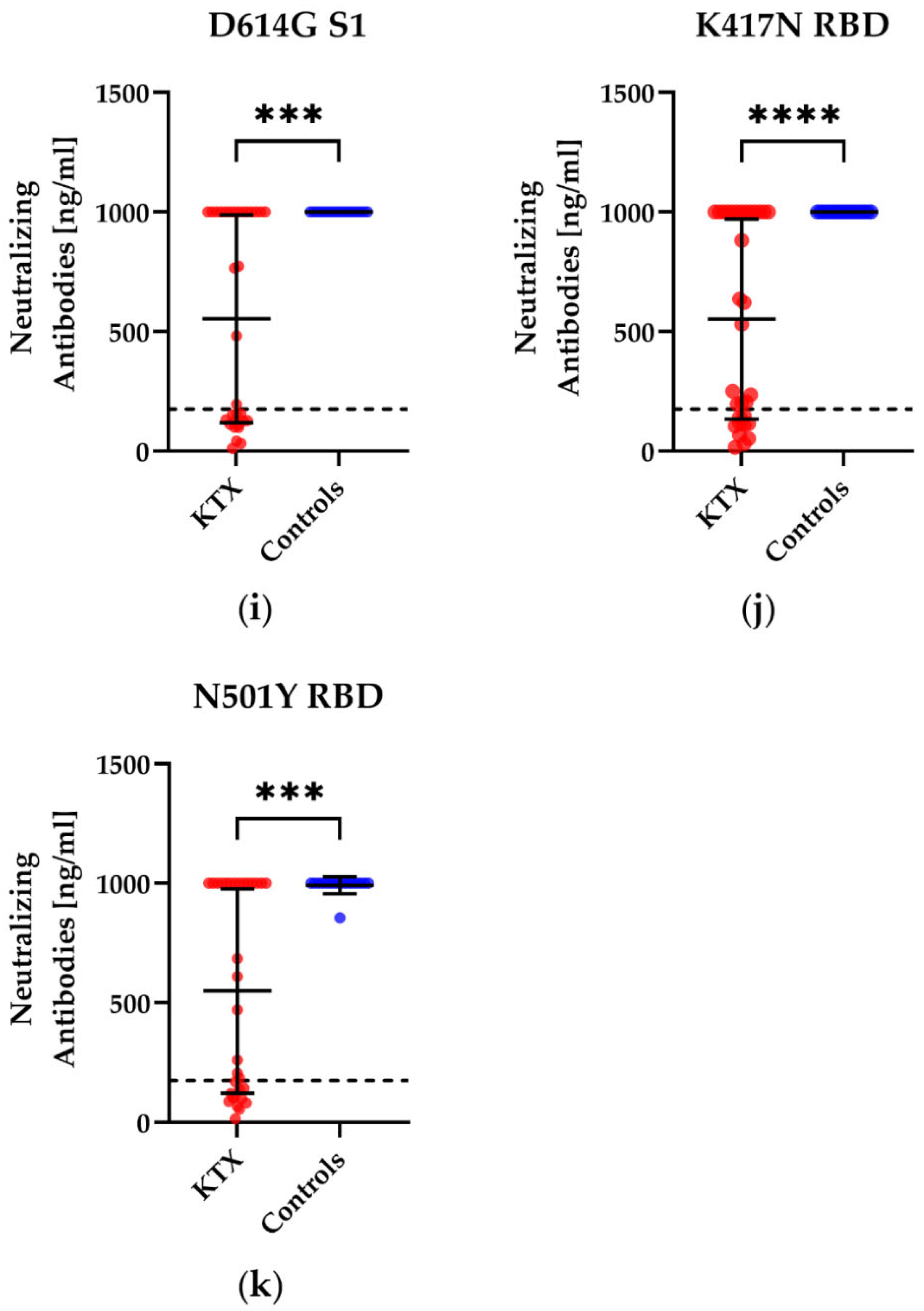
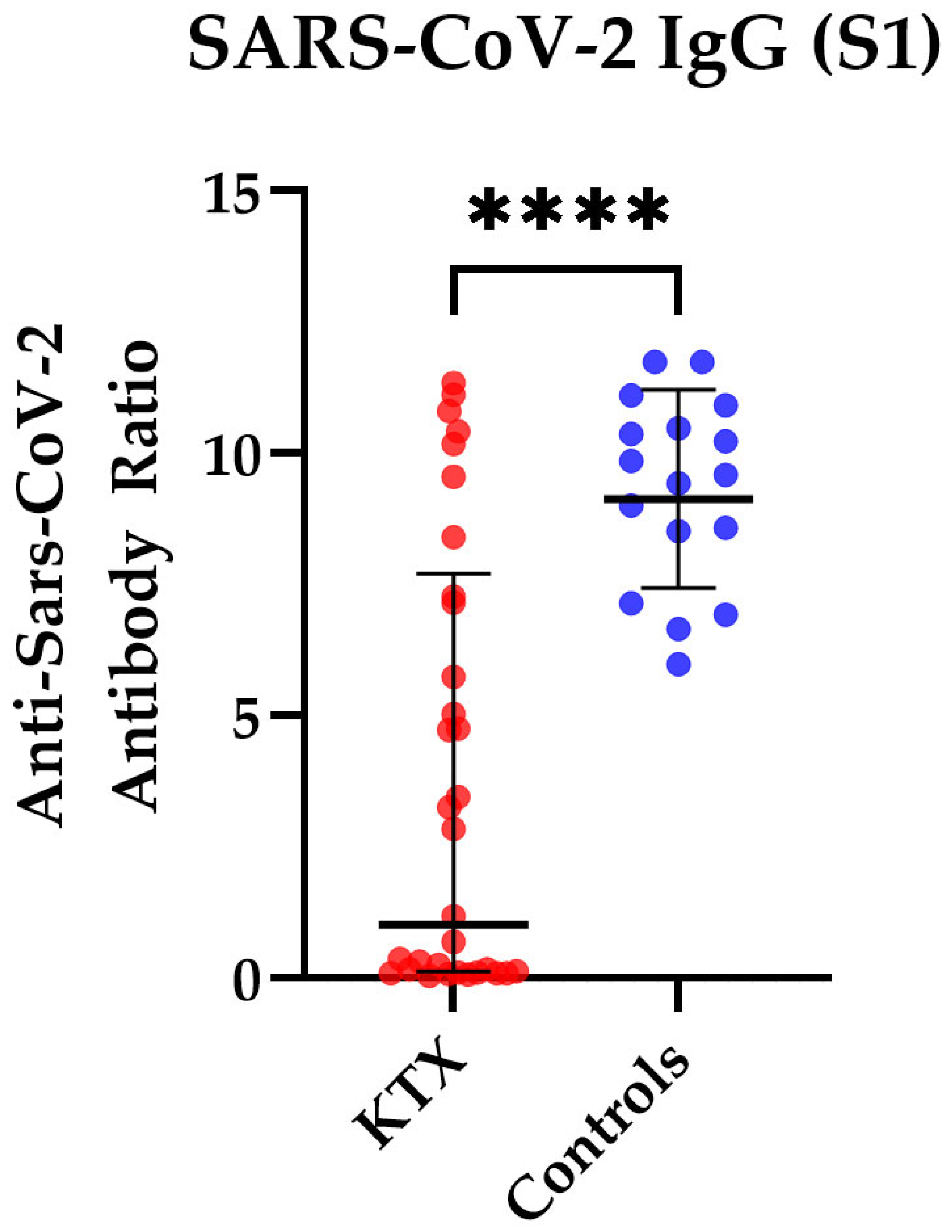
3.2. Humoral Immunity in Kidney Transplant Patients and Healthy Controls
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. 2022. Available online: https://covid19.who.int/ (accessed on 15 July 2022).
- Caillard, S.; Chavarot, N.; Francois, H.; Matignon, M.; Greze, C.; Kamar, N.; Gatault, P.; Thaunat, O.; Legris, T.; Frimat, L.; et al. Is COVID-19 infection more severe in kidney transplant recipients? Am. J. Transplant. 2021, 21, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Van der Straten, K.; van Gils, M.J.; de Taeye, S.W.; de Bree, G.J. Optimization of Anti-SARS-CoV-2 Neutralizing Antibody Therapies: Roadmap to Improve Clinical Effectiveness and Implementation. Front. Med. Technol. 2022, 4, 867982. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.D.; Ding, M.; Dong, X.; Zhang, J.-J.; Azkur, A.K.; Azkur, D.; Gan, H.; Sun, Y.-L.; Fu, W.; Li, W.; et al. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy 2021, 76, 428–455. [Google Scholar] [CrossRef]
- Giesen, N.; Sprute, R.; Rüthrich, M.; Khodamoradi, Y.; Mellinghoff, S.C.; Beutel, G.; Lueck, C.; Koldehoff, M.; Hentrich, M.; Sandherr, M.; et al. Evidence-based management of COVID-19 in cancer patients: Guideline by the Infectious Diseases Working Party (AGIHO) of the German Society for Haematology and Medical Oncology (DGHO). Eur. J. Cancer 2020, 140, 86–104. [Google Scholar] [CrossRef] [PubMed]
- Giesen, N.; Sprute, R.; Rüthrich, M.; Khodamoradi, Y.; Mellinghoff, S.C.; Beutel, G.; Lueck, C.; Koldehoff, M.; Hentrich, M.; Sandherr, M.; et al. 2021 update of the AGIHO guideline on evidence-based management of COVID-19 in patients with cancer regarding diagnostics, viral shedding, vaccination, and therapy. Eur. J. Cancer 2021, 147, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, C.S.; Balletto, E.; Cornberg, M.; Mikulska, M. Coronavirus disease 2019 vaccination in transplant recipients. Curr. Opin. Infect. Dis. 2021, 34, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Benotmane, I.; Gautier, G.; Perrin, P.; Olagne, J.; Cognard, N.; Fafi-Kremer, S.; Caillard, S. Antibody Response after a Third Dose of the mRNA-1273 SARS-CoV-2 Vaccine in Kidney Transplant Recipients with Minimal Serologic Response to 2 Doses. JAMA 2021, 326, 1063–1065. [Google Scholar] [CrossRef]
- Grupper, A.; Rabinowich, L.; Schwartz, D.; Schwartz, I.F.; Ben-Yehoyada, M.; Shashar, M.; Katchman, E.; Halperin, T.; Turner, D.; Goykhman, Y.; et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am. J. Transplant. 2021, 21, 2719–2726. [Google Scholar] [CrossRef]
- Hall, V.G.; Ferreira, V.H.; Ku, T.; Ierullo, M.; Majchrzak-Kita, B.; Chaparro, C.; Selzner, N.; Schiff, J.; McDonald, M.; Tomlinson, G.; et al. Randomized Trial of a Third Dose of mRNA-1273 Vaccine in Transplant Recipients. N. Engl. J. Med. 2021, 385, 1244–1246. [Google Scholar] [CrossRef]
- Werbel, W.A.; Boyarsky, B.J.; Ou, M.T.; Massie, A.B.; Tobian, A.A.R.; Garonzik-Wang, J.M.; Segev, D.L. Safety and Immunogenicity of a Third Dose of SARS-CoV-2 Vaccine in Solid Organ Transplant Recipients: A Case Series. Ann. Intern. Med. 2021, 174, 1330–1332. [Google Scholar] [CrossRef]
- Peled, Y.; Ram, E.; Lavee, J.; Segev, A.; Matezki, S.; Wieder-Finesod, A.; Halperin, R.; Mandelboim, M.; Indenbaum, V.; Levy, I.; et al. Third dose of the BNT162b2 vaccine in heart transplant recipients: Immunogenicity and clinical experience. J. Heart Lung Transplant. 2022, 41, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Thümmler, L.; Koldehoff, M.; Fisenkci, N.; Brochhagen, L.; Horn, P.A.; Krawczyk, A.; Lindemann, M. Cellular and Humoral Immunity after the Third Vaccination against SARS-CoV-2 in Hematopoietic Stem-Cell Transplant Recipients. Vaccines 2022, 10, 972. [Google Scholar] [CrossRef] [PubMed]
- Schwarzkopf, S.; Krawczyk, A.; Knop, D.; Klump, H.; Heinold, A.; Heinemann, F.M.; Thümmler, L.; Temme, C.; Breyer, M.; Witzke, O.; et al. Cellular Immunity in COVID-19 Convalescents with PCR-Confirmed Infection but with Undetectable SARS-CoV-2-Specific IgG. Emerg. Infect. Dis. 2021, 27, 122–129. [Google Scholar] [CrossRef]
- World Health Organization. Tracking SARS-CoV-2 Variants. 2022. Available online: https://www.who.int/activities/tracking-SARS-CoV-2-variants (accessed on 15 July 2022).
- Heilingloh, C.S.; Aufderhorst, U.W.; Schipper, L.; Dittmer, U.; Witzke, O.; Yang, D.; Zheng, X.; Sutter, K.; Trilling, M.; Alt, M.; et al. Susceptibility of SARS-CoV-2 to UV irradiation. Am. J. Infect. Control. 2020, 48, 1273–1275. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, M.; Klisanin, V.; Thümmler, L.; Fisenkci, N.; Tsachakis-Mück, N.; Ditschkowski, M.; Schwarzkopf, S.; Klump, H.; Reinhardt, H.C.; Horn, P.A.; et al. Humoral and Cellular Vaccination Responses against SARS-CoV-2 in Hematopoietic Stem Cell Transplant Recipients. Vaccines 2021, 9, 1075. [Google Scholar] [CrossRef] [PubMed]
- Widera, M.; Wilhelm, A.; Toptan, T.; Raffel, J.M.; Kowarz, E.; Roesmann, F.; Grözinger, F.; Siemund, A.L.; Luciano, V.; Külp, M.; et al. Generation of a Sleeping Beauty Transposon-Based Cellular System for Rapid and Sensitive Screening for Compounds and Cellular Factors Limiting SARS-CoV-2 Replication. Front. Microbiol. 2021, 12, 701198. [Google Scholar] [CrossRef] [PubMed]
- Diagnostik, I. IDK® anti-SARS-CoV-2 IgG ELISA. 2021. Available online: https://www.immundiagnostik.com/media/pages/testkits/k-5004/e859ef5a82-1656295290/k5004_2021-08-10_idk_sars-cov-2-igg.pdf (accessed on 15 July 2022).
- Schrezenmeier, E.; Rincon-Arevalo, H.; Stefanski, A.; Potekhin, A.; Staub-Hohenbleicher, H.; Choi, M.; Bachmann, F.; Proβ, V.; Hammett, C.; Schrezenmeier, H.; et al. B and T Cell Responses after a Third Dose of SARS-CoV-2 Vaccine in Kidney Transplant Recipients. J. Am. Soc. Nephrol. 2021, 32, 3027–3033. [Google Scholar] [CrossRef]
- Cao, Y.; Yisimayi, A.; Jian, F.; Song, W.; Xiao, T.; Wang, L.; Du, S.; Wang, J.; Li, Q.; Chen, X.; et al. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. Nature 2022. [Google Scholar] [CrossRef]
- Stumpf, J.; Siepmann, T.; Lindner, T.; Karger, C.; Schwöbel, J.; Anders, L.; Faulhaber-Walter, R.; Schewe, J.; Martin, H.; Schirutschke, H.; et al. Humoral and cellular immunity to SARS-CoV-2 vaccination in renal transplant versus dialysis patients: A prospective, multicenter observational study using mRNA-1273 or BNT162b2 mRNA vaccine. Lancet Reg. Health Eur. 2021, 9, 100178. [Google Scholar] [CrossRef]
- Atanackovic, D.; Luetkens, T.; Avila, S.V.; Hardy, N.M.; Lutfi, F.; Sanchez-Petitto, G.; Vander Mause, E.; Glynn, N.; Mannuel, H.D.; Alkhaldi, H.; et al. Anti-SARS-CoV-2 Immune Responses in Patients Receiving an Allogeneic Stem Cell or Organ Transplant. Vaccines 2021, 9, 737. [Google Scholar] [CrossRef]
- Lederer, K.; Bettini, E.; Parvathaneni, K.; Painter, M.M.; Agarwal, D.; Lundgreen, K.A.; Weirick, M.; Muralidharan, K.; Castaño, D.; Goel, R.R.; et al. Germinal center responses to SARS-CoV-2 mRNA vaccines in healthy and immunocompromised individuals. Cell 2022, 185, 1008–1024.e15. [Google Scholar] [CrossRef] [PubMed]
- Rincon-Arevalo, H.; Choi, M.; Stefanski, A.; Halleck, F.; Weber, U.; Szelinski, F.; Jahrsdörfer, B.; Schrezenmeier, H.; Ludwig, C.; Sattler, A.; et al. Impaired humoral immunity to SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients and dialysis patients. Sci. Immunol. 2021, 6, eabj1031. [Google Scholar] [CrossRef] [PubMed]
- Schrezenmeier, E.; Rincon-Arevalo, H.; Jens, A.; Stefanski, A.; Hammett, C.; Osmanodja, B.; Koch, N.; Zukunft, B.; Beck, J.; Oellerich, M.; et al. Temporary antimetabolite treatment hold boosts SARS-CoV-2 vaccination-specific humoral and cellular immunity in kidney transplant recipients. JCI Insight 2022, 7, e157836. [Google Scholar] [CrossRef] [PubMed]
- Addeo, A.; Shah, P.K.; Bordry, N.; Hudson, R.D.; Albracht, B.; di Marco, M.; Kaklamani, V.; Dietrich, P.; Taylor, B.S.; Simand, P.; et al. Immunogenicity of SARS-CoV-2 messenger RNA vaccines in patients with cancer. Cancer Cell 2021, 39, 1091–1098.e2. [Google Scholar] [CrossRef]
- Robinson, A.; Mazurek, A.; Xu, M.; Gong, Y. Quantitative Analysis of SARS-CoV-2 Antibody Status between Patients with Cancer and Healthy Individuals with Extended Vaccination Dosing Intervals in Canada. Curr. Oncol. 2021, 29, 68–76. [Google Scholar] [CrossRef]
- Zeng, C.; Evans, J.P.; Chakravarthy, K.; Qu, P.; Reisinger, S.; Song, N.; Rubinstein, M.P.; Shields, P.G.; Li, Z.; Liu, S. COVID-19 mRNA booster vaccines elicit strong protection against SARS-CoV-2 Omicron variant in patients with cancer. Cancer Cell 2022, 40, 117–119. [Google Scholar] [CrossRef]
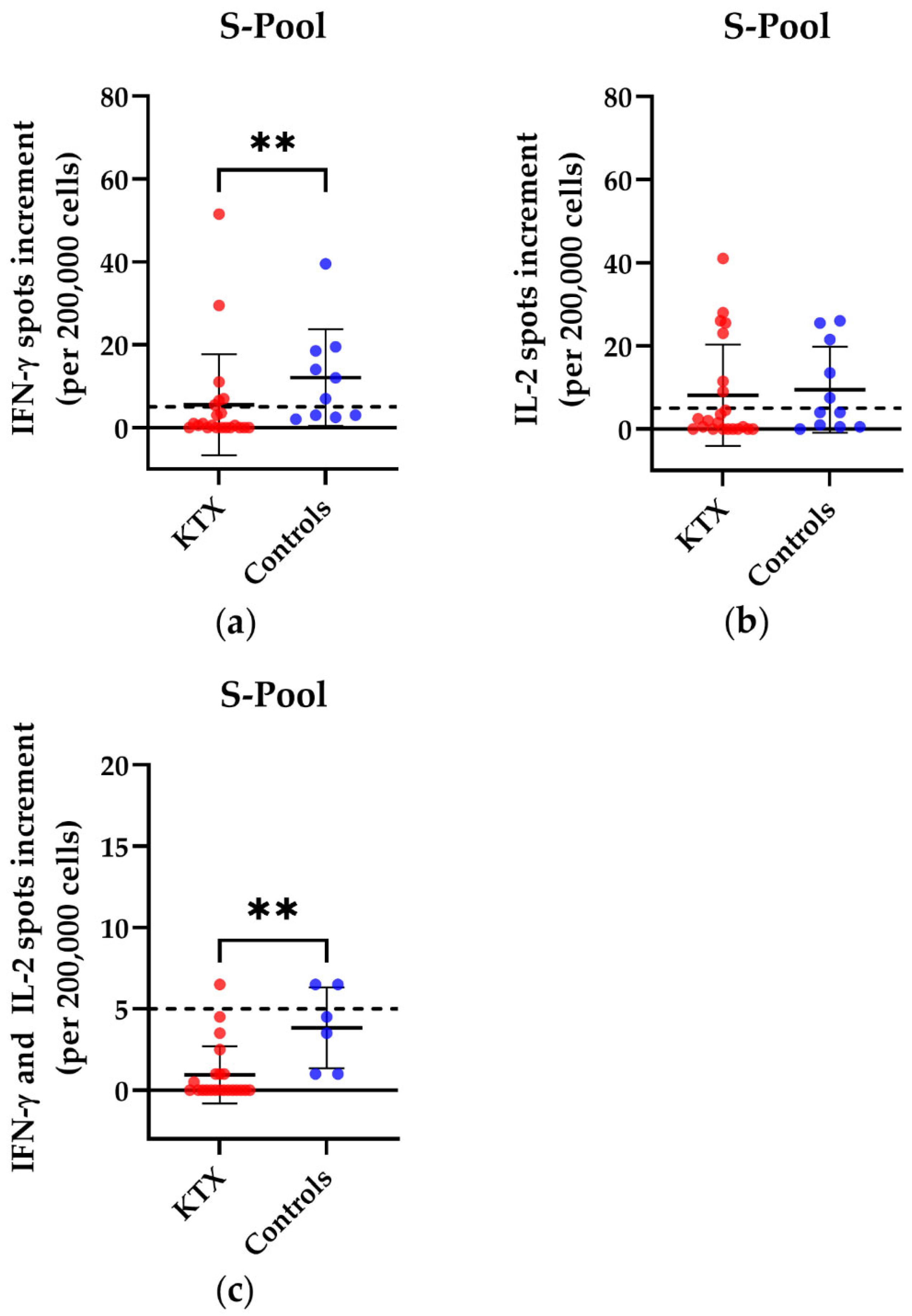
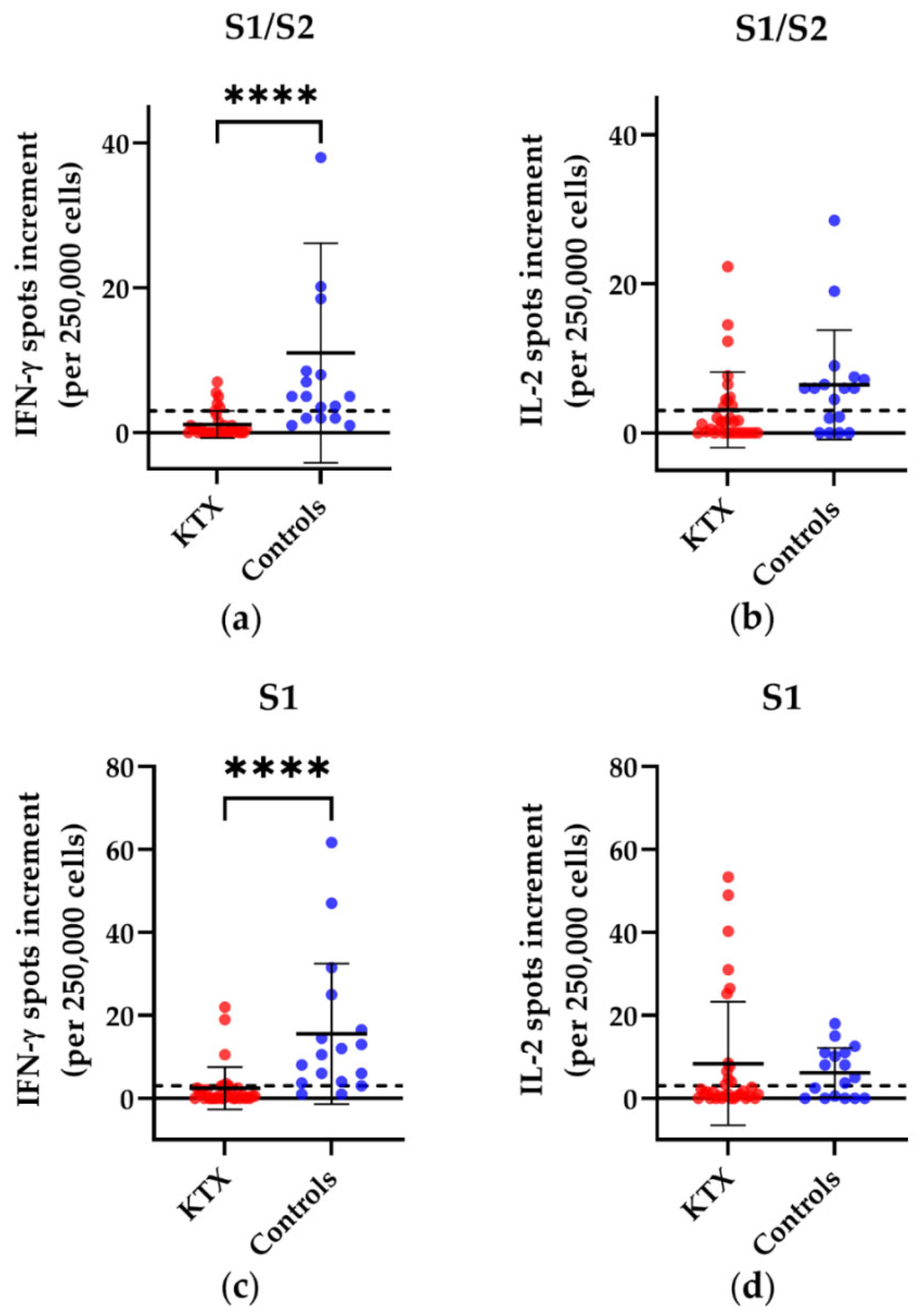
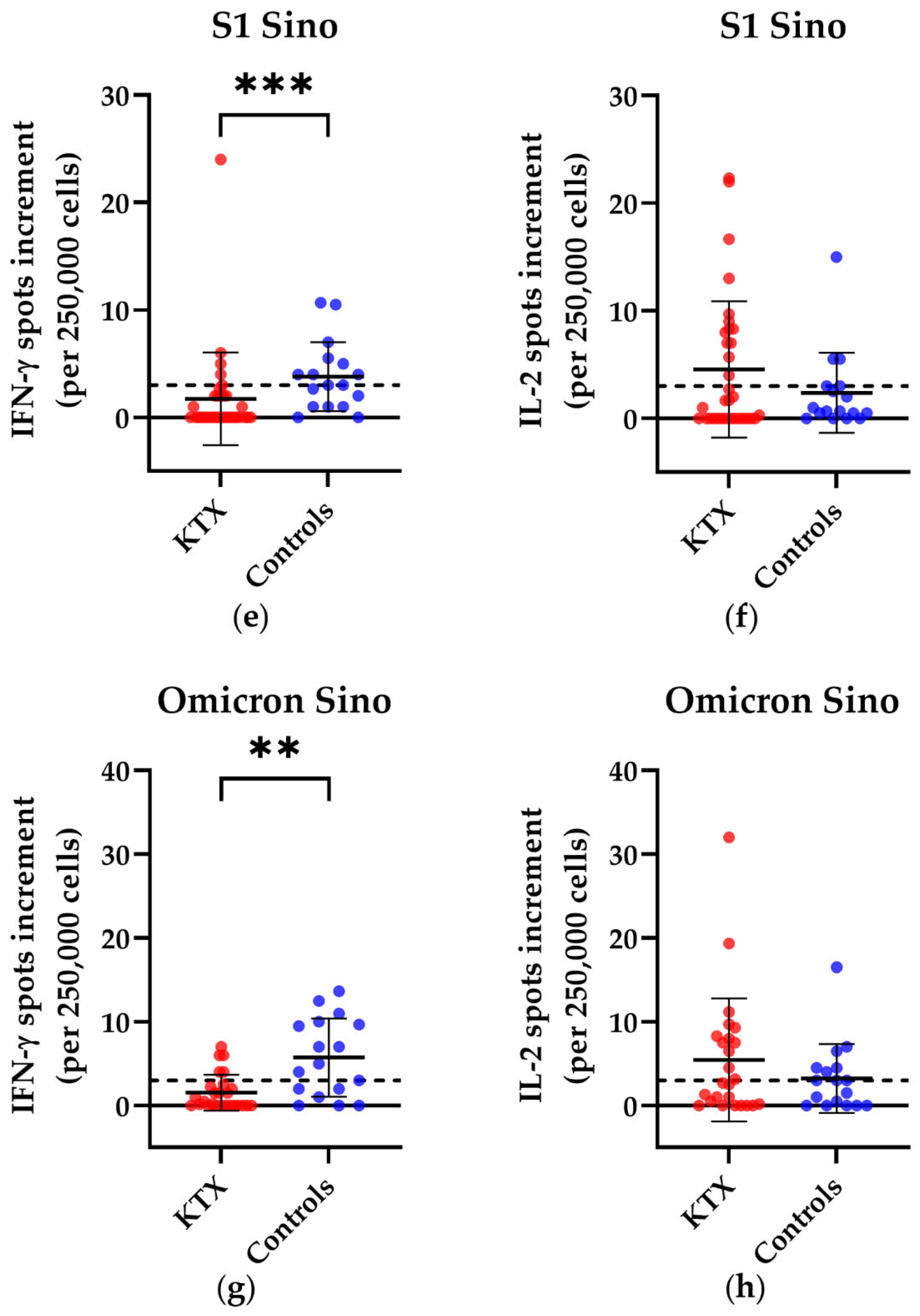
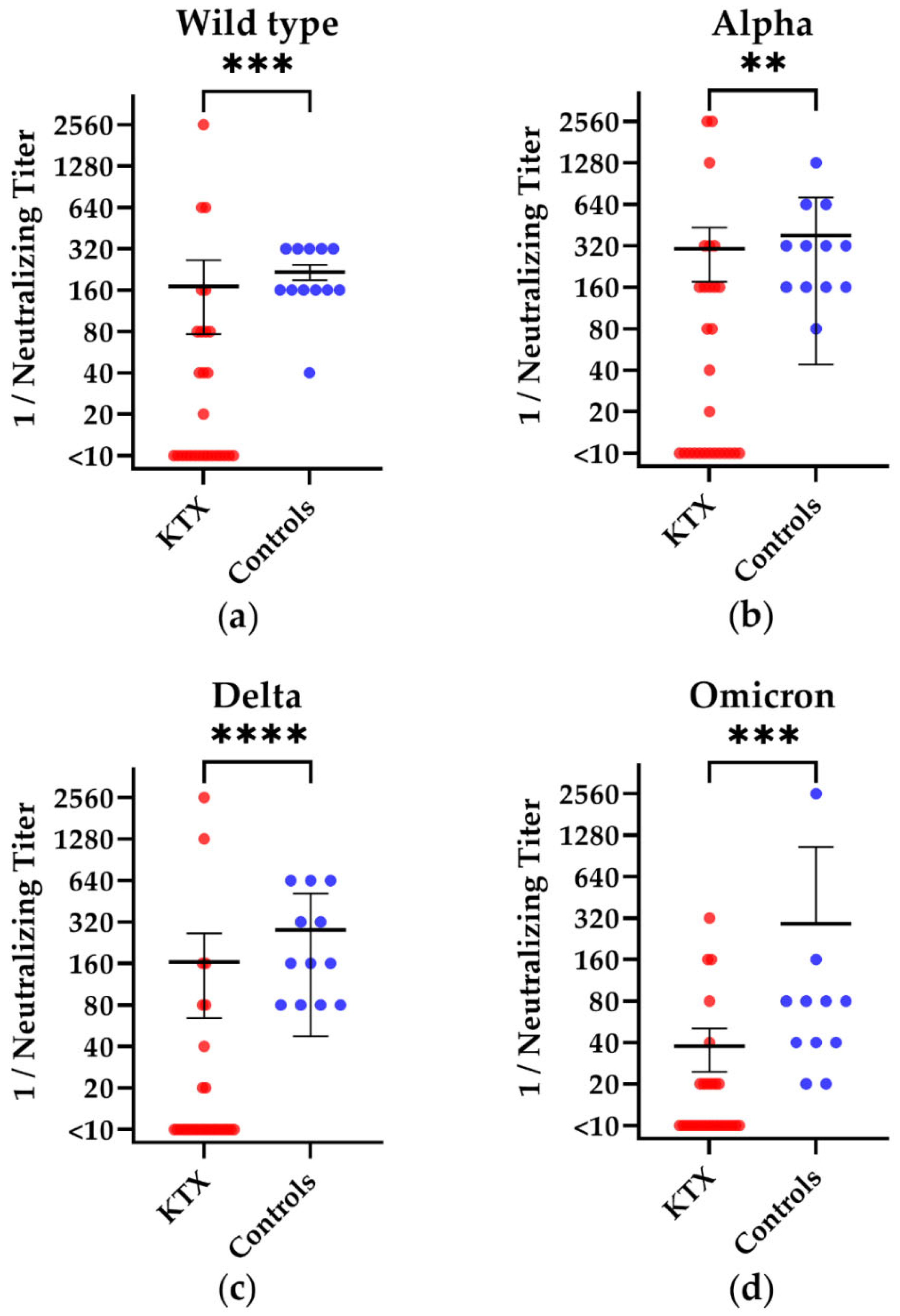
| Characteristics 1 | Kidney Transplant Recipients | Healthy Controls |
|---|---|---|
| sex | 12 males 20 females | 5 males 12 females |
| age, y | 54 (21–76) | 53 (35–65) |
| tacrolimus | 32 (100%) | Ø |
| mycophenolate | 26 (81%) | Ø |
| belatacept | 2 (6%) | Ø |
| prednisone | 32 (100%) | Ø |
| interval kidney transplantation—blood collection | 2 years (0.4–11.8) | Ø |
| interval vaccination—blood collection | 111 days (43–212) | 182 days (69–213) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thümmler, L.; Gäckler, A.; Bormann, M.; Ciesek, S.; Widera, M.; Rohn, H.; Fisenkci, N.; Otte, M.; Alt, M.; Dittmer, U.; et al. Cellular and Humoral Immunity against Different SARS-CoV-2 Variants Is Detectable but Reduced in Vaccinated Kidney Transplant Patients. Vaccines 2022, 10, 1348. https://doi.org/10.3390/vaccines10081348
Thümmler L, Gäckler A, Bormann M, Ciesek S, Widera M, Rohn H, Fisenkci N, Otte M, Alt M, Dittmer U, et al. Cellular and Humoral Immunity against Different SARS-CoV-2 Variants Is Detectable but Reduced in Vaccinated Kidney Transplant Patients. Vaccines. 2022; 10(8):1348. https://doi.org/10.3390/vaccines10081348
Chicago/Turabian StyleThümmler, Laura, Anja Gäckler, Maren Bormann, Sandra Ciesek, Marek Widera, Hana Rohn, Neslinur Fisenkci, Mona Otte, Mira Alt, Ulf Dittmer, and et al. 2022. "Cellular and Humoral Immunity against Different SARS-CoV-2 Variants Is Detectable but Reduced in Vaccinated Kidney Transplant Patients" Vaccines 10, no. 8: 1348. https://doi.org/10.3390/vaccines10081348
APA StyleThümmler, L., Gäckler, A., Bormann, M., Ciesek, S., Widera, M., Rohn, H., Fisenkci, N., Otte, M., Alt, M., Dittmer, U., Horn, P. A., Witzke, O., Krawczyk, A., & Lindemann, M. (2022). Cellular and Humoral Immunity against Different SARS-CoV-2 Variants Is Detectable but Reduced in Vaccinated Kidney Transplant Patients. Vaccines, 10(8), 1348. https://doi.org/10.3390/vaccines10081348







