Dynamics of Antibody and T Cell Immunity against SARS-CoV-2 Variants of Concern and the Impact of Booster Vaccinations in Previously Infected and Infection-Naïve Individuals
Abstract
1. Introduction
2. Materials and Methods
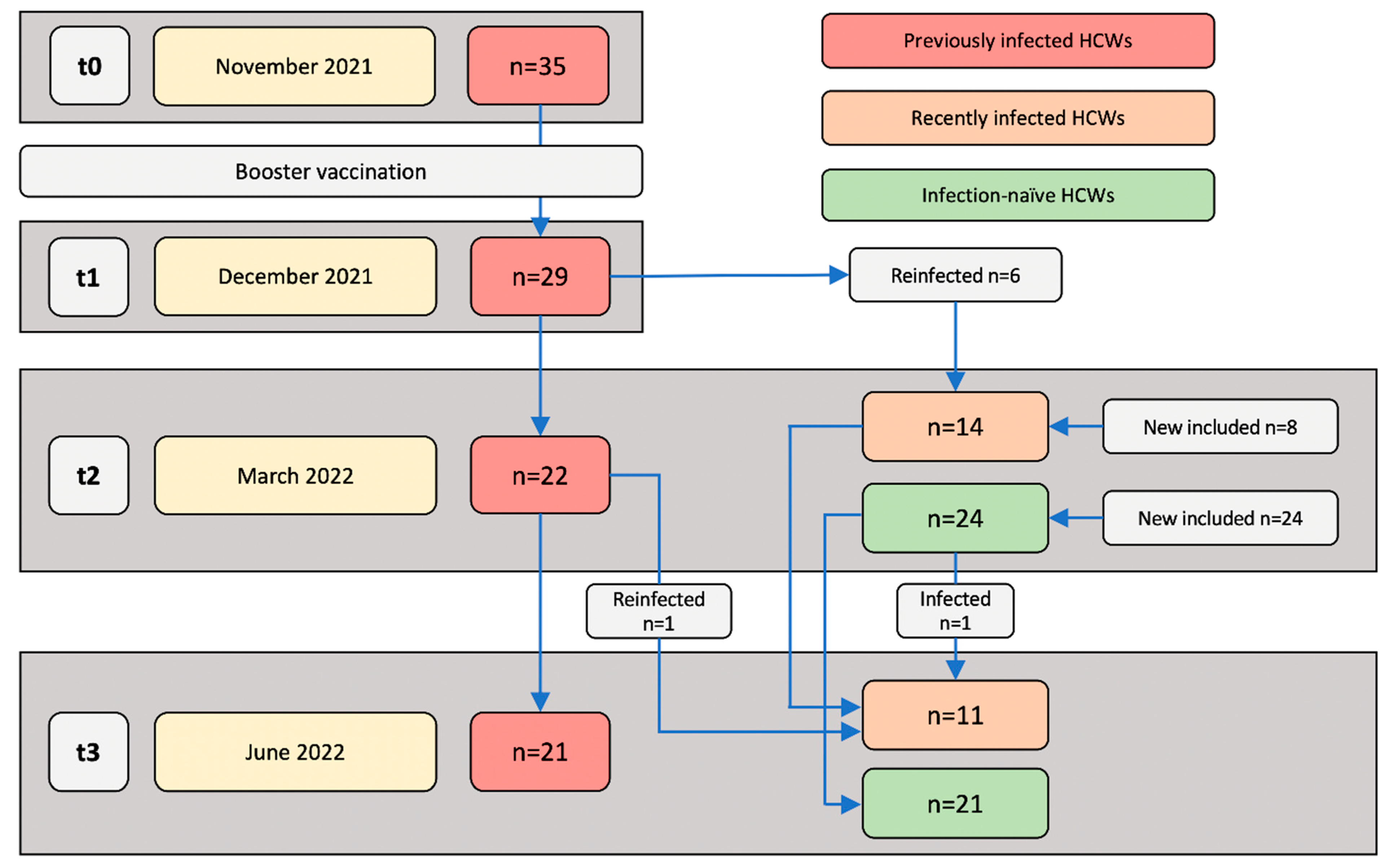
2.1. Study Design
2.2. PBMC and Serum Isolation
2.3. SARS-CoV-2 S1 and N Interferon-Gamma (IFN-γ) ELIspot
2.4. SARS-CoV-2 Variant IFN-γ ELIspot
2.5. ELIspot Image Processing and Spot Quantification
2.6. SARS-CoV-2 Anti-RBD IgG Quantitative ELISA
2.7. SARS-CoV-2 IgG Surrogate Virus Neutralization Assay
2.8. SARS-CoV-2 Anti-N IgG Qualitative CMIA
2.9. Statistical Analysis
3. Results
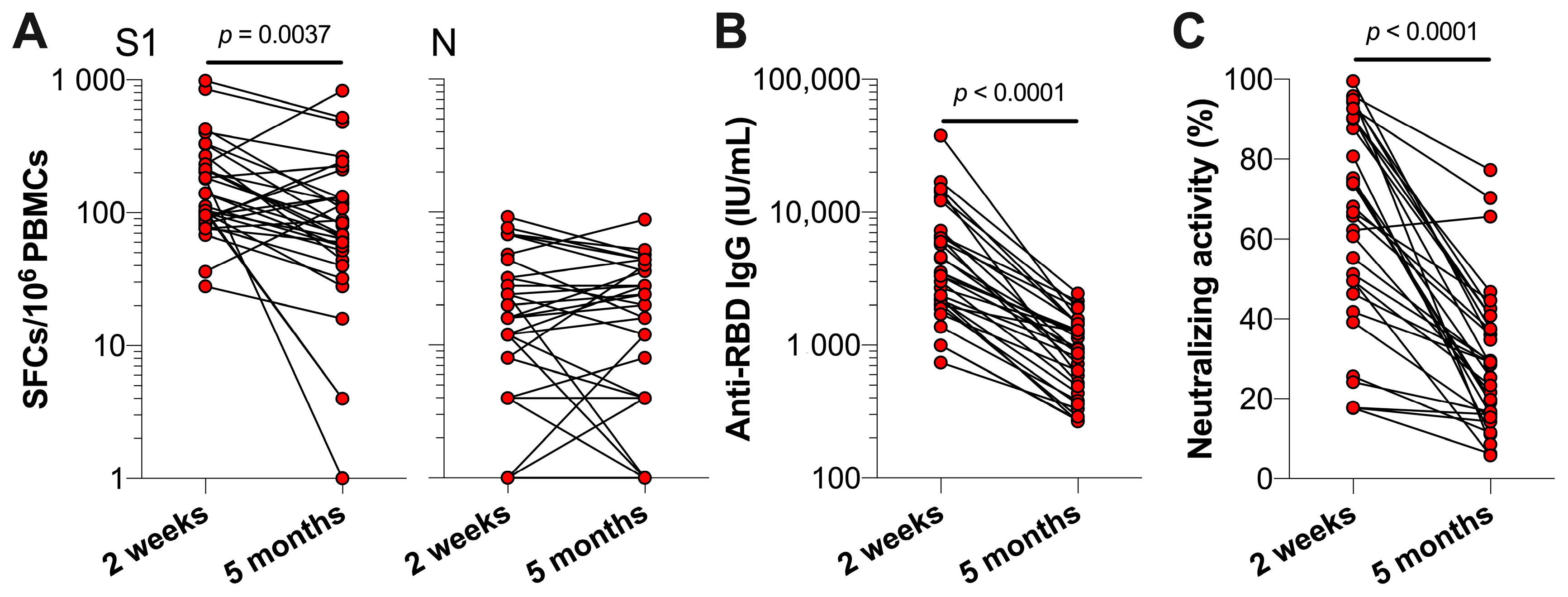
3.1. SARS-CoV-2 Specific T Cell and Antibody Responses Five Months Post-Primary COVID-19 Vaccinations
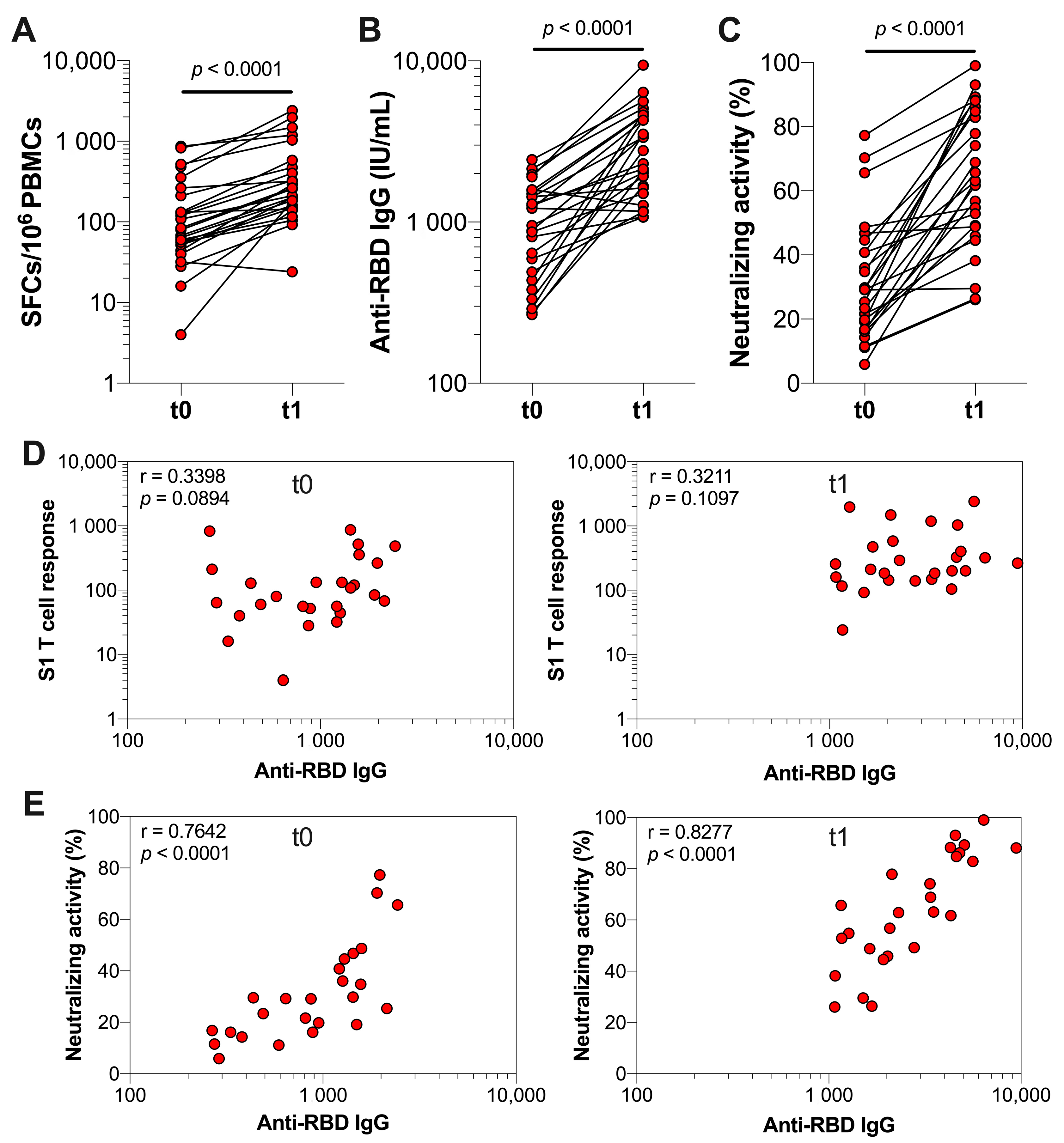
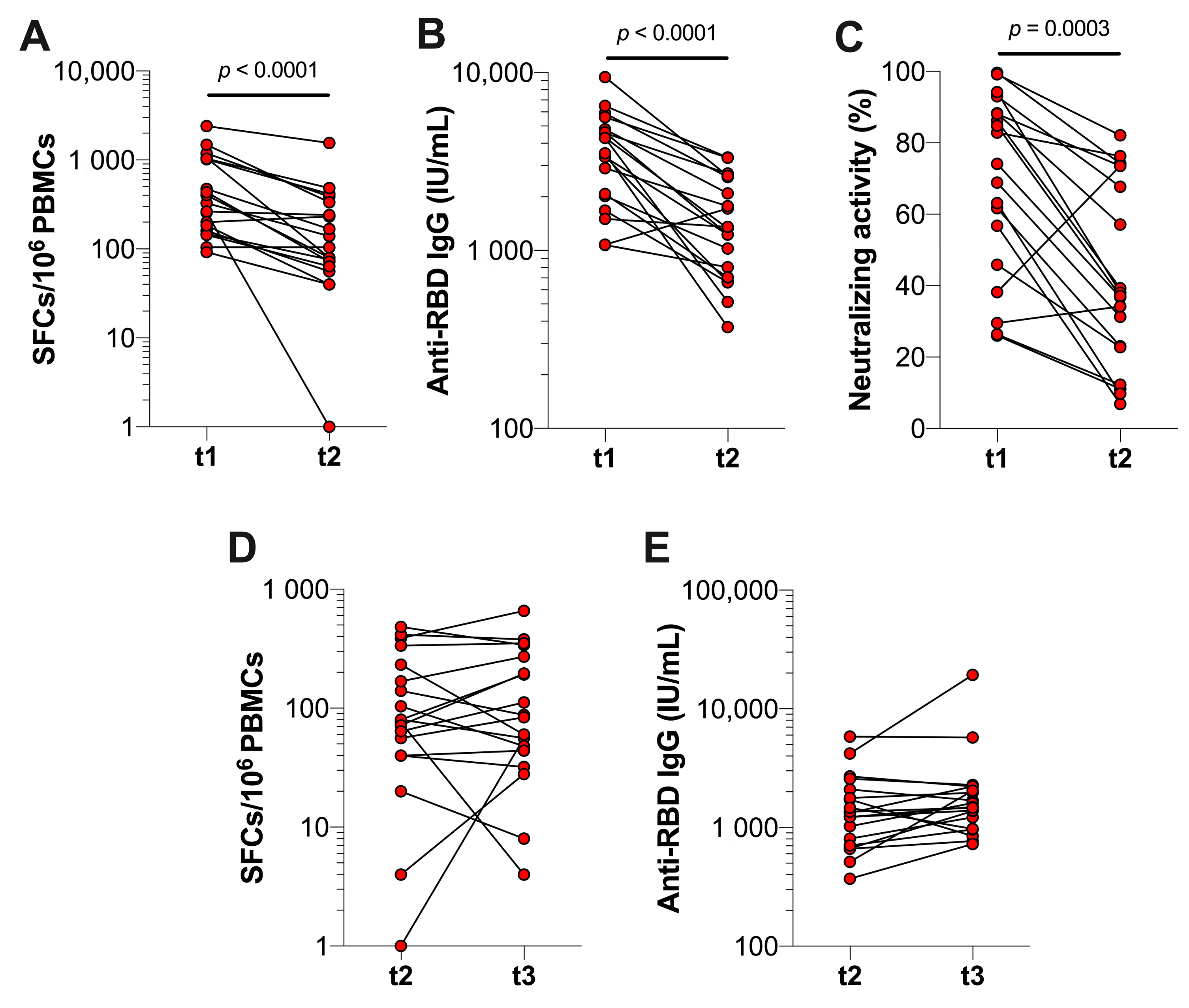
3.2. The Effect of Booster Vaccination in Previously Infected Individuals
3.3. Durability of SARS-CoV-2-Specific T Cell and Antibody Responses after Booster Vaccination in Previously Infected Individuals
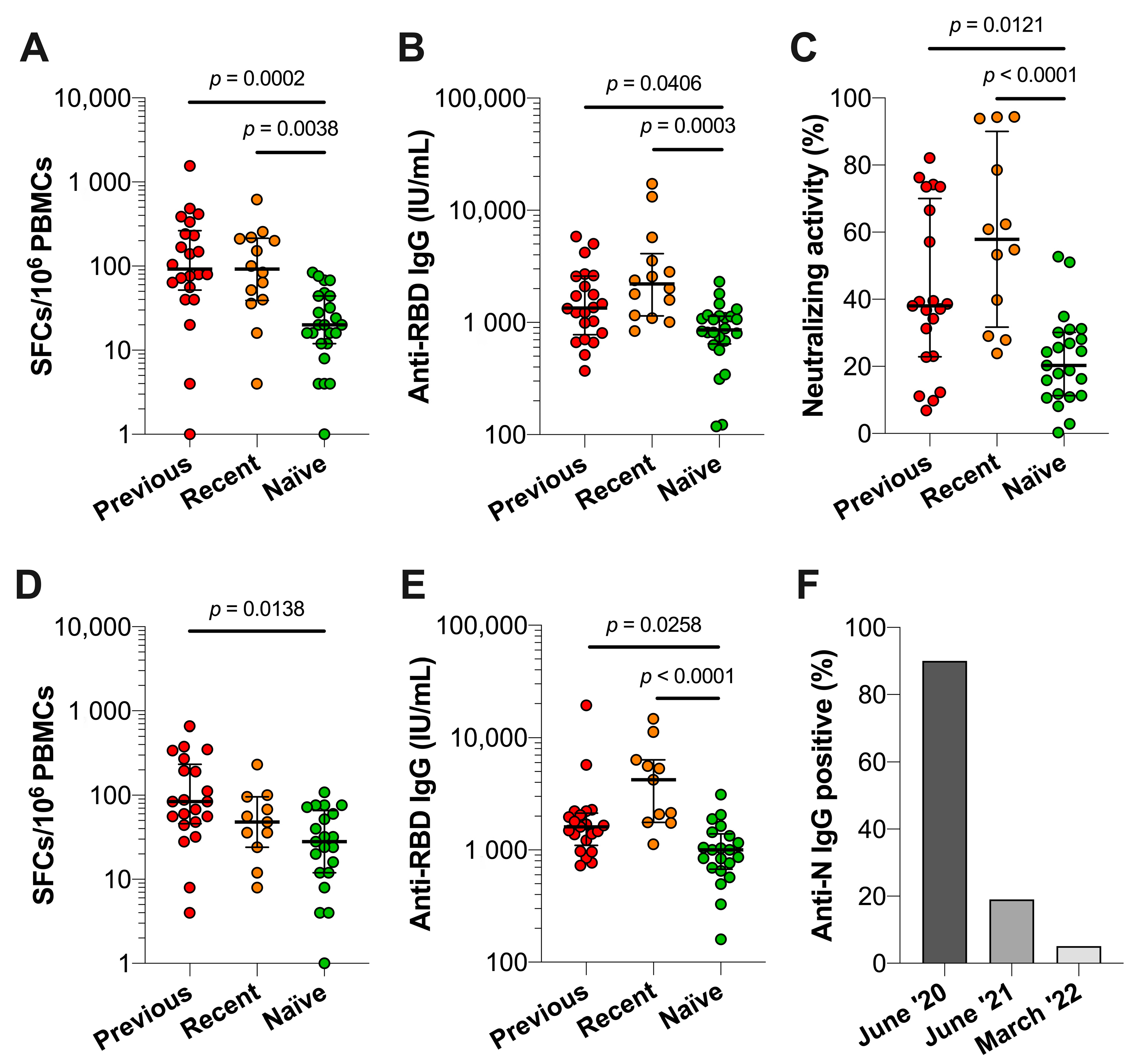
3.4. Comparison of Immune Responses between Previously Infected, Recently Infected and Infection-Naïve Individuals
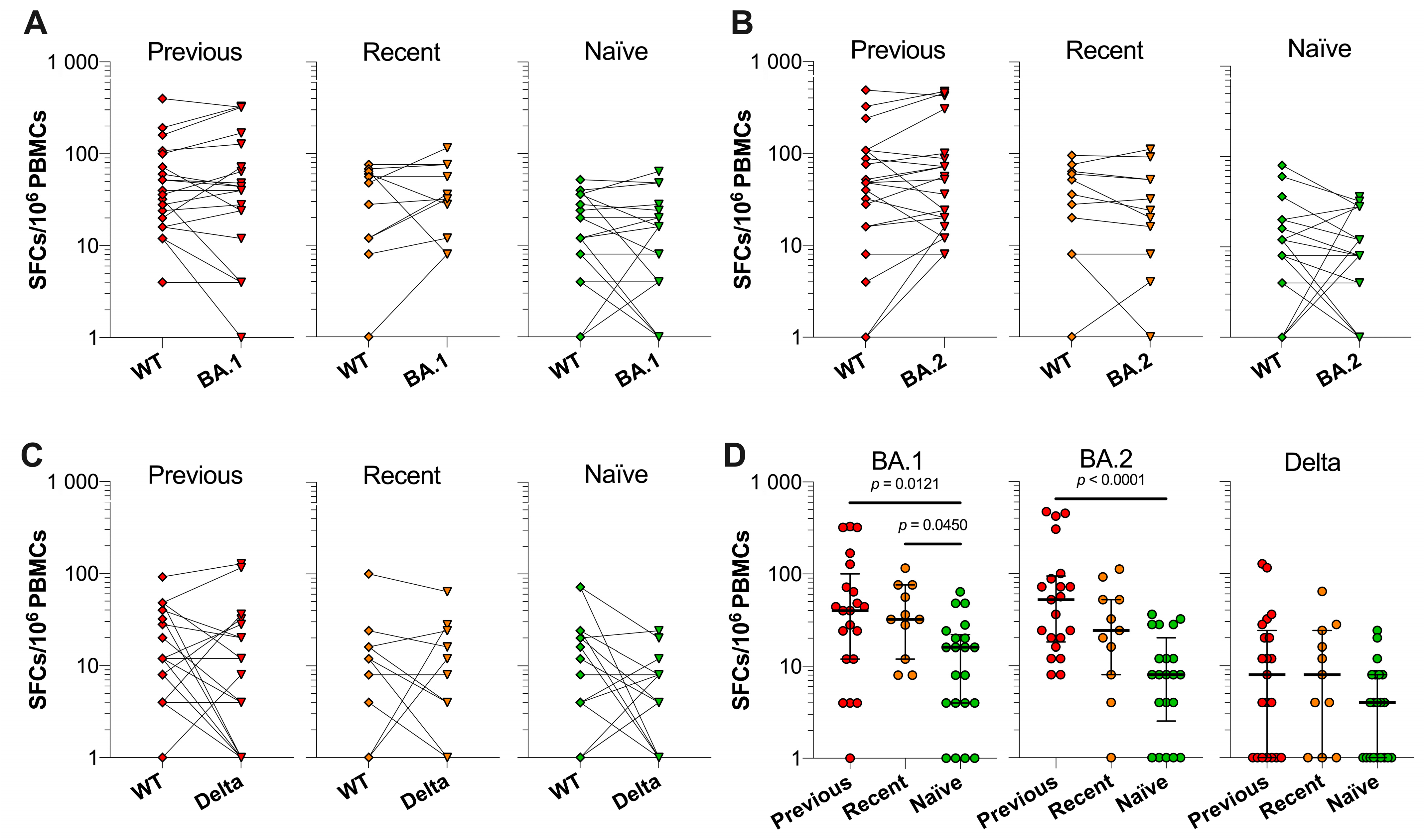
3.5. SARS-CoV-2 VOC Spike-Specific T Cell Responses in Previously Infected, Recently Infected and Infection-Naïve Individuals
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. General’s Opening Remarks at the Media Briefing on COVID-19. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19 (accessed on 1 November 2022).
- Hacisuleyman, E.; Hale, C.; Saito, Y.; Blachere, N.E.; Bergh, M.; Conlon, E.G.; Schaefer-Babajew, D.J.; DaSilva, J.; Muecksch, F.; Gaebler, C.; et al. Vaccine Breakthrough Infections with SARS-CoV-2 Variants. N. Engl. J. Med. 2021, 384, 2212–2218. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. COVID-19 Weekly Epidemiological Update, 105th ed.; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Nordström, P.; Ballin, M.; Nordström, A. Risk of SARS-CoV-2 Reinfection and COVID-19 Hospitalisation in Individuals with Natural and Hybrid Immunity: A Retrospective, Total Population Cohort Study in Sweden. Lancet Infect. Dis. 2022, 22, 781–790. [Google Scholar] [CrossRef]
- Lau, E.H.Y.; Tsang, O.T.Y.; Hui, D.S.C.; Kwan, M.Y.W.; Chan, W.-H.; Chiu, S.S.; Ko, R.L.W.; Chan, K.H.; Cheng, S.M.S.; Perera, R.A.P.M.; et al. Neutralizing Antibody Titres in SARS-CoV-2 Infections. Nat. Commun. 2021, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ong, D.S.Y.; Fragkou, P.C.; Schweitzer, V.A.; Chemaly, R.F.; Moschopoulos, C.D.; Skevaki, C. How to Interpret and Use COVID-19 Serology and Immunology Tests. Clin. Microbiol. Infect. 2021, 27, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Liu, B.; Wang, X.; Zhang, L. The Humoral Response and Antibodies against SARS-CoV-2 Infection. Nat. Immunol. 2022, 23, 1008–1020. [Google Scholar] [CrossRef]
- Moss, P. The T Cell Immune Response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef]
- Mak, W.A.; Koeleman, J.G.M.; van der Vliet, M.; Keuren, F.; Ong, D.S.Y. SARS-CoV-2 Antibody and T Cell Responses One Year after COVID-19 and the Booster Effect of Vaccination: A Prospective Cohort Study. J. Infect. 2022, 84, 171–178. [Google Scholar] [CrossRef]
- Demaret, J.; Lefèvre, G.; Vuotto, F.; Trauet, J.; Duhamel, A.; Labreuche, J.; Varlet, P.; Dendooven, A.; Stabler, S.; Gachet, B.; et al. Severe SARS-CoV-2 Patients Develop a Higher Specific T-Cell Response. Clin. Transl. Immunol. 2020, 9, 1–14. [Google Scholar] [CrossRef]
- Yao, L.; Wang, G.-L.; Shen, Y.; Wang, Z.-Y.; Zhan, B.-D.; Duan, L.-J.; Lu, B.; Shi, C.; Gao, Y.-M.; Peng, H.-H.; et al. Persistence of Antibody and Cellular Immune Responses in Coronavirus Disease 2019 Patients Over Nine Months After Infection. J. Infect. Dis. 2021, 224, 586–594. [Google Scholar] [CrossRef]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological Memory to SARS-CoV-2 Assessed for up to 8 Months after Infection. Science 2021, 371, 1–22. [Google Scholar] [CrossRef]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489–1501. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Alós, L.; Armenteros, J.J.A.; Madsen, J.R.; Hansen, C.B.; Jarlhelt, I.; Hamm, S.R.; Heftdal, L.D.; Pries-Heje, M.M.; Møller, D.L.; Fogh, K.; et al. Modeling of Waning Immunity after SARS-CoV-2 Vaccination and Influencing Factors. Nat. Commun. 2022, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Marcotte, H.; Piralla, A.; Zuo, F.; Du, L.; Cassaniti, I.; Wan, H.; Kumagai-Braesh, M.; Andréll, J.; Percivalle, E.; Sammartino, J.C.; et al. Immunity to SARS-CoV-2 up to 15 Months after Infection. iScience 2022, 25, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Muecksch, F.; Schaefer-Babajew, D.; Finkin, S.; Viant, C.; Gaebler, C.; Hoffmann, H.H.; Barnes, C.O.; Cipolla, M.; Ramos, V.; et al. Naturally Enhanced Neutralizing Breadth against SARS-CoV-2 One Year after Infection. Nature 2021, 595, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Eythorsson, E.; Runolfsdottir, H.L.; Ingvarsson, R.F.; Sigurdsson, M.I.; Palsson, R. Rate of SARS-CoV-2 Reinfection During an Omicron Wave in Iceland. JAMA Netw. Open 2022, 5, 1–4. [Google Scholar] [CrossRef]
- Pilz, S.; Theiler-Schwetz, V.; Trummer, C.; Krause, R.; Ioannidis, J.P.A. SARS-CoV-2 Reinfections: Overview of Efficacy and Duration of Natural and Hybrid Immunity. Environ. Res. 2022, 209, 1–10. [Google Scholar] [CrossRef]
- Jeffery-Smith, A.; Rowland, T.A.J.; Patel, M.; Whitaker, H.; Iyanger, N.; Williams, S.V.; Giddings, R.; Thompson, L.; Zavala, M.; Aiano, F.; et al. Reinfection with New Variants of SARS-CoV-2 after Natural Infection: A Prospective Observational Cohort in 13 Care Homes in England. Lancet Health Longev. 2021, 2, e811–e819. [Google Scholar] [CrossRef]
- Bernal, J.L.; Andrews, N.; Gower, C.; Gallagher, E.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; Groves, N.; Dabrera, G.; et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. N. Engl. J. Med. 2021, 385, 585–594. [Google Scholar] [CrossRef]
- Planas, D.; Veyer, D.; Baidaliuk, A.; Staropoli, I.; Guivel-Benhassine, F.; Rajah, M.M.; Planchais, C.; Porrot, F.; Robillard, N.; Puech, J.; et al. Reduced Sensitivity of SARS-CoV-2 Variant Delta to Antibody Neutralization. Nature 2021, 596, 276–280. [Google Scholar] [CrossRef]
- Geers, D.; Shamier, M.C.; Bogers, S.; Den Hartog, G.; Gommers, L.; Nella, N.; Schmitz, K.S.; Rijsbergen, L.C.; Van Osch, J.A.T.; Dijkhuizen, E.; et al. SARS-CoV-2 Variants of Concern Partially Escape Humoral but Not T-Cell Responses in COVID-19 Convalescent Donors and Vaccinees. Sci. Immunol. 2021, 6, 1–22. [Google Scholar] [CrossRef]
- Khan, K.; Karim, F.; Ganga, Y.; Bernstein, M.; Jule, Z.; Reedoy, K.; Cele, S.; Lustig, G.; Amoako, D.; Wolter, N.; et al. Omicron BA. 4/BA.5 Escape Neutralizing Immunity Elicited by BA.1 Infection. Nat. Commun. 2022, 13, 1–7. [Google Scholar] [CrossRef]
- Kliker, L.; Zuckerman, N.; Atari, N.; Barda, N.; Gilboa, M.; Nemet, I.; Abd Elkader, B.; Fratty, I.S.; Jaber, H.; Mendelson, E.; et al. COVID-19 Vaccination and BA.1 Breakthrough Infection Induce Neutralising Antibodies Which Are Less Efficient against BA.4 and BA.5 Omicron Variants, Israel, March to June 2022. Eurosurveillance 2022, 27, 2200559. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Zhu, K.-L.; Jiang, X.-L.; Wang, X.-J.; Zhan, B.-D.; Gao, H.-X.; Geng, X.-Y.; Duan, L.-J.; Dai, E.-H.; Ma, M.-J. Omicron Subvariants Escape Antibodies Elicited by Vaccination and BA.2.2 Infection. Lancet Infect. Dis. 2022, 22, 1116–1117. [Google Scholar] [CrossRef]
- Altarawneh, H.N.; Chemaitelly, H.; Ayoub, H.H.; Hasan, M.R.; Coyle, P.; Yassine, H.M.; Al-Khatib, H.A.; Smatti, M.K.; Al-Kanaani, Z.; Al-Kuwari, E.; et al. Protective Effect of Previous SARS-CoV-2 Infection against Omicron BA.4 or BA.5 Subvariants. N. Engl. J. Med. 2022, 387, 1618–1620. [Google Scholar] [CrossRef]
- Ong, D.S.Y.; Keuren, F.; van der Vliet, M.; Boxma-de Klerk, B.M.; Koeleman, J.G.M. SARS-CoV-2 Antibody Response Dynamics and Heterogeneous Diagnostic Performance of Four Serological Tests and a Neutralization Test in Symptomatic Healthcare Workers with Non-Severe COVID-19. J. Clin. Virol. 2021, 141, 104904. [Google Scholar] [CrossRef] [PubMed]
- Mak, W.A.; Koeleman, J.G.M.; Ong, D.S.Y. Development of an In-House SARS-CoV-2 Interferon-Gamma ELISpot and Plate Reader-Free Spot Detection Method. J. Virol. Methods 2022, 300, 114398. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Evans, J.P.; Zeng, C.; Carlin, C.; Lozanski, G.; Saif, L.J.; Oltz, E.M.; Gumina, R.J.; Liu, S. Neutralizing Antibody Responses Elicited by SARS-CoV-2 MRNA Vaccination Wane over Time and Are Boosted by Breakthrough Infection. Sci. Transl. Med. 2022, 14, eabn8057. [Google Scholar] [CrossRef]
- Busà, R.; Sorrentino, M.C.; Russelli, G.; Amico, G.; Miceli, V.; Miele, M.; Di Bella, M.; Timoneri, F.; Gallo, A.; Zito, G.; et al. Specific Anti-SARS-CoV-2 Humoral and Cellular Immune Responses After Booster Dose of BNT162b2 Pfizer-BioNTech MRNA-Based Vaccine: Integrated Study of Adaptive Immune System Components. Front. Immunol. 2022, 13, 856657. [Google Scholar] [CrossRef]
- Takeuchi, J.S.; Fukunaga, A.; Yamamoto, S.; Tanaka, A.; Matsuda, K.; Kimura, M.; Kamikawa, A.; Kito, Y.; Maeda, K.; Ueda, G.; et al. SARS-CoV-2 Specific T Cell and Humoral Immune Responses upon Vaccination with BNT162b2: A 9 Months Longitudinal Study. Sci. Rep. 2022, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gilboa, M.; Regev-yochay, G.; Mandelboim, M.; Indenbaum, V.; Asraf, K.; Fluss, R.; Amit, S.; Mendelson, E.; Doolman, R.; Afek, A.; et al. Durability of Immune Response After COVID-19 Booster Vaccination and Association With COVID-19 Omicron Infection. JAMA Netw. Open 2022, 5, e2231778. [Google Scholar] [CrossRef] [PubMed]
- Alidjinou, E.K.; Demaret, J.; Faure, K.; Deplanque, D.; Bocket, L.; Duhamel, A.; Sobaszek, A.; Hober, D.; Hisbergues, M.; Puisieux, F.; et al. Immunogenicity of BNT162b2 Vaccine Booster against SARS-CoV-2 Delta and Omicron Variants in Nursing Home Residents: A Prospective Observational Study in Older Adults Aged from 68 to 98 Years. lancet Reg. Health 2022, 17, 100385. [Google Scholar] [CrossRef] [PubMed]
- Lipsitch, M.; Krammer, F.; Regev-yochay, G.; Lustig, Y.; Balicer, R.D. SARS-CoV-2 Breakthrough Infections in Vaccinated Individuals: Measurement, Causes and Impact. Nat. Rev. Immunol. 2022, 22, 57–65. [Google Scholar] [CrossRef]
- Kuhlmann, C.; Mayer, C.K.; Claassen, M.; Maponga, T.; Burgers, W.A.; Keeton, R.; Riou, C.; Sutherland, A.D.; Suliman, T.; Shaw, M.L.; et al. Breakthrough Infections with SARS-CoV-2 Omicron despite MRNA Vaccine Booster Dose. Lancet 2022, 399, 625–626. [Google Scholar] [CrossRef]
- Tarke, A.; Coelho, C.H.; Zhang, Z.; Dan, J.M.; Yu, E.D.; Methot, N.; Bloom, N.I.; Goodwin, B.; Phillips, E.; Mallal, S.; et al. SARS-CoV-2 Vaccination Induces Immunological T Cell Memory Able to Cross-Recognize Variants from Alpha to Omicron. Cell 2022, 185, 847–859. [Google Scholar] [CrossRef]
- Keeton, R.; Tincho, M.B.; Ngomti, A.; Baguma, R.; Benede, N.; Suzuki, A.; Khan, K.; Cele, S.; Bernstein, M.; Karim, F.; et al. T Cell Responses to SARS-CoV-2 Spike Cross-Recognize Omicron. Nature 2022, 603, 488–492. [Google Scholar] [CrossRef]
- GeurtsvanKessel, C.H.; Geers, D.; Schmitz, K.S.; Mykytyn, A.Z.; Lamers, M.M.; Bogers, S.; Scherbeijn, S.; Gommers, L.; Sablerolles, R.S.G.; Nieuwkoop, N.N.; et al. Divergent SARS-CoV-2 Omicron-Reactive T and B Cell Responses in COVID-19 Vaccine Recipients. Sci. Immunol. 2022, 7, eabo2202. [Google Scholar] [CrossRef]
- Gao, Y.; Cai, C.; Grifoni, A.; Müller, T.R.; Niessl, J.; Olofsson, A.; Humbert, M.; Hansson, L.; Österborg, A.; Bergman, P.; et al. Ancestral SARS-CoV-2-Specific T Cells Cross-Recognize the Omicron Variant. Nat. Med. 2022, 28, 472–476. [Google Scholar] [CrossRef]
- Karsten, H.; Cords, L.; Westphal, T.; Knapp, M.; Brehm, T.T.; Hermanussen, L.; Omansen, T.F.; Schmiedel, S.; Woost, R.; Ditt, V.; et al. High-resolution Analysis of Individual Spike Peptide-specific CD4+ T-cell Responses in Vaccine Recipients and COVID-19 Patients. Clin. Transl. Immunol. 2022, 11, e1410. [Google Scholar] [CrossRef]
- Gallais, F.; Gantner, P.; Bruel, T.; Velay, A.; Planas, D.; Wendling, M.J.; Bayer, S.; Solis, M.; Laugel, E.; Reix, N.; et al. Evolution of Antibody Responses up to 13 Months after SARS-CoV-2 Infection and Risk of Reinfection. EBioMedicine 2021, 71, 103561. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Stellas, D.; Rosati, M.; Sergentanis, T.N.; Hu, X.; Politou, M.; Pappa, V.; Ntanasis-stathopoulos, I.; Karaliota, S.; Bear, J.; et al. SARS-CoV-2 Antibody Kinetics Eight Months from COVID-19 Onset: Persistence of Spike Antibodies but Loss of Neutralizing Antibodies in 24% of Convalescent Plasma Donors. Eur. J. Intern. Med. 2021, 89, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Ortega, N.; Ribes, M.; Vidal, M.; Rubio, R.; Aguilar, R.; Williams, S.; Barrios, D.; Alonso, S.; Hernández-luis, P.; Mitchell, R.A.; et al. Seven-Month Kinetics of SARS-CoV-2 Antibodies and Role of Pre-Existing Antibodies to Human Coronaviruses. Nat. Commun. 2021, 12, 1–10. [Google Scholar] [CrossRef]
- Villemonteix, J.; Cohen, L.; Guihot, A.; Guérin, V.; Bonacorsi, S.; Carcelain, G. Comparison between Enzyme-linked Immunospot Assay and Intracellular Cytokine Flow Cytometry Assays for the Evaluation of T Cell Response to SARS-CoV-2 after Symptomatic COVID-19. Immun. Inflamm. Dis. 2022, 10, e617. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Cao, Y.; Zhang, S.; Zhang, Z. Single-Cell Analysis of the Adaptive Immune Response to SARS-CoV-2 Infection and Vaccination. Front. Immunol. 2022, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faas, M.R.; Mak, W.A.; Markus, H.Y.; van der Zwan, E.M.; van der Vliet, M.; Koeleman, J.G.M.; Ong, D.S.Y. Dynamics of Antibody and T Cell Immunity against SARS-CoV-2 Variants of Concern and the Impact of Booster Vaccinations in Previously Infected and Infection-Naïve Individuals. Vaccines 2022, 10, 2132. https://doi.org/10.3390/vaccines10122132
Faas MR, Mak WA, Markus HY, van der Zwan EM, van der Vliet M, Koeleman JGM, Ong DSY. Dynamics of Antibody and T Cell Immunity against SARS-CoV-2 Variants of Concern and the Impact of Booster Vaccinations in Previously Infected and Infection-Naïve Individuals. Vaccines. 2022; 10(12):2132. https://doi.org/10.3390/vaccines10122132
Chicago/Turabian StyleFaas, Michel R., Willem A. Mak, Hilde Y. Markus, Ellen M. van der Zwan, Marijke van der Vliet, Johannes G. M. Koeleman, and David S. Y. Ong. 2022. "Dynamics of Antibody and T Cell Immunity against SARS-CoV-2 Variants of Concern and the Impact of Booster Vaccinations in Previously Infected and Infection-Naïve Individuals" Vaccines 10, no. 12: 2132. https://doi.org/10.3390/vaccines10122132
APA StyleFaas, M. R., Mak, W. A., Markus, H. Y., van der Zwan, E. M., van der Vliet, M., Koeleman, J. G. M., & Ong, D. S. Y. (2022). Dynamics of Antibody and T Cell Immunity against SARS-CoV-2 Variants of Concern and the Impact of Booster Vaccinations in Previously Infected and Infection-Naïve Individuals. Vaccines, 10(12), 2132. https://doi.org/10.3390/vaccines10122132








