Outcomes Assessment of Sustainable and Innovatively Simple Lifestyle Modification at the Workplace-Drinking Electrolyzed-Reduced Water (OASIS-ERW): A Randomized, Double-Blind, Placebo-Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of the Study Design
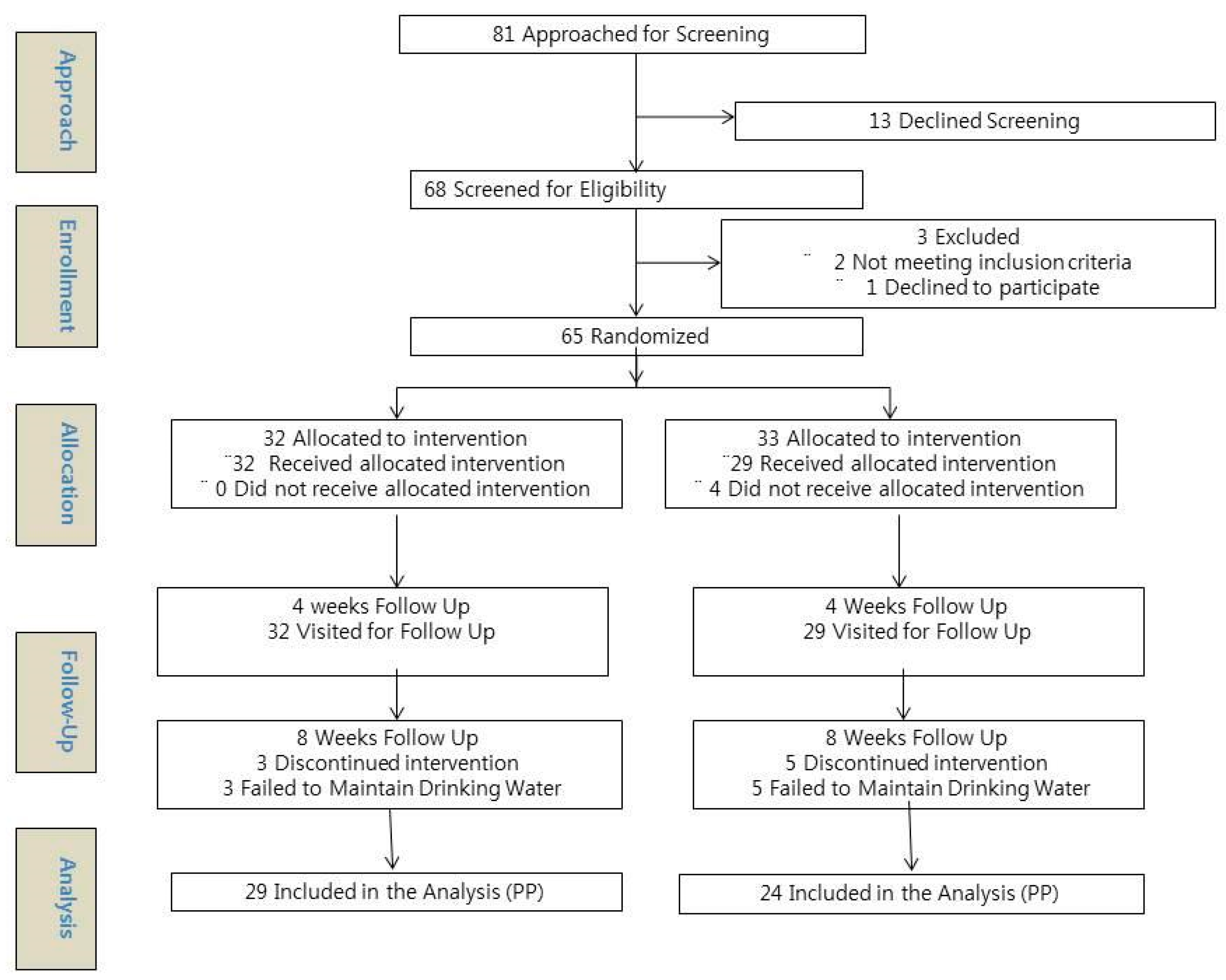
2.2. Participants, Eligibility Criteria, and Recruitment
2.3. Study Setting and Interventions
2.4. Outcome Variables
2.5. Sample Size
2.6. Randomization, Allocation, and Blinding
2.7. Measurement of Outcome Variables
2.7.1. Biomarkers of Oxidative Stress
2.7.2. Biochemistry Parameters
2.7.3. Activity of NK Cells
2.7.4. Advanced Glycation End Products (AGEs)
2.7.5. Cardio-Ankle Vascular Index (CAVI)
2.7.6. Heart Rate Variability (HRV)
2.7.7. PhA and Body Composition
2.7.8. Blood Pressure and Pulse Rate
2.7.9. Questionnaires to Evaluate Stress, Fatigue, and HRQoL
2.8. Data Analysis
3. Results
3.1. Baseline Characteristics of Study Participants
3.2. Effect of Treatment on the Primary Outcome Variables: Biomarkers of Oxidative Stress
3.3. Effect of Treatment on the Secondary Outcome Variables: Biochemistry Parameters
3.4. Effect of Treatment on the Secondary Outcome Variables: Body Composition, CAVI, HRV, and PhA
3.5. Effect of Treatment on the Secondary Outcome Variables, BEPSI-K, BFI, FSS, and SF-36 Score
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Commoner, B.; Townsend, J.; Pake, G.E. Free radicals in biological materials. Nature 1954, 174, 689–691. [Google Scholar] [CrossRef]
- Powers, S.K.; Jackson, M.J. Exercise-Induced Oxidative Stress: Cellular Mechanisms and Impact on Muscle Force Production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative Stress: An Essential Factor in the Pathogenesis of Gastrointestinal Mucosal Diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef]
- Poljsak, B.; Šuput, D.; Milisav, I. Achieving the Balance between ROS and Antioxidants: When to Use the Synthetic Antioxidants. Oxidative Med. Cell. Longev. 2013, 2013, 1–11. [Google Scholar] [CrossRef]
- Hohn, A.; Weber, D.; Jung, T.; Ott, C.; Hugo, M.; Kochlik, B.; Kehm, R.; Konig, J.; Grune, T.; Castro, J.P. Happily (n)ever after: Aging in the context of oxidative stress, proteostasis loss and cellular senescence. Redox Biol. 2017, 11, 482–501. [Google Scholar] [CrossRef]
- Chandrasekaran, A.; Idelchik, M.; Melendez, J.A. Redox control of senescence and age-related disease. Redox Biol. 2017, 11, 91–102. [Google Scholar] [CrossRef]
- Kochlik, B.; Grune, T.; Weber, D. New findings of oxidative stress biomarkers in nutritional research. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 349–359. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Venkataraman, K.; Khurana, S.; Tai, T.C. Oxidative stress in aging--matters of the heart and mind. Int. J. Mol. Sci. 2013, 14, 17897–17925. [Google Scholar] [CrossRef]
- Therond, P.; Bonnefont-Rousselot, D.; Davit-Spraul, A.; Conti, M.; Legrand, A. Biomarkers of oxidative stress: An analytical approach. Curr. Opin. Clin. Nutr. Metab. Care 2000, 3, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Shirahata, S.; Hamasaki, T.; Teruya, K. Advanced research on the health benefit of reduced water. Trends Food Sci. Technol. 2012, 23, 124–131. [Google Scholar] [CrossRef]
- Hanaoka, K.; Sun, D.; Lawrence, R.; Kamitani, Y.; Fernandes, G. The mechanism of the enhanced antioxidant effects against superoxide anion radicals of reduced water produced by electrolysis. Biophys. Chem. 2004, 107, 71–82. [Google Scholar] [CrossRef]
- Shirahata, S.; Murakami, E.; Kusumoto, K.-i.; Yamashita, M.; Oda, M.; Temya, K.; Kabayama, S.; Otsubo, K.; Morisawa, S.; Hayashi, H. Telomere shortening in cancer cells by electrolyzed-reduced water. In Animal Cell Technology: Challenges for the 21st Century; Springer: Dordrecht, The Netherlands, 2002; pp. 355–359. [Google Scholar]
- Shirahata, S.; Kabayama, S.; Nakano, M.; Miura, T.; Kusumoto, K.; Gotoh, M.; Hayashi, H.; Otsubo, K.; Morisawa, S.; Katakura, Y. Electrolyzed-reduced water scavenges active oxygen species and protects DNA from oxidative damage. Biochem. Biophys. Res. Commun. 1997, 234, 269–274. [Google Scholar] [CrossRef]
- Franceschelli, S.; Gatta, D.M.; Pesce, M.; Ferrone, A.; Patruno, A.; de Lutiis, M.A.; Grilli, A.; Felaco, M.; Croce, F.; Speranza, L. New Approach in Translational Medicine: Effects of Electrolyzed Reduced Water (ERW) on NF-kappaB/iNOS Pathway in U937 Cell Line under Altered Redox State. Int. J. Mol. Sci. 2016, 17, 1461. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Yan, H.; Hamasaki, T.; Kinjo, T.; Nakamichi, N.; Teruya, K.; Kabayama, S.; Shirahata, S. Electrochemically reduced water protects neural cells from oxidative damage. Oxid Med. Cell. Longev. 2014, 2014, 869121. [Google Scholar] [CrossRef]
- Ye, J.; Li, Y.; Hamasaki, T.; Nakamichi, N.; Komatsu, T.; Kashiwagi, T.; Teruya, K.; Nishikawa, R.; Kawahara, T.; Osada, K.; et al. Inhibitory effect of electrolyzed reduced water on tumor angiogenesis. Biol. Pharm. Bull. 2008, 31, 19–26. [Google Scholar] [CrossRef]
- Tsai, C.-F.; Hsu, Y.-W.; Chen, W.-K.; Ho, Y.-C.; Lu, F.-J. Enhanced Induction of Mitochondrial Damage and Apoptosis in Human Leukemia HL-60 Cells Due to Electrolyzed-Reduced Water and Glutathione. Biosci. Biotechnol. Biochem. 2009, 73, 280–287. [Google Scholar] [CrossRef]
- Shirahata, S. Reduced water for prevention of diseases. In Animal cell technology: Basic & Applied Aspects; Springer: Dordrecht, The Netherlands, 2002; pp. 25–30. [Google Scholar]
- Kinjo, T.; Ye, J.; Yan, H.; Hamasaki, T.; Nakanishi, H.; Toh, K.; Nakamichi, N.; Kabayama, S.; Teruya, K.; Shirahata, S. Suppressive effects of electrochemically reduced water on matrix metalloproteinase-2 activities and in vitro invasion of human fibrosarcoma HT1080 cells. Cytotechnology 2012, 64, 357–371. [Google Scholar] [CrossRef]
- Hamasaki, T.; Nakamichi, N.; Teruya, K.; Shirahata, S. Removal efficiency of radioactive cesium and iodine ions by a flow-type apparatus designed for electrochemically reduced water production. PLoS ONE 2014, 9, e102218. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, H.K. Anti-diabetic effects of electrolyzed reduced water in streptozotocin-induced and genetic diabetic mice. Life Sci. 2006, 79, 2288–2292. [Google Scholar] [CrossRef]
- Abe, M.; Sato, S.; Toh, K.; Hamasaki, T.; Nakamichi, N.; Teruya, K.; Katakura, Y.; Morisawa, S.; Shirahata, S. Suppressive effect of ERW on lipid peroxidation and plasma triglyceride level. In Animal Cell Technology: Basic & Applied Aspects; Springer: Dordrecht, The Netherlands, 2010; pp. 315–321. [Google Scholar]
- Spulber, S.; Edoff, K.; Hong, L.; Morisawa, S.; Shirahata, S.; Ceccatelli, S. Molecular hydrogen reduces LPS-induced neuroinflammation and promotes recovery from sickness behaviour in mice. PLoS ONE 2012, 7, e42078. [Google Scholar] [CrossRef]
- Cheng, T.C.; Hsu, Y.W.; Lu, F.J.; Chen, Y.Y.; Tsai, N.M.; Chen, W.K.; Tsai, C.F. Nephroprotective effect of electrolyzed reduced water against cisplatin-induced kidney toxicity and oxidative damage in mice. J. Chin. Med. Assoc. 2018, 81, 119–126. [Google Scholar] [CrossRef]
- Yan, H.; Tian, H.; Kinjo, T.; Hamasaki, T.; Tomimatsu, K.; Nakamichi, N.; Teruya, K.; Kabayama, S.; Shirahata, S. Extension of the Lifespan of Caenorhabditis elegansby the Use of Electrolyzed Reduced Water. Biosci. Biotechnol. Biochem. 2010, 74, 2011–2015. [Google Scholar] [CrossRef]
- Magro, M.; Corain, L.; Ferro, S.; Baratella, D.; Bonaiuto, E.; Terzo, M.; Corraducci, V.; Salmaso, L.; Vianello, F. Alkaline Water and Longevity: A Murine Study. Evid. Based Complement. Altern. Med. 2016, 2016, 3084126. [Google Scholar] [CrossRef]
- Huang, K.-C.; Yang, C.-C.; Lee, K.-T.; Chien, C.-T. Reduced hemodialysis-induced oxidative stress in end-stage renal disease patients by electrolyzed reduced water. Kidney Int. 2003, 64, 704–714. [Google Scholar] [CrossRef]
- Huang, K.C.; Yang, C.C.; Hsu, S.P.; Lee, K.T.; Liu, H.W.; Morisawa, S.; Otsubo, K.; Chien, C.T. Electrolyzed-reduced water reduced hemodialysis-induced erythrocyte impairment in end-stage renal disease patients. Kidney Int. 2006, 70, 391–398. [Google Scholar] [CrossRef]
- Huang, K.C.; Hsu, S.P.; Yang, C.C.; Ou-Yang, P.; Lee, K.T.; Morisawa, S.; Otsubo, K.; Chien, C.T. Electrolysed-reduced water dialysate improves T-cell damage in end-stage renal disease patients with chronic haemodialysis. Nephrol. Dial. Transplant. 2010, 25, 2730–2737. [Google Scholar] [CrossRef]
- Li, Y.; Hamasaki, T.; Teruya, K.; Nakamichi, N.; Gadek, Z.; Kashiwagi, T.; Yan, H.; Kinjo, T.; Komatsu, T.; Ishii, Y.; et al. Suppressive effects of natural reduced waters on alloxan-induced apoptosis and type 1 diabetes mellitus. Cytotechnology 2012, 64, 281–297. [Google Scholar] [CrossRef]
- Masuda, K.; Tanaka, Y.; Kanehisa, M.; Ninomiya, T.; Inoue, A.; Higuma, H.; Kawashima, C.; Nakanishi, M.; Okamoto, K.; Akiyoshi, J. Natural reduced water suppressed anxiety and protected the heightened oxidative stress in rats. Neuropsychiatr. Dis. Treat. 2017, 13, 2357–2362. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Liu, C.; Ding, X. The influence of natural mineral water on aquaporin water permeability and human natural killer cell activity. Biochem. Biophys. Res. Commun. 2011, 409, 40–45. [Google Scholar] [CrossRef]
- Wynn, E.; Krieg, M.A.; Aeschlimann, J.M.; Burckhardt, P. Alkaline mineral water lowers bone resorption even in calcium sufficiency: Alkaline mineral water and bone metabolism. Bone 2009, 44, 120–124. [Google Scholar] [CrossRef]
- Chycki, J.; Kurylas, A.; Maszczyk, A.; Golas, A.; Zajac, A. Alkaline water improves exercise-induced metabolic acidosis and enhances anaerobic exercise performance in combat sport athletes. PLoS ONE 2018, 13, e0205708. [Google Scholar] [CrossRef] [PubMed]
- Nakao, A.; Toyoda, Y.; Sharma, P.; Evans, M.; Guthrie, N. Effectiveness of hydrogen rich water on antioxidant status of subjects with potential metabolic syndrome-an open label pilot study. J. Clin. Biochem. Nutr. 2010, 46, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Lancet Public Health. Public health and the workplace: A new era dawns. Public health and the workplace: A new era dawns. Lancet Public Health 2018, 3, e508. [Google Scholar] [CrossRef]
- Banerjee, P.; Gavaravarapu, S.M. Wellness programmes in the workplace in India. Lancet Public Health 2018, 3, e515. [Google Scholar] [CrossRef]
- Bergman, F.; Wahlström, V.; Stomby, A.; Otten, J.; Lanthén, E.; Renklint, R.; Waling, M.; Sörlin, A.; Boraxbekk, C.-J.; Wennberg, P.; et al. Treadmill workstations in office workers who are overweight or obese: A randomised controlled trial. Lancet Public Health 2018, 3, e523–e535. [Google Scholar] [CrossRef]
- Haufe, S.; Kerling, A.; Protte, G.; Bayerle, P.; Stenner, H.T.; Rolff, S.; Sundermeier, T.; Kuck, M.; Ensslen, R.; Nachbar, L.; et al. Telemonitoring-supported exercise training, metabolic syndrome severity, and work ability in company employees: A randomised controlled trial. Lancet Public Health 2019, 4, e343–e352. [Google Scholar] [CrossRef]
- Van Den Brand, F.A.; Nagelhout, G.E.; Winkens, B.; Chavannes, N.H.; Van Schayck, O.C.P. Effect of a workplace-based group training programme combined with financial incentives on smoking cessation: A cluster-randomised controlled trial. Lancet Public Health 2018, 3, e536–e544. [Google Scholar] [CrossRef]
- Fineberg, H.V. A Successful and Sustainable Health System—How to Get There from Here. N. Engl. J. Med. 2012, 366, 1020–1027. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Friedman, L.M.; Furberg, C.; DeMets, D.L.; Reboussin, D.M.; Granger, C.B. Fundamentals of Clinical Trials; Springer: New York, NY, USA, 2010; Volume 4. [Google Scholar]
- Sakpal, T.V. Sample size estimation in clinical trial. Perspect. Clin. Res. 2010, 1, 67–69. [Google Scholar]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: http.www.R-Project.Org (accessed on 22 February 2019).
- Kotani, K.; Sakane, N. C-reactive protein and reactive oxygen metabolites in subjects with metabolic syndrome. J. Int. Med. Res. 2012, 40, 1074–1081. [Google Scholar] [CrossRef]
- Sugiura, T.; Dohi, Y.; Takase, H.; Yamashita, S.; Tanaka, S.; Kimura, G. Increased reactive oxygen metabolites is associated with cardiovascular risk factors and vascular endothelial damage in middle-aged Japanese subjects. Vasc Health Risk Manag. 2011, 7, 475–482. [Google Scholar] [CrossRef]
- Yagi, H.; Sumino, H.; Yoshida, K.; Aoki, T.; Tsunekawa, K.; Araki, O.; Kimura, T.; Nara, M.; Nakajima, K.; Murakami, M. Biological antioxidant potential negatively correlates with carotid artery intima-media thickness. Int. Heart J. 2016, 57, 220–225. [Google Scholar] [CrossRef]
- Cesarone, M.R.; Belcaro, G.; Carratelli, M.; Cornelli, U.; De Sanctis, M.T.; Incandela, L.; Barsotti, A.; Terranova, R.; Nicolaides, A. A simple test to monitor oxidative stress. Int. Angiol. 1999, 18, 127–130. [Google Scholar]
- Fattoretti, P.; Malavolta, M.; Fabbietti, P.; Papa, R.; Giacconi, R.; Costarelli, L.; Galeazzi, R.; Paoloni, C.; Postacchini, D.; Lattanzio, F.; et al. Oxidative Stress in Elderly with Different Cognitive Status: My Mind Project. J. Alzheimers Dis. 2018, 63, 1405–1414. [Google Scholar] [CrossRef]
- Celi, P.; Sullivan, M.; Evans, D. The stability of the reactive oxygen metabolites (d-ROMs) and biological antioxidant potential (BAP) tests on stored horse blood. Vet. J. 2010, 183, 217–218. [Google Scholar] [CrossRef]
- Kitada, M.; Ogura, Y.; Maruki-Uchida, H.; Sai, M.; Suzuki, T.; Kanasaki, K.; Hara, Y.; Seto, H.; Kuroshima, Y.; Monno, I.; et al. The Effect of Piceatannol from Passion Fruit (Passiflora edulis) Seeds on Metabolic Health in Humans. Nutrients 2017, 9, 1142. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Dokumacioglu, E.; Demiray, O.; Dokumacioglu, A.; Sahin, A.; Sen, T.M.; Cankaya, S. Measuring urinary 8-hydroxy-2′-deoxyguanosine and malondialdehyde levels in women with overactive bladder. Investig. Clin. Urol. 2018, 59, 252–256. [Google Scholar] [CrossRef]
- Basavaraj, K.H.; Devaraju, P.V.; Rao, K.S. Studies on serum 8-hydroxy guanosine (8-OHdG) as reliable biomarker for psoriasis. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 655–657. [Google Scholar] [CrossRef]
- Sarmiento, A.; Diaz-Castro, J.; Pulido-Moran, M.; Moreno-Fernandez, J.; Kajarabille, N.; Chirosa, I.; Guisado, I.M.; Javier Chirosa, L.; Guisado, R.; Ochoa, J.J. Short-term ubiquinol supplementation reduces oxidative stress associated with strenuous exercise in healthy adults: A randomized trial. BioFactors 2016, 42, 612–622. [Google Scholar] [CrossRef]
- Wheeler, C.R.; Salzman, J.A.; Elsayed, N.M.; Omaye, S.T.; Korte, D.W., Jr. Automated assays for superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase activity. Anal. Biochem. 1990, 184, 193–199. [Google Scholar] [CrossRef]
- Zabłocka-Słowińska, K.; Płaczkowska, S.; Prescha, A.; Pawełczyk, K.; Porębska, I.; Kosacka, M.; Pawlik-Sobecka, L.; Grajeta, H. Serum and whole blood Zn, Cu and Mn profiles and their relation to redox status in lung cancer patients. J. Trace Elem. Med. Biol. 2018, 45, 78–84. [Google Scholar] [CrossRef]
- Zhang, F.; Ren, Y.; Fu, W.; Wang, Y.; Qian, J.; Tao, C.; You, C.; Yang, M. Association between neutrophil to lymphocyte ratio and blood glucose level at admission in patients with spontaneous intracerebral hemorrhage. Sci. Rep. 2019, 9, 1–9. [Google Scholar]
- Hitsumoto, T. Association of hemorheology with high-sensitivity cardiac troponin T levels in patients with type 2 diabetes mellitus assessed by microchannel array flow analyzer. Cardiol. Res. 2017, 8, 304. [Google Scholar]
- Jobin, G.; Rodriguez-Suarez, R.; Betito, K. Association Between Natural Killer Cell Activity and Colorectal Cancer in High-Risk Subjects Undergoing Colonoscopy. Gastroenterology 2017, 153, 980–987. [Google Scholar] [CrossRef]
- van Waateringe, R.P.; Slagter, S.N.; van Beek, A.P.; van der Klauw, M.M.; van Vliet-Ostaptchouk, J.V.; Graaff, R.; Paterson, A.D.; Lutgers, H.L.; Wolffenbuttel, B.H.R. Skin autofluorescence, a non-invasive biomarker for advanced glycation end products, is associated with the metabolic syndrome and its individual components. Diabetol. Metab. Syndr. 2017, 9, 42. [Google Scholar] [CrossRef]
- Meerwaldt, R.; Graaff, R.; Oomen, P.H.N.; Links, T.P.; Jager, J.J.; Alderson, N.L.; Thorpe, S.R.; Baynes, J.W.; Gans, R.O.B.; Smit, A.J. Simple non-invasive assessment of advanced glycation endproduct accumulation. Diabetologia 2004, 47, 1324–1330. [Google Scholar] [CrossRef]
- van Waateringe, R.P.; Fokkens, B.T.; Slagter, S.N.; van der Klauw, M.M.; van Vliet-Ostaptchouk, J.V.; Graaff, R.; Paterson, A.D.; Smit, A.J.; Lutgers, H.L.; Wolffenbuttel, B.H.R. Skin autofluorescence predicts incident type 2 diabetes, cardiovascular disease and mortality in the general population. Diabetologia 2019, 62, 269–280. [Google Scholar] [CrossRef]
- Morioka, N.; Funada, J.; Takata, Y.; Hashida, H.; Iwata, T.; Higaki, J.; Okayama, H. Influence of meal intake on pulse wave indices in type 2 diabetes. Hypertens. Res. 2010, 33, 743–747. [Google Scholar] [CrossRef]
- Shirai, K.; Utino, J.; Otsuka, K.; Takata, M. A Novel Blood Pressure-independent Arterial Wall Stiffness Parameter; Cardio-Ankle Vascular Index (CAVI). J. Atheroscler. Thromb. 2006, 13, 101–107. [Google Scholar] [CrossRef]
- Wakabayashi, I.; Masuda, H. Association of acute-phase reactants with arterial stiffness in patients with type 2 diabetes mellitus. Clin. Chim. Acta 2006, 365, 230–235. [Google Scholar] [CrossRef]
- Saiki, A.; Sato, Y.; Watanabe, R.; Watanabe, Y.; Imamura, H.; Yamaguchi, T.; Ban, N.; Kawana, H.; Nagumo, A.; Nagayama, D.; et al. The Role of a Novel Arterial Stiffness Parameter, Cardio-Ankle Vascular Index (CAVI), as a Surrogate Marker for Cardiovascular Diseases. J. Atheroscler. Thromb 2016, 23, 155–168. [Google Scholar] [CrossRef]
- Shirai, K.; Hiruta, N.; Song, M.; Kurosu, T.; Suzuki, J.; Tomaru, T.; Miyashita, Y.; Saiki, A.; Takahashi, M.; Suzuki, K.; et al. Cardio-Ankle Vascular Index (CAVI) as a Novel Indicator of Arterial Stiffness: Theory, Evidence and Perspectives. J. Atheroscler. Thromb. 2011, 18, 924–938. [Google Scholar] [CrossRef]
- Jong, G.-J.; Horng, G.-J. The PPG Physiological Signal for Heart Rate Variability Analysis. Wirel. Pers. Commun. 2017, 97, 5229–5276. [Google Scholar] [CrossRef]
- Park, S.K.; Tucker, K.L.; O’Neill, M.S.; Sparrow, D.; Vokonas, P.S.; Hu, H.; Schwartz, J. Fruit, vegetable, and fish consumption and heart rate variability: The Veterans Administration Normative Aging Study. Am. J. Clin. Nutr. 2009, 89, 778–786. [Google Scholar] [CrossRef]
- Tomeleri, C.M.; Cavaglieri, C.R.; De Souza, M.F.; Cavalcante, E.F.; Antunes, M.; Nabbuco, H.C.G.; Venturini, D.; Barbosa, D.S.; Silva, A.M.; Cyrino, E.S. Phase angle is related with inflammatory and oxidative stress biomarkers in older women. Exp. Gerontol. 2018, 102, 12–18. [Google Scholar] [CrossRef]
- Tan, R.S.; Liang, D.H.; Liu, Y.; Zhong, X.S.; Zhang, D.S.; Ma, J. Bioelectrical Impedance Analysis-Derived Phase Angle Predicts Protein-Energy Wasting in Maintenance Hemodialysis Patients. J. Ren. Nutr. 2019, 29, 295–301. [Google Scholar] [CrossRef]
- Frank, S.H.; Zyzanski, S.J. Stress in the clinical setting: The Brief Encounter Psychosocial Instrument. J. Fam Pract. 1988, 26, 533–539. [Google Scholar]
- Yim, J.; Bae, J.; Choi, S.; Kim, S.; Hwang, H.; Huh, B. The validity of modified Korean-translated BEPSI (Brief Encounter Psychosocial Instrument) as instrument of stress measurement in outpatient clinic. J. Korean Acad. Fam. Med. 1996, 17, 42–53. [Google Scholar]
- Mendoza, T.R.; Wang, X.S.; Cleeland, C.S.; Morrissey, M.; Johnson, B.A.; Wendt, J.K.; Huber, S.L. The rapid assessment of fatigue severity in cancer patients: Use of the Brief Fatigue Inventory. Cancer 1999, 85, 1186–1196. [Google Scholar] [CrossRef]
- Krupp, L.B.; LaRocca, N.G.; Muir-Nash, J.; Steinberg, A.D. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch. Neurol. 1989, 46, 1121–1123. [Google Scholar] [CrossRef]
- Ware, J.E., Jr.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef]
- Hone, T.; Macinko, J.; Millett, C.J.T.L. Revisiting Alma-Ata: What is the role of primary health care in achieving the Sustainable Development Goals? Lancet 2018, 392, 1461–1472. [Google Scholar] [CrossRef]
- Kluge, H.; Kelley, E.; Barkley, S.; Theodorakis, P.N.; Yamamoto, N.; Tsoy, A.; Aiypkhanova, A.; Ganesh, V.; Hipgrave, D.B.; Peterson, S.S. How primary health care can make universal health coverage a reality, ensure healthy lives, and promote wellbeing for all. Lancet 2018, 392, 1372–1374. [Google Scholar] [CrossRef]
- World Health Organization. Astana Declaration of Primary Health Care: From Alm-Ata towards Universal Health Coverage and Sustainable Development Goals. 2018. 2019; World Health Organization: Geneva, Switzerlands, 2018. [Google Scholar]
- Marrocco, I.; Altieri, F.; Peluso, I. Measurement and Clinical Significance of Biomarkers of Oxidative Stress in Humans. Oxidative Med. Cell. Longev. 2017, 2017, 6501046. [Google Scholar] [CrossRef]
- Tang, K.W.; Toh, Q.C.; Teo, B.W. Normalisation of urinary biomarkers to creatinine for clinical practice and research--when and why. Singap. Med. J. 2015, 56, 7–10. [Google Scholar] [CrossRef]
- Nakamura, K.; Matsunaga, K. Susceptibility of natural killer (NK) cells to reactive oxygen species (ROS) and their restoration by the mimics of superoxide dismutase (SOD). Cancer Biother. Radiopharm. 1998, 13, 275–290. [Google Scholar] [CrossRef]
- Harguindey, S.; Reshkin, S.J. “The new pH-centric anticancer paradigm in Oncology and Medicine”; SCB, 2017. Semin. Cancer Biol. 2017, 43, 1–4. [Google Scholar] [CrossRef]
- Dumas, J.F.; Brisson, L.; Chevalier, S.; Maheo, K.; Fromont, G.; Moussata, D.; Besson, P.; Roger, S. Metabolic reprogramming in cancer cells, consequences on pH and tumour progression: Integrated therapeutic perspectives with dietary lipids as adjuvant to anticancer treatment. Semin. Cancer Biol. 2017, 43, 90–110. [Google Scholar] [CrossRef]
- Kim, Y.S.; Sayers, T.J.; Colburn, N.H.; Milner, J.A.; Young, H.A. Impact of dietary components on NK and Treg cell function for cancer prevention. Mol. Carcinog. 2015, 54, 669–678. [Google Scholar] [CrossRef]
- Leischner, C.; Burkard, M.; Pfeiffer, M.M.; Lauer, U.M.; Busch, C.; Venturelli, S. Nutritional immunology: Function of natural killer cells and their modulation by resveratrol for cancer prevention and treatment. Nutr. J. 2015, 15, 47. [Google Scholar] [CrossRef]
- Grudzien, M.; Rapak, A. Effect of natural compounds on NK cell activation. J. Immunol. Res. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Johnson, S.A.; Feresin, R.G.; Navaei, N.; Figueroa, A.; Elam, M.L.; Akhavan, N.S.; Hooshmand, S.; Pourafshar, S.; Payton, M.E.; Arjmandi, B.H.; et al. Effects of daily blueberry consumption on circulating biomarkers of oxidative stress, inflammation, and antioxidant defense in postmenopausal women with pre-and stage 1-hypertension: A randomized controlled trial. Food Funct. 2017, 8, 372–380. [Google Scholar] [CrossRef]
- Kowalska, K.; Olejnik, A.; Rychlik, J.; Grajek, W. Cranberries (Oxycoccus quadripetalus) inhibit lipid metabolism and modulate leptin and adiponectin secretion in 3T3-L1 adipocytes. Food Chem. 2015, 185, 383–388. [Google Scholar] [CrossRef]
- Kowalska, K.; Olejnik, A.; Szwajgier, D.; Olkowicz, M. Inhibitory activity of chokeberry, bilberry, raspberry and cranberry polyphenol-rich extract towards adipogenesis and oxidative stress in differentiated 3T3-L1 adipose cells. PLoS ONE 2017, 12, e0188583. [Google Scholar] [CrossRef]
- Martino, H.S.D.; Dias, M.M.d.S.; Noratto, G.; Talcott, S.; Mertens-Talcott, S.U. Anti-lipidaemic and anti-inflammatory effect of açai (Euterpe oleracea Martius) polyphenols on 3T3-L1 adipocytes. J. Funct. Foods 2016, 23, 432–443. [Google Scholar] [CrossRef]
- Stern, J.H.; Rutkowski, J.M.; Scherer, P.E. Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk. Cell Metab. 2016, 23, 770–784. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Cantone, E.; Cassarano, S.; Tuccinardi, D.; Barrea, L.; Savastano, S.; Colao, A. Gut microbiota: A new path to treat obesity. Int. J. Obes. Suppl. 2019, 9, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Parekh, P.J.; Balart, L.A.; Johnson, D.A. The influence of the gut microbiome on obesity, metabolic syndrome and gastrointestinal disease. Clin. Transl. Gastroenterol. 2015, 6, e91. [Google Scholar] [CrossRef]
- Tseng, C.-H.; Wu, C.-Y. The gut microbiome in obesity. J. Formos. Med. Assoc. 2019, 118, S3–S9. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027. [Google Scholar] [CrossRef] [PubMed]
- Vorobjeva, N.V. Selective stimulation of the growth of anaerobic microflora in the human intestinal tract by electrolyzed reducing water. Med. Hypotheses 2005, 64, 543–546. [Google Scholar] [CrossRef]
- Hamasaki, T.; Harada, G.; Nakamichi, N.; Kabayama, S.; Teruya, K.; Fugetsu, B.; Gong, W.; Sakata, I.; Shirahata, S. Electrochemically reduced water exerts superior reactive oxygen species scavenging activity in HT1080 cells than the equivalent level of hydrogen-dissolved water. PLoS ONE 2017, 12, e0171192. [Google Scholar] [CrossRef]
- Ganesan, P.; Sivanantham, A.; Shanmugam, S. Nanostructured Nickel-Cobalt-Titanium Alloy Grown on Titanium Substrate as Efficient Electrocatalyst for Alkaline Water Electrolysis. ACS Appl. Mater. Interfaces 2017, 9, 12416–12426. [Google Scholar] [CrossRef]
| Characteristic | ERW (n = 29) | MW (n = 24) | p-Value | |
|---|---|---|---|---|
| Demographic | years | |||
| Age | 39.3 ± 8.9 | 41.0 ± 9.3 | 0.523 * | |
| Sex | no. (%) | 1.000 † | ||
| Female | 25 (86.2%) | 21 (87.5%) | ||
| Male | 4 (13.8%) | 3 (12.5%) | ||
| Marital status | no. (%) | 0.378 ‡ | ||
| Married | 18 (62.1%) | 12 (50.0%) | ||
| Single | 11 (37.9%) | 12 (50.0%) | ||
| Educational level | no. (%) | 0.701 § | ||
| High school | 3 (10.3%) | 2 (8.3%) | ||
| College/University | 21 (72.4%) | 17 (70.8%) | ||
| Postgraduate | 5 (17.2%) | 5 (20.8%) | ||
| Occupation | no. (%) | 0.886 § | ||
| Professionals | 6 (20.7%) | 5 (20.8%) | ||
| White collar | 20 (69.0%) | 17 (70.8%) | ||
| Blue collar | 3 (10.3%) | 2 (8.3%) | ||
| Clinical | ||||
| d-ROMs | U.CARR | 301.3 ± 68.6 | 347.3 ± 94.9 | 0.046 * |
| BAP | umol/L | 2027.4 ± 226.9 | 2015.7 ± 274.8 | 0.734 ‖ |
| TBARS(MDA) | μM | 9.88 ± 4.67 | 8.90 ± 5.98 | 0.506 * |
| 8-OHdG | ng/mL | 14.33 ± 6.88 | 10.90 ± 6.42 | 0.069 * |
| oxLDL | U/L | 52.43 ± 17.38 | 49.38 ± 13.61 | 0.520 ‖ |
| GPx | nmol/min/mL | 151.4 ± 31.1 | 140.1 ± 34.9 | 0.180 ‖ |
| Body Mass Index | kg/m2 | 22.2 ± 3.1 | 23.0 ± 3.1 | 0.357 * |
| Blood pressure | mmHg | |||
| Systolic | 116.3 ± 10.6 | 116.3 ± 9.5 | 0.943 ‖ | |
| Diastolic | 72.0 ± 11.6 | 72.4 ± 9.5 | 0.971 ‖ | |
| Glucose | mg/dL | 92.8 ± 7.5 | 92.3 ± 6.9 | 0.805 * |
| ERW (n = 29) | MW (n = 24) | p | |||
|---|---|---|---|---|---|
| Outcome Variables | Means ±SD | Means ±SD | Time | Group | Time * Group |
| d-ROMs(U.CARR) | |||||
| Baseline | 301.3 ± 68.6 | 347.3 ± 94.9 | |||
| 4 weeks | 286.7 ± 45.1 | 306.3 ± 54.8 | 0.004 | 0.007 | 0.044 |
| 8 weeks | 288.0 ± 50.0 | 349.6 ± 62.7 | |||
| BAP (umol/L) | |||||
| Baseline | 2027.4 ± 226.9 | 2015.7 ± 274.8 | |||
| 4 weeks | 2585.9 ± 258.8 | 2504.8 ± 187.8 | 0.000 | 0.875 | 0.045 |
| 8 weeks | 2603.9 ± 255.9 | 2670.4 ± 180.2 | |||
| TBARS (MDA) (μM) | |||||
| Baseline | 9.88 ± 4.67 | 8.90 ± 5.98 | |||
| 4 weeks | 6.84 ± 3.99 | 8.95 ± 6.14 | 0.003 | 0.809 | 0.091 |
| 8 weeks | 6.97 ± 4.51 | 6.63 ± 4.47 | |||
| 8-OHdG (ng/mL) | |||||
| Baseline | 14.33 ± 6.88 | 10.90 ± 6.42 | |||
| 4 weeks | 13.87 ± 8.31 | 15.63 ± 11.18 | 0.033 | 0.594 | 0.138 |
| 8 weeks | 11.87 ± 7.90 | 10.87 ± 7.00 | |||
| oxLDL (U/L) | |||||
| Baseline | 52.43 ± 17.38 | 49.38 ± 13.61 | |||
| 4 weeks | 53.60 ± 15.32 | 52.00 ± 12.94 | 0.332 | 0.679 | 0.534 |
| 8 weeks | 52.24 ± 15.97 | 52.1 ± 12.99 | |||
| GPx (nmol/min/mL) | |||||
| Baseline | 151.4 ± 31.1 | 140.1 ± 34.9 | |||
| 4 weeks | 162.8 ± 36.5 | 161.7 ± 37.9 | 0.000 | 0.639 | 0.412 |
| 8 weeks | 185.8 ± 30.9 | 189.6 ± 23.6 | |||
| ERW (n = 29) | MW (n = 24) | p | |||
|---|---|---|---|---|---|
| Outcome Variables | Means ±SD | Means ±SD | Time | Group | Time * Group |
| NK Cell Activity(pg/mL) | |||||
| Baseline | 949.0 ± 810.7 | 1263.4 ± 905.2 | |||
| 4 weeks | 1943.5 ± 1109.2 | 1829.0 ± 1136.7 | 0.000 | 0.528 | 0.289 |
| 8 weeks | 1814.2 ± 1091.8 | 2042.0 ± 964.9 | |||
| Glucose (mg/dL) | |||||
| Baseline | 92.8 ± 7.5 | 92.3 ± 6.9 | |||
| 4 weeks | 92.8 ± 8.2 | 92.1 ± 6.0 | 0.978 | 0.640 | 0.916 |
| 8 weeks | 93.0 ± 5.9 | 91.8 ± 6.2 | |||
| HbA1c(mg/dL) | |||||
| Baseline | 5.2 ± 0.3 | 5.3 ± 0.3 | |||
| 4 weeks | 5.3 ± 0.3 | 5.4 ± 0.2 | 0.000 | 0.228 | 0.971 |
| 8 weeks | 5.3 ± 0.3 | 5.4 ± 0.3 | |||
| Insulin (uU/mL) | |||||
| Baseline | 8.65 ± 3.78 | 8.21 ± 10.31 | |||
| 4 weeks | 6.66 ± 3.19 | 7.18 ± 4.76 | 0.091 | 0.729 | 0.401 |
| 8 weeks | 7.15 ± 3.19 | 8.50 ± 6.78 | |||
| HOMA-IR | |||||
| Baseline | 2.21 ± 0.93 | 1.95 ± 2.74 | |||
| 4 weeks | 1.55 ± 0.80 | 1.65 ± 1.15 | 0.112 | 0.739 | 0.575 |
| 8 weeks | 1.68 ± 0.80 | 1.98 ± 1.61 | |||
| A.G.E. (AU) | |||||
| Baseline | 2.05 ± 0.26 | 2.04 ± 0.27 | |||
| 4 weeks | 1.83 ± 0.32 | 1.82 ± 0.23 | 0.000 | 0.767 | 0.246 |
| 8 weeks | 1.82 ± 0.34 | 1.91 ± 0.33 | |||
| Cortisol(ng/mL) | |||||
| Baseline | 80.81 ± 35.53 | 81.78 ± 25.30 | |||
| 4 weeks | 76.95 ± 27.36 | 79.06 ± 41.63 | 0.743 | 0.443 | 0.308 |
| 8 weeks | 72.63 ± 27.47 | 85.70 ± 26.27 | |||
| Lactic Acid(mmol/L) | |||||
| Baseline | 1.76 ± 0.86 | 1.52 ± 0.55 | |||
| 4 weeks | 1.65 ± 0.65 | 1.58 ± 0.61 | 0.454 | 0.699 | 0.093 |
| 8 weeks | 1.44 ± 0.45 | 1.61 ± 0.47 | |||
| ERW (n = 29) | MW (n = 24) | p | |||
|---|---|---|---|---|---|
| Outcome Variables | Means ± SD | Means ± SD | Time | Group | Time * Group |
| Fat Mass-Total (kg) | |||||
| Baseline | 16.21 ± 4.88 | 17.30 ± 5.03 | |||
| 4 weeks | 16.18 ± 5.00 | 16.55 ± 4.90 | 0.000 | 0.678 | 0.041 |
| 8 weeks | 15.86 ± 4.86 | 16.10 ± 5.31 | |||
| Fat Mass-Visceral (kg) | |||||
| Baseline | 1.86 ± 0.96 | 2.00 ± 0.95 | |||
| 4 weeks | 1.87 ± 1.00 | 1.87 ± 0.82 | 0.000 | 0.846 | 0.027 |
| 8 weeks | 1.79 ± 0.94 | 1.80 ± 0.93 | |||
| Fat Mass-Subcutaneous (kg) | |||||
| Baseline | 14.36 ± 3.96 | 15.30 ± 4.12 | |||
| 4 weeks | 14.30 ± 4.04 | 14.69 ± 4.11 | 0.000 | 0.644 | 0.047 |
| 8 weeks | 14.07 ± 3.96 | 14.30 ± 4.41 | |||
| CAVI-Rt | |||||
| Baseline | 6.44 ± 0.80 | 6.36 ± 1.06 | |||
| 4 weeks | 6.32 ± 0.80 | 6.36 ± 0.73 | 0.627 | 0.738 | 0.615 |
| 8 weeks | 6.18 ± 0.69 | 6.36 ± 0.86 | |||
| CAVI-Lt | |||||
| Baseline | 6.52 ± 0.77 | 6.48 ± 1.11 | |||
| 4 weeks | 6.38 ± 0.83 | 6.48 ± 0.76 | 0.444 | 0.577 | 0.677 |
| 8 weeks | 6.23 ± 0.69 | 6.42 ± 0.81 | |||
| HRV-PSI | |||||
| Baseline | 71.92 ± 81.46 | 52.85 ± 31.23 | |||
| 4 weeks | 71.54 ± 59.39 | 54.99 ± 33.62 | 0.825 | 0.182 | 0.765 |
| 8 weeks | 63.09 ± 41.19 | 54.68 ± 38.06 | |||
| HRV-TP (ms2) | |||||
| Baseline | 913.43 ± 613.60 | 1071.63 ± 876.97 | |||
| 4 weeks | 985.86 ± 1030.49 | 1041.21 ± 1223.80 | 0.502 | 0.795 | 0.851 |
| 8 weeks | 1185.43 ± 1570.90 | 1115.73 ± 973.82 | |||
| HRV-LF/HF | |||||
| Baseline | 1.52 ± 1.30 | 2.01 ± 2.14 | |||
| 4 weeks | 1.64 ± 1.41 | 1.64 ± 1.79 | 0.629 | 0.958 | 0.197 |
| 8 weeks | 2.19 ± 2.43 | 1.64 ± 1.34 | |||
| Phase Angle (° ) | |||||
| Baseline | 5.89 ± 0.85 | 5.83 ± 0.85 | |||
| 4 weeks | 5.63 ± 0.75 | 5.67 ± 0.78 | 0.000 | 0.952 | 0.566 |
| 8 weeks | 5.62 ± 0.67 | 5.60 ± 0.84 | |||
| ERW (n = 29) | MW (n = 24) | p | |||
|---|---|---|---|---|---|
| Outcome Variables | Mean ± SD | Mean ± SD | Time | Group | Time * Group |
| BEPSI-K | |||||
| Baseline | 1.82 ± 0.65 | 1.73 ± 0.51 | |||
| 4 weeks | 1.71 ± 0.72 | 1.73 ± 0.51 | 0.553 | 0.907 | 0.511 |
| 8 weeks | 1.76 ± 0.73 | 1.78 ± 0.56 | |||
| BFI | |||||
| BFI Global | |||||
| Baseline | 4.14 ± 2.09 | 4.01 ± 2.27 | |||
| 4 weeks | 3.60 ± 1.98 | 3.56 ± 2.09 | 0.016 | 0.964 | 0.901 |
| 8 weeks | 3.28 ± 2.29 | 3.38 ± 2.28 | |||
| BFI Severity | |||||
| Baseline | 5.69 ± 2.06 | 5.36 ± 2.45 | |||
| 4 weeks | 5.49 ± 1.77 | 5.14 ± 2.28 | 0.049 | 0.566 | 0.956 |
| 8 weeks | 4.90 ± 2.32 | 4.71 ± 2.40 | |||
| BFI Interference | |||||
| Baseline | 3.36 ± 2.39 | 3.33 ± 2.38 | |||
| 4 weeks | 2.66 ± 2.37 | 2.76 ± 2.30 | 0.028 | 0.845 | 0.895 |
| 8 weeks | 2.47 ± 2.46 | 2.72 ± 2.41 | |||
| FSS | |||||
| Baseline | 3.58 ± 1.58 | 3.65 ± 1.68 | |||
| 4 weeks | 3.15 ± 1.53 | 3.40 ± 1.67 | 0.172 | 0.928 | 0.489 |
| 8 weeks | 3.39 ± 1.81 | 3.17 ± 1.65 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.A.; Lee, D.H.; Cho, D.-Y.; Lee, Y.-J. Outcomes Assessment of Sustainable and Innovatively Simple Lifestyle Modification at the Workplace-Drinking Electrolyzed-Reduced Water (OASIS-ERW): A Randomized, Double-Blind, Placebo-Controlled Trial. Antioxidants 2020, 9, 564. https://doi.org/10.3390/antiox9070564
Choi YA, Lee DH, Cho D-Y, Lee Y-J. Outcomes Assessment of Sustainable and Innovatively Simple Lifestyle Modification at the Workplace-Drinking Electrolyzed-Reduced Water (OASIS-ERW): A Randomized, Double-Blind, Placebo-Controlled Trial. Antioxidants. 2020; 9(7):564. https://doi.org/10.3390/antiox9070564
Chicago/Turabian StyleChoi, Young Ah, Dong Hyeon Lee, Doo-Yeoun Cho, and Yong-Jae Lee. 2020. "Outcomes Assessment of Sustainable and Innovatively Simple Lifestyle Modification at the Workplace-Drinking Electrolyzed-Reduced Water (OASIS-ERW): A Randomized, Double-Blind, Placebo-Controlled Trial" Antioxidants 9, no. 7: 564. https://doi.org/10.3390/antiox9070564
APA StyleChoi, Y. A., Lee, D. H., Cho, D.-Y., & Lee, Y.-J. (2020). Outcomes Assessment of Sustainable and Innovatively Simple Lifestyle Modification at the Workplace-Drinking Electrolyzed-Reduced Water (OASIS-ERW): A Randomized, Double-Blind, Placebo-Controlled Trial. Antioxidants, 9(7), 564. https://doi.org/10.3390/antiox9070564





