Gallic Acid-Dextran Conjugate: Green Synthesis of a Novel Antioxidant Molecule
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
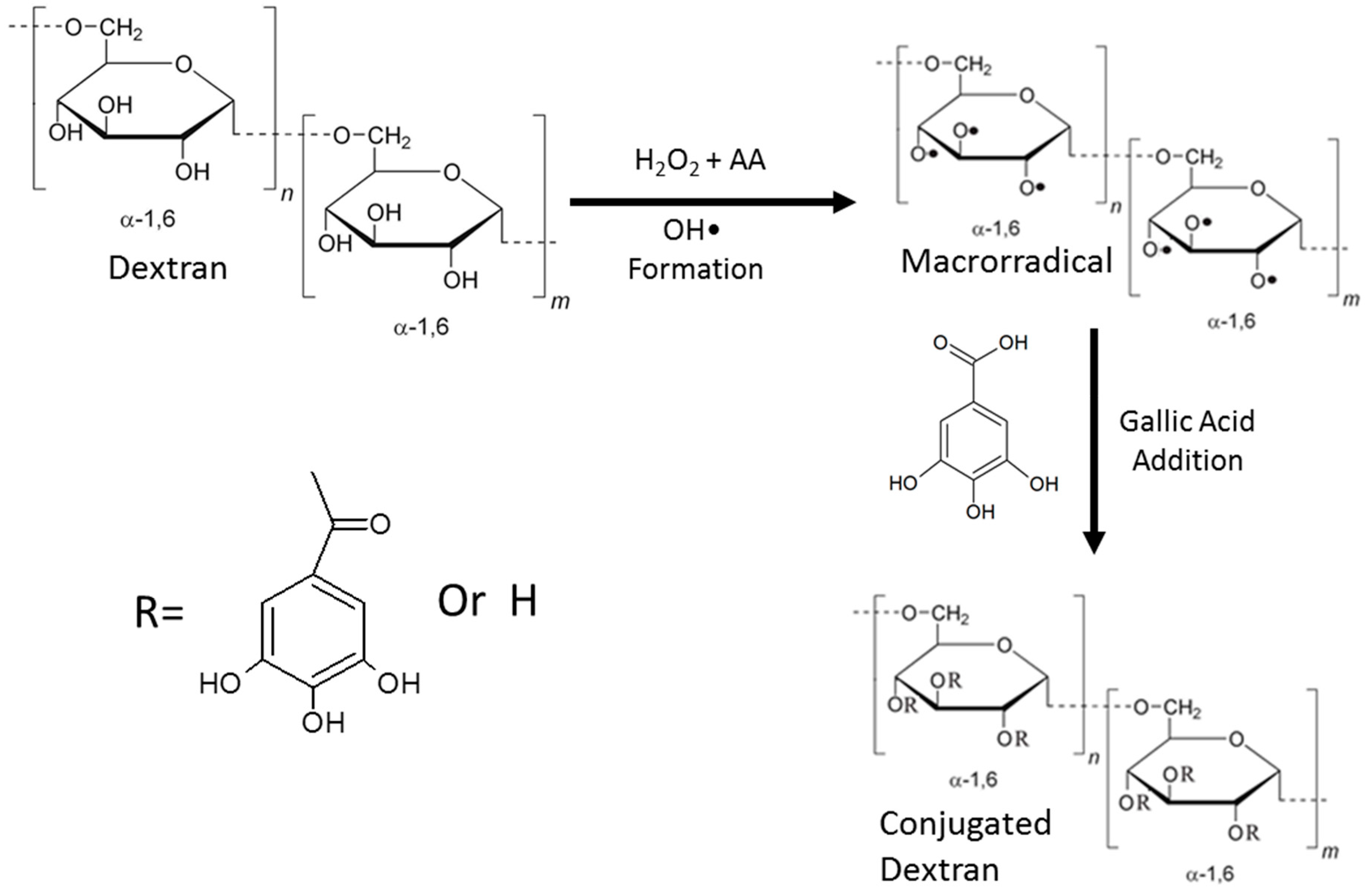
2.2. Conjugation of GA to Dextran
2.3. Structural Analysis and Characterization of GA-dextran Conjugate
2.3.1. Quantification of Phenolic Compounds
2.3.2. Determination of Molecular Weight of Dextran
2.3.3. Fourier Transformed Infrared (FTIR) Spectroscopy
2.3.4. Nuclear Magnetic Resonance (NMR) Spectroscopy
2.4. Antioxidant Tests
2.4.1. Determination of Total Antioxidant Capacity (TAC)
2.4.2. Superoxide Radical-Scavenging Assay
2.4.3. Reducing Power
2.4.4. Iron Chelating Assay
2.5. Statistical Analysis
3. Results and Discussion
3.1. Characterization
3.1.1. GA Dosage and Determination of Apparent Molecular Weight
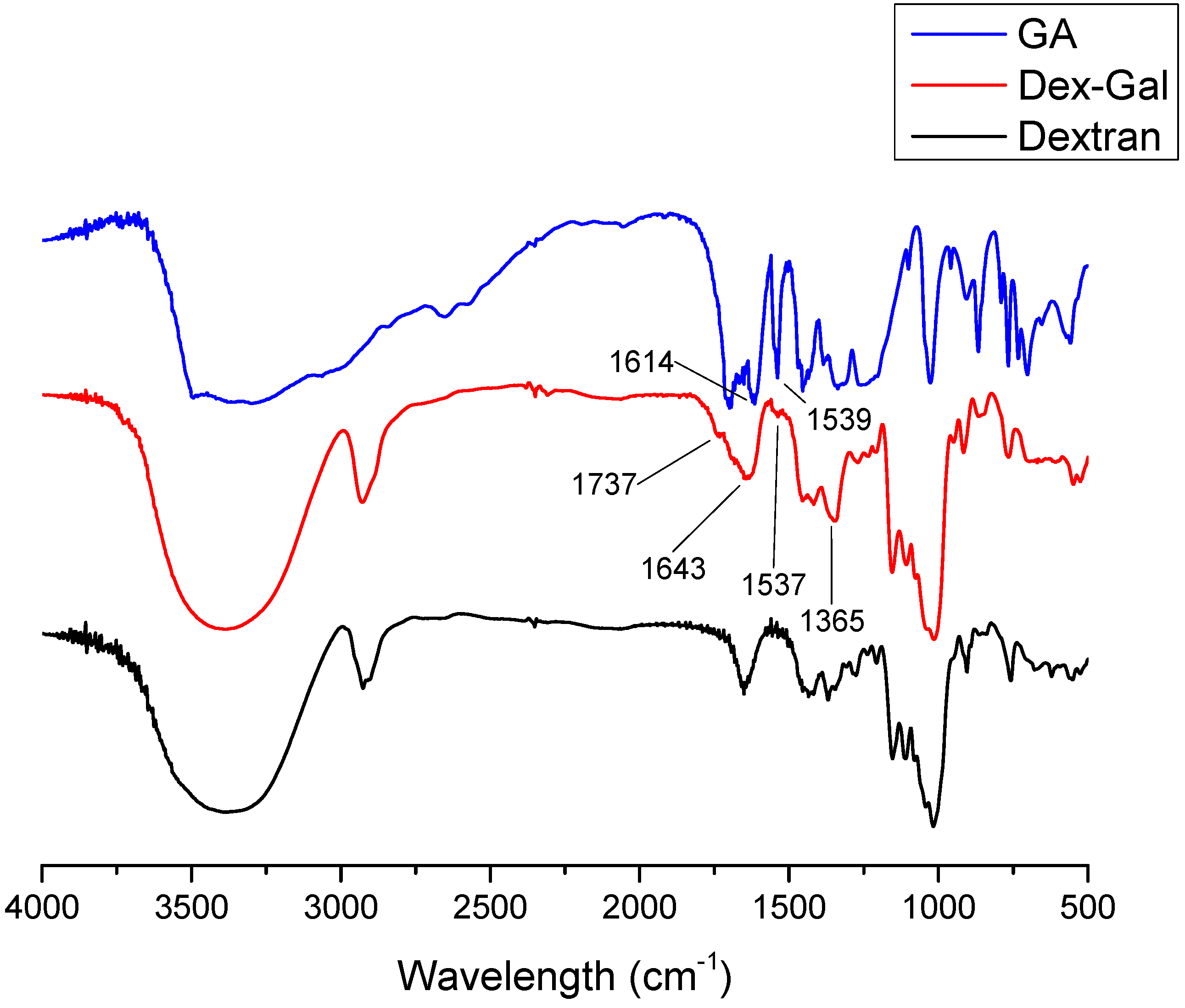
3.1.2. FTIR Analysis
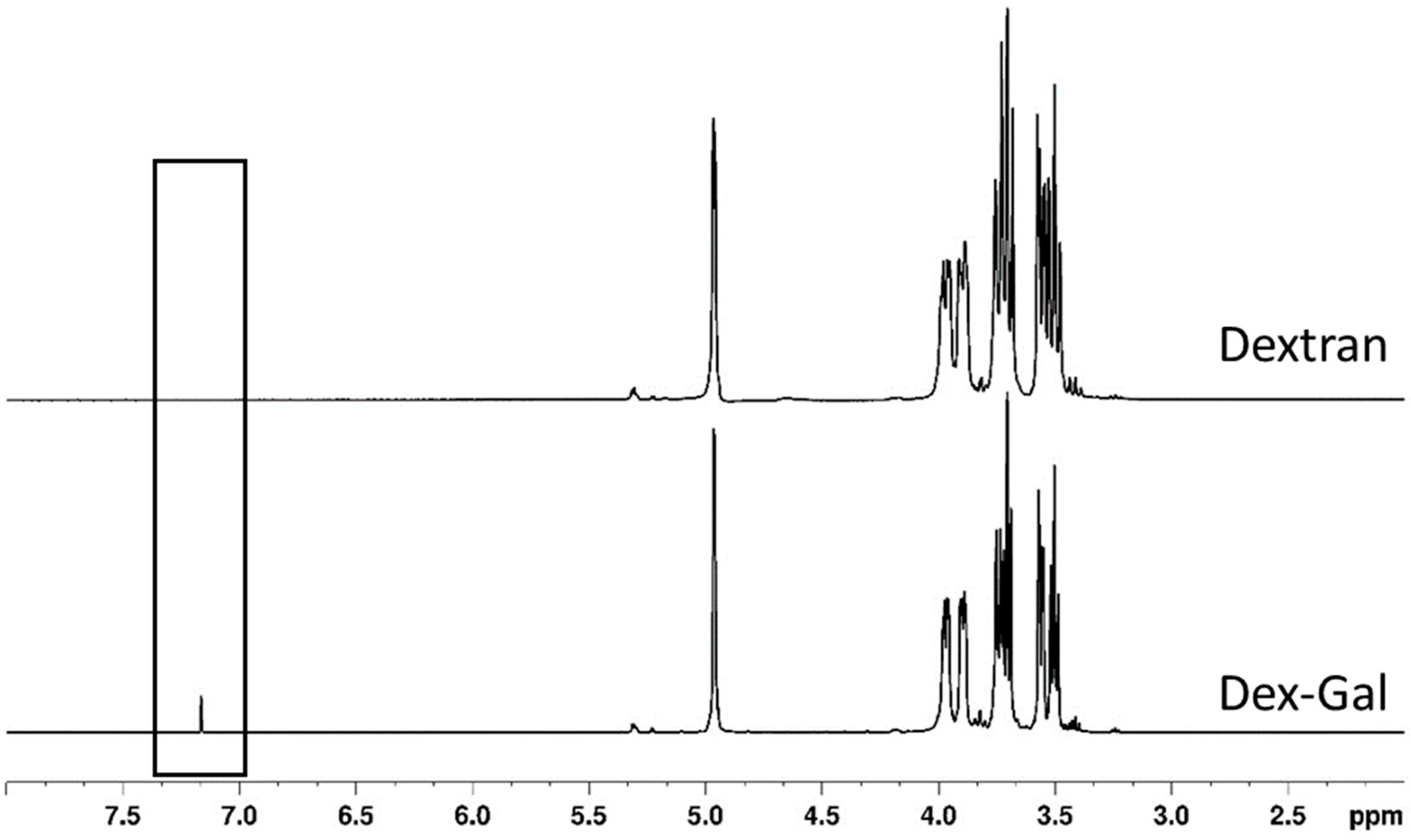
3.1.3. NMR Analyses
3.2. Antioxidant Activities
3.2.1. Ferric Chelating Activity
3.2.2. Superoxide Radical-scavenging Activity
3.2.3. Total Antioxidant Capacity (TAC)
3.2.4. Reducing Power
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cheng, H.N.; Smith, P.B.; Gross, R.A. Green Polymer Chemistry: A Brief Review; ACS Symposium Series; Cheng, H.N., Smith, P.B., Gross, R.A., Eds.; ACS Publications: Washington, DC, USA, 2013; Volume 1144, pp. 1–12. [Google Scholar]
- Vettori, M.H.P.B.; Blanco, K.C.; Cortezi, M.; Lima, C.J.B.; Contiero, J. Dextran: Effect of process parameters on production, purification and molecular weight and recent applications. Diálogos Ciênc. 2012, 2012, 171–186. [Google Scholar] [CrossRef]
- Varshosaz, J. Dextran conjugates in drug delivery. Expert Opin. Drug Deliv. 2012, 9, 509–523. [Google Scholar] [CrossRef]
- Gil, E.C.; Colarte, A.I.; El Ghzaoui, A.; Durand, D.; Delarbre, J.L.; Bataille, B. A sugar cane native dextran as an innovative functional excipient for the development of pharmaceutical tablets. Eur. J. Pharm. Biopharm. 2008, 68, 319–329. [Google Scholar] [CrossRef]
- Bhavani, A.L.; Nisha, J. Dextran-The polysaccharide with versatile uses. Int. J. Pharm. Bio Sci. 2010, 1, 569–573. [Google Scholar]
- Yi, J.; Liu, Y.; Zhang, Y.; Gao, L. Fabrication of Resveratrol-Loaded Whey Protein-Dextran Colloidal Complex for the Stabilization and Delivery of β-Carotene Emulsions. J. Agric. Food Chem. 2018, 66, 9481–9489. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Yi, J.; Zhang, Y.; Yokoyama, W. Fabrication of curcumin-loaded bovine serum albumin (BSA)-dextran nanoparticles and the cellular antioxidant activity. Food Chem. 2018, 239, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Zuluaga, M.; Gregnanin, G.; Cencetti, C.; Di Meo, C.; Gueguen, V.; Letourneur, D.; Meddahi-Pellé, A.; Pavon-Djavid, G.; Matricardi, P. PVA/Dextran hydrogel patches as delivery system of antioxidant astaxanthin: A cardiovascular approach. Biomed. Mater. 2018, 13, 015020. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.S.; Hu, L.L.; Wang, Z.D.; Li, Z.P.; Wang, A.W.; Liu, J. Resveratrol-loaded folic acid-grafted dextran stearate submicron particles exhibits enhanced antitumor efficacy in non-small cell lung cancers. Mater. Sci. Eng. C 2017, 72, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Soeiro, V.; Melo, K.; Alves, M.; Medeiros, M.; Grilo, M.; Almeida-Lima, J.; Pontes, D.; Costa, L.; Rocha, H. Dextran: Influence of Molecular Weight in Antioxidant Properties and Immunomodulatory Potential. Int. J. Mol. Sci. 2016, 17, 1340. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Qiao, X.; Zhao, F.; Song, Q.; Zhou, Q.; Wang, Y.; Pan, L.; Han, Y.; Zhou, Z. Purification, characterization and antioxidant activity of dextran produced by Leuconostoc pseudomesenteroides from homemade wine. Carbohydr. Polym. 2018, 198, 529–536. [Google Scholar] [CrossRef]
- Ye, G.; Chen, Y.; Wang, C.; Yang, R.; Bin, X. Purification and characterization of exopolysaccharide produced by Weissella cibaria YB-1 from pickle Chinese cabbage. Int. J. Biol. Macromol. 2018, 120, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Domnina, N.; Aref’ev, D.; Komarova, E.; Bilibin, A. Dextran as antioxidant’s activity carrier. Macromol. Symp. 1999, 144, 339–350. [Google Scholar] [CrossRef]
- Vittorio, O.; Cirillo, G.; Iemma, F.; Di Turi, G.; Jacchetti, E.; Curcio, M.; Barbuti, S.; Funel, N.; Parisi, O.I.; Puoci, F.; et al. Dextran-Catechin Conjugate: A Potential Treatment Against the Pancreatic Ductal Adenocarcinoma. Pharm. Res. 2012, 29, 2601–2614. [Google Scholar] [CrossRef] [PubMed]
- Oliver, S.; Yee, E.; Kavallaris, M.; Vittorio, O.; Boyer, C. Water Soluble Antioxidant Dextran–Quercetin Conjugate with Potential Anticancer Properties. Macromol. Biosci. 2018, 18, 1–11. [Google Scholar] [CrossRef]
- Lu, Z.; Nie, G.; Belton, P.S.; Tang, H.; Zhao, B. Structure-activity relationship analysis of antioxidant ability and neuroprotective effect of gallic acid derivatives. Neurochem. Int. 2006, 48, 263–274. [Google Scholar] [CrossRef]
- Ji, H.F.; Zhang, H.Y.; Shen, L. Proton dissociation is important to understanding structure-activity relationships of gallic acid antioxidants. Bioorg. Med. Chem. Lett. 2006, 16, 4095–4098. [Google Scholar] [CrossRef]
- Chandramohan Reddy, T.; Bharat Reddy, D.; Aparna, A.; Arunasree, K.M.; Gupta, G.; Achari, C.; Reddy, G.V.; Lakshmipathi, V.; Subramanyam, A.; Reddanna, P. Anti-leukemic effects of gallic acid on human leukemia K562 cells: Downregulation of COX-2, inhibition of BCR/ABL kinase and NF-κB inactivation. Toxicol. Vitr. 2012, 26, 396–405. [Google Scholar] [CrossRef]
- Panich, U.; Onkoksoong, T.; Limsaengurai, S.; Akarasereenont, P.; Wongkajornsilp, A. UVA-induced melanogenesis and modulation of glutathione redox system in different melanoma cell lines: The protective effect of gallic acid. J. Photochem. Photobiol. B Biol. 2012, 108, 16–22. [Google Scholar] [CrossRef]
- Ferk, F.; Chakraborty, A.; Jäger, W.; Kundi, M.; Bichler, J.; Mišík, M.; Wagner, K.H.; Grasl-Kraupp, B.; Sagmeister, S.; Haidinger, G.; et al. Potent protection of gallic acid against DNA oxidation: Results of human and animal experiments. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 2011, 715, 61–71. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chemie-Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef]
- Pasanphan, W.; Chirachanchai, S. Conjugation of gallic acid onto chitosan: An approach for green and water-based antioxidant. Carbohydr. Polym. 2008, 72, 169–177. [Google Scholar] [CrossRef]
- Curcio, M.; Puoci, F.; Iemma, F.; Parisi, O.I.; Cirillo, G.; Spizzirri, U.G.; Picci, N. Covalent insertion of antioxidant molecules on chitosan by a free radical grafting procedure. J. Agric. Food Chem. 2009, 57, 5933–5938. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S.; Kim, S.K.; Ahn, C.B.; Je, J.Y. Preparation, characterization, and antioxidant properties of gallic acid-grafted-chitosans. Carbohydr. Polym. 2011, 83, 1617–1622. [Google Scholar] [CrossRef]
- Xie, M.; Hu, B.; Wang, Y.; Zeng, X. Grafting of gallic acid onto chitosan enhances antioxidant activities and alters rheological properties of the copolymer. J. Agric. Food Chem. 2014, 62, 9128–9136. [Google Scholar] [CrossRef]
- Queiroz, M.F.; Melo, K.R.T.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O.; Costa, L.S. Gallic Acid-Chitosan Conjugate Inhibits the Formation of Calcium Oxalate Crystals. Molecules 2019, 24, 2074. [Google Scholar] [CrossRef]
- Wong-Paz, J.E.; Muñiz-Márquez, D.B.; Aguilar-Zárate, P.; Rodríguez-Herrera, R.; Aguilar, C.N. Microplate quantification of total phenolic content from plant extracts obtained by conventional and ultrasound methods. Phytochem. Anal. 2014, 25, 439–444. [Google Scholar] [CrossRef]
- Presa, F.B.; Marques, M.L.M.; Viana, R.L.S.; Nobre, L.T.D.B.; Costa, L.S.; Rocha, H.A.O. The Protective role of sulfated polysaccharides from green seaweed Udotea flabellum in cells exposed to oxidative damage. Mar. Drugs 2018, 16, 1–16. [Google Scholar] [CrossRef]
- Wu, C.; Tian, J.; Li, S.; Wu, T.; Hu, Y.; Chen, S.; Sugawara, T.; Ye, X. Structural properties of films and rheology of film-forming solutions of chitosan gallate for food packaging. Carbohydr. Polym. 2016, 146, 10–19. [Google Scholar] [CrossRef]
- Oliver, S.; Thomas, D.S.; Kavallaris, M.; Vittorio, O.; Boyer, C. Efficient functionalisation of dextran-aldehyde with catechin: Potential applications in the treatment of cancer. Polym. Chem. 2016, 7, 2542–2552. [Google Scholar] [CrossRef]
- Liu, J.; Lu, J.F.; Kan, J.; Jin, C.H. Synthesis of chitosan-gallic acid conjugate: Structure characterization and in vitro anti-diabetic potential. Int. J. Biol. Macromol. 2013, 62, 321–329. [Google Scholar] [CrossRef]
- Wolkers, W.F.; Oliver, A.E.; Tablin, F.; Crowe, J.H. A Fourier-transform infrared spectroscopy study of sugar glasses. Carbohydr. Res. 2004, 339, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.R.F.; Dore, C.M.P.G.; Marques, C.T.; Nascimento, M.S.; Benevides, N.M.B.; Rocha, H.A.O.; Chavante, S.F.; Leite, E.L. Anticoagulant activity, paw edema and pleurisy induced carrageenan: Action of major types of commercial carrageenans. Carbohydr. Polym. 2010, 79, 26–33. [Google Scholar] [CrossRef]
- Melo-Silveira, R.F.; Fidelis, G.P.; Pereira Costa, M.S.S.; Telles, C.B.S.; Dantas-Santos, N.; de Oliveira Elias, S.; Ribeiro, V.B.; Barth, A.L.; Macedo, A.J.; Leite, E.L.; et al. In vitro antioxidant, anticoagulant and antimicrobial activity and in inhibition of cancer cell proliferation by xylan extracted from corn cobs. Int. J. Mol. Sci. 2012, 13, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Feng, F.; Yang, Y.; Zhao, F.; Du, R.; Zhou, Z.; Han, Y. Characterization of a dextran produced by Leuconostoc pseudomesenteroides XG5 from homemade wine. Int. J. Biol. Macromol. 2018, 107, 2234–2241. [Google Scholar] [CrossRef] [PubMed]
- Rosca, I.; Petrovici, A.R.; Peptanariu, D.; Nicolescu, A.; Dodi, G.; Avadanei, M.; Ivanov, I.C.; Bostanaru, A.C.; Mares, M.; Ciolacu, D. Biosynthesis of dextran by Weissella confusa and its In Vitro functional characteristics. Int. J. Biol. Macromol. 2018, 107, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, S.K.; Chand, N.; Tiwari, S.; Soni, S. Swelling behavior of cross-linked dextran hydrogels and preliminary gliclazide release behavior. Int. J. Biol. Macromol. 2016, 93, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Babior, B.M. Superoxide: A two-edged sword. Brazilian J. Med. Biol. Res. 1997, 30, 141–155. [Google Scholar] [CrossRef]
- Maity, P.; Nandi, A.K.; Manna, D.K.; Pattanayak, M.; Sen, I.K.; Bhanja, S.K.; Samanta, S.; Panda, B.C.; Paloi, S.; Acharya, K.; et al. Structural characterization and antioxidant activity of a glucan from Meripilus giganteus. Carbohydr. Polym. 2017, 157, 1237–1245. [Google Scholar] [CrossRef]
- Giese, E.C.; Gascon, J.; Anzelmo, G.; Barbosa, A.M.; da Cunha, M.A.A.; Dekker, R.F.H. Free-radical scavenging properties and antioxidant activities of botryosphaeran and some other β-D-glucans. Int. J. Biol. Macromol. 2015, 72, 125–130. [Google Scholar] [CrossRef]
- Sellimi, S.; Maalej, H.; Rekik, D.M.; Benslima, A.; Ksouda, G.; Hamdi, M.; Sahnoun, Z.; Li, S.; Nasri, M.; Hajji, M. Antioxidant, antibacterial and In Vivo wound healing properties of laminaran purified from Cystoseira barbata seaweed. Int. J. Biol. Macromol. 2018, 119, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Huang, G.; Zhao, F.; Zhou, L.; Huang, S.; Li, H. The antioxidant activities of six (1 → 3)-β-D-glucan derivatives prepared from yeast cell wall. Int. J. Biol. Macromol. 2017, 98, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Theis, T.V.; Queiroz Santos, V.A.; Appelt, P.; Barbosa-Dekker, A.M.; Vetvicka, V.; Dekker, R.F.H.; Cunha, M.A.A. Fungal Exocellular (1-6)-β-d-glucan: Carboxymethylation, Characterization, and Antioxidant Activity. Int. J. Mol. Sci. 2019, 20, 2337. [Google Scholar] [CrossRef] [PubMed]
- Villaño, D.; Fernández-Pachón, M.S.; Moyá, M.L.; Troncoso, A.M.; García-Parrilla, M.C. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta 2007, 71, 230–235. [Google Scholar] [CrossRef]
- Gow-Chin, Y.; Pin-Der, D.; Hui-Ling, T.; Yen, G.C.; der Duh, P.; Tsai, H.L. Antioxidant and pro-oxidant properties of ascorbic acid and gallic acid. Food Chem. 2002, 79, 307–313. [Google Scholar]
- Scorsatto, M.; de Pimentel, A.C.; da Silva, A.J.R.; Sabally, K.; Rosa, G.; de Oliveira, G.M.M. Assessment of Bioactive Compounds, Physicochemical Composition, and In Vitro Antioxidant Activity of Eggplant Flour. Int. J. Cardiovasc. Sci. 2017, 30, 235–242. [Google Scholar] [CrossRef]

| Sample | MW (kDa) | GA Contend (mg/g) | TAC (mg/g) |
|---|---|---|---|
| Dextran | 15.5 | ND | ND |
| Dex−Gal | 11.23 | 36.8 ± 1.4 | 14.8 ± 2.47 |
| Band (cm−1) | Correlation |
|---|---|
| 3400 | OH vibration |
| 2900 | C–H vibration |
| 1421 | C–O vibration |
| 1016 | α-(1→6) glicosidic linkage |
| 1153 | C–O–C glicosidic linkage |
| 906 | Piranose |
| 850 | α-D-glucose |
| 1537 | C C aromatic ring |
| 1643 | C=C aromatic ring |
| 1737 | C=O ester |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Queiroz, M.F.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O.; Costa, L.S. Gallic Acid-Dextran Conjugate: Green Synthesis of a Novel Antioxidant Molecule. Antioxidants 2019, 8, 478. https://doi.org/10.3390/antiox8100478
Queiroz MF, Sabry DA, Sassaki GL, Rocha HAO, Costa LS. Gallic Acid-Dextran Conjugate: Green Synthesis of a Novel Antioxidant Molecule. Antioxidants. 2019; 8(10):478. https://doi.org/10.3390/antiox8100478
Chicago/Turabian StyleQueiroz, Moacir Fernandes, Diego Araujo Sabry, Guilherme Lanzi Sassaki, Hugo Alexandre Oliveira Rocha, and Leandro Silva Costa. 2019. "Gallic Acid-Dextran Conjugate: Green Synthesis of a Novel Antioxidant Molecule" Antioxidants 8, no. 10: 478. https://doi.org/10.3390/antiox8100478
APA StyleQueiroz, M. F., Sabry, D. A., Sassaki, G. L., Rocha, H. A. O., & Costa, L. S. (2019). Gallic Acid-Dextran Conjugate: Green Synthesis of a Novel Antioxidant Molecule. Antioxidants, 8(10), 478. https://doi.org/10.3390/antiox8100478






