Anthocyanins Protect Hepatocytes against CCl4-Induced Acute Liver Injury in Rats by Inhibiting Pro-inflammatory mediators, Polyamine Catabolism, Lipocalin-2, and Excessive Proliferation of Kupffer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Bilberry Fruit Extract Preparation and HPLC Analysis
2.3. Animals and Protocol
2.4. Experimental Groups
2.5. Sample Preparation for Biochemical Analyses
2.6. Determination of Glutamate Dehydrogenase (GLDH) Activity
2.7. Determination of Sorbitol Dehydrogenase (SDH) Activity
2.8. Determination of Malate Dehydrogenase (MDH) Activity
2.9. Determination of (TOS) Total Oxidative Status
2.10. Determination of (TAC) Total Antioxidant Capacity
2.11. Determination of Conjugated Dienes (CD) Concentration
2.12. Determination of Lipid Hydroperoxide (LOOH) Concentration
2.13. Determination of Thiobarbituric Acid Reactive Substances (TBARS) Concentration
2.14. Determination of Advanced Oxidation Protein Products (AOPP) Concentration
2.15. Determination of Hydrogen Peroxide (H2O2) Concentration
2.16. Determination of NADPH Oxidase Activity
2.17. Determination of Oxidized Glutathione (GSSG) Concentration
2.18. Determination of Nitrite (NO2−) Determination
2.19. Determination of Myeloperoxidase (MPO) Activity
2.20. Determination of Arginase Activity
2.21. Determination of Citrulline Concentration
2.22. Determination of Putrescine Oxidase (PutOX) Activity
2.23. Determination of Spermine Oxidase (SpmOX) Activity
2.24. Determination of Reduced Glutathione (GSH) Concentration
2.25. Determination of Glutathione S-transferase (GST) Activity
2.26. Determination of Quinone Reductase (QR) Activity
2.27. Determination of Lipocalin-2 (NGAL, LCN2), CD68, TNF-α, IL-4, IL-6, and IL-13 Concentration
2.28. Determination of Protein Concentration
2.29. Histopathological Examinations and Morphometric Analysis
2.30. Immunohistochemical Analysis
2.31. Statistical Analysis
3. Results
3.1. Bilberry Fruit Extract Composition Analysis
3.2. The Impact of CCl4 and the Bilberry Fruit Extract on the Biochemical Markers of Liver Damage
3.3. The Impact of CCl4 and the Bilberry Fruit Extract on the Total Oxidative Status (TOS) and Total Antioxidant Capacity (TAC)
3.4. The Impact of CCl4 and the Bilberry Fruit Extract on Pro-Oxidative Markers
3.5. The Impact of CCl4 and the Bilberry Fruit Extract on Pro-Inflammatory Mediators
3.6. The Impact of CCl4 and Bilberry Fruit Extract on the Arginine and Polyamine Catabolism
3.7. The Impact of CCl4 and the Bilberry Fruit Extract on Antioxidative Liver Markers
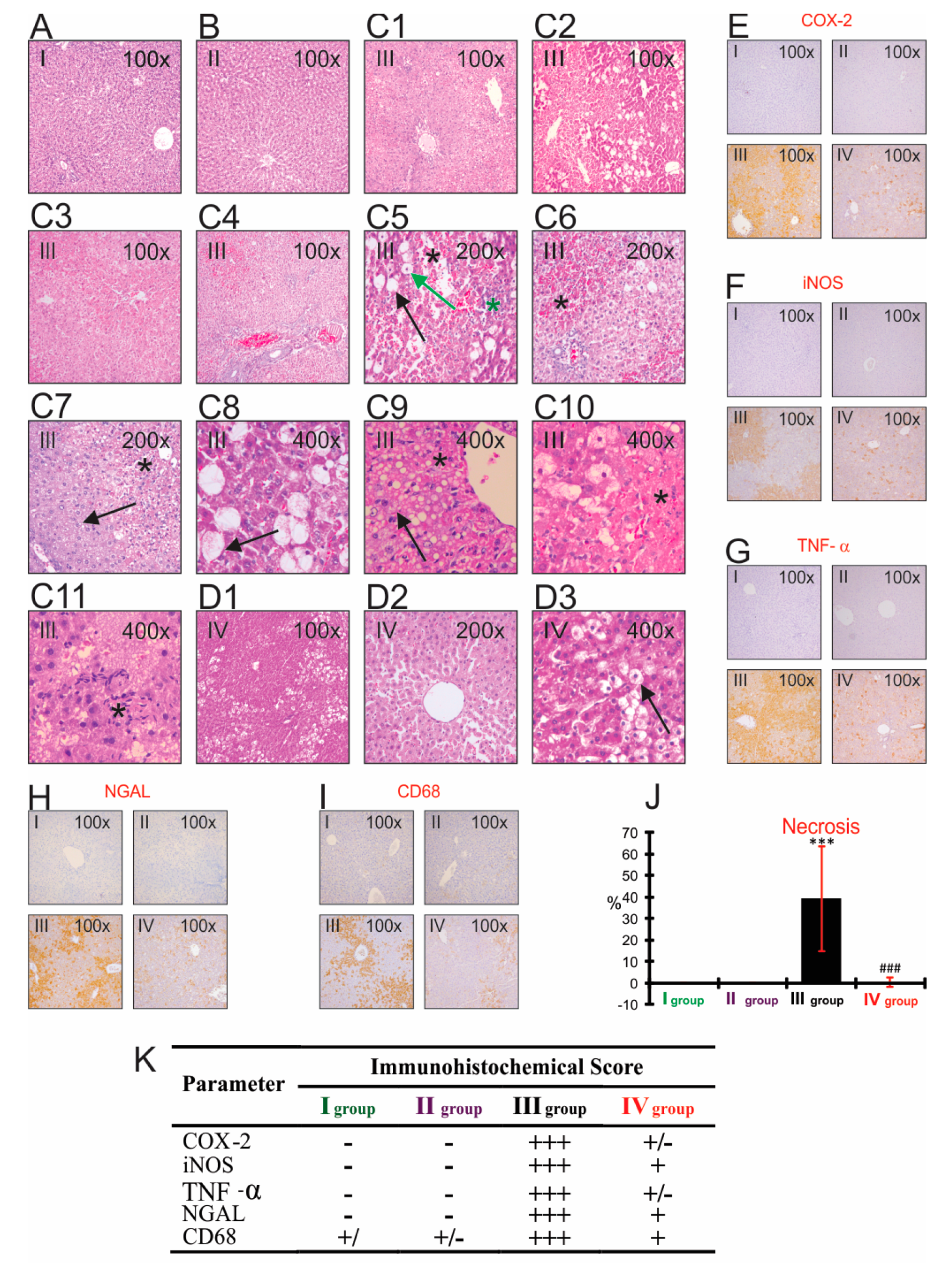
3.8. The Impact of CCl4 and Bilberry Fruit Extract on the Histopathological and Immunohistochemical Analyses
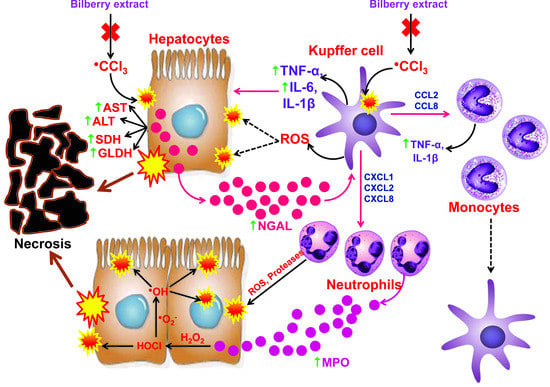
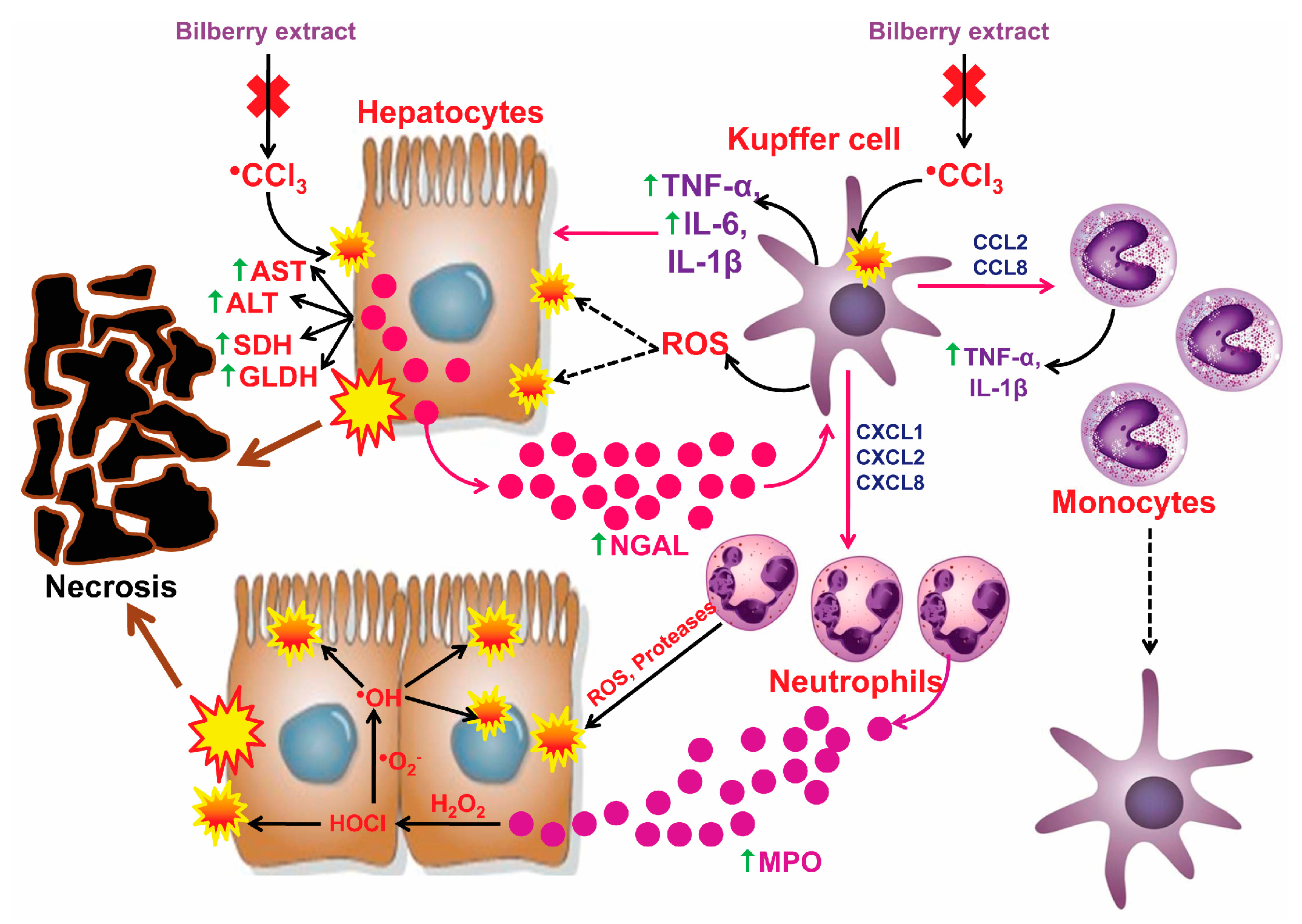
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carakostas, M.C.; Gossett, K.A.; Church, G.E.; Cleghorn, B.L. Evaluating toxin-induced hepatic injury in rats by laboratory results and discriminant analysis. Vet. Pathol. 1986, 23, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.M.; Subeq, Y.M.; Lee, R.P.; Chiou, T.W.; Hsu, B.G. Single dose intravenous thioacetamide administration as a model of acute liver damage in rats. Int. J. Exp. Pathol. 2008, 89, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Ren, N.; Gao, H.; Lei, X.; Zheng, J.; Cao, W. Antioxidant and hepatoprotective effects of Schisandra chinensis pollen extract on CCl4-induced acute liver damage in mice. Food Chem. Toxicol. 2013, 55, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zhang, J.; Guo, F.; Wei, L.; Zhou, Q. Oxidative stress and inflammatory responses in the liver of swamp eel (Monopterus albus) exposed to carbon tetrachloride. Aquaculture 2018, 496, 232–238. [Google Scholar] [CrossRef]
- Ahn, M.; Kim, J.; Bang, H.; Moon, J.; Kim, G.O.; Shin, T. Hepatoprotective effects of allyl isothiocyanate against carbon tetrachloride-induced hepatotoxicity in rat. Chem. Biol. Interact. 2016, 254, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Ritesh, K.R.; Suganya, A.; Dileepkumar, H.V.; Rajashekar, Y.; Shivanandappa, T. A single acute hepatotoxic dose of CCl4 causes oxidative stress in the rat brain. Toxicol. Rep. 2015, 2, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Bhadauria, M.; Nirala, S.K.; Shukla, S. Multiple treatment of propolis extract ameliorates carbon tetrachloride induced liver injury in rats. Food Chem. Toxicol. 2008, 46, 2703–2712. [Google Scholar] [CrossRef]
- Valentová, K.; Ulrichová, J.; Cvak, L.; Šimánek, V. Cytoprotective effect of a bilberry extract against oxidative damage of rat hepatocytes. Food Chem. 2007, 101, 912–917. [Google Scholar] [CrossRef]
- Može, S.; Polak, T.; Gasperlin, L.; Koron, D.; Vanzo, A.; Ulrih, N.P.; Abram, V. Phenolics in Slovenian bilberries (Vaccinium myrtillus L.) and blueberries (Vaccinium corymbosum L.). J. Agric. Food Chem. 2011, 59, 6998–7004. [Google Scholar] [CrossRef]
- Popović, D.; Đukić, D.; Katić, V.; Jović, Z.; Jović, M.; Lalić, J.; Golubović, I.; Stojanović, S.; Ulrih, N.P.; Stanković, M.; et al. Antioxidant and proapoptotic effects of anthocyanins from bilberry extract in rats exposed to hepatotoxic effects of carbon tetrachloride. Life Sci. 2016, 157, 168–177. [Google Scholar] [CrossRef]
- Szymanowska, U.; Baraniak, B. Antioxidant and Potentially Anti-Inflammatory Activity of Anthocyanin Fractions from Pomace Obtained from Enzymatically Treated Raspberries. Antioxidants 2019, 8, 299. [Google Scholar] [CrossRef] [PubMed]
- Torres, L.R.; Santana, F.C.; Torres-Leal, F.L.; Melo, L.L.; Yoshime, L.T.; Matos-Neto, E.M.; Seelaender, M.C.; Araújo, C.M.; Cogliati, B.; Mancini-Filho, J. Pequi (Caryocar brasiliense Camb.) almond oil attenuates carbon tetrachloride-induced acute hepatic injury in rats: Antioxidant and anti-inflammatory effects. Food Chem. Toxicol. 2016, 97, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, M.T.; Cohen, J.I.; Roychowdhury, S.; Pratt, B.T.; Nagy, L.E. Early growth response-1 attenuates liver injury and promotes hepatoprotection after carbon tetrachloride exposure in mice. J. Hepatol. 2010, 53, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Asimakopoulou, A.; Weiskirchen, S.; Weiskirchen, R. Lipocalin2 (LCN2) Expression in Hepatic Malfunction and Therapy. Front. Physiol. 2016, 7, 430. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, O.A.; Bartosch, B.; Zakirova, N.F.; Kochetkov, S.N.; Ivanov, A.V. Polyamine Metabolism and Oxidative Protein Folding in the ER as ROS-Producing Systems Neglected in Virology. Int. J. Mol. Sci. 2018, 19, 1219. [Google Scholar] [CrossRef] [PubMed]
- Popović, D.; Kocić, G.; Katić, V.; Jović, Z.; Zarubica, A.; Veličković, L.J.; Nikolić, V.; Jović, A.; Kundalić, B.; Rakić, V.; et al. Protective effects of anthocyanins from bilberry extract in rats exposed to nephrotoxic effects of carbon tetrachloride. Chem. Biol. Interact. 2019, 304, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Kuratsu, T.; Hirata, F. Purification and some properties of glutamate dehydrogenase from Proteus inconstans. J. Ferment. Technol. 1979, 57, 428–433. [Google Scholar]
- Rose, C.L.; Henderson, A.R. Reaction-rate assay of serum sorbitol dehydrogenase activity at 37 C. Clin. Chem. 1975, 21, 1619–1626. [Google Scholar]
- Žewski, M.; Świerczyński, J. Malic enzyme in human liver Intracellular distribution, purification and properties of cytosolic isozyme. Eur. J. Biochem. 1991, 201, 339–345. [Google Scholar] [CrossRef]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Devasagayam, T.P.A.; Boloor, K.K.; Ramasarma, T. Methods for estimating lipid peroxidation: An analysis of merits and demerits. Indian J. Biochem. Biophys. 2003, 40, 300–308. [Google Scholar] [PubMed]
- Hermes-Lima, M.; Willmore, W.G.; Storey, K.B. Quantification of lipid peroxidation in tissue extracts based on Fe(III)xylenol orange complex formation. Free Radic. Biol. Med. 1995, 19, 271–280. [Google Scholar] [CrossRef]
- Sahreen, S.; Khan, M.R.; Khan, R.A. Hepatoprotective effects of methanol extract of Carissa opaca leaves on CCl4-induced damage in rat. BMC Complement. Altern. Med. 2011, 11, 48. [Google Scholar] [CrossRef] [PubMed]
- Witko-Sarsat, V.; Friedlander, M.; Khoa, T.N.; Capeillère-Blandin, C.; Nguyen, A.T.; Canteloup, S.; Dayer, J.M.; Jungers, P.; Drüeke, T.; Descamps-Latscha, B. Advanced Oxidation Protein Products as Novel Mediators of Inflammation and Monocyte Activation in Chronic Renal Failure. J. Immunol. 1998, 161, 2524–2532. [Google Scholar]
- Pick, E.; Keisari, Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J. Immunol. Methods 1980, 38, 161–170. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, J. Involvement of plasma-membrane NADPH oxidase in abscisic acid-and water stress-induced antioxidant defense in leaves of maize seedlings. Planta 2002, 215, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2006, 1, 3159–3165. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.; Khan, M.R.; Shah, N.A.; Ullah, S.; Younis, T.; Majid, M.; Ahmad, B.; Nigussie, D. Proficiencies of Artemisia Scoparia against CCl4 induced DNA damages and renal toxicity in rat. BMC Complement. Altern. Med. 2016, 16, 149. [Google Scholar] [CrossRef] [PubMed]
- Desser, R.K.; Himmelhoch, S.R.; Evans, W.H.; Januska, M.; Mage, M.; Shelton, E. Guinea pig heterophil and eosinophil peroxidase. Arch.Biochem. Biophys. 1972, 148, 452–465. [Google Scholar] [CrossRef]
- Porembska, Z.; Kedra, M. Early diagnosis of myocardial infarction by arginase activity determination. Clin. Chim. Acta 1975, 60, 355–361. [Google Scholar] [CrossRef]
- Knipp, M.; Vašák, M. A Colorimetric 96-Well Microtiter Plate Assay for the Determination of Enzymatically Formed Citrulline. Anal. Biochem. 2000, 286, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Quash, G.; Gresland, L.; Delain, E.; Huppert, J. Antipolyamine antibodies and cell lysis: The Inhibitory Effect of Putrescine. Exp. Cell Res. 1972, 75, 363–368. [Google Scholar] [CrossRef]
- Simons, P.C.; Vander Jagt, D.L. Purification of glutathione S-transferases from human liver by glutathione-affinity chromatography. Anal. Biochem. 1977, 82, 334–341. [Google Scholar] [CrossRef]
- Shah, M.D.; Gnanaraj, C.; Haque, A.E.; Iqbal, M. Antioxidative and chemopreventive effects of Nephrolepis biserrata against carbon tetrachloride (CCl4)-induced oxidative stress and hepatic dysfunction in rats. Pharm. Biol. 2015, 53, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, W.; Kim, Y.H.; Lee, Y.W. Chlorella vulgaris extract ameliorates carbon tetrachloride-induced acute hepatic injury in mice. Exp. Toxicol. Pathol. 2013, 65, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Kondeva-Burdina, M.; Shkondrov, A.; Simeonova, R.; Vitcheva, V.; Krasteva, I.; Ionkova, I. In vitro/in vivo antioxidant and hepatoprotective potential of defatted extract and flavonoids isolated from Astragalus spruneri Boiss. (Fabaceae). Food Chem. Toxicol. 2018, 111, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Domitrović, R.; Rashed, K.; Cvijanović, O.; Vladimir-Knežević, S.; Škoda, M.; Višnić, A. Myricitrin exhibits antioxidant, anti-inflammatory and antifibrotic activity in carbon tetrachloride-intoxicated mice. Chem. Biol. Interact. 2015, 230, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Kemelo, M.K.; Pierzynová, A.; Canová, N.K.; Kučera, T.; Farghali, H. The involvement of sirtuin 1 and heme oxygenase 1 in the hepatoprotective effects of quercetin against carbon tetrachloride-induced sub-chronic liver toxicity in rats. Chem. Biol. Interact. 2017, 269, 1–8. [Google Scholar] [CrossRef]
- Yamaji, K.; Ochiai, Y.; Ohnishi, K.I.; Yawata, A.; Chikuma, T.; Hojo, H. Up-regulation of hepatic heme oxygenase-1 expression by locally induced interleukin-6 in rats administered carbon tetrachloride intraperitoneally. Toxicol. Lett. 2008, 179, 124–129. [Google Scholar] [CrossRef]
- Ma, J.Q.; Ding, J.; Xiao, Z.H.; Liu, C.M. Puerarin ameliorates carbon tetrachloride-induced oxidative DNA damage and inflammation in mouse kidney through ERK/Nrf2/ARE pathway. Food Chem. Toxicol. 2014, 71, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Żebrowska, E.; Maciejczyk, M.; Żendzian-Piotrowska, M.; Zalewska, A.; Chabowski, A. High Protein Diet Induces Oxidative Stress in Rat Cerebral Cortex and Hypothalamus. Int. J. Mol. Sci. 2019, 20, 1547. [Google Scholar] [CrossRef] [PubMed]
- Veal, E.A.; Toone, W.M.; Jones, N.; Morgan, B.A. Distinct roles for glutathione S-transferases in the oxidative stress response in Schizosaccharomyces pombe. J. Biol. Chem. 2002, 277, 35523–35531. [Google Scholar] [CrossRef] [PubMed]
- Mileo, A.M.; Miccadei, S. Polyphenols as Modulator of Oxidative Stress in Cancer Disease: New Therapeutic Strategies. Oxid. Med. Cell. Longev. 2016, 2016, 6475624. [Google Scholar] [CrossRef] [PubMed]
- Korkina, L.G. Phenylpropanoids as naturally occurring antioxidants: From plant defense to human health. Cell. Mol. Biol. 2007, 53, 15–25. [Google Scholar]
- Weisel, T.; Baum, M.; Eisenbrand, G.; Dietrich, H.; Will, F.; Stockis, J.P.; Kulling, S.; Rüfer, C.; Johannes, C.; Janzowski, C. An anthocyanin/polyphenolic-rich fruit juice reduces oxidative DNA damage and increases glutathione level in healthy probands. Biotechnol. J. 2006, 1, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Knockaert, L.; Berson, A.; Ribault, C.; Prost, P.E.; Fautrel, A.; Pajaud, J.; Lepage, S.; Lucas-Clerc, C.; Bégué, J.M.; Fromenty, B.; et al. Carbon tetrachloride-mediated lipid peroxidation induces early mitochondrial alterations in mouse liver. Lab. Investig. 2012, 92, 396. [Google Scholar] [CrossRef]
- Bami, E.; Ozakpınar, O.B.; Ozdemir-Kumral, Z.N.; Köroglu, K.; Ercan, F.; Cirakli, Z.; Sekerler, T.; Izzettin, F.V.; Sancar, M.; Okuyan, B. Protective effect of ferulic acid on cisplatin induced nephrotoxicity in rats. Environ. Toxicol. Pharmacol. 2017, 54, 105–111. [Google Scholar] [CrossRef]
- DeCicco, L.A.; Rikans, L.E.; Tutor, C.G.; Hornbrook, K.R. Serum and liver concentrations of tumor necrosis factor α and interleukin-1β following administration of carbon tetrachloride to male rats. Toxicol. Lett. 1998, 98, 115–121. [Google Scholar] [CrossRef]
- Teschke, R.; Vierke, W.; Goldermann, L. Carbon tetrachloride (CCl4) levels and serum activities of liver enzymes following acute CCl4 intoxication. Toxicol. Lett. 1983, 17, 175–180. [Google Scholar] [CrossRef]
- Schomaker, S.; Warner, R.; Bock, J.; Johnson, K.; Potter, D.; Van Winkle, J.; Aubrecht, J. Assessment of Emerging Biomarkers of Liver Injury in Human Subjects. Toxicol. Sci. 2013, 132, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Ozer, J.; Ratner, M.; Shawc, M.; Bailey, W.; Schomaker, S. The current state of serum biomarkers of hepatotoxicity. Toxicology 2008, 245, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.O.; Ryu, H.W.; So, Y.; Jin, C.H.; Baek, J.Y.; Park, K.H.; Byun, E.H.; Jeong, I.Y. Hepatoprotective effect of 2,3-dehydrosilybin on carbon tetrachloride-induced liver injury in rats. Food Chem. 2013, 138, 107–115. [Google Scholar] [CrossRef] [PubMed]
- El-Haskoury, R.; Al-Waili, N.; Kamoun, Z.; Makni, M.; Al-Waili, H.; Lyoussi, B. Antioxidant Activity and Protective Effect of Carob Honey in CCl4-induced Kidney and Liver Injury. Arch. Med Res. 2018, 49, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Ljubuncic, P.; Tanne, Z.; Bomzon, A. Evidence of a systemic phenomenon for oxidative stress in cholestatic liver disease. Gut 2000, 47, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Mubarak, O.B.; Morsi, N.F.; Hamama, M. Roles of H. rosa sinensis extract in the treatment of liver against CCl4-induced hepatic oxidative stress and antioxidants disorder in male albino rats. Int. J. Med. Plants Res. 2016, 5, 209–220. [Google Scholar]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Deng, J.S.; Chang, Y.C.; Wenc, C.L.; Liao, J.C.; Hou, W.C.; Amagaya, S.; Huang, S.S.; Huang, G.J. Hepatoprotective effect of the ethanol extract of Vitisthun bergii on carbon tetrachloride-induced acute hepatotoxicity in rats through anti-oxidative activities. J. Ethnopharmacol. 2012, 142, 795–803. [Google Scholar] [CrossRef]
- Chan, C.C.; Lee, K.C.; Huang, Y.H.; Chou, C.K.; Lin, H.C.; Lee, F.Y. Regulation by resveratrol of the cellular factors mediating liver damage and regeneration after acute toxic liver injury. J.Gastroenterol. Hepatol. 2014, 29, 603–613. [Google Scholar] [CrossRef]
- Dong, X.; Liu, J.; Xu, Y.; Cao, H. Role of macrophages in experimental liver injury and repair in mice. Exp. Ther. Med. 2019, 17, 3835–3847. [Google Scholar] [CrossRef]
- Dixon, L.J.; Barnes, M.; Tang, H.; Pritchard, M.T.; Nagy, L.E. Kupffer Cells in the Liver. Compr. Physiol. 2013, 3, 785–797. [Google Scholar] [PubMed]
- Bocsan, I.C.; Milaciu, M.V.; Pop, R.M.; Vesa, S.C.; Ciumarnean, L.; Matei, D.M.; Buzoianu, A.D. Cytokines Genotype-Phenotype Correlation in Nonalcoholic Steatohepatitis. Oxid. Med. Cell Longev. 2017, 2017, 4297206. [Google Scholar] [CrossRef] [PubMed]
- Borkham-Kamphorst, E.; Drews, F.; Weiskirchen, R. Induction of lipocalin-2 expression in acute and chronic experimental liver injury moderated by pro-inflammatory cytokines interleukin-1β through nuclear factor-κB activation. Liver Int. 2011, 31, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Dahl, S.L.; Woodworth, J.S.; Lerche, C.J.; Cramer, E.P.; Nielsen, P.R.; Moser, C.; Thomsen, A.R.; Borregaard, N.; Cowland, J.B. Lipocalin-2 Functions as Inhibitor of Innate Resistance to Mycobacterium tuberculosis. Front. Immunol. 2018, 9, 2717. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz-Ozden, T.; Can, A.; Karatug, A.; Pala-Kara, Z.; Okyar, A.; Bolkent, S. Carbon tetrachloride-induced kidney damage and protective effect of Amaranthus lividus L. in rats. Toxicol. Ind. Health 2016, 32, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Vijaya, S.; Nagarajan, B. Arginine metabolism in rat liver after hepatic damage. Biochem. Med. 1982, 27, 86–94. [Google Scholar] [CrossRef]
- Zahedi, K.; Barone, S.L.; Xu, J.; Steinbergs, N.; Schuster, R.; Lentsch, A.B.; Amlal, H.; Wang, J.; Casero, R.A., Jr.; Soleimani, M. Hepatocyte-specific ablation of spermine/spermidine-N1-acetyltransferase gene reduces the severity of CCl4-induced acute liver injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, 546–560. [Google Scholar] [CrossRef]
- Hesse, M.; Modolell, M.; La Flamme, A.C.; Schito, M.; Fuentes, J.M.; Cheever, A.W.; Pearce, E.J.; Wynn, T.A. Differential Regulation of Nitric Oxide Synthase-2 and Arginase-1 by Type 1/Type 2 Cytokines in Vivo: Granulomatous Pathology Is Shaped by the Pattern of L-Arginine Metabolism. J. Immunol. 2001, 167, 6533–6544. [Google Scholar] [CrossRef] [PubMed]
- Laskin, D.L.; Laskin, J.D. Role of macrophages and inflammatory mediators in chemically induced toxicity. Toxicology 2001, 160, 111–118. [Google Scholar] [CrossRef]
- Bhatnagar, P.; Minocha, R.; Minocha, S.C. Genetic Manipulation of the Metabolism of Polyamines in Poplar Cells. The Regulation of Putrescine Catabolism. Plant Physiol. 2002, 128, 1455–1469. [Google Scholar] [CrossRef][Green Version]
- Nikolic, J.; Stojanovic, I.; Pavlovic, R.; Sokolovic, D.; Bjelakovic, G.; Beninati, S. The role of L-arginine in toxic liver failure: Interrelation of arginase, polyamine catabolic enzymes and nitric oxide synthase. Amino Acids 2007, 32, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; You, Y.; Yoon, H.G.; Lee, Y.H.; Kim, K.; Lee, J.; Kim, M.S.; Kim, J.S.; Jun, W. Hepatoprotective effects of fermented Curcuma longa L. on carbon tetrachloride-induced oxidative stress in rats. Food Chem. 2014, 151, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, M.; Akdogan, M.; Keskin, I.; Kisioglu, A.N.; Oztas, S.; Yildiz, K. Effect of Silybum marianum on acute hepatic damage caused by carbon tetrachloride in rats. Biomed. Res. 2012, 23, 268–274. [Google Scholar]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, R.; Zhao, H.; Chen, G.; Jiang, Y.; Lyu, X.; Wu, T. Reduction of Aging-Induced Oxidative Stress and Activation of Autophagy by Bilberry Anthocyanin Supplementation via the AMPK−mTOR Signaling Pathway in Aged Female Rats. J. Agric. Food Chem. 2019, 67, 7832–7843. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cao, G.; Prior, R.L. Oxygen Radical Absorbing Capacity of Anthocyanins. J. Agric.Food Chem. 1997, 45, 304–309. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Heinonen, M. Antioxidant activity of anthocyanins and their Aglycons. J. Agric.Food Chem. 2003, 51, 628–633. [Google Scholar] [CrossRef] [PubMed]

| Individual Anthocyanins in the Bilberry Extract | Concentration in Extract (mg/L) | Percentage of Anthocyanins Compounds in the Extract (%) | |
|---|---|---|---|
| Anthocyanins | Delphinidin 3-galactoside | 634.36 | 14.16 |
| Delphinidin 3-glucoside | 628.26 | 14.02 | |
| Delphinidin3-arabinoside | 578.08 | 12.90 | |
| Cyanidin 3-galactoside | 497.41 | 11.10 | |
| Cyanidin 3-glucoside | 489.76 | 10.93 | |
| Cyanidin3-arabinoside | 393.90 | 8.79 | |
| Petunidin 3-glucoside | 345.05 | 7.70 | |
| Petunidin 3-galactoside | 169.84 | 3.79 | |
| Petunidin3-arabinoside | 88.73 | 1.98 | |
| Peonidin 3-glucoside | 183.73 | 4.10 | |
| Peonidin 3-galactoside | 43.78 | 0.98 | |
| Peonidin3-arabinoside | 15.55 | 0.35 | |
| Malvidin 3-glucoside | 264.39 | 5.90 | |
| Malvidin 3-galactoside | 89.18 | 1.99 | |
| Malvidin3-arabinoside | 59.60 | 1.33 | |
| Total Anthocyanins | 4481.62 | 100.0 | |
| Group | GLDH (U/L) | SDH (U/L) | MDH (U/L) |
|---|---|---|---|
| I (not treated) | 46.16 ± 5.48 | 31.51 ± 9.49 | 102.17 ± 28.85 |
| II (treated b) | 42.16 ± 5.48 | 36.29 ± 13.22 | 96.49 ± 29.46 |
| III (CCl4) | 182.69 ± 17.14 *** | 239.23 ± 27.95 *** | 310.83 ± 63.28 *** |
| IV (treated b + CCl4) | 91.60 ± 6.51 ### | 73.89 ± 10.35 ### | 117.50 ± 14.46 ### |
| Group | TOS (nM/mg Protein) | TAC (nM/mg Protein) |
|---|---|---|
| I (not treated) | 4.32 ± 1.16 | 27.93 ± 2.88 |
| II (treated b) | 4.54 ± 1.23 | 35.44 ± 3.92 §§ |
| III (CCl4) | 16.63 ± 4.19 *** | 13.22 ± 2.47 *** |
| IV (treated b + CCl4) | 9.53 ± 1.58 ## | 23.07 ± 3.81 ### |
| Group | CD (nM/mg Protein) | LOOH (nM/mg Protein) | TBARS (nM/mg Protein) | AOPP (nM/mg Protein) |
|---|---|---|---|---|
| I (not treated) | 10.36 ± 1.98 | 16.18 ± 1.37 | 2.58 ± 0.56 | 32.77 ± 4.13 |
| II (treated b) | 10.12 ± 1.69 | 17.04 ± 1.99 | 2.55 ± 0.41 | 31.67 ± 3.34 |
| III (CCl4) | 20.23 ± 3.28 *** | 34.12 ± 5.22 *** | 5.89 ± 0.58 *** | 54.68 ± 10.61 *** |
| IV (treated b + CCl4) | 13.98 ± 2.25 ### | 22.98 ± 3.28 ## | 4.07 ± 0.81 ### | 43.9 ± 3.30 # |
| Group | H2O2 (nM/mg Protein) | GSSG (nM/mg Protein) | NADPH Oxidase (nM/mg Protein) |
|---|---|---|---|
| I (not treated) | 12.80 ± 2.32 | 2.51 ± 0.69 | 12.73 ± 1.43 |
| II (treated b) | 12.91 ± 2.89 | 2.39 ± 0.48 | 12.51 ± 2.91 |
| III (CCl4) | 27.97 ± 2.95 *** | 6.14 ± 0.63 *** | 45.69 ± 4.72 *** |
| IV (treated b + CCl4) | 18.89 ± 2.85 ### | 4.39 ± 1.24 # | 23.01 ± 3.15 ### |
| Group | TNF-α (pg/mg Protein) | IL-6 (pg/mg Protein) | Nitrite (μM/mg Protein) | MPO (U/mg Protein) | CD68 (ng/mg Protein) | NGAL (pg/mg Protein) |
|---|---|---|---|---|---|---|
| I (not treated) | 163.56 ± 33.43 | 15.09 ± 0.78 | 3.88 ± 0.26 | 1.07 ± 0.08 | 3.12 ± 0.69 | 25.48 ± 10.52 |
| II (treated b) | 173.13 ± 35.86 | 15.42 ± 1.49 | 4.04 ± 0.39 | 1.02 ± 0.12 | 3.06 ± 0.82 | 23.14 ± 5.92 |
| III (CCl4) | 440.75 ± 54.33 *** | 46.27 ± 2.43*** | 9.03 ± 1.27 *** | 2.99 ± 0.33 *** | 18.27 ± 4.28 *** | 102.21 ± 20.02 *** |
| IV (treated b + CCl4) | 297.89 ± 36.32 ### | 33.25 ± 2.39 ### | 5.39 ± 0.84 ### | 1.68 ± 0.23 ### | 5.91 ± 1.04 ### | 58.55 ± 15.34 ## |
| Group | Arginase c (μmol/L) | Arginase d (μmol/mg Protein) | Citrulline d (μmol/mg Protein) | IL-4 d (ng/mg Protein) | IL-13 d (pg/mg Protein) |
|---|---|---|---|---|---|
| I (not treated) | 79.58 ± 15.65 | 0.63 ± 0.07 | 0.4 ± 0.06 | 1.14 ± 0.13 | 35.78 ± 1.78 |
| II (treated b) | 83.72 ± 23.38 | 0.65 ± 0.08 | 0.39 ± 0.05 | 1.13 ± 0.18 | 35.57 ± 1.79 |
| III (CCl4) | 1295.82 ± 282.73 *** | 0.20 ± 0.06 *** | 0.97 ± 0.19 *** | 0.62 ± 0.17 *** | 20.41 ± 2.39 *** |
| IV (treated b + CCl4) | 290.26 ± 26.98 ### | 0.37 ± 0.05 ### | 0.61 ± 0.06 ## | 0.87 ± 0.12 ## | 25.65 ± 2.12 ## |
| Group | PutOX (nM/mg Protein) | SpmOX (nM/mg Protein) |
|---|---|---|
| I (not treated) | 3.34 ± 0.84 | 3.10 ± 0.89 |
| II (treated b) | 3.36 ± 0.69 | 3.58 ± 1.10 |
| III (CCl4) | 7.98 ± 1.49 *** | 10.34 ± 1.51 *** |
| IV (treated b + CCl4) | 5.74 ± 0.57 ## | 7.47 ± 1.09 ## |
| Group | GST (nM/mg Protein) | QR (nM/mg Protein) | GSH (nM/mg Protein) |
|---|---|---|---|
| I (not treated) | 176.54 ± 17.95 | 34.22 ± 3.46 | 58.83.± 5.65 |
| II (treated b) | 222.14 ± 19.28 §§ | 40.54 ± 1.79 §§ | 66.73 ± 3.09 § |
| III (CCl4) | 76.59 ± 9.54 *** | 24.14 ± 6.63 *** | 22.99 ± 5.35 *** |
| IV (treated b + CCl4) | 127.89 ± 16.89 ### | 33.79 ± 4.96 # | 44.42± 7.37 ### |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popović, D.; Kocić, G.; Katić, V.; Zarubica, A.; Janković Veličković, L.; Ničković, V.P.; Jović, A.; Veljković, A.; Petrović, V.; Rakić, V.; et al. Anthocyanins Protect Hepatocytes against CCl4-Induced Acute Liver Injury in Rats by Inhibiting Pro-inflammatory mediators, Polyamine Catabolism, Lipocalin-2, and Excessive Proliferation of Kupffer Cells. Antioxidants 2019, 8, 451. https://doi.org/10.3390/antiox8100451
Popović D, Kocić G, Katić V, Zarubica A, Janković Veličković L, Ničković VP, Jović A, Veljković A, Petrović V, Rakić V, et al. Anthocyanins Protect Hepatocytes against CCl4-Induced Acute Liver Injury in Rats by Inhibiting Pro-inflammatory mediators, Polyamine Catabolism, Lipocalin-2, and Excessive Proliferation of Kupffer Cells. Antioxidants. 2019; 8(10):451. https://doi.org/10.3390/antiox8100451
Chicago/Turabian StylePopović, Dejan, Gordana Kocić, Vuka Katić, Aleksandra Zarubica, Ljubinka Janković Veličković, Vanja P. Ničković, Andrija Jović, Andrej Veljković, Vladimir Petrović, Violeta Rakić, and et al. 2019. "Anthocyanins Protect Hepatocytes against CCl4-Induced Acute Liver Injury in Rats by Inhibiting Pro-inflammatory mediators, Polyamine Catabolism, Lipocalin-2, and Excessive Proliferation of Kupffer Cells" Antioxidants 8, no. 10: 451. https://doi.org/10.3390/antiox8100451
APA StylePopović, D., Kocić, G., Katić, V., Zarubica, A., Janković Veličković, L., Ničković, V. P., Jović, A., Veljković, A., Petrović, V., Rakić, V., Jović, Z., Poklar Ulrih, N., Sokolović, D., Stojanović, M., Stanković, M., Radenković, G., Nikolić, G. R., Lukač, А., Milosavljević, A., & Sokolović, D. (2019). Anthocyanins Protect Hepatocytes against CCl4-Induced Acute Liver Injury in Rats by Inhibiting Pro-inflammatory mediators, Polyamine Catabolism, Lipocalin-2, and Excessive Proliferation of Kupffer Cells. Antioxidants, 8(10), 451. https://doi.org/10.3390/antiox8100451