Abstract
Physiological levels of reactive oxygen species (ROS) play a crucial role as intracellular signaling molecules, helping to maintain cellular homeostasis. However, when ROS accumulate excessively, they become toxic to cells, leading to damage to lipids, proteins, and DNA. This oxidative stress can impair cellular function and lead to various forms of cell death, including apoptosis, necroptosis, ferroptosis, pyroptosis, paraptosis, parthanatos, and oxeiptosis. Despite their significance, the role of ROS in autosis (an autophagy-dependent form of cell death) remains largely unexplored. In this review, we gather current knowledge on autotic cell death and summarize how oxidative stress influences the activity of Beclin-1 and the Na+,K+-ATPase pump, both of which are critical effectors of this pathway. Finally, we discuss the theoretical potential for ROS to modulate this type of cell death, proposing a possible dual role for these species in autosis regulation through effectors such as HIF-1α, TFEB, or the FOXO family, and highlighting the need to experimentally address cellular redox status when working on autotic cell death.
1. Introduction
The oxidoreduction (redox) state of a cell is the center of its metabolism. Almost all metabolic pathways include redox reactions, which affect the redox state of the cell and, at the same time, are influenced by it [1]. The redox state of cells is primarily established by the ratios of the cofactors NADH/NAD+, NADPH/NADP+, and glutathione (GSH)/glutathione disulfide (GSSG), as well as the balance between reactive oxygen species (ROS) and antioxidants [2]. Maintaining these ratios is essential for cellular homeostasis, which is critical for its correct function and adaptation to environmental stressors. Interestingly, moderate levels of ROS are beneficial, as they preserve, modulate, and regulate cellular functions. However, excessive production of ROS causes oxidative stress, which provokes cellular damage and dysfunction, affecting different cellular mechanisms, including autophagy [3].
Autophagy is an evolutionarily well-conserved pathway essential for cell survival, characterized by the delivery of intracellular components to the lysosome for degradation and recycling. To date, three major forms of autophagy have been described: macroautophagy, microautophagy, and chaperone-mediated autophagy [4]. Amongst them, macroautophagy remains the most studied autophagy variant, and it is defined by the formation of double-membraned vesicles (termed “autophagosomes”) that sequester cellular cargo. These autophagosomes eventually fuse with lysosomes, where pH-sensitive hydrolases mediate degradation of the enclosed material [5]. Autophagy is essential to maintain cellular homeostasis and viability, as it avoids the accumulation of damaged intracellular components and ensures the metabolic needs of the cell during stress and nutrient starvation conditions [6]. Furthermore, it also plays an important role in additional processes, such as intercellular communication, modulation of immune cell functions, and maintenance of tissue barrier integrity [4]. However, autophagy dysregulation or excessive autophagic flux can lead to autophagy-mediated cell death, as it occurs with autosis [7].
In this review, we summarize our current knowledge on autotic cell death and how oxidative stress influences the activity of Beclin-1 and the Na+,K+-ATPase pump, crucial mediators of autosis. Finally, we discuss the potential for ROS to modulate this type of cell death, hypothesizing a possible dual role for these species in autosis regulation and highlighting the need to address redox status when working on autotic cell death.
2. ROS and Oxidative Stress
Oxidative stress is a cellular phenomenon characterized by an imbalance between the production of ROS and the antioxidant defence mechanisms. Under normal physiological conditions, the presence of antioxidants neutralizes these reactive species, maintaining cellular homeostasis. However, some factors, such as radiation (e.g., UV), inflammation, and different metabolic processes, can disrupt this balance and lead to excessive ROS production [8]. These species are highly reactive and can cause damage to cellular components such as DNA, proteins, and lipids. These aggressions can lead to mutations in critical genes, alterations in signaling pathways, and impaired cellular functions [9,10]. For instance, the accumulation of DNA damage induced by ROS in cancer can contribute to genetic instability and the development of malignant tumors, promoting proliferation, angiogenesis, and resistance to cell death, providing favorable conditions for tumor growth and metastasis [11,12]. In addition to ROS, reactive nitrogen species (RNS) also play a significant role in oxidative stress, contributing to cellular and DNA damage and affecting mitochondrial functions [13].
Mitochondria are the primary intracellular source of reactive oxygen species (ROS). During oxidative phosphorylation, complexes I and III of the mitochondrial electron transport chain generate superoxide anions (O2•—) as byproducts of electron leakage. These anions can be converted into hydrogen peroxide (H2O2), which, in the presence of transition metal ions such as Fe2+ or Cu+, leads to the formation of highly reactive hydroxyl radicals (•OH) through the Fenton reaction [14]. In addition to this canonical pathway, mitochondria can also produce ROS via reverse electron transport (RET), a process in which electrons flow backwards from complex II to complex I. This phenomenon occurs under specific metabolic conditions, such as high substrate availability and elevated mitochondrial membrane potential, and results in a burst of superoxide production. RET has gained attention as a potent and regulated source of mitochondrial ROS, with implications in both physiological signaling and pathological oxidative damage [15]. Beyond mitochondria, several non-mitochondrial sources contribute to the intracellular generation of reactive oxygen species. One of the most prominent is NADPH oxidase (NOX), a key enzyme involved in inflammatory responses, particularly in immune cells such as neutrophils and macrophages, where it produces superoxide during the respiratory burst [16]. Furthermore, xanthine oxidase, involved in purine metabolism, also generates superoxide as a byproduct of its enzymatic activity [17]. Under pathological conditions, nitric oxide synthase (NOS) can shift from its normal function and contribute to oxidative stress by producing peroxynitrite (ONOO—) through the reaction of nitric oxide with superoxide [18]. Additionally, enzymes in the endoplasmic reticulum, such as members of the cytochrome P450 family, can generate ROS during the metabolism of xenobiotics, especially when ER stress or substrate overload occurs [19]. While all of these systems play essential roles in normal cellular physiology, their dysregulation can lead to excessive ROS accumulation and significant oxidative damage.
Oxidative stress has been related to many pathological conditions, such as cancer, cardiovascular diseases, diabetes, and neurodegenerative diseases [20,21,22,23]. In fact, it has been identified as a key mediator of cell death processes in several of these diseases. For example, oxidative stress can contribute to the emergence of ferroptosis cell death in some cardiovascular diseases, such as atherosclerosis or ischemia [24,25]. In addition, several cell death mechanisms that have been demonstrated to be related to oxidative stress, like apoptosis, necroptosis, or autophagy-dependent cell death, are also relevant in neurodegenerative diseases [26,27,28]. Cells activate different pathways when they detect ROS accumulation to prevent further damage, namely NF-κB, MAPK/p38, or JNK (c-Jun N-terminal kinase), which may induce apoptosis if necessary [29]. A less dramatic response can be mediated by autophagic activation, which would be induced by different pro-survival mediators, such as AMPK or nuclear factor erythroid 2-related factor 2 (NRF2), or directly activating autophagic proteins like ATG4 [30,31,32]. But autophagy can also result in cellular death when exacerbated [33], which could happen in response to uncontrolled oxidative stress. Thus, it is necessary to evaluate the possible interplay between ROS and autophagic cell death and clarify how these species regulate autophagic cell death modes such as autosis.
3. Autotic Cell Death
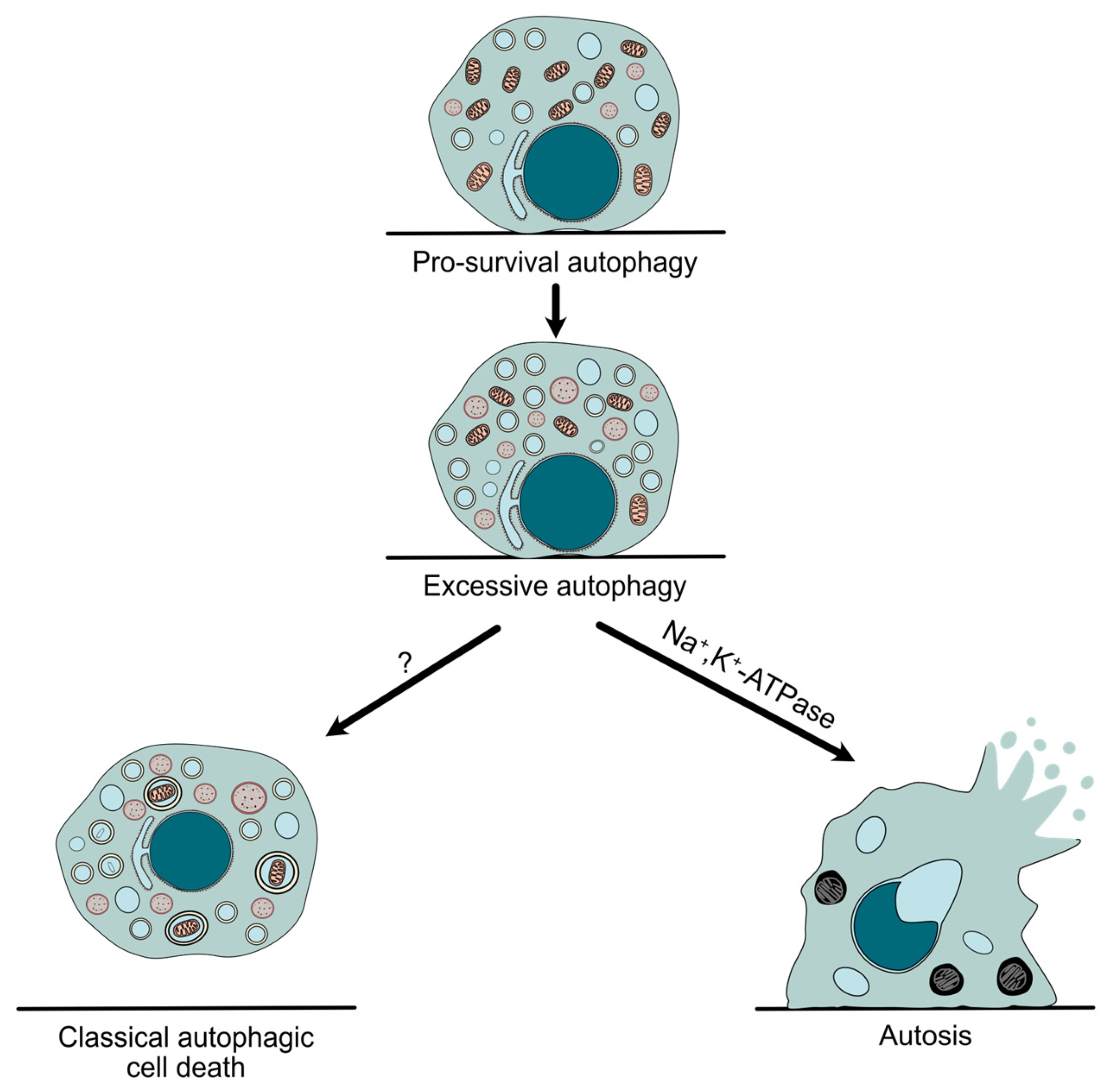
Autophagy is a conserved catabolic pathway that mediates the lysosomal degradation of intracellular threats and long-lived organelles, enabling cells to adapt to environmental stresses such as nutrient deprivation or hypoxia [34]. Even though autophagy has been mainly described as a pro-survival cellular mechanism, it was linked to cell death long ago. Historically, the classification of cell death has been based on morphological traits, distinguishing type I (apoptosis), type II (autophagic cell death), and type III (necrosis) [35]. Accordingly, the term “autophagic cell death” was first used upon ultrastructural features such as the accumulation of autophagosomes. However, autophagy can sometimes be induced during cell death as a protective mechanism, without being responsible for the final demise of the cell. For this reason, the Nomenclature Committee on Cell Death (NCCD) recommendation is to use the term “autophagic cell death” only when the causative role of autophagy has been proven, showing that it can be blocked by genetic or chemical inhibition of at least two components of the autophagic machinery [33] (Figure 1).
Figure 1.
Autophagy in cell survival and cell death. Although autophagy often acts as a protective mechanism, its dysregulation can lead to an exacerbated response that results in autophagy-mediated cell death, such as autosis, which differs from other possible types of autophagic cell death due to its unique morphological features, as well as its dependence on Na+,K+-ATPase.
Autosis, a new form of cell demise that differs from “classical” autophagic cell death (Table 1), was originally described in 2013 following the aforementioned criteria. Moreover, pharmacological and genetic approaches demonstrated its high dependence on the Na+,K+-ATPase pump and the autophagy machinery. However, it is important to note that autosis was only inhibited when early stages of autophagy (but not autophagosome–lysosome fusion) were blocked. Interestingly, no autosis reduction was detected when either apoptosis or necroptosis was repressed, showing its independence from other types of cell death [7]. As previously mentioned, the biochemical signature of this process is its dependence on Na+/K+-ATPase. Accordingly, a large chemical screening identified cardiac glycosides, known natural antagonists of the pump, as potent inhibitors of autosis [7,36]. Further studies, such as a genome-wide siRNA screening, confirmed the implication of Na+,K+-ATPase in autosis and identified potential new drivers that need to be investigated in the future. Additionally, these studies have also shown that the physical interaction between the pump and Beclin-1, a key autophagy protein, plays a crucial role in autosis [37,38].

Table 1.
Morphological differences between autophagy-dependent cell death and autosis.
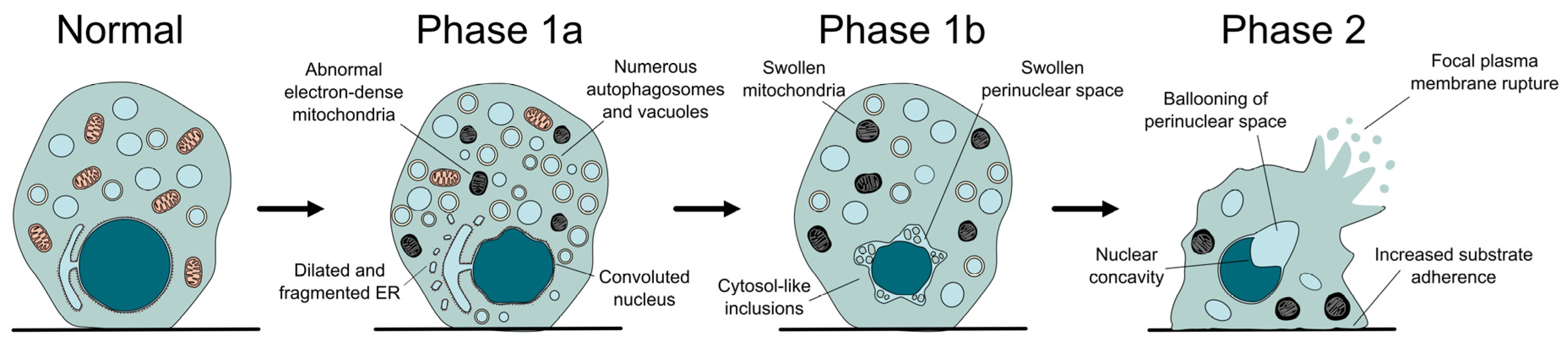
From a morphological point of view, autosis can be divided into three phases, characterized by distinctive ultrastructural changes (Figure 2). In what has been called phase 1a, a gradual increase in vacuolar dynamics is observed, with autophagosome accumulation, endoplasmic reticulum dilation and fragmentation, and changes in mitochondria morphology. Later on, during phase 1b, the external and internal membranes separate, with this newly formed perinuclear space occasionally containing inclusions with density and granularity resembling the cytosol. Finally, phase 2 can be described as a rapid and abrupt collapse of the cell, characterized by the shrinkage of the nucleus, which acquires a concave morphology due to the ballooning of the perinuclear space where the nuclear membranes show their maximal separation [7]. Besides these unique features, the dying cell resembles a necrotic one, with swollen mitochondria, absence of the endoplasmic reticulum, and final rupture of the plasma membrane [7,36]. Interestingly, cells become more adherent during autosis and remain on the plate (not floating away) when growing in vitro. Even though light microscopy allows the observation of some of these features, electron microscopy remains the gold standard for the identification of autotic cells (for example, to correctly identify the separation between nuclear membranes) [39].
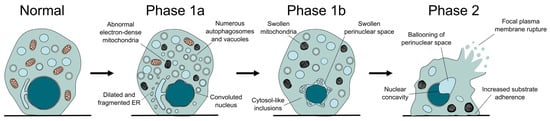
Figure 2.
The different phases of autotic cell death. During autosis, the cell undergoes several morphological changes, some of which are distinctive of this process, like the separation of the nuclear membranes, the increased attachment to the substrate, or the formation of a balloon and a concavity in the surface of the nucleus.
The first observation of this type of death occurred in HeLa cells exposed to increasing doses of autophagy-inducing peptides such as Tat-beclin-1 and Tat-vFLIP α2, showing a time-dose dependent induction of cell death [7]. Additionally, this process has also been observed in other contexts, such as in vitro nutrient starvation and hypoxia, or different models of ischemia [7,37,40,41]. Interestingly, autosis has also been identified in the livers of patients with severe anorexia nervosa [42], as well as different immune and cancer cells [43,44,45,46,47]. Even in other vertebrates, like zebrafish, features similar to autosis have also been observed; however, they were not defined as such at that moment [48].
4. Oxidative Stress and Autosis
Even though it was first reported that antioxidants that block ROS-mediated cell death cannot repress autosis [7], recent reports have shown that the activity of essential autotic proteins (mainly, Beclin-1 and the Na+,K+-ATPase pump) can be altered by oxidative stress, as it is explained in the next subsections.
4.1. Beclin-1 and Oxidative Stress
Beclin-1 is an essential protein required for the formation of autophagosomes during macroautophagy. This process is initiated when the ULK1 complex, formed by ULK1/ATG13/ATG101/RB1CC1 (FIP200) proteins, is activated in membranes containing ATG9 [49,50,51]. ULK1, acting with the protein SRC, phosphorylates ATG9, promoting the translocation of ATG9-containing vesicles to the autophagy initiation sites [52]. This allows the incorporation of phospholipids from sources such as the endoplasmic reticulum (ER), recycling endosomes, and mitochondria, initiating the elongation of pre-autophagosomal membranes [53]. A complex with Class III phosphatidylinositol 3-kinase (PI3K) activity, containing Beclin-1/PK3C3 (VPS34)/PI3R4 (VPS15) proteins [54,55], is then recruited to these membranes by ATG14 and NRBF2 [56,57], resulting in the production of phosphatidylinositol 3-phosphate (PI3P) by PK3C3 (VPS34). The action of members of the WIPI family and the union of PI3P-binding proteins to these autophagosomal membranes sustain their expansion until their closure [58].
Recent studies have described that Beclin-1 modulation may influence cellular response towards oxidative stress. For instance, Guo et al. demonstrated that with prolonged glucose starvation and hypoxia, ROS levels increase and activate the ATM/CHK2/Beclin-1 axis, promoting autophagy to control excessive ROS accumulation, clear damaged mitochondria, and inhibit apoptosis. During this process, CHK2 phosphorylates Beclin-1 at Ser90/93 residues, which are located in the Bcl-2 binding domain. Thus, this phosphorylation disrupts the interaction between Beclin-1 and Bcl-2, promoting autophagy under oxidative stress [59]. In hepatocytes, the inhibition of PSMD14 (RPN11) deubiquitinase protects against hepatic steatosis and insulin resistance induced by a high-fat diet. This is caused, in part, by the stabilization of Beclin-1, supporting autophagy and decreasing oxidative stress in hepatic cells [60]. Another study shows that a sustained period of oxidative stress in the mammary glands of dairy cows suffering from ketosis is a major cause of injury during early lactation. The presence of oxidative stress is attributed to the supraphysiological circulating concentrations of non-esterified fatty acids (NEFA), which also enhance autophagy as a counteracting response [61]. Moreover, silencing of Beclin-1 attenuated autophagy activity and increased the levels of ROS, which once again suggests that Beclin-1 plays an important role against oxidative stress [62]. However, it has also been proven that ROS can inhibit autophagy initiation, specifically repressing Beclin-1 activity. For example, they can trigger TRPM2-dependent Ca2+ influx, which activates CAMK2 at both phosphorylation and oxidation levels, and subsequently phosphorylates Beclin-1 on Ser295. This phosphorylation, in turn, decreased the association of Beclin-1 with PK3C3 (VPS34) while increasing its interaction with BCL2, and thus inhibiting autophagy [63].
The relationship between Beclin-1 and oxidative stress is already being explored to develop therapeutic strategies against some diseases, such as cancer. An example is the antineoplastic agent cannabidiol, which promotes the dissociation of Beclin-1 and BCL2 (enhancing its interaction with PK3C3 (VPS34)) through ROS-induced autophagy [64,65].
4.2. Na+,K+-ATPase and Oxidative Stress
Na+,K+-ATPase is a fundamental enzyme for the maintenance of ionic homeostasis in cells and the regulation of the membrane potential [66]. This pump is a multimeric complex consisting of three subunits: α, β, and γ [67]. The α-subunit is the catalytic component, responsible for ATP hydrolysis and ion transport, while the β-subunit has no catalytic activity but regulates the enzymatic function of the pump and confers stability to the α-subunit [68]. There are four isoforms of the α-subunit in humans: α1 is the dominant isoform and is widely expressed in almost all cell types [66], α2-isoform is predominantly produced in both cardiac and skeletal muscle as well as in the brain (in astrocytes and glia cells) [66,69], α3 is mainly located in neurons and cardiac cells (with gender-specific differences in the latter) [70,71], and α4-isoform is only expressed in the testes, where it has been associated with sperm motility [72]. There are also three isoforms of the β-subunit [73]: β1 is found in most tissues [66], while β2 is expressed in the colon, neurons, and cardiac cells [69,74,75], and β3 is detected in the testes [76]. Finally, mammals express up to seven isoforms of the γ-subunit (also known as the FXYD family), and they are responsible for the regulation of the affinity of the enzyme to different ligands [77]. Besides modulating the ionic gradient of Na+ and K+, the pump also forms complexes with proteins from the plasma membrane, like caveolin, SRC, EGFR, or GPR35, which enable it to participate in different signaling pathways [78,79]. Furthermore, the pump can also modulate adhesion and migration between cells [80].
Many stimuli induce specific modifications in Na+,K+-ATPase and can change its activity [81,82], ROS being one of them. For example, Na+,K+-ATPase α1-subunit has been observed to be degraded during hypoxia-induced pulmonary edema due to mitochondria-generated ROS and the participation of the ubiquitin-conjugating system [83]. During some neurodegenerative diseases, such as Alzheimer’s, trans fatty acids like linoelaidic acid enhance oxidative stress and decrease Na+,K+-ATPase activity. Furthermore, it highly reacts with amyloid β (Aβ) depositions, causing even more severe oxidative damage [84]. Also, long-term exposure to Aβ can change the thiol redox status of human neuroblastoma cells, inhibiting the pump and leading to the induction of glutathionylation of the α-subunit [85]. S-glutathionylation is, in fact, one of the most documented oxidative modifications of Na+,K+-ATPase. In the case of cardiac sarcolemma, the activation of the renin–angiotensin system also leads to the glutathionylation of the β1 subunit of the pump, causing its inhibition [86]. Similarly, oxidative stress from placental ischemia/reperfusion and hypoxia in preeclampsia also causes S-glutathionylation of the β1 subunit, hampering its function [87]. S-glutathionylation of cysteine residues of the α1 and β1 subunits can also reduce the α1/β1 association, causing conformational changes and blocking the α1 subunit’s intracellular ATP-binding site, leading to the inhibition of its activity [88,89]. Nevertheless, the oxidative modifications of Na+,K+-ATPase appear to be reversible [90,91], which means its activity can be regulated in a redox-sensitive manner. Furthermore, members of the FXYD family can also reverse oxidative stress-induced inhibition of the pump activity by deglutathionylation of the β1 subunit [91]. Still, the exposure of the pump to free radicals increases its susceptibility to degradation by proteolytic enzymes [92,93].
Apart from ROS, RNS can also modify Na+,K+-ATPase activity. Peroxynitrite, for example, is produced when nitric oxide and superoxide react [94], and it can act as a potent irreversible inhibitor of the pump activity by both nitrating tyrosine residues in all three subunits and modifying cysteine residues [95]. Furthermore, the free radical nitric oxide (•NO) can also regulate Na+,K+-ATPase through the activation of soluble guanylate cyclase and cGMP in the central nervous system, modulating cerebral blood flow and synaptic transmission [96,97]. The regulation of the pump activity by •NO is also known in other tissues, such as the choroid plexus [98].
Interestingly, oxidative stress can also modulate the interaction of the pump with other proteins, like the Na+,K+-ATPase/SRC axis. The α1-subunit serves as a scaffold for proto-oncogene tyrosine kinase c-SRC, interacting with it and inhibiting its function [99]. However, when cardiac glucosides bind to the extracellular domain of the pump, a conformational change is produced, allowing SRC to be activated. In turn, it transactivates EGFR, which then activates RAS [100]. This can lead to the production of ROS by the mitochondria, resulting in the activation of nuclear factor kappa B (NF-κB) and initiating the MAPK cascade [101,102]. Furthermore, SRC may also be regulated by ROS through a reversible oxidation of cysteine residues, generating a positive feedback loop [90]. Some of these oxidized residues are located where the interaction between Na+,K+-ATPase and SRC takes place [99], inducing changes in either the pump, SRC, or both, which in turn might affect the interaction between them and, consequently, the downstream kinase cascade [103].
5. A New Layer in Autosis Regulation
A decade has passed since autosis was first described, yet our understanding of this specific type of cell death remains limited. While the Na+,K+-ATPase pump and certain components of the autophagy machinery are crucial for initiating this cellular response, the complete molecular pathway and its regulatory mechanisms are still largely unexplored. Most research has concentrated on potential ionic imbalances caused by dysregulated activity of the pump, as well as oxygen and nutrient deprivation, since both hypoxia and starvation can trigger autosis. However, in this review, we have displayed recent studies that indicate that ROS can also affect the function of autotic components. Therefore, we propose that the redox status of the cell should be considered when investigating the molecular and regulatory mechanisms of autosis.
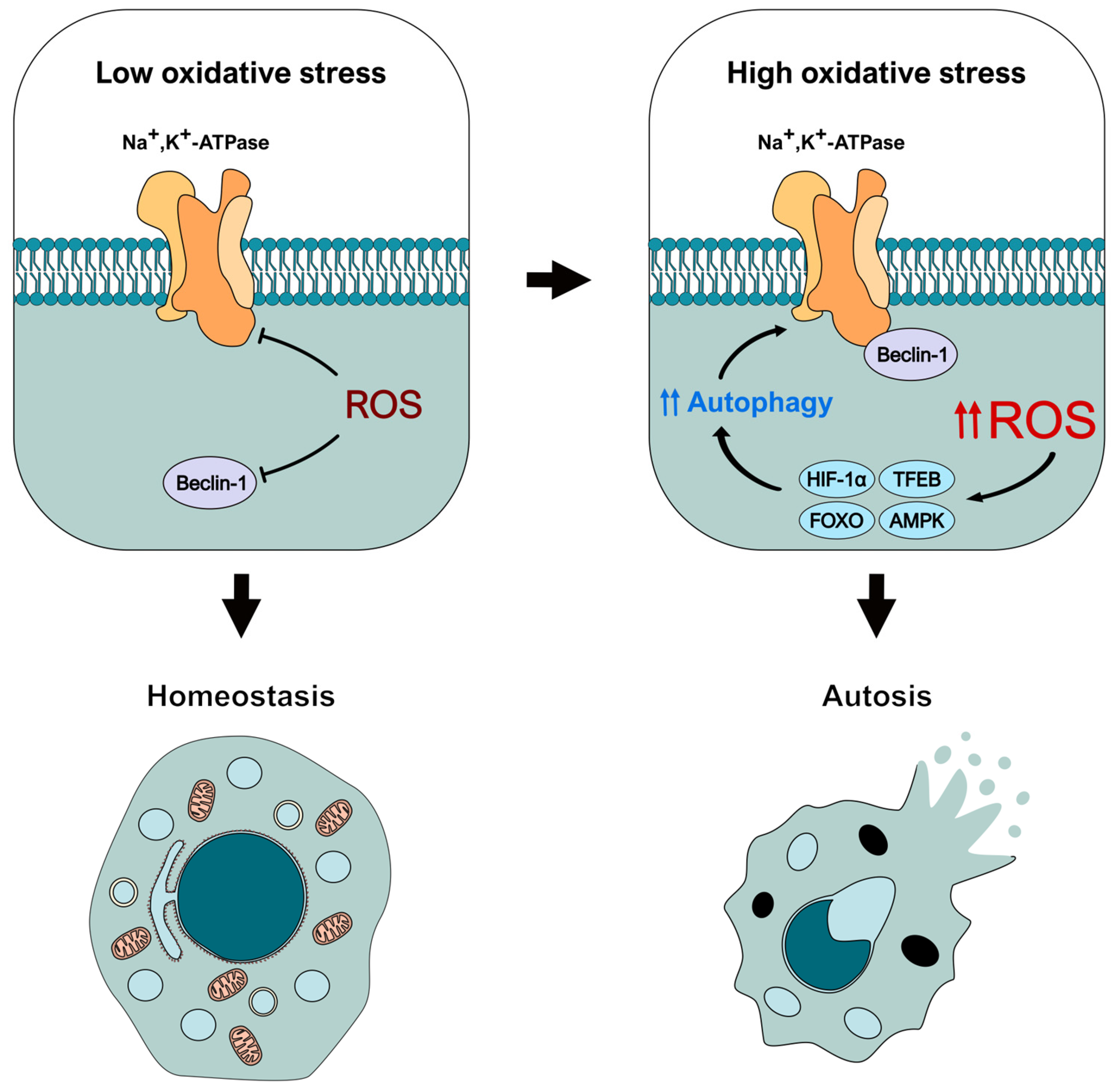
In this regard, it is noteworthy that oxidative stress can inhibit both Beclin-1 and Na+,K+-ATPase [63,88,104,105,106,107,108], for example, by modifications such as S-glutathionylation, tyrosine nitration, and others [89,95,109]. This suggests that these species may actually prevent autotic induction at first, rather than promote it, as the repression of Beclin-1 and pump activities can effectively block autosis [7]. Depending on the context, ROS can act as important signaling molecules instead of toxins, helping to maintain cellular homeostasis by allowing cells to rapidly respond to various conditions for survival. In this sense, ROS may first negatively affect Beclin-1 and the pump, reducing their activity, preventing their binding, and hindering the autotic response. However, an overwhelming increase in ROS production could concurrently promote an exacerbated autophagic response that is triggered as an attempt to counteract oxidative stress [110], but that can ultimately lead to the initiation of autosis. This could be particularly relevant, for example, under hypoxic conditions, which are associated with both elevated ROS generation [111,112] and autotic cell death [7,37,38], potentially through HIF-1α. This is an essential transcription factor that can be activated and stabilized in response to hypoxia-induced ROS [113], resulting in the promotion of autophagy [114,115]. Another transcription factor that could be important in this process is TFEB, which is also activated under oxidative stress [116,117] and promotes the expression of autophagic and lysosomal genes [118]. Other interesting effectors that can activate autophagy in response to ROS (even under hypoxic conditions) are components of the FOXO family, such as FOXO1 and FOXO3 [119,120,121,122,123]. But how much oxidative stress would be needed to activate autosis? What is the threshold for this switch in the ROS-autosis interplay? These are complex questions that would need to be experimentally addressed. Nevertheless, it seems it could greatly depend on the context. For example, several studies have described that AMPK, one of the main autophagy regulators, can be both repressed and stimulated by oxidative stress, depending on whether ROS mediates the phosphorylation (positive regulation) or the oxidation (negative regulation) of its residues [124].
In conclusion, based on our current understanding of ROS and autotic-related proteins, we suggest that these species could have a dual role in autosis (Figure 3). First, ROS may function as molecular brakes for this type of cell death by inhibiting the activity of Beclin-1 and the Na+,K+-ATPase pump. However, when present in high concentrations, ROS can activate different autophagy-inducing signaling pathways (through mediators such as HIF-1α, TFEB, FOXO, or AMPK), exacerbating the autophagy response and leading to autosis initiation. Nevertheless, this conceptual model must be experimentally confirmed in the future to fully unveil the role of ROS and oxidative stress in this type of autophagy-dependent cell death. Therefore, we believe it is crucial to consider the redox status of the cell when examining the molecular and regulatory mechanisms of autosis, as it can influence the function of key effectors in the pathway.
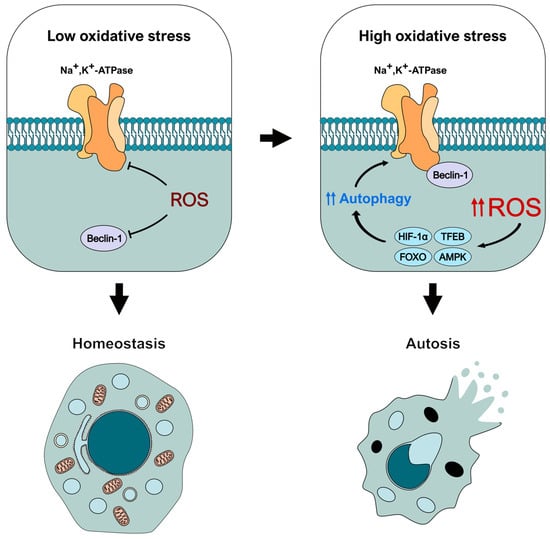
Figure 3.
Theoretical model of the dual regulation of autosis-related effectors by oxidative stress. ROS would block autosis initiation early by inhibiting the activity of Beclin-1 and Na+,K+-ATPase. However, increased oxidative stress would trigger an intense autophagic response mediated by different effectors (such as HIF-1 α, TFEB, or AMPK) that could lead to autosis initiation.
Author Contributions
Conceptualization, M.G.-M. and Á.F.F.; writing—original draft preparation, M.G.-A., I.M.-R., A.P.-M. and E.L.-F.; writing—review and editing, M.G.-M. and Á.F.F.; supervision, M.G.-M. and Á.F.F. All authors have read and agreed to the published version of the manuscript.
Funding
M.G.A.’s contract is funded by a predoctoral grant from the University of Oviedo (PAPI-22-PF-21). E.L.F. has an FPI contract (PRE2023-138478) funded by the grant PID2022-138478OA-100. M.G.M. is a Ramón y Cajal researcher (grant RYC2021-033684-I, funded by MICIU/AEI/10.13039/501100011033 and by European Union NextGenerationEU/PRTR), and her research is funded by an Internationalization Project “CL-EI-2021-08-IBFG Unit of Excellence” from the Spanish National Research Council (CSIC), which is funded by the Junta de Castilla y León and co-financed by the European Regional Development Fund (ERDF “Europe drives our growth”) and the grant PID2022-138478OA-100, funded by MICIU/AEI/10.13039/501100011033 and by FEDER, UE. Á.F.F.’s work is supported by grants CNS2024-154954 and PID2021-127534OB-I00, funded by MICIU/AEI/10.13039/501100011033 and by ERDF/EU.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We are members of and thank Women in Autophagy (WIA), Sociedad Española de Bioquímica y Biología Molecular (SEBBM), and Sociedad Española de Autofagia (SEFAGIA).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| Aβ | Amyloid β |
| AMPK | AMP-activated protein kinase |
| ATG9 | Autophagy-related protein 9 |
| ATG13 | Autophagy-related protein 13 |
| ATG14 | Autophagy-related protein 14 |
| ATG101 | Autophagy-related protein 101 |
| ATM | Serine protein kinase ATM |
| BCL2 | Apoptosis regulator Bcl-2 |
| CAMK2 | Calcium/calmodulin-dependent protein kinase type II |
| cGMP | Cyclic guanosine monophosphate |
| CHK2 | Serine/threonine-protein kinase Chk2 |
| EGFR | Epidermal growth factor receptor |
| FIP200 FOXO | FAK family kinase-interacting protein of 200 kDa Forkhead box proteins |
| FXYD | FXYD domain-containing ion transport regulator |
| GPR35 | G-protein coupled receptor 35 |
| GSH | Glutathione |
| GSSG | Glutathione disulfide |
| H2O2 | Hydrogen peroxide |
| HIF-1α JNK | Hypoxia-inducible factor 1-alpha c-Jun N-terminal kinase |
| MAPK | Mitogen-activated protein kinase |
| NAD | Reactive oxygen species |
| NADP | Reactive nitrogen species |
| NCCD | Nomenclature Committee on Cell Death |
| NEFA | Non-esterified fatty acids |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NOS | Nitric oxide synthase |
| NOX | NADPH oxidase |
| NRBF2 | Nuclear receptor-binding factor 2 |
| O2•— | Superoxide anion |
| ONOO— | Peroxynitrite |
| PI3K | Class III phosphatidylinositol 3-kinase |
| PI3P | Phosphatidylinositol 3-phosphate |
| PI3R4 | Phosphoinositide 3-kinase regulatory subunit 4 |
| PK3C3 | Phosphatidylinositol 3-kinase catalytic subunit type 3 |
| PSMD14 | 26S proteasome non-ATPase regulatory subunit 14 |
| RAS | Rat sarcoma virus small GTPase |
| RB1CC1 | RB1-inducible coiled-coil protein 1 |
| RET | Reverse electron transport |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| RPN11 | 26S proteasome regulatory subunit RPN11 |
| SiRNA | Small interfering RNA |
| SRC TFEB | Proto-oncogene tyrosine-protein kinase Src Transcription factor EB |
| TRPM2 | Transient receptor potential cation channel subfamily M member 2 |
| ULK1 | Serine/threonine-protein kinase ULK1 |
| UV | Ultraviolet |
| VPS15 | Vacuolar protein sorting 15 |
| VPS34 | Vacuolar protein sorting 34 |
| WIPI | WD repeat domain phosphoinositide-interacting |
| •OH | Hydroxyl radical |
| •NO | Nitric oxide |
References
- Lennicke, C.; Cochemé, H.M. Redox Metabolism: ROS as Specific Molecular Regulators of Cell Signaling and Function. Mol. Cell 2021, 81, 3691–3707. [Google Scholar] [CrossRef]
- Zoccarato, A.; Nabeebaccus, A.A.; Oexner, R.R.; Santos, C.X.C.; Shah, A.M. The Nexus between Redox State and Intermediary Metabolism. FEBS J. 2022, 289, 5440–5462. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive Oxygen Species, Toxicity, Oxidative Stress, and Antioxidants: Chronic Diseases and Aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Petroni, G.; Amaravadi, R.K.; Baehrecke, E.H.; Ballabio, A.; Boya, P.; Pedro, J.M.B.; Cadwell, K.; Cecconi, F.; Choi, A.M.K.; et al. Autophagy in Major Human Diseases. EMBO J. 2021, 40, e108863. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.R.; Thorburn, A. Autophagy and Organelle Homeostasis in Cancer. Dev. Cell 2021, 56, 906–918. [Google Scholar] [CrossRef] [PubMed]
- Rakesh, R.; PriyaDharshini, L.C.; Sakthivel, K.M.; Rasmi, R.R. Role and Regulation of Autophagy in Cancer. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166400. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shoji-Kawata, S.; Sumpter, R.M.; Wei, Y.; Ginet, V.; Zhang, L.; Posner, B.; Tran, K.A.; Green, D.R.; Xavier, R.J.; et al. Autosis Is a Na+,K+-ATPase-Regulated Form of Cell Death Triggered by Autophagy-Inducing Peptides, Starvation, and Hypoxia-Ischemia. Proc. Natl. Acad. Sci. USA 2013, 110, 20364–20371. [Google Scholar] [CrossRef]
- Iqbal, M.J.; Kabeer, A.; Abbas, Z.; Siddiqui, H.A.; Calina, D.; Sharifi-Rad, J.; Cho, W.C. Interplay of Oxidative Stress, Cellular Communication and Signaling Pathways in Cancer. Cell Commun. Signal. 2024, 22, 7. [Google Scholar] [CrossRef]
- Stefanou, D.T.; Kouvela, M.; Stellas, D.; Voutetakis, K.; Papadodima, O.; Syrigos, K.; Souliotis, V.L. Oxidative Stress and Deregulated DNA Damage Response Network in Lung Cancer Patients. Biomedicines 2022, 10, 1248. [Google Scholar] [CrossRef]
- Foret, M.K.; Orciani, C.; Welikovitch, L.A.; Huang, C.; Cuello, A.C.; Do Carmo, S. Early Oxidative Stress and DNA Damage in Aβ-Burdened Hippocampal Neurons in an Alzheimer’s-like Transgenic Rat Model. Commun. Biol. 2024, 7, 861. [Google Scholar] [CrossRef]
- Bardelčíková, A.; Šoltys, J.; Mojžiš, J. Oxidative Stress, Inflammation and Colorectal Cancer: An Overview. Antioxidants 2023, 12, 901. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhou, L.; Duan, J.; Qin, S.; Jiang, J.; Chen, H.; Wang, K.; Liu, R.; Yuan, M.; Tang, X.; et al. Oxidative Stress Promotes Liver Cancer Metastasis via RNF25-Mediated E-Cadherin Protein Degradation. Adv. Sci. 2024, 11, e2306929. [Google Scholar] [CrossRef] [PubMed]
- Zaric, B.L.; Macvanin, M.T.; Isenovic, E.R. Free Radicals: Relationship to Human Diseases and Potential Therapeutic Applications. Int. J. Biochem. Cell Biol. 2023, 154, 106346. [Google Scholar] [CrossRef] [PubMed]
- Scialo, F.; Sanz, A. Coenzyme Q Redox Signalling and Longevity. Free Radic. Biol. Med. 2021, 164, 187–205. [Google Scholar] [CrossRef]
- Scialò, F.; Fernández-Ayala, D.J.; Sanz, A. Role of Mitochondrial Reverse Electron Transport in ROS Signaling: Potential Roles in Health and Disease. Front. Physiol. 2017, 8, 273331. [Google Scholar] [CrossRef]
- Taylor, J.P.; Tse, H.M. The Role of NADPH Oxidases in Infectious and Inflammatory Diseases. Redox Biol. 2021, 48, 102159. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Koshiishi, I.; Inoguchi, T.; Nawata, H.; Utsumi, H. Confirmation of Superoxide Generation via Xanthine Oxidase in Streptozotocin-Induced Diabetic Mice. Free Radic. Res. 2003, 37, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Radi, R. Peroxynitrite, a Stealthy Biological Oxidant. J. Biol. Chem. 2013, 288, 26464–26472. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, A.J.P.O.; De Oliveira, J.C.P.L.; Da Silva Pontes, L.V.; De Souza Júnior, J.F.; Gonçalves, T.A.F.; Dantas, S.H.; De Almeida Feitosa, M.S.; Silva, A.O.; De Medeiros, I.A. ROS: Basic Concepts, Sources, Cellular Signaling, and Its Implications in Aging Pathways. Oxid. Med. Cell Longev. 2022, 2022, 1225578. [Google Scholar] [CrossRef]
- Moon, D.O.; Kim, M.O.; Choi, Y.H.; Hyun, J.W.; Chang, W.Y.; Kim, G.Y. Butein Induces G2/M Phase Arrest and Apoptosis in Human Hepatoma Cancer Cells through ROS Generation. Cancer Lett. 2010, 288, 204–213. [Google Scholar] [CrossRef]
- Dai, D.F.; Hsieh, E.J.; Liu, Y.; Chen, T.; Beyer, R.P.; Chin, M.T.; MacCoss, M.J.; Rabinovitch, P.S. Mitochondrial Proteome Remodelling in Pressure Overload-Induced Heart Failure: The Role of Mitochondrial Oxidative Stress. Cardiovasc. Res. 2012, 93, 79–88. [Google Scholar] [CrossRef]
- Chatterjee, K.; Pal, A.; Padhy, D.S.; Saha, R.; Chatterjee, A.; Bharadwaj, M.; Sarkar, B.; Mazumder, P.M.; Banerjee, S. Vitamin K2 Ameliorates Diabetes-Associated Cognitive Decline by Reducing Oxidative Stress and Neuroinflammation. J. Neuroimmune Pharmacol. 2024, 19, 56. [Google Scholar] [CrossRef]
- Guan, L.; Mao, Z.; Yang, S.; Wu, G.; Chen, Y.; Yin, L.; Qi, Y.; Han, L.; Xu, L. Dioscin Alleviates Alzheimer’s Disease through Regulating RAGE/NOX4 Mediated Oxidative Stress and Inflammation. Biomed. Pharmacother. 2022, 152, 113248. [Google Scholar] [CrossRef] [PubMed]
- Hofmans, S.; Berghe, T.V.; Devisscher, L.; Hassannia, B.; Lyssens, S.; Joossens, J.; Van Der Veken, P.; Vandenabeele, P.; Augustyns, K. Novel Ferroptosis Inhibitors with Improved Potency and ADME Properties. J. Med. Chem. 2016, 59, 2041–2053. [Google Scholar] [CrossRef] [PubMed]
- Baba, Y.; Higa, J.K.; Shimada, B.K.; Horiuchi, K.M.; Suhara, T.; Kobayashi, M.; Woo, J.D.; Aoyagi, H.; Marh, K.S.; Kitaoka, H.; et al. Protective Effects of the Mechanistic Target of Rapamycin against Excess Iron and Ferroptosis in Cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H659–H668. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, D.; Tian, R.; Yan, X.; Zhou, Y. Resveratrol Alleviates Amyloid β-Induced Neuronal Apoptosis, Inflammation, and Oxidative and Endoplasmic Reticulum Stress by Circ_0050263/MiR-361-3p/PDE4A Axis during Alzheimer’s Disease. Chem. Biol. Drug Des. 2023, 102, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Thadathil, N.; Nicklas, E.H.; Mohammed, S.; Lewis, T.L.; Richardson, A.; Deepa, S.S. Necroptosis Increases with Age in the Brain and Contributes to Age-Related Neuroinflammation. Geroscience 2021, 43, 2345. [Google Scholar] [CrossRef]
- Zheng, L.; Terman, A.; Hallbeck, M.; Dehvari, N.; Cowburn, R.F.; Benedikz, E.; Kågedal, K.; Cedazo-Minguez, A.; Marcusson, J. Macroautophagy-Generated Increase of Lysosomal Amyloid β-Protein Mediates Oxidant-Induced Apoptosis of Cultured Neuroblastoma Cells. Autophagy 2011, 7, 1528–1545. [Google Scholar] [CrossRef]
- Shi, S.; Chen, Y.; Luo, Z.; Nie, G.; Dai, Y. Role of Oxidative Stress and Inflammation-Related Signaling Pathways in Doxorubicin-Induced Cardiomyopathy. Cell Commun. Signal. 2023, 21, 61. [Google Scholar] [CrossRef]
- Li, L.; Chen, Y.; Gibson, S.B. Starvation-Induced Autophagy Is Regulated by Mitochondrial Reactive Oxygen Species Leading to AMPK Activation. Cell. Signal. 2013, 25, 50–65. [Google Scholar] [CrossRef]
- Komatsu, M.; Kurokawa, H.; Waguri, S.; Taguchi, K.; Kobayashi, A.; Ichimura, Y.; Sou, Y.S.; Ueno, I.; Sakamoto, A.; Tong, K.I.; et al. The Selective Autophagy Substrate P62 Activates the Stress Responsive Transcription Factor Nrf2 through Inactivation of Keap1. Nat. Cell Biol. 2010, 12, 213–223. [Google Scholar] [CrossRef]
- Scherz-Shouval, R.; Shvets, E.; Fass, E.; Shorer, H.; Gil, L.; Elazar, Z. Reactive Oxygen Species Are Essential for Autophagy and Specifically Regulate the Activity of Atg4. EMBO J. 2007, 26, 1749–1760. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular Mechanisms of Cell Death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Kroemer, G. Biological Functions of Autophagy Genes: A Disease Perspective. Cell 2019, 176, 11–42. [Google Scholar] [CrossRef] [PubMed]
- Schweichel, J.-U.; Merker, H.-J. The Morphology of Various Types of Cell Death in Prenatal Tissues. Teratology 1973, 7, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Levine, B. Autosis and Autophagic Cell Death: The Dark Side of Autophagy. Cell Death Differ. 2015, 22, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Fernández, Á.F.; Liu, Y.; Ginet, V.; Shi, M.; Nah, J.; Zou, Z.; Zhou, A.; Posner, B.A.; Xiao, G.; Tanguy, M.; et al. Interaction between the Autophagy Protein Beclin 1 and Na+,K+-ATPase during Starvation, Exercise, and Ischemia. JCI Insight 2020, 5, e133282. [Google Scholar] [CrossRef] [PubMed]
- Depierre, P.; Ginet, V.; Truttmann, A.C.; Puyal, J. Neuronal Autosis Is Na+/K+-ATPase Alpha 3-Dependent and Involved in Hypoxic-Ischemic Neuronal Death. Cell Death Dis. 2024, 15, 363. [Google Scholar] [CrossRef] [PubMed]
- Kaludercic, N.; Maiuri, M.C.; Kaushik, S.; Fernández, Á.F.; De Bruijn, J.; Castoldi, F.; Chen, Y.; Ito, J.; Mukai, R.; Murakawa, T.; et al. Comprehensive Autophagy Evaluation in Cardiac Disease Models. Cardiovasc. Res. 2020, 116, 483–504. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Henson, E.S.; Xiao, W.; Huang, D.; McMillan-Ward, E.M.; Israels, S.J.; Gibson, S.B. Tyrosine Kinase Receptor EGFR Regulates the Switch in Cancer Cells between Cell Survival and Cell Death Induced by Autophagy in Hypoxia. Autophagy 2016, 12, 1029–1046. [Google Scholar] [CrossRef]
- Nah, J.; Zhai, P.; Huang, C.Y.; Fernández, Á.F.; Mareedu, S.; Levine, B.; Sadoshima, J. Upregulation of Rubicon Promotes Autosis during Myocardial Ischemia/Reperfusion Injury. J. Clin. Investig. 2020, 130, 2978–2991. [Google Scholar] [CrossRef] [PubMed]
- Kheloufi, M.; Boulanger, C.M.; Codogno, P.; Rautou, P.E. Autosis Occurs in the Liver of Patients with Severe Anorexia Nervosa. Hepatology 2015, 62, 657–658. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Luk, B.T.; Wei, X.; Campbell, G.R.; Fang, R.H.; Zhang, L.; Spector, S.A. Selective Cell Death of Latently HIV-Infected CD4+ T Cells Mediated by Autosis Inducing Nanopeptides. Cell Death Dis. 2019, 10, 419. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Fang, J.; Xue, G.; Wang, Z.; Li, X.; Zhou, M.; Jin, G.; Rahman, M.M.; McFadden, G.; Lu, Y. Induction of Tumor Cell Autosis by Myxoma Virus-Infected CAR-T and TCR-T Cells to Overcome Primary and Acquired Resistance. Cancer Cell 2022, 40, 973–985.e7. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Li, X.; Wang, K.; Zhu, D.; Meng, B.; Liu, J.; Liang, X.; Jin, Y.; Liu, X.; Wen, Q.; et al. PURPL Represses Autophagic Cell Death to Promote Cutaneous Melanoma by Modulating ULK1 Phosphorylation. Cell Death Dis. 2021, 12, 1070. [Google Scholar] [CrossRef] [PubMed]
- Camuzard, O.; Trojani, M.-C.; Santucci-Darmanin, S.; Pagnotta, S.; Breuil, V.; Carle, G.; Pierrefite-Carle, V. Autophagy in Osteosarcoma Cancer Stem Cells Is Critical Process Which Can Be Targeted by the Antipsychotic Drug Thioridazine. Cancers 2020, 12, 3675. [Google Scholar] [CrossRef]
- Zhang, X.; Shan, G.; Li, N.; Chen, J.; Ji, C.; Li, X.; Jiang, L.; Kin, T.; Lee, W.; Keng, V.W.; et al. An Autophagy-Inducing Stapled Peptide Induces Mitochondria Dysfunction and Triggers Autotic Cell Death in Triple-Negative Breast Cancer. Cell Death Discov. 2023, 9, 303. [Google Scholar] [CrossRef]
- Santos-Ledo, A.; Garcia-Macia, M.; Campbell, P.D.; Gronska, M.; Marlow, F.L. Kinesin-1 Promotes Chondrocyte Maintenance during Skeletal Morphogenesis. PLoS Genet. 2017, 13, e1006918. [Google Scholar] [CrossRef]
- Hosokawa, N.; Sasaki, T.; Iemura, S.I.; Natsume, T.; Hara, T.; Mizushima, N. Atg101, a Novel Mammalian Autophagy Protein Interacting with Atg13. Autophagy 2009, 5, 973–979. [Google Scholar] [CrossRef]
- Ganley, I.G.; Lam, D.H.; Wang, J.; Ding, X.; Chen, S.; Jiang, X. ULK1.ATG13.FIP200 Complex Mediates MTOR Signaling and Is Essential for Autophagy. J. Biol. Chem. 2009, 284, 12297–12305. [Google Scholar] [CrossRef] [PubMed]
- Karanasios, E.; Walker, S.A.; Okkenhaug, H.; Manifava, M.; Hummel, E.; Zimmermann, H.; Ahmed, Q.; Domart, M.C.; Collinson, L.; Ktistakis, N.T. Autophagy Initiation by ULK Complex Assembly on ER Tubulovesicular Regions Marked by ATG9 Vesicles. Nat. Commun. 2016, 7, 12420. [Google Scholar] [CrossRef]
- Zhou, C.; Ma, K.; Gao, R.; Mu, C.; Chen, L.; Liu, Q.; Luo, Q.; Feng, D.; Zhu, Y.; Chen, Q. Regulation of MATG9 Trafficking by Src- and ULK1-Mediated Phosphorylation in Basal and Starvation-Induced Autophagy. Cell Res. 2016, 27, 184–201. [Google Scholar] [CrossRef]
- Galluzzi, L.; Baehrecke, E.H.; Ballabio, A.; Boya, P.; Bravo-San Pedro, J.M.; Cecconi, F.; Choi, A.M.; Chu, C.T.; Codogno, P.; Colombo, M.I.; et al. Molecular Definitions of Autophagy and Related Processes. EMBO J. 2017, 36, 1811–1836. [Google Scholar] [CrossRef] [PubMed]
- Kihara, A.; Noda, T.; Ishihara, N.; Ohsumi, Y. Two Distinct Vps34 Phosphatidylinositol 3-Kinase Complexes Function in Autophagy and Carboxypeptidase Y Sorting in Saccharomyces Cerevisiae. J. Cell Biol. 2001, 152, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Kihara, A.; Kabeya, Y.; Ohsumi, Y.; Yoshimori, T. Beclin-Phosphatidylinositol 3-Kinase Complex Functions at the Trans-Golgi Network. EMBO Rep. 2001, 2, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Itakura, E.; Kishi, C.; Inoue, K.; Mizushima, N. Beclin 1 Forms Two Distinct Phosphatidylinositol 3-Kinase Complexes with Mammalian Atg14 and UVRAG. Mol. Biol. Cell 2008, 19, 5360–5372. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; He, L.; Behrends, C.; Araki, M.; Araki, K.; Jun Wang, Q.; Catanzaro, J.M.; Friedman, S.L.; Zong, W.X.; Fiel, M.I.; et al. NRBF2 Regulates Autophagy and Prevents Liver Injury by Modulating Atg14L-Linked Phosphatidylinositol-3 Kinase III Activity. Nat. Commun. 2014, 5, 3920. [Google Scholar] [CrossRef]
- Dooley, H.C.; Razi, M.; Polson, H.E.J.; Girardin, S.E.; Wilson, M.I.; Tooze, S.A. WIPI2 Links LC3 Conjugation with PI3P, Autophagosome Formation, and Pathogen Clearance by Recruiting Atg12–5-16L1. Mol. Cell 2014, 55, 238. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, S.; Zhang, S.; Xu, H.; Li, X.; Guan, Y.; Yi, F.; Zhou, T.; Jiang, B.; Bai, N.; et al. ATM-CHK2-Beclin 1 Axis Promotes Autophagy to Maintain ROS Homeostasis under Oxidative Stress. EMBO J. 2020, 39, e103111. [Google Scholar] [CrossRef]
- Zhou, B.; Luo, Y.; Bi, H.; Zhang, N.; Ma, M.; Dong, Z.; Ji, N.; Zhang, S.; Wang, X.; Liu, Y.; et al. Amelioration of Nonalcoholic Fatty Liver Disease by Inhibiting the Deubiquitylating Enzyme RPN11. Cell Metab. 2024, 36, 2228–2244.e7. [Google Scholar] [CrossRef]
- Chang, R.; Sun, X.; Jia, H.; Xu, Q.; Dong, Z.; Tang, Y.; Luo, S.; Jiang, Q.; Loor, J.J.; Xu, C. Inhibiting Nuclear Factor Erythroid 2 Related Factor 2-Mediated Autophagy in Bovine Mammary Epithelial Cells Induces Oxidative Stress in Response to Exogenous Fatty Acids. J. Anim. Sci. Biotechnol. 2022, 13, 48. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Dong, Y.; Cao, L.; Li, G.; Yang, Z.; Luo, J.; Lei, L.; Du, X.; Song, Y.; Usman, M.; et al. Caveolin 1 Ameliorates Nonesterified Fatty Acid-Induced Oxidative Stress via the Autophagy Regulator Beclin 1 in Bovine Mammary Gland Epithelial Cells. J. Dairy Sci. 2024, 108, 928–942. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Guo, W.; Hao, B.; Shi, X.; Lu, Y.; Wong, C.W.M.; Ma, V.W.S.; Yip, T.T.C.; Au, J.S.K.; Hao, Q.; et al. Mechanistic Study of TRPM2-Ca2+-CAMK2-BECN1 Signaling in Oxidative Stress-Induced Autophagy Inhibition. Autophagy 2016, 12, 1340–1354. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Kuzontkoski, P.M.; Groopman, J.E.; Prasad, A. Cannabidiol Induces Programmed Cell Death in Breast Cancer Cells by Coordinating the Cross-Talk between Apoptosis and Autophagy. Mol. Cancer Ther. 2011, 10, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.Y.; Shivanne Gowda, S.G.; Lee, S.G.; Sethi, G.; Ahn, K.S. Cannabidiol Induces ERK Activation and ROS Production to Promote Autophagy and Ferroptosis in Glioblastoma Cells. Chem. Biol. Interact. 2024, 394, 110995. [Google Scholar] [CrossRef] [PubMed]
- Clausen, M.V.; Hilbers, F.; Poulsen, H. The Structure and Function of the Na,K-ATPase Isoforms in Health and Disease. Front. Physiol. 2017, 8, 257141. [Google Scholar] [CrossRef]
- Mobasheri, A.; Avila, J.; Cózar-Castellano, I.; Brownleader, M.D.; Trevan, M.; Francis, M.J.O.; Lamb, J.F.; Martín-Vasallo, P. Na+, K+-ATPase Isozyme Diversity; Comparative Biochemistry and Physiological Implications of Novel Functional Interactions. Biosci. Rep. 2000, 20, 51–91. [Google Scholar] [CrossRef]
- Da Silva, C.I.; Gonçalves-de-Albuquerque, C.F.; De Moraes, B.P.; Garcia, D.G.; Burth, P. Na/K-ATPase: Their Role in Cell Adhesion and Migration in Cancer. Biochimie 2021, 185, 1–8. [Google Scholar] [CrossRef]
- Zlokovic, B.V.; Mackic, J.B.; Wang, L.; McComb, J.G.; McDonough, A. Differential Expression of Na,K-ATPase Alpha and Beta Subunit Isoforms at the Blood-Brain Barrier and the Choroid Plexus. J. Biol. Chem. 1993, 268, 8019–8025. [Google Scholar] [CrossRef]
- Gaborit, N.; Varro, A.; Le Bouter, S.; Szuts, V.; Escande, D.; Nattel, S.; Demolombe, S. Gender-Related Differences in Ion-Channel and Transporter Subunit Expression in Non-Diseased Human Hearts. J. Mol. Cell Cardiol. 2010, 49, 639–646. [Google Scholar] [CrossRef]
- Bøttger, P.; Tracz, Z.; Heuck, A.; Nissen, P.; Romero-Ramos, M.; Lykke-Hartmann, K. Distribution of Na/K-ATPase Alpha 3 Isoform, a Sodium-Potassium P-Type Pump Associated with Rapid-Onset of Dystonia Parkinsonism (RDP) in the Adult Mouse Brain. J. Comp. Neurol. 2011, 519, 376–404. [Google Scholar] [CrossRef]
- Jimenez, T.; Sanchez, G.; McDermott, J.P.; Nguyen, A.N.; Kumar, T.R.; Blanco, G. Increased Expression of the Na,K-ATPase Alpha4 Isoform Enhances Sperm Motility in Transgenic Mice. Biol. Reprod. 2011, 84, 153–161. [Google Scholar] [CrossRef]
- Geering, K. Functional Roles of Na,K-ATPase Subunits. Curr. Opin. Nephrol. Hypertens. 2008, 17, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Baker Bechmann, M.; Rotoli, D.; Morales, M.; Maeso, M.d.C.; García, M.d.P.; Ávila, J.; Mobasheri, A.; Martín-Vasallo, P. Na,K-ATPase Isozymes in Colorectal Cancer and Liver Metastases. Front. Physiol. 2016, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Habeck, M.; Tokhtaeva, E.; Nadav, Y.; Zeev, E.B.; Ferris, S.P.; Kaufman, R.J.; Bab-Dinitz, E.; Kaplan, J.H.; Dada, L.A.; Farfel, Z.; et al. Selective Assembly of Na,K-ATPase A2β2 Heterodimers in the Heart: Distinct Functional Properties And Isoform-Selective Inhibitors. J. Biol. Chem. 2016, 291, 23159–23174. [Google Scholar] [CrossRef] [PubMed]
- Arystarkhova, E.; Sweadner, K.J. Tissue-Specific Expression of the Na,K-ATPase Beta3 Subunit. The Presence of Beta3 in Lung and Liver Addresses the Problem of the Missing Subunit. J. Biol. Chem. 1997, 272, 22405–22408. [Google Scholar] [CrossRef]
- Mishra, N.K.; Peleg, Y.; Cirri, E.; Belogus, T.; Lifshitz, Y.; Voelker, D.R.; Apell, H.J.; Garty, H.; Karlish, S.J.D. FXYD Proteins Stabilize Na,K-ATPase: Amplification of Specific Phosphatidylserine-Protein Interactions. J. Biol. Chem. 2011, 286, 9699–9712. [Google Scholar] [CrossRef]
- Cui, X.; Xie, Z. Protein Interaction and Na/K-ATPase-Mediated Signal Transduction. Molecules 2017, 22, 990. [Google Scholar] [CrossRef]
- Schneditz, G.; Elias, J.E.; Pagano, E.; Zaeem Cader, M.; Saveljeva, S.; Long, K.; Mukhopadhyay, S.; Arasteh, M.; Lawley, T.D.; Dougan, G.; et al. GPR35 Promotes Glycolysis, Proliferation, and Oncogenic Signaling by Engaging with the Sodium Potassium Pump. Sci. Signal. 2019, 12, eaau9048. [Google Scholar] [CrossRef]
- Li, L.; Feng, R.; Xu, Q.; Zhang, F.; Liu, T.; Cao, J.; Fei, S. Expression of the Β3 Subunit of Na+/K+-ATPase Is Increased in Gastric Cancer and Regulates Gastric Cancer Cell Progression and Prognosis via the PI3/AKT Pathway. Oncotarget 2017, 8, 84285. [Google Scholar] [CrossRef]
- Chkadua, G.; Nozadze, E.; Tsakadze, L.; Shioshvili, L.; Leladze, M.; Arutinova, N.; Dzneladze, S.; Javakhishvili, M.; Kupradze, S. Some Kinetic Features of Na,K-ATPase and Sensitivity to Noradrenaline. Cell Biochem. Biophys. 2022, 80, 23–29. [Google Scholar] [CrossRef]
- Mohan, S.; Tiwari, M.N.; Biala, Y.; Yaari, Y. Regulation of Neuronal Na+/K+-ATPase by Specific Protein Kinases and Protein Phosphatases. J. Neurosci. 2019, 39, 5440. [Google Scholar] [CrossRef]
- Comellas, A.P.; Dada, L.A.; Lecuona, E.; Pesce, L.M.; Chandel, N.S.; Quesada, N.; Budinger, G.R.S.; Strous, G.J.; Ciechanover, A.; Sznajder, J.I. Hypoxia-Mediated Degradation of Na,K-ATPase via Mitochondrial Reactive Oxygen Species and the Ubiquitin-Conjugating System. Circ. Res. 2006, 98, 1314–1322. [Google Scholar] [CrossRef]
- Tsai, S.J.; Liu, W.H.; Yin, M.C. Trans Fatty Acids Enhanced Beta-Amyloid Induced Oxidative Stress in Nerve Growth Factor Differentiated PC12 Cells. Neurochem. Res. 2012, 37, 786–794. [Google Scholar] [CrossRef]
- Lakunina, V.A.; Petrushanko, I.Y.; Burnysheva, K.M.; Mitkevich, V.A.; Makarov, A.A. Alzheimer’s Disease Aβ42 Peptide Induces an Increase in Na,K-ATPase Glutathionylation. Dokl. Biochem. Biophys. 2017, 473, 114–117. [Google Scholar] [CrossRef]
- Rasmussen, H.H.; Hamilton, E.J.; Liu, C.C.; Figtree, G.A. Reversible Oxidative Modification: Implications for Cardiovascular Physiology and Pathophysiology. Trends Cardiovasc. Med. 2010, 20, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Zhang, Y.; Kim, Y.J.; Hamilton, E.J.; Xu, B.; Limas, J.; McCracken, S.A.; Morris, J.M.; Makris, A.; Hennessy, A.; et al. B3-Adrenergic Agonist Counters Oxidative Stress and Na1 -K1 Pump Inhibitory S-Glutathionylation of Placental Cells: Implications for Preeclampsia. Am. J. Physiol. Cell Physiol. 2025, 328, C27–C39. [Google Scholar] [CrossRef] [PubMed]
- Petrushanko, I.Y.; Yakushev, S.; Mitkevich, V.A.; Kamanina, Y.V.; Ziganshin, R.H.; Meng, X.; Anashkina, A.A.; Makhro, A.; Lopina, O.D.; Gassmann, M.; et al. S-Glutathionylation of the Na,K-ATPase Catalytic α Subunit Is a Determinant of the Enzyme Redox Sensitivity. J. Biol. Chem. 2012, 287, 32195–32205. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Garcia, A.; Mahmmoud, Y.A.; Hamilton, E.J.; Galougahi, K.K.; Fry, N.A.S.; Figtree, G.A.; Cornelius, F.; Clarke, R.J.; Rasmussen, H.H. Susceptibility of Β1 Na+-K+ Pump Subunit to Glutathionylation and Oxidative Inhibition Depends on Conformational State of Pump. J. Biol. Chem. 2012, 287, 12353–12364. [Google Scholar] [CrossRef]
- Yan, Y.; Shapiro, A.P.; Haller, S.; Katragadda, V.; Liu, L.; Tian, J.; Basrur, V.; Malhotra, D.; Xie, Z.J.; Abraham, N.G.; et al. Involvement of Reactive Oxygen Species in a Feed-Forward Mechanism of Na/K-ATPase-Mediated Signaling Transduction. J. Biol. Chem. 2013, 288, 34249–34258. [Google Scholar] [CrossRef]
- Figtree, G.A.; Liu, C.C.; Bibert, S.; Hamilton, E.J.; Garcia, A.; White, C.N.; Chia, K.K.M.; Cornelius, F.; Geering, K.; Rasmussen, H.H. Reversible Oxidative Modification: A Key Mechanism of Na+-K+ Pump Regulation. Circ. Res. 2009, 105, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Thévenod, F.; Friedmann, J.M. Cadmium-Mediated Oxidative Stress in Kidney Proximal Tubule Cells Induces Degradation of Na+/K(+)-ATPase through Proteasomal and Endo-/Lysosomal Proteolytic Pathways. FASEB J. 1999, 13, 1751–1761. [Google Scholar] [CrossRef] [PubMed]
- Zolotarjova, N.; Ho, C.; Mellgren, R.L.; Askari, A.; Huang, W. hsiung Different Sensitivities of Native and Oxidized Forms of Na+/K(+)-ATPase to Intracellular Proteinases. Biochim. Biophys. Acta 1994, 1192, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Prolo, C.; Piacenza, L.; Radi, R. Peroxynitrite: A Multifaceted Oxidizing and Nitrating Metabolite. Curr. Opin. Chem. Biol. 2024, 80, 102459. [Google Scholar] [CrossRef] [PubMed]
- Reifenberger, M.S.; Arnett, K.L.; Gatto, C.; Milanick, M.A. The Reactive Nitrogen Species Peroxynitrite Is a Potent Inhibitor of Renal Na-K-ATPase Activity. Am. J. Physiol. Renal Physiol. 2008, 295, F1191–F1198. [Google Scholar] [CrossRef] [PubMed]
- Mckee, M.; Scavone, C.; Nathanson, J.A. Nitric Oxide, CGMP, and Hormone Regulation of Active Sodium Transport. Proc. Natl. Acad. Sci. USA 1994, 91, 12056–12060. [Google Scholar] [CrossRef] [PubMed]
- Ellis, D.Z.; Rabe, J.; Sweadner, K.J. Global Loss of Na,K-ATPase and Its Nitric Oxide-Mediated Regulation in a Transgenic Mouse Model of Amyotrophic Lateral Sclerosis. J. Neurosci. 2003, 23, 43–51. [Google Scholar] [CrossRef]
- Ellis, D.Z.; Nathanson, J.A.; Sweadner, K.J. Carbachol Inhibits Na(+)-K(+)-ATPase Activity in Choroid Plexus via Stimulation of the NO/CGMP Pathway. Am. J. Physiol. Cell Physiol. 2000, 279. [Google Scholar] [CrossRef]
- Nie, Y.; Bai, F.; Chaudhry, M.A.; Pratt, R.; Shapiro, J.I.; Liu, J. The Na/K-ATPase A1 and c-Src Form Signaling Complex under Native Condition: A Crosslinking Approach. Sci. Rep. 2020, 10, 6006. [Google Scholar] [CrossRef]
- Haas, M.; Wang, H.; Tian, J.; Xie, Z. Src-Mediated Inter-Receptor Cross-Talk between the Na+/K+-ATPase and the Epidermal Growth Factor Receptor Relays the Signal from Ouabain to Mitogen-Activated Protein Kinases. J. Biol. Chem. 2002, 277, 18694–18702. [Google Scholar] [CrossRef]
- Schoner, W.; Scheiner-Bobis, G. Endogenous Cardiac Glycosides: Hormones Using the Sodium Pump as Signal Transducer. Semin. Nephrol. 2005, 25, 343–351. [Google Scholar] [CrossRef]
- Cai, L.; Pessoa, M.T.; Gao, Y.; Strause, S.; Banerjee, M.; Tian, J.; Xie, Z.; Pierre, S.V. The Na/K-ATPase A1/Src Signaling Axis Regulates Mitochondrial Metabolic Function and Redox Signaling in Human IPSC-Derived Cardiomyocytes. Biomedicines 2023, 11, 3207. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ye, Q.; Liu, C.; Xie, J.X.; Yan, Y.; Lai, F.; Duan, Q.; Li, X.; Tian, J.; Xie, Z. Involvement of Na/K-ATPase in Hydrogen Peroxide-Induced Activation of the Src/ERK Pathway in LLC-PK1 Cells. Free Radic. Biol. Med. 2014, 71, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Bogdanova, A.; Petrushanko, I.; Boldyrev, A.; Gassmann, M. Oxygen- and Redox-Induced Regulation of the Na/K ATPase. Curr. Enzym. Inhib. 2006, 2, 37–59. [Google Scholar] [CrossRef]
- Dobrota, D.; Matejovicova, M.; Kurella, E.G.; Boldyrev, A.A. Na/K-ATPase under Oxidative Stress: Molecular Mechanisms of Injury. Cell. Mol. Neurobiol. 1999, 19, 141–149. [Google Scholar] [CrossRef]
- Kurella, E.G.; Tyulina, O.V.; Boldyrev, A.A. Oxidative Resistance of Na/K-ATPase. Cell Mol. Neurobiol. 1999, 19, 133–140. [Google Scholar] [CrossRef]
- Liu, J.; Nie, Y.; Chaudhry, M.; Bai, F.; Chuang, J.; Sodhi, K.; Shapiro, J.I. The Redox-Sensitive Na/K-ATPase Signaling in Uremic Cardiomyopathy. Int. J. Mol. Sci. 2020, 21, 1256. [Google Scholar] [CrossRef]
- De Melo, A.D.; Freire, V.A.F.; Diogo, Í.L.; Santos, H.d.L.; Barbosa, L.A.; De Carvalho, L.E.D. Antioxidant Therapy Reduces Oxidative Stress, Restores Na,K-ATPase Function and Induces Neuroprotection in Rodent Models of Seizure and Epilepsy: A Systematic Review and Meta-Analysis. Antioxidants 2023, 12, 1397. [Google Scholar] [CrossRef]
- Hill, S.M.; Wrobel, L.; Rubinsztein, D.C. Post-Translational Modifications of Beclin 1 Provide Multiple Strategies for Autophagy Regulation. Cell Death Differ. 2018, 26, 617–629. [Google Scholar] [CrossRef]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative Stress and Autophagy: The Clash between Damage and Metabolic Needs. Cell Death Differ. 2014, 22, 377–388. [Google Scholar] [CrossRef]
- Pialoux, V.; Mounier, R. Hypoxia-Induced Oxidative Stress in Health Disorders. Oxid. Med. Cell Longev. 2012, 2012, 940121. [Google Scholar] [CrossRef]
- Tafani, M.; Sansone, L.; Limana, F.; Arcangeli, T.; De Santis, E.; Polese, M.; Fini, M.; Russo, M.A. The Interplay of Reactive Oxygen Species, Hypoxia, Inflammation, and Sirtuins in Cancer Initiation and Progression. Oxid. Med. Cell Longev. 2016, 2016, 3907147. [Google Scholar] [CrossRef] [PubMed]
- Hielscher, A.; Gerecht, S. Hypoxia and Free Radicals: Role in Tumor Progression and the Use of Engineering-Based Platforms to Address These Relationships. Free Radic. Biol. Med. 2015, 79, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Asgari, R.; Yarani, R.; Mohammadi, P.; Emami Aleagha, M.S. HIF-1α in the Crosstalk Between Reactive Oxygen Species and Autophagy Process: A Review in Multiple Sclerosis. Cell Mol. Neurobiol. 2022, 42, 2121–2129. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Long, M.; Zhang, S.; Cheng, Z.; Zhao, X.; He, F.; Liu, H.; Ming, L. Hypoxia Inducible Factor-1α Regulates Autophagy via the P27-E2F1 Signaling Pathway. Mol. Med. Rep. 2017, 16, 2107–2112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cheng, X.; Yu, L.; Yang, J.; Calvo, R.; Patnaik, S.; Hu, X.; Gao, Q.; Yang, M.; Lawas, M.; et al. MCOLN1 Is a ROS Sensor in Lysosomes That Regulates Autophagy. Nat. Commun. 2016, 7, 12109. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Wan, X.; Zou, X.; Sun, S.; Hao, X.; Liang, C.; Zhang, Z.; Zhang, F.; Sun, B.; Li, H.; et al. Arsenic Trioxide Induces Macrophage Autophagy and Atheroprotection by Regulating ROS-Dependent TFEB Nuclear Translocation and AKT/MTOR Pathway. Cell Death Dis. 2021, 12, 88. [Google Scholar] [CrossRef] [PubMed]
- Settembre, C.; Di Malta, C.; Polito, V.A.; Arencibia, M.G.; Vetrini, F.; Erdin, S.; Erdin, S.U.; Huynh, T.; Medina, D.; Colella, P.; et al. TFEB Links Autophagy to Lysosomal Biogenesis. Science 2011, 332, 1429–1433. [Google Scholar] [CrossRef]
- Klotz, L.O.; Sánchez-Ramos, C.; Prieto-Arroyo, I.; Urbánek, P.; Steinbrenner, H.; Monsalve, M. Redox Regulation of FoxO Transcription Factors. Redox Biol. 2015, 6, 51–72. [Google Scholar] [CrossRef]
- Murtaza, G.; Khan, A.K.; Rashid, R.; Muneer, S.; Hasan, S.M.F.; Chen, J. FOXO Transcriptional Factors and Long-Term Living. Oxid. Med. Cell Longev. 2017, 2017, 3494289. [Google Scholar] [CrossRef]
- Akasaki, Y.; Alvarez-Garcia, O.; Saito, M.; Caramés, B.; Iwamoto, Y.; Lotz, M.K. FoxO Transcription Factors Support Oxidative Stress Resistance in Human Chondrocytes. Arthritis Rheumatol. 2014, 66, 3349–3358. [Google Scholar] [CrossRef]
- Cheng, Z. The FoxO–Autophagy Axis in Health and Disease. Trends Endocrinol. Metab. 2019, 30, 658–671. [Google Scholar] [CrossRef]
- Chi, Y.; Shi, C.; Zhao, Y.; Guo, C. Forkhead Box O (FOXO) 3 Modulates Hypoxia-Induced Autophagy through AMPK Signalling Pathway in Cardiomyocytes. Biosci. Rep. 2016, 36, 345. [Google Scholar] [CrossRef]
- Agostini, F.; Bisaglia, M.; Plotegher, N. Linking ROS Levels to Autophagy: The Key Role of AMPK. Antioxidants 2023, 12, 1406. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).