Carbon Dots-Enhanced Soy Protein Isolate/Polyvinyl Alcohol Composite Film for Active Preservation of Oxidation-Sensitive Foods
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of CDs
2.2.1. Antibacterial Activity of CDs
Minimum Inhibitory Concentration (MIC) and Growth Curve
Integrity of Cell Membrane
Permeability of Cell Wall
2.2.2. Antioxidant Activity of CDs
2.3. Preparation of Composite Film
2.3.1. Characterization of Films
2.3.2. Properties of Films
Light Transmittance
Water Vapor Transmission Rate (WVTR) and Water Vapor Permeability (WVP)
Water Contact Angle (WCA)
Water Absorption Rate
Antioxidant Activity
Antibacterial Activity
Release Kinetics
2.4. Packaging Test
2.4.1. Green Jujube Storage Test
2.4.2. Meatball Storage Test
2.4.3. Oil Storage Test
2.5. Statistical Analysis
3. Results and Discussion
3.1. Morphology of CDs
3.2. Characterization of Films
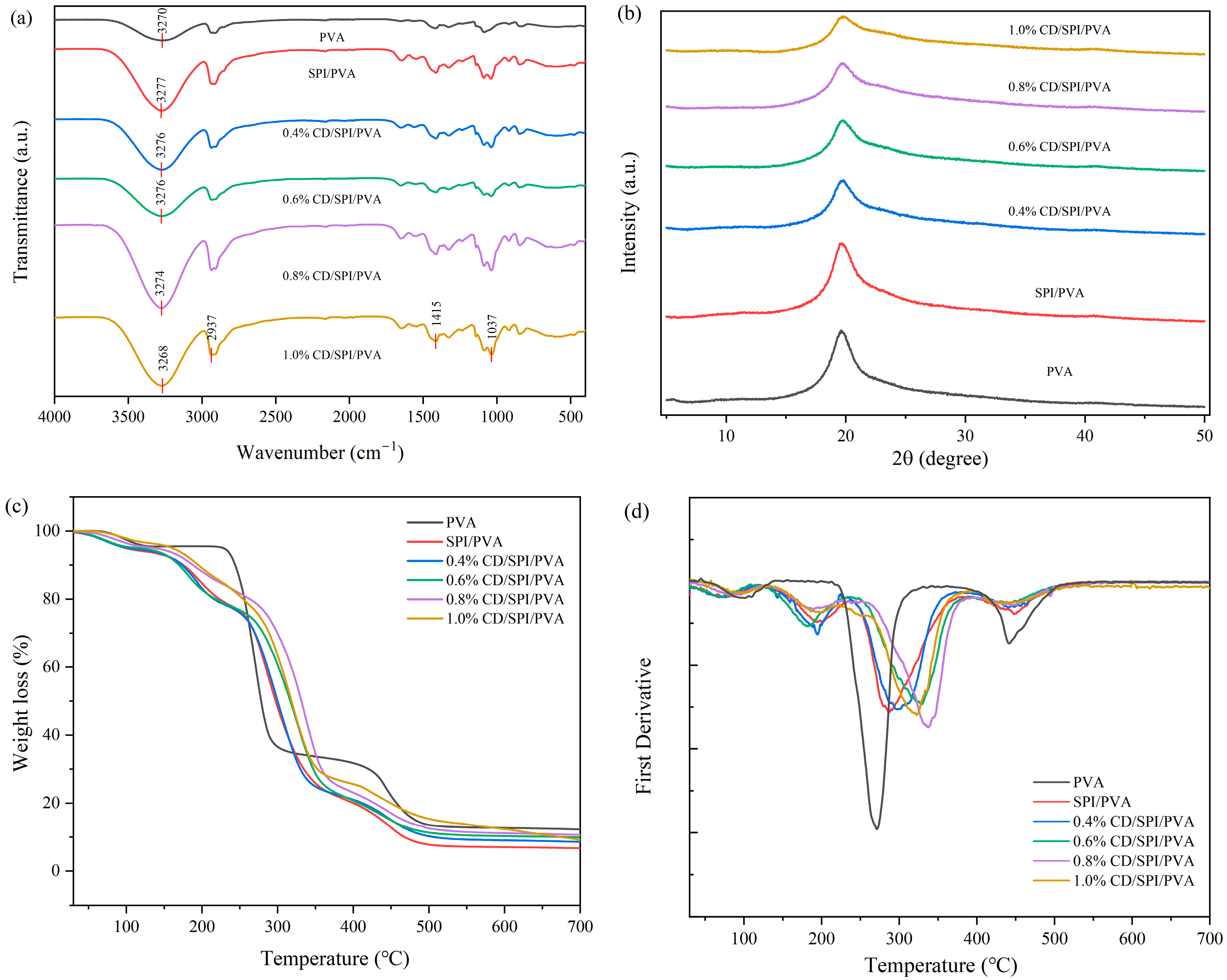
3.2.1. FTIR Analysis
3.2.2. XRD Analysis
3.2.3. Thermogravimetric Analysis
3.3. Film Properties
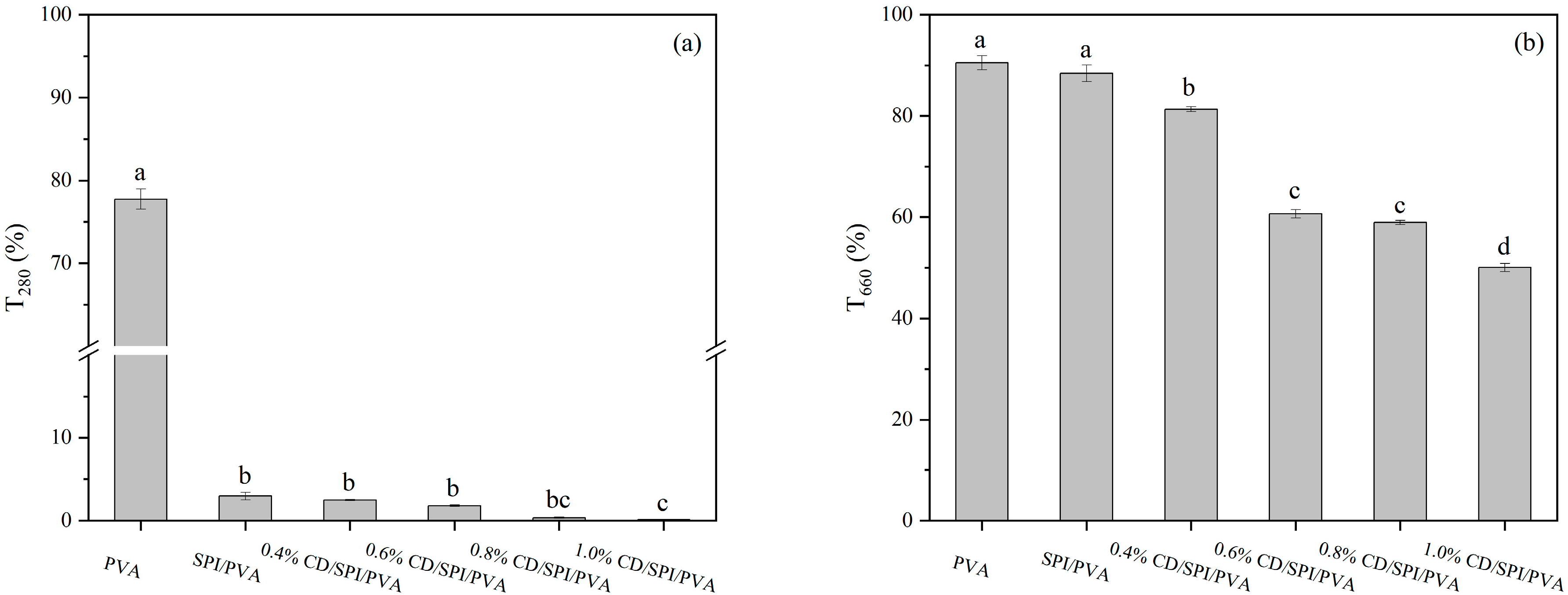
3.3.1. Light Transmittance
3.3.2. WVP, WVTR, WCA, and Water Absorption Rate
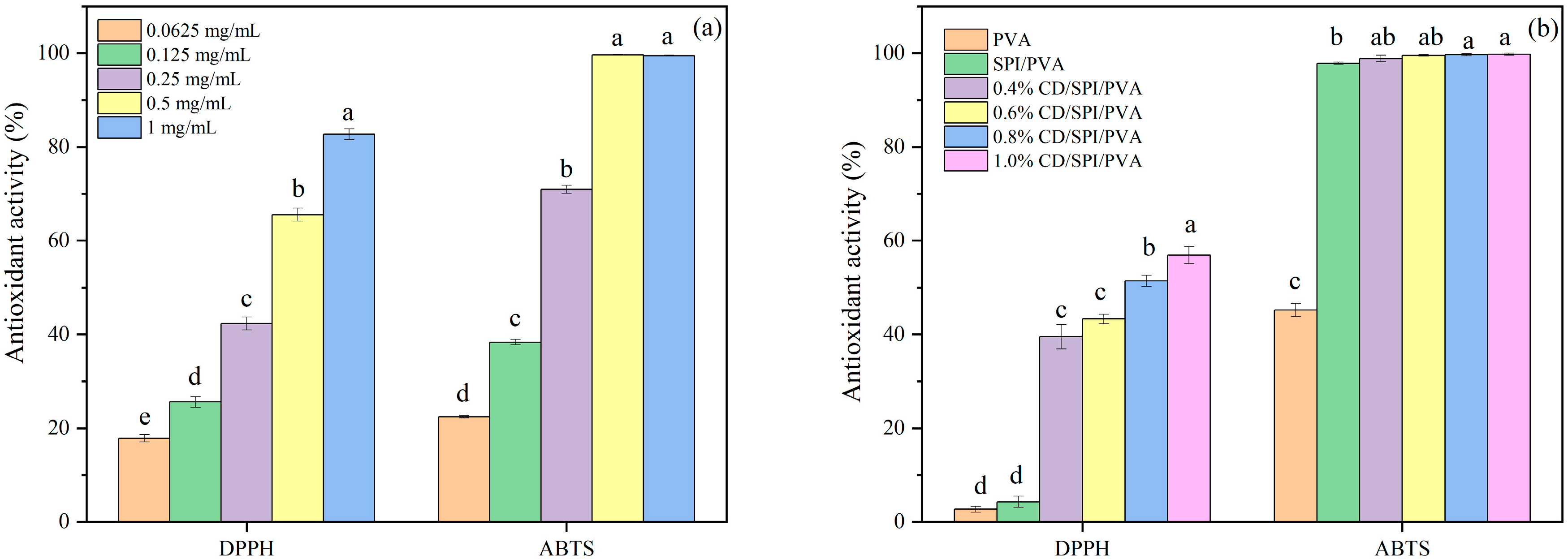
3.4. Antioxidant Activity of CDs and Films
3.5. Antibacterial Properties of CDs and Films
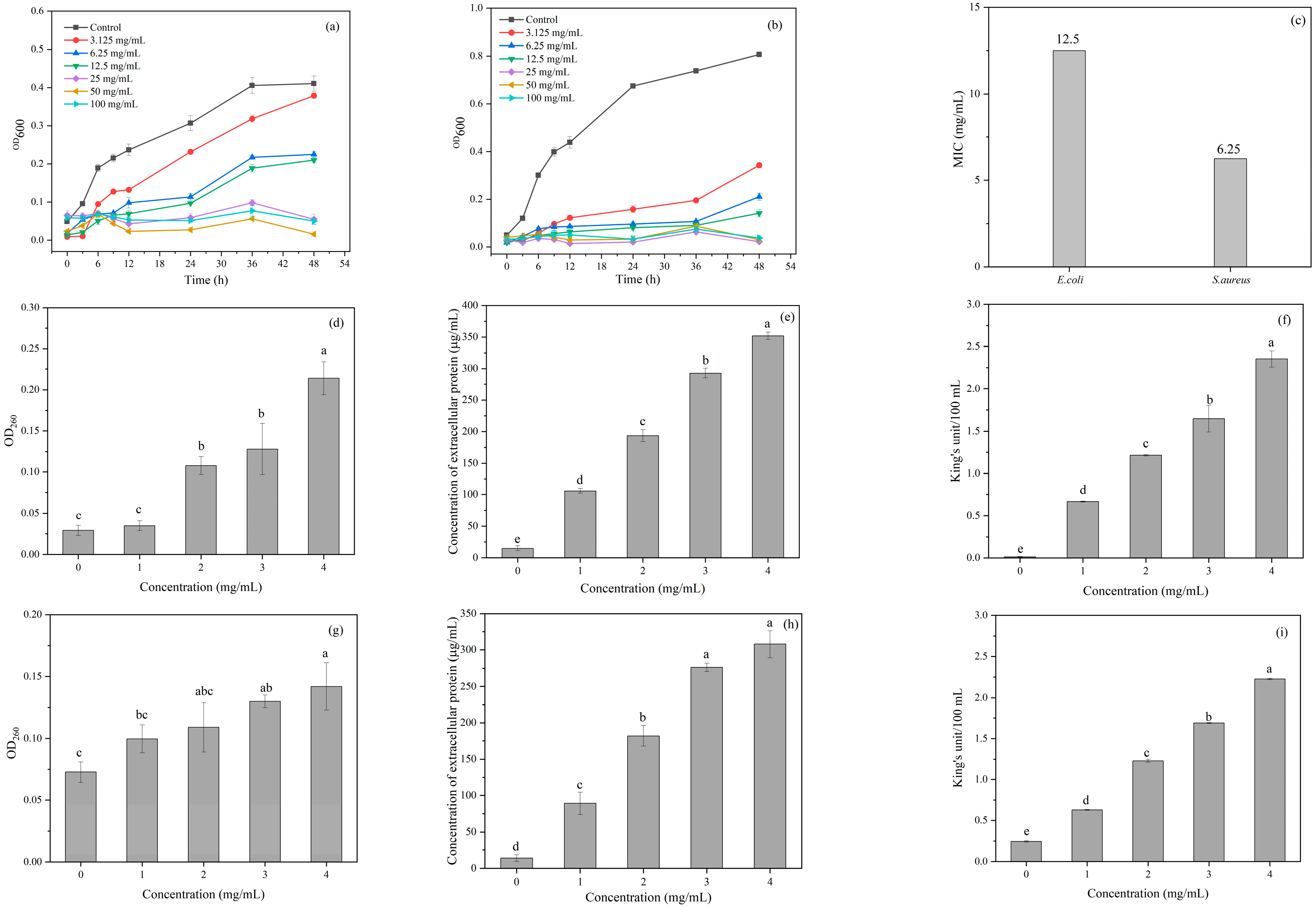
3.5.1. Antibacterial Activity of CDs
3.5.2. Inhibitory Mechanism of CDs
Integrity of Cell Membrane
Permeability of Cell Wall
3.5.3. Antibacterial Activity of Films
3.6. Release Kinetics
3.7. Effect of Active Film on Food Sample Storage
3.7.1. Green Jujubes
3.7.2. Meatballs
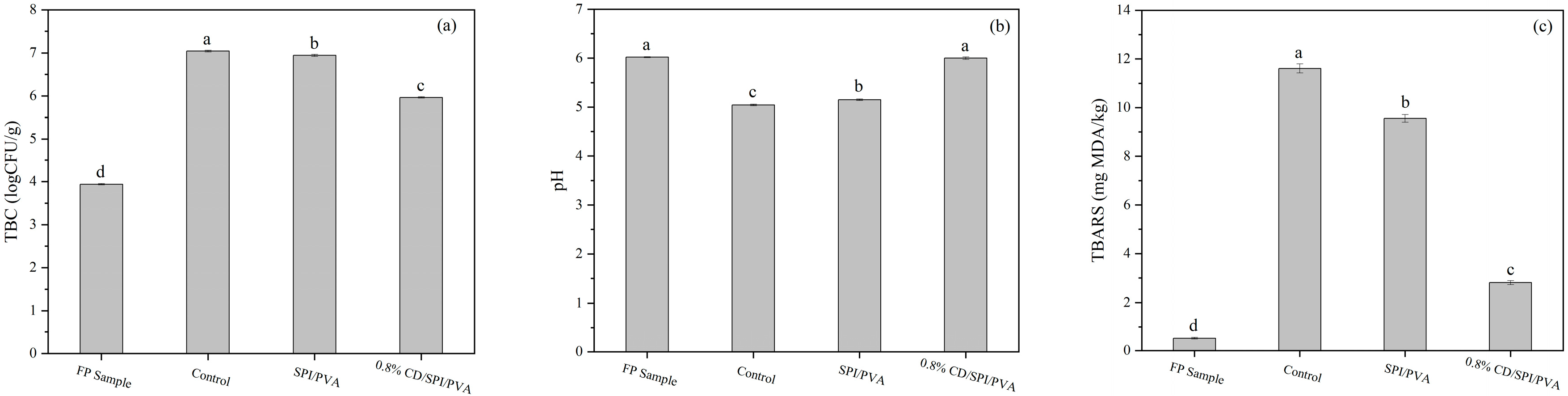
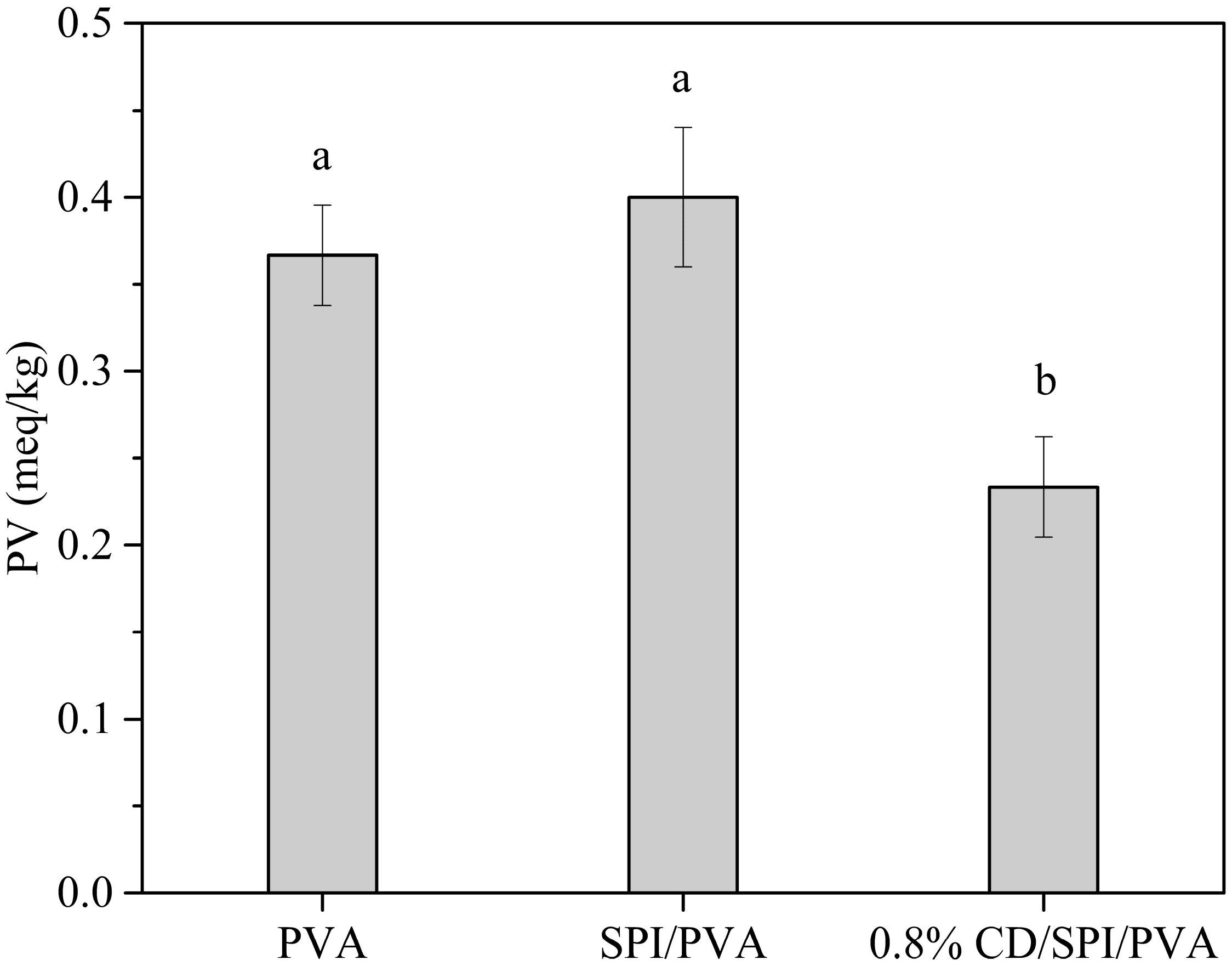
3.7.3. Soybean Oil
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gaikwad, K.K.; Singh, S.; Negi, Y.S. Ethylene scavengers for active packaging of fresh food produce. Environ. Chem. Lett. 2020, 18, 269–284. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, M.; Mujumdar, A.S.; Adhikari, B.; Wang, H. Preparation of a novel carbon dot/polyvinyl alcohol composite film and its application in food preservation. ACS Appl. Mater. Interfaces 2022, 14, 37528–37539. [Google Scholar] [CrossRef] [PubMed]
- Piergiovanni, L.; Limbo, S. Plastic packaging materials. In Food Packaging Materials; Springer: Cham, Switzerland, 2016; pp. 33–49. [Google Scholar] [CrossRef]
- Roy, S.; Kim, H.C.; Panicker, P.S.; Rhim, J.-W.; Kim, J. Cellulose nanofiber-based nanocomposite films reinforced with zinc oxide nanorods and grapefruit seed extract. Nanomaterials 2021, 11, 877. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Daver, F.; Ivanova, E.P.; Adhikari, B. Synthesis of green hybrid materials using starch and non-isocyanate polyurethanes. Carbohydr. Polym. 2020, 229, 115535. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, Y.; Pan, H.; Xu, N.; Mei, C.; Mao, H.; Zhang, W.; Cai, J.; Xu, C. Preparation and performance of radiata-pine-derived polyvinyl alcohol/carbon quantum dots fluorescent films. Materials 2019, 13, 67. [Google Scholar] [CrossRef]
- Yang, W.; Owczarek, J.; Fortunati, E.; Kozanecki, M.; Mazzaglia, A.; Balestra, G.M.; Kenny, J.M.; Torre, L.; Puglia, D. Antioxidant and antibacterial lignin nanoparticles in polyvinyl alcohol/chitosan films for active packaging. Ind. Crops Prod. 2016, 94, 800–811. [Google Scholar] [CrossRef]
- Chen, C.W.; Xie, J.; Yang, F.X.; Zhang, H.L.; Xu, Z.W.; Liu, J.L.; Chen, Y.J. Development of moisture-absorbing and antioxidant active packaging film based on poly (vinyl alcohol) incorporated with green tea extract and its effect on the quality of dried eel. J. Food Process. Preserv. 2017, 42, e13374. [Google Scholar] [CrossRef]
- Su, J.-F.; Huang, Z.; Liu, K.; Fu, L.-L.; Liu, H.-R. Mechanical properties, biodegradation and water vapor permeability of blend films of soy protein isolate and poly (vinyl alcohol) compatibilized by glycerol. Polym. Bull. 2007, 58, 913–921. [Google Scholar] [CrossRef]
- Insaward, A.; Duangmal, K.; Mahawanich, T. Mechanical, optical, and barrier properties of soy protein film as affected by phenolic acid addition. J. Agric. Food Chem. 2015, 63, 9421–9426. [Google Scholar] [CrossRef]
- Phuhongsung, P.; Zhang, M.; Bhandari, B. 4D printing of products based on soy protein isolate via microwave heating for flavor development. Food Res. Int. 2020, 137, 109605. [Google Scholar] [CrossRef]
- Guo, G.; Zhang, C.; Du, Z.; Zou, W.; Li, H. Structure and properties of poly (vinyl alcohol)/soy protein isolate blend film fabricated through melt processing. J. Polym. Environ. 2015, 23, 183–189. [Google Scholar] [CrossRef]
- Li, C.; Fan, L.; Zhu, R.; Li, X.; Wen, P.; Zhao, X.; Wang, G.; Zou, J.; Kim, F. Adjusting channel size within PVA-based hydrogels via ice templating for enhanced solar steam generation. ACS Appl. Energy Mater. 2020, 3, 9216–9225. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, L. New evidences of glass transitions and microstructures of soy protein plasticized with glycerol. Macromol. Biosci. 2005, 5, 237–245. [Google Scholar] [CrossRef]
- Liang, W.; Liu, X.; Zheng, J.; Zhao, W.; Su, C.; Ge, X.; Shen, H.; Zhang, X.; Lu, Y.; Muratkhan, M. Insight into crosslinked chitosan/soy protein isolate/PVA plastics by revealing its structure, physicochemical properties, and biodegradability. Ind. Crops Prod. 2022, 187, 115548. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Lee, J.H.; Ng, P.K. Mechanical and barrier properties of biodegradable soy protein isolate-based films coated with polylactic acid. LWT-Food Sci. Technol. 2007, 40, 232–238. [Google Scholar] [CrossRef]
- Wareing, T.C.; Gentile, P.; Phan, A.N. Biomass-based carbon dots: Current development and future perspectives. ACS Nano 2021, 15, 15471–15501. [Google Scholar] [CrossRef]
- Sri, S.; Kumar, R.; Panda, A.K.; Solanki, P.R. Highly biocompatible, fluorescence, and zwitterionic carbon dots as a novel approach for bioimaging applications in cancerous cells. ACS Appl. Mater. Interfaces 2018, 10, 37835–37845. [Google Scholar] [CrossRef]
- Wei, X.; Li, L.; Liu, J.; Yu, L.; Li, H.; Cheng, F.; Yi, X.; He, J.; Li, B. Green synthesis of fluorescent carbon dots from gynostemma for bioimaging and antioxidant in zebrafish. ACS Appl. Mater. Interfaces 2019, 11, 9832–9840. [Google Scholar] [CrossRef]
- Li, H.; Huang, J.; Song, Y.; Zhang, M.; Wang, H.; Lu, F.; Huang, H.; Liu, Y.; Dai, X.; Gu, Z.; et al. Degradable carbon dots with broad-spectrum antibacterial activity. ACS Appl. Mater. Interfaces 2018, 10, 26936–26946. [Google Scholar] [CrossRef]
- Ezati, P.; Rhim, J.-W.; Molaei, R.; Priyadarshi, R.; Roy, S.; Min, S.; Kim, Y.H.; Lee, S.-G.; Han, S. Preparation and characterization of B, S, and N-doped glucose carbon dots: Antibacterial, antifungal, and antioxidant activity. Sustain. Mater. Technol. 2022, 32, e00397. [Google Scholar] [CrossRef]
- Gautam, S.; Sharma, B.; Jain, P. To investigate interfacial interaction between soy protein isolate biocomposite thin films reinforced with poly (vinyl alcohol) matrix. Polym. Compos. 2021, 42, 3114–3124. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, M.; Mujumdar, A.S.; Wang, H. Application of carbon dots in food preservation: A critical review for packaging enhancers and food preservatives. Crit. Rev. Food Sci. Nutr. 2023, 63, 6738–6756. [Google Scholar] [CrossRef] [PubMed]
- Mehta, V.N.; Jha, S.; Kailasa, S.K. One-pot green synthesis of carbon dots by using Saccharum officinarum juice for fluorescent imaging of bacteria (Escherichia coli) and yeast (Saccharomyces cerevisiae) cells. Mater. Sci. Eng. C 2014, 38, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, M.; Wang, H.; Devahastin, S. Effects of carbon dots in combination with rosemary-inspired carnosic acid on oxidative stability of deep frying oils. Food Control 2021, 125, 107968. [Google Scholar] [CrossRef]
- Ke, Y.; Ding, B.; Zhang, M.; Dong, T.; Fu, Y.; Lv, Q.; Ding, W.; Wang, X. Study on inhibitory activity and mechanism of chitosan oligosaccharides on Aspergillus Flavus and Aspergillus Fumigatus. Carbohydr. Polym. 2022, 275, 118673. [Google Scholar] [CrossRef]
- Lin, Y.; Tang, X.; Xu, L.; Wang, S. Antibacterial properties and possible action mechanism of chelating peptides-zinc nanocomposite against Escherichia coli. Food Control 2019, 106, 106675. [Google Scholar] [CrossRef]
- Li, X.; Lin, J.; Gao, Y.; Han, W.; Chen, D. Antioxidant activity and mechanism of Rhizoma Cimicifugae. Chem. Cent. J. 2012, 6, 140. [Google Scholar] [CrossRef]
- Jamróz, E.; Kopel, P.; Tkaczewska, J.; Dordevic, D.; Jancikova, S.; Kulawik, P.; Milosavljevic, V.; Dolezelikova, K.; Smerkova, K.; Svec, P. Nanocomposite furcellaran films—The influence of nanofillers on functional properties of furcellaran films and effect on linseed oil preservation. Polymers 2019, 11, 2046. [Google Scholar] [CrossRef]
- Huang, X.; Zhou, X.; Dai, Q.; Qin, Z. Antibacterial, antioxidation, UV-blocking, and biodegradable soy protein isolate food packaging film with mangosteen peel extract and ZnO nanoparticles. Nanomaterials 2021, 11, 3337. [Google Scholar] [CrossRef]
- Maciel, F.S.; Assis, R.Q.; Rios, A.d.O.; Pertuzatti, P.B. Açaí powder-enriched biodegradable starch films: Characterization, release in food simulants and protective effect in photodegradation system. Int. J. Biol. Macromol. 2025, 308, 142420. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Ahmed, S.R.; Sherazee, M.; Margel, S.; Gedanken, A.; Srinivasan, S.; Rajabzadeh, A.R. Carbon dot biopolymer-based flexible functional films for antioxidant and food monitoring applications. ACS Appl. Polym. Mater. 2022, 4, 9323–9340. [Google Scholar] [CrossRef]
- Yuan, H.; Li, W.; Chen, C.; Yu, H.; Huang, J.; Lou, X.; Tian, H. The role of bacterial nanocellulose mats encapsulated with cinnamaldehyde on chilled meat preservation. Int. J. Food Sci. Technol. 2022, 58, 880–889. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, L.; Ding, W. Eugenol nanocapsules embedded with gelatin-chitosan for chilled pork preservation. Int. J. Biol. Macromol. 2020, 158, 837–844. [Google Scholar] [CrossRef]
- Rakariyatham, K.; Teerawutgulrag, A.; Laokuldilok, T.; Osiriphun, S.; Ackcharoensuk, N.; Tanbamrung, W. Combinatorial effects of longan (Dimocarpus longan) peel extract and lecithin on stability of soybean oil and the oxidative stability of fried shrimp crackers during storage. LWT 2024, 198, 116065. [Google Scholar] [CrossRef]
- Mathew, S.; Snigdha, S.; Mathew, J.; Radhakrishnan, E. Biodegradable and active nanocomposite pouches reinforced with silver nanoparticles for improved packaging of chicken sausages. Food Packag. Shelf Life 2019, 19, 155–166. [Google Scholar] [CrossRef]
- Khan, A.; Ezati, P.; Rhim, J.-W. Chitosan/starch-based active packaging film with N, P-doped carbon dots for meat packaging. ACS Appl. Bio Mater. 2023, 6, 1294–1305. [Google Scholar] [CrossRef]
- Dai, S.; Xu, T.; Yuan, Y.; Fang, Q.; Lian, Z.; Tian, T.; Tong, X.; Jiang, L.; Wang, H. Combination and precipitation mechanism of soy protein and tea polyphenols. Food Hydrocoll. 2024, 146, 109197. [Google Scholar] [CrossRef]
- Ezati, P.; Rhim, J.-W. Fabrication of quercetin-loaded biopolymer films as functional packaging materials. ACS Appl. Polym. Mater. 2021, 3, 2131–2137. [Google Scholar] [CrossRef]
- Riahi, Z.; Rhim, J.-W.; Bagheri, R.; Pircheraghi, G.; Lotfali, E. Carboxymethyl cellulose-based functional film integrated with chitosan-based carbon quantum dots for active food packaging applications. Prog. Org. Coat. 2022, 166, 106794. [Google Scholar] [CrossRef]
- Ezati, P.; Rhim, J.-W.; Molaei, R.; Rezaei, Z. Carbon quantum dots-based antifungal coating film for active packaging application of avocado. Food Packag. Shelf Life 2022, 33, 100878. [Google Scholar] [CrossRef]
- Ezati, P.; Rhim, J.-W.; Moradi, M.; Tajik, H.; Molaei, R. CMC and CNF-based alizarin incorporated reversible pH-responsive color indicator films. Carbohydr. Polym. 2020, 246, 116614. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, J.; Guo, H. Research progress of polyvinyl alcohol water-resistant film materials. Membranes 2022, 12, 347. [Google Scholar] [CrossRef]
- Liang, S.; Wang, L. A natural antibacterial-antioxidant film from soy protein isolate incorporated with cortex phellodendron extract. Polymers 2018, 10, 71. [Google Scholar] [CrossRef]
- Ezati, P.; Rhim, J.-W.; Molaei, R.; Priyadarshi, R.; Han, S. Cellulose nanofiber-based coating film integrated with nitrogen-functionalized carbon dots for active packaging applications of fresh fruit. Postharvest Biol. Technol. 2022, 186, 111845. [Google Scholar] [CrossRef]
- Du, J.; Ni, Z.-J.; Wang, W.; Thakur, K.; Ma, R.-H.; Ma, W.-P.; Wei, Z.-J. Carbon dot-mediated photodynamic treatment improves the quality attributes of post-harvest goji berries (Lycium barbarum L.) via regulating the antioxidant system. Foods 2024, 13, 955. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.S.; Tomar, M.; Punia, S.; Kukula-Koch, W.; Kumar, M. Enhancing the functionality of chitosan-and alginate-based active edible coatings/films for the preservation of fruits and vegetables: A review. Int. J. Biol. Macromol. 2020, 164, 304–320. [Google Scholar] [CrossRef]
- Min, S.; Ezati, P.; Yoon, K.S.; Rhim, J.-W. Gelatin/poly (vinyl alcohol)-based functional films integrated with spent coffee ground-derived carbon dots and grapefruit seed extract for active packaging application. Int. J. Biol. Macromol. 2023, 231, 123493. [Google Scholar] [CrossRef] [PubMed]
- Krishnaveni, T.; Ramasubbu, A. Synthesis and characterization of biomimetic hydroxy apatite-silver impregnated soy protein isolate nanocomposites for dental implantations. Asian J. Chem. 2017, 29, 2634–2638. [Google Scholar] [CrossRef]
- Su, J.-F. Biodegradable soy protein isolate/poly (vinyl alcohol) packaging films. In Handbook of Composites from Renewable Materials; Scrivener Publishing LLC: Austin, TX, USA, 2017; Volume 30, p. 587. [Google Scholar] [CrossRef]
- Min, S.; Ezati, P.; Rhim, J.-W. Gelatin-based packaging material incorporated with potato skins carbon dots as functional filler. Ind. Crops Prod. 2022, 181, 114820. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Jiang, H.; Han, Z.; Gu, W.; Chitrakar, B.; Meng, X. Carbon Dots-Enhanced Soy Protein Isolate/Polyvinyl Alcohol Composite Film for Active Preservation of Oxidation-Sensitive Foods. Antioxidants 2025, 14, 669. https://doi.org/10.3390/antiox14060669
Zhao L, Jiang H, Han Z, Gu W, Chitrakar B, Meng X. Carbon Dots-Enhanced Soy Protein Isolate/Polyvinyl Alcohol Composite Film for Active Preservation of Oxidation-Sensitive Foods. Antioxidants. 2025; 14(6):669. https://doi.org/10.3390/antiox14060669
Chicago/Turabian StyleZhao, Linlin, Huinan Jiang, Zhengxuan Han, Wenqin Gu, Bimal Chitrakar, and Xiangren Meng. 2025. "Carbon Dots-Enhanced Soy Protein Isolate/Polyvinyl Alcohol Composite Film for Active Preservation of Oxidation-Sensitive Foods" Antioxidants 14, no. 6: 669. https://doi.org/10.3390/antiox14060669
APA StyleZhao, L., Jiang, H., Han, Z., Gu, W., Chitrakar, B., & Meng, X. (2025). Carbon Dots-Enhanced Soy Protein Isolate/Polyvinyl Alcohol Composite Film for Active Preservation of Oxidation-Sensitive Foods. Antioxidants, 14(6), 669. https://doi.org/10.3390/antiox14060669





