Abstract
This study examined the protective impact of tributyrin on heat-stressed Taihe silky fowls, providing insight into oxidative status, inflammatory response, and mucosal barrier function. Three hundred chicks were randomly assigned to 6 treatments: control (CON, 24 ± 1 °C) fed with basal diet and 5 heat stress (HS) treatments (34 ± 1 °C for 8 h/d) fed with basal diet containing 0, 0.04, 0.08, 0.16, and 0.32% tributyrin. Heat stress elevated serum malondialdehyde (MDA), tumor necrosis factor α (TNF-α), interleukin 1β (IL-1β), D-lactate, and diamine oxidase levels, and decreased total antioxidant capacity (T-AOC) and superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), interleukin 4 (IL-4), interleukin 10 (IL-10), and immunoglobulin G (IgG) levels (p < 0.05). Compared with HS treatment, tributyrin reversed these serum changes (p < 0.05). Moreover, HS elevated jejunal and ileal MDA content and IL-1β mRNA abundance, decreased GSH-Px activity, villus height (VH), VH: crypt depth ratio, and mRNA abundance of IL-10, occludin, and zonula occludens-1 (ZO-1), and decreased cecal butyrate content (p < 0.05). Compared with HS treatment, tributyrin reduced jejunal and ileal MDA content and IL-1β mRNA abundance, increased GSH-Px activity, VH, and mRNA abundance of IL-4, IL-10, occludin, and ZO-1, and increased cecal butyrate content (p < 0.05). In conclusion, tributyrin enhanced antioxidant capacity, attenuated inflammatory responses, increased cecal butyrate content, and improved intestinal morphology and mucosal barrier function in cyclic heat-stressed Taihe silky fowls.
1. Introduction
With global climate warming and the increasing density of intensive livestock farming, poultry are generally subjected to heat stress [1]. Heat stress adversely impacts poultry health and production, causing economic losses in the industry [2]. Numerous studies have shown that heat stress can trigger various physiological disorders in poultry, including endocrine function disorders, immunosuppression, oxidative stress, and intestinal dysfunction [3,4,5].
The small intestine is essential for nutrient digestion and absorption, while also maintaining the mucosal barrier function and regulating signal transduction [6]. However, the small intestine is prone to being affected by heat stress [7]. Heat stress could weaken the transmembrane barrier function of intestinal epithelial cells in broilers and increase intestinal permeability, thereby damaging intestinal integrity [8,9]. The disruption of the intestinal epithelial barrier integrity can allow toxic substances to invade the intestinal mucosa and trigger an increase in inflammatory cytokines, thereby leading to an imbalance in immune function [10]. Numerous research studies have reported strategies for alleviating heat stress, in which dietary supplementation additives are considered an effective solution [11,12,13,14]. Butyrate has attracted widespread attention because of its nutritional and physiological functions [15].
Butyrate is a short-chain fatty acid that can be rapidly absorbed by epithelial cells through the action of butyrate transporters, providing energy for the intestinal epithelium [16]. Nevertheless, the unpleasant odor and rapid metabolism of butyrate or sodium butyrate have restricted its use in poultry production [17]. Tributyrin is a butyrate prodrug formed through the esterification of one molecule of glycerol with three molecules of butyrate [18]. In the intestines of animals, tributyrin decomposes into butyrate, which is rapidly absorbed and utilized by intestinal epithelial cells [14]. It reported that tributyrin plays a crucial role in regulating immune responses, antioxidant capacity, and anti-inflammatory effects [19,20,21]. Li et al. (2015) also demonstrated that tributyrin improved antioxidant capacity and decrease the secretion of pro-inflammatory cytokines in lipopolysaccharide (LPS)-challenged chickens [22]. Our previous research found that tributyrin improved growth performance and meat quality in heat-stressed Taihe silky fowls [23]. Furthermore, compared to commercial broiler chickens, Taihe silky fowls have poor heat resistance due to their unique filamentous feathers and black skin, which hinder heat dissipation [24,25]. To date, the alleviating effect of dietary tributyrin on intestinal damage in heat-stressed broilers is still unclear. Intestinal damage was highly related to inflammation response and oxidative status [26].
Accordingly, we selected the native Taihe silky fowls (Gallus domesticus) as representative models to examine the protective impact of tributyrin on oxidative status, inflammatory responses, and mucosal barrier function in heat-stressed Taihe silky fowls, and to provide practical nutritional strategies for alleviating intestinal damage.
2. Materials and Methods
2.1. Experiment Design
Four hundred 1-day-old Taihe silky fowls were obtained from a commercial hatchery and provided with a standard commercial diet (Taihe County, Jiangxi Province, China). At 120 days of age, 300 birds (average BW = 915.29 ± 7.99 g) were assigned to 6 dietary treatments, each of which was replicated as five cages with ten broilers per cage. Control treatment (CON, basal diet): birds were raised in a thermoneutral temperature (24 ± 1 °C); heat stress treatment (HS, basal diet): birds were subjected to cyclic heat stress (34 ± 1 °C from 9:30 to 17:30 and 24 ± 1 °C for the remainder of the time); HS + tributyrin treatments (HST1, HST2, HST3, and HST4): birds received the basal diet supplemented with 0.04%, 0.08%, 0.16%, and 0.32% tributyrin (ProPhorce SR 130, butyrate content ≥ 51.1%, Perstorp Waspik B.V., Waspik, The Netherlands) and were reared under cyclic heat stress (CHS) conditions. The dose selection referred to the standards of the previous studies [22,27]. Birds were housed in three-tier wire cages (length = 125 cm, width = 65 cm, and height = 50 cm). As recommended by the Feeding Standard of Chicken, China (NY/T 33-2004), the basal diet (Table 1) was created. The 28-day trial was conducted with birds provided feed and water ad libitum throughout. Additionally, two birds per cage were chosen at random on days 1, 7, 14, 21, and 28, and an electronic thermometer was used to recorded their rectal temperature.

Table 1.
Composition and nutrient contents of basal diets.
2.2. Sample Collection
A bird was randomly chosen from each cage after a 12 h fast, after 28 days of heat exposure. Blood samples were taken from the wing vein and then centrifuged (3500 rpm, 10 min, at 4 °C) to harvest serum. The birds were then euthanized using cervical dislocation followed by exsanguination. The entire gastrointestinal tract was immediately excised, and 2 cm jejunal and ileal mid-sections were separated and preserved in 4% (w/v) paraformaldehyde for morphology analysis. Mucosal samples from the jejunum and ileum were scraped with a sterile microscope slide. Additionally, cecal digesta was collected. The serum and intestinal samples were stored at −80 °C.
2.3. Serum Indicators
According to the manufacturers’ instructions, the levels of total antioxidant capacity (T-AOC, No. A015-2), glutathione peroxidase (GSH-Px, No. A005-1), superoxide dismutase (SOD, No. A001-3), and malondialdehyde (MDA, No. A003-1) were measured with commercial kits (Nanjing Jiancheng Institute of Biotechnology, Nanjing, China). In addition, ELISA kits (Shanghai Enzyme-linked Biotechnology, Shanghai, China) were used to detect the levels of D-lactate (No. ml08770), diamine oxidase (DAO, No. ml036981), immunoglobulins (IgA (No. ml002792), IgG (No. ml042771), and IgM (No. ml890233)) and inflammatory cytokines (TNF-α (No. ml002790), IL-1β (No. ml059835), IL-6 (No. ml059839), IL-4 (No. ml059838), and IL-10 (No. ml059830)). These ELISA kits are only used for the determination of relevant indicators in chicken samples, and the wavelength was 450 nm.
2.4. Intestinal Morphology
The samples of jejunum and ileum were preserved in 4% paraformaldehyde. Following a series of graded ethanol dehydration, they were embedded in paraffin and cut with a microtome to a 4 μm thickness. After staining the section for two minutes with hematoxylin and forty seconds with eosin, it was dehydrated and mounted. After that, the stained section was examined and photographed under an optical microscope to assess the morphological changes in the intestine. Using ImageJ software (version 1.54), the villus height (VH) and crypt depth (CD) were determined, and the VH/CD was computed.
2.5. Intestinal Mucosa Oxidative Status
The samples of jejunal and ileal mucosa, each weighing 1 g, were carefully homogenized with 9 mL of PBS. Then, the homogenate was centrifuged (3000 rpm, 10 min, at 4 °C) to collect the supernatant for further analysis. Following this, commercial assay kits from the Nanjing Jiancheng Institute were used to measure the levels of antioxidant indicators (T-AOC, GSH-Px, SOD, and MDA) and total protein.
2.6. Real-Time qPCR Assay
The Ultrapure RNA kit was used to extract RNA from the jejunum and ileum. The expression levels of related genes were determined using the real-time qPCR technique with the specific primers presented in Table 2. The Real-time qPCR was conducted on a QuantStudioTM 5 Real-Time PCR instrument equipped with a 384-well block (Thermo Scientific, Waltham, MA, USA). In our study, the 10 µL qPCR reaction comprised 5 µL 2 × SuperStar Blue Universal SYBR Master Mix, 0.2 µL each of forward and reverse primers, 1 µL template cDNA, and 3.6 µL ddH2O. The above kits were purchased from Kangwei Century Biotechnology (Taizhou, China). The thermal cycling conditions are shown in Table 3. The mRNA abundances were calculated as per the 2−∆∆Ct method.

Table 2.
Sequences for real-time qPCR PCR primers.

Table 3.
Real-time qPCR thermal cycling conditions.
2.7. Short Chain Fatty Acids (SCFAs) in Cecal Chyme
The SCFAs content in cecal chyme was measured as per a method previously described [28]. Briefly, 0.5 g of chyme was homogenized with 3 mL of distilled water and then centrifuged (10,000 rpm, 10 min, at 4 °C) to harvest supernatant, which was combined with 25% metaphosphate (w/v, 1:5) and centrifuged again under the same conditions. Finally, 1 mL of the supernatant was placed in a screw-cap vial for detection. A GC-8860 gas chromatograph (Agilent, Santa Clara, CA, USA) equipped with a 30 m × 0.25 mm × 0.25 μm capillary column (DB-FastFAME, Agilent) and a flame ionization detector were utilized to analyze the samples. The SCFAs content in each sample was calculated by integrating the peak areas of the standard solution and the sample for comparison.
2.8. Statistical Analysis
All experimental data were analyzed using SPSS version 25.0 (Chicago, IL, USA). We utilized one-way ANOVA followed by Tukey’s multiple range test to assess differences among treatments. Results are presented as means ± SEM, with significance defined as p < 0.05. The analyses were conducted with the cage as the experimental unit (n = 5).
3. Results
3.1. Rectal Temperature
As seen in Table 4, the rectal temperature of Taihe silky fowls in the HS treatment was higher than that in the CON treatment after heat exposure on days 1, 7, 14, 21, and 28 of heat exposure (p < 0.05).

Table 4.
Effects of tributyrin on cloacal temperatures in cyclic heat-stressed Taihe silky fowls, °C.
3.2. Serum Antioxidant Parameters
The effect of tributyrin on serum antioxidant parameters in cyclic heat-stressed Taihe silky fowls is exhibited in Figure 1. Relative to the CON treatment, heat stress significantly decreased serum T-AOC, SOD, and GSH-Px activities and concurrently elevated MDA concentration (p < 0.05). Compared with HS treatment, dietary supplementation with 0.08%, 0.16%, and 0.32% tributyrin significantly elevated serum SOD and GSH-Px activities (p < 0.05); supplementation with 0.16% and 0.32% tributyrin significantly increased serum T-AOC activity and decreased MDA content in cyclic heat-stressed Taihe silky fowls (p < 0.05).
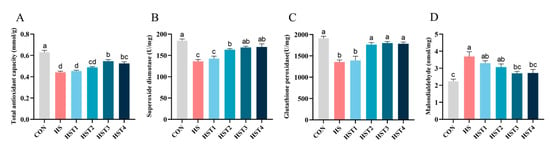
Figure 1.
Effect of tributyrin on serum antioxidant parameters in cyclic heat-stressed Taihe silky fowls. (A) Total antioxidant capacity, (B) superoxide dismutase, (C) glutathione peroxidase, and (D) malondialdehyde. CON group = basal diet + thermoneutral temperature (24 ± 1 °C); HS group = basal diet + cyclic heat stress (CHS) conditions (34 ± 1 °C from 9: 30 to 17: 30 and 24 ± 1 °C for the rest of the time); HS + tributyrin groups (HST1, HST2, HST3, and HST4), basal diet + 0.04%, 0.08%, 0.16%, and 0.32% tributyrin + CHS conditions. a–d Groups without common letters differ significantly (p < 0.05). n = 5.
3.3. Serum Immunoglobulins
Serum immunoglobulin concentrations were quantified to evaluate the impact of tributyrin on the immune function in the serum of Taihe silky fowls under CHS conditions (Figure 2). Heat stress reduced serum IgG concentration relative to the CON treatment (p < 0.05). Compared with HS treatment, dietary addition of 0.16% and 0.32% tributyrin markedly elevated serum IgG content in cyclic heat-stressed Taihe silky fowls (p < 0.05). No significant difference was observed in serum IgA and IgM levels among treatments (p > 0.05).
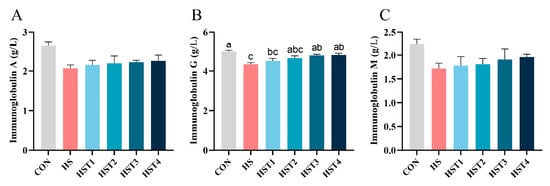
Figure 2.
Effect of tributyrin on serum immunoglobulins in cyclic heat-stressed Taihe silky fowls. (A) Immunoglobulin A, (B) immunoglobulin G, and (C) immunoglobulin M. CON group = basal diet + thermoneutral temperature (24 ± 1 °C); HS group = basal diet + cyclic heat stress (CHS) conditions (34 ± 1 °C from 9: 30 to 17: 30 and 24 ± 1 °C for the rest of the time); HS + tributyrin groups (HST1, HST2, HST3, and HST4), basal diet + 0.04%, 0.08%, 0.16%, and 0.32% tributyrin + CHS conditions. a–c Groups without common letters differ significantly (p < 0.05). n = 5.
3.4. Serum Inflammatory Cytokines
Relative to the CON treatment, heat stress elevated serum TNF-α and IL-1β concentrations, as well as reducing IL-4 and IL-10 concentrations (p < 0.05, Figure 3). Compared with HS treatment, dietary supplementation with 0.04%, 0.08%, 0.16%, and 0.32% tributyrin significantly increased serum IL-10 concentration (p < 0.05); supplementation with 0.08%, 0.16 and 0.32% tributyrin significantly enhanced serum IL-4 concentration (p < 0.05); supplementation with 0.16% and 0.32% tributyrin significantly reduced serum TNF-α and IL-1β concentrations (p < 0.05) in cyclic heat-stressed Taihe silky fowls. However, no significant difference in serum IL-6 was observed among treatments (p > 0.05).
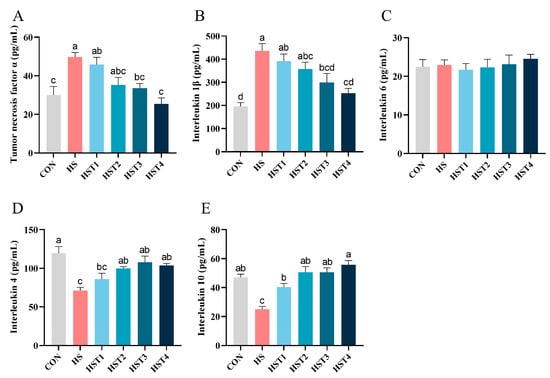
Figure 3.
Effect of tributyrin on serum cytokines in cyclic heat-stressed Taihe silky fowls. (A) Tumor necrosis factor α, (B) interleukin 1β, (C) interleukin 6, (D) interleukin-4, and (E) interleukin-10. CON group = basal diet + thermoneutral temperature (24 ± 1 °C); HS group = basal diet + cyclic heat stress (CHS) conditions (34 ± 1 °C from 9: 30 to 17: 30 and 24 ± 1 °C for the rest of the time); HS + tributyrin groups (HST1, HST2, HST3, and HST4), basal diet + 0.04%, 0.08%, 0.16%, and 0.32% tributyrin + CHS conditions. a–d Groups without common letters differ significantly (p < 0.05). n = 5.
3.5. Serum D-Lactate and DAO
The concentrations of serum D-lactate and DAO were measured to assess the impact of tributyrin on intestinal permeability of Taihe silky fowls under CHS conditions (Figure 4). Heat stress elevated serum D-lactate and DAO concentrations relative to the CON treatment (p < 0.05). Compared with HS treatment, dietary supplementation with 0.04%, 0.08%, 0.16%, and 0.32% tributyrin markedly reduced serum D-lactate concentration (p < 0.05); supplementation with 0.08%, 0.16%, and 0.32% tributyrin significantly decreased serum DAO activity in cyclic heat-stressed Taihe silky fowls (p < 0.05).
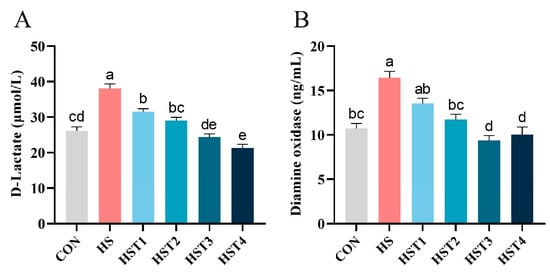
Figure 4.
Effect of tributyrin on serum (A) D-lactate and (B) diamine oxidase in cyclic heat-stressed Taihe silky fowls. CON group = basal diet + thermoneutral temperature (24 ± 1 °C); HS group = basal diet + cyclic heat stress (CHS) conditions (34 ± 1 °C from 9: 30 to 17: 30 and 24 ± 1 °C for the rest of the time); HS + tributyrin groups (HST1, HST2, HST3, and HST4), basal diet + 0.04%, 0.08%, 0.16%, and 0.32% tributyrin + CHS conditions. a–e Groups without common letters differ significantly (p < 0.05). n = 5.
3.6. Intestinal Morphology
Figure 5 showed that heat stress reduced VH and VH/CD in the jejunum and ileum relative to the CON treatment (p < 0.05). Compared with HS treatment, dietary supplementation with 0.16% and 0.32% tributyrin significantly elevated jejunal and ileal VH, as well as jejunal VH/CD in cyclic heat-stressed Taihe silky fowls (p < 0.05).
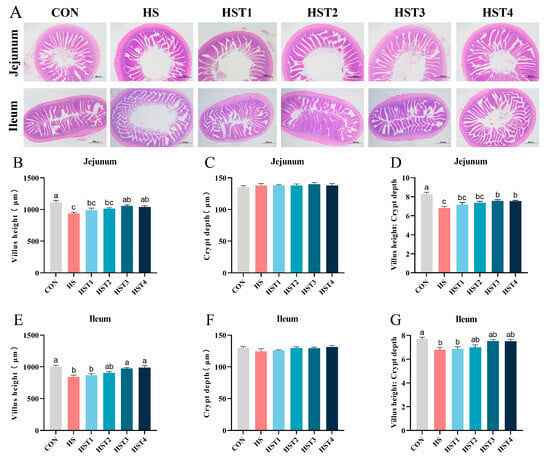
Figure 5.
Effect of tributyrin on intestinal morphology in cyclic heat-stressed Taihe silky fowls. (A) A representative hematoxylin and eosin (H&E) image of the jejunum and ileum was observed at a magnification of 20×. Villus height of jejunum (B) and ileum (E). Crypt depth of jejunum (C) and ileum (F). Villus height: Crypt depth of jejunum (D) and ileum (G). CON group = basal diet + thermoneutral temperature (24 ± 1 °C); HS group = basal diet + cyclic heat stress (CHS) conditions (34 ± 1 °C from 9: 30 to 17: 30 and 24 ± 1 °C for the rest of the time); HS + tributyrin groups (HST1, HST2, HST3, and HST4), basal diet + 0.04%, 0.08%, 0.16%, and 0.32% tributyrin + CHS conditions. a–c Groups without common letters differ significantly (p < 0.05). n = 5.
3.7. Intestinal Mucosa Antioxidant Parameters
Figure 6 summarizes the effects of tributyrin on antioxidant parameters in the intestinal mucosa of Taihe silky fowls under CHS conditions. Compared with the CON treatment, heat stress markedly decreased GSH-Px activity and increased MDA concentration in both jejunal and ileal mucosa (p < 0.05). Compared with HS treatment, dietary supplementation with 0.16% and 0.32% tributyrin significantly increased GSH-Px activity in the jejunal mucosa and decreased MDA content in the ileal mucosa (p < 0.05); supplementation with 0.16% tributyrin also decreased jejunal mucosal MDA content (p < 0.05).
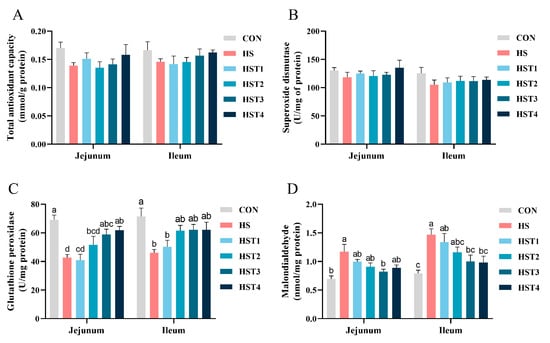
Figure 6.
Effect of tributyrin on the antioxidant parameters in both jejunal and ileal mucosa in cyclic heat-stressed Taihe silky fowls. (A) Total antioxidant capacity, (B) superoxide dismutase, (C) glutathione peroxidase, and (D) malondialdehyde. CON group = basal diet + thermoneutral temperature (24 ± 1 °C); HS group = basal diet + cyclic heat stress (CHS) conditions (34 ± 1 °C from 9: 30 to 17: 30 and 24 ± 1 °C for the rest of the time); HS + tributyrin groups (HST1, HST2, HST3, and HST4), basal diet + 0.04%, 0.08%, 0.16%, and 0.32% tributyrin + CHS conditions. a–d Groups without common letters differ significantly (p < 0.05). n = 5.
3.8. Relative mRNA Expression of Jejunal and Ileal Mucosa
Compared with the CON treatment, heat stress significantly elevated the mRNA abundances of TNF-α, IL-1β, and IL-6 in the jejunal mucosa (p < 0.05, Figure 7). Compared with HS treatment, dietary supplementation with 0.04%, 0.08%, 0.16%, and 0.32% tributyrin significantly decreased the mRNA abundances of TNF-α and IL-1β in the jejunal mucosa (p < 0.05); supplementation with 0.08% and 0.16% tributyrin decreased ileal mucosal IL-1β mRNA abundance (p < 0.05). Additionally, heat stress significantly decreased the mRNA abundances of IL-4, IL-10, occludin, and ZO-1 in the jejunal mucosa (p < 0.05), as well as decreased the mRNA abundances of IL-10, occludin, claudin-1, and ZO-1 in the ileal mucosa (p < 0.05). Compared with HS treatment, dietary supplementation with 0.04%, 0.08%, 0.16%, and 0.32% tributyrin significantly increased ileal mucosal IL-4 mRNA abundance (p < 0.05). Additionally, supplementation with 0.08%, 0.16%, and 0.32% tributyrin significantly elevated jejunal mucosal ZO-1 and ileal mucosal IL-10, occludin, and claudin-1 mRNA abundances (p < 0.05); supplementation with 0.16% and 0.32% tributyrin also increased jejunal mucosal IL-4 and occludin mRNA abundances together with ileal mucosal ZO-1 mRNA abundance (p < 0.05); and supplementation with 0.32% tributyrin significantly raised jejunal mucosal IL-10 mRNA abundance (p < 0.05).
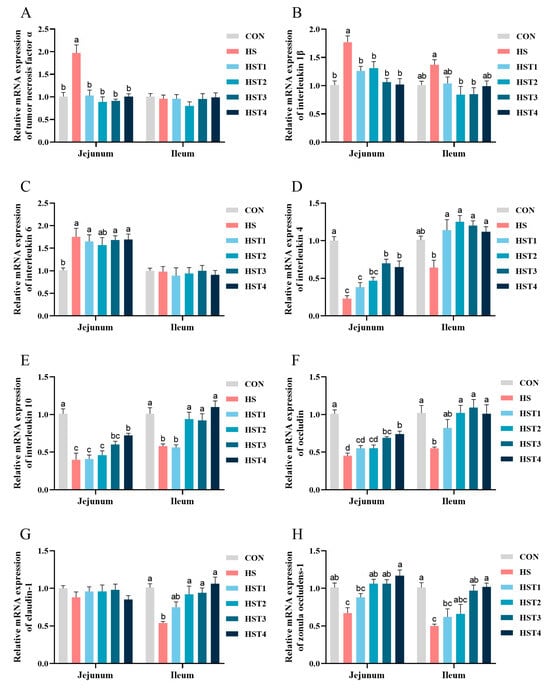
Figure 7.
Effect of tributyrin on the mRNA abundance of jejunal and ileal mucosa in cyclic heat-stressed Taihe silky fowls. (A) Tumor necrosis factor α, (B) interleukin 1β, (C) interleukin 6, (D) interleukin-4, (E) interleukin-10, (F) occludin, (G) claudin-1, and (H) zonula occludens-1. CON group = basal diet + thermoneutral temperature (24 ± 1 °C); HS group = basal diet + cyclic heat stress (CHS) conditions (34 ± 1 °C from 9: 30 to 17: 30 and 24 ± 1 °C for the rest of the time); HS + tributyrin groups (HST1, HST2, HST3, and HST4), basal diet + 0.04%, 0.08%, 0.16%, and 0.32% tributyrin + CHS conditions. a–d Groups without common letters differ significantly (p < 0.05). n = 5.
3.9. SCFAs in Cecal Chyme
The concentration of SCFAs (acetic acid, propanoic acid, butyric acid, isobutyric acid, valeric acid, and isovaleric acid) among the treatments was analyzed and exhibited in Figure 8. Compared with the CON treatment, the HS treatment lowered cecal butyric acid concentration (p < 0.05). Compared with HS treatment, dietary addition of 0.16% and 0.32% tributyrin increased cecal butyric acid concentration in cyclic heat-stressed Taihe silky fowls (p < 0.05).
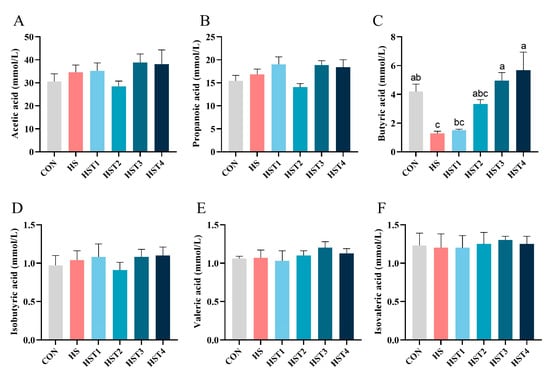
Figure 8.
Effect of tributyrin on SCFAs content of cecal digesta in cyclic heat-stressed Taihe silky fowls. (A) acetic acid, (B) propanoic acid, (C) butyric acid, (D) isobutyric acid, (E) valeric acid, and (F) isovaleric acid. CON group = basal diet + thermoneutral temperature (24 ± 1 °C); HS group = basal diet + cyclic heat stress (CHS) conditions (34 ± 1 °C from 9: 30 to 17: 30 and 24 ± 1 °C for the rest of the time); HS + tributyrin groups (HST1, HST2, HST3, and HST4), basal diet + 0.04%, 0.08%, 0.16%, and 0.32% tributyrin + CHS conditions. a–c Groups without common letters differ significantly (p < 0.05). n = 5.
4. Discussion
The long-term exposure to elevated temperatures renders poultry particularly susceptible to HS due to their higher metabolic rate and lack of sweat glands [2]. Ambient temperatures above the broiler’s thermoneutral zone (16~26 °C) impose HS, rapidly manifesting as hyperthermia and a spectrum of physiological disturbances [29]. Throughout the experiment, Taihe silky fowls exposed to HS exhibited an increase in rectal temperature at all five sampling time points, confirming the successful establishment of the heat-stressed model, consistent with previous research findings [30].
Generally, HS can lead to oxidative stress, particularly when the antioxidant defense system is dysregulated [31]. Research has demonstrated that HS can generate excessive free radicals, cause lipid peroxidation, and disrupt the redox balance, ultimately resulting in oxidative damage to tissue [14,26]. Our findings indicated that HS elevated serum and mucosal (jejunum and ileum) MDA concentrations, while simultaneously decreasing serum T-AOC, SOD, and GSH-Px levels, as well as mucosal GSH-Px activities in the jejunum and ileum. Recent studies have suggested that dietary tributyrin acts as an antioxidant by boosting GSH-Px and SOD activity, while inhibiting lipid peroxidation in animals [22,32,33]. Similarly, supplementation with tributyrin led to increases in serum T-AOC, SOD, and GSH-Px levels, as well as elevated GSH-Px activities in the mucosal tissues of the jejunum and ileum. At the same time, tributyrin reduced MDA content, supporting its role as an antioxidant found in previous research. Our results suggested that tributyrin can improve the redox status of intestinal mucosa in heat-stressed Taihe silky fowls by enhancing the activity of key antioxidant enzymes.
Inflammatory responses frequently accompany oxidative stress [34]. The levels of inflammatory cytokines can serve as indirect indicators of oxidative damage [35]. In our study, HS elevated the concentrations of serum TNF-α and IL-1β, while decreasing the levels of IL-4 and IL-10. It has been reported that butyric acid can directly suppress macrophage-mediated anti-inflammatory and modulate immune responses [36]. Our research found that dietary supplementation with tributyrin decreased the concentrations of serum IL-1β and TNF-α, while increasing the levels of IL-4, IL-10, and IgG in cyclic heat-stressed Taihe silky fowls. Zou et al. (2019) also reported that sodium butyrate lowered serum levels of TNF-α and IL-1β, while raising IL-10 in chickens [37]. Tang and Chen (2016) found that γ-aminobutyric acid increased plasma concentrations of IgA, IgG, and IgM in heat-stressed broilers [38]. Moreover, tributyrin down-regulated the mRNA abundance of IL-1β in the jejunal and ileal mucosa, while up-regulating the mRNA abundances of IL-4 and IL-10 in the same segments. Thus, dietary tributyrin supplementation could enhance immunity and alleviate inflammation in cyclic heat-stressed Taihe silky fowls.
Acting as a vital protective barrier, the intestinal mucosa not only aids in nutrient digestion and absorption but also serves as an important indicator for assessing the integrity of intestinal development and function [39]. Numerous studies have shown that HS induces lipid peroxidation damage, which impairs the intestinal mucosa, causing the VH to become shorter and the CD to become shallower [6,13]. As expected, HS results in a decrease in VH in the jejunum and a reduced VH/CD. Dietary supplementation with tributyrin has been shown to enhance intestinal architecture by promoting mucosal repair and improving intestinal morphology, which in turn increases nutrient absorption efficiency [27]. It is undoubtedly that tributyrin supplementation can improve intestinal morphology of chickens under normal conditions [40]. Furthermore, previous studies have reported that adding butyrate and its derivatives, such as γ-aminobutyric acid and tributyrin, to the diet can effectively reduce damage to the intestinal morphology of poultry following post-Eimeria challenges or HS [33,41,42]. Our data showed that adding tributyrin to the feed effectively improved intestinal morphology in Taihe silky fowls under CHS conditions.
The DAO is an intracellular enzyme mainly produced by epithelial cells located at the tips of intestinal villi [43]. D-lactate is primarily generated by gut bacteria through the fermentation of carbohydrates [44]. Elevated levels of serum DAO activity and D-lactate suggest increased intestinal permeability, which serves as a valuable indicator for assessing the integrity of the intestinal barrier [45]. In our study, we observed that HS led to increases in serum DAO and D-lactate levels, which aligns with previous findings [26,46].
The tight junctions (TJs) of the gut epithelium, which consist of the adaptor protein ZO-1 and the transmembrane proteins occludin and claudins, are indispensable for preserving mucosa barrier function [47]. Cheng et al. (2019) demonstrated that HS reduced the mRNA abundances of occludin in jejunal mucosa, as well as ZO-1 and occludin in the ileal mucosa of chickens [30]. Deng et al. (2023) also observed that HS decreased the mRNA abundance of occludin and ZO-1 the ileal mucosa of broilers [48]. In this study, we found that HS reduced the mRNA abundances of occludin and ZO-1 in both the jejunal and ileal mucosa, and it also suppressed claudin-1 mRNA in the ileum. These findings suggest that HS may disrupt intestinal barrier integrity, which could explain the concurrent increase in serum DAO activity and D-lactate concentration. Tributyrin has been shown to positively influence mucosal barrier function in weaned pigs [32]. Additionally, tributyrin can enhance the expression of TJs in broilers affected by Eimeria maxima induced by coccidiosis [49]. In our study, dietary tributyrin decreased serum D-lactate level and DAO activity. Moreover, tributyrin improved mucosa barrier integrity by up-regulating the mRNA abundance of occludin and ZO-1 in the jejunal and ileal mucosa, as well as claudin-1 in the ileal mucosa. These results indicated that tributyrin could effectively protect against HS-induced mucosal barrier dysfunction in broilers.
The SCFAs play an important role in maintaining normal pH values in the intestine, promoting the proliferation of beneficial microbiota, suppressing pathogen colonization, and thereby reinforcing epithelial barrier integrity [50]. In poultry, the concentration of SCFAs in the cecum increases consistently with higher dietary levels of tributyrin or sodium butyrate [40,51]. Furthermore, under CHS conditions, the levels of butyric acid in the cecum significantly rise, with high doses of tributyrin being the most effective in this study. This aligned with tributyrin’s role in improving intestinal morphology in the present study. These findings suggested that tributyrin might enhance intestinal development by increasing the content of butyric acid.
Gut microbiota plays a pivotal role in maintaining intestinal homeostasis [52]. Previous work showed that tributyrin elevated cecal Lactobacillus abundance in broilers kept under thermoneutral conditions [40]. The Lactobacillus secretes various antibacterial substances and mucins that reinforce the epithelial barrier, exclude pathogens, and promote the proliferation of the beneficial microbial community [53]. Our earlier study demonstrated that dietary tributyrin increased the relative abundance of cecal Rikenellaceae_RC9_gut_group and tended to raise (p = 0.096) Lactobacillus, while improving growth performance in cyclic heat-stressed Taihe silky fowls [23]. The Rikenellaceae_RC9_gut_group is a fiber-degrading, SCFAs-producing bacterial group within the Bacteroidota phylum that contributes to gut health [54]. Thus, tributyrin might boost the content of butyric acid by regulating the abundance of microbiota.
In this study, tributyrin supplementation decreased MDA concentrations and increased GSH-Px activity, accompanied by reduced TNF-α and IL-1β mRNA expression and diminished inflammatory cytokine release. However, the major limitations of the present study are that the key signaling pathways, such as nuclear factor erythroid 2-related factor 2 (Nrf2) and nuclear factor κB (NF-κB), were not validated. In the lipopolysaccharide-induced inflammation model, supplementation with butyrate reduced inflammatory markers, including NF-κB and IL-1β [55]. Moreover, butyrate also increased the mRNA expression of Nrf2 in the colon piglets [56]. Similarly, sodium butyrate bolstered antioxidant defenses and suppressed inflammatory markers (IL-6, IL-1β, TNF-α) by activating Nrf2 while inhibiting the NF-κB pathway, thereby protecting bovine mammary epithelial cells from LPS-induced injury [57]. As a butyrate precursor, tributyrin has likewise been shown to suppress inflammation and elevate antioxidant capacity [22,58]. Thus, whether tributyrate alleviates the HS-induced oxidative damage and inflammation by modulating the Nrf2 and NF-κB pathways still requires further in vivo and in vitro studies to validate. In addition, no significant differences in IgA, IgM, IL-6, and SCFAs (acetic acid, propanoic acid, butyric acid, isobutyric acid, valeric acid, and isovaleric acid) between the different concentrations of tributyrin in this study. This aspect could be explained based on a relatively insufficient number of observations, as well as the fact that heat stress lasted only 28 days. Therefore, enhancing the sample size of the experiment and extending the trial period. These are essential for future research.
5. Conclusions
In summary, the conclusion could be reached that, as shown in Figure 9, dietary tributyrin increased antioxidant capacity and mitigated inflammatory responses in heat-stressed Taihe silky fowls. Additionally, tributyrin effectively alleviated intestinal damage by increasing cecal butyrate content and improving both intestinal morphology and mucosal barrier function.
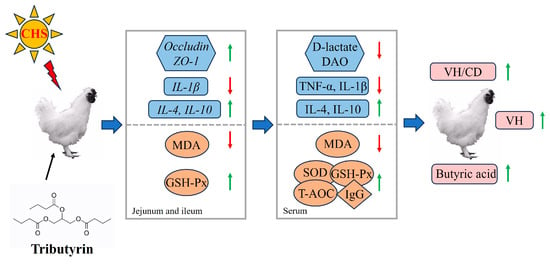
Figure 9.
Schematic model illustrating the mechanism of tributyrin alleviates cyclic heat stress-induced inflammation and oxidative damage of Taihe silky fowls. The red color arrow indicated a decrease in the parameters, while the green color arrow represented an increase in the parameters.
Author Contributions
C.C.: writing—original draft, methodology, data curation, formal analysis. M.Q.: writing—review and editing, supervision, conceptualization. G.L.: writing—review and editing, methodology, conceptualization. G.W.: software, visualization. H.L.: formal analysis. W.Z.: data curation. L.X.: writing—review and editing, validation, resources, project administration, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Grant Numbers: 32260852, 32060760) and Graduate Student Innovation Special Fund project of Jiangxi Province (Grant Numbers: YC2024-B120).
Institutional Review Board Statement
In this work, all birds-handling procedures were approved by the Animal Care and Use Committee of Jiangxi Agricultural University (application reference number: JXAULL-20210913, 13 September 2021).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors are thankful to staff of the Jiangxi Province Key Laboratory of Animal Nutrition and Feed for their support throughout the research.
Conflicts of Interest
The authors declare no potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| T-AOC | Total antioxidant capacity |
| GSH-Px | Glutathione peroxidase |
| SOD | Superoxide dismutase |
| DAO | Diamine oxidase |
| MDA | Malondialdehyde |
| IgA | Immunoglobulin A |
| IgG | Immunoglobulin G |
| IgM | Immunoglobulin M |
| TNF-α | Tumor necrosis factor α |
| IL-1β | Interleukin 1β |
| IL-6 | Interleukin 6 |
| IL-4 | Interleukin 4 |
| IL-10 | Interleukin 10 |
| ZO-1 | Zonula occludens-1 |
| VH | Villus height |
| CD | Crypt depth |
References
- Rostagno, M.H. Effects of heat stress on the gut health of poultry. J. Anim. Sci. 2020, 98, skaa090. [Google Scholar] [CrossRef]
- Oluwagbenga, E.M.; Fraley, G.S. Heat stress and poultry production: A comprehensive review. Poult. Sci. 2023, 102, 103141. [Google Scholar] [CrossRef]
- Brugaletta, G.; Teyssier, J.; Rochell, S.J.; Dridi, S.; Sirri, F. A review of heat stress in chickens. Part I: Insights into physiology and gut health. Front. Physiol. 2022, 13, 934381. [Google Scholar] [CrossRef] [PubMed]
- Barekatain, R.; Inhuber, V.; Sharma, N.; Nowland, T.; Van, T.T.H.; Moore, R.J.; Cadogan, D. Intestinal barrier function, caecal microbiota and growth performance of thermoneutral or heat stressed broiler chickens fed reduced crude protein diets supplemented with guanidinoacetic acid. Poult. Sci. 2025, 104, 104792. [Google Scholar] [CrossRef] [PubMed]
- Elokil, A.; Li, S.; Chen, W.; Farid, O.; Abouelezz, K.; Zohair, K.; Nassar, F.; El-Komy, E.; Farag, S.; Elattrouny, M. Ethoxyquin attenuates enteric oxidative stress and inflammation by promoting cytokine expressions and symbiotic microbiota in heat-stressed broilers. Poult. Sci. 2024, 103, 103761. [Google Scholar] [CrossRef]
- Peng, X.Y.; Xing, T.; Li, J.L.; Zhang, L.; Jiang, Y.; Gao, F. Guanidinoacetic acid supplementation improves intestinal morphology, mucosal barrier function of broilers subjected to chronic heat stress. J. Anim. Sci. 2023, 101, skac355. [Google Scholar] [CrossRef]
- He, X.; Lu, Z.; Ma, B.; Zhang, L.; Li, J.; Jiang, Y.; Zhou, G.; Gao, F. Chronic heat stress damages small intestinal epithelium cells associated with the adenosine 5′-monophosphate-activated protein kinase pathway in broilers. J. Agric. Food Chem. 2018, 66, 7301–7309. [Google Scholar] [CrossRef]
- Nanto-Hara, F.; Kikusato, M.; Ohwada, S.; Toyomizu, M. Heat stress directly affects intestinal integrity in broiler chickens. J. Poult. Sci. 2020, 57, 284–290. [Google Scholar] [CrossRef]
- Ghulam Mohyuddin, S.; Khan, I.; Zada, A.; Qamar, A.; Arbab, A.A.I.; Ma, X.; Yu, Z.; Liu, X.; Yong, Y.; Ju, X.H.; et al. Influence of heat stress on intestinal epithelial barrier function, tight junction protein, and immune and reproductive physiology. Biomed. Res. Int. 2022, 2022, 8547379. [Google Scholar] [CrossRef] [PubMed]
- Omonijo, F.A.; Liu, S.; Hui, Q.; Zhang, H.; Lahaye, L.; Bodin, J.; Gong, J.; Nyachoti, M.; Yang, C. Thymol improves barrier function and attenuates inflammatory responses in porcine intestinal epithelial cells during lipopolysaccharide (LPS)-induced inflammation. J. Agric. Food Chem. 2019, 67, 615–624. [Google Scholar] [CrossRef]
- He, S.; Yu, Q.; He, Y.; Hu, R.; Xia, S.; He, J. Dietary resveratrol supplementation inhibits heat stress-induced high-activated innate immunity and inflammatory response in spleen of yellow-feather broilers. Poult. Sci. 2019, 98, 6378–6387. [Google Scholar] [CrossRef]
- Shakeri, M.; Cottrell, J.J.; Wilkinson, S.; Zhao, W.; Le, H.H.; McQuade, R.; Furness, J.B.; Dunshea, F.R. Dietary betaine improves intestinal barrier function and ameliorates the impact of heat stress in multiple vital organs as measured by Evans blue dye in broiler chickens. Animals 2019, 10, 38. [Google Scholar] [CrossRef]
- Wu, Q.J.; Liu, N.; Wu, X.H.; Wang, G.Y.; Lin, L. Glutamine alleviates heat stress-induced impairment of intestinal morphology, intestinal inflammatory response, and barrier integrity in broilers. Poult. Sci. 2018, 97, 2675–2683. [Google Scholar] [CrossRef]
- Hu, H.; Dai, S.; Li, J.; Wen, A.; Bai, X. Glutamine improves heat stress-induced oxidative damage in the broiler thigh muscle by activating the nuclear factor erythroid 2-related 2/Kelch-like ECH-associated protein 1 signaling pathway. Poult. Sci. 2020, 99, 1454–1461. [Google Scholar] [CrossRef]
- Sikandar, A.; Zaneb, H.; Younus, M.; Masood, S.; Aslam, A.; Khattak, F.; Ashraf, S.; Yousaf, M.S.; Rehman, H. Effect of sodium butyrate on performance, immune status, microarchitecture of small intestinal mucosa and lymphoid organs in broiler chickens. Asian-Australas. J. Anim. Sci. 2017, 30, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A.O.; Eweedah, N.M.; Elbialy, Z.I.; Abdelhamid, A.I. Dietary sodium butyrate ameliorated the blood stress biomarkers, heat shock proteins, and immune response of Nile tilapia (Oreochromis niloticus) exposed to heat stress. J. Therm. Biol. 2020, 88, 102500. [Google Scholar] [CrossRef]
- Gu, T.; Duan, M.; Liu, J.; Chen, L.; Tian, Y.; Xu, W.; Zeng, T.; Lu, L. Effects of tributyrin supplementation on liver fat deposition, lipid levels and lipid metabolism-related gene expression in broiler chickens. Genes 2022, 13, 2219. [Google Scholar] [CrossRef]
- Murray, R.L.; Zhang, W.; Liu, J.; Cooper, J.; Mitchell, A.; Buman, M.; Song, J.; Stahl, C.H. Tributyrin, a butyrate pro-drug, primes satellite cells for differentiation by altering the epigenetic landscape. Cells 2021, 10, 3475. [Google Scholar] [CrossRef]
- Guo, W.; Liu, J.; Yang, Y.; Ma, H.; Gong, Q.; Kan, X.; Ran, X.; Cao, Y.; Wang, J.; Fu, S.; et al. Rumen-bypassed tributyrin alleviates heat stress by reducing the inflammatory responses of immune cells. Poult. Sci. 2021, 100, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cao, S.; Zhang, Q.; Shen, Z.; Feng, J.; Hong, Q.; Lu, J.; Xie, F.; Peng, Y.; Hu, C. Dietary tributyrin attenuates intestinal inflammation, enhances mitochondrial function, and induces mitophagy in piglets challenged with diquat. J. Agric. Food Chem. 2019, 67, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Bach Knudsen, K.E.; Lærke, H.N.; Hedemann, M.S.; Nielsen, T.S.; Ingerslev, A.K.; Gundelund Nielsen, D.S.; Theil, P.K.; Purup, S.; Hald, S.; Schioldan, A.G.; et al. Impact of diet-modulated butyrate production on intestinal barrier function and inflammation. Nutrients 2018, 10, 1499. [Google Scholar] [CrossRef]
- Li, J.; Hou, Y.; Yi, D.; Zhang, J.; Wang, L.; Qiu, H.; Ding, B.; Gong, J. Effects of tributyrin on intestinal energy status, antioxidative capacity and immune response to lipopolysaccharide challenge in broilers. Asian-Australas. J. Anim. Sci. 2015, 28, 1784–1793. [Google Scholar] [CrossRef]
- Chen, C.; Qu, M.; Li, G.; Wan, G.; Liu, P.; Omar, S.M.; Mei, W.; Hu, Z.; Zhou, Q.; Xu, L. Dietary tributyrin improves growth performance, meat quality, muscle oxidative status, and gut microbiota in Taihe silky fowls under cyclic heat stress. Animals 2024, 14, 3041. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Shi, X.; Hu, H.; Han, X.; Jiang, K.; Liu, Y.; Xiong, G. Comparative metabolomics analysis reveals the unique nutritional characteristics of breed and feed on muscles in Chinese Taihe black-bone silky fowl. Metabolites 2022, 12, 914. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.L.; He, J.H.; Xie, H.B.; Yang, Y.S.; Li, J.C.; Zou, Y. Resveratrol induces antioxidant and heat shock protein mRNA expression in response to heat stress in black-boned chickens. Poult. Sci. 2014, 93, 54–62. [Google Scholar] [CrossRef]
- Lan, R.; Li, Y.; Chang, Q.; Zhao, Z. Dietary chitosan oligosaccharides alleviate heat stress-induced intestinal oxidative stress and inflammatory response in yellow-feather broilers. Poult. Sci. 2020, 99, 6745–6752. [Google Scholar] [CrossRef]
- Wang, C.; Cao, S.; Shen, Z.; Hong, Q.; Feng, J.; Peng, Y.; Hu, C. Effects of dietary tributyrin on intestinal mucosa development, mitochondrial function and AMPK-mTOR pathway in weaned pigs. J. Anim. Sci. Biotechnol. 2019, 10, 93. [Google Scholar] [CrossRef] [PubMed]
- Lourenco, J.M.; Kieran, T.J.; Seidel, D.S.; Glenn, T.C.; Silveira, M.F.D.; Callaway, T.R.; Stewart, R.L. Comparison of the ruminal and fecal microbiotas in beef calves supplemented or not with concentrate. PLoS ONE 2020, 15, e0231533. [Google Scholar] [CrossRef]
- Farag, M.R.; Alagawany, M. Physiological alterations of poultry to the high environmental temperature. J. Therm. Biol. 2018, 76, 101–106. [Google Scholar] [CrossRef]
- Cheng, Y.F.; Chen, Y.P.; Chen, R.; Su, Y.; Zhang, R.Q.; He, Q.F.; Wang, K.; Wen, C.; Zhou, Y.M. Dietary mannan oligosaccharide ameliorates cyclic heat stress-induced damages on intestinal oxidative status and barrier integrity of broilers. Poult. Sci. 2019, 98, 4767–4776. [Google Scholar] [CrossRef]
- Chauhan, S.S.; Rashamol, V.P.; Bagath, M.; Sejian, V.; Dunshea, F.R. Impacts of heat stress on immune responses and oxidative stress in farm animals and nutritional strategies for amelioration. Int. J. Biometeorol. 2021, 65, 1231–1244. [Google Scholar] [CrossRef]
- Wang, C.; Shen, Z.; Cao, S.; Zhang, Q.; Peng, Y.; Hong, Q.; Feng, J.; Hu, C. Effects of tributyrin on growth performance, intestinal microflora and barrier function of weaned pigs. Anim. Feed Sci. Technol. 2019, 258, 114311. [Google Scholar] [CrossRef]
- Wang, J.; Fu, S.; Zou, X.; Luo, C.; Shu, D.; Qu, H. The impact of tributyrin on performance and intestinal health of broiler chickens post coccidiosis vaccination. Avian Dis. 2021, 65, 493–499. [Google Scholar] [CrossRef]
- Biswas, S.K. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid. Med. Cell. Longev. 2016, 2016, 5698931. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Ali, S.; Shi, X.; Cao, G.; Feng, J.; Yang, C.; Zhang, R. Protective impacts of bamboo leaf flavonoids in stressed broilers induced by diquat: Insight of antioxidant, immune response and intestinal barrier function. Anim. Nutr. 2024, 20, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; He, X.; Huang, J.; Yu, S.; Cui, M.; Gao, M.; Liu, L.; Qian, Y.; Xie, Y.; Hui, M.; et al. Short-chain fatty acid-butyric acid ameliorates granulosa cells inflammation through regulating METTL3-mediated N6-methyladenosine modification of FOSL2 in polycystic ovarian syndrome. Clin. Epigenetics 2023, 15, 86. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Ji, J.; Qu, H.; Wang, J.; Shu, D.M.; Wang, Y.; Liu, T.F.; Li, Y.; Luo, C.L. Effects of sodium butyrate on intestinal health and gut microbiota composition during intestinal inflammation progression in broilers. Poult. Sci. 2019, 98, 4449–4456. [Google Scholar] [CrossRef]
- Tang, J.; Chen, Z. The protective effect of γ-aminobutyric acid on the development of immune function in chickens under heat stress. J. Anim. Physiol. Anim. Nutr. 2016, 100, 768–777. [Google Scholar] [CrossRef]
- Farré, R.; Fiorani, M.; Abdu Rahiman, S.; Matteoli, G. Intestinal Permeability, Inflammation and the Role of Nutrients. Nutrients 2020, 12, 1185. [Google Scholar] [CrossRef]
- Hu, Q.; Yin, F.; Li, B.; Guo, Y.; Yin, Y. Dietary tributyrin administration improves intestinal morphology and selected bacterial and short-chain fatty acid profiles in broilers under an isocaloric feeding regime. Front. Microbiol. 2021, 12, 715712. [Google Scholar] [CrossRef]
- Abdelqader, A.M.; Abuajamieh, M.; Hammad, H.M.; Al Fataftah, A.R.A. Effects of dietary butyrate supplementation on intestinal integrity of heat-stressed cockerels. J. Anim. Physiol. Anim. Nutr. 2017, 101, 1115–1121. [Google Scholar] [CrossRef]
- Al Wakeel, R.A.; Shukry, M.; Abdel Azeez, A.; Mahmoud, S.; Saad, M.F. Alleviation by gamma amino butyric acid supplementation of chronic heat stress-induced degenerative changes in jejunum in commercial broiler chickens. Stress 2017, 20, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Fukudome, I.; Kobayashi, M.; Dabanaka, K.; Maeda, H.; Okamoto, K.; Okabayashi, T.; Baba, R.; Kumagai, N.; Oba, K.; Fujita, M.; et al. Diamine oxidase as a marker of intestinal mucosal injury and the effect of soluble dietary fiber on gastrointestinal tract toxicity after intravenous 5-fluorouracil treatment in rats. Med. Mol. Morphol. 2014, 47, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Uehara, A.; Maekawa, M.; Nakagawa, K. Enhanced intestinal barrier function as the mechanism of antibiotic growth promoters in feed additives. Biosci. Biotechnol. Biochem. 2023, 87, 1381–1392. [Google Scholar] [CrossRef]
- Duan, Y.; Huang, J.; Sun, M.; Jiang, Y.; Wang, S.; Wang, L.; Yu, N.; Peng, D.; Wang, Y.; Chen, W.; et al. Poria cocos polysaccharide improves intestinal barrier function and maintains intestinal homeostasis in mice. Int. J. Biol. Macromol. 2023, 249, 125953. [Google Scholar] [CrossRef]
- Alhotan, R.A.; Al Sulaiman, A.R.; Alharthi, A.S.; Abudabos, A.M. Protective influence of betaine on intestinal health by regulating inflammation and improving barrier function in broilers under heat stress. Poult. Sci. 2021, 100, 101337. [Google Scholar] [CrossRef] [PubMed]
- Awad, W.A.; Hess, C.; Hess, M. Enteric pathogens and their toxin-induced disruption of the intestinal barrier through alteration of tight junctions in chickens. Toxins 2017, 9, 60. [Google Scholar] [CrossRef]
- Deng, C.; Zheng, J.; Zhou, H.; You, J.; Li, G. Dietary glycine supplementation prevents heat stress-induced impairment of antioxidant status and intestinal barrier function in broilers. Poult. Sci. 2023, 102, 102408. [Google Scholar] [CrossRef]
- Hansen, V.L.; Kahl, S.; Proszkowiec-Weglarz, M.; Jiménez, S.C.; Vaessen, S.F.C.; Schreier, L.L.; Jenkins, M.C.; Russell, B.; Miska, K.B. The effects of tributyrin supplementation on weight gain and intestinal gene expression in broiler chickens during Eimeria maxima-induced coccidiosis. Poult. Sci. 2021, 100, 100984. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Wu, W.; Xiao, Z.; An, W.; Dong, Y.; Zhang, B. Dietary sodium butyrate improves intestinal development and function by modulating the microbial community in broilers. PLoS ONE 2018, 13, e0197762. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, H.; Zhang, H.; Zhao, Q.; Si, W.; Qin, Y.; Zhang, J. Dietary Epimedium extract supplementation improves intestinal functions and alters gut microbiota in broilers. J. Anim. Sci. Biotechnol. 2023, 14, 14. [Google Scholar] [CrossRef]
- Saeed, M.; Al-Khalaifah, H.; Al-Nasser, A.; Al-Surrayai, T. Feeding the future: A new potential nutritional impact of Lactiplantibacillus plantarum and its promising interventions in future for poultry industry. Poult. Sci. 2025, 104, 105130. [Google Scholar] [CrossRef]
- Sebastià, C.; Folch, J.M.; Ballester, M.; Estellé, J.; Passols, M.; Muñoz, M.; García-Casco, J.M.; Fernández, A.I.; Castelló, A.; Sánchez, A.; et al. Interrelation between gut microbiota, SCFA, and fatty acid composition in pigs. mSystems 2024, 9, e01049-23. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Tang, C.; Zhao, Q.; Fan, S.; Yang, P.; Zhang, J. Butyrate mitigates lipopolysaccharide-induced intestinal morphological changes in weanling piglets by regulating the microbiota and energy metabolism, and alleviating inflammation and apoptosis. Microorganisms 2022, 10, 2001. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lan, T.; Ding, Q.; Ren, Z.; Tang, Z.; Tang, Q.; Peng, X.; Xu, Y.; Sun, Z. Effect of low protein diets supplemented with sodium butyrate, medium-chain fatty acids, or n-3 polyunsaturated fatty acids on the growth performance, immune function, and microbiome of weaned piglets. Int. J. Mol. Sci. 2023, 24, 17592. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Li, C.; Kuang, M.; Shah, A.U.; Shafiq, M.; Ahmad, M.A.; Abdalmegeed, D.; Li, L.; Wang, G. Nrf2 Activation and NF-Kb & caspase/bax signaling inhibition by sodium butyrate alleviates LPS-induced cell injury in bovine mammary epithelial cells. Mol. Immunol. 2022, 148, 54–67. [Google Scholar] [CrossRef]
- Liu, S.; Wu, J.; Wu, Z.; Alugongo, G.M.; Zahoor Khan, M.; Li, J.; Xiao, J.; He, Z.; Ma, Y.; Li, S.; et al. Tributyrin administration improves intestinal development and health in pre-weaned dairy calves fed milk replacer. Anim. Nutr. 2022, 10, 399–411. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).