Abstract
Oxidative stress is a key contributor to the onset and progression of diverse pathological conditions, including metabolic dysfunction-associated steatotic liver disease (MASLD), neurodegeneration, cardiovascular disorders, and cancer. Conventional antioxidant therapies, such as small-molecule scavengers or systemic enzyme administration, are limited by poor stability, inefficient delivery, and off-target effects. Extracellular vesicles (EVs), particularly exosomes, are increasingly recognized as natural carriers of antioxidant enzymes (AOEs), including catalase, superoxide dismutases, glutathione peroxidases, peroxiredoxins, and thioredoxin. These vesicles not only protect enzymes from degradation but also enable targeted delivery to recipient cells, where they can actively modulate redox homeostasis. In this review, we summarize current evidence for AOEs as bona fide EV cargo, outline mechanisms that govern their selective packaging and transfer, and highlight their roles in intercellular communication under physiological and pathological conditions. We also discuss emerging therapeutic applications of both natural and engineered EVs for redox modulation, along with the challenges of quantifying enzymatic activity, ensuring reproducibility, and scaling clinical translation. By integrating insights from cell biology, redox signaling, and translational research, we propose that EV-mediated AOE delivery represents a promising next-generation strategy for combating oxidative stress-related diseases.
1. Introduction
Reactive oxygen species (ROS) are continuously generated as byproducts of normal cellular metabolism and signaling [1]. Under physiological conditions, redox homeostasis is tightly regulated by antioxidant defense systems composed of enzymatic and non-enzymatic components [1,2]. However, excessive ROS production or impaired antioxidant defense capacity leads to oxidative stress, a critical factor implicated in the onset and progression of various diseases, including metabolic dysfunction-associated steatotic liver disease (MASLD), cardiovascular disorders, neurodegenerative diseases, and cancer [3,4,5,6]. In these contexts, oxidative stress not only induces direct macromolecular damage but also acts as a signaling hub that exacerbates inflammation, apoptosis, and cellular senescence, thereby perpetuating tissue dysfunction [3,7,8].
Over the past decades, antioxidant therapies have been extensively explored to mitigate oxidative damage [4,9,10]. Classical strategies, such as small-molecule scavengers (e.g., N-acetylcysteine, vitamin E, vitamin A) or systemic administration of antioxidant enzymes (AOEs) like catalase and superoxide dismutase (SOD), have demonstrated limited clinical efficacy due to poor bioavailability, short half-life, and inadequate tissue targeting [11,12]. Additionally, nonspecific systemic delivery may disrupt physiological redox signaling, highlighting the need for more selective and efficient approaches to restore redox balance in diseased tissues [10,12].
Extracellular vesicles (EVs) are lipid bilayer-enclosed nanoparticles secreted by virtually all cell types and have emerged as promising natural carriers for therapeutic cargo [13,14,15]. Their intrinsic stability, low immunogenicity, and cell type-specific tropism give EVs clear advantages over synthetic nanocarriers for delivering bioactive molecules [15]. Recent studies have revealed that EVs can encapsulate a range of AOEs, including catalase, SODs, glutathione peroxidases (GPXs), peroxiredoxins (PRDXs), and thioredoxin (TXN), either passively reflecting cellular content or through selective sorting mechanisms. Once delivered to recipient cells, these enzymes can modulate local redox homeostasis, influence signaling cascades, and even alter disease trajectories [1,10,16,17,18,19].
This review aims to provide a comprehensive overview of current evidence for AOEs as bona fide EV cargo, emphasizing the mechanisms underlying their selective packaging, secretion, and functional transfer. We further discuss how EV-associated AOEs contribute to intercellular redox communication under physiological and pathological conditions. Finally, we explore translational strategies using natural and engineered EVs for therapeutic redox modulation, while addressing challenges in enzyme quantification, reproducibility, and clinical scalability. Through this integrative perspective, we aim to elucidate how EV-mediated AOEs delivery may offer a next-generation paradigm for combating oxidative stress-related diseases.
2. Antioxidant Enzymes (AOEs) in EVs
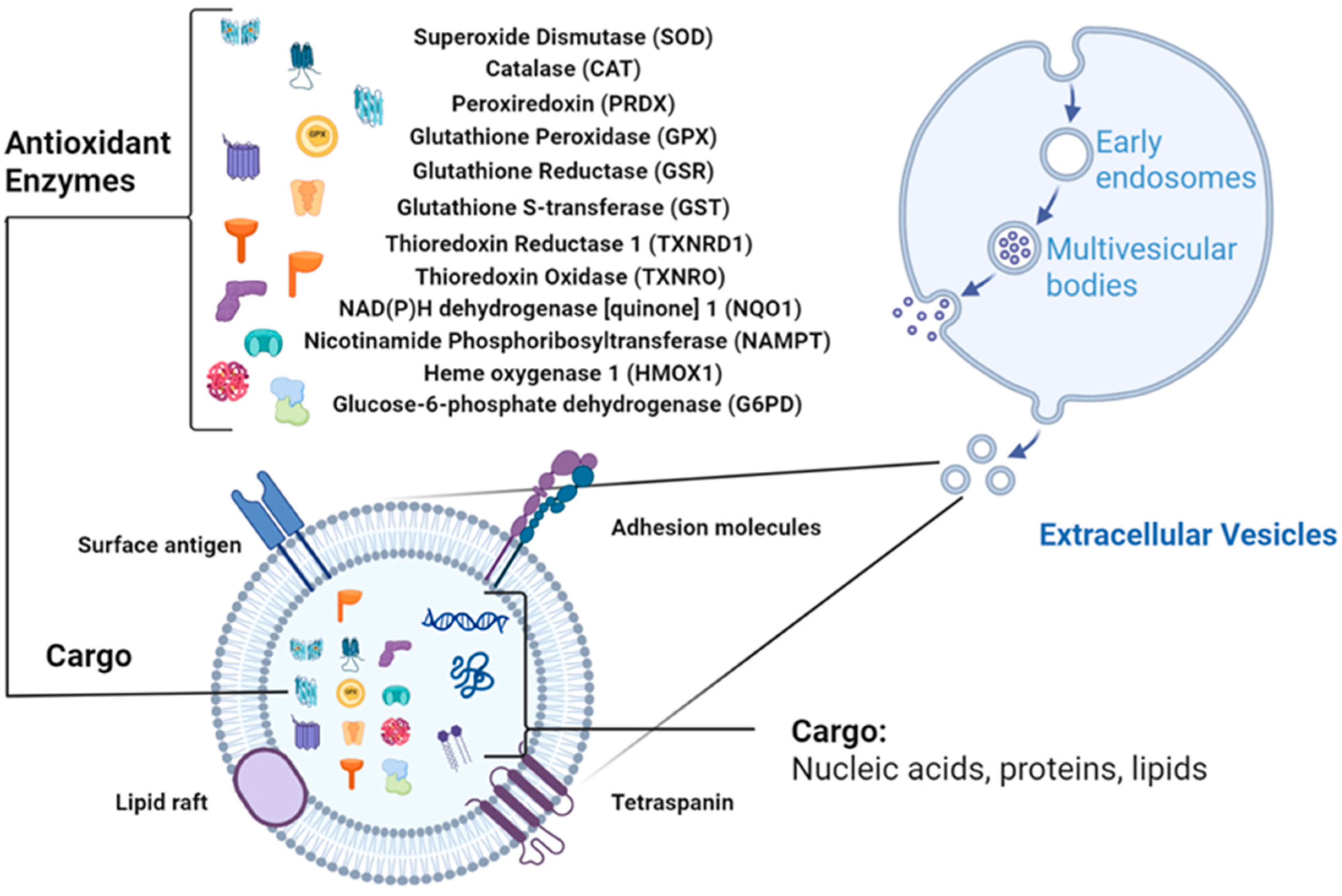
EVs are increasingly recognized as carriers of redox-regulatory enzymes that actively shape intercellular oxidative balance. Proteomic and biochemical evidence across multiple species show that AOEs are present in EVs and retain catalytic activity upon transfer to recipient cells (Figure 1). The following sections summarize major antioxidant enzyme families identified in EVs, drawing upon curated data from PubMed, ProteomeXchange, Vesiclepedia, and ExoCarta, and highlight representative findings summarized in Table 1.
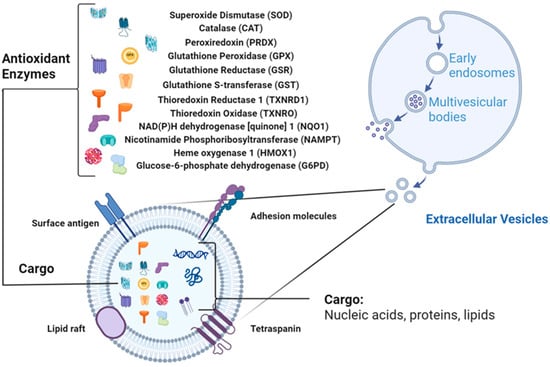
Figure 1.
EV structure with antioxidant enzymes (AOEs) as cargo (Created in BioRender. Wang, J. (2026) https://BioRender.com/t0f0rdi, accessed on 10 October 2025).

Table 1.
Antioxidant enzymes (AOEs) are reported in EVs.
2.1. Superoxide Dismutases (SOD1/2/3)
Superoxide dismutases (SODs) catalyze the conversion of superoxide (O2−•) into hydrogen peroxide (H2O2) and oxygen, forming the first enzymatic defense line against ROS. All three major isoforms, cytosolic SOD1, mitochondrial SOD2, and extracellular SOD3, have been consistently detected in EVs from stem cells, adipose tissue, skeletal muscle, macrophages, and plasma [17,19,20,21,22,23,24,25,27,28,29,30,33,34,35,36]. Multiple studies confirm that EV-associated SODs retain catalytic activity, enabling functional delivery to recipient cells [16,17,25,27,28,30,33,35,36]. Exercise markedly increases SOD enrichment in circulating EVs, particularly those originating from contracting skeletal muscle, thereby enhancing systemic oxidative stress resistance and contributing to cardioprotection and neuroprotection [24,35]. Genetic overexpression of SOD3 in MSCs further augments the antioxidative and immunomodulatory effects of their secreted EVs, demonstrating that vesicular SOD3 can be functionally potentiated for therapeutic use [30]. EVs from several plant sources also contain active SODs, expanding the diversity of biological systems in which SOD loading occurs [26,37,38,39,40].
2.2. Catalase
Catalase decomposes H2O2 into water and molecular oxygen, preventing toxic H2O2 accumulation [51]. It is a frequent and functionally validated component of EVs from plasma, cardiac tissue, adipose-derived vesicles, and macrophages [17,20,22,23,24,27,29,34,36,41,52]. Catalase-loaded EVs protect hepatocytes and cardiomyocytes from oxidative injury and appear dynamically regulated by physiological states, for example, exercise increases both catalase abundance and activity in circulating EVs [41]. Catalase is also present in bacterial and plant-derived EVs, where it can modulate host or environmental redox states, for instance, KatB-containing bacterial EVs suppress ROS in plant tissues to promote pathogen survival [26,38,39,42].
2.3. Peroxiredoxins (PRDXs)
PRDXs (1–6) reduce H2O2 and organic hydroperoxides via the thioredoxin system. Multiple PRDX isoforms have been identified in plasma- and stem cell-derived EVs, and mitochondrial-derived vesicles [17,19,23,24,25,34,43,44,45,46]. PRDX abundance within EVs varies with external stimuli, indicating redox-responsive packaging, although the enzymatic activity of EV-associated PRDXs is less frequently verified experimentally [19,45].
2.4. Glutathione Peroxidases (GPXs)
GPXs 1/3/4 reduce H2O2 and lipid hydroperoxides using glutathione (GSH) as a cofactor. GPX proteins and activity have been detected in EVs from stem cells, cardiac tissue, adipose tissue, and plasma [20,22,24,36,47,48]. GPX-positive EVs protect recipient cells against oxidative and lipid peroxidation-associated damage [36]. Interestingly, GPX loading into adipose-derived EVs appears selectively regulated, as high-fat diet increased catalase but not GPX activity [36].
2.5. Glutathione System Enzymes and Glutathione S-Transferase (GST)
Key glutathione cycle enzymes, including glutathione reductase (GSR) and glutathione S-transferase (GST), are present in plasma- and cardiac-derived EVs [21,22,24,41]. The co-presence of these enzymes suggests that EVs may operate as self-contained glutathione-regenerating units, sustaining redox cycling and enhancing antioxidant capacity in recipient cells.
2.6. Thioredoxin (TXN) and Related Enzymes
Thioredoxin (TXN) and thioredoxin reductase (TXNRD1), central mediators of thiol redox balance, have been consistently identified in plasma- and mitochondrial-derived EVs [16,17,24,25,27,41]. Exercise enhances TXN abundance in circulating EVs, indicating that vesicular thiol-reducing capacity contributes to systemic adaptation to oxidative challenge [16,24,27,41]. These findings support a functional role for EV-associated TXN in intercellular redox regulation under both homeostatic and stress conditions.
2.7. Additional Redox-Active Enzymes
Several other redox-modulating enzymes have been identified in EVs, including NAD(P)H dehydrogenase [quinone] 1 (NQO1), nicotinamide phosphoribosyltransferase (NAMPT), heme oxygenase 1 (HMOX1), and glucose-6-phosphate dehydrogenase (G6PD) [17,21,24,35,41,49,50]. These enzymes are functionally linked to NAD(P)H regeneration, quinone detoxification, and heme catabolism, thereby connecting vesicular redox buffering to broader metabolic and cytoprotective pathways.
2.8. Functional Evidence of Enzymatic Activity
Beyond proteomic and Western blotting identification, several studies have provided functional evidence that EV-associated AOEs retain catalytic activity after secretion. Catalase- and GPX-containing EVs from adipose tissue, as well as SOD-enriched vesicles released from human neutrophils, exhibit measurable enzymatic activity in standard redox assays [33,36]. SOD-loaded EVs from human umbilical cord MSCs (hUC-MSCs) effectively alleviate hepatic ischemia–reperfusion injury in rats by reducing oxidative stress and attenuating neutrophil-mediated inflammation [28]. Plasma-derived EVs released during exercise or inflammatory stimulation show sustained GSR, catalase, and NAMPT activity, contributing to tissue protection and systemic redox buffering [41,49]. Comparable catalytic activities have also been documented in plant-derived EVs, particularly for SOD and catalase [37,38,39,40]. Collectively, these findings confirm that EVs function as active vectors of antioxidant enzymatic activity, shaping redox signaling and enhancing cytoprotection in recipient cells.
3. Mechanisms of Antioxidant Enzymes (AOEs) Packaging and Transfer
The selective incorporation and intercellular transfer of AOEs via EVs are key determinants of their functional impact. Although the underlying mechanisms are not fully understood, evidence suggests that both canonical vesicle biogenesis pathways and redox-dependent regulatory processes influence the loading, release, and uptake of enzymatically active antioxidant cargo (Figure 2). While AOE-containing EVs have also been reported in plants and bacteria, this review focuses on the mechanisms of AOE packaging and transfer in human and animal physiological and pathological contexts.
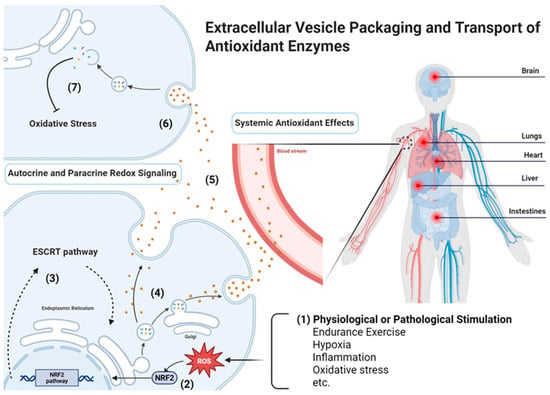
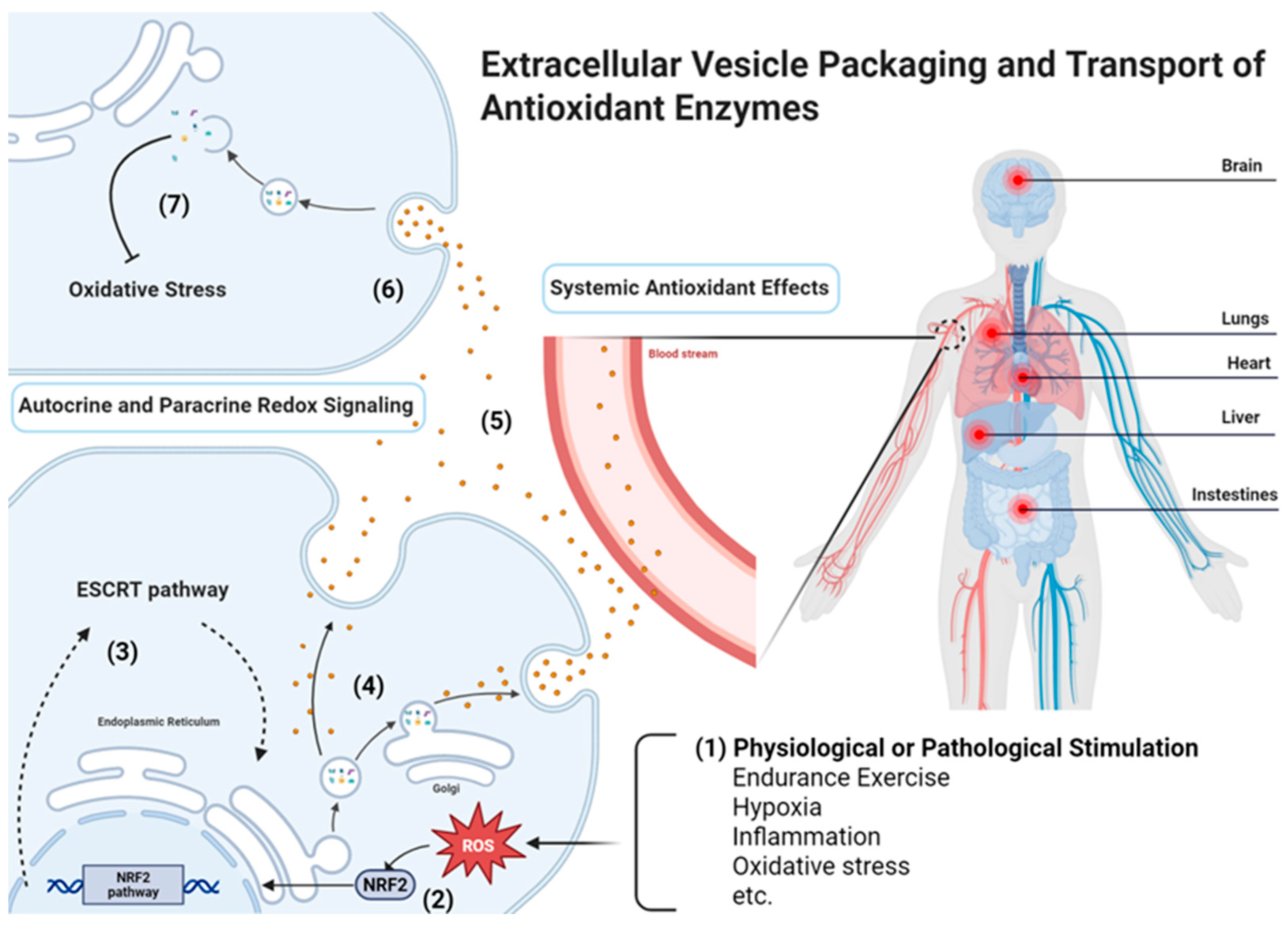
Figure 2.
Mechanisms of antioxidant enzymes (AOEs)-loading EV packaging and delivery (Created in BioRender. Wang, J. (2026) https://BioRender.com/t0f0rdi, accessed on 10 October 2025). EV-mediated antioxidant defense involves several coordinated steps: (1) Cargo incorporation occurs either passively under basal conditions or is enhanced by stress-induced enrichment; (2) Redox-related signaling pathways, such as the Nrf2 pathway, regulate the expression and sorting of AOEs; (3) Endosomal sorting can proceed through the endosomal sorting complex required for transport (ESCRT)-dependent or ESCRT-independent mechanisms, involving tetraspanins and ceramide-mediated pathways; (4) AOEs (e.g., catalase, SOD, GPX) are selectively packaged into EVs; (5) EVs are released and transported locally via autocrine and paracrine redox signaling or systemically through circulating EVs that mediate organism-wide redox adaptation; (6) Recipient cells internalize EVs through receptor-mediated uptake, endocytosis, or membrane fusion; (7) The delivered AOEs restore redox homeostasis and exert protective effects within recipient cells.
3.1. Cellular Origins of Antioxidant Enzymes (AOEs) in EVs
The origin of AOE-containing EVs varies depending on the physiological context and the cell types involved. Diverse AOEs have been identified in EVs secreted by multiple cell types, including stem cells, immune cells, and cardiomyocytes (Table 1), supporting the notion that EV-mediated redox modulation is a conserved intercellular mechanism. To date, however, no study has conclusively demonstrated that specific AOEs are selectively packaged into EVs by defined cell types or under strictly controlled conditions. Instead, most available evidence highlights dynamic changes in AOE abundance within EVs in response to physiological or pathological stimuli (Table 1). Under homeostatic conditions, metabolically active tissues such as skeletal muscle and heart continuously release low levels of AOE-loaded EVs [22,35], and EV-derived AOEs further increase following exercise training [41]. In contrast, during oxidative or inflammatory stress, immune cells, cardiomyocytes, and adipocytes become major contributors to the circulating EV pool [9,19,25,41,49]. These stress-responsive cells upregulate vesicle biogenesis and selectively enrich antioxidant cargo, thereby coupling intracellular redox dynamics to systemic oxidative balance.
3.2. Passive Incorporation Versus Stress-Induced Enrichment
AOEs loading into EVs can occur passively, reflecting the intracellular redox state, or actively in response to physiological stress. Exercise, hypoxia, and inflammation cues commonly increase EV secretion and modify their redox cargo. Conversely, severe oxidative or metabolic stress may reduce AOE content per vesicle, suggesting altered loading efficiency. Notably, EV-associated AOEs can increase following exercise training. Sagini et al. reported that elevated TXN in plasma EVs from endurance-trained females following acute exercise [16]. Exercise also enhances NAMPT release in circulating EVs, influencing NAD+ metabolism in recipient cells [49], and stimulates Nrf2-dependent antioxidant gene expression that becomes enriched in circulating EVs [35]. Although multiple studies have documented stress-related changes in AOE content in EVs from skeletal muscle, adipose tissue, and heart, the precise cellular origins of these EVs remain incompletely defined. Immune cells also actively secrete AOE-rich EVs. Iversen et al. showed that SOD3 is stored in secretory vesicles of neutrophils and released upon stimulation [33]. Likewise, macrophage-derived EVs deliver PRDX6 to tumor cells, promoting ferroptosis resistance [46]. Stem cell-derived EVs contain multiple AOEs even without external stimulation [19,20,28,30,44], suggesting a constant baseline source of circulating antioxidant activity. Collectively, current evidence supports that stress-induced AOE enrichment in EVs represents an adaptive mechanism to enhance antioxidant defense during periods of elevated oxidative load.
3.3. Sorting Pathways Influencing Antioxidant Enzymes (AOEs) Loading
Immune and stem cells can markedly increase AOE loading or the number of AOE-rich EVs under oxidative stress, reflecting both passive and active sorting mechanisms. AOE incorporation into EVs can be passive or stress-driven. Oxidative stress-responsive pathways, particularly Nrf2, influence EV cargo composition. Gao et al. proposed that Nrf2 activation upregulates cytoprotective proteins, including AOEs, which are subsequently released via EVs and transferred to distant tissues [35]. Although direct mechanistic studies remain limited, other stress-related pathways such as HIF-1, MAPK, and NF-κB may also contribute [2,35]. Mechanisms governing selective AOE packaging and the fate of EV-delivered AOEs remain unclear and represent a major knowledge gap. EV biogenesis involves multiple routes. The ESCRT-dependent pathway recruits specific cytosolic proteins, including potentially redox-active enzymes, into intraluminal vesicles via components such as TSG101, ALIX, and VPS4 [53,54,55,56]. ESCRT-independent mechanisms, including ceramide-driven budding and tetraspanin-enriched microdomains (CD9, CD63, CD81), may also support AOE incorporation [57,58]. The cellular redox state may further modify sorting efficiency, as oxidative stress can alter membrane lipid composition and induce post-translational modifications (e.g., S-glutathionylation, nitrosylation) that influence AOE localization and vesicular packaging [59,60].
3.4. Uptake Mechanisms in Recipient Cells
Upon release, EVs interact with recipient cells through multiple entry routes, including clathrin- or caveolin-mediated endocytosis, macropinocytosis, phagocytosis, receptor-mediated internalization, or direct membrane fusion [61,62]. Despite significant progress in elucidating the molecular mechanisms underlying EV uptake, the question of how specificity in EV–cell interactions are achieved remains largely unresolved. The route of uptake is influenced by factors such as vesicle size, lipid composition, and the presence of specific surface ligands [61,62,63]. For instance, tetraspanins (e.g., CD9, CD63, CD81) and integrins on EV membranes can mediate docking and internalization by hepatocytes, cardiomyocytes, or endothelial cells [15,61,63]. In particular, no studies to date have clearly identified the specific target cell types for AOE-enriched EVs or elucidated the precise uptake mechanisms by which these vesicles are internalized by recipient cells.
3.5. Functional Evidence of Enzyme Transfer
Growing functional evidence shows that EV-mediated transfer of AOEs can directly modulate redox status in recipient cells. Catalase-rich EVs from adipose tissue reduce intracellular ROS levels in hepatocytes and improve cell survival during oxidative stress, demonstrating that vesicle-delivered catalase remains enzymatically active after uptake [36]. Similarly, AOE-enriched EVs reduced oxidative damage in human iPSC-derived cardiomyocytes at baseline and after H2O2 exposure, producing a pronounced cardioprotective effect [41]. SOD-loaded EVs from stem cells exert pronounced antioxidant and anti-inflammatory effects by catalyzing superoxide dismutation and limiting oxidative tissue injury [30]. EV-associated TXN further protects cardiomyocytes from H2O2-induced oxidative stress and helps preserve thiol–redox balance during exercise- or inflammation-associated conditions [16]. These findings collectively indicate that EVs act as functional vectors of antioxidant capacity, enabling intercellular redox coordination and metabolic resilience.
3.6. Outstanding Questions and Knowledge Gaps
Despite these advances, several unresolved questions limit mechanistic insight. It remains unclear whether AOEs are selectively packaged into EVs via defined sorting signals or are incorporated passively based on cellular abundance. The contribution of post-translational modifications, protein–lipid interactions, or redox-sensitive motifs to sorting specificity also requires clarification. In recipient cells, the stability, trafficking routes, and catalytic lifespan of EV-derived enzymes, particularly within endosomal or lysosomal compartments, are still poorly characterized. Addressing these gaps will be essential to harness EV-mediated antioxidant delivery for therapeutic applications, enabling controlled modulation of redox signaling and improved translational reproducibility.
4. Functional Roles of EV-Derived Antioxidant Enzymes (AOEs)
EVs carrying AOEs exert multifaceted effects that extend beyond intracellular redox regulation, influencing both local microenvironments and systemic physiological processes. Through the transfer of catalytically active enzymes, EVs can buffer oxidative stress, modulate cell signaling, and shape disease outcomes across various biological contexts (Figure 2).
4.1. Autocrine and Paracrine Redox Signaling
EV-derived AOEs modulate redox homeostasis in neighboring or originating cells by regulating extracellular and intracellular ROS levels. Stem cell- and tumor-derived EVs can reduce oxidative burden in target cells [21,44,48]. Liu et al. showed that stem cell-derived EVs alleviate senescence by reducing oxidative stress, restoring mitochondrial function, and enhancing proliferation [44]. Similarly, hypoxic glioblastoma cells release GPx1-enriched EVs that buffer H2O2 in surrounding tumor cells, illustrating EV-mediated autocrine and paracrine protection [48]. In the cardiac microenvironment, EVs from cardiac stromal cells or MSCs deliver catalase and GPX to protect cardiomyocytes from oxidative injury [28,64]. Neutrophil-derived EVs containing SOD modulate cytokine responses and help limit inflammation [31,33]. In the central nervous system, glia-derived EVs reduce neuronal ROS and protect against oxidative and mitochondrial dysfunction [65,66]. In the liver, EV-mediated redox signaling may influence hepatocyte stress responses, hepatic stellate cell (HSC) activation, fibrosis progression, and MASLD pathogenesis [13,67,68,69,70,71,72,73]. AOE-enriched EVs can protect hepatocytes from H2O2- or lipotoxicity-induced apoptosis [36], and reduce ROS accumulation in liver endothelial cells [31]. Given that oxidative stress drives both steatosis and fibrogenesis, EV-based AOE transfer may function as an intrinsic mechanism limiting HSC activation and fibrotic remodeling. Clarifying these EV-mediated redox interactions may reveal new therapeutic opportunities for restoring redox balance in chronic liver disease.
4.2. Systemic Antioxidant Effects
Circulating EVs also serve as vehicles for systemic redox adaptation. Exercise-induced EVs enriched in SOD, catalase, NAMPT, and TXN contribute to whole-body oxidative stress resistance by transferring antioxidant capacity to distant tissues such as the myocardium and central nervous system [16,35,41]. Abdelsaid et al. demonstrated that exercise improves the angiogenic function of circulating exosomes in type 2 diabetes by enriching them with extracellular SOD3; these SOD3-loaded exosomes attenuate oxidative stress, restore endothelial nitric oxide signaling, and promote vascular repair [32]. Bao et al. similarly reported that neutrophils alleviate sepsis-associated coagulopathy by releasing EVs enriched with mitochondrial SOD2, which reduce oxidative stress and endothelial injury, thereby preserving vascular integrity in a murine model of lipopolysaccharide (LPS)-induced sepsis [31]. Other circulating EV populations carrying AOEs have likewise been shown to confer antioxidant protection in recipient cells, contributing to systemic defense against oxidative stress [16,35,45,49]. Stem cell-derived EVs containing multiple AOEs effectively reduce ROS accumulation and improve metabolic recovery in models of ischemia, inflammation, and aging [28,30,44]. These findings highlight that EV-mediated enzyme transfer constitutes a physiological mechanism for inter-organ redox communication and systemic cytoprotection.
5. Therapeutic Applications
5.1. Natural EVs
5.1.1. Stem Cell-Derived EVs
Stem cell-derived EVs have shown remarkable potential in protecting cells from oxidative stress, primarily due to the presence of AOEs. For instance, EVs from hUC-MSCs alleviated hepatic ischemia-reperfusion injury by reducing oxidative stress and inflammatory responses, with key AOEs like SOD playing a vital role [28]. Similarly, stem cell-derived EVs have been shown to improve aging cellular phenotypes, largely due to the abundance of AOEs they carry [44]. These findings highlight the therapeutic potential of stem cell-derived EVs, with AOEs being a critical component in mitigating oxidative stress.
In addition to their inherent antioxidant properties, the production of AOEs within stem cell-derived EVs can be further enhanced through various methods. For example, MSCs preconditioned with LPS, which increase the release of small EVs that effectively treat periodontitis through ROS-mediated antioxidant effects [21]. Additionally, overexpressing AOEs in MSCs can enhance the immunomodulatory abilities of their derived EVs, improving their therapeutic efficacy in models of inflammation and oxidative stress [30]. Moreover, hypoxic preconditioning of hUC-MSCs increases the expression of exosomal TXN-1, which has been shown to inhibit ferroptosis in doxorubicin-induced cardiotoxicity through mTORC1 signaling [74]. Quercetin, a natural antioxidant, enhances the antioxidant capacity of stem cells and their EVs by upregulating SOD1, thereby boosting their anti-inflammatory and antioxidant properties [19]. Developing strategies to enhance the antioxidant capacity of stem cell-derived EVs will improve their effectiveness in treating oxidative stress-related diseases.
5.1.2. Immune Cell-Derived EVs
In addition to stem cells, immune cells represent another natural source of EVs rich in AOEs. Proteomic analyses have identified TXN II within dendritic cell-derived exosomes, suggesting its functional contribution to oxidative balance [75]. Extracellular SOD is stored in secretory vesicles of neutrophils and released into the extracellular space upon cellular activation [33]. In a mouse model of sepsis, circulating neutrophils secrete mitochondria-containing EVs highly enriched with SOD2 [31]. These vesicles effectively reduce endothelial ROS accumulation and alleviate disseminated intravascular coagulation [31]. Although direct evidence is limited, macrophage-derived exosomes are often engineered to enhance antioxidant properties, highlighting their potential as ROS-scavenging carriers for inflammatory disease treatment, tissue repair, and oxidative stress mitigation. Overall, the abundance of AOEs in immune cell-derived EVs likely reflects the activation and oxidative state of parent cells, offering insights for disease monitoring.
5.1.3. Plant-Derived EVs
In recent years, plant-derived EVs have attracted growing attention due to their excellent biocompatibility and distinctive bioactivity, particularly in antioxidant and anti-inflammatory applications [76]. Numerous studies have identified high levels of key antioxidant enzyme families in plant-derived EVs isolated from sources including Perilla frutescens, various vegetables and fruits, Aloe vera peels, and microalgae [26,37,38,39,40,76,77]. These findings strongly support that AOEs are integral to the protein cargo of plant-derived EVs, providing a molecular foundation for their antioxidant functionality. These EVs are also widely used as delivery systems due to their low cost and abundant availability [78]. Nevertheless, evidence regarding the effective delivery of these enzymes to recipient cells remains limited, and their specific contribution to the overall antioxidant capacity of plant-derived EVs is still unclear.
5.1.4. Milk-Derived EVs
Milk is a complex biological fluid containing various endogenous antioxidant enzymes, such as SOD, catalase, and GPX [79]. These enzymes are essential for neutralizing free radicals, protecting milk lipids from oxidative damage, and maintaining the nutritional and sensory quality of dairy products. Recent studies indicate that milk-derived EVs (MEVs) remain structurally stable under simulated gastrointestinal conditions [80,81] and exhibit antioxidant properties, including the ability to neutralize ROS and reduce oxidative stress [82,83]. However, research on the antioxidant function of MEVs is still limited. There is a lack of proteomic validation of the vesicle cargo and uncertainty about the localization of antioxidant enzymes, whether inside the vesicles or on their surface [84]. Future research should focus on providing direct and quantitative data to confirm the role of MEVs as natural carriers of antioxidant enzymes.
5.2. Engineered EVs
The use of engineered EVs containing AOEs has emerged as a promising strategy in modern therapeutics [85]. These natural EVs can be modified and enriched with AOEs using multiple engineering strategies. Common post-isolation loading techniques include sonication, electroporation, and mechanical extrusion. In parallel, genetic modification of donor cells represents an equally important strategy: by overexpressing specific AOEs in the parental cells, the intracellular abundance of these enzymes is increased, resulting in their efficient and physiologically compatible incorporation into secreted EVs [30]. This overexpression-based method avoids harsh physical manipulation and enables more controlled enrichment of therapeutic enzymes within EVs. Engineered EVs exhibit potent antioxidant effects, reducing oxidative stress, lipid peroxidation, and neuroinflammation, while also restoring mitochondrial function. They have demonstrated therapeutic potential in various disease models, especially for cardiovascular disease, cancer, and neurodegenerative disorders [86,87,88]. The key benefit of engineering EVs lies in their ability to enhance the natural properties of EVs, such as improved targeting capabilities and biocompatibility, and the ability to carry a variety of therapeutic payloads while minimizing potential off-target effects, which is a significant advantage over traditional drug delivery systems [89]. Table 2 summarizes the representative engineered AOE-loading EVs, including those generated by AOE overexpression in donor cells and those produced through exogenous enzyme encapsulation strategies. Despite their promise, important questions remain regarding the long-term safety, biodistribution, and potential immunogenicity of engineered EVs. As research advances, engineered EVs containing AOEs show great promise as a next-generation therapeutic platform.

Table 2.
Representative examples of engineered antioxidant enzyme (AOE)-loaded EVs.
5.3. Nanozymes
Unlike natural AOEs encapsulated in EVs, nanozymes are inorganic or nanostructured catalysts that functionally imitate AOE activity but are not biological proteins. As shown previously, natural AOEs rely on defined amino-acid sequences and metal/cofactor-based active centers, and their activity is highly specific, regulated by cellular context, and subject to denaturation or proteolytic degradation. In contrast, nanozymes generate enzyme-like effects through surface redox reactions, catalytic metal centers, or defect-rich lattices. Their activity is often broader and less substrate-selective, but it is more resistant to harsh chemical or thermal conditions [95,96]. These nanozymes offer promising alternatives to traditional enzyme therapies, as summarized in several reviews [97,98,99,100]. The most common antioxidant nanozymes are metal oxide nanoparticles (CeO2, MnO2, Fe3O4), which can mimic SOD, catalase, and even PRDX activities [101]. Single-atom catalysts, through precise design of their metal centers, can mimic several natural AOEs simultaneously, making them a current research hotspot [102]. Carbon-based nanozymes and metal–organic framework (MOF)-based nanozymes, with adjustable surface functional groups and pore structures, can perform multiple antioxidant functions and are applicable in biosensing, drug delivery, and disease treatment [103,104,105]. Overall, nanozymes complement natural AOEs by providing high stability, scalable synthesis, and tunable catalytic performance, while EV-delivered natural enzymes offer higher biological specificity and compatibility. Combining these two strategies may therefore yield synergistic antioxidant therapies.
5.4. Diagnostic Value of Antioxidant Enzymes (AOEs) in EVs
EVs are increasingly recognized as promising diagnostic tools because they function as a “liquid biopsy” that reflects physiological and pathological status. The content of EVs, including AOEs, can provide valuable insights about disease mechanisms. In amyotrophic lateral sclerosis (ALS), for example, SOD1 found in circulating EVs has been proposed as a potential biomarker, offering diagnostic and prognostic value for ALS progression [106]. Similarly, in echinococcosis, a parasitic infection caused by Echinococcus species, high levels of TXN in EVs serve as an early diagnostic indicator, detectable as early as 10 days post-infection [107]. Moreover, mitochondria-derived EVs also carry enzyme cargo that may be implicated in diseases associated with mitochondrial dysfunction [25]. These EVs, particularly under oxidative stress, contain key AOEs and redox-active proteins, which could offer diagnostic value for early detection and monitoring of disease progression in mitochondrial disorders or diseases related to oxidative stress, like cardiovascular diseases or neurodegenerative diseases. Together, these findings highlight the potential of EV cargo profiling as a non-invasive approach for early detection and disease monitoring, although further validation is still required.
6. Challenges and Limitations
EV-based delivery of AOEs holds significant promise for treating diseases driven by oxidative stress. However, substantial scientific and translational challenges remain, particularly due to the limited understanding of AOE-specific EV sorting mechanisms. These challenges extend across several key dimensions, including large-scale production, enzyme loading efficiency, stability, targeting, characterization, and regulatory issues.
6.1. Large-Scale Production and Quality Standards
One major barrier in EV-mediated enzyme delivery is the low yield and high cost associated with current EV isolation methods. Traditional techniques such as ultracentrifugation and density gradient centrifugation generate low yields and require labor-intensive workflows, which cannot meet the demand for clinical applications [108]. Another significant challenge regarding the EV isolation methods lies in the contamination with non-EV-derived proteins, including enzymes. These contaminants may result in false-positive detection of antioxidant enzyme activity, thus complicating the interpretation of experimental outcomes. Furthermore, batch-to-batch heterogeneity poses a significant issue. The EVs isolated from the same cell line show variability in size, membrane proteins, and lipid compositions, which affects the loading efficiency of enzymes and their therapeutic efficacy [109]. Variability also arises from biological factors such as cell state, nutrient composition, and culture stress, all of which reshape EV content. The absence of standardized processes for Good Manufacturing Practice (GMP) production further complicates the clinical translation of EV-based therapies. Currently, no unified purification or sterilization standard is recognized by regulatory agencies [110,111], leaving EV-based therapeutics without a clear manufacturing framework.
Moreover, regarding the detection of EV-derived AOEs, many studies evaluating the enzymatic activity of enzymes delivered by EVs employ insufficiently rigorous methodologies. Due to their low abundance and susceptibility to inactivation, simple enzyme activity assays can be unreliable, thereby obscuring the true biological relevance of the findings. To address these challenges, future research should prioritize the development of standardized protocols for EV isolation to ensure sample purity and consistency. Additionally, more accurate and sensitive assays for evaluating enzymatic activity are essential to obtain reliable and reproducible results.
6.2. Enzyme Loading Efficiency and Activity Preservation
Efficient enzyme loading of AOEs into EVs remains difficult. Common techniques such as sonication, extrusion, and electroporation achieve only modest loading efficiency for large enzymes like catalase and SOD, which are insufficient to meet therapeutic dosages [112]. These high-energy techniques can lead to structural damage to the enzymes, decreasing their biological activity. The reproducibility of loading methods is another issue, as identical procedures often yield different results when performed across different labs or batches of EVs [113]. Reported efficiencies vary widely across studies because “loading” is often quantified by total protein rather than by enzyme activity, which frequently overestimates the true functional payload. The stability of the enzyme-EV complex is also a concern, as enzymes might detach from the EVs rapidly in vivo due to non-specific adsorption, potentially leading to the premature clearance of free enzymes by the immune system [113]. Recent studies have shown that MOFs offer a promising solution to enhance the stability and loading efficiency of enzymes into EVs. MOFs, with their tunable porosity and high surface area, can provide a more stable and controlled environment for enzyme encapsulation, improving both loading efficiency and the in vivo retention of the enzyme-EV complex [112,114]. However, their long-term biodegradability, metal-ion residue, and biosafety remain insufficiently evaluated.
6.3. Stability and Storage Challenges
EVs are sensitive to freezing, thawing, and mechanical stress, resulting in membrane rupture, aggregation, or cargo loss. EVs, especially when loaded with enzymes, face stability challenges during storage and transportation. Cryopreservation at temperatures as low as −80 °C can cause membrane rupture and aggregation, compromising the protective role of EVs for the enzyme cargo [115]. The storage medium also plays a crucial role, as different buffers and media (e.g., PBS, glycosylation buffers) can significantly affect both the integrity of EVs and the activity of the enzymes encapsulated within them [115]. Additionally, the need for cold-chain logistics to maintain the stability of EVs adds substantial costs, limiting large-scale commercialization and clinical use. The field currently lacks systematic comparisons of storage conditions or mechanistic studies explaining how storage-induced biophysical changes affect in vivo efficacy. Until such evidence emerges, reliable long-term storage of EV-enzyme formulations remains an unmet challenge.
6.4. Targeted Delivery and Tissue Penetration
Targeting specific tissues with EVs remains a significant challenge in enzyme delivery. After systemic administration, the majority of EVs are quickly captured by macrophages in the liver and spleen, leading to low accumulation in target tissues like the brain, heart, or eyes [116]. Although surface engineering of EVs with peptides like LAMP2B has been shown to improve brain delivery, efficiency remains suboptimal [117]. Furthermore, the lack of efficient targeting ligands, such as folate receptor or heart-specific peptides, makes tissue-specific targeting costly and inefficient [118]. Additionally, the mechanisms by which EVs enter target cells, whether via fusion, endocytosis, or membrane interaction, are not fully understood, hindering the precise control of delivery efficiency [111]. Another risk involves off-target oxidation or reduction, which can occur if the enzyme is unevenly loaded or if its release is not properly controlled, resulting in oxidative damage in non-target tissues [119]. More mechanistic work is required to define safe and effective targeting strategies.
6.5. Analytical Characterization and Quality Control
Characterization of EVs and their enzymatic cargo suffers from a lack of standardized measurement criteria. Various metrics, including size, concentration, protein/lipid composition, and enzyme activity, are measured using different methods, making it difficult to compare results across labs [109,115]. Furthermore, the absence of reference materials for EVs complicates the calibration of instruments and the validation of enzyme loading efficiencies [110]. High-resolution techniques such as flow cytometry, cryo-electron microscopy, and mass spectrometry are often too costly for routine quality control, limiting widespread adoption in EV-based therapeutic development [115]. The absence of reference materials further hinders instrument calibration and assay validation.
6.6. Regulatory and Clinical Translation
Regulatory uncertainty remains a major obstacle [111]. Globally, there is no unified classification, approval pathway, or quality standard for EV-based medicines. This regulatory uncertainty complicates clinical trial design and delays market approval. Although some early-phase clinical trials have been conducted, the safety data are limited, and long-term risks, such as immune responses and potential carcinogenesis, have not been systematically evaluated [120]. Additionally, cost-effectiveness is difficult to evaluate, as EV-AOE therapies have not yet demonstrated clear superiority over existing antioxidants or recombinant enzymes. Future clinical development will require well-defined endpoints, standardized dosing strategies, and disease indications where EV delivery provides a measurable advantage.
7. Future Directions
Future research on AOE-loaded EVs should aim to deepen mechanistic understanding, optimize therapeutic design, and accelerate clinical translation. To address current mechanistic gaps, future studies should explore how selective AOE packaging occurs at the molecular level, including the roles of ESCRT-dependent pathways, lipid raft-driven sorting, post-translational modifications, and organelle contact sites in cargo recruitment and vesicle formation. Targeted investigations of the intracellular fate of EV-delivered AOEs, including endosomal escape, lysosomal degradation, and cytosolic release, are critically needed. Furthermore, elucidating downstream signaling pathways modulated by EV-borne AOEs, such as effects on redox-sensitive transcription factors (e.g., Nrf2), mitochondrial homeostasis, inflammatory signaling (NF-κB), and cell survival pathways, represents a major unmet need. First, high-resolution proteomic and lipidomic profiling is needed to map the diversity and abundance of antioxidant cargo within EVs derived from different cellular and physiological sources. Such mapping will clarify how oxidative or inflammatory stress influences AOE packaging and release. Integrating these analyses with functional assays and redox-sensitive biosensors could allow real-time tracking of EV function, intracellular antioxidant activity, and downstream signaling in recipient cells, facilitating the establishment of quantitative redox pharmacokinetics. Second, rational engineering approaches, including genetic modification, preconditioning, and biohybrid nanostructure integration, should be employed to enhance both the antioxidant payload and tissue specificity of EVs. Combining MOF-based nanocarriers or synthetic membranes with natural EVs may also improve enzyme stability, loading efficiency, and controlled release. Third, the integration of redox-sensitive biosensors into experimental models could allow real-time tracking of EV function and intracellular antioxidant activity in vivo, facilitating the establishment of quantitative redox pharmacokinetics. Fourth, expanding disease-specific profiling of EVs, particularly in metabolic, inflammatory, and degenerative diseases such as MASLD, cardiovascular disease, and neurodegeneration, could reveal diagnostic and prognostic EV signatures. Finally, advancing clinical translation will require the development of standardized GMP-grade production pipelines, validated analytical methods, and regulatory frameworks. These steps could ultimately enable the use of EVs as a novel form of enzyme replacement therapy for oxidative stress-related disorders.
8. Conclusions
EVs represent a natural, biocompatible, and multifunctional platform for the delivery of AOEs. Evidence from stem cell-, immune cell-, and plant-derived EVs highlights their intrinsic ability to modulate redox homeostasis under both physiological and pathological conditions. Engineered EVs further extend these advantages by enabling precise control over cargo composition, targeting, and therapeutic efficacy. Despite current limitations in production scalability, loading efficiency, and clinical standardization, ongoing progress in EV engineering, characterization, and regulatory harmonization holds strong promise. With continued refinement, EV-mediated AOEs delivery could emerge as a next-generation redox-based therapeutic strategy, bridging the gap between natural defense mechanisms and advanced nanomedicine.
Author Contributions
Conceptualization, J.W. and Y.L.; software, J.W.; writing—original draft preparation, J.W. and Y.L.; writing—review and editing, J.W., Y.L., R.P.F.D. and P.O. and H.M.; visualization, J.W.; supervision, R.P.F.D. and P.O. and H.M.; project administration, P.O. and H.M.; funding acquisition, J.W., Y.L., R.P.F.D., P.O. and H.M. All authors have read and agreed to the published version of the manuscript.
Funding
This project is financially supported by the China Scholarship Council (File No. 202006250036 (J.W.) File No. 202206210134 (Y.L.)) and the De Cock-Hadders Foundation (File No. 2022-43 (J.W.) & No. 2024-18 (J.W.) & No. 2025-47 (J.W.) & No. 2025-28 (Y.L.)).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
During the preparation of this manuscript, the author(s) used ChatGPT-5 (San Francisco, USA) for the purposes of improving the language and clarity of the text. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AOE | Antioxidant enzyme |
| AML12 | Alpha mouse liver 12 cells |
| ALS | Amyotrophic lateral sclerosis |
| DFSCs | Dental follicle stem cells |
| ESCRT | Endosomal sorting complex required for transport |
| EV | Extracellular vesicle |
| ExT | Exercise training |
| EEx | Endurance exercise |
| G6PD | Glucose-6-phosphate dehydrogenase |
| GMP | Good Manufacturing Practice |
| GPX | Glutathione peroxidase |
| GSH | Glutathione |
| GSR | Glutathione reductase |
| GST | Glutathione S-transferase |
| GSSG | Oxidized glutathione |
| H2O2 | Hydrogen peroxide |
| HMOX1 | Heme oxygenase 1 |
| hUC-MSC | Human umbilical cord–derived mesenchymal stem cell |
| hMNCs | Human mononu-clear cells |
| HDF | Human Dermal Fibroblast |
| HIIT | High-intensity interval training |
| LPS | Lipopolysaccharide |
| MASLD | Metabolic dysfunction–associated steatotic liver disease |
| MEV | Milk-derived extracellular vesicle |
| Mn-SOD | Manganese superoxide dismutase |
| MOF | Metal–organic framework |
| MSC | Mesenchymal stem cell |
| mTORC1 | Mechanistic target of rapamycin complex 1 |
| NADPH | Nicotinamide adenine dinucleotide phosphate (reduced form) |
| NAMPT | Nicotinamide phosphoribosyltransferase |
| NQO1 | NAD(P)H dehydrogenase [quinone] 1 |
| O2−• | Superoxide anion |
| OR | Oxidative stress |
| PRDX | Peroxiredoxin |
| PDLSCs | Periodontal ligament stem cells |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| SOD1 | Cytosolic Cu/Zn-superoxide dismutase |
| SOD2 | Mitochondrial Mn-superoxide dismutase |
| SOD3 | Extracellular superoxide dismutase |
| TRXNRD1 | Thioredoxin reductase 1 |
| TXN | Thioredoxin |
| TXNRO | Thioredoxin oxidase |
References
- Lennicke, C.; Cocheme, H.M. Redox metabolism: ROS as specific molecular regulators of cell signaling and function. Mol. Cell 2021, 81, 3691–3707. [Google Scholar] [CrossRef]
- Li, B.; Ming, H.; Qin, S.; Nice, E.C.; Dong, J.; Du, Z.; Huang, C. Redox regulation: Mechanisms, biology and therapeutic targets in diseases. Signal Transduct. Target. Ther. 2025, 10, 72. [Google Scholar] [CrossRef]
- Arroyave-Ospina, J.C.; Wu, Z.; Geng, Y.; Moshage, H. Role of Oxidative Stress in the Pathogenesis of Non-Alcoholic Fatty Liver Disease: Implications for Prevention and Therapy. Antioxidants 2021, 10, 174. [Google Scholar] [CrossRef]
- Chandimali, N.; Bak, S.G.; Park, E.H.; Lim, H.J.; Won, Y.S.; Kim, E.K.; Park, S.I.; Lee, S.J. Free radicals and their impact on health and antioxidant defenses: A review. Cell Death Discov. 2025, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.M.D. Oxidative Stress and Antioxidants in Neurodegenerative Disorders. Antioxidants 2023, 12, 517. [Google Scholar] [CrossRef]
- Zhou, N.J.; Bao, W.Q.; Zhang, C.F.; Jiang, M.L.; Liang, T.L.; Ma, G.Y.; Liu, L.; Pan, H.D.; Li, R.Z. Immunometabolism and oxidative stress: Roles and therapeutic strategies in cancer and aging. NPJ Aging 2025, 11, 59. [Google Scholar] [CrossRef]
- Stojanovic, B.; Jovanovic, I.; Dimitrijevic Stojanovic, M.; Stojanovic, B.S.; Kovacevic, V.; Radosavljevic, I.; Jovanovic, D.; Miletic Kovacevic, M.; Zornic, N.; Arsic, A.A.; et al. Oxidative Stress-Driven Cellular Senescence: Mechanistic Crosstalk and Therapeutic Horizons. Antioxidants 2025, 14, 987. [Google Scholar] [CrossRef]
- Liu, S.; Liu, J.; Wang, Y.; Deng, F.; Deng, Z. Oxidative Stress: Signaling Pathways, Biological Functions, and Disease. MedComm (2020) 2025, 6, e70268. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, I. Antioxidants: A comprehensive review. Arch. Toxicol. 2025, 99, 1893–1997. [Google Scholar] [CrossRef]
- Uti, D.E.; Atangwho, I.J.; Alum, E.U.; Ntaobeten, E.; Obeten, U.N.; Bawa, I.; Agada, S.A.; Ukam, C.I.; Egbung, G.E. Antioxidants in cancer therapy mitigating lipid peroxidation without compromising treatment through nanotechnology. Discov. Nano 2025, 20, 70. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Xia, M.; Salas, S.S.; Trillos-Almanza, M.C.; Aguilar, M.M.; Arroyave-Ospina, J.C.; Wang, J.; Arrese, M.; Sydor, S.; Bechmann, L.P.; et al. Extracellular vesicles in metabolic dysfunction associated fatty liver disease: Mechanisms, diagnostic and therapeutic implications. Explor. Dig. Dis. 2022, 4–20. [Google Scholar] [CrossRef]
- Wang, J.; Lei, J.; Harmsen, M.C.; Moshage, H. From cooperation to collapse: Systemic failure in liver disease through a sociological lens. Explor. Dig. Dis. 2025, 4, 100580. [Google Scholar] [CrossRef]
- Kumar, M.A.; Baba, S.K.; Sadida, H.Q.; Marzooqi, S.A.; Jerobin, J.; Altemani, F.H.; Algehainy, N.; Alanazi, M.A.; Abou-Samra, A.B.; Kumar, R.; et al. Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduct. Target. Ther. 2024, 9, 27. [Google Scholar] [CrossRef]
- Sagini, K.; Selvarajah, M.; Romero, S.; Bassols-Citores, E.; Martin-Gracia, B.; Ramirez-Garrastacho, M.; Rise, F.; Endzelins, E.; Sadovska, L.; Skorinkina, D.; et al. The Redox Enzyme Thioredoxin Is Increased in Plasma Extracellular Vesicles From Endurance-Trained Females in Response to Acute Exercise. FASEB J. 2025, 39, e70927. [Google Scholar] [CrossRef]
- Lisi, V.; Senesi, G.; Balbi, C. Converging protective pathways: Exploring the linkage between physical exercise, extracellular vesicles and oxidative stress. Free Radic. Biol. Med. 2023, 208, 718–727. [Google Scholar] [CrossRef]
- Bodega, G.; Alique, M.; Puebla, L.; Carracedo, J.; Ramirez, R.M. Microvesicles: ROS scavengers and ROS producers. J. Extracell. Vesicles 2019, 8, 1626654. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Liu, X.; Chang, L.; Liu, B.; Zhang, M.; Mao, Y.; Shen, X. Exosomes Derived from Rejuvenated Stem Cells Inactivate NLRP3 Inflammasome and Pyroptosis of Nucleus Pulposus Cells via the Transfer of Antioxidants. Tissue Eng. Regen. Med. 2024, 21, 1061–1077. [Google Scholar] [CrossRef]
- Mas-Bargues, C.; Sanz-Ros, J.; Romero-Garcia, N.; Huete-Acevedo, J.; Dromant, M.; Borras, C. Small extracellular vesicles from senescent stem cells trigger adaptive mechanisms in young stem cells by increasing antioxidant enzyme expression. Redox Biol. 2023, 62, 102668. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, Q.; Liu, L.; Huo, F.; Guo, S.; Tian, W. Lipopolysaccharide-Preconditioned Dental Follicle Stem Cells Derived Small Extracellular Vesicles Treating Periodontitis via Reactive Oxygen Species/Mitogen-Activated Protein Kinase Signaling-Mediated Antioxidant Effect. Int. J. Nanomed. 2022, 17, 799–819. [Google Scholar] [CrossRef] [PubMed]
- Claridge, B.; Rai, A.; Fang, H.; Matsumoto, A.; Luo, J.; McMullen, J.R.; Greening, D.W. Proteome characterisation of extracellular vesicles isolated from heart. Proteomics 2021, 21, e2100026. [Google Scholar] [CrossRef]
- Apostolopoulou, M.; Mastrototaro, L.; Hartwig, S.; Pesta, D.; Strassburger, K.; de Filippo, E.; Jelenik, T.; Karusheva, Y.; Gancheva, S.; Markgraf, D.; et al. Metabolic responsiveness to training depends on insulin sensitivity and protein content of exosomes in insulin-resistant males. Sci. Adv. 2021, 7, eabi9551. [Google Scholar] [CrossRef] [PubMed]
- McIlvenna, L.C.; Whitham, M. Exercise, healthy ageing, and the potential role of small extracellular vesicles. J. Physiol. 2023, 601, 4937–4951. [Google Scholar] [CrossRef]
- Vasam, G.; Nadeau, R.; Cadete, V.J.J.; Lavallee-Adam, M.; Menzies, K.J.; Burelle, Y. Proteomics characterization of mitochondrial-derived vesicles under oxidative stress. FASEB J. 2021, 35, e21278. [Google Scholar] [CrossRef]
- Garaeva, L.; Tolstyko, E.; Putevich, E.; Kil, Y.; Spitsyna, A.; Emelianova, S.; Solianik, A.; Yastremsky, E.; Garmay, Y.; Komarova, E.; et al. Microalgae-Derived Vesicles: Natural Nanocarriers of Exogenous and Endogenous Proteins. Plants 2025, 14, 2354. [Google Scholar] [CrossRef]
- Lisi, V.; Moulton, C.; Fantini, C.; Grazioli, E.; Guidotti, F.; Sgro, P.; Dimauro, I.; Capranica, L.; Parisi, A.; Di Luigi, L.; et al. Steady-state redox status in circulating extracellular vesicles: A proof-of-principle study on the role of fitness level and short-term aerobic training in healthy young males. Free Radic. Biol. Med. 2023, 204, 266–275. [Google Scholar] [CrossRef]
- Yao, J.; Zheng, J.; Cai, J.; Zeng, K.; Zhou, C.; Zhang, J.; Li, S.; Li, H.; Chen, L.; He, L.; et al. Extracellular vesicles derived from human umbilical cord mesenchymal stem cells alleviate rat hepatic ischemia-reperfusion injury by suppressing oxidative stress and neutrophil inflammatory response. FASEB J. 2019, 33, 1695–1710. [Google Scholar] [CrossRef]
- Haque, S.; Kodidela, S.; Sinha, N.; Kumar, P.; Cory, T.J.; Kumar, S. Differential packaging of inflammatory cytokines/ chemokines and oxidative stress modulators in U937 and U1 macrophages-derived extracellular vesicles upon exposure to tobacco constituents. PLoS One 2020, 15, e0233054. [Google Scholar] [CrossRef]
- Yang, J.W.; Seo, Y.; Shin, T.H.; Ahn, J.S.; Oh, S.J.; Shin, Y.Y.; Kang, M.J.; Lee, B.C.; Lee, S.; Kang, K.S.; et al. Extracellular Vesicles from SOD3-Transduced Stem Cells Exhibit Improved Immunomodulatory Abilities in the Murine Dermatitis Model. Antioxidants 2020, 9, 1165. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Xing, H.; Cao, S.; Long, X.; Liu, H.; Ma, J.; Guo, F.; Deng, Z.; Liu, X. Neutrophils restrain sepsis associated coagulopathy via extracellular vesicles carrying superoxide dismutase 2 in a murine model of lipopolysaccharide induced sepsis. Nat. Commun. 2022, 13, 4583. [Google Scholar] [CrossRef] [PubMed]
- Abdelsaid, K.; Sudhahar, V.; Harris, R.A.; Das, A.; Youn, S.W.; Liu, Y.; McMenamin, M.; Hou, Y.; Fulton, D.; Hamrick, M.W.; et al. Exercise improves angiogenic function of circulating exosomes in type 2 diabetes: Role of exosomal SOD3. FASEB J. 2022, 36, e22177. [Google Scholar] [CrossRef]
- Iversen, M.B.; Gottfredsen, R.H.; Larsen, U.G.; Enghild, J.J.; Praetorius, J.; Borregaard, N.; Petersen, S.V. Extracellular superoxide dismutase is present in secretory vesicles of human neutrophils and released upon stimulation. Free Radic. Biol. Med. 2016, 97, 478–488. [Google Scholar] [CrossRef]
- Haque, S.; Sinha, N.; Ranjit, S.; Midde, N.M.; Kashanchi, F.; Kumar, S. Monocyte-derived exosomes upon exposure to cigarette smoke condensate alter their characteristics and show protective effect against cytotoxicity and HIV-1 replication. Sci. Rep. 2017, 7, 16120. [Google Scholar] [CrossRef]
- Gao, L.; Wang, H.J.; Tian, C.; Zucker, I.H. Skeletal Muscle Nrf2 Contributes to Exercise-Evoked Systemic Antioxidant Defense Via Extracellular Vesicular Communication. Exerc. Sport. Sci. Rev. 2021, 49, 213–222. [Google Scholar] [CrossRef]
- Jeong, I.; Lee, J.; Park, S.J.; Kim, O.K. High fat diet enhances catalase loading into adipose tissue derived extracellular vesicles with limited effect on oxidative stress. Sci. Rep. 2025, 15, 31010. [Google Scholar] [CrossRef]
- Kim, M.K.; Choi, Y.C.; Cho, S.H.; Choi, J.S.; Cho, Y.W. The Antioxidant Effect of Small Extracellular Vesicles Derived from Aloe vera Peels for Wound Healing. Tissue Eng. Regen. Med. 2021, 18, 561–571. [Google Scholar] [CrossRef]
- Logozzi, M.; Di Raimo, R.; Mizzoni, D.; Fais, S. Nanovesicles from Organic Agriculture-Derived Fruits and Vegetables: Characterization and Functional Antioxidant Content. Int. J. Mol. Sci. 2021, 22, 8170. [Google Scholar] [CrossRef]
- Di Raimo, R.; Mizzoni, D.; Aloi, A.; Pietrangelo, G.; Dolo, V.; Poppa, G.; Fais, S.; Logozzi, M. Antioxidant Effect of a Plant-Derived Extracellular Vesicles’ Mix on Human Skin Fibroblasts: Induction of a Reparative Process. Antioxidants 2024, 13, 1373. [Google Scholar] [CrossRef] [PubMed]
- Azizi, F.; Shiri, E.; Azadian, Z.; Piryaei, F.; Jalali, A.; Nouri, F.; Dalirfardouei, R. Preparation of Allium cepa-derived exosome-like nanovesicles and their anti-inflammatory potential in a skin wound healing mouse model. Mol. Biol. Rep. 2025, 52, 769. [Google Scholar] [CrossRef] [PubMed]
- Lisi, V.; Senesi, G.; Bertola, N.; Pecoraro, M.; Bolis, S.; Gualerzi, A.; Picciolini, S.; Raimondi, A.; Fantini, C.; Moretti, E.; et al. Plasma-derived extracellular vesicles released after endurance exercise exert cardioprotective activity through the activation of antioxidant pathways. Redox Biol. 2023, 63, 102737. [Google Scholar] [CrossRef]
- Deng, J.; Li, W.; Wang, Z.; Zeng, J.; Cai, Q. KatB, a bacterial extracellular vesicles (EVs)-secreted catalase, detoxifies reactive oxygen species (ROS) and promotes pathogen proliferation in plants. J. Integr. Plant Biol. 2025, 67, 1928–1946. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Eguchi, A.; Tamai, Y.; Fukuda, S.; Tempaku, M.; Izuoka, K.; Iwasa, M.; Takei, Y.; Togashi, K. Protein Composition of Circulating Extracellular Vesicles Immediately Changed by Particular Short Time of High-Intensity Interval Training Exercise. Front. Physiol. 2021, 12, 693007. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Mahairaki, V.; Bai, H.; Ding, Z.; Li, J.; Witwer, K.W.; Cheng, L. Highly Purified Human Extracellular Vesicles Produced by Stem Cells Alleviate Aging Cellular Phenotypes of Senescent Human Cells. Stem Cells 2019, 37, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Warnier, G.; van Doorslaer de Ten Ryen, S.; Lannoy, C.; Mahy, T.; Antoine, N.; Boyer, E.; Kienlen-Campard, P.; Verboven, K.; Copine, S.; Francaux, M.; et al. Effect of a 12-Week Endurance Training Program on Circulating Extracellular Vesicle Proteome in Sedentary Adults With Obesity. J. Extracell. Biol. 2025, 4, e70087. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Li, F.; Huang, Q.; Huang, X.; Maj, T. Macrophages and macrophage extracellular vesicles confer cancer ferroptosis resistance via PRDX6-mediated mitophagy inhibition. Redox Biol. 2025, 86, 103826. [Google Scholar] [CrossRef]
- Huang, J.; Li, S.; Yang, Y.; Li, C.; Zuo, Z.; Zheng, R.; Chai, J.; Jiang, S. GPX5-Enriched Exosomes Improve Sperm Quality and Fertilization Ability. Int. J. Mol. Sci. 2024, 25, 569. [Google Scholar] [CrossRef]
- Lei, F.J.; Chiang, J.Y.; Chang, H.J.; Chen, D.C.; Wang, H.L.; Yang, H.A.; Wei, K.Y.; Huang, Y.C.; Wang, C.C.; Wei, S.T.; et al. Cellular and exosomal GPx1 are essential for controlling hydrogen peroxide balance and alleviating oxidative stress in hypoxic glioblastoma. Redox Biol. 2023, 65, 102831. [Google Scholar] [CrossRef]
- Chong, M.C.; Silva, A.; James, P.F.; Wu, S.S.X.; Howitt, J. Exercise increases the release of NAMPT in extracellular vesicles and alters NAD(+) activity in recipient cells. Aging Cell 2022, 21, e13647. [Google Scholar] [CrossRef]
- Bryl-Gorecka, P.; Sathanoori, R.; Al-Mashat, M.; Olde, B.; Jogi, J.; Evander, M.; Laurell, T.; Erlinge, D. Effect of exercise on the plasma vesicular proteome: A methodological study comparing acoustic trapping and centrifugation. Lab. Chip 2018, 18, 3101–3111. [Google Scholar] [CrossRef]
- Glorieux, C.; Buc Calderon, P. Targeting catalase in cancer. Redox Biol. 2024, 77, 103404. [Google Scholar] [CrossRef]
- Hayes, S.H.; Liu, Q.; Selvakumaran, S.; Haney, M.J.; Batrakova, E.V.; Allman, B.L.; Walton, P.A.; Kiser, P.; Whitehead, S.N. Brain Targeting and Toxicological Assessment of the Extracellular Vesicle-Packaged Antioxidant Catalase-SKL Following Intranasal Administration in Mice. Neurotox. Res. 2021, 39, 1418–1429. [Google Scholar] [CrossRef]
- Wang, W.; Qiao, S.; Kong, X.; Zhang, G.; Cai, Z. The role of exosomes in immunopathology and potential therapeutic implications. Cell Mol. Immunol. 2025, 22, 975–995. [Google Scholar] [CrossRef]
- Yu, J.; Sane, S.; Kim, J.E.; Yun, S.; Kim, H.J.; Jo, K.B.; Wright, J.P.; Khoshdoozmasouleh, N.; Lee, K.; Oh, H.T.; et al. Biogenesis and delivery of extracellular vesicles: Harnessing the power of EVs for diagnostics and therapeutics. Front. Mol. Biosci. 2023, 10, 1330400. [Google Scholar] [CrossRef]
- Pust, S.; Brech, A.; Wegner, C.S.; Stenmark, H.; Haglund, K. Vesicle-mediated transport of ALIX and ESCRT-III to the intercellular bridge during cytokinesis. Cell Mol. Life Sci. 2023, 80, s00018–s00023. [Google Scholar] [CrossRef]
- Stoten, C.L.; Carlton, J.G. ESCRT-dependent control of membrane remodelling during cell division. Semin. Cell Dev. Biol. 2018, 74, 50–65. [Google Scholar] [CrossRef] [PubMed]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal 2021, 19, 47. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.F.; Li, W.J.; Hu, K.S.; Gao, J.; Zhai, W.L.; Yang, J.H.; Zhang, S.J. Exosome biogenesis: Machinery, regulation, and therapeutic implications in cancer. Mol. Cancer 2022, 21, 207. [Google Scholar] [CrossRef]
- Karkossa, I.; Furst, S.; Grosskopf, H.; von Bergen, M.; Schubert, K. Oxidation is an underappreciated post-translational modification in the regulation of immune responses associated with changes in phosphorylation. Front. Immunol. 2023, 14, 1244431. [Google Scholar] [CrossRef] [PubMed]
- Vrettou, S.; Wirth, B. S-Glutathionylation and S-Nitrosylation in Mitochondria: Focus on Homeostasis and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 15849. [Google Scholar] [CrossRef]
- Kwok, Z.H.; Wang, C.; Jin, Y. Extracellular Vesicle Transportation and Uptake by Recipient Cells: A Critical Process to Regulate Human Diseases. Processes 2021, 9, 273. [Google Scholar] [CrossRef]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef]
- Rubinstein, E.; Thery, C.; Zimmermann, P. Tetraspanins affect membrane structures and the trafficking of molecular partners: What impact on extracellular vesicles? Biochem. Soc. Trans. 2025, 0, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Czosseck, A.; Chen, M.M.; Hsu, C.C.; Shamrin, G.; Meeson, A.; Oldershaw, R.; Nguyen, H.; Livkisa, D.; Lundy, D.J. Extracellular vesicles from human cardiac stromal cells up-regulate cardiomyocyte protective responses to hypoxia. Stem Cell Res. Ther. 2024, 15, 363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Hong, J.; Zhang, H.; Zheng, W.; Yang, Y. Astrocyte-derived exosomes protect hippocampal neurons after traumatic brain injury by suppressing mitochondrial oxidative stress and apoptosis. Aging 2021, 13, 21642–21658. [Google Scholar] [CrossRef]
- Pistono, C.; Bister, N.; Stanova, I.; Malm, T. Glia-Derived Extracellular Vesicles: Role in Central Nervous System Communication in Health and Disease. Front. Cell Dev. Biol. 2020, 8, 623771. [Google Scholar] [CrossRef] [PubMed]
- Boonkaew, B.; Charoenthanakitkul, D.; Suntornnont, N.; Ariyachet, C.; Tangkijvanich, P. Extracellular vesicles in metabolic dysfunction-associated steatotic liver disease: From intercellular signaling to clinical translation. World J. Hepatol. 2025, 17, 108259. [Google Scholar] [CrossRef]
- Liu, G.; Yin, X.M. The Role of Extracellular Vesicles in Liver Pathogenesis. Am. J. Pathol. 2022, 192, 1358–1367. [Google Scholar] [CrossRef]
- Damba, T.; Bourgonje, A.R.; Abdulle, A.E.; Pasch, A.; Sydor, S.; van den Berg, E.H.; Gansevoort, R.T.; Bakker, S.J.L.; Blokzijl, H.; Dullaart, R.P.F.; et al. Oxidative stress is associated with suspected non-alcoholic fatty liver disease and all-cause mortality in the general population. Liver Int. 2020, 40, 2148–2159. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Buist-Homan, M.; Harmsen, M.C.; Moshage, H. Extracellular vesicle-dependent crosstalk between hepatic stellate cells and Kupffer cells promotes their mutual activation. Biochim. Biophys. Acta Mol. Basis Dis. 2025, 1871, 167914. [Google Scholar] [CrossRef]
- Geng, Y.; Wang, J.; Serna-Salas, S.A.; Villanueva, A.H.; Buist-Homan, M.; Arrese, M.; Olinga, P.; Blokzijl, H.; Moshage, H. Hepatic stellate cells induce an inflammatory phenotype in Kupffer cells via the release of extracellular vesicles. J. Cell Physiol. 2023, 238, 2293–2303. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Z.; Xia, M.; Salas, S.S.; Ospina, J.A.; Buist-Homan, M.; Harmsen, M.C.; Moshage, H. Extracellular vesicles derived from liver sinusoidal endothelial cells inhibit the activation of hepatic stellate cells and Kupffer cells in vitro. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 167020. [Google Scholar] [CrossRef]
- Wu, Z.; Xia, M.; Wang, J.; Aguilar, M.M.; Buist-Homan, M.; Moshage, H. Extracellular vesicles originating from steatotic hepatocytes promote hepatic stellate cell senescence via AKT/mTOR signaling. Cell Biochem. Funct. 2024, 42, e4077. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wu, T.; Lu, Y.; Zhao, W.; Zhang, J.; Chen, Q.; Ge, G.; Hua, Y.; Chen, K.; Ullah, I.; et al. Exosomal thioredoxin-1 from hypoxic human umbilical cord mesenchymal stem cells inhibits ferroptosis in doxorubicin-induced cardiotoxicity via mTORC1 signaling. Free Radic. Biol. Med. 2022, 193, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Boussac, M.; Véron, P.; Ricciardi-Castagnoli, P.; Raposo, G.a.; Garin, J.m.; Amigorena, S. Proteomic Analysis of Dendritic Cell-Derived Exosomes: A Secreted Subcellular Compartment Distinct from Apoptotic Vesicles1. J. Immunol. 2001, 166, 7309–7318. [Google Scholar] [CrossRef]
- Huang, J.; Chen, L.; Li, W.; Chang, C.J. Anti-inflammatory and antioxidative effects of Perilla frutescens-derived extracellular vesicles: Insights from Zebrafish models. Mol. Immunol. 2025, 182, 126–138. [Google Scholar] [CrossRef]
- Xu, R.; Lu, Y.; Cai, L.; Zhang, L. Utilizing Extracellular Vesicles from Phaeodactylum tricornutum as a Novel Approach for Protecting the Skin from Oxidative Damage. ACS Biomater. Sci. Eng. 2025, 11, 3400–3415. [Google Scholar] [CrossRef]
- Zhang, X.; Ye, X.; Xie, Y.; Yang, Z.; Spanos, M.; Guo, Z.; Jin, Y.; Li, G.; Lei, Z.; Schiffelers, R.M.; et al. GEV (Sod2) Powder: A Modified Product Based on Biovesicles Functioned in Air Pollution PM2.5-Induced Cardiopulmonary Injury. Research 2025, 8, 0609. [Google Scholar] [CrossRef]
- Gaweł, P.; Karcz, K.; Zaręba-Wdowiak, N.; Królak-Olejnik, B. Antioxidant Capacity of Colostrum of Mothers with Gestational Diabetes Mellitus-A Cross-Sectional Study. Nutrients 2025, 17, 3324. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Zhang, S.; Liu, Q.; Huang, C.; Hao, H.; Tan, M.S.; Yu, X.; Lou, C.K.L.; Huang, R.; Zhang, Z.; et al. Milk-derived extracellular vesicles protect intestinal barrier integrity in the gut-liver axis. Sci. Adv. 2023, 9, eade5041. [Google Scholar] [CrossRef]
- Muttiah, B.; Law, J.X. Milk-derived extracellular vesicles and gut health. NPJ Sci. Food 2025, 9, 12. [Google Scholar] [CrossRef]
- Kong, C.; Huang, L.B.; Yang, M.F.; Yue, N.N.; Zhang, Y.; Tian, C.M.; Wang, Y.H.; Wei, D.R.; Shi, R.Y.; Liang, Y.J.; et al. Milk-derived extracellular vesicles: Nature’s nanocarriers for drug delivery and therapeutics. Front. Pharmacol. 2025, 16, 1595891. [Google Scholar] [CrossRef]
- Prasadani, M.; Kodithuwakku, S.; Pennarossa, G.; Fazeli, A.; Brevini, T.A.L. Therapeutic Potential of Bovine Milk-Derived Extracellular Vesicles. Int. J. Mol. Sci. 2024, 25, 5543. [Google Scholar] [CrossRef] [PubMed]
- Ribas-Maynou, J.; Parra, A.; Martínez-Díaz, P.; Rubio, C.P.; Lucas, X.; Yeste, M.; Roca, J.; Barranco, I. Protective role of extracellular vesicles against oxidative DNA damage. Biol. Res. 2025, 58, 14. [Google Scholar] [CrossRef]
- Francesco, D.D.; Mantovani, D.; Hussey, G.; Boccafoschi, F. Matrix bound nanovesicles: A great promise for TERM in less than a decade of research. Matrix Biol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, H.; Chen, Y.; He, J.; Zhao, L.; Huang, Y.; Zhao, F.; Jiang, Y.; Fu, S.; Hong, Z. Improving therapeutic effects of exosomes encapsulated gelatin methacryloyl/hyaluronic acid blended and oxygen releasing injectable hydrogel by cardiomyocytes induction and vascularization in rat myocardial infarction model. Int. J. Biol. Macromol. 2024, 271, 132412. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, Z.; Li, D.; Ruan, X.; Yang, J.; Chen, S.; Li, X.; Ling, W. Integrating oxygen-boosted sonodynamic therapy and ferroptosis via engineered exosomes for effective cancer treatment. Theranostics 2025, 15, 68–85. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Liu, Y.; Cao, Y.; Liu, Z. Engineering Macrophage Exosome Disguised Biodegradable Nanoplatform for Enhanced Sonodynamic Therapy of Glioblastoma. Adv. Mater. 2022, 34, e2110364. [Google Scholar] [CrossRef]
- Cheng, Q.; Dai, Z.; Shi, X.; Duan, X.; Wang, Y.; Hou, T.; Zhang, Y. Expanding the toolbox of exosome-based modulators of cell functions. Biomaterials 2021, 277, 121129. [Google Scholar] [CrossRef]
- Cao, H.; Li, W.; Zhang, H.; Hong, L.; Feng, X.; Gao, X.; Li, H.; Lv, N.; Liu, M. Bio-nanoparticles loaded with synovial-derived exosomes ameliorate osteoarthritis progression by modifying the oxidative microenvironment. J. Nanobiotechnology 2024, 22, 271. [Google Scholar] [CrossRef]
- Shao, X.; Zhang, M.; Chen, Y.; Sun, S.; Yang, S.; Li, Q. Exosome-mediated delivery of superoxide dismutase for anti-aging studies in Caenorhabditis elegans. Int. J. Pharm. 2023, 641, 123090. [Google Scholar] [CrossRef]
- Shao, X.; Feng, X.; Feng, J.; Qiu, T.; Yang, S.; Li, Q. Exosome-mediated co-delivery of superoxide dismutase and chondroitinase ABC for multiple sclerosis therapy. Int. J. Biol. Macromol. 2025, 327, 147438. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, J.; Li, Z.; Yang, Y.; Li, L.; Zhao, Y.; Zhao, J. Enzyme-Loaded Catalytic Macrophage Vesicles with Cascade Amplification of Tumor-Targeting for Oxygenated Photodynamic Therapy. Int. J. Nanomed. 2021, 16, 7801–7812. [Google Scholar] [CrossRef]
- Xu, Z.; Hong, W.; Mo, Y.; Shu, F.; Liu, Y.; Cheng, Y.; Tan, N.; Jiang, L. Stem cells derived exosome laden oxygen generating hydrogel composites with good electrical conductivity for the tissue-repairing process of post-myocardial infarction. J. Nanobiotechnology 2025, 23, 213. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhang, R.; Yan, X.; Fan, K. Superoxide dismutase nanozymes: An emerging star for anti-oxidation. J. Mater. Chem. B 2021, 9, 6939–6957. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yan, X.; Gao, L.; Fan, K. Nanozymes expanding the boundaries of biocatalysis. Nat. Commun. 2025, 16, 6817. [Google Scholar] [CrossRef]
- Zamanian Dastmalchi, H.; Dashtestani, F.; Ghourchian, H. Superoxide dismutase-mimetic nanozymes: A promising alternative to natural superoxide dismutases for biomedical and industrial applications. Colloids Surf. B Biointerfaces 2025, 257, 115138. [Google Scholar] [CrossRef]
- Shen, J.; Pan, Y.; Han, L.; Luo, L.; Sun, T.; Yu, Y. Nanozymes as next-generation ROS scavengers: Design strategies, catalytic mechanisms, and therapeutic frontiers. J. Mater. Chem. B 2025, 13, 8286–8297. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Wang, Y.; Jiang, Y.; Li, T.; Qiu, X. Advances in total antioxidant capacity detection based on nanozyme. Talanta 2025, 292, 127941. [Google Scholar] [CrossRef]
- Tagaras, N.; Song, H.; Sahar, S.; Tong, W.; Mao, Z.; Buerki-Thurnherr, T. Safety Landscape of Therapeutic Nanozymes and Future Research Directions. Adv. Sci. 2024, 11, e2407816. [Google Scholar] [CrossRef]
- Liang, M.; Yan, X. Nanozymes: From New Concepts, Mechanisms, and Standards to Applications. Acc. Chem. Res. 2019, 52, 2190–2200. [Google Scholar] [CrossRef]
- Zeng, Q.; Zhong, H.; Liao, J.; Huo, Q.; Miao, B.; Zeng, L.; Zhang, B.; Nie, G. Antioxidant activities of metal single-atom nanozymes in biomedicine. Biomater. Sci. 2024, 12, 5150–5163. [Google Scholar] [CrossRef]
- Zhao, H.; Serre, C.; Steunou, N. Metal-Organic Frameworks for the Therapy of Inflammatory Diseases. Adv. Healthc. Mater. 2025, 14, e2404334. [Google Scholar] [CrossRef]
- Ameen, S.S.M.; Omer, K.M. Metal-organic framework-based nanozymes for water-soluble antioxidants and Total antioxidant capacity detection: Principles and applications. Food Chem. 2025, 479, 143876. [Google Scholar] [CrossRef]
- Ye, H.; Lai, Y.; Wu, Z.; Li, G.; Hua, Q.; Zhu, W. Carbon-based nanozymes: Catalytic mechanisms, performance tuning, and environmental and biomedical applications. Anal. Methods 2025, 17, 6264–6281. [Google Scholar] [CrossRef] [PubMed]
- Darabi, S.; Ariaei, A.; Rustamzadeh, A.; Afshari, D.; Charkhat Gorgich, E.A.; Darabi, L. Cerebrospinal fluid and blood exosomes as biomarkers for amyotrophic lateral sclerosis; a systematic review. Diagn. Pathol. 2024, 19(47), s13000–s13024. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, S.; Zhang, J.; Li, R.; Zhang, Y.; Wang, Z.; Kong, Q.; Cho, W.C.; Ju, X.; Shen, Y.; et al. Proteomic profiling of serum extracellular vesicles identifies diagnostic markers for echinococcosis. PLoS Negl. Trop. Dis. 2022, 16, e0010814. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; He, C.; Hao, Y.; Wang, L.; Li, L.; Zhu, G. Prospects and challenges of extracellular vesicle-based drug delivery system: Considering cell source. Drug Deliv. 2020, 27, 585–598. [Google Scholar] [CrossRef]
- de Jong, O.G.; Kooijmans, S.A.A.; Murphy, D.E.; Jiang, L.; Evers, M.J.W.; Sluijter, J.P.G.; Vader, P.; Schiffelers, R.M. Drug Delivery with Extracellular Vesicles: From Imagination to Innovation. Acc. Chem. Res. 2019, 52, 1761–1770. [Google Scholar] [CrossRef]
- Nelson, B.C.; Maragh, S.; Ghiran, I.C.; Jones, J.C.; DeRose, P.C.; Elsheikh, E.; Vreeland, W.N.; Wang, L. Measurement and standardization challenges for extracellular vesicle therapeutic delivery vectors. Nanomedicine 2020, 15, 2149–2170. [Google Scholar] [CrossRef]
- Teng, F.; Fussenegger, M. Shedding Light on Extracellular Vesicle Biogenesis and Bioengineering. Adv. Sci. 2020, 8, 2003505. [Google Scholar] [CrossRef]
- Cheng, G.; Li, W.; Ha, L.; Han, X.; Hao, S.; Wan, Y.; Wang, Z.; Dong, F.; Zou, X.; Mao, Y.; et al. Self-Assembly of Extracellular Vesicle-like Metal-Organic Framework Nanoparticles for Protection and Intracellular Delivery of Biofunctional Proteins. J. Am. Chem. Soc. 2018, 140, 7282–7291. [Google Scholar] [CrossRef]
- Sharma, S.; Masud, M.K.; Kaneti, Y.V.; Rewatkar, P.; Koradia, A.; Hossain, M.S.A.; Yamauchi, Y.; Popat, A.; Salomon, C. Extracellular Vesicle Nanoarchitectonics for Novel Drug Delivery Applications. Small 2021, 17, e2102220. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, B.; Zhang, S.; Cao, C.; Qi, L. Fabrication of Hierarchically Mesoporous Multishelled Metal-Organic Frameworks via Template-Induced Assembly/Grinding Strategy for Immobilization of Enzyme and Enhancing Catalytic Performance. ACS Appl. Mater. Interfaces 2025. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.I.; Amorim, M.G.; Gadelha, C.; Milic, I.; Welsh, J.A.; Freitas, V.M.; Nawaz, M.; Akbar, N.; Couch, Y.; Makin, L.; et al. Technical challenges of working with extracellular vesicles. Nanoscale 2018, 10, 881–906. [Google Scholar] [CrossRef]
- Hoshino, A.; Costa-Silva, B.; Shen, T.L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef]
- Kang, J.Y.; Mun, D.; Chun, Y.; Park, D.S.; Kim, H.; Yun, N.; Joung, B. Engineered small extracellular vesicle-mediated NOX4 siRNA delivery for targeted therapy of cardiac hypertrophy. J. Extracell. Vesicles 2023, 12, e12371. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Zhou, L.; Zhi, K.; Raji, B.; Pernell, S.; Tadrous, E.; Kodidela, S.; Nookala, A.; Kochat, H.; Kumar, S. Challenges in Biomaterial-Based Drug Delivery Approach for the Treatment of Neurodegenerative Diseases: Opportunities for Extracellular Vesicles. Int. J. Mol. Sci. 2020, 22, 138. [Google Scholar] [CrossRef] [PubMed]
- Claridge, B.; Lozano, J.; Poh, Q.H.; Greening, D.W. Development of Extracellular Vesicle Therapeutics: Challenges, Considerations, and Opportunities. Front. Cell Dev. Biol. 2021, 9, 734720. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).