Abstract
Our study aimed to test the hypothesis that supplementation with a combination of dry grape extracts (DGS) and olive-originated hydroxityrosol (HxT) polyphenols during ovine gestation under moderate nutritional restriction allows for the improvement of the birth weight and size of newborn lambs, especially in more demanding pregnancies such as twins. Twenty-six pregnant ewes (fourteen ewes with singleton pregnancies and twelve ewes with twin pregnancies) were divided in two equal groups, treated or not with the polyphenols, and involved in the study. The results indicate that supplementation with the combination of polyphenols, without modifying maternal body weight and body condition score, increased the maternal total antioxidant capacity (p < 0.05) and, interacting with pregnancy rank, improved maternal metabolic status by reducing β-hydroxybutyrate and non-esterified fatty acids (p < 0.05). Afterwards, supplementation improved birth weight and size of newborns in the most compromised pregnancies (i.e., twin pregnancies; twins lambs born to supplemented ewes were significantly heavier than those born to control ewes (3.3 ± 0.13 vs. 2.8 ± 0.23 kg, respectively; p < 0.05).
1. Introduction
Sheep production is usually associated with harsh environments (either semiarid or dry areas and/or at high altitude), causing undernutrition, due to the limited capacity to produce forage in adequate quantity and quality to meet the demands of sheep flocks [1,2]. During pregnancy, maternal undernutrition or exposure to high altitudes evokes fetal hypoxemia and oxidative stress [3,4]. Both circumstances affect the adequate development of pregnancy, leading to restricted intrauterine growth and fetal re-programming [5], which are even more boosted in the case of twin pregnancies [4,5]. Antioxidant supplementation in pregnant ewes in such disadvantageous environments, has been shown to have beneficial effects by improving the birth weight of the offspring and their survival [3,6].
The European Mediterranean countries are characterized by producing and consuming significant quantities of meat and dairy products from sheep origin [7]. The so-called Mediterranean Diet also includes other products such as fish, vegetables, fruits, olive oil, and wine [7]. Recent studies suggest that olive oil and wine production will increase in response to growing global demand, a trend that will be driven not only by traditional Mediterranean producers but also by new entrants to the market [8,9]. However, the production of both olive oil and wine is controversial for the environment, due to generation of a large number of residues that are potentially polluting the surroundings [10,11]. On the other hand, such residues are, in fact, by-products which contain antioxidant polyphenolic compounds that can be extracted and used as nutritional supplements in case of risk of increased oxidative stress [12,13,14,15]. Hence, the use of antioxidants generated from olives and grapes by-products may be highly beneficial for animal production, and specifically sheep production, which would be an outstanding example of sustainability and circular economy.
In fact, polyphenolic compounds from different plant origin have been tested in different livestock species [15,16,17,18]. These compounds are currently extracted, characterized and marketed as nutritional additives. Specifically, dry grape extracts (DGS; >80% polyphenols) and hydroxityrosol (HxT; derived from olive by-products and leaf) are authorized in the European Union for their use as nutritional additives [19,20]. Thus, DGS supplementation in well-nourished sheep during the first 47 days of lactation significantly increased live weight and carcass weight of the lambs at 115 days of age [21]. Moreover, weaned lambs, consuming a balanced diet supplemented with DGS for 60 days, resulted in a significant increase in antioxidant status, daily weight gain, feed conversion efficiency, and immune status [18]. On the other hand, HxT has not been tested in sheep or other ruminants. However, in monogastric farm animals such as pigs, HxT supplementation has shown interesting results. Sows treated with HxT from day 35 of gestation showed increased antioxidant status, birth weight and size, and postnatal development of their piglets [22].
In the present study, we tested the hypothesis that supplementation with a combination of DGS and HxT during ovine gestation under moderate nutritional restriction, improves the birth weight and size of newborn lambs, especially in more demanding pregnancies such as twins.
2. Materials and Methods
2.1. Ethics, Animals, and General Management
The study was carried out at the experimental farm of the Faculty of Veterinary Medicine, CEU Cardenal Herrera University (Alfara del Patriarca, Valencia, Spain; February to July 2024) according to the European Union Directive and the Spanish Policy for Animal Protection RD53/2013.
Thirty Segureña ewes, 3–4 years old, 54.5 ± 1.7 Kg of body weight (BW) and 3.5 ± 0.2 of body condition score (BCS) in accordance with Jefferies scale [23], were used. The animals were kept in a common, roofed paddock (160 m2), with daily access to an open paddock (700 m2), receiving alfalfa hay, oat straw, and water ad libitum. Ewes were subjected for estrous synchronization using progesterone intrauterine devices (CIDR OVIS®, 0.35 g progesterone; Zoetis, Spain), for 12 days. After devices removal, ewes were hand-mated with three rams of proven fertility.
Forty days after mating, pregnancy and number of embryos were determined by transrectal ultrasonography (M-Turbo, FUJIFILM Sonosite, Inc., 21919 30th Drive SE Bothell, WA 98021, USA). Twenty-six ewes became pregnant, and fourteen ewes with singleton pregnancies and twelve ewes with twin pregnancies were involved in the study. Immediately after the ultrasound examination and until lambing, these ewes were separated by gestation rank (singleton and twin) in adjacent paddocks and subjected to a permanent regime of moderate nutritional restriction (~20% energy/protein restriction), according to the differential requirements by stage of gestation and rank [24]. Diets consisted of alfalfa hay and oat straw in amounts adjusted to provide protein and energy meeting approximately 80% of the daily requirements of ewes carrying single (82 g CP; 8.4 MJ/kg ME) or twin fetuses (99 g CP; 10.3 MJ/kg ME) until day 90 of gestation. From day 91 until parturition, daily dietary allowances were increased to 108 g CP and 10.4 MJ/kg ME for single-bearing ewes, and to 132 g CP and 13.2 MJ/kg ME for twin-bearing ewes. Each group of gestational rank was divided into two equal treatment groups, half supplemented with antioxidants (AOx), and half controls without AOx. Hence, four groups were finally considered as follows: single pregnancies supplemented with AOx (n = 7); single control pregnancies (n = 7); twin pregnancies supplemented with AOx (n = 6); and twin control pregnancies (n = 6). From the start of treatment until the end of gestation, weight and BCS of each ewe were biweekly assessed. The allocation of treatment (AOx or control) within each gestational rank was carried out using block randomization based on ewe body weight.
AOx supplementation, started simultaneously with the nutritional restriction. It was performed with DGS (0.8 mg Kg−1 ewe live weight, Nuxafen®, Alvinesa, Spain) and HxT (0.01 mg Kg−1 ewe live weight, Olive Fruit Extract liquid®, Genosa, Alvinesa, Spain). Since no information was available on the combined use of both products in ruminants, the doses used in this study were estimated according to the following criteria. For DGS, we consider its antioxidant power to be equivalent to 10 times that of pure vitamin E, as indicated by the manufacturers. Additionally, improvements in antioxidant status and lambs birth weight (mainly twins) was observed when pregnant ewes received a daily dose of 7–8 mg Kg−1 body weight of vitamin E [25], suggesting that 0.8 mg Kg−1 of DGS could mimic the antioxidant capacity achieved with vitamin E. In the case of HxT, no studies on supplementation in small ruminants during gestation were found. According to studies in pregnant sows under nutritional restriction, an approximate daily dose of 0.02 mg Kg−1 body weight, supplemented during the last trimester of gestation, significantly increased the antioxidant status and birth weight of piglets [22]. Therefore, to enhance the effect of DGS, we decided to include a dose of 0.01 mg Kg−1 body weight of HxT. Both products were mixed and suspended in physiological solution to prepare and administrate a dosage of 1 mL of suspension for each 10 kg of ewe’s body weight. The volume of solution, adjusted to the body weight of each pregnant ewe, was daily administered directly into the mouth of each treated animal with a syringe and, in order to maintain the same management, the control ewes received orally an equivalent volume of physiological solution. The dose administered was adjusted biweekly according to the body weight of each animal. One week before the estimated date of delivery, blood samples (5 mL) were taken with heparinized syringes from the jugular vein of the ewes for evaluation of antioxidant status and biochemical parameters. The blood was centrifuged at 1300× g for 5 min and the plasma obtained was stored at −80 °C until analysis. At delivery, both nutritional restriction and AOx or physiological solution supplementation were suspended and the ewes returned to their full diet.
Approximately 4 h after delivery, the newborn lambs were assessed for sex, birth weight, and body size (body length, fore- and hindlimb length, and thoracic and abdominal circumference). Simultaneously, a colostrum sample (20 mL) was collected from each ewe, immediately labeled, and stored at −20 °C until compositional analysis.
2.2. Analysis of Blood and Colostrum Samples
Oxidative stress in blood plasma samples was evaluated through measurement of total antioxidant capacity (TAC) and malondialdehyde (MDA, a lipid oxidation biomarker) by enzyme immunoassay (ELISA). TAC was measured in 10 µL of blood plasma using the Antioxidant Assay Kit® (Cayman Chemical Company, Ann Arbor, MI, USA). MDA was measured in 100 µL of blood plasma using the TBARS Assay Kit (Cayman Chemical Company, Ann Arbor, MI, USA). Absorbance for MDA and TAC were assessed in a microplate reader (FLUOstar Omega BMG LABTECH GmbH, Ortenberg, Germany) at 540 and 405 nm, respectively. All the assays were performed in duplicate in accordance with the supplier instructions and has been previously adapted and tested for sheep [3,5].
Blood biochemistry analysis consisted of the measurement of β-hydroxybutyrate (BHB), glucose, lactate, non-esterified fatty acids (NEFAs), triglycerides, protein, and urea, using a clinical analyzer (Konelab 20i, Thermo Fisher Scientific, Madrid, Spain).
The colostrum samples were diluted in deionized distilled water (1:1 v/v), quickly homogenized and assayed at 23 °C for evaluation of the fat, protein, lactose, total solids, and non-fat solids content using an IR spectrophotometer (MilkoScan ™ 7RM, Foss Iberia, Barcelona, Spain).
2.3. Statistical Analysis
Data were checked for normality and homoscedasticity and transformed when necessary. For traits measured once, a 2 × 2 factorial design was used with pregnancy rank (single vs. twin) and treatment (Control vs. AOx) as fixed factors. Maternal and newborn traits were analyzed by two-way analysis of variance using the general linear model (GLM) procedure from the GraphPad Prism 10 statistical software (GraphPad Software, Inc., Boston, MA, USA) to evaluate the main effects of pregnancy rank and treatment. No interaction between factors was observed for any of the variables analyzed; therefore, interaction terms are not included in the tables. Maternal BW and BCS, which were measured repeatedly over time, were analyzed separately from the factorial model. Because the biological interest was to characterize temporal patterns within each pregnancy rank, longitudinal changes in these variables were evaluated using repeated-measures ANOVA conducted independently for single- and twin-bearing ewes. To complement this analysis, mean differences between pregnancy ranks at each gestational time point were assessed using independent-samples t-tests (cross-sectional comparisons). A probability of p ≤ 0.05 was considered statistically significant. Results are expressed as mean ± SEM.
3. Results
3.1. Maternal Traits
3.1.1. Changes in Maternal BW and BCS over Time of Pregnancy
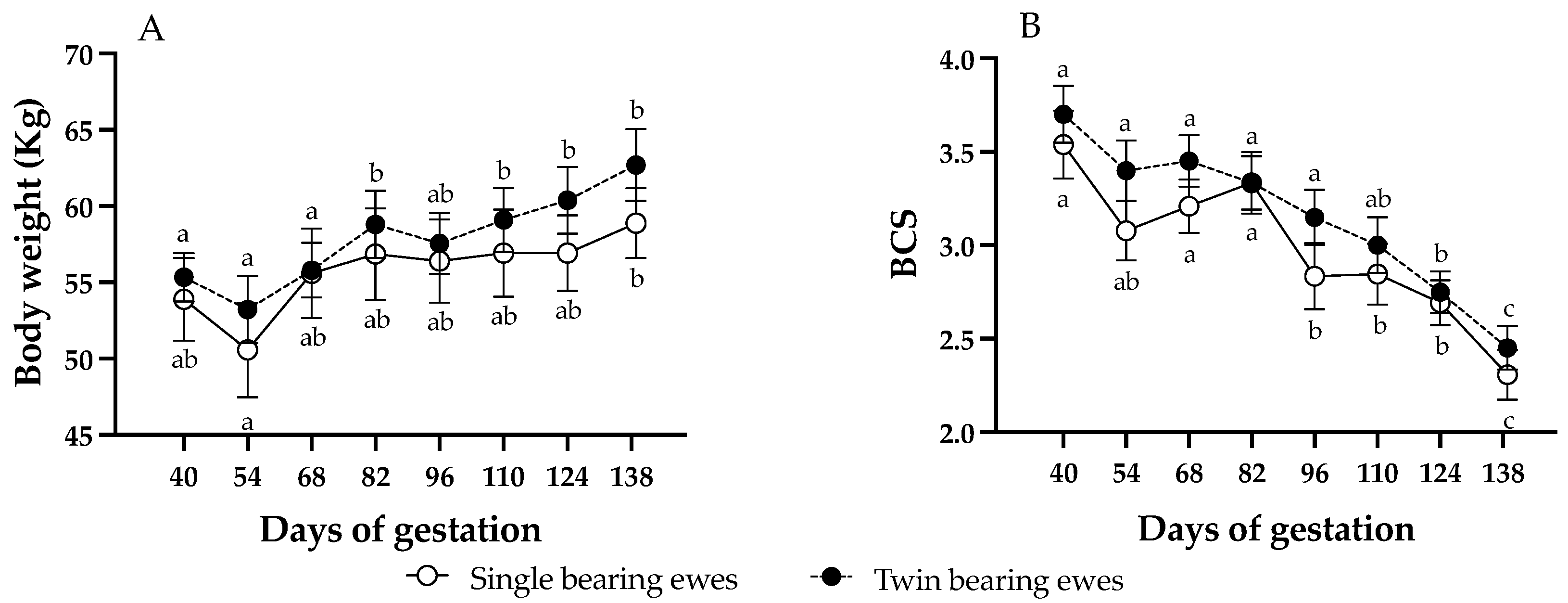
Body weight and BCS were not affected by the AOx treatment or by the gestational rank (p > 0.05). Therefore, no differences in mean BW (single bearing ewes, control: 57.1 ± 0.8, AOx: 55.8 ± 0.9; twin-bearing ewes, control: 57.6 ± 0.7, AOx: 59.1 ± 1.3; p = 0.062) or BCS (single bearing ewes, control: 3.1 ± 0.1, AOx: 2.8 ± 0.1; twin-bearing ewes, control: 3.2.6 ± 0.2, AOx: 3.1 ± 0.1; p = 0.30) between groups were detected. Then, Figure 1 presents them as twin and singleton pregnancies, without differentiating between those supplemented with AOx or not supplemented. Body weight of the ewes increased progressively over time of pregnancy, without significant differences between pregnancy ranks. Ewes carrying singletons gained around 5.3 kg during gestation, with significant differences only between days 54 and 138; meanwhile ewes carrying twins gained around 7.4 kg, showing significant increases from day 82 of gestation. Conversely, BCS showed an inverse pattern, with a marked decrease along gestation and again without significant differences between pregnancy ranks, being significantly lower from day 96 of gestation in singleton ewes and from day 124 in twin ewes. Ewes carrying singletons decreased BCS by 1.2 points, while those with twins by 1.3 points but without significant differences.
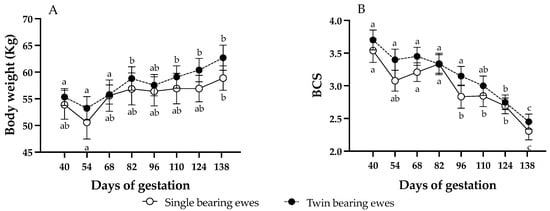
Figure 1.
Changes (mean ± SEM) in ewe’s body weight (panel A) and BCS (panel B) throughout pregnancy. Different letters within each pregnancy rank indicate significant differences between gestation times (p < 0.05; longitudinal comparisons). No differences were detected in body weight or BCS between ewes with single or twin fetuses at each time point (cross-sectional comparisons).
3.1.2. Assessment of Maternal Antioxidant/Oxidative Status at 140 Days of Pregnancy
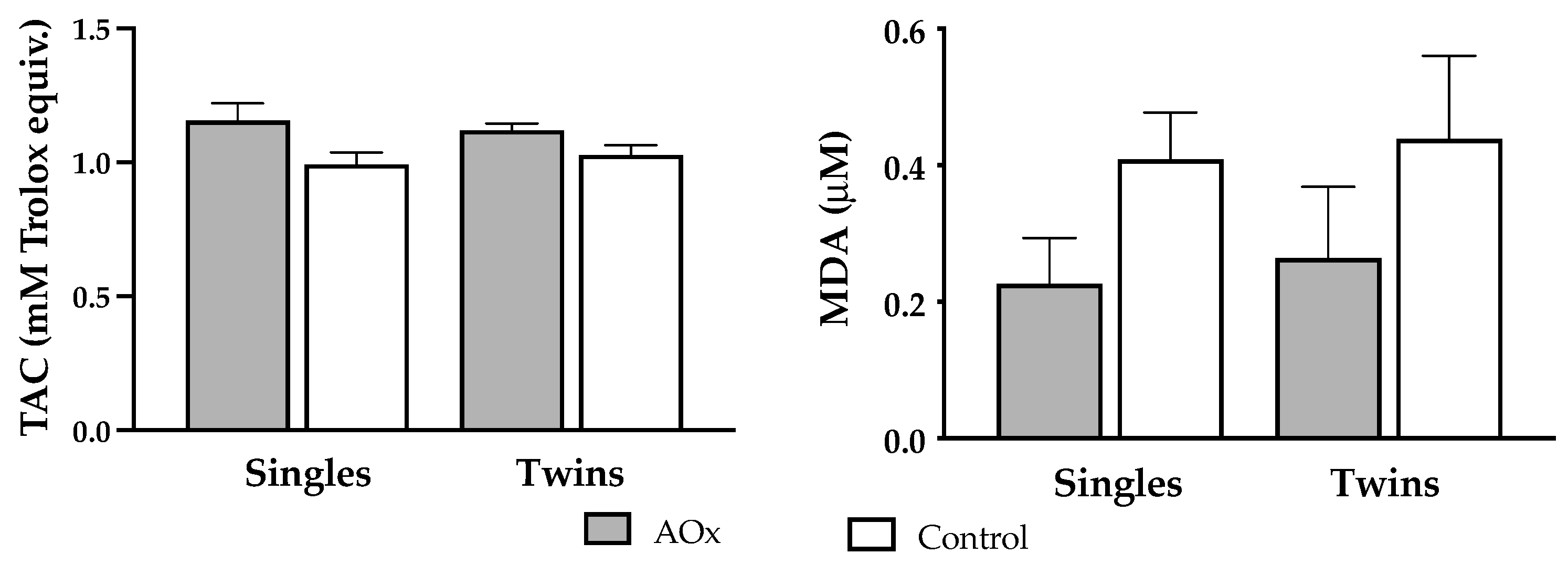
Supplementation with antioxidants induced a significant increase (p = 0.004) in plasma TAC at 140 days of pregnancy in both single and twin pregnant ewes (Figure 2). Conversely, concentrations of MDA were lowered in singleton and twin pregnant sheep receiving antioxidants supplementation, but the difference did not reach significance.
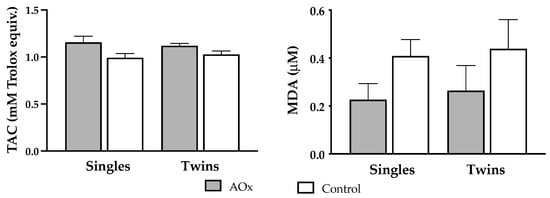
Figure 2.
Plasma levels (mean ± SEM) of biomarkers of antioxidant/oxidative status. Left graph depicts total antioxidant capacity (TAC; Rank p = 0.995, AOx p = 0.004) and right graph depicts malondialdehyde (MDA; Rank p = 0.212, AOx p = 0.443) concentrations at 140 days of gestation, in ewes carrying singleton and twin pregnancies and supplemented or not with AOx. There was no interaction between the factors in any of the two variables.
3.1.3. Assessment of Maternal Metabolic Biomarkers
There were no major changes in plasma biomarkers of metabolic status at 140 days of pregnancy, excepting BHB and NEFAs which were affected by pregnancy rank (Table 1), with twin-bearing control ewes showing the highest values. Additionally, twin-bearing ewes showed a marked trend towards higher blood lactate concentrations compared to those carrying singletons.

Table 1.
Plasma concentrations (mean ± SEM) of biomarkers of metabolic status at 140 days of gestation in ewes carrying singleton and twin pregnancies with (AOx) or without (Con) antioxidants supplementation.
3.1.4. Assessment of Colostrum Composition
There were no effects from either number of fetuses or antioxidant supplementation on the colostrum nutritional composition (Table 2).

Table 2.
Colostrum composition (%) immediately after parturition in ewes carrying singleton and twin pregnancies with (AOx) or without (Con) antioxidants supplementation.
3.2. Newborn Traits
3.2.1. Birth Weight
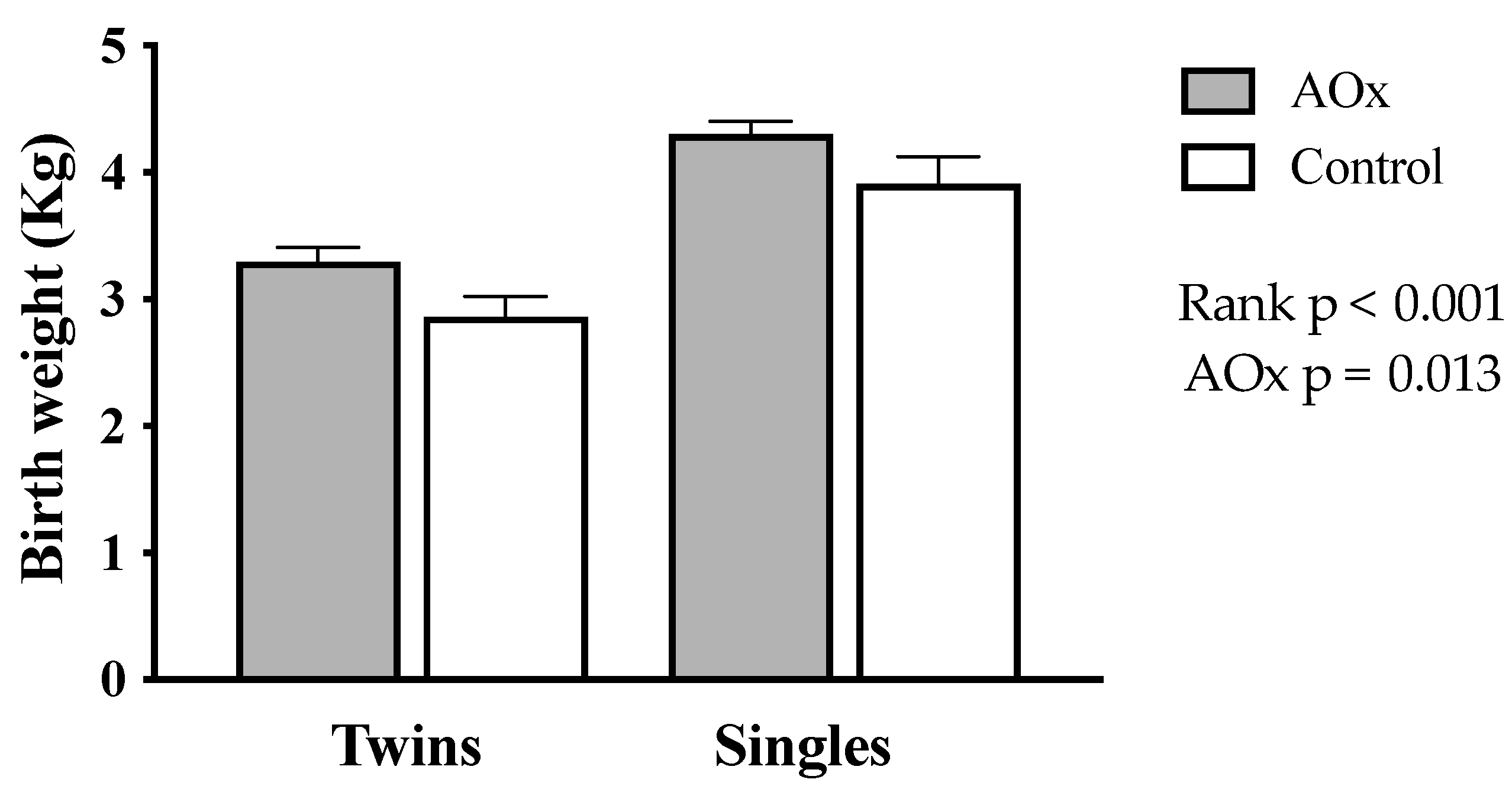
Birth weight of the lambs is shown in Figure 3. It was significantly higher in singleton than in twin lambs (4.0 ± 0.15 vs. 3.1 ± 0.11 Kg, respectively; p < 0.001). The supplementation with antioxidants also increases the birth weight of lambs (p = 0.013), an effect that was greater in twins (AOx: 3.3 ± 0.13 Kg, control: 2.8 ± 0.23 Kg) than singles (AOx: 4.1 ± 0.21 Kg, control: 3.9 ± 0.22 Kg).
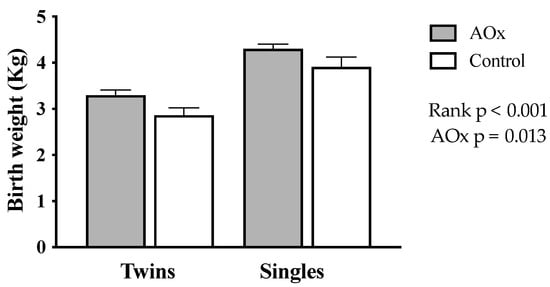
Figure 3.
Body weight at birth from lamb’s born to single- and twin-bearing ewes supplemented or not with AOx.
3.2.2. Body Size at Birth
Body measurements of newborn lambs are reported in Table 3. The body-length and the thoracic circumference of the newborn lambs were not significantly affected by pregnancy rank or treatment, although AOx supplementation showed a trend to promote thoracic perimeter. The abdominal perimeter, on the other hand, was affected by both the pregnancy rank and the AOx supplementation and twins had a smaller abdominal perimeter than singletons, but both singleton and twins born to ewes supplemented with AOX showed highest values than their control counterparts. Finally, both forelimb and hindlimb lengths were greater in singleton lambs than in twins, with no AOx effects.

Table 3.
Biometrical traits at birth of singleton and twin lambs born to ewes carrying singleton and twin pregnancies with (AOx) or without (Con) antioxidants supplementation.
4. Discussion
The results of the present study support the idea that twinning in sheep under moderately challenged pregnancies (i.e., 20% nutritional restriction) is associated with maternal metabolic stress, as indicated by significantly higher plasma BHB and NEFA concentrations and numerically higher lactate levels, while differences in body weight (BW) and body condition score (BCS) were observed only during the last month of gestation. Compared with singletons, these ewes give birth to lambs with lower birth weight and smaller body size. Supplementation of underfed pregnant ewes with a combination of polyphenols (DGS and HxT) improves their antioxidant status and, without altering BW, BCS, or metabolic traits, also enhances the birth weight and size of the offspring, particularly in the most compromised pregnancies (i.e., twin pregnancies).
4.1. Changes in Maternal Body Weight, Body Condition, and Metabolic Status
The assessment of changes in BW and BCS throughout gestation confirmed the undernourished state of the ewes in our study, as evidenced by a smaller increase in BW compared with values typically reported for well-nourished pregnant ewes (approximately 10% of BW in singleton-bearing ewes and about 18% in twin-bearing ewes. Concomitantly, the BCS should not decrease more than 0.5 points in both singleton and twin pregnancies but, in our study, BCS decreased by 1.2–1.3 points [26]. These changes were similar in both singleton and twin pregnancies, and no significant differences in BW or BCS were detected between pregnancy ranks.
Conversely, pregnancy rank affected the metabolic status of the dams, with twin-bearing ewes showing higher plasma concentrations of BHB and NEFAs, and a trend for higher lactate concentrations, than sheep carrying singletons. BHB, NEFAs, and lactate reflect the level of lipid catabolism, and their concentration increases when glucose metabolism is deficient [27]. Thus, the higher plasma concentrations of BHB and NEFAs, along with the upward trend in lactate, reflect increased metabolic stress and impaired energy balance in twin-bearing ewes, resulting in intensified lipomobilization, as previously shown in cows [28], and similar to previous studies in both meat and dairy sheep [29]. Moreover, we have to note that both BHB and NEFAs levels in control sheep with twin pregnancies remained above physiological levels for the species (0.36–0.80 and 0.18–0.68 mmol/L, respectively) [30,31,32], which is indicative of a considerable metabolic challenge in these sheep.
In our study, supplementation with polyphenolic compounds did not affect maternal BW and condition, as previously described for other AOx therapies in pregnant sheep under more severe conditions of nutritional restriction [5]. However, it improved the antioxidant/oxidative status, as evidence by the significant increase in TAC and the reduction in MDA plasma concentrations.
The increase in TAC is a protective mechanism particularly relevant in multiple pregnancies, where oxidative stress is more pronounced [5]. This stage is characterized by the progressive rise in energy metabolism to sustain rapid fetal growth, placental function, and maternal metabolic demands [33,34]. Consequently, the elevated metabolic activity accelerates the production of reactive oxygen species (ROS) and enhances oxidative damage [35,36], which, if not adequately counteracted, may compromise cellular integrity, impair fetal development, and predispose animals to various diseases [37], ultimately affecting future productive [38] and reproductive [39] performance. Oxidative stress states, resulting from elevated ROS concentrations in ewes, have been associated with restricted fetal growth [4,35,40].
Lipid peroxidation generates a wide range of aldehydes, among which malondialdehyde (MDA) is recognized as a specific biomarker of oxidative stress [41]. The increase in oxidative stress during gestation is associated with elevated MDA concentrations [42]. To maintain redox balance, the organism relies on several endogenous defense mechanisms based on enzymatic scavenging systems, among which superoxide dismutase (SOD), catalase (CAT), and peroxidases are particularly prominent. However, under certain conditions such as gestation, these systems may be overwhelmed, leading to a state of oxidative stress. To counteract this deficit, the use of exogenous antioxidants has been shown to be an effective strategy to reinforce the organism’s defensive capacity, thereby reducing MDA levels [4].
In our study, supplementation with DGS and HxT was associated with a trend towards reduced MDA concentrations, as previously reported in other studies [5,43], although this decrease did not reach the expected level of statistical significance. This suggests that, while antioxidant supplementation contributed to strengthening endogenous systemic defenses, the degree of lipid peroxidation in these females may not have been sufficiently pronounced to allow the detection of a significant reduction with a clear effect within this period. Nevertheless, studies in dairy cows during late gestation have not revealed significant changes in MDA concentrations. This apparent absence of variation may largely be explained by the high individual variability in this biomarker, which could mask physiologically relevant changes [44]. Therefore, supplementation with polyphenolic compounds enhances the antioxidant capacity of ewes during the final third of gestation, irrespective of whether they are carrying singletons or twins. At the same time, this positive effect on oxidative status occurs without substantially altering the body reserves of the ewes.
4.2. Changes in Newborn Lambs’ Body Weight and Size
Maternal undernourishment is known to induce intrauterine growth restriction and, thus, low birth weight of the newborns [45], even more in case of twinning [4]. Lamb birth weight and morphometric measurements are indicators of intrauterine growth rate and can be influenced by maternal factors (age and parity), placental factors (size and blood flow), fetal factors (number and sex), and environmental conditions (nutritional status and hypobaric hypoxia) [3,46]. In our study, singleton lambs had a mean birth weight of 4.0 kg, whereas twins averaged 3.1 kg. This difference represents an approximate reduction of 0.9 kg, equivalent to around 22% less weight in twins compared with singletons, directly reflecting that multiple pregnancies increase intrauterine competition for nutrients and oxygen, thereby limiting fetal growth [4,47,48].
Previous studies have also shown that intrauterine growth restriction is related to impaired supplies of nutrients and oxygen and, consequently, a weakened antioxidant defense system [49,50,51]. Ewes under extensive rearing are prone to undernourishment and intrauterine growth restriction, mainly in case of twin pregnancies, which is associated with the occurrence of an oxidative stress condition [4]. In these sheep, the effects of intrauterine growth restriction may be alleviated by the maternal supplementation with the antioxidant vitamins C and E, which ameliorate the antioxidant/oxidative ratio, improve the placental function, and increase the weight and viability of the newborn [3,6,25]. In the present study, similarly to these previous data in sheep affected by undernourishment and/or hypoxia and treated with vitamins C and E, the use of antioxidant polyphenols (olive and grape extracts) induced a non-significant increase of around 200 g in the birth weight of singletons but a significant increase of around 500 g in the birth weight of twins.
The lack of effect of maternal antioxidant (AOx) supplementation on the birth weight of singleton lambs, indicates that in single-fetus pregnancies, under a maternal 20% food restriction, nutrient availability is sufficient to sustain optimal growth irrespective of supplementation [4]. In contrast, twin lambs born to AOx-supplemented ewes were significantly heavier compared with those born to control ewes (3.3 kg vs. 2.8 kg; p = 0.035). This suggests that AOx supplementation may mitigate oxidative stress or the metabolic challenges associated with multiple pregnancies, allowing for improved nutrient utilization or enhanced placental efficiency [6]. The data indicates that antioxidant support becomes particularly relevant under conditions of increased fetal competition, when the risk of intrauterine growth restriction rises as litter size increases [52] and AOx supplementation counteracts this negative effect, and could potentially improve lamb survival, which is highly correlated to lamb weight at birth [53].
Morphometric measurements not only reflect physical conformation, carcass structure, growth conditions, and the relationship of development among tissues and organs, but also provide additional information on growth patterns during gestation and postnatal development of the offspring [54]. Body length and thoracic circumference of newborn lambs were not affected by type of pregnancy or treatment, although AOx supplementation showed a tendency to promote thoracic girth. These findings are consistent with previous reports, where no marked differences in these morphometric traits were observed [3].
Abdominal circumference of lambs was influenced by both type of pregnancy and maternal supplementation. Twins presented significantly lower values compared with singletons; however, in both groups, this measure was greater when the ewes received AOx. Since abdominal circumference constitutes an indirect indicator of visceral development and gastrointestinal capacity, this finding suggests that AOx supplementation favors intra-abdominal growth, which may enhance metabolic and digestive adaptation in early life. Similarly, singleton lambs exhibited longer forelimbs and hindlimbs, a development consistent with their higher birth weight (4.0 ± 0.15 kg) compared with their twin counterparts. This pattern aligns with their greater body size and growth potential during gestation, reflecting differences in nutrient availability throughout this stage. Although AOx supplementation did not significantly influence these fetal traits, the evidence indicates that its administration during gestation does not alter the linear dimensions of the limbs but rather exerts a more direct effect on overall birth weight and other body measurements.
4.3. Changes in Colostrum
Colostrum composition, including fat, protein, and lactose levels may vary according to several factors, such as parity, litter size, nutrition, and potentially breed [55]. In the present study, the percentages of fat, protein, lactose, total solids, and non-fat solids remained within the physiological ranges reported for this species [55] and neither the rank, nor antioxidant supplementation, exerted a significant effect on the nutritional composition of colostrum. This contrasts with what was observed in sheep under more severe undernutrition, where those that gave birth to a single lamb have more protein and lactose than those that gave birth to twins [56].
The literature on the effects of antioxidants on the nutritional quality of the sheep colostrum is scarce. However, melatonin implanted in ewes at 3 months of pregnancy increases fat in colostrum [57]. On the other hand, maternal antioxidant supplementation in well-fed dams during the final third of gestation has been shown to improve colostrum composition, reflected in increased concentrations of fat, protein, and immunoglobulins in small ruminants [58,59]. Therefore, difference in colostrum composition supports the need for further studies to evaluate the factors that may cause variations in colostrum composition under conditions of antioxidant supplementation in nutritionally restricted ewes.
5. Conclusions
Supplementation with a combination of dry grape extract (DGS) and olive-derived hydroxytyrosol (HxT) polyphenols during gestation in sheep under mild nutritional restriction, without affecting maternal body weight or condition, enhances maternal antioxidant defenses and metabolic status, as well as the birth weight and size of the lambs—particularly in more demanding pregnancies such as twins. The use of such agro-industrial by-products may offer benefits not only from an environmental perspective but also from a productive one, as promoting circular economy practices could improve performance in the most compromised gestations, such as twin pregnancies, where perinatal mortality remains one of the main limitations to sheep production worldwide. A limitation of this study is the use of a single concentration for each of the evaluated products, so further studies aiming to identify the most cost-efficient doses are foreseen. In addition, the promising results obtained under the conditions of this study should be confirmed in future experiments involving a larger number of animals under real production conditions.
Author Contributions
Conceptualization, V.H.P., P.M.-R. and A.G.-B.; methodology, V.H.P., P.M.-R., A.G.-B. and J.L.P.; formal analysis, V.H.P., F.S. and M.D.l.R.; investigation, V.H.P., J.L.P., P.M.-R., N.M.H., N.Y.-V. and M.C.L.-M.; resources, P.M.-R.; writing—original draft preparation, V.H.P., F.S. and A.G.-B.; writing—review and editing, V.H.P., A.G.-B., J.L.P., N.M.H., P.M.-R., M.D.l.R. and L.A.R.; project administration, P.M.-R.; funding acquisition, P.M.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Universidad Cardenal Herrera GIR23/54.
Institutional Review Board Statement
The Committee of Ethics in Animal Research of Universidad CEU-Cardenal Herrera approved the experiment (report CEEA 24/15 2024-VSC-PEA-0103). Date of approval of the experimental protocol: 24 April 2024.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy.
Acknowledgments
We would like to thank the students of the Small Ruminant Research Practices and the PhD student Angela Nakafeero from the Faculty of Veterinary at CEU Cardenal Herrera University, as well as technicians Violeta Pérez and Ángel Mora, for their valuable assistance in handling the sheep during the experiment. We would also like to thank Technologist Marta Grajales for her assistance in the laboratory analysis. We are also grateful to Fundación Carolina for supporting the short postdoctoral stay (JLP). Finally, we would like to thank the company Alvinesa (Spain) for donating the antioxidants used in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Iniguez, L. The challenges of research and development of small ruminant production in dry areas. Small Rumin. Res. 2011, 98, 12–20. [Google Scholar] [CrossRef]
- Sun, Y.; Angerer, J.P.; Hou, J. Effects of grazing systems on herbage mass and liveweight gain of Tibetan sheep in Eastern Qinghai-Tibetan Plateau, China. Rangel. J. 2015, 37, 181–190. [Google Scholar] [CrossRef]
- Parraguez, V.H.; Atlagich, M.; Araneda, O.; García, C.; Muñoz, A.; De los Reyes, M.; Urquieta, B. Effects of antioxidant vitamins on newborn and placental traits in gestations at high altitude: Comparative study in high and low altitude native sheep. Reprod. Fertil. Dev. 2011, 23, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Sales, F.; Peralta, O.A.; Narbona, E.; McCoard, S.; De los Reyes, M.; González-Bulnes, A.; Parraguez, V.H. Hypoxia and oxidative stress are associated with reduced fetal growth in twin and undernourished sheep pregnancies. Animals 2018, 8, 217. [Google Scholar] [CrossRef]
- Parraguez, V.H.; Sales, F.; Peralta; De los Reyes, M.; González-Bulnes, A. Oxidative stress and fetal growth restriction set up earlier in undernourished sheep twin pregnancies: Prevention with antioxidant and nutritional supplementation. Antioxidants 2022, 11, 1287. [Google Scholar] [CrossRef]
- Parraguez, V.H.; Sales, F.; Peralta, O.A.; Narbona, E.; Lira, R.; De los Reyes, M.; González-Bulnes, A. Supplementation of underfed twin-bearing ewes with herbal vitamins C and E: Impacts on birth weight, postnatal growth, and pre-weaning survival of the lambs. Animals 2020, 10, 652. [Google Scholar] [CrossRef]
- Análisis del Consumo en la Unión Europea. Ministerio de Agricultura, Pesca y Alimentación. Gobierno de España. Available online: https://www.mapa.gob.es/en/alimentacion/temas/consumo-tendencias/cap_02_tcm38-89428.pdf (accessed on 17 January 2025).
- Mili, S.; Bouhaddane, M. Forecasting global developments and challenges in olive oil supply and demand: A Delphi survey from Spain. Agriculture 2021, 11, 191. [Google Scholar] [CrossRef]
- Ohana-Levi, N.; Netzer, Y.Y. Long-term trends of global wine market. Agriculture 2023, 13, 224. [Google Scholar] [CrossRef]
- Dahdouh, A.; Khay, I.; Le Brech, Y.; El Maakoul, A.; Bakhouya, M. Olive oil industry: A review of waste stream composition, environmental impacts, and energy valorization paths. Environ. Sci. Pollut. Res. 2023, 30, 45473–45497. [Google Scholar] [CrossRef]
- Jorge, N.; Teixeira, A.R.; Gomes, A.; Peres, J.A.; Lucas, M.S. Winery Wastewater: Challenges and Perspectives. Eng. Proc. 2023, 56, 267. [Google Scholar]
- Tapia-Quirós, P.; Montenegro-Landívar, M.F.; Reig, M.; Vecino, X.; Alvarino, T.; Cortina, J.L.; Saurina, J.; Granados, M. Olive mill and winery wastes as viable sources of bioactive compounds: A study on polyphenols recovery. Antioxidants 2020, 9, 1074. [Google Scholar] [CrossRef] [PubMed]
- Enaime, G.; Dababat, S.; Wichern, M.; Lübken, M. Olive mill wastes: From wastes to resources. Environ. Sci. Pollut. Res. 2024, 31, 20853–20880. [Google Scholar] [CrossRef]
- Evtuguin, D.; Aniceto, J.P.S.; Marques, R.; Portugal, I.; Silva, C.M.; Serafim, L.S.; Xavier, A.M.R.B. Obtaining value from wine wastes: Paving the way for sustainable development. Fermentation 2024, 10, 24. [Google Scholar] [CrossRef]
- Waqas, M.; Salman, M.; Sharif, M.S. Application of polyphenolic compounds in animal nutrition and their promising effects. J. Anim. Feed Sci. 2023, 32, 233–256. [Google Scholar] [CrossRef]
- Makri, S.; Kafantaris, I.; Savva, S.; Ntanou, P.; Stagos, D.; Argyroulis, I.; Kotsampasi, B.; Christodoulou, V.; Gerasopoulos, K.; Petrotos, K.; et al. Novel feed including olive oil mill wastewater bioactive compounds enhanced the redox status of lambs. In Vivo 2018, 32, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Correddu, F.; Lunesu, M.F.; Buffa, G.; Atzori, A.S.; Nudda, A.; Battacone, G.; Pulina, G. Can agro-industrial by-products rich in polyphenols be advantageously used in the feeding and nutrition of dairy small ruminants? Animals 2020, 10, 131. [Google Scholar] [CrossRef]
- Jian, M.; Tao, L.; Lu, L.; Yuezhang, L.; Xi, C.; Sibing, L.; Chunmei, D.; Chen, W.; Fuquan, Y.; Shangquan, G. Effects of grape seed extract supplementation on the growth performance, nutrients digestion and immunity of weaned lambs. Front. Vet. Sci. 2024, 11, 1402637. [Google Scholar]
- European Food Safety Authority (EFSA). Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). Safety and efficacy of dry grape extract when used as a feed flavouring for all animal species and categories. EFSA 2016, 14, 4476. [Google Scholar]
- European Food Safety Authority (EFSA). Panel on Dietetic Products, Nutrition and Allergies (NDA). Safety of hydroxytyrosol as a novel food pursuant to Regulation (EC) No 258/97. EFSA 2017, 15, 4728. [Google Scholar]
- Giller, K.; Sinz, S.; Messadene-Chelali, J.; Marquardt, S. Maternal and direct dietary polyphenol supplementation affect growth, carcass and meat quality of sheep and goats. Animal 2021, 15, 100333. [Google Scholar] [CrossRef]
- Gómez, G.; Laviano, H.D.; García-Casco, J.M.; Escudero, R.; Muñoz, M.; Heras-Molina, A.; González-Bulnes, A.; Óvilo, C.; López-Bote, C.; Rey, A.I. Different effect of vitamin E or hydroxytyrosol supplementation to sow’s diet on oxidative status and performances of weaned piglets. Antioxidants 2023, 12, 1504. [Google Scholar] [CrossRef]
- Jefferies, B.C. Body condition scoring and its use in management. Tasman. J. Agric. 1961, 32, 19–21. [Google Scholar]
- NRC. Nutrient requirements of small ruminants: Sheep, goats, cervids, and new world camelids. In National Research Council, Committee on the Nutrient Requirements of Small Ruminants, Board on Agriculture, Division on Earth, and Life Studies NRC; The National Academic Press: Washington, DC, USA, 2007; pp. 244–270. [Google Scholar]
- Sales, F.; Peralta, O.A.; Narbona, E.; McCoard, S.; Lira, R.; De los Reyes, M.; González-Bulnes, A.; Parraguez, V.H. Maternal supplementation with antioxidant vitamins in sheep results in increased transfer to the fetus and improvement of fetal antioxidant status and development. Antioxidants 2019, 8, 59. [Google Scholar] [CrossRef]
- Russel, A.J.F. Means of assessing the adequacy of nutrition of pregnant ewes. Livest. Prod. Sci. 1984, 11, 429–436. [Google Scholar] [CrossRef]
- Pesántez-Pacheco, J.L.; Heras-Molina, A.; Torres-Rovira, L.; Sanz-Fernández, M.V.; García-Contreras, C.; Vázquez-Gómez, M.; Feyjoo, P.; Cáceres, E.; Frías-Mateo, M.; Hernández, F.; et al. Maternal metabolic demands caused by pregnancy and lactation: Association with productivity and offspring phenotype in high-yielding dairy ewes. Animals 2019, 9, 295. [Google Scholar] [CrossRef]
- Douglas, G.N.; Overton, T.R.; Bateman, H.G.; Dann, H.M.; Drackley, J.K. Prepartal plane of nutrition, regardless of dietary energy source, affects periparturient metabolism and dry matter intake in Holstein cows. J. Dairy Sci. 2006, 89, 2141–2157. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; McCutcheon, S.N.; Parker, W.J.; Walsh, P.A. Blood metabolite levels in late pregnant ewes as indicators of their nutritional status. N. Z. J. Agric. Res. 1990, 33, 63–68. [Google Scholar] [CrossRef]
- Harmeyer, J.; Schlumbohm, C. Pregnancy impairs ketone body disposal in late gestating ewes: Implications for onset of pregnancy toxaemia. Res. Vet. Sci. 2006, 81, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Cal-Pereyra, L.; Benech, A.; González-Montaña, J.R.; Acosta-Dibarrat, J.; Da Silva, S.; Martin, A. Changes in the metabolic profile of pregnant ewes to an acute feed restriction in late gestation. N. Z. Vet. J. 2015, 63, 141–146. [Google Scholar] [CrossRef]
- Durak, M.H.; Altiner, A. Effect of energy deficiency during late pregnancy in Chios ewes on free fatty acids, hydroxybutyrate and urea metabolites. Turkish J. Vet. Anim. Sci. 2006, 30, 497–502. [Google Scholar]
- Toschi, P.; Baratta, M. Ruminant placental adaptation in early maternal undernutrition: An overview. Front. Vet. Sci. 2021, 8, 755034. [Google Scholar] [CrossRef]
- Plante-Dubé, M.; Bourassa, R.; Luimes, P.; Buczinski, S.; Castonguay, F.; Gervais, R. Peripartum energy metabolism of prolific ewes and their progeny in response to prepartum feeding and litter size. Animals 2025, 19, 101382. [Google Scholar] [CrossRef]
- Gao, F.; Liu, Y.; Zhang, C.; Zhang, Z.; Song, S. Effect of intrauterine growth restriction during late pregnancy on the growth performance, blood components, immunity and anti-oxidation capability of ovine fetus. Livest. Sci. 2013, 155, 435–441. [Google Scholar] [CrossRef]
- Grzeszczak, K.; Åanocha-Arendarczyk, N.; Malinowski, W.; Ziƒôtek, P.; Kosik-Bogacka, D. Oxidative stress in pregnancy. Biomolecules 2023, 13, 1768. [Google Scholar] [CrossRef] [PubMed]
- Marseglia, L.; D’Angelo, G.; Manti, S.; Arrigo, T.; Barberi, I.; Reiter, R.J.; Gitto, E. Oxidative stress-mediated aging during the fetal and perinatal periods. Oxidative Med. Cell. Longev. 2014, 2024, 358375. [Google Scholar] [CrossRef]
- Gundersen, K.; Anas, M. Developmental programming and postnatal modulations of muscle development in ruminants. Biology 2025, 14, 929. [Google Scholar] [CrossRef] [PubMed]
- Chavatte-Palmer, P.; Dupont, C.; Debus, N.; Camous, S. Nutritional programming and the reproductive function of the offspring. Anim. Prod. Sci. 2014, 54, 1166–1176. [Google Scholar] [CrossRef]
- Yawno, T.; Sutherland, A.E.; Pham, Y.; Castillo-Melendez, M.; Jenkin, G.; Miller, S.L. Fetal growth restriction alters cerebellar development in fetal and neonatal sheep. Front. Physiol. 2019, 10, 560. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Nawito, M.F.; Hameed, A.R.; Sosa, A.S.; Mahmoud, K.G. Impact of pregnancy and nutrition on oxidant/antioxidant balance in sheep and goats reared in South Sinai, Egypt. Vet. World 2016, 9, 801–805. [Google Scholar] [CrossRef]
- Guimarães, L.J.; Da Silva, I.G.; Sena, G.C.; Lourenço, G.; Azoia, I.P.; Simonelli, S.M.; Rego, F.C.D.A.; Damasceno, D.C.; Zundt, M. Annatto concentrate reduces oxidative stress in ewes during metabolic challenge stages. Anim. Feed Sci. Technol. 2025, 325, 116353. [Google Scholar] [CrossRef]
- Castillo, C.; Hernández, J.; Valverde, I.; Pereira, V.; Sotillo, J.; Alonso, M.L.; Benedito, J.L. Plasma malonaldehyde (MDA) and total antioxidant status (TAS) during lactation in dairy cows. Res. Vet. Sci. 2006, 80, 133–139. [Google Scholar] [CrossRef]
- Osgerby, J.C.; Wathes, D.C.; Howard, D.; Gadd, T.S. The effect of maternal undernutrition on ovine fetal growth. Int. J. Endocrinol. 2002, 173, 131–141. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Wallace, J.M.; Spencer, T.E. Board-invited review: Intrauterine growth retardation: Implications for the animal sciences. J. Anim. Sci. 2006, 84, 2316–2337. [Google Scholar] [CrossRef]
- Freetly, H.C.; Leymaster, K.A. Relationship between litter birth weight and litter size in six breeds of sheep. J. Anim. Sci. 2004, 82, 612–618. [Google Scholar] [CrossRef]
- Wilson, C.S.; Cherry, N.L.; Taylor, J.B. Lamb survivability: A new approach to an old problem. Front. Anim. Sci. 2025, 6, 1497380. [Google Scholar] [CrossRef]
- Biri, A.; Bozkurt, N.; Turp, A.; Kavutcu, M.; Himmetoglu, O.; Durak, I. Role of oxidative stress in intrauterine growth restriction. Gynecol. Obstet. Investig. 2007, 64, 187–192. [Google Scholar] [CrossRef]
- Gupta, P.; Narang, M.; Banerjee, B.D.; Basu, S. Oxidative stress in term small for gestational age neonates born to undernourished mothers: A case control study. BMC Pediatr. 2004, 4, 14. [Google Scholar] [CrossRef]
- Kamath, U.; Rao, G.; Kamath, S.U.; Rai, L. Maternal and fetal indicators of oxidative stress during intrauterine growth retardation (IUGR). Indian J. Clin. Biochem. 2006, 21, 111–115. [Google Scholar] [CrossRef]
- Vazquez-Gomez, M.; Heras-Molina, A.; Garcia-Contreras, C.; Pesantez-Pacheco, J.L.; Torres-Rovira, L.; Martinez-Fernandez, B.; Gonzalez, J.; Encinas, T.; Astiz, S.; Ovilo, C.; et al. Polyphenols and IUGR pregnancies: Effects of maternal hydroxytyrosol supplementation on postnatal growth, metabolism and body composition of the offspring. Antioxidants 2019, 8, 535. [Google Scholar] [CrossRef]
- Besufkad, S.; Abebe, A.; Getachew, T.; Goshme, S.; Bisrat, A.; Abebe, A.; Zewdie, T.; Alemayehu, L.; Kebede, A.; Gizaw, S. Survival analysis of genetic and non-genetic factors influencing lamb survival of different sheep breeds. Small Rumin. Res. 2024, 232, 107206. [Google Scholar] [CrossRef]
- Qin, Q.; Dai, D.; Zhang, C.; Zhao, C.; Liu, Z.; Xu, X.; Lan, M.; Wang, Z.; Zhang, Y.; Su, R.; et al. Identification of body size characteristic points based on the Mask R-CNN and correlation with body weight in Ujumqin sheep. Front. Vet. Sci. 2022, 9, 995724. [Google Scholar] [CrossRef]
- Kessler, E.C.; Bruckmaier, R.M.; Gross, J.J. Immunoglobulin G content and colostrum composition of different goat and sheep breeds in Switzerland and Germany. J. Dairy Sci. 2019, 102, 5542–5549. [Google Scholar] [CrossRef]
- Turín, J.; Sales, F.; Peralta, O.A.; De los Reyes, M.; Borie, C.; Carrasco, A.; González-Bulnes, A.; Parraguez, V.H. Colostrum traits and newborn body weight and growth: Comparison between single and twin underfed sheep pregnancies. Front. Vet. Sci. 2023, 10, 1256989. [Google Scholar] [CrossRef]
- Abecia, J.A.; Garrido, C.; Gave, M.; García, A.I.; López, D.; Luis, S.; Valares, J.A.; Mata, L. Exogenous melatonin and male foetuses improve the quality of sheep colostrum. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1305–1309. [Google Scholar] [CrossRef]
- Mehmood, N.; Hussain, T.; Hameed, A.; Yaseen, A.; Ahmed, A.; Hussain, M.; Al Jbawi, E. Prospective effect of vitamin E and selenium supplementation on colostrum quality in Beetal goats. Cogent Food Agric. 2023, 9, 2197711. [Google Scholar] [CrossRef]
- Long, S.; Wu, D.; He, T.; Piao, X. Dietary supplementation with Forsythia suspensa extract during late gestation improves reproductive performance, colostrum composition, antioxidant status, immunoglobulin, and inflammatory cytokines in sows and newborn piglets. Anim. Feed Sci. Technol. 2021, 271, 114700. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).