There was a mistake in the published paper [1] in Figure 1A (under “MA + PTE,” Pterostilbene 100 mg qD for 3 weeks, “qD” should be “BID”). This error was inadvertently missed by all authors/editors.
The same change was made in the Graphical Abstract, so the Graphical Abstract has also been updated.
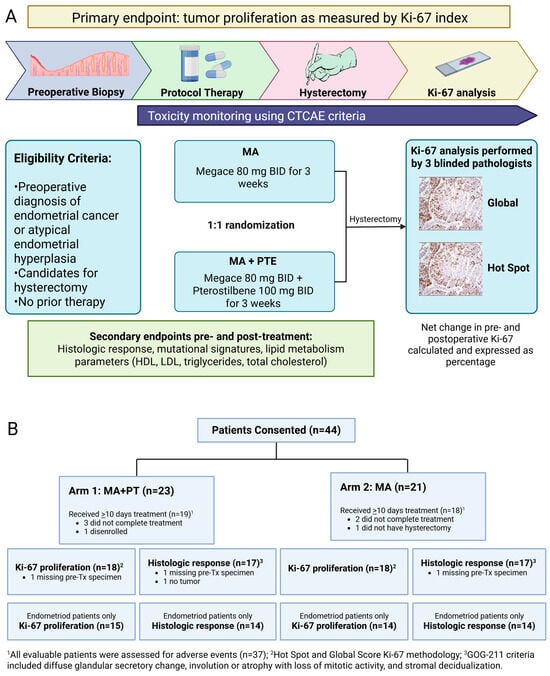
Figure 1.
(A) Study schema of phase II randomized 2-arm study with primary endpoint Ki-67 index. Baseline endometrial biopsy was obtained as block or slides from initial diagnosis, or as new office endometrial biopsy. Intra-operative endometrial biopsy at time of hysterectomy was obtained if feasible. Post-treatment endometrial tumor was also obtained as block/slides if surgery was performed at a hospital outside of COH. (B) Flow diagram showing patients included in the study grouped by Arm 1 (n = 19) and Arm 2 (n = 18). Seven patients were not evaluable (5 received <10 days of treatment, 1 did not have hysterectomy, and 1 disenrolled). AE, adverse event; MA, megestrol acetate; PT, pterostilbene; Tx, treatment.
Under Section 2. Materials and Methods, Study design, the authors added the dosage of “100 mg twice a day” after “oral PT”. This error was inadvertently missed by all authors/editors.
The paragraph should be read as follows:
Study Design: The planned accrual for this study was 36 patients (Arm 1, n = 18 and Arm 2, n = 18). Patients were randomized in a 1:1 ratio to either Arm 1 (oral PT 100 mg twice a day + MA twice a day for three weeks), or Arm 2 (oral MA twice a day for three weeks). Therapy continued in the absence of unacceptable toxicity or progression of disease.
Under Section 3.5, Biomarkers of Response by IHC, “progression/regression” needs to be changed to “estrogen receptor/progesterone receptor.” This error was inadvertently missed by all authors/editors.
The paragraph was also updated.
3.5. Biomarkers of Response by IHC
Molecular markers of all eligible patients (n = 37) were analyzed at the beginning and completion of treatment to evaluate cell cycle regulation and cell survival (CDK4, Cyclin D1, BCL2, p-STAT3, and p-ERK1/2) and estrogen receptor/progesterone receptor (ER, PR). The results showed no significant differences between Arm 1 (n = 19) and Arm 2 (n = 18).
The correction has been approved by the Academic Editor. These changes do not affect the conclusions of the paper.
Reference
- Senguttuvan, R.N.; Cho, H.; Wu, X.; Frankel, P.H.; Ruel, N.; Yost, S.E.; Kebria, M.; Han, E.; Song, M.; de Leon, M.; et al. The Anti-Cancer Role of Pterostilbene in Endometrial Cancer: A Phase II Prospective, Randomized, Window-of-Opportunity Clinical Trial with Megestrol Acetate. Antioxidants 2025, 14, 345. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).