Abstract
Developmental programming, shaped by environmental and lifestyle stressors during prenatal life, is increasingly recognized as a major contributor to non-communicable diseases (NCDs) later in life. Oxidative stress, one of key mechanisms linking these stressors to fetal metabolomic reprogramming and disease pathogenesis, leaves measurable metabolomic signatures that reflect disrupted redox balance. Alterations in glucose, lipid, and amino acid metabolism and antioxidant response could reveal the main pathways driving NCD development. This review summarizes epidemiological studies that have investigated biochemical responses of the prenatal exposure to metals, air pollution, and tobacco smoke and e-cigarette vapor in maternal–placental–fetal compartments using a metabolomic approach. Summarized studies indicate that maternal exposure to metals primarily disrupts amino acid pathways related to one-carbon metabolism, glutathione synthesis, and oxidative stress defense, while air pollution, particularly fine particulate matter, mainly affects lipid oxidation, fatty acid β-oxidation, and amino acid and carbohydrate metabolism. Tobacco smoke and e-cigarette vapor induce widespread disturbances involving reduced citric acid cycle intermediates, altered acylcarnitines and phospholipids, and impaired antioxidant capacity, collectively promoting oxidative damage and inflammatory signaling. The identification of these metabolome alterations might contribute to a deeper understanding of the toxicity and biological impact of environmental stressors on offspring health. These results may eventually lead to the identification of early biomarkers and to the development of therapeutic strategies aimed at reducing NCD risk.
1. Introduction
During pregnancy, the production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) naturally increases as a result of enhanced metabolism, heightened placental mitochondrial activity, and elevated oxygen consumption. In order to ensure optimal fetal growth and development, the maternal body undergoes systemic adaptations that prioritize nutrient delivery to the fetus through regulation of amino acid, lipid, and energy metabolism, contributing to elevated ROS/RNS generation [,,,]. This physiological rise in ROS and RNS plays a crucial role in signaling pathways that are essential for proper embryogenesis, placental development, and vascular remodeling [,,,,]. The maintenance of this sensitive dynamic redox balance is required to support healthy pregnancy. When the uncontrolled increase of ROS and RNS exceeds the capacity of antioxidant defense, oxidative damage of nucleic acids, lipids, and proteins can occur []. Such molecular damage contributes to dysfunction of placenta and related pregnancy complications such as fetal growth restriction, preterm birth, and small-for-gestational-age or large-for-gestational-age delivery [,,,,,]. Moreover, suboptimal fetal development may increase susceptibility to various diseases that may become evident later in life [,,,].
Exposure to environmental pollutants, including toxic metals, particulate matter, nitrogen oxides, and tobacco and nicotine-containing products, can further exacerbate oxidative stress and metabolic dysregulation in maternal–placental–fetal units, altering pathways such as lipid peroxidation, amino acid metabolism, and mitochondrial energy production, which are critical for nutrient transport and fetal growth [,,]. These metabolic perturbations reflect alterations in cellular homeostasis that can manifest as measurable changes in specific maternal and fetal metabolites, termed metabolome [,,]. As the metabolome reflects the end products of endogenous biological processes influenced by both genetic and environmental factors, it can serve as a valuable tool for investigating how various exposures contribute to diseases development. Metabolomics, the comprehensive analysis of these metabolites and their dynamic fluctuations within biological systems, enables detailed characterization of metabolic alterations at the time of sampling. This approach links environmental exposure to internal dose, physiological response, and the underlying mechanisms of disease development [,]. An overview of key metabolomics terminology is provided in the Glossary (Table A1) in Appendix A.
Recently, an increasing number of studies have investigated how environmental stressors modify alterations in the maternal–fetal metabolome during pregnancy. These studies often highlight changes in primary metabolic pathways, such as glycolysis, aerobic respiration, the citric acid cycle, fatty acid oxidation, and gluconeogenesis, which are essential for energy production and metabolic homeostasis. Disruption of these pathways may reflect dynamic interactions between cellular redox imbalance, mitochondrial dysfunction and external stressors, ultimately contributing to long-term disease risk []. Using different high-throughput analytical techniques such as NMR spectroscopy and mass spectrometry in a variety of biospecimens, metabolomics enables the identification and quantification of redox sensitive metabolites [,], providing a valuable insight into the biological impact of prenatal environmental exposures. This approach offers the potential to identify biomarkers indicative of oxidative damage and adaptive responses.
This narrative review aims to summarize recent epidemiological studies published since 2016 regarding the metabolomics response to prenatal exposure to metals, air pollution, and tobacco and nicotine-containing products in maternal–placental–fetal compartments. The primary focus is on exposure-related metabolome alterations related to oxidative stress pathways. A better understanding of these metabolomic responses may help clarify how environmental exposures to these pollutants disrupt metabolic homeostasis and contribute to oxidative stress-related disease mechanisms.
2. A Short Overview of Oxidative Stress in Normal (Healthy) Pregnancy
In a healthy pregnancy, oxidative stress results from increased metabolic demands and dynamic oxygen fluctuations that are essential for fetal development. During early gestation, the hypoxic environment of the placental bed supports embryogenesis and trophoblast invasion. At this stage, ROS generation is minimal to protect cells against teratogenic effects [,]. As pregnancy progresses, the development of uteroplacental circulation raises oxygen tension. This drives mitochondrial electron transport activity in placental cells and elevates ROS production []. The oxidative environment is counterbalanced by a coordinated activation of antioxidant defenses including glutathione (GSH), superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT). These antioxidants help neutralize excess ROS to prevent oxidative damage. At the same time, they allow ROS to continue their important roles in redox signaling processes essential for placental vascular remodeling, angiogenesis, and immune cells activation [,,].
The interplay between ROS and antioxidants evolves across trimesters, reflecting the metabolic adaptations that occur through gestation. During the second trimester, rising maternal oxygen consumption and lipid catabolism increase hydrogen peroxide generation. Insulin resistance and fatty acid mobilization further amplify ROS production during the third trimester [,]. Placental mitochondria generate superoxide anions that participate in signaling pathways necessary for vascular remodeling and syncytiotrophoblast differentiation [,]. Clinical studies demonstrate that physiological oxidative stress peaks near term, when placental tissue and maternal serum exhibit the highest levels of antioxidant enzymes [,]. This delicate balance ensures that ROS contribute to vital processes such as cervical ripening and prostaglandin synthesis without causing pathological inflammation or DNA damage []. However, environmental and lifestyle stressors can disrupt this balance.
3. Metabolic Pathways in Redox Regulation During Pregnancy
Pregnancy involves substantial metabolic adaptations in protein, carbohydrate, and lipid metabolism to meet the continuous demand for nutrients and other substrates essential for placental development and fetal growth. These changes are necessary for creating an optimal intrauterine environment while simultaneously supporting the increased physiological demands of the mother []. Tightly regulated changes in these pathways help maintain energy balance and redox homeostasis through gestation, as each plays a key role in both the generation and regulation of ROS.
Carbohydrate metabolism adapts by increasing maternal glucose production and inducing insulin resistance, which helps to direct more glucose to the fetus [,]. Dysregulation of glycolysis or mitochondrial dysfunction in trophoblasts can reduce ATP production, impair nutrient transport, and increase ROS production, contributing to conditions like gestational diabetes mellitus [], and preeclampsia []. Carbohydrate metabolism, including the pentose phosphate pathway, is also critical for generating NADPH that is essential for the regeneration of GSH and maintaining cellular redox balance [].
Lipids and fatty acids are sources of energy, structural components, and signaling molecules [,]. The lipid profile varies considerably throughout pregnancy, from lipid synthesis and fat storage in early pregnancy to lipolysis and fatty acid oxidation later, generating ATP, but also ROS, particularly in the presence of polyunsaturated fatty acids (PUFAs)like docosahexaenoic acid (DHA) and arachidonic acid (AA) [,,].
Amino acids are essential for protein synthesis and organogenesis, vascular development, and antioxidant defense with GSH, a tripeptide composed of cysteine, glycine, and glutamate, tryptophan, and arginine metabolites playing key roles in redox homeostasis [,,,].
Disruption of these pathways, whether by environmental pollutants or poor lifestyle habits, compromises antioxidant defenses and increases oxidative stress, contributing to placental dysfunction and pregnancy complications. The following sections explore how environmental stressors drive these metabolomic alterations, providing insights into potential biomarkers and intervention strategies to mitigate oxidative stress.
4. Environmental Pollutants and Their Effects on Metabolomic Profiles and Pathways During Pregnancy
There is a growing concern about the impact of environmental pollutants and lifestyle factors on pregnancy outcomes. Some of them can induce or mediate the formation of oxidative stress, thereby disrupting key metabolic pathways such as energy, carbohydrate, lipid, and amino acid metabolism in the maternal–placental–fetal unit [,,,], increasing susceptibility to risk of maternal and fetal complications [,]. Understanding the mechanisms by which environmental stressors lead to oxidative stress and affect metabolic pathways in mother and her offspring is crucial for elucidating how these stressors modulate maternal–child health.
4.1. Metal/Metalloid Exposure
Exposure to metals such as cadmium, lead, mercury, and metalloids such as arsenic remains a significant global health concern due to their persistent presence in the environment and their documented associations with numerous adverse health outcomes [,]. Pregnant women are primarily exposed through the consumption of contaminated food, inhalation of active and passive tobacco smoke, and, in the case of inorganic arsenic, the use of contaminated groundwater. These toxic elements tend to bioaccumulate in the maternal body over a lifespan, leading to systemic toxicity and inducing adverse health effects, even at low-level exposure within environmentally relevant concentrations. Lead, arsenic, and mercury readily cross the placenta, thereby increasing the fetal body burden, while cadmium tends to accumulate in the placenta with limited transfer to the fetus, impairing function and nutrient transport [,,,].
These prenatal exposures have been associated with redox imbalance [,,], impaired steroidogenesis [], epigenetic changes [,], and adverse outcomes for the mother and her child [,]. While the adverse effects of exposure to high levels of toxic metals are well recognized, the impact of low-level metal exposure, particularly during pregnancy, remains insufficiently understood. Recently, several studies investigated effects of low-level metal(loid) exposure during pregnancy on metabolome alterations in mothers and their offspring. Table S1 summarizes these studies.
4.1.1. Cadmium
Environmental cadmium exposure in Chinese pregnant women has been linked to alterations in urinary metabolites, indicating effects on oxidative stress and nephrotoxicity. A comparison of metabolomic profiles between women in the lowest and highest tertiles of urinary cadmium during the first trimester of pregnancy in Wuhan revealed increased levels of L-cystine, reflecting an adaptive upregulation of antioxidant defenses required for glutathione synthesis and elevated levels of L-tyrosine and its oxidized derivative dityrosine, indicating protein oxidation []. Decreased levels of histamine and uric acid were suggested to possibly reflect early nephrotoxic effects of cadmium, indicating alterations in purine metabolism and impaired renal reabsorption.
In another study of 103 pregnant women from Taiyuan, the combined evaluation of cadmium and zinc exposure identified 51 significantly differently expressed metabolites implicating alterations across several biochemical pathways []. Activation of amino acids pathways, including arginine and proline metabolism, important in nitric oxide production and redox balance, may link metal exposure to greater metabolic risks, such as obesity, hyperlipidemia, and hypertriglyceridemia. It was suggested that higher levels of bovinic acid, a conjugated linoleic acid known for its anti-inflammatory and anticancer properties, may be indicative of possible protective effects of zinc against oxidative stress and inflammation. Authors also highlighted octadecylamine, a long-chain fatty amine, as a potential biomarker for cadmium exposure and noted that its levels may decrease with zinc supplementation, possibly reflecting an antagonistic interaction between these metals [].
4.1.2. Lead
Metabolomic alterations associated with lead exposure were investigated in the Programming Research in Obesity, Growth, Environment, and Social Stressors (PROGRESS) cohort in Mexico City, Mexico, during third trimester of pregnancy []. Untargeted metabolomic analysis identified 31 serum metabolites significantly associated with blood lead levels, demonstrating significant effects of short-term lead exposure on metabolites classified as fatty acids and conjugates, amino acids and peptides, and purines. Changes in 2-hydroxybutyrate and metabolites from the alpha-linoleic and linoleic acid pathways point to possible links between recent lead exposure, disrupted glucose metabolism, increased risk for gestational diabetes, and potential effects on the maternal gut microbiome. Elevated bone (patella and tibia) lead in this study [] was associated with higher betaine levels, suggesting an influence on methylation and epigenetic regulation. These findings highlight multiple pathways through which both acute and chronic lead exposure may adversely affect maternal and fetal health.
4.1.3. Arsenic
Studies in pregnant women from China revealed alterations in urinary metabolomic profiles associated with low-level arsenic exposure, reflecting oxidative stress and potential liver and kidney metabolic disturbances [,]. A comparison of metabolomic profiles between women in the lowest and highest tertiles of urinary arsenic identified significant changes []. Elevated levels of two metabolites indicated enhanced leukotriene E4 (LTE4) production via the arachidonic acid pathway, while increased thiocysteine suggested dysregulation of thiol redox metabolism, consistent with the known ability of arsenic to bind to sulfhydryl groups, impairing protein folding and amplifying oxidative stress []. On the other hand, several antioxidant metabolites such as glutathione, cystathionine ketimine and 1-(beta-d-ribofuranosyl)-1,4-dihydronicotinamide were significantly decreased, probably due to their consumption in response to elevated ROS. Additionally, decreased LysoPC (14:0) and increased p-cresol glucuronide and vanillactic acid may reflect arsenic-induced metabolic dysfunction in the liver and kidneys [].
Metabolic alterations associated with urinary arsenic species and their potential link to gestational diabetes mellitus (GDM) were examined using the meet-in-metabolite analysis (MIMA) strategy []. Key joint metabolites mediating these associations were thiosulfate and phosphoroselenoic acid, which are both essential for cysteine/selenocysteine biosynthesis, and pyridoxamine 5′-phosphate, an active form of vitamin B6. These metabolites are involved in purine and amino acid metabolism, one-carbon metabolism (OCM), and glycometabolism, related to antioxidant defense and arsenic methylation, which transforms inorganic arsenic into less toxic species, facilitating excretion and reducing toxicity []. The authors reported that a positive association of As5+ with these potentially “preventive” (protective) metabolites suggests As5+ may help attenuate the risk of GDM, while As3+ appears to contribute to GDM risk by impairing glucose regulation and disrupting purine metabolism and OCM pathways [].
The impact of prenatal exposure to inorganic arsenic (iAs) on neonatal metabolite alterations was investigated in the Biomarkers of Exposure to ARsenic (BEAR) pregnancy cohort in Gómez Palacio, Mexico, by analyzing the cord serum metabolome of 50 neonates []. Multivariate analyses identified 10 cord serum metabolites significantly associated with maternal urinary levels of iAs and/or its metabolites, and 17 cord serum metabolites significantly associated with iAs and/or its metabolites in cord serum. Eight metabolites were significantly associated with both maternal exposure and biotransformation indicators in urine and neonatal cord serum measures of iAs and its metabolites, including glutamate, glycine, isoleucine, mannose, methionine, O-acethylcholine, taurine, and tyrosine. These metabolites demonstrated that prenatal arsenic exposure perturbs metabolism of amino acids, vitamin B6, and the citric acid cycle. For example, elevated maternal urinary arsenic correlated with disrupted energy metabolism (e.g., increased mannose and succinate) and amino acid metabolism (e.g., decreased tyrosine and isoleucine). Similarly, higher total arsenic in cord serum was significantly correlated with increased mannose, and decreased glutamate, isoleucine, methionine, O-acetylcholine and tyrosine, further implicating adverse effects of prenatal arsenic exposure on neonatal energy and amino acid metabolism.
4.1.4. Metallomics—Effects of Metal Mixtures on the Metabolome
Metallomics, which examines multiple metals and metalloids simultaneously, offers a more realistic assessment of maternal exposure because pregnant women are usually exposed to complex metal mixtures rather than single elements. Since metals can interact with each other in synergistic, additive, or antagonistic ways [], analyzing mixtures allows researchers to detect both cumulative and specific effects that may be overlooked in single-metal studies []. Recent metabolomics studies support this approach, linking metal mixtures to alterations in metabolic networks relevant to fetal development and maternal health.
A metabolome-wide association study (MWAS) within the Boston Birth Cohort (MA, USA) evaluated the impact of in utero exposure to lead, mercury, cadmium, selenium, and manganese on fetal metabolic programming []. Data from 670 mother–child pairs identified 25 cord blood metabolites, including xenobiotics, lipids, amino acids, and nucleotides that were significantly associated with at least one of these metals measured in maternal red blood cells (RBCs). The strong positive association between cadmium and nicotine metabolites, cotinine, and hydroxycotinine confirmed existing evidence identifying tobacco smoke as a major source of cadmium exposure. An inverse association between lead and piperine, a major component of black pepper with demonstrated antioxidant properties, indicates reduced antioxidant capacity and enhanced oxidative damage, consistent with experimental findings that piperine reduces insulin resistance, inflammation, and oxidative stress []. Mercury exposure was significantly associated with lipid metabolites in both positive and inverse directions, consistent with mercury’s suspected cardiotoxic effects mediated through inflammation, oxidative stress, and endothelial dysfunction [,].
Another US-based study performed within a subset of the Puerto Rico Testsite for Exploring Contamination Threats (PROTECT) cohort investigated association between maternal blood concentrations of multiple metals/metalloids and alterations in plasma lipidomic profiles in 83 pregnant women, of whom 23 experienced preterm birth []. Higher maternal manganese and zinc showed an inverse association with plasmenyl-phosphatidylethanolamine (PLPE), particularly species containing polyunsaturated fatty acids (PUFA) chains, while arsenic and mercury showed positive associations with PLPE and plasmenyl-phosphatidylcholine (PLPC), respectively. These findings are somewhat unexpected, given that PLPE and PLCE are key components of cell membranes with proposed antioxidant and anti-inflammatory functions. The authors note that the mechanisms underlying these associations remain unclear. However, they may reflect compensatory metabolic responses to metal-induced oxidative stress rather than direct protective effects, particularly in the case of arsenic and mercury. It is also important to note that this was a relatively small study, and samples were collected during the late second trimester (at approximately 26th weeks of gestation), when decomposition of lipid storage may occur, which may affect the observed lipidomic patterns.
Further study performed in the Nanjing cohort (China) related maternal metal exposure during the second trimester to birth outcomes in newborns []. Arsenic exposure was associated with reduced birth weight and shorter gestational age, whereas mercury exposure correlated with increased birth weight and longer gestation. Metabolomic analyses identified 55 metabolites significantly associated with exposure to arsenic, mercury, cadmium, and cobalt, implicating disruption in lipid, carbohydrate, and amino acid metabolism. Key altered metabolites included decreased levels of 2-hydroxycaproic acid and 7b-hydroxycholesterol (lipid metabolism), lower rhamnose and succinic acid (carbohydrate metabolism), and changes in amino acid metabolites such as 3-methyladenine, which was found to mediate the negative effects of arsenic on birth weight.
In addition, prenatal exposure to metals has been linked to impaired neurodevelopment in children. In a large Wuhan Healthy Baby Cohort (N = 1088), Wuhan, China, maternal urinary levels of eleven metals including manganese, nickel, aluminum, lead, cadmium, and arsenic measured in early pregnancy were associated with cognitive scores []. Metabolomics analysis identified perturbations in amino acid metabolism (particularly beta-alanine and histidine pathways), riboflavin metabolism, purine and pyrimidine metabolism, and lipid metabolism including phospholipids and sphingolipids. These pathways mediated the link between prenatal metal exposure and reduced neurodevelopmental scores, implicating oxidative stress and disrupted energy metabolism as key mechanisms. Carnosine, a neuroprotective dipeptide with antioxidant properties, and glutamine, critical for neurotransmitter cycling and ammonia homeostasis, were among key metabolites mediating neurotoxic effects. Additionally, enhanced riboflavin metabolism may partially mitigate cognitive impairments, reflecting compensatory antioxidant responses.
Similarly, studies in the PHIME and HERACLES European cohorts [] demonstrated that prenatal exposure to metals, including mercury, lead, arsenic, manganese, and cadmium, perturbed mitochondrial respiration-related pathways such as glycolysis, gluconeogenesis, and the urea cycle. Dysregulation of amino acid metabolism, including pathways involved in neurotransmitter and nitric oxide synthesis (arginine, proline, histidine), as well as lipid metabolism pathways related to membrane integrity and myelination, were also identified. Dietary antioxidants such as lycopene from tomatoes and omega-3 fatty acids from fish appeared to mitigate these impacts.
Trimester-specific changes in metal exposure and amino acid metabolism were investigated in a longitudinal cohort of 232 healthy pregnant women from Wuhan, China []. The results demonstrated significant associations between urinary levels of seven metals (cadmium, cobalt, copper, cesium, manganese, thallium, and vanadium) and intermediates of arginine and proline, tryptophan, tyrosine, and lysine metabolism. Cadmium showed significant positive correlation with 2-Oxoarginine, N2-Succinyl-L-glutamic acid 5-semialdehyde, and N-Succinyl-L,L-2,6-diaminopimelate, and negative association with creatinine, indole, and N-Methyltryptamine. These findings underline oxidative stress and mitochondrial dysfunction as central mechanisms by which prenatal metal exposures impair child neurodevelopment, mediated through alterations in amino acid, lipid, carbohydrate, and energy metabolism detectable via metabolomics in urine.
Finally, a two-year prospective study from Nanjing, China, investigated the impact of prenatal exposure to multiple metals on the metabolic profile of pregnant women and examined how these exposures relate to the timing of primary tooth eruption and the number of teeth erupted by age one in their infants []. Higher maternal urinary cobalt concentrations were significantly associated with later eruption of primary teeth and a reduced number of teeth at age one. Using MWAS approach, glycine was identified as the key metabolite mediating these effects. Glycine, a non-essential amino acid with known anti-inflammatory and antioxidant properties, is essential for the formation of dentin and enamel. Thus, authors suggested that decreased glycine associated with elevated cobalt exposure may impair teeth development. Metabolomic pathway analysis supported perturbations in amino acid metabolism (particularly glycine, serine, and threonine metabolism), energy metabolism via citric acid cycle, and other pathways related to oxidative stress defense. Although cobalt is essential for human health, these findings highlight the importance of controlling cobalt exposure during pregnancy, particularly in regions where industrial activities such as smelting operations and widespread use of cobalt-containing compounds contribute to increased maternal exposure.
4.2. Air Pollution
Air pollution is a complex mixture of solid particles, liquid droplets, and gases suspended in the atmosphere. Air pollutants such as particulate matter (PM), carbon monoxide (CO), nitrogen oxides (NOx), ozone (O3), and sulfur dioxide (SO2) have been most strongly linked to public health risks. Variability in particle size, surface area, chemical composition, and presence of redox-active compounds contributes to differences in oxidative potential of air pollutants and their ability to induce oxidative stress and inflammation [,,]. A major source of urban air pollution is traffic related air pollution (TRAP), a complex mix of gaseous and particulate matter emissions from motor vehicles, and the wear of brakes and tires. The following paragraphs and Table S2 summarize published findings on air pollution-associated metabolomic alterations in lipid, carbohydrate, energy, and amino acid metabolism, with a particular focus on pathways linked to oxidative stress and its potential impacts during pregnancy.
Several studies suggest that PM2.5 exposure during pregnancy disrupts energy metabolism, contributing to adverse birth outcomes, particularly preterm birth. In 52 pregnant women from Fuzhou, Fujian Province, China [], relatively low-level PM2.5 exposure during early pregnancy was associated with mitochondrial dysfunction via oxidative phosphorylation pathway, implicating impaired energy metabolism, increased inflammatory responses and preterm birth risk. Similar findings were reported among African American women [], with increased maternal serum levels of carnitine and adenosine triphosphate (ATP), and decreased levels of adenosine, along with disruptions in aromatic amino acid metabolism, affecting neurotransmitter synthesis, redox homeostasis, and immune regulation []. A positive association with lysophosphatidylethanolamine (lysoPE) (20:3) further indicated adverse effects on cell membrane integrity, lipid signaling and immune response [].
Consistently, the Shanghai Maternal-Child Pairs Cohort prospective study identified the second and third trimesters as critical windows for PM2.5-induced placental toxicity []. Elevated PM2.5 exposure in non-smoking pregnant women were linked to fatty acids, bile acids, carbohydrate, and amino acids, reflecting compromised energy supply, oxidative stress and inflammation that impair placental and fetal development. All these findings suggest that PM2.5-induced metabolic and inflammatory disruptions are critical contributors to risks of premature delivery.
Research conducted in regions with high air pollution exposure has further investigated its effects on maternal and fetal health. In 160 mothers from California’s Central Valley, USA [], first trimester traffic-related exposure to CO, NOx, and PM2.5 was associated with widespread serum metabolome disruption in mid-pregnancy. Air pollution exposure correlated with decreased levels of fatty acids such as linoleic acid and heptadecanoic acid, and amino acids such as methionine, cysteine, L-histidine, and serine. Pathway enrichment analysis revealed substantial alterations in lipid, eicosanoid, and carbohydrate metabolism, including glycolysis, gluconeogenesis, and the citric acid cycle.
Another study conducted in the same region further explored whether the impact of air pollution exposures on the maternal serum metabolome differs in mothers whose children later develop autism spectrum disorders (ASD) []. Compared to controls, mothers of ASD children had higher hypotaurine and lower valine and isoleucine intermediates, lysine, phenylalanine, and asparagine, which dysregulation may reflect increased oxidative stress and inflammatory response [,]. Impairment of lipid metabolism included changes in fatty acid metabolism, carnitine shuttle and bile acid synthesis, pathways crucial for mitochondrial production of energy. In addition, changes in glycolysis, gluconeogenesis, fructose and mannose, galactose and pyruvate metabolism, and the pentose phosphate pathway, indicated impaired energy production and redox balance. Increased levels of uric acid in mothers with ASD children further suggested a pro-inflammatory and oxidative environment potentially contributing to ASD development.
In addition, a study performed in Mexican cohort exposed to high ambient PM2.5 during early to mid-pregnancy revealed increased bile acids and amino acids, decreased fatty acids (including linoleic acid), and dysregulated glycerophospholipids, indicating disrupted adipogenesis, peroxisome proliferator-activated receptor (PPAR)-α signaling, glucagon-like peptide-1 (GLP-1), and incretin hormone pathways []. These changes suggested impaired energy metabolism, fatty acid oxidation, and glycemic control, likely originating from pregnancy-related inflammatory and metabolic adaptations.
Further studies show that maternal exposure to PM2.5 during mid-pregnancy disrupts metabolic and lipid pathways, particularly in women with overweight or hyperlipidemia. The Maternal and Developmental Risks from Environmental and Social Stressors (MADRES) pregnancy cohort study linked PM2.5 exposure during the second trimester to disruption in vitamin A and D3, tyrosine, pyrimidine and ascorbate-aldarate pathways, bile acid and C-21 steroid hormone biosynthesis (e.g., progesterone depletion) [], contributing to placental dysfunction and fetal growth restriction. Authors suggested that overweight or obese women may be at greater risk to metabolic disturbances due to pre-existing redox imbalances.
Similarly, a lipidomic study in mother–newborn pairs from Beijing cohort, China [], found that maternal PM2.5 exposure during the second trimester increased cord serum levels of several lipid species and inflammatory adipokines (e.g., angiopoietin-like 4 (ANGPTL4), insulin-like growth factor binding-protein 2 (IGFBP), interleukin IL-12p40, and tumor necrosis factor receptor II (NRF-RII)) in hyperlipidemic mothers. Perturbations in phosphatidylcholines, triacylglycerides, and phosphatidylinositols created a pro-oxidant environment linked to increasing risk of adverse birth outcomes and cardiovascular disease development in later life.
A neonatal metabolomics study in California, USA, further demonstrated disruptions in lipid metabolism pathways associated with maternal PM2.5 exposure during the third trimester []. Using neonatal blood spots, the study identified consistently affected pathways including fatty acid activation, biosynthesis, and metabolism, the carnitine shuttle, and glycerophospholipid metabolism. These disruptions point to the impairment of cell membrane integrity and energy production [,]. Elevated pro-inflammatory arachidonic acid (precursor to prostaglandin and leukotriene), and decreased anti-inflammatory linolenic acid reflected disrupted inflammatory signaling and redox balance, contributing to abnormal placental and uterine blood flow []. Alterations in metabolism of sulfur-containing amino acids methionine and cysteine further suggested impaired methylation and antioxidant defense.
Recent studies by Holzhausen et al. [,] identified distinct metabolic signatures of prenatal and early-life exposure to ambient air pollutants, particularly traffic-related PM10, PM2.5, NOx, and O3 in minority populations. In the Southern California Mother’s Milk Study (Latino cohort) [], prenatal exposure was significantly associated with changes in vitamin B6, pyridine, tyrosine, fatty acid, and nucleotide metabolism at one month of age, while postnatal exposures affected histidine, bile acid, and energy metabolism throughout infancy showing sex-specific differences. Pathway enrichment analyses consistently implicated disturbances in amino acid metabolism, including cysteine, methionine, histidine, and tryptophan pathways, as well as lipid metabolic pathways such as fatty acid activation, biosynthesis, and glycerophospholipid metabolism. A complementary multicohort analysis of the Atlanta African American mother–newborn pairs (N = 205) and the Latino mother–child pairs (N = 122) found that higher maternal NOx exposure during pregnancy led to significant metabolic alterations in amino acid metabolism (e.g., isoleucine, phenylalanine, histidine, asparagine, and glutamic acid), lipid metabolism (including stearidonic acid, and sphinganine), and carbohydrate metabolism in neonates and infants []. Pathway analyses across both cohorts indicated a central role for methionine, cysteine, and tryptophan metabolism that are involved in redox-sensitive processes, and linked these alterations to oxidative stress and inflammatory pathways.
Together, these studies provide evidence that prenatal and early-life exposure to air pollutants, particularly components of traffic-related air pollution, induces persistent disturbances in metabolic pathways essential for maintaining redox balance, energy homeostasis, and cellular defense mechanisms during early development.
4.3. Exposure to Tobacco Smoke and E-Cigarette Vapor
Tobacco smoke is a complex aerosol mixture containing over 9500 chemicals, including thousands of toxic compounds such as nicotine, polycyclic aromatic hydrocarbons, volatile organic compounds, metals like cadmium and lead, nitrosamines, numerous oxygen-containing compounds, and free radicals [,]. These constituents contribute to the generation of ROS, and exacerbate oxidative stress in both pregnant women and their offspring by overwhelming endogenous antioxidant defenses, including key enzymes like superoxide dismutase and glutathione peroxidase [,]. This imbalance disrupts redox homeostasis and damages lipids, proteins, and DNA. Maternal tobacco smoking during pregnancy significantly increases the exposure of both mother and fetus to these toxic substances, many of which readily cross the placental barrier, alter steroid hormone levels [], and directly affect placental and fetal development. Epidemiological and experimental studies link in utero and early-life tobacco smoke exposure to fetal growth restriction, preterm birth, and increased risk of metabolic, respiratory, cardiovascular, and neurodevelopmental disorders later in life [,]. Furthermore, second-hand tobacco smoke exposure during pregnancy has also been associated with adverse maternal and fetal health outcomes, underscoring the importance of comprehensive exposure assessment. Moreover, the increasing use of e-cigarettes and other electronic nicotine delivery systems has raised concerns regarding their potential health risks, particularly to vulnerable populations [,]. Despite perceptions that e-cigarettes are a safer alternative to conventional cigarettes, their aerosols contain nicotine as well as numerous toxic substances that may have adverse effects on health [,]. Recent evidence suggests that even secondhand exposure to e-cigarette vapor presents serious developmental and metabolic risks during critical windows such as pregnancy. Several metabolomic studies have investigated the metabolic perturbations associated with prenatal tobacco smoke exposure, highlighting alterations in lipid, carbohydrate, energy, and amino acid metabolism linked to oxidative stress pathways. Table S3 summarizes these studies.
In the Generation R Study cohort in Rotterdam, the Netherlands, cord blood metabolite profiles from neonates exposed to maternal smoking showed decreased levels of mono-unsaturated acyl-alkyl-phosphatidylcholines and lysophosphatidylcholines, implicating altered lipid metabolism []. Metabolic adaptations differed according to the duration of tobacco exposure, with the first trimester smoking only affecting neonatal metabolites involved in the citric acid cycle and oxidative stress pathways, whereas continuous smoking throughout pregnancy was linked to increased inflammation responses, activation of transulfuration pathway, and insulin resistance. Sex-specific differences in neonatal metabolic response to tobacco exposure were also noted, particularly affecting lipid metabolites in girls.
Similarly, targeted analysis in two multiethnic maternal–child cohorts from Tennessee, USA, the Pregnancy Risk Assessment Monitoring System (PRAMS, N = 8600) and the Infant Susceptibility to Pulmonary Infections and Asthma following RSV Exposure (INSPIRE, N = 1918), showed that smoking during third trimester was associated with higher concentrations of free carnitine, glycine, and leucine in newborns of smoking mothers then in control []. Smoking cessation during pregnancy contributed to lower metabolite concentrations, comparable to those observed in newborns of non-smoking mothers, suggesting reversibility of metabolic perturbations.
Assessment of tobacco exposure via nicotine-related metabolite measurements directly in biological samples in diverse cohorts has consistently revealed disruptions in metabolic pathways essential for urea cycle and citric acid cycle linking lipid, carbohydrate, amino acid, and energy metabolism to the toxic effects of tobacco smoke [,,,]. The European LINA (Lifestyle and Environmental Factors and their Influence on Newborn Allergy risk) study in 35 mother–child pairs from Leipzig, Germany, showed that maternal smoking, confirmed through questionnaires and urinary biomarkers resulted with opposite effects on lipid and amino acid metabolism in mothers and newborns []. Metabolites involved in lipid metabolism such as diacyl- and acyl-ether phosphatidylcholines and sphingomyelins were downregulated in smoking mothers and upregulated in their newborns, except acylcarnitines, which were decreased in exposed newborns. Amino acid metabolism was increased in smoking mothers but decreased in exposed newborns, particularly for glutamine and glycine. Decreased lipid-soluble antioxidant capacity in the serum of smoking mothers, indicated increased oxidative damage related to tobacco smoke.
Similarly, a study from Greenwood, SC, USA, used second-trimester maternal serum cotinine as a measure of low-level nicotine exposure to assess metabolic profiles in 65 mother–child pairs from karyotypically normal pregnancies []. The results demonstrated significant metabolic perturbations in amniotic fluid with dysregulated pathways including aspartate and asparagine metabolism, pyrimidine metabolism, urea/amino group metabolism, and arginine and proline metabolism. Additionally, analysis of maternal serum identified leukotriene metabolism, linoleates, and eicosapentaenoic acid derived metabolites as the most affected pathways, reflecting smoking-related increased activation of inflammatory processes. The study found that even low-level recent nicotine exposure, indicated by maternal serum cotinine levels below 2 ng/mL, was associated with distinct perturbations in both maternal and fetal metabolomes supporting the role of smoking in promotion of oxidative stress and inflammation.
In the African American cohort [], metabolomic analysis of maternal serum (N = 105) confirmed perturbations in 47 metabolites that closely relate maternal urinary cotinine levels with preterm birth, and shorter gestational age during early (8–14 weeks gestation) and late (24–30 weeks gestation) pregnancy. Lipid metabolism showed significant alterations in fatty acid and glycerophospholipid pathways. Cotinine was positively associated with proinflammatory lysophosphatidylcholines (LysoPCs), as well as with 17-hydroxyprogesterone and acylcarnitine, involved in fatty acid oxidation, which indicated disrupted energy production and lipid metabolism in smokers. A positive association was found with glutamate, serine, choline, and taurine, amino acids that play roles in regulating oxidative stress, inflammation, and vascular function [,,]. Cotinine-related changes in carbohydrate metabolism, including glucose-6-phosphate, suggest that tobacco smoke may impair glucose metabolism and tolerance during pregnancy, contributing to adverse outcomes [].
A large population-based untargeted MWAS of newborn dried blood spots (N = 899) in California, USA, also linked maternal perinatal smoking, defined by self-report, and cotinine/hydroxycotinine levels in newborn blood to widespread perturbations in metabolomic profiles []. Key affected metabolic pathways included vitamin A (retinol) metabolism, tryptophan and kynurenine metabolism, and arachidonic acid metabolism. Disruption of these pathways was associated with inflammatory responses, oxidative stress, and biological mechanisms implicated in chronic diseases of the lung and central nervous system [,].
Extending concerns about tobacco smoke, recent study has also investigated an impact of second-hand exposure to e-cigarettes vapors on maternal metabolome alterations in an ongoing prospective longitudinal cohort, the New York University Children’s Health and Environment Study (NYU CHES) []. This small sample-size preliminary study, with exposure assessed via self-report of “living with someone who smoked e-cigarettes during pregnancy” revealed significant differences in maternal urinary metabolic profiles between exposed and unexposed groups, including alterations in lipid, fatty acid, and amino acid metabolism. Several lipid-related metabolites, such as palmitamide, glycerol trihexanoate, 2,4-undecadiene-8,10-diynoic acid isobutylamide were enriched in the exposed group, whereas levels of 1,2,3-propanetriol triacetate was lower compared to control group. Altered levels of sedoheptulose-7-phosphate, a metabolite in the pentose phosphate pathway essential for NADPH generation and redox homeostasis, indicate disruption in energy production and potential oxidative stress. Moreover, amino acid metabolism was also perturbed, as indicated by altered levels of N-acetylputrescine, and tryptophan pathway metabolites 5-methoxytryptophan and 5-hydroxy-L-tryptophan. Despite its limitations, this study highlights the importance of protecting pregnant women and other vulnerable groups from exposure to tobacco products, even in indirect, second-hand contexts.
In summary, all these findings highlight that environmental pollutants, including metals, air pollution, and tobacco smoke and e-cigarette vapor, disrupt key metabolic pathways involving carbohydrates, lipids, and amino acids. These disruptions often lead to mitochondrial dysfunction and oxidative stress, which may mediate their adverse effects on maternal and fetal health. A schematic overview of key metabolic perturbations linked to pollutant exposure during pregnancy is illustrated in Figure 1.
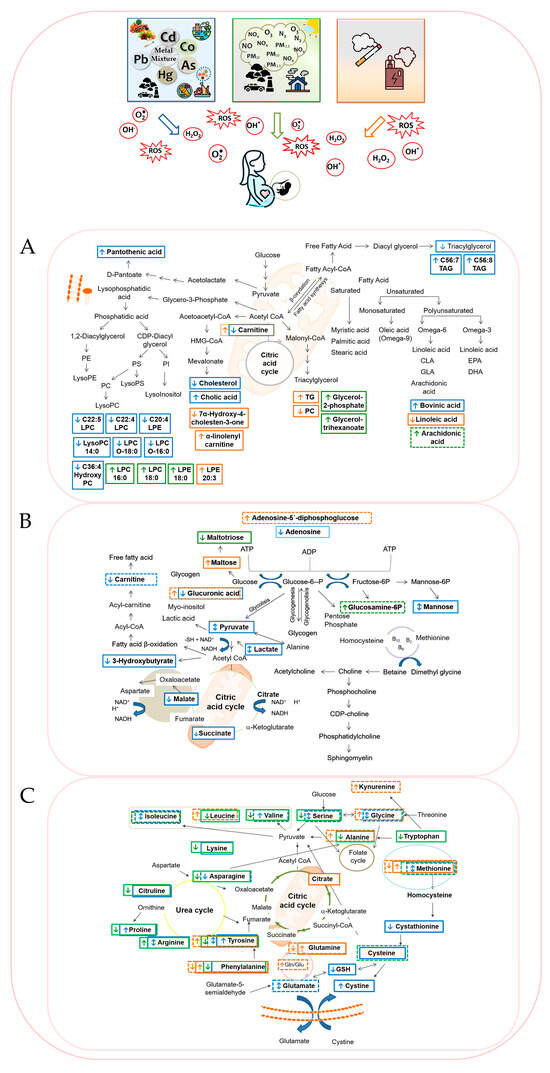
Figure 1.
Integrated schematic overview of pollutant-related disruptions in (A). lipid, (B). carbohydrate, and (C). amino acid metabolism linked to oxidative stress. Exposure to metals/metalloid (blue), air pollution (green), and cigarette smoke/e-cigarette vapor (orange) are shown with their corresponding increases or decreases in specific metabolites. Maternal metabolome changes are marked with solid-line rectangles and offspring changes with dashed-line rectangles.
5. Environmental Exposures and Metabolomic Disruptions in Maternal and Offspring Health
Maternal and fetal metabolism play a central role in maintaining health during pregnancy, operating as an integral network where redox balance, energy production, inflammation, hormonal signaling, and biosynthesis are intricately linked. Environmental pollutants have the potential to disrupt this delicate metabolic balance, as shown by metabolomic studies examining effects of toxic metals, air pollutants, and tobacco smoke and e-cigarette vapor. Maternal metabolic alterations, whether induced by metal mixtures, airborne pollutants, or nicotine, have direct consequences on fetal development. These changes alter nutrient transport, hormone levels (e.g., progesterone, bile acids, vitamins), and placental function, resulting in reduced fetal nutrient availability and compromised intrauterine growth [,]. It has been indicated that environmental exposure to toxic metals, air pollutants, and tobacco smoke commonly interferes with the body’s ability to control oxidative stress, regulate energy metabolism, and maintain optimal levels of amino acids, lipids, and carbohydrates [,,].
Metals and metalloids like lead, cadmium, mercury, and arsenic have been shown in cohort and cross-sectional studies to disrupt key metabolic pathways related to amino acid metabolism (particularly arginine, valine, and methionine), lipid peroxidation, energy metabolism, and oxidative stress (Figure 1). These disruptions may be explained by the high affinity of these metals for sulfhydryl groups in amino acids, enzymes, and other biomolecules, which can impair protein function, enzyme activity, and antioxidant defenses [,]. Observed molecular perturbations have linked metal exposure in utero to impaired placental function, fetal growth restriction, and impaired neurodevelopment [,,,].
Similarly, air pollution exposure studies have documented metabolic alterations associated with PM2.5, NOx, and O3 with pathways involving oxidative phosphorylation, glutathione metabolism, and amino acid metabolism frequently affected [,] (Figure 1). Maternal serum and cord blood metabolomics reveal disruptions in lipid signaling molecules such as lysophosphatidylethanolamines and bile acids, implicating inflammation and oxidative stress in observed pregnancy complications such as low birth weight, small-for-gestational-age, and preterm birth [,,,]. These metabolic alterations likely reflect mitochondrial dysfunction and impaired antioxidant defenses [].
In tobacco smoke exposure, metabolomic studies in maternal serum and neonatal blood spots show activation of detoxification pathways, increased oxidative stress markers, and alterations in carnitine and fatty acid metabolism [,,] (Figure 1). Such perturbations correspond with risks for birth weight reductions and altered neonatal neurobehavioral outcomes []. In addition, nicotine exposure during gestation disrupts metabolic profiles in amniotic fluid associated with energy and amino acid pathways indicative of oxidative damage outcomes []. Emerging evidence also suggests that second-hand exposure to e-cigarette vapor may induce similar metabolic disruptions in homeostasis of redox-related metabolites in pregnant women, underscoring the need to further investigate the effects of presumed low-risk nicotine products [].
Exposure-specific metabolite signatures, such as perturbed purine metabolism from metal exposure [,,], disrupted bile acid and phospholipid profiles from PM2.5 [,], or altered tryptophan-kynurenine metabolism from tobacco smoke [,] underscore the mechanistic diversity of toxicant effects. These observed metabolomic alterations contribute to adverse pregnancy outcomes by inducing oxidative stress-mediated damage. Oxidative stress has been indicated as a key mechanism by which environmental pollutants disrupt placental nutrient and oxygen transfer, mitochondrial activity, and fetal metabolic programming. Disrupted amino acid metabolism and lipid peroxidation products reflect cellular injury and energy imbalance, potentially contributing to growth restrictions and neurodevelopmental impairments. Moreover, epigenetic modifications influenced by these metabolic changes may underlie the developmental origins of adult diseases. To illustrate these complex mechanisms, Table 1 summarizes the predominant metabolic pathways affected and associated health risks in mothers and offspring.

Table 1.
Main metabolic pathways affected by metal, air pollution, tobacco smoke and e-cigarette vapor exposure during pregnancy.
6. Strengths, Current Gaps and Challenges
Metabolomics enables comprehensive characterization of small-molecule metabolites that reflect physiological and pathological states in the body [,]. In toxicology, metabolomics facilitates the elucidation of mechanistic pathways through which pollutants induce biological effects, such as oxidative stress, inflammation, and metabolic dysregulation, particularly during sensitive windows like pregnancy [,].
Recent advances in high-resolution metabolomics have enabled the simultaneous measurement of thousands of metabolic features, providing a comprehensive view of the biochemical pathways affected by maternal environmental exposures. Both untargeted and targeted mass spectrometry-based approach have been widely applied to identify alterations in lipid, carbohydrate, energy, and amino acid metabolism, many of which are associated with oxidative stress processes. The integration of MWAS approach with pathway enrichment, bioinformatics tools, and existing biological knowledge has substantially advanced our understanding of exposure-related perturbations.
These achievements are particularly noteworthy given the inherent challenges of conducting research during pregnancy, including ethical considerations, limited access to suitable biospecimens, and the complexity of accurately assessing exposures that may occur before conception or during the earliest stages of gestation, when embryo is most vulnerable. Despite these barriers, researchers have made substantial progress in elucidating mechanisms underlying environmentally induced non-communicable diseases (NCDs), reflecting a strong commitment to advancing this complex area of study.
However, several important limitations remain [,,]. Many studies use cross-sectional designs with relatively small sample sizes, sometimes fewer than 100 participants, and rely on single time-point exposure assessment. These design limitations make it difficult to determine cause-and-effect relationships and to capture dynamic changes in both exposure and metabolomic profiles over time. Accurate exposure assessment also remains a challenge. Metals are frequently measured directly in biospecimens such as blood, and urine, providing a direct measure of internal dose, integrating exposure from multiple sources and routes (ingestion, inhalation, dermal). These levels typically reflect only recent exposure, and may not capture long-term or cumulative exposure.
The placenta, although increasingly recognized as a valuable biomarker of in utero environmental exposure [,], remains underutilized in exposure assessment and is rarely incorporated in metabolomic studies. This limits the understanding of tissue-specific metabolic changes and the ability to directly link maternal exposures to fetal responses. Challenges related to placental sampling, biological variability, and analytical complexity likely contribute to its limited use in current metabolomic research.
Exposure to air pollution and tobacco smoke is typically estimated indirectly through external modeling or biomarkers with short half-lives, which increases the potential for exposure misclassification. Pollutant levels are often approximated using ambient air quality data modeled at residential locations or collected from fixed-site monitoring stations, failing to account for individual activity patterns and varying microenvironments, such as indoor versus outdoor settings or workplace exposures [].
Most epidemiologic studies have focused on single pollutants, overlooking the reality of simultaneous exposure to multiple pollutants. However, an increasing number of metallomic studies have investigated the joint effects of multiple elements. This reflects a growing recognition of the need to capture the complexity of real-life scenario, in line with the exposome paradigm, which emphasizes the cumulative and interactive nature of environmental impacts on human health. Continued development of multi-pollutant approaches is essential for advancing comprehensive risk assessments and informing effective public health strategies.
Methodological heterogeneity across studies further complicates data interpretation and comparison. A persistent challenge is the limited availability of authentic chemical standards, which hampers confident metabolite identification and validation. Most cited studies used untargeted metabolomics that offers broad biochemical coverage, but provides only relative quantification, and is subject to variability in detection and limited reproducibility. Targeted metabolomics approaches allow for more precise quantification, typically supported by authentic standards, but are limited by preselection of metabolites. Liquid chromatography-mass spectrometry (LC-MS) remains the dominant platform due to its wide coverage of polar and semi-polar metabolites, while gas chromatography-mass spectrometry (GC-MS) retains indispensable utility for detecting volatile and thermally stable metabolites, providing complementary insights into metabolic alterations that LC-MS alone may overlook.
Overall, limited quantification accuracy and a lack of standardization across analytical platforms, methods and study designs make it difficult to replicate findings, identify reliable biomarkers or apply findings clinically. Ongoing efforts to overcome methodological barriers, along with continued technical advances and cross-disciplinary collaboration, are steadily refining our understanding and laying the foundation for future discoveries.
7. Opportunities and Future Directions
Recognizing these challenges, future studies should focus on improving exposure assessment methods and adopting longitudinal designs to capture critical windows throughout pregnancy [,]. Longitudinal sampling and data collection at multiple time points with repeated measures of exposure that begin before conception and follow participants throughout pregnancy into early childhood is essential to clarify the cause-and-effect relationships and their temporal dynamics. Expanding the use of biospecimens such as placenta, maternal and cord blood, will facilitate more precise exposure characterization, comprehensive biochemical profiling, and identification of tissue-specific mechanisms underlying adverse pregnancy outcomes. Investigation of sex-specific metabolic responses is also essential, as fetal sex may influence susceptibility to pollutant-induced metabolic disruption and shape both maternal and fetal adaptive responses. Paired sampling from maternal and fetal compartments can further enable direct comparisons and improve understanding of transplacental metabolic alterations.
Standardization and harmonization of metabolite identification and quantification protocols are essential to enhance reproducibility and comparability across studies [,]. Developing frameworks for absolute quantification and thorough method validation will foster biomarker utility in clinical and public health settings. Addressing real-world complex exposures requires multipollutant and exposome-wide approaches employing advanced statistical and computational tools to elucidate effects of chemical mixtures. Use of personal exposure monitoring technologies and refined exposure models that incorporate individual behaviors will reduce misclassification and better estimate internal dose. Integration of cutting-edge technologies such as artificial intelligence and systems biology will facilitate multidimensional data integration and novel hypothesis generation. Interdisciplinary collaboration across epidemiology, toxicology, biochemistry, and clinical sciences is crucial for accelerating progress and translating findings into preventive strategies. Although this review focuses on epidemiologic evidence of oxidative stress-related metabolomic alterations, these findings provide a foundation for future research exploring antioxidant-based prevention and therapeutic interventions to reduce exposure-related oxidative damage in vulnerable maternal–fetal populations. Leveraging these advances can deepen understanding of how prenatal exposures disrupt metabolic pathways through oxidative stress, ultimately improving maternal and fetal health outcomes. Ensuring adequate dietary intake, including diets rich in antioxidants and omega-3 fatty acids from diverse vegetables, fruits, fatty fish, and nuts, offers promising, practical opportunities to support maternal antioxidant defense and help mitigate oxidative stress, improve placental function, and decrease the risk of adverse pregnancy outcomes [,]. These dietary strategies could complement efforts to minimize environmental exposures. However, it is important to avoid excess nutritional interventions or supplementation that may carry risks of toxicity or contribute to oxidative stress [,].
8. Conclusions
Prenatal exposure to metals, air pollution, and tobacco smoke trigger a cascade of oxidative stress and metabolic dysfunction in both mother and fetus, contributing to adverse pregnancy outcomes and heightened early-life health risks. Metabolomic studies indicated that these exposures alter key biochemical pathways such as amino acid metabolism, lipid peroxidation, and mitochondrial energy production, compromising nutrient transport and redox balance. Specifically, toxic metals interfered with amino acid metabolism pathways involving glutamine, glycine, methionine, cysteine, and arginine, as well as impacting lipid and energy metabolism. Air pollution, especially PM2.5 and TRAP, was primarily linked to perturbations of lipid metabolism, including phospholipids and fatty acids, while tobacco smoke and e-cigarette vapor were associated with disturbances in mitochondrial dysfunction, lipid and bile acid signaling, and amino acid metabolism. These metabolic disturbances were linked to adverse pregnancy outcomes, including fetal growth restriction, preterm birth, and impaired neurodevelopment, and may influence long-term disease risk in offspring. Differences between mothers and offspring include somewhat opposing metabolomic responses, such as increased lipid metabolites in exposed newborns versus decreased in mothers, highlighting the value of paired biospecimen analyses.
Recognizing these findings, future studies should focus on improving exposure assessment, adopting longitudinal sampling to capture critical gestational windows, standardizing metabolomic approaches, and investigating sex-specific responses. The integration of multipollutant or exposome-wide modeling remains limited, but it is important for showing real-world exposure and clarifying interaction effects among chemicals. Mixed exposure scenario reflects real-life conditions but also presents analytical challenges that require sophisticated statistical and systems biology tools. Such advances will facilitate more accurate and reliable causal interpretation in environmental health outcomes and identify biomarkers predictive of fetal and child health risks. Given the substantial resources needed, it is essential that future funding mechanisms explicitly support and enable this type of comprehensive research.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox14121442/s1, Table S1: Overview of studies on metal/metalloid pollution-induced metabolomic changes in lipid, carbohydrate, and amino acid metabolism related to oxidative stress pathways; Table S2: Overview of studies on air pollution-induced metabolomic changes in lipid, carbohydrate, and amino acid metabolism related to oxidative stress pathways; Table S3: Overview of studies on tobacco smoke- and e-cigarette vapor-induced metabolomic changes in lipid, carbohydrate, and amino acid metabolism related to oxidative stress pathways.
Funding
This study was supported by the Croatian Science Foundation as part of the research project “Assessment of Daily Exposure to Metals and Maternal Individual Susceptibility as Factors of Developmental Origins of Health and Disease, METALORIGINS” (grant HRZZ-IP-2016-06-1998), and the European Union—Next Generation EU, “Interactions between Human and Environmental Health: Determinants of Health Preservation” (HumEnHealth), Program Contract of 8 December 2023, Class: 643-02/23-01/00016, Reg. no. 533-03-23-0006.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This study was performed using the facilities and equipment funded within the European Regional Development Fund project KK.01.1.1.02.0007 “Research and Education Centre of Environmental Health and Radiation Protection—Reconstruction and Expansion of the Institute for Medical Research and Occupational Health”. A graphical abstract and figures were created using publicly available vectors from Freepik (www.freepik.com), used in accordance with the Freepik Free License.
Conflicts of Interest
The author declares no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| Asn | Asparagine |
| Asp | Aspartic acid |
| BEAR Cohort | Biomarkers of Exposure to Arsenic |
| CALINE4 | The California Line Source Dispersion Model, version 4 |
| EWAS | Environment, and Social stressors |
| FIA-MS/MS | Flow injection analysis coupled with tandem mass spectrometry |
| GDM | Gestational diabetes mellitus |
| Gln | Glutamine |
| Glu | Glutamic acid |
| HMDB | Human Metabolome Database |
| iAs | Inorganic arsenic |
| INSPIRE Cohort | Infant Susceptibility to Pulmonary Infections and Asthma following Respiratory Syncytial Virus (RSV) Exposure |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LC-HRMS | Liquid Chromatography—High Resolution Mass Spectrometry |
| LINA Study | Lifestyle and Environmental Factors and their Influence on Newborn Allergy Risk |
| LPA | Lysophosphatidic acid |
| LPC | Lysophosphatidylcholine |
| LPG | Lysophosphatidylglycerol |
| Lyso.PC. | Acyl-lysophosphatidylcholines |
| Lyso.PC.e | Alkyl-lysophosphatidylcholines |
| MADRES study | The Maternal and Developmental Risks from Environmental and Social Stressors |
| MIMA | Meet-in-metabolite-analysis |
| MITM | Meet-in-the-middle approach |
| MSE | Mass Spectrometry with Elevated Energy, untargeted fragmentation of all ions using alternating collision energies for wide metabolite coverage |
| MS/MS | Tandem mass spectrometry with targeted fragmentation of selected molecules to help identify compounds |
| MWAS | Metabolome-wide association approach |
| NIST | National Institute of Standards and Technology |
| PCaa | Diacyl-phosphatidylcholine |
| PCae | Acyl-alkyl-phosphatidylcholine |
| PHIME Cohort | Public Health Impact of long-term, low-level Mixed Element exposure in susceptible population strata |
| PRAMS | Tennessee Pregnancy Risk Assessment Monitoring System |
| PROGRESS Cohort | Programming Research in Obesity, Growth, Environment and Social Stressors |
| Pro | Proline |
| PROTECT Cohort | Puerto Rico Testsite for Exploring Contamination Threats |
| PTB | Pre-term birth |
| SM | Sphingomyelins |
| TAP data | Tracking Air Pollution in China (a combined geographic–statistical model utilizing satellite remote sensing data) |
| tAs | Total arsenic |
Appendix A

Table A1.
Key Metabolomics Terminology Glossary.
Table A1.
Key Metabolomics Terminology Glossary.
| Metabolomic Features: |
| Distinct metabolic signals or peaks detected by analytical platforms (e.g., mass spectrometry), representing putative metabolites that may be identified or unknown. |
| Targeted Metabolomics: |
| Quantitative analysis of a predefined set of known metabolites with high sensitivity, focused on specific biochemi-cal pathways or hypotheses. |
| Untargeted Metabolomics: |
| Global, unbiased profiling aiming to detect as many metabolites as possible, enabling discovery of novel metabolic changes and pathways. |
| Pathway Enrichment Analysis: |
| Statistical methods that identify biochemical pathways significantly impacted based on altered metabolite profiles, linking molecular changes to biological functions. |
| Mummichog-based Metabolic Pathway Analysis: |
| A computational strategy enabling pathway-level interpretation from untargeted metabolomic data without re-quiring full metabolite identification, facilitating rapid biological insights. |
| Metabolome-wide association study (MWAS) approach: |
| Systemic, high-throughput analysis that assesses associations between a broad spectrum of metabolite profiles and phenotypes, exposures, or disease outcomes, enabling unbiased identification of metabolic biomarkers or pathways linked to health and disease risk. |
| Meet-in-metabolite-analysis (MIMA): |
| A strategy used to reconstitute the adverse outcome pathways (AOPs) by identifying metabolomic linkages be-tween environmental exposures and associated health outcomes or diseases. |
| Meet-in-the-middle approach (MITM): |
| A research method that identifies biological markers linking environmental exposure to disease by finding meta-bolic changes associated with both the exposure and the health outcome. |
| Exposome-wide association study (EWAS): |
| A research approach that systematically screens a large number of environmental exposures to identify which are statistically linked to specific health outcome, similar to how genome-wide association studies identify genetic as-sociations. |
Note: Definitions in this glossary were adapted from [,,].
References
- Talebi, S.; Kianifar, H.R.; Mehdizadeh, A. Nutritional requirements in pregnancy and lactation. Clin. Nutr. ESPEN 2024, 64, 400–410. [Google Scholar] [CrossRef]
- Clarke, G.S.; Gatford, K.L.; Young, R.L.; Grattan, D.R.; Ladyman, S.R.; Page, A.J. Maternal adaptations to food intake across pregnancy: Central and peripheral mechanisms. Obesity 2021, 29, 1813–1824. [Google Scholar] [CrossRef]
- Morrison, J.L.; Regnault, T.R. Nutrition in pregnancy: Optimising maternal diet and fetal adaptations to altered nutrient supply. Nutrients 2016, 8, 342. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Hsu, C.N. Amino acids during pregnancy and offspring cardiovascular-kidney-metabolic health. Nutrients 2024, 16, 1263. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The effects of oxidative stress on female reproduction: A review. Reprod. Biol. Endocrinol. 2012, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Myatt, L.; Cui, X. Oxidative stress in the placenta. Histochem. Cell Biol. 2004, 122, 369–382. [Google Scholar] [CrossRef]
- Vornic, I.; Buciu, V.; Furau, C.G.; Gaje, P.N.; Ceausu, R.A.; Dumitru, C.S.; Barb, A.C.; Novacescu, D.; Cumpanas, A.A.; Latcu, S.C.; et al. Oxidative stress and placental pathogenesis: A contemporary overview of potential biomarkers and emerging therapeutics. Int. J. Mol. Sci. 2024, 25, 12195. [Google Scholar] [CrossRef]
- Wu, F.; Tian, F.J.; Lin, Y.; Xu, W.M. Oxidative stress: Placenta function and dysfunction. Am. J. Reprod. Immunol. 2016, 76, 258–271. [Google Scholar] [CrossRef]
- Ruano, C.S.M.; Miralles, F.; Méhats, C.; Vaiman, D. The impact of oxidative stress of environmental origin on the onset of placental diseases. Antioxidants 2022, 11, 106. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, P.; Ramiro-Cortijo, D.; Reyes-Hernández, C.G.; López de Pablo, A.L.; González, M.C.; Arribas, S.M. Implication of oxidative stress in fetal programming of cardiovascular disease. Front. Physiol. 2018, 9, 602. [Google Scholar] [CrossRef]
- Ferguson, K.K.; O’Neill, M.S.; Meeker, J.D. Environmental contaminant exposures and preterm birth: A comprehensive review. J. Toxicol. Environ. Health B Crit. Rev. 2013, 16, 69–113. [Google Scholar] [CrossRef] [PubMed]
- Mallozzi, M.; Bordi, G.; Garo, C.; Caserta, D. The effect of maternal exposure to endocrine disrupting chemicals on fetal and neonatal development: A review on the major concerns. Birth Defects Res. Part C Embryo Today 2016, 108, 224–242. [Google Scholar] [CrossRef] [PubMed]
- Street, M.E.; Bernasconi, S. Endocrine-disrupting chemicals in human fetal growth. Int. J. Mol. Sci. 2020, 21, 1430. [Google Scholar] [CrossRef] [PubMed]
- Zejnullahu, V.A.; Zejnullahu, V.A.; Kosumi, E. The role of oxidative stress in patients with recurrent pregnancy loss: A review. Reprod. Health 2021, 18, 207. [Google Scholar] [CrossRef]
- Grzeszczak, K.; Łanocha-Arendarczyk, N.; Malinowski, W.; Ziętek, P.; Kosik-Bogacka, D. Oxidative stress in pregnancy. Biomolecules 2023, 13, 1768. [Google Scholar] [CrossRef]
- Brooker, I.A.; Fisher, J.J.; Sutherland, J.M.; Pringle, K.G. Understanding the impact of placental oxidative and nitrative stress in pregnancies complicated by fetal growth restriction. Placenta 2024, 158, 318–328. [Google Scholar] [CrossRef]
- Perera, F.; Herbstman, J. Prenatal environmental exposures, epigenetics, and disease. Reprod. Toxicol. 2011, 31, 363–373. [Google Scholar] [CrossRef]
- Thompson, L.P.; Al-Hasan, Y. Impact of oxidative stress in fetal programming. J. Pregnancy 2012, 2012, 582748. [Google Scholar] [CrossRef]
- Singh, S.; Goel, I.; Tripathi, S.; Ahirwar, A.; Kumar, M.; Rana, A.; Dhar, R.; Karmakar, S. Effect of environmental air pollutants on placental function and pregnancy outcomes: A molecular insight. Environ. Sci. Pollut. Res. Int. 2024, 31, 59819–59851. [Google Scholar] [CrossRef]
- Sun, C.; Shen, J.; Fang, R.; Huang, H.; Lai, Y.; Hu, Y.; Zheng, J. The impact of environmental and dietary exposure on gestational diabetes mellitus: A comprehensive review emphasizing the role of oxidative stress. Front. Endocrinol. 2025, 16, 1393883. [Google Scholar] [CrossRef]
- Chavan-Gautam, P.; Rani, A.; Freeman, D.J. Distribution of fatty acids and lipids during pregnancy. Adv. Clin. Chem. 2018, 84, 209–239. [Google Scholar] [CrossRef]
- Duttaroy, A.K.; Basak, S. Maternal fatty acid metabolism in pregnancy and its consequences in the feto-placental development. Front. Physiol. 2022, 12, 787848. [Google Scholar] [CrossRef]
- Dai, Y.; Huo, X.; Cheng, Z.; Faas, M.M.; Xu, X. Early-life exposure to widespread environmental toxicants and maternal-fetal health risk: A focus on metabolomic biomarkers. Sci. Total Environ. 2020, 739, 139626. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Qiu, C.; Hao, J.; Liao, J.; Lurmann, F.; Pavlovic, N.; Habre, R.; Jones, D.P.; Bastain, T.M.; Breton, C.V.; et al. Maternal metabolomics linking prenatal exposure to fine particulate matter and birth weight: A cross-sectional analysis of the MADRES cohort. Environ. Health 2025, 24, 14. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xie, P.; Cao, G.; Ran, J.; Xu, S.; Xia, W.; Cai, Z. Metabolic signatures of prenatal exposure to ‘cocktails’ of benzotriazoles and benzothiazoles and its health implications. J. Hazard. Mater. 2024, 473, 134717. [Google Scholar] [CrossRef] [PubMed]
- Orešič, M.; McGlinchey, A.; Wheelock, C.E.; Hyötyläinen, T. Metabolic signatures of the exposome—Quantifying the impact of exposure to environmental chemicals on human health. Metabolites 2020, 10, 454. [Google Scholar] [CrossRef]
- Inoue, K.; Yan, Q.; Arah, O.A.; Paul, K.; Walker, D.I.; Jones, D.P.; Ritz, B. Air pollution and adverse pregnancy and birth outcomes: Mediation analysis using metabolomic profiles. Curr. Environ. Health Rep. 2020, 7, 231–242. [Google Scholar] [CrossRef]
- Liu, J.; Litt, L.; Segal, M.R.; Kelly, M.J.; Pelton, J.G.; Kim, M. Metabolomics of oxidative stress in recent studies of endogenous and exogenously administered intermediate metabolites. Int. J. Mol. Sci. 2011, 12, 6469–6501. [Google Scholar] [CrossRef]
- Hajnajafi, K.; Iqbal, M.A. Mass-spectrometry based metabolomics: An overview of workflows, strategies, data analysis and applications. Proteome Sci. 2025, 23, 5. [Google Scholar] [CrossRef]
- Andrisic, L.; Dudzik, D.; Barbas, C.; Milkovic, L.; Grune, T.; Zarkovic, N. Short overview on metabolomics approach to study pathophysiology of oxidative stress in cancer. Redox Biol. 2018, 14, 47–58. [Google Scholar] [CrossRef]
- DeFreitas, M.J.; Katsoufis, C.P.; Benny, M.; Young, K.; Kulandavelu, S.; Ahn, H.; Sfakianaki, A.; Abitbol, C.L. Educational review: The impact of perinatal oxidative stress on the developing kidney. Front. Pediatr. 2022, 10, 853722. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Murtaza, G.; Metwally, E.; Kalhoro, D.H.; Kalhoro, M.S.; Rahu, B.A.; Sahito, R.G.A.; Yin, Y.; Yang, H.; Chughtai, M.I.; et al. The role of oxidative stress and antioxidant balance in pregnancy. Mediat. Inflamm. 2021, 2021, 9962860. [Google Scholar] [CrossRef] [PubMed]
- Mendes, S.; Timóteo-Ferreira, F.; Almeida, H.; Silva, E. New insights into the process of placentation and the role of oxidative uterine microenvironment. Oxid. Med. Cell. Longev. 2019, 2019, 9174521. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.J.; Bartho, L.A.; Perkins, A.V.; Holland, O.J. Placental mitochondria and reactive oxygen species in the physiology and pathophysiology of pregnancy. Clin. Exp. Pharmacol. Physiol. 2020, 47, 176–184. [Google Scholar] [CrossRef]
- Yüksel, S.; Yiğit, A.A. Malondialdehyde and nitric oxide levels and catalase, superoxide dismutase, and glutathione peroxidase levels in maternal blood during different trimesters of pregnancy and in the cord blood of newborns. Turk. J. Med. Sci. 2015, 45, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Guo, Y.; Yang, Y.; Du, Z.; Fan, Y.; Zhao, Y.; Yuan, S. Oxidative stress on vessels at the maternal-fetal interface for female reproductive system disorders: Update. Front. Endocrinol. 2023, 14, 1118121. [Google Scholar] [CrossRef]
- Hadden, D.R.; McLaughlin, C. Normal and abnormal maternal metabolism during pregnancy. Semin. Fetal Neonatal Med. 2009, 14, 66–71. [Google Scholar] [CrossRef]
- Parrettini, S.; Caroli, A.; Torlone, E. Nutrition and metabolic adaptations in physiological and complicated pregnancy: Focus on obesity and gestational diabetes. Front. Endocrinol. 2020, 11, 611929. [Google Scholar] [CrossRef]
- Lain, K.Y.; Catalano, P.M. Metabolic changes in pregnancy. Clin. Obstet. Gynecol. 2007, 50, 938–948. [Google Scholar] [CrossRef]
- Butte, N.F. Carbohydrate and lipid metabolism in pregnancy: Normal compared with gestational diabetes mellitus. Am. J. Clin. Nutr. 2000, 71 (Suppl. 5), 1256S–1261S. [Google Scholar] [CrossRef]
- Li, S.; Zhu, J.; Zhao, Y.; An, P.; Zhao, H.; Xiong, Y. Metabolic disorder of nutrients—An emerging field in the pathogenesis of preeclampsia. Front. Nutr. 2025, 12, 1560610. [Google Scholar] [CrossRef]
- Wild, R.; Feingold, K.R. Effect of pregnancy on lipid metabolism and lipoprotein levels. In Endotext [Internet]; Feingold, K.R., Ahmed, S.F., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK498654/ (accessed on 9 October 2025). [PubMed]
- Cao, X.Y.; Li, M.Y.; Shao, C.X.; Shi, J.L.; Zhang, T.; Xie, F.; Peng, T.; Li, M.Q. Fatty acid metabolism disruptions: A subtle yet critical factor in adverse pregnancy outcomes. Int. J. Biol. Sci. 2024, 20, 6018–6037. [Google Scholar] [CrossRef]
- Dominko, K.; Đikić, D. Glutathionylation: A regulatory role of glutathione in physiological processes. Arh. Hig. Rada Toksikol. 2018, 69, 1–24. [Google Scholar] [CrossRef]
- Pizent, A.; Lazarus, M.; Kovačić, J.; Tariba Lovaković, B.; Brčić Karačonji, I.; Živković Semren, T.; Sekovanić, A.; Orct, T.; Branović-Čakanić, K.; Brajenović, N.; et al. Cigarette smoking during pregnancy: Effects on antioxidant enzymes, metallothionein and trace elements in mother-newborn pairs. Biomolecules 2020, 10, 892. [Google Scholar] [CrossRef] [PubMed]
- Haidar, Z.; Fatema, K.; Shoily, S.S.; Sajib, A.A. Disease-associated metabolic pathways affected by heavy metals and metalloid. Toxicol. Rep. 2023, 10, 554–570. [Google Scholar] [CrossRef] [PubMed]
- Pizent, A.; Tariba, B.; Živković, T. Reproductive toxicity of metals in men. Arh. Hig. Rada Toksikol. 2012, 63 (Suppl. 1), 35–46. [Google Scholar] [CrossRef] [PubMed]
- Gundacker, C.; Hengstschläger, M. The role of the placenta in fetal exposure to heavy metals. Wien. Med. Wochenschr. 2012, 162, 201–206. [Google Scholar] [CrossRef]
- Rísová, V. The pathway of lead through the mother’s body to the child. Interdiscip. Toxicol. 2019, 12, 1–6. [Google Scholar] [CrossRef]
- Dack, K.; Fell, M.; Taylor, C.M.; Havdahl, A.; Lewis, S.J. Prenatal mercury exposure and neurodevelopment up to the age of 5 years: A systematicrReview. Int. J. Environ. Res. Public Health 2022, 19, 1976. [Google Scholar] [CrossRef]
- Rychlik, K.A.; Illingworth, E.J.; Sillé, F.C.M. Arsenic and the placenta: A review with emphasis on the immune system. Placenta 2025, 160, 73–81. [Google Scholar] [CrossRef]
- Kim, S.S.; Meeker, J.D.; Keil, A.P.; Aung, M.T.; Bommarito, P.A.; Cantonwine, D.E.; McElrath, T.F.; Ferguson, K.K. Exposure to 17 trace metals in pregnancy and associations with urinary oxidative stress biomarkers. Environ. Res. 2019, 179 Pt B, 108854. [Google Scholar] [CrossRef]
- Piasek, M.; Škrgatić, L.; Sulimanec, A.; Orct, T.; Sekovanić, A.; Kovačić, J.; Katić, A.; Branović Čakanić, K.; Pizent, A.; Brajenović, N.; et al. Effects of maternal cigarette smoking on trace element levels and steroidogenesis in the maternal-placental-fetal unit. Toxics 2023, 11, 714. [Google Scholar] [CrossRef] [PubMed]
- Fragou, D.; Fragou, A.; Kouidou, S.; Njau, S.; Kovatsi, L. Epigenetic mechanisms in metal toxicity. Toxicol. Mech. Methods 2011, 21, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Kurita, H.; Ohuchi, K.; Inden, M. Effects of environmental non-essential toxic heavy metals on epigenetics during development. Toxics 2025, 13, 167. [Google Scholar] [CrossRef] [PubMed]
- Dettwiler, M.; Flynn, A.C.; Rigutto-Farebrother, J. Effects of non-essential “toxic” trace elements on pregnancy outcomes: A narrative overview of recent literature syntheses. Int. J. Environ. Res. Public Health 2023, 20, 5536. [Google Scholar] [CrossRef]
- Issah, I.; Duah, M.S.; Arko-Mensah, J.; Bawua, S.A.; Agyekum, T.P.; Fobil, J.N. Exposure to metal mixtures and adverse pregnancy and birth outcomes: A systematic review. Sci. Total Environ. 2024, 908, 168380. [Google Scholar] [CrossRef]
- Li, H.; Huang, K.; Jin, S.; Peng, Y.; Liu, W.; Wang, M.; Zhang, H.; Zhang, B.; Xia, W.; Li, Y.; et al. Environmental cadmium exposure induces alterations in the urinary metabolic profile of pregnant women. Int. J. Hyg. Environ. Health 2019, 222, 556–562. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Liu, J.; Wu, M.; Li, C.; Yang, J.; Wang, L. Environmental concentrations of cadmium and zinc and associating metabolomics profile alternations in urine of pregnant women in the first trimester: A prospective cohort study in Taiyuan, North China. Ecotoxicol. Environ. Saf. 2023, 267, 115611. [Google Scholar] [CrossRef]
- Niedzwiecki, M.M.; Eggers, S.; Joshi, A.; Dolios, G.; Cantoral, A.; Lamadrid-Figueroa, H.; Amarasiriwardena, C.; Téllez-Rojo, M.M.; Wright, R.O.; Petrick, L. Lead exposure and serum metabolite profiles in pregnant women in Mexico City. Environ. Health 2021, 20, 125. [Google Scholar] [CrossRef]
- Li, H.; Wang, M.; Liang, Q.; Jin, S.; Sun, X.; Jiang, Y.; Pan, X.; Zhou, Y.; Peng, Y.; Zhang, B.; et al. Urinary metabolomics revealed arsenic exposure related to metabolic alterations in general Chinese pregnant women. J. Chromatogr. A 2017, 1479, 145–152. [Google Scholar] [CrossRef]
- Zhang, Q.; Tian, M.; Zhang, X.; Zhang, X.; Yang, X.; Lu, Y.Y.; Li, S.; Liu, L.; Li, J.; Hassanian-Moghaddam, H.; et al. Metabolic biomarkers linking urinary arsenic species to gestational diabetes mellitus: A cross-sectional study in Chinese pregnant women. Sci. Total Environ. 2023, 892, 164761. [Google Scholar] [CrossRef]
- Shen, S.; Li, X.F.; Cullen, W.R.; Weinfeld, M.; Le, X.C. Arsenic binding to proteins. Chem. Rev. 2013, 113, 7769–7792. [Google Scholar] [CrossRef]
- Saxena, R.; Bozack, A.K.; Gamble, M.V. Nutritional influences on one-carbon metabolism: Effects on arsenic methylation and toxicity. Annu. Rev. Nutr. 2018, 38, 401–429. [Google Scholar] [CrossRef] [PubMed]
- Laine, J.E.; Bailey, K.A.; Olshan, A.F.; Smeester, L.; Drobná, Z.; Stýblo, M.; Douillet, C.; García-Vargas, G.; Rubio-Andrade, M.; Pathmasiri, W.; et al. Neonatal metabolomic profiles related to prenatal arsenic exposure. Environ. Sci. Technol. 2017, 51, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Telišman, S. Interactions of essential and/or toxic metals and metalloid regarding interindividual differences in susceptibility to various toxicants and chronic diseases in man. Arh. Hig. Rada Toksikol. 1995, 46, 459–476. [Google Scholar] [PubMed]
- Shih, Y.H.; Chen, H.Y.; Christensen, K.; Handler, A.; Turyk, M.E.; Argos, M. Prenatal exposure to multiple metals and birth outcomes: An observational study within the National Children’s Study cohort. Environ. Int. 2021, 147, 106373. [Google Scholar] [CrossRef]
- Zhang, M.; Buckley, J.P.; Liang, L.; Hong, X.; Wang, G.; Wang, M.C.; Wills-Karp, M.; Wang, X.; Mueller, N.T. A metabolome-wide association study of in utero metal and trace element exposures with cord blood metabolome profile: Findings from the Boston Birth Cohort. Environ. Int. 2022, 158, 106976. [Google Scholar] [CrossRef]
- Derosa, G.; Maffioli, P.; Sahebkar, A. Piperine and its role in chronic diseases. Adv. Exp. Med. Biol. 2016, 928, 173–184. [Google Scholar] [CrossRef]
- Houston, M.C. Role of mercury toxicity in hypertension, cardiovascular disease, and stroke. J. Clin. Hypertens. 2011, 13, 621–627. [Google Scholar] [CrossRef]
- Amin, A.; Saadatakhtar, M.; Mohajerian, A.; Marashi, S.M.; Zamanifard, S.; Keshavarzian, A.; Molaee, P.; Keshmiri, M.S.; Nikdoust, F. Mercury-mediated cardiovascular toxicity: Mechanisms and remedies. Cardiovasc. Toxicol. 2025, 25, 507–522. [Google Scholar] [CrossRef]
- Kim, C.; Ashrap, P.; Watkins, D.J.; Mukherjee, B.; Rosario-Pabón, Z.Y.; Vélez-Vega, C.M.; Alshawabkeh, A.N.; Cordero, J.F.; Meeker, J.D. Maternal metals/metalloid blood levels are associated with lipidomic profiles among pregnant women in Puerto Rico. Front. Public Health 2022, 9, 754706. [Google Scholar] [CrossRef]
- Zhao, S.; Yang, X.; Xu, Q.; Li, H.; Su, Y.; Xu, Q.; Li, Q.; Xia, Y.; Shen, R. Association of maternal metals exposure, metabolites and birth outcomes in newborns: A prospective cohort study. Environ. Int. 2023, 179, 108183. [Google Scholar] [CrossRef]
- Xie, Y.; Xiao, H.; Zheng, D.; Mahai, G.; Li, Y.; Xia, W.; Xu, S.; Zhou, A. Associations of prenatal metal exposure with child neurodevelopment and mediation by perturbation of metabolic pathways. Nat. Commun. 2025, 16, 2089. [Google Scholar] [CrossRef]
- Anesti, O.; Papaioannou, N.; Gabriel, C.; Karakoltzidis, A.; Dzhedzheia, V.; Petridis, I.; Stratidakis, A.; Dickinson, M.; Horvat, M.; Snoj Tratnik, J.; et al. An exposome connectivity paradigm for the mechanistic assessment of the effects of prenatal and early life exposure to metals on neurodevelopment. Front. Public Health 2023, 10, 871218. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xia, W.; Liu, H.; Liu, F.; Li, H.; Chang, H.; Sun, J.; Liu, W.; Sun, X.; Jiang, Y.; et al. Urinary metabolomics reveals novel interactions between metal exposure and amino acid metabolic stress during pregnancy. Toxicol. Res. 2018, 7, 1164–1172. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Xu, B.; Guan, Y.; Chen, T.; Huang, R.; Zhang, T.; Sun, R.; Xie, K.; Chen, M. A metabolomic study on the association of exposure to heavy metals in the first trimester with primary tooth eruption. Sci. Total Environ. 2020, 723, 138107. [Google Scholar] [CrossRef] [PubMed]
- Aust, A.E.; Ball, J.C.; Hu, A.A.; Lighty, J.S.; Smith, K.R.; Straccia, A.M.; Veranth, J.M.; Young, W.C. Particle characteristics responsible for effects on human lung epithelial cells. Res. Rep. Health Eff. Inst. 2002, 110, 1–65. [Google Scholar]
- Mukherjee, A.; Agrawal, M. A global perspective of fine particulate matter pollution and its health effects. Rev. Environ. Contam. Toxicol. 2018, 244, 5–51. [Google Scholar] [CrossRef]
- Farahani, V.J.; Altuwayjiri, A.; Pirhadi, M.; Verma, V.; Ruprecht, A.A.; Diapouli, E.; Eleftheriadis, K.; Sioutas, C. The oxidative potential of particulate matter (PM) in different regions around the world and its relation to air pollution sources. Environ. Sci. Atmos. 2022, 2, 1076–1086. [Google Scholar] [CrossRef]
- Zheng, L.; Zhou, J.; Zhu, L.; Xu, X.; Luo, S.; Xie, X.; Li, H.; Lin, S.; Luo, J.; Wu, S. Associations of air pollutants and related metabolites with preterm birth during pregnancy. Sci. Total Environ. 2024, 951, 175542. [Google Scholar] [CrossRef]
- Li, Z.; Dunlop, A.L.; Sarnat, J.A.; Hüls, A.; Eick, S.M.; Gaskins, A.; Chang, H.; Russell, A.; Tan, Y.; Cheng, H.; et al. Unraveling the molecular links between fine particulate matter exposure and early birth risks in African American mothers: A metabolomics study in the Atlanta African American Maternal-Child Cohort. Environ. Sci. Technol. 2025, 59, 10905–10918. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, A.; Cross, P.J.; Dobson, R.C.J.; Adams, L.E.; Savka, M.A.; Hudson, A.O. A three-ring circus: Metabolism of the three proteogenic aromatic amino acids and their role in the health of plants and animals. Front. Mol. Biosci. 2018, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, L.; Li, Q.; Xu, S.; Wang, P.; Shi, H.; Zhang, Y.; Li, J. The effect of PM2.5 exposure on placenta and its associated metabolites: A birth cohort study. Ecotoxicol. Environ. Saf. 2025, 292, 117891. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Liew, Z.; Uppal, K.; Cui, X.; Ling, C.; Heck, J.E.; von Ehrenstein, O.S.; Wu, J.; Walker, D.I.; Jones, D.P.; et al. Maternal serum metabolome and traffic-related air pollution exposure in pregnancy. Environ. Int. 2019, 130, 104872. [Google Scholar] [CrossRef]
- Kim, J.H.; Yan, Q.; Uppal, K.; Cui, X.; Ling, C.; Walker, D.I.; Heck, J.E.; von Ehrenstein, O.S.; Jones, D.P.; Ritz, B. Metabolomics analysis of maternal serum exposed to high air pollution during pregnancy and risk of autism spectrum disorder in offspring. Environ. Res. 2021, 196, 110823. [Google Scholar] [CrossRef]
- De Simone, R.; Vissicchio, F.; Mingarelli, C.; De Nuccio, C.; Visentin, S.; Ajmone-Cat, M.A.; Minghetti, L. Branched-chain amino acids influence the immune properties of microglial cells and their responsiveness to pro-inflammatory signals. Biochim. Biophys. Acta 2013, 1832, 650–659. [Google Scholar] [CrossRef]
- Zheng, H.F.; Wang, W.Q.; Li, X.M.; Rauw, G.; Baker, G.B. Body fluid levels of neuroactive amino acids in autism spectrum disorders: A review of the literature. Amino Acids 2017, 49, 57–65. [Google Scholar] [CrossRef]
- India-Aldana, S.; Petrick, L.; Niedzwiecki, M.M.; Valvi, D.; Just, A.C.; Gutiérrez-Avila, I.; Kloog, I.; Barupal, D.K.; Téllez-Rojo, M.M.; Wright, R.O.; et al. Pregnancy as a susceptible period to ambient air pollution exposure on the maternal postpartum metabolome. Environ. Sci. Technol. 2025, 59, 6400–6413. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, G.; Liang, S.; Liu, J.; Zhang, J.; Shen, H.; Chen, Y.; Duan, J.; Sun, Z. PM2.5 exposure exaggerates the risk of adverse birth outcomes in pregnant women with pre-existing hyperlipidemia: Modulation role of adipokines and lipidome. Sci. Total Environ. 2021, 787, 147604. [Google Scholar] [CrossRef]
- Ritz, B.; Yan, Q.; He, D.; Wu, J.; Walker, D.I.; Uppal, K.; Jones, D.P.; Heck, J.E. Child serum metabolome and traffic-related air pollution exposure in pregnancy. Environ. Res. 2022, 203, 111907. [Google Scholar] [CrossRef]
- Szczuko, M.; Kikut, J.; Komorniak, N.; Bilicki, J.; Celewicz, Z.; Ziętek, M. The role of arachidonic and linoleic acid derivatives in pathological pregnancies and the human reproduction process. Int. J. Mol. Sci. 2020, 21, 9628. [Google Scholar] [CrossRef]
- Holzhausen, E.A.; Chalifour, B.N.; Tan, Y.; Young, N.; Lurmann, F.; Jones, D.P.; Sarnat, J.A.; Chang, H.H.; Goran, M.I.; Liang, D.; et al. Prenatal and early life exposure to ambient air pollutants is associated with the fecal metabolome in the first two years of life. Environ. Sci. Technol. 2024, 58, 14121–14134. [Google Scholar] [CrossRef]
- Holzhausen, E.A.; Tan, Y.; Young, N.; Jones, R.B.; Tang, Z.; Sarnat, J.A.; Lurmann, F.; Chang, H.H.; Tran, V.; Jones, D.P.; et al. Prenatal nitrogen oxide (NOx) and its potential impact on infant metabolism during the first month of life: Evidence from two distinct cohorts—The Atlanta African American Maternal-Child Cohort and the Southern California Mother’s Milk Study. Environ. Sci. Technol. 2025, 59, 19131–19145. [Google Scholar] [CrossRef]
- Talhout, R.; Schulz, T.; Florek, E.; van Benthem, J.; Wester, P.; Opperhuizen, A. Hazardous compounds in tobacco smoke. Int. J. Environ. Res. Public Health 2011, 8, 613–628. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hecht, S.S. Carcinogenic components of tobacco and tobacco smoke: A 2022 update. Food Chem. Toxicol. 2022, 165, 113179. [Google Scholar] [CrossRef] [PubMed]
- Mund, M.; Louwen, F.; Klingelhoefer, D.; Gerber, A. Smoking and pregnancy—A review on the first major environmental risk factor of the unborn. Int. J. Environ. Res. Public Health 2013, 10, 6485–6499. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, C.T.; Spindel, E.R. Pulmonary effects of maternal smoking on the fetus and child: Effects on lung development, respiratory morbidities, and lifelong lung health. Paediatr. Respir. Rev. 2017, 21, 27–33. [Google Scholar] [CrossRef]
- Gordon, T.; Karey, E.; Rebuli, M.E.; Escobar, Y.H.; Jaspers, I.; Chen, L.C. E-Cigarette Toxicology. Annu. Rev. Pharmacol. Toxicol. 2022, 62, 301–322. [Google Scholar] [CrossRef]
- Patanavanich, R.; Thatasawakul, C.; Youngcharoen, K.; Soponvashira, V.; Pichetsin, P. Health Effects from Secondhand Exposure to E-Cigarettes: A Systematic Review of Peer-Reviewed Articles from 2004–2024. Int. J. Environ. Res. Public Health 2025, 22, 1408. [Google Scholar] [CrossRef]
- Zečić, A.; Vazdar, B.; Slišković, L.; Sutlović, D. Urine Levels of Nicotine and Its Metabolites in Young Population Exposed to Second-Hand Smoke in Nightclubs: A Pilot Study. Arh. Hig. Rada Toksikol. 2024, 75, 211–216. [Google Scholar] [CrossRef]
- Rodriguez, J.; Silverstein, D.; Mutic, A.; Liang, D.; Peterson, S.; Yang, I. Passive electronic cigarette vapor exposure in children: A systematic review. Biol. Res. Nurs. 2025, 10998004251357832. [Google Scholar] [CrossRef]
- Cajachagua-Torres, K.N.; Blaauwendraad, S.M.; El Marroun, H.; Demmelmair, H.; Koletzko, B.; Gaillard, R.; Jaddoe, V.W.V. Fetal exposure to maternal smoking and neonatal metabolite profiles. Metabolites 2022, 12, 1101. [Google Scholar] [CrossRef]
- Snyder, B.M.; Nian, H.; Miller, A.M.; Ryckman, K.K.; Li, Y.; Tindle, H.A.; Ammar, L.; Ramesh, A.; Liu, Z.; Hartert, T.V.; et al. Associations between smoking and smoking cessation during pregnancy and newborn metabolite concentrations: Findings from PRAMS and INSPIRE birth cohorts. Metabolites 2023, 13, 1163. [Google Scholar] [CrossRef]
- Rolle-Kampczyk, U.E.; Krumsiek, J.; Otto, W.; Röder, S.W.; Kohajda, T.; Borte, M.; Theis, F.; Lehmann, I.; von Bergen, M. Metabolomics reveals effects of maternal smoking on endogenous metabolites from lipid metabolism in cord blood of newborns. Metabolomics 2016, 12, 76. [Google Scholar] [CrossRef]
- Fischer, S.T.; Lili, L.N.; Li, S.; Tran, V.T.; Stewart, K.B.; Schwartz, C.E.; Jones, D.P.; Sherman, S.L.; Fridovich-Keil, J.L. Low-level maternal exposure to nicotine associates with significant metabolic perturbations in second-trimester amniotic fluid. Environ. Int. 2017, 107, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Barr, D.B.; Ryan, P.B.; Fedirko, V.; Sarnat, J.A.; Gaskins, A.J.; Chang, C.J.; Tang, Z.; Marsit, C.J.; Corwin, E.J.; et al. High-resolution metabolomics of exposure to tobacco smoke during pregnancy and adverse birth outcomes in the Atlanta African American maternal-child cohort. Environ. Pollut. 2022, 292 Pt A, 118361. [Google Scholar] [CrossRef] [PubMed]
- Abebe, W.; Mozaffari, M.S. Role of taurine in the vasculature: An overview of experimental and human studies. Am. J. Cardiovasc. Dis. 2011, 1, 293–311. [Google Scholar] [PubMed]
- Park, S.; Park, J.Y.; Lee, J.H.; Kim, S.H. Plasma levels of lysine, tyrosine, and valine during pregnancy are independent risk factors of insulin resistance and gestational diabetes. Metab. Syndr. Relat. Disord. 2015, 13, 64–70. [Google Scholar] [CrossRef]
- Kwan, S.T.C.; King, J.H.; Yan, J.; Jiang, X.; Wei, E.; Fomin, V.G.; Roberson, M.S.; Caudill, M.A. Maternal choline supplementation during murine pregnancy modulates placental markers of inflammation, apoptosis and vascularization in a fetal sex-dependent manner. Placenta 2017, 53, 57–65. [Google Scholar] [CrossRef]
- Scholl, T.O.; Chen, X.; Khoo, C.S.; Lenders, C. The dietary glycemic index during pregnancy: Influence on infant birth weight, fetal growth, and biomarkers of carbohydrate metabolism. Am. J. Epidemiol. 2004, 159, 467–474. [Google Scholar] [CrossRef]
- He, D.; Yan, Q.; Uppal, K.; Walker, D.I.; Jones, D.P.; Ritz, B.; Heck, J.E. An untargeted metabolome-wide association study of maternal perinatal tobacco smoking in newborn blood spots. Metabolomics 2025, 21, 30. [Google Scholar] [CrossRef]
- Xue, Y.; Harris, E.; Wang, W.; Baybutt, R.C. Vitamin A depletion induced by cigarette smoke is associated with an increase in lung cancer-related markers in rats. J. Biomed. Sci. 2015, 22, 84. [Google Scholar] [CrossRef] [PubMed]
- Lovelace, M.D.; Varney, B.; Sundaram, G.; Lennon, M.J.; Lim, C.K.; Jacobs, K.; Guillemin, G.J.; Brew, B.J. Recent evidence for an expanded role of the kynurenine pathway of tryptophan metabolism in neurological diseases. Neuropharmacology 2017, 112 Pt B, 373–388. [Google Scholar] [CrossRef] [PubMed]
- Cavalier, H.; Long, S.E.; Rodrick, T.; Siu, Y.; Jacobson, M.H.; Afanasyeva, Y.; Sherman, S.; Liu, M.; Kahn, L.G.; Jones, D.R.; et al. Exploratory Untargeted Metabolomics Analysis Reveals Differences in Metabolite Profiles in Pregnant People Exposed vs. Unexposed to E-Cigarettes Secondhand in the NYU Children’s Health and Environment Study. Metabolomics 2025, 21, 92. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Nepovimova, E.; Kuca, K.; Valko, M. Heavy metals: Toxicity and human health effects. Arch. Toxicol. 2025, 99, 153–209. [Google Scholar] [CrossRef]
- Liang, D.; Li, Z.; Vlaanderen, J.; Tang, Z.; Jones, D.P.; Vermeulen, R.; Sarnat, J.A. A state-of-the-science review on high-resolution metabolomics application in air pollution health research: Current progress, analytical challenges, and recommendations for future direction. Environ. Health Perspect. 2023, 131, 56002. [Google Scholar] [CrossRef]
- Syama, K.P.; Blais, E.; Kumarathasan, P. Maternal mechanisms in air pollution exposure-related adverse pregnancy outcomes: A systematic review. Sci. Total Environ. 2025, 970, 178999. [Google Scholar] [CrossRef]
- Hood, R.B.; Moyd, S.; Hoffman, S.; Chow, S.S.; Tan, Y.; Bhanushali, P.; Wang, Y.; Sivalogan, K.; Gaskins, A.J.; Liang, D. Metabolomics application in understanding the link between air pollution and infant health outcomes: A narrative review. Curr. Pollut. Rep. 2024, 10, 786–798. [Google Scholar] [CrossRef]
- Bedia, C. Metabolomics in environmental toxicology: Applications and challenges. Trends Environ. Anal. Chem. 2022, 34, e00161. [Google Scholar] [CrossRef]
- Piasek, M.; Mikolić, A.; Sekovanić, A.; Sulimanec Grgec, A.; Jurasović, J. Cadmium in placenta—A valuable biomarker of exposure during pregnancy in biomedical research. J. Toxicol. Environ. Health A 2014, 77, 1071–1074. [Google Scholar] [CrossRef]
- Piasek, M.; Jurasović, J.; Sekovanić, A.; Brajenović, N.; Brčić Karačonji, I.; Mikolić, A.; Grgec, A.S.; Stasenko, S. Placental cadmium as an additional noninvasive bioindicator of active maternal tobacco smoking. J. Toxicol. Environ. Health A 2016, 79, 443–446. [Google Scholar] [CrossRef]
- Lovrić, M.; Gajski, G.; Fernández-Agüera, J.; Pöhlker, M.; Gursch, H.; EDIAQI Consortium; Borg, A.; Switters, J.; Mureddu, F. Evidence driven indoor air quality improvement: An innovative and interdisciplinary approach to improving indoor air quality. Biofactors 2025, 51, e2126. [Google Scholar] [CrossRef] [PubMed]
- Soni, V.; Bartelo, N.; Schweickart, A.; Chawla, Y.; Dutta, A.; Jain, S. Future Perspectives of Metabolomics: Gaps, Planning, and Recommendations. In Metabolomics; Soni, V., Hartman, T.E., Eds.; Springer: Cham, Switzerland, 2023; pp. 479–512. [Google Scholar] [CrossRef]
- Hsu, C.N.; Lin, Y.J.; Hou, C.Y.; Chen, Y.W.; Chang-Chien, G.P.; Lin, S.F.; Tain, Y.L. Antioxidants, gut microbiota, and cardiovascular programming: Unraveling a triad of early-life interactions. Antioxidants 2025, 14, 1049. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.; Wright, C. Safety and efficacy of supplements in pregnancy. Nutr. Rev. 2020, 78, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Kluz, T.; Costa, M. Toxic element contaminations of prenatal vitamins. Toxicol. Appl. Pharmacol. 2023, 477, 116670. [Google Scholar] [CrossRef]
- Vineis, P.; Perera, F. Molecular epidemiology and biomarkers in etiologic cancer research: The new in light of the old. Cancer Epidemiol. Biomarkers Prev. 2007, 16, 1954–1965. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Holmes, E.; Elliott, P. The metabolome-wide association study: A new look at human disease risk factors. J. Proteome Res. 2008, 7, 3637–3638. [Google Scholar] [CrossRef]
- Liu, X.; Locasale, J.W. Metabolomics: A primer. Trends Biochem. Sci. 2017, 42, 274–284. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).