Dehydroascorbic Acid Induces Cell Death in Sarcoma Stem Cells Under bFGF-Mediated Stemness-Supporting Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.1.1. Establishing Parental Sarcoma Cell Culture
2.1.2. Sarcoma Stem Cell Isolation
2.2. Sarcoma Stem Cell Characterization
2.2.1. Hoechst 33342 Dye Efflux Assay
2.2.2. qPCR Stemness Marker Analysis
2.3. MTT Viability Assay
2.4. Assessment of Changes in Metabolism, Response to Oxidative Stress, and Differentiation
2.4.1. qPCR
2.4.2. Western Blot
2.5. Somatic Mutation Gene Panels
3. Results
3.1. Sarcoma Cancer Stem Cells Isolation and Characterization
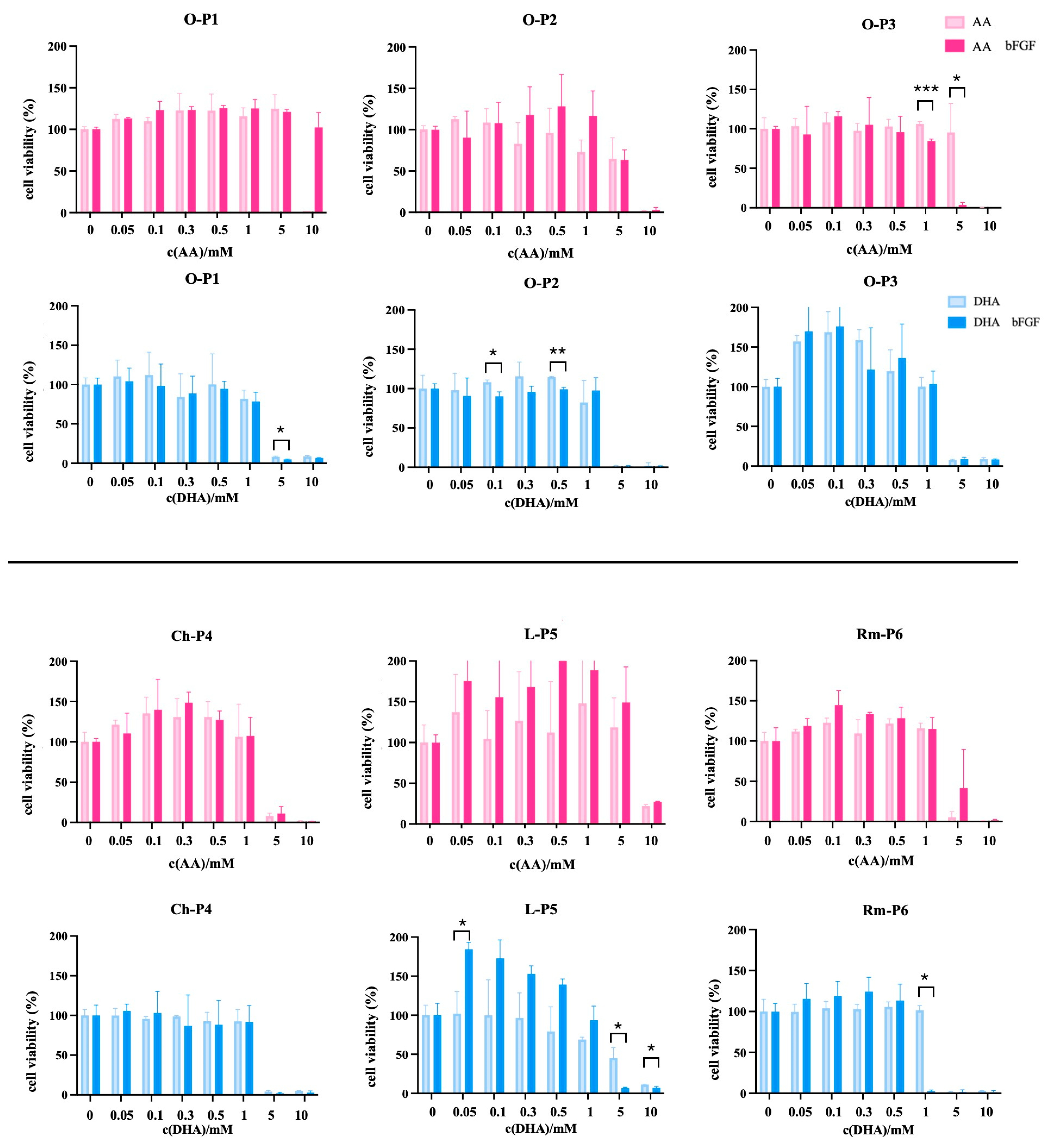
3.2. Differential Viability Responses to Ascorbate Derivatives in Normal Cells, Osteosarcoma Cell Line, and Sarcoma CSCs
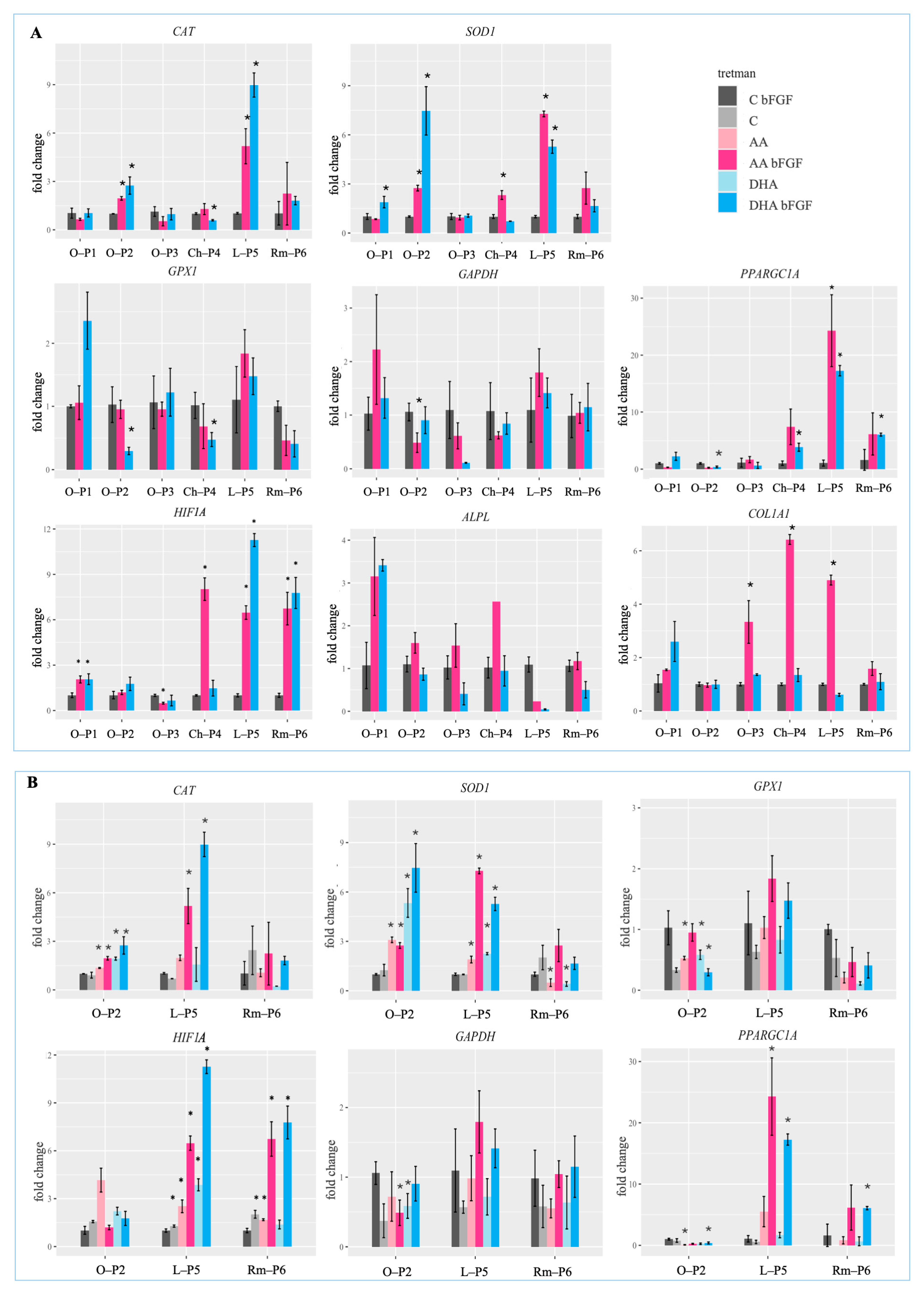
3.3. Modulation of Gene Expression Related to Oxidative Stress, Metabolism, and Differentiation in Sarcoma CSCs by Ascorbate Treatments in the Presence of bFGF
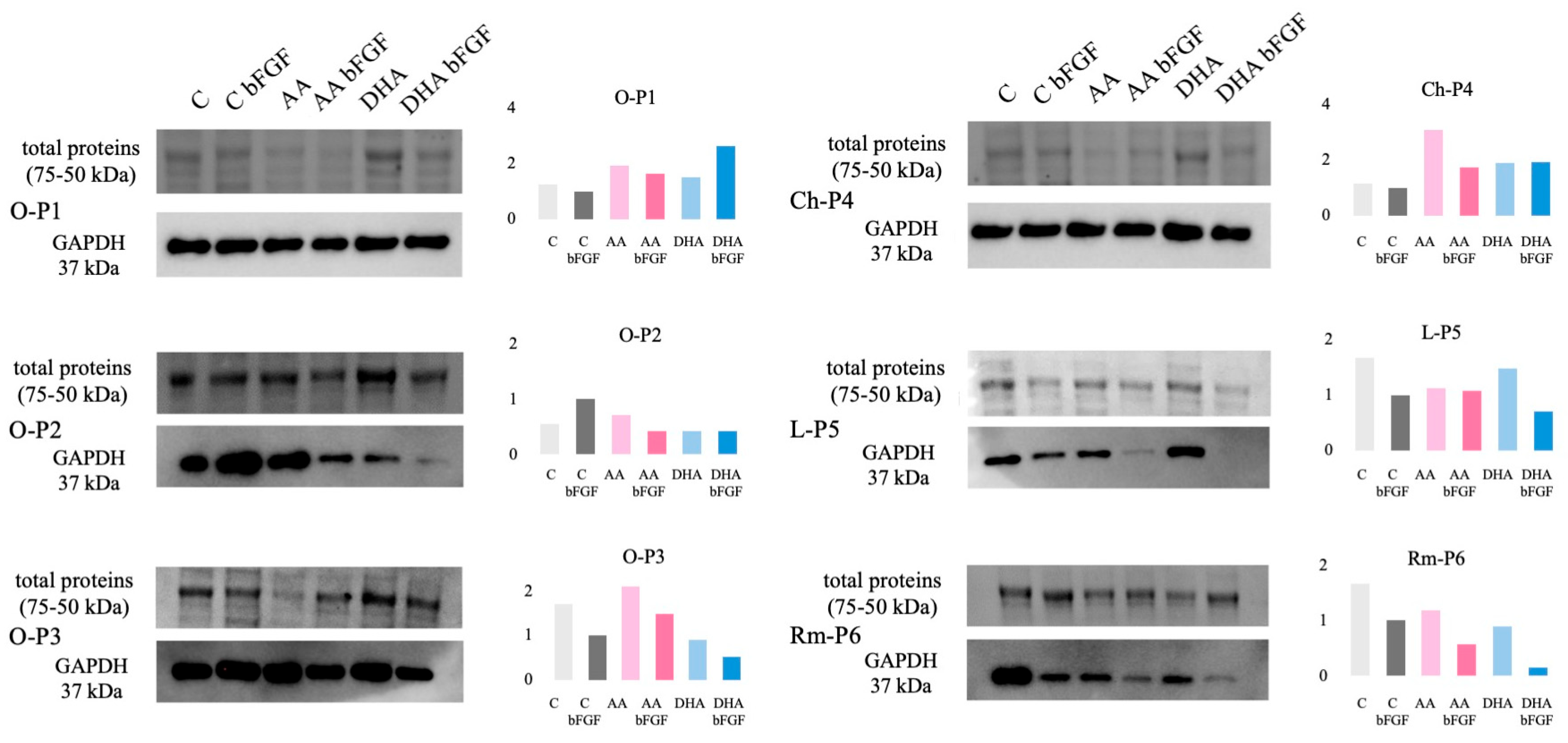
3.4. GAPDH Protein Expression in Sarcoma CSCs Treated with AA and DHA
3.5. Additional qPCR Analysis Reveals bFGF-Dependent Modulation of Oxidative Stress and Metabolic Genes in Sarcoma CSCs Treated with Ascorbate
3.6. Mutation Analysis of Oncogenes and Tumor Suppressor Genes in Sarcoma CSCs Responsive to Ascorbate Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grünewald, T.G.; Alonso, M.; Avnet, S.; Banito, A.; Burdach, S.; Cidre-Aranaz, F.; Di Pompo, G.; Distel, M.; Dorado-Garcia, H.; Garcia-Castro, J.; et al. Sarcoma Treatment in the Era of Molecular Medicine. EMBO Mol. Med. 2020, 12, e11131. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, C.D.M.; Krishnan, K.; Mertens, U.F. Pathology and Genetics of Tumours of Soft Tissue and Bone; World Health Organization: Lyon, France, 2002. [Google Scholar]
- Hatina, J.; Kripnerova, M.; Houfkova, K.; Pesta, M. Sarcoma Stem Cell Heterogeneity; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Martínez-Delgado, P.; Lacerenza, S.; Obrador-Hevia, A.; Lopez-Alvarez, M.; Mondaza-Hernandez, J.L.; Blanco-Alcaina, E.; Sanchez-Bustos, P.; Hindi, N.; Moura, D.S.; Martin-Broto, J. Cancer Stem Cells in Soft-Tissue Sarcomas. Cells 2020, 9, 1449. [Google Scholar] [CrossRef]
- Aponte, P.M.; Caicedo, A. Stemness in Cancer: Stem Cells, Cancer Stem Cells, and Their Microenvironment. Stem Cells Int. 2017, 2017, 5619472. [Google Scholar] [CrossRef] [PubMed]
- Peiris-Pagès, M.; Martinez-Outschoorn, U.E.; Pestell, R.G.; Sotgia, F.; Lisanti, M.P. Cancer Stem Cell Metabolism. Breast Cancer Res. 2016, 18, 55. [Google Scholar] [CrossRef]
- Pattabiraman, D.R.; Weinberg, R.A. Tackling the Cancer Stem Cells-What Challenges Do They Pose? Nat. Rev. Drug Discov. 2014, 13, 497–512. [Google Scholar] [CrossRef]
- Najafi, M.; Farhood, B.; Mortezaee, K. Cancer Stem Cells (CSCs) in Cancer Progression and Therapy. J. Cell. Physiol. 2019, 234, 8381–8395. [Google Scholar] [CrossRef]
- Ghanbari, Z.; Rastegari-Pouyani, M. Cancer Cells Change Their Glucose Metabolism to Overcome Increased ROS: One Step from Cancer Cell to Cancer Stem Cell? Biomed. Pharmacother. 2019, 112, 108690. [Google Scholar] [CrossRef] [PubMed]
- Chae, Y.C.; Kim, J.H. Cancer Stem Cell Metabolism: Target for Cancer Therapy. BMB Rep. 2018, 51, 319–326. [Google Scholar] [CrossRef]
- Sancho, P.; Barneda, D.; Heeschen, C. Hallmarks of Cancer Stem Cell Metabolism. Br. J. Cancer 2016, 114, 1305–1312. [Google Scholar] [CrossRef]
- Satheesh, N.J.; Samuel, S.M.; Büsselberg, D. Combination Therapy with Vitamin C Could Eradicate Cancer Stem Cells. Biomolecules 2020, 10, 79. [Google Scholar] [CrossRef]
- Ngo, B.; Van Riper, J.M.; Cantley, L.C.; Yun, J. Targeting Cancer Vulnerabilities with High-Dose Vitamin C. Nat. Rev. Cancer 2019, 19, 271–282. [Google Scholar] [CrossRef]
- Zhitkovich, A. Nuclear and Cytoplasmic Functions of Vitamin C. Chem. Res. Toxicol. 2020, 33, 2515–2526. [Google Scholar] [CrossRef]
- Vissers, M.C.M.; Das, A.B. Potential Mechanisms of Action for Vitamin C in Cancer: Reviewing the Evidence. Front. Physiol. 2018, 9, 809. [Google Scholar] [CrossRef]
- Lee, Y. Role of Vitamin C in Targeting Cancer Stem Cells and Cellular Plasticity. Cancers 2023, 15, 5657. [Google Scholar] [CrossRef] [PubMed]
- Uetaki, M.; Tabata, S.; Nakasuka, F.; Soga, T.; Tomita, M. Metabolomic Alterations in Human Cancer Cells by Vitamin C-Induced Oxidative Stress. Sci. Rep. 2015, 5, 13896. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Mullarky, E.; Lu, C.; Bosch, K.N.; Kavalier, A.; Rivera, K.; Roper, J.; Chio, I.I.C.; Giannopoulou, E.G.; Rago, C.; et al. Vitamin C Selectively Kills KRAS and BRAF Mutant Colorectal Cancer Cells by Targeting GAPDH. Science 2015, 350, 1391–1396. [Google Scholar] [CrossRef]
- Mossahebi-Mohammadi, M.; Quan, M.; Zhang, J.S.; Li, X. FGF Signaling Pathway: A Key Regulator of Stem Cell Pluripotency. Front. Cell Dev. Biol. 2020, 8, 79. [Google Scholar] [CrossRef]
- Gotoh, N. Control of Stemness by Fibroblast Growth Factor Signaling in Stem Cells and Cancer Stem Cells. Curr. Stem Cell Res. Ther. 2009, 4, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, A.; Ostler, A.E.; Jones, R.L.; Huang, P.H. Fibroblast Growth Factor Receptor (Fgfr) Signaling in Gist and Soft Tissue Sarcomas. Cells 2021, 10, 1533. [Google Scholar] [CrossRef]
- Coutu, D.L.; Galipeau, J. Roles of FGF Signaling in Stem Cell Self-Renewal, Senescence and Aging. Aging 2011, 3, 920–933. [Google Scholar] [CrossRef]
- OriGene Technologies. Available online: https://www.origene.com (accessed on 1 September 2025).
- Dahn, M.L.; Dean, C.A.; Jo, D.B.; Coyle, K.M.; Marcato, P. Human-Specific GAPDH QRT-PCR Is an Accurate and Sensitive Method of Xenograft Metastasis Quantification. Mol. Ther. Methods Clin. Dev. 2021, 20, 398–408. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, H.; You, Y.; Zhang, J.; Chen, R. High Gpx1 Expression Predicts Poor Survival in Laryngeal Squamous Cell Carcinoma. Auris Nasus Larynx 2018, 45, 13–19. [Google Scholar] [CrossRef]
- Palmini, G.; Zonefrati, R.; Mavilia, C.; Aldinucci, A.; Luzi, E.; Marini, F.; Franchi, A.; Capanna, R.; Tanini, A.; Brandi, M.L. Establishment of Cancer Stem Cell Cultures from Human Conventional Osteosarcoma. J. Vis. Exp. 2016, 2016, e53884. [Google Scholar] [CrossRef]
- Martins-Neves, S.R.; Lopes, Á.O.; do Carmo, A.; Paiva, A.A.; Simões, P.C.; Abrunhosa, A.J.; Gomes, C.M.F. Therapeutic Implications of an Enriched Cancer Stem-like Cell Population in a Human Osteosarcoma Cell Line. BMC Cancer 2012, 12, 139. [Google Scholar] [CrossRef]
- Kim, H.S.; Ahn, S.H.; Kim, H.J.; Park, J.W.; Han, I. Delta-like Factor 1 as a Possible Therapeutic Target for Sarcomas. CiOS Clin. Orthop. Surg. 2020, 12, 404–412. [Google Scholar] [CrossRef]
- Stratford, E.W.; Castro, R.; Wennerstrøm, A.; Holm, R.; Munthe, E.; Lauvrak, S.; Bjerkehagen, B.; Myklebost, O. Liposarcoma Cells with Aldefluor and CD133 Activity Have a Cancer Stem Cell Potential. Clin. Sarcoma Res. 2011, 1, 8. [Google Scholar] [CrossRef]
- Seigel, G.M.; Campbell, L.M. High-Throughput Microtiter Assay for Hoechst 33342 Dye Uptake. Cytotechnology 2004, 45, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wei, Q.; Utomo, V.; Nadesan, P.; Whetstone, H.; Kandel, R.; Wunder, J.S.; Alman, B.A. Side Population Cells Isolated from Mesenchymal Neoplasms Have Tumor Initiating Potential. Cancer Res. 2007, 67, 8216–8222. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yan, M.; Zhang, R.; Li, J.; Luo, Z. Side Population Cells Isolated from Human Osteosarcoma Are Enriched with Tumor-Initiating Cells. Cancer Sci. 2011, 102, 1774–1781. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.J.; Zhao, Y.H.; Qiao, L.X.; Jin, C.L.; Tian, J.; Li, Q.S. Aberrant Wnt/β-Catenin Signaling and Elevated Expression of Stem Cell Proteins Are Associated with Osteosarcoma Side Population Cells of High Tumorigenicity. Mol. Med. Rep. 2015, 12, 5042–5048. [Google Scholar] [CrossRef][Green Version]
- Lv, H.; Wang, C.; Fang, T.; Li, T.; Lv, G.; Han, Q.; Yang, W.; Wang, H. Vitamin C Preferentially Kills Cancer Stem Cells in Hepatocellular Carcinoma via SVCT-2. Nat. Precis. Oncol. 2018, 2, 1. [Google Scholar] [CrossRef]
- Giansanti, M.; Karimi, T.; Faraoni, I.; Graziani, G. High-Dose Vitamin C: Preclinical Evidence for Tailoring Treatment in Cancer Patients. Cancers 2021, 13, 1428. [Google Scholar] [CrossRef]
- Wechtersbach, L.; Polak, T.; Ulrih, N.P.; Cigić, B. Stability and Transformation of Products Formed from Dimeric Dehydroascorbic Acid at Low PH. Food Chem. 2011, 129, 965–973. [Google Scholar] [CrossRef]
- Villagran, M.; Ferreira, J.; Martorell, M.; Mardones, L. The Role of Vitamin C in Cancer Prevention and Therapy: A Literature Review. Antioxidants 2021, 10, 1894. [Google Scholar] [CrossRef]
- Chen, Q.; Espey, M.G.; Krishna, M.C.; Mitchell, J.B.; Corpe, C.P.; Buettner, G.R.; Shacter, E.; Levine, M. Pharmacologic Ascorbic Acid Concentrations Selectively Kill Cancer Cells: Action as a pro-Drug to Deliver Hydrogen Peroxide to Tissues. Proc. Natl. Acad. Sci. USA 2005, 102, 13604–13609. [Google Scholar] [CrossRef] [PubMed]
- Valenti, M.T.; Zanatta, M.; Donatelli, L.; Viviano, G.; Cavallini, C.; Scupoli, M.T.; Carbonare, L.D. Ascorbic Acid Induces Either Differentiation or Apoptosis in MG-63 Osteosarcoma Lineage. Anticancer Res. 2014, 34, 1617–1628. [Google Scholar] [PubMed]
- Vaishampayan, P.; Lee, Y. Redox-Active Vitamin C Suppresses Human Osteosarcoma Growth by Triggering Intracellular ROS-Iron–Calcium Signaling Crosstalk and Mitochondrial Dysfunction. Redox Biol. 2024, 75, 103288. [Google Scholar] [CrossRef] [PubMed]
- Jovičić, M.Š.; Pušić, M.; Antunović, M.; Ledinski, M.; Librenjak, L.; Kolundžić, R.; Ribičić, T.; Trkulja, V.; Urlić, I. In Vitro Effects of Ascorbic Acid on Viability and Metabolism of Patients’ Osteosarcoma Stem Cells. Acta Pharm. 2022, 72, 599–613. [Google Scholar] [CrossRef]
- Wan, J.; Zhou, J.; Fu, L.; Li, Y.; Zeng, H.; Xu, X.; Lv, C.; Jin, H. Ascorbic Acid Inhibits Liver Cancer Growth and Metastasis in Vitro and in Vivo, Independent of Stemness Gene Regulation. Front. Pharmacol. 2021, 12, 726015. [Google Scholar] [CrossRef]
- Bonuccelli, G.; De Francesco, E.M.; de Boer, R.; Tanowitz, H.B.; Lisanti, M.P. NADH Autofluorescence, a New Metabolic Biomarker for Cancer Stem Cells: Identification of Vitamin C and CAPE as Natural Products Targeting “Stemness”. Oncotarget 2017, 8, 20667–20678. [Google Scholar] [CrossRef]
- Sen, U.; Chaudhury, D.; Shenoy, P.S.; Bose, B. Differential Sensitivities of Triple-Negative Breast Cancer Stem Cell towards Various Doses of Vitamin C: An Insight into the Internal Antioxidant Systems. J. Cell. Biochem. 2021, 122, 349–366. [Google Scholar] [CrossRef]
- Seyama, Y.; Sudo, K.; Yamada, T.; Tsuchiya, K.; Nakamura, Y. Ascorbic Acid Predominantly Kills Cancer Stem Cell-like Cells in the Hepatocellular Carcinoma Cell Line Li-7 and Is More Effective at Low Cell Density and in Small Spheroids. Biochem. Biophys. Res. Commun. 2024, 709, 149816. [Google Scholar] [CrossRef]
- Kim, T.J.; Byun, J.S.; Kwon, H.S.; Kim, D.Y. Cellular Toxicity Driven by High-Dose Vitamin C on Normal and Cancer Stem Cells. Biochem. Biophys. Res. Commun. 2018, 497, 347–353. [Google Scholar] [CrossRef]
- Rodríguez-Enríquez, S.; Marín-Hernández, Á.; Gallardo-Pérez, J.C.; Pacheco-Velázquez, S.C.; Belmont-Díaz, J.A.; Robledo-Cadena, D.X.; Vargas-Navarro, J.L.; de la Peña, N.A.C.; Saavedra, E.; Moreno-Sánchez, R. Transcriptional Regulation of Energy Metabolism in Cancer Cells. Cells 2019, 8, 1225. [Google Scholar] [CrossRef] [PubMed]
- Bost, F.; Kaminski, L. The Metabolic Modulator PGC-1α in Cancer. Am. J. Cancer Res. 2019, 9, 198–211. [Google Scholar] [PubMed]
- Lebleu, V.S.; O’Connell, J.T.; Gonzalez Herrera, K.N.; Wikman, H.; Pantel, K.; Haigis, M.C.; De Carvalho, F.M.; Damascena, A.; Domingos Chinen, L.T.; Rocha, R.M.; et al. PGC-1α Mediates Mitochondrial Biogenesis and Oxidative Phosphorylation in Cancer Cells to Promote Metastasis. Nat. Cell Biol. 2014, 16, 992–1003. [Google Scholar] [CrossRef]
- Rao, Z.; Shen, D.; Chen, J.; Jin, L.; Wu, X.; Chen, M.; Li, L.; Chu, M.; Lin, J. Basic Fibroblast Growth Factor Attenuates Injury in Myocardial Infarction by Enhancing Hypoxia-Inducible Factor-1 Alpha Accumulation. Front. Pharmacol. 2020, 11, 1193. [Google Scholar] [CrossRef]
- Kim, J.H.; Hwang, S.; Lee, J.H.; Im, S.S.; Son, J. Vitamin C Suppresses Pancreatic Carcinogenesis through the Inhibition of Both Glucose Metabolism and Wnt Signaling. Int. J. Mol. Sci. 2022, 23, 12249. [Google Scholar] [CrossRef]
- Mo, Y.; Wang, Y.; Zhang, L.; Yang, L.; Zhou, M.; Li, X.; Li, Y.; Li, G.; Zeng, Z.; Xiong, W.; et al. The Role of Wnt Signaling Pathway in Tumor Metabolic Reprogramming. J. Cancer 2019, 10, 3789–3797. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, E.; Tran, T.; Vranic, S.; Levy, A.; Bonfil, R.D. Role and Significance of C-KIT Receptor Tyrosine Kinase in Cancer: A Review. Bosn. J. Basic Med. Sci. 2022, 22, 683–698. [Google Scholar] [CrossRef]
- Zhou, S.; Abdihamid, O.; Tan, F.; Zhou, H.; Liu, H.; Li, Z.; Xiao, S.; Li, B. KIT Mutations and Expression: Current Knowledge and New Insights for Overcoming IM Resistance in GIST. Cell Commun. Signal. 2024, 22, 153. [Google Scholar] [CrossRef]
- Sattler, M.; Salgia, R. Targeting C-Kit Mutations: Basic Science to Novel Therapies. Leuk. Res. 2004, 28, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Pećina-Šlaus, N. Merlin, the NF2 Gene Product. Pathol. Oncol. Res. 2013, 19, 365–373. [Google Scholar] [CrossRef]
- Schroeder, R.D.; Angelo, L.S.; Kurzrock, R. NF2/Merlin in Hereditary Neurofibromatosis 2 versus Cancer: Biologic Mechanisms and Clinical Associations. Oncotarget 2013, 5, 67. [Google Scholar] [CrossRef] [PubMed]
- Petrilli, A.M.; Fernández-Valle, C. Role of Merlin/NF2 Inactivation in Tumor Biology. Oncogene 2016, 35, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.H.; Bernards, R. Playing Cancer at Its Own Game: Activating Mitogenic Signaling as a Paradoxical Intervention. Mol. Oncol. 2021, 15, 1975–1985. [Google Scholar] [CrossRef]
- Dias, M.H.; Friskes, A.; Wang, S.; Fernandes Neto, J.M.; van Gemert, F.; Mourragui, S.; Papagianni, C.; Kuiken, H.J.; Mainardi, S.; Alvarez-Villanueva, D.; et al. Paradoxical Activation of Oncogenic Signaling as a Cancer Treatment Strategy. Cancer Discov. 2024, 14, 1276–1301. [Google Scholar] [CrossRef]


| Gene | Gene Accession Number | Primer | Primer Sequence |
|---|---|---|---|
| ACT | NM_001101.5 | upstream | CACCATTGGCAATGAGCGGTTC |
| downstream | AGGTCTTTGCGGATGTCCACG | ||
| SLC2A1 | NM_006516 | upstream | TTGCAGGCTTCTCCAACTGGAC |
| downstream | CAGAACCAGGAGCACAGTGAAG | ||
| SLC23A2 | NM_203327.2 | upstream | CCAAGAAAGGATGGACTGCGTAC |
| downstream | CATCTGTGCGAGCATAGAAGCC | ||
| NANOG | NM_024865 | upstream | CTCCAACATCCTGAACCTCAGC |
| downstream | CGTCACACCATTGCTATTCTTCG | ||
| POU5F1 | NM_002701 | upstream | CCTGAAGCAGAAGAGGATCACC |
| downstream | AAAGCGGCAGATGGTCGTTTGG | ||
| NES | NM_006617.2 | upstream | TCAAGATGTCCCTCAGCCTGGA |
| downstream | AAGCTGAGGGAAGTCTTGGAGC |
| Gene | Gene Accession Number | Primer | Primer Sequence |
|---|---|---|---|
| ACT | NM_001101.5 | upstream | CACCATTGGCAATGAGCGGTTC |
| downstream | AGGTCTTTGCGGATGTCCACG | ||
| SOD1 | NM_000454.5 | upstream | CTCACTCTCAGGAGACCATTGC |
| downstream | CCACAAGCCAAACGACTTCCAG | ||
| CAT | NM_001752.4 | upstream | GTGCGGAGATTCAACACTGCCA |
| downstream | CGGCAATGTTCTCACACAGACG | ||
| GPX1 | NM_001329502.2 | upstream | GCGGGGCAAGGTACTACTTA |
| downstream | CTCTTCGTTCTTGGCGTTCT | ||
| HIF1A | NM_001530 | upstream | TATGAGCCAGAAGAACTTTTAGGC |
| downstream | CACCTCTTTTGGCAAGCATCCTG | ||
| GAPDH | NM_002046.7 | upstream | TCAAGGCTGAGAACGGGAAG |
| downstream | CGCCCCACTTGATTTTGGAG | ||
| PPARGC1A | NM_013261 | upstream | CCAAAGGATGCGCTCTCGTTCA |
| downstream | CGGTGTCTGTAGTGGCTTGACT | ||
| ALPL | NM_000478 | upstream | GCTGTAAGGACATCGCCTACCA |
| downstream | CCTGGCTTTCTCGTCACTCTCA | ||
| COL1A1 | NM_000088 | upstream | GATTCCCTGGACCTAAAGGTGC |
| downstream | AGCCTCTCCATCTTTGCCAGCA |
| Gene | COSMIC ID | nt Change | AA Change | O-P2 | L-P5 | Rm-P6 |
|---|---|---|---|---|---|---|
| CTNNB1 | 5663 | c.133T>C | p.S45P | Mutant | WT | WT |
| CTNNB1 | 5738 | c.61G>A | p.A21T | WT | Mutant | WT |
| CTNNB1 | 5661 | c.94G>T | p.D32Y | WT | WT | WT |
| KIT | 28026 | c.1621A>C | p.M541L | WT | WT | Mutant |
| KIT | 1221 | c.1669T>G | p.W557G | WT | Mutant | WT |
| KIT | 18896 | c.1673_168del15 | p.K558_E562del | WT | Mutant | WT |
| KIT | 1255 | c.1676T>C | p.V559A | Mutant | Mutant | WT |
| KIT | 1290 | c.1727T>C | p.L576P | WT | Mutant | WT |
| NF2 | 22240 | c.634C>T | p.Q212 * | WT | WT | Mutant |
| NF2 | 22454 | c.655G>A | p.V219M | WT | Mutant | WT |
| NF2 | 22000 | c.784C>T | p.R262 * | WT | WT | WT |
| TP53 | 10758 | c.659A>G | p.Y220C | WT | WT | WT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ledinski, M.; Caput Mihalić, K.; Šimić Jovičić, M.; Ostojić, K.; Škibola, Z.; Kolundžić, R.; Urlić, I. Dehydroascorbic Acid Induces Cell Death in Sarcoma Stem Cells Under bFGF-Mediated Stemness-Supporting Conditions. Antioxidants 2025, 14, 1376. https://doi.org/10.3390/antiox14111376
Ledinski M, Caput Mihalić K, Šimić Jovičić M, Ostojić K, Škibola Z, Kolundžić R, Urlić I. Dehydroascorbic Acid Induces Cell Death in Sarcoma Stem Cells Under bFGF-Mediated Stemness-Supporting Conditions. Antioxidants. 2025; 14(11):1376. https://doi.org/10.3390/antiox14111376
Chicago/Turabian StyleLedinski, Maja, Katarina Caput Mihalić, Marijana Šimić Jovičić, Karla Ostojić, Zara Škibola, Robert Kolundžić, and Inga Urlić. 2025. "Dehydroascorbic Acid Induces Cell Death in Sarcoma Stem Cells Under bFGF-Mediated Stemness-Supporting Conditions" Antioxidants 14, no. 11: 1376. https://doi.org/10.3390/antiox14111376
APA StyleLedinski, M., Caput Mihalić, K., Šimić Jovičić, M., Ostojić, K., Škibola, Z., Kolundžić, R., & Urlić, I. (2025). Dehydroascorbic Acid Induces Cell Death in Sarcoma Stem Cells Under bFGF-Mediated Stemness-Supporting Conditions. Antioxidants, 14(11), 1376. https://doi.org/10.3390/antiox14111376








