Correlation of Antioxidant and Antibacterial Activities of the Aqueous Pinus pinaster Aiton Bark Extract Within a Cytocompatible Concentration Range
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Plant Material—Pinus pinaster Bark
2.2.1. Sample Preparation and Characterization
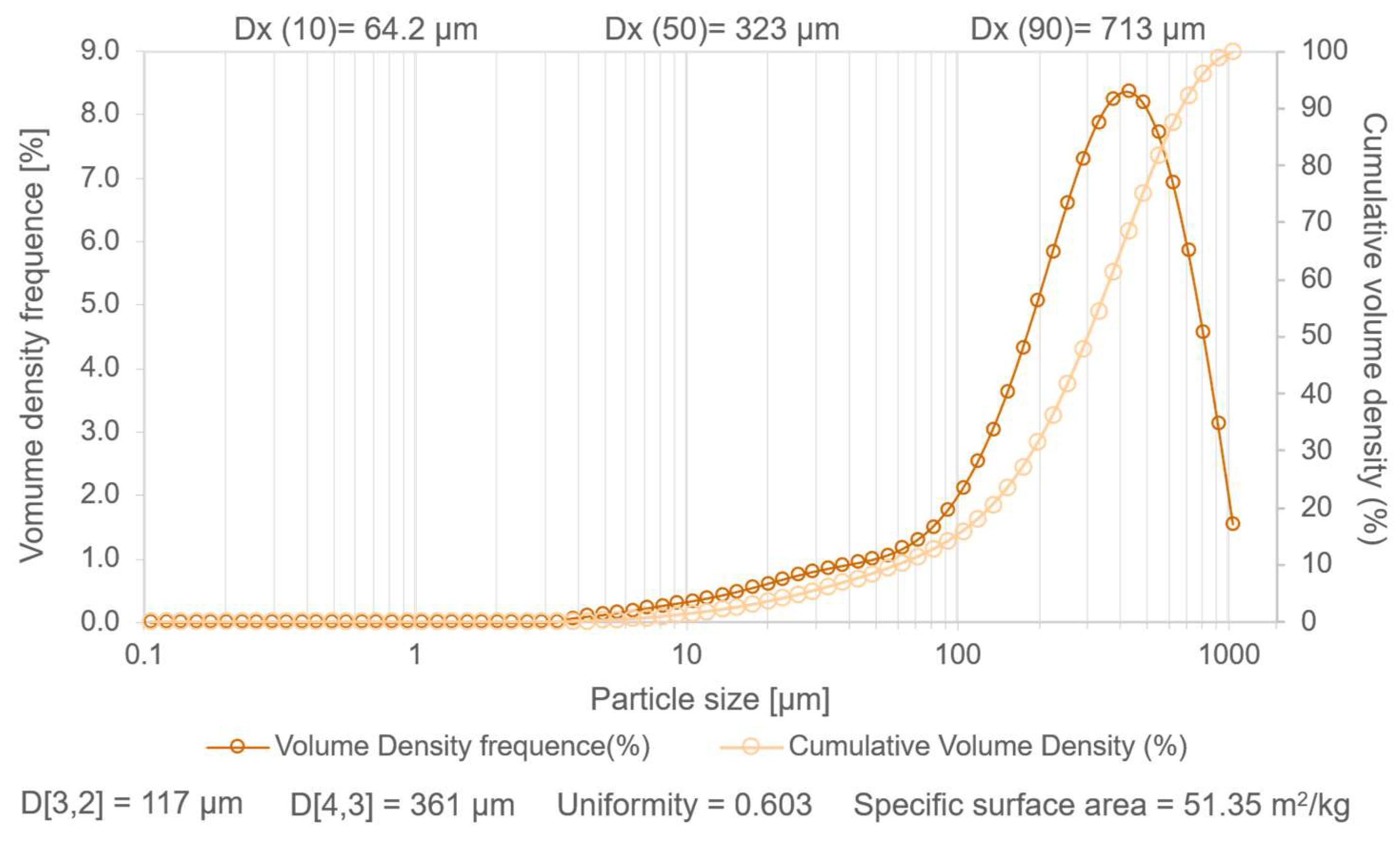
2.2.2. Granulometry
2.2.3. Chemical Analyses
2.3. Pine pinaster Bark Extracts
2.3.1. Microwave-Assisted Extraction (MAE) Processes
2.3.2. Extraction Yield
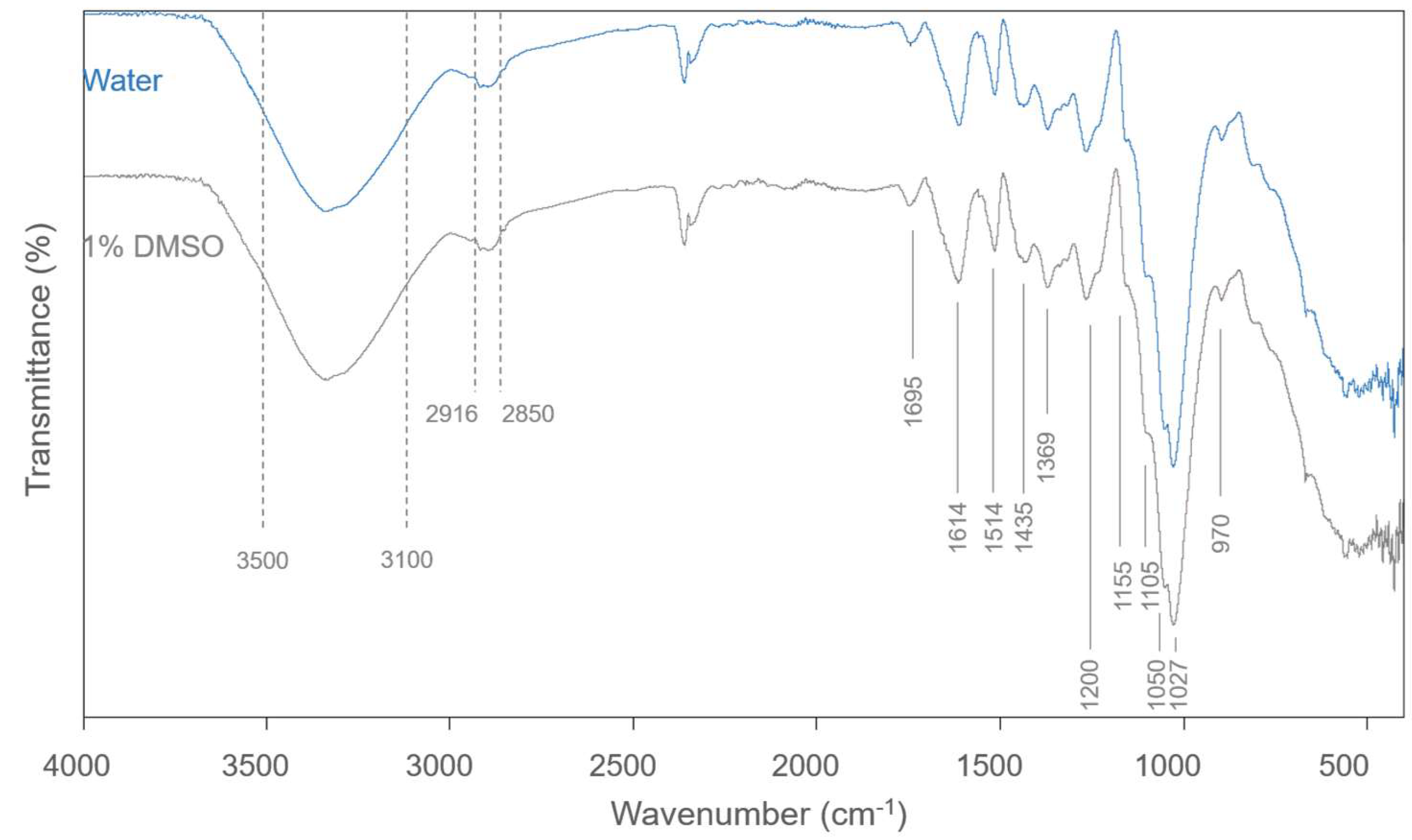
2.3.3. Fourier Transform Infrared Spectroscopy
2.3.4. Total Polyphenols and Flavonoids Contents, and Antioxidant Activity
2.3.5. Extracts’ Phenolic Compound Profile Using HPLC-DAD Analysis
2.4. Biological Profile of Pine Bark Extracts
2.4.1. Antibacterial Susceptibility Testing
2.4.2. Cell Culture and Cytotoxicity Assay
2.5. Data Analysis
3. Results and Discussion
3.1. Pine Bark (PB) Characterization
3.1.1. Particle Size Distribution
3.1.2. Chemical Characterization
3.2. Chemical Characterization of Pine Bark Extract (PBE)
3.2.1. Extraction Yield
3.2.2. FTIR Spectroscopy
3.2.3. Content of Total Polyphenol and Total Flavonoid
3.2.4. Antioxidant Activity
3.2.5. Assessment of Extract Similarities
3.2.6. Phenolic Compound Profile by HPLC-DAD Analysis
3.3. Antibacterial Activity and Cytotoxicity of the Aqueous Extract
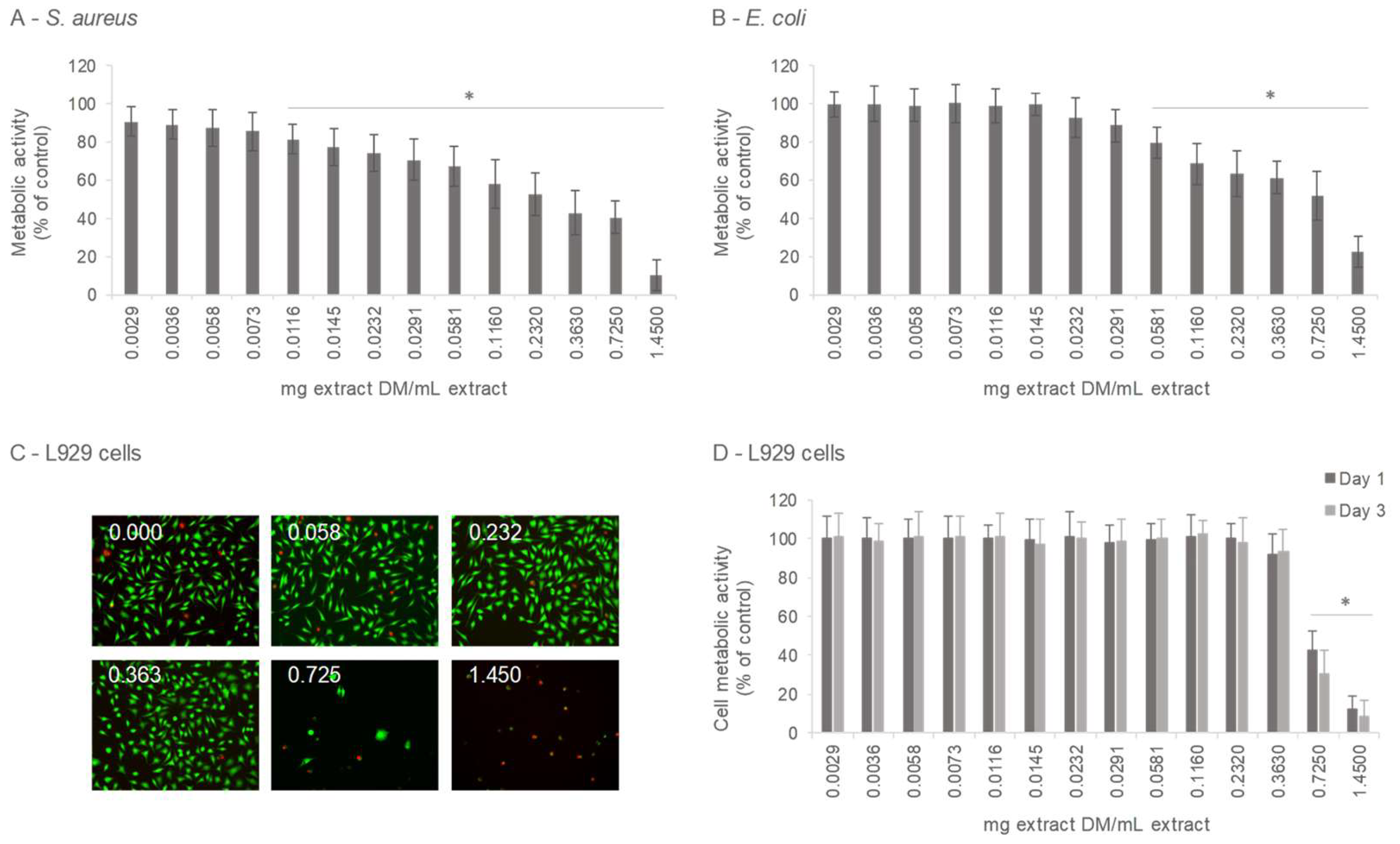
3.3.1. Antibacterial Activity
3.3.2. Cytotoxicity/Cytocompatibility
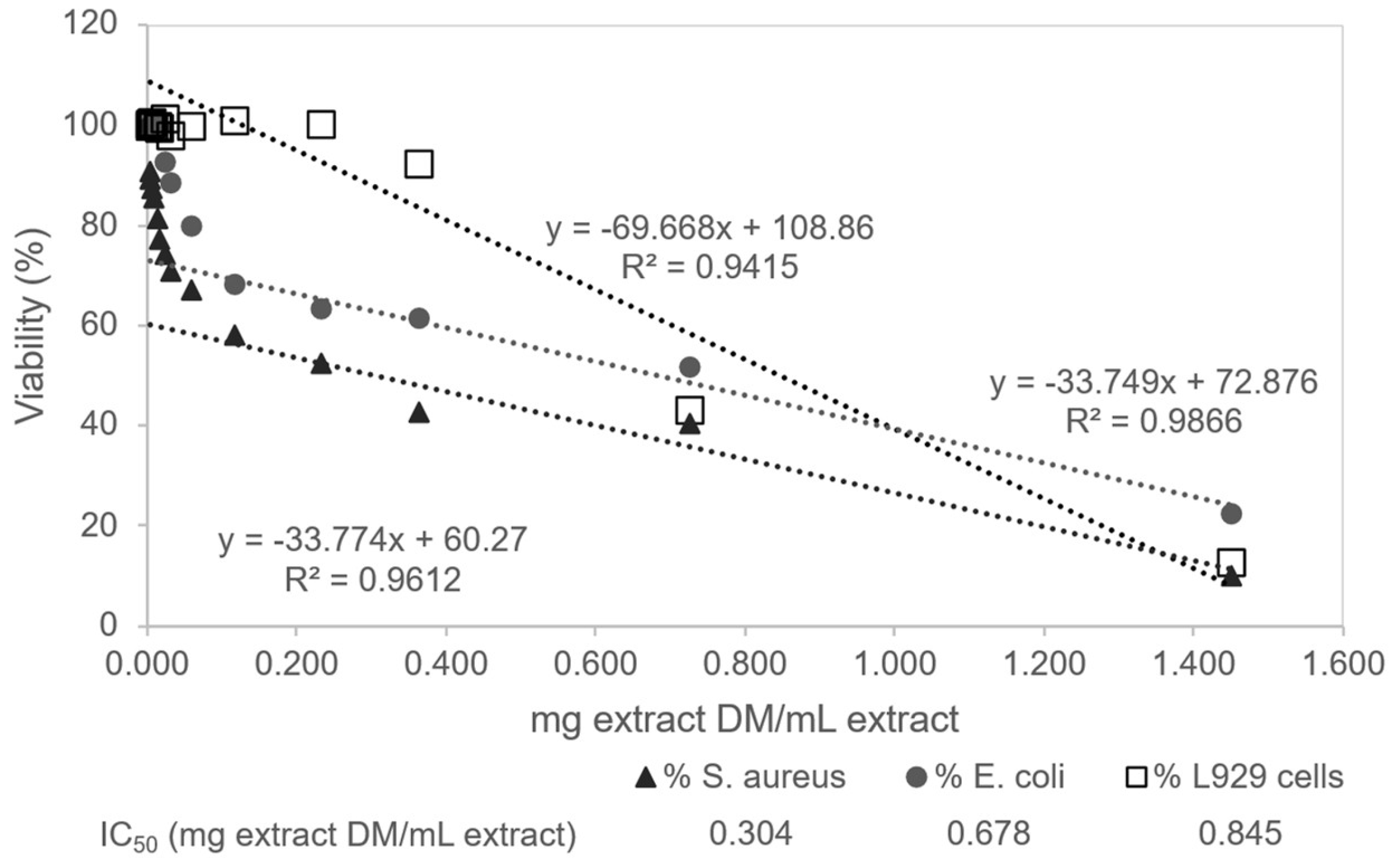
3.3.3. Correlation Between Antioxidant Activity and Biological Effects
3.4. Limitations of This Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alegria, C.; Roque, N.; Albuquerque, T.; Fernandez, P.; Ribeiro, M.M. Modelling Maritime Pine (Pinus pinaster Aiton) Spatial Distribution and Productivity in Portugal: Tools for Forest Management. Forests 2021, 12, 368. [Google Scholar] [CrossRef]
- da Costa, T.P.; Quinteiro, P.; Arroja, L.; Dias, A.C. Environmental comparison of forest biomass residues application in Portugal: Electricity, heat and biofuel. Renew. Sustain. Energy Rev. 2020, 134, 110302. [Google Scholar] [CrossRef]
- Dedrie, M.; Jacquet, N.; Bombeck, P.-L.; Hébert, J.; Richel, A. Oak barks as raw materials for the extraction of polyphenols for the chemical and pharmaceutical sectors: A regional case study. Ind. Crops Prod. 2015, 70, 316–321. [Google Scholar] [CrossRef]
- Dróżdż, P.; Pyrzynska, K. Extracts from pine and oak barks: Phenolics, minerals and antioxidant potential. International J. Environ. Anal. Chem. 2021, 101, 464–472. [Google Scholar] [CrossRef]
- Duarte, H.; Gomes, V.; Aliaño-González, M.J.; Faleiro, L.; Romano, A.; Medronho, B. Ultrasound-Assisted Extraction of Polyphenols from Maritime Pine Residues with Deep Eutectic Solvents. Foods 2022, 11, 3754. [Google Scholar] [CrossRef] [PubMed]
- Nisca, A.; Tanase, C. Approaches to Extracting Bioactive Compounds from Bark of Various Plants: A Brief Review. Plants 2025, 14, 2929. [Google Scholar] [CrossRef] [PubMed]
- Bayer, J.; Högger, P. Review of the pharmacokinetics of French maritime pine bark extract (Pycnogenol®) in humans. Front. Nutr. 2024, 11, 1389422. [Google Scholar] [CrossRef]
- Alonso-Esteban, J.I.; Carocho, M.; Barros, D.; Velho, M.V.; Heleno, S.; Barros, L. Chemical composition and industrial applications of Maritime pine (Pinus pinaster Ait.) bark and other non-wood parts. Rev. Environ. Sci. Bio./Technol. 2022, 21, 583–633. [Google Scholar] [CrossRef]
- Ramos, P.A.B.; Pereira, C.; Gomes, A.P.; Neto, R.T.; Almeida, A.; Santos, S.A.O.; Silva, A.M.S.; Silvestre, A.J.D. Chemical Characterisation, Antioxidant and Antibacterial Activities of Pinus pinaster Ait. and Pinus pinea L. Bark Polar Extracts: Prospecting Forestry By-Products as Renewable Sources of Bioactive Compounds. Appl. Sci. 2022, 12, 784. [Google Scholar] [CrossRef]
- Lee, E.L.; Barnes, J. Pine bark. J. Prim. Health Care 2023, 15, 192–194. [Google Scholar] [CrossRef]
- Mohammadi, S.; Fulop, T.; Khalil, A.; Ebrahimi, S.; Hasani, M.; Ziaei, S.; Farsi, F.; Mirtaheri, E.; Afsharianfar, M.; Heshmati, J. Does Supplementation with Pine Bark Extract Improve Cardiometabolic Risk Factors? A Systematic Review and Meta-Analysis. BMC Complement. Med. Ther. 2025, 25, 71. [Google Scholar] [CrossRef]
- Duarte, H.; Gomes, V.; Aliaño-González, M.J.; Faleiro, L.; Romano, A.; Medronho, B. Optimization of the extraction of polyphenols from Pinus pinaster residues using deep eutectic solvents: A sustainable approach. Wood Sci. Technol. 2023, 57, 1175–1196. [Google Scholar] [CrossRef]
- Ku, C.S.; Mun, S.P.; Jang, J. Effects of Water Extraction Temperatures on the Yield, Molecular Weight, and Antioxidant Activity of Proanthocyanidins Extracted from Pinus radiata Bark. For. Prod. J. 2011, 61, 321–325. [Google Scholar] [CrossRef]
- Ku, C.S.; Jang, J.P.; Mun, S.P. Exploitation of polyphenol-rich pine barks for potent antioxidant activity. J. Wood Sci. 2007, 53, 524–528. [Google Scholar] [CrossRef]
- D’Andrea, G. Pycnogenol: A blend of procyanidins with multifaceted therapeutic applications? Fitoterapia 2010, 81, 724–736. [Google Scholar] [CrossRef]
- Mármol, I.; Quero, J.; Jiménez-Moreno, N.; Rodríguez-Yoldi, M.J.; Ancín-Azpilicueta, C. A systematic review of the potential uses of pine bark in food industry and health care. Trends Food Sci. Technol. 2019, 88, 558–566. [Google Scholar] [CrossRef]
- Dziedziński, M.; Kobus-Cisowska, J.; Stachowiak, B. Pinus Species as Prospective Reserves of Bioactive Compounds with Potential Use in Functional Food—Current State of Knowledge. Plants 2021, 10, 1306. [Google Scholar] [CrossRef]
- Barros, D.; Fernandes, É.; Jesus, M.; Barros, L.; Alonso-Esteban, J.I.; Pires, P.; Vaz Velho, M. The Chemical Characterisation of the Maritime Pine Bark Cultivated in Northern Portugal. Plants 2023, 12, 3940. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Rockville, MD, USA, 1995. [Google Scholar]
- D1105-96; Standard Test Method for Preparation of Extractive-Free Wood. ASTM: West Conshohocken, PA, USA, 2001.
- Ona, T.; Sonoda, T.; Shibata, M.; Fukazawa, K. Small-Scale Method to Determine the Content of Wood Components from Multiple Eucalyptus Samples. Tappi J. 1995, 78, 121–126. [Google Scholar]
- APHA/AWWA/WEF. Standard Methods for the Examination of Water and Wastewater; American Water Works Association: Denver, CO, USA, 2017. [Google Scholar]
- Barros, D.; Pereira-Pinto, R.; Fernandes, É.; Pires, P.; Vaz-Velho, M. Microwave-Assisted Extraction for the Sustainable Recovery and Valorization of Phenolic Compounds from Maritime Pine Bark. Sustain. Chem. 2025, 6, 26. [Google Scholar] [CrossRef]
- Sládková, A.; Benedeková, M.; Stopka, J.; Surina, I.; Haz, A.; Strižincová, P.; Kučíková, K.; Butor Skulcova, A.; Burčová, Z.; Kreps, F.; et al. Yield of Polyphenolic Substances Extracted From Spruce (Picea abies) Bark by Microwave-Assisted Extraction. Bioresources 2016, 11, 9912–9921. [Google Scholar] [CrossRef]
- Jurić, M.; Golub, N.; Galić, E.; Radić, K.; Maslov Bandić, L.; Vitali Čepo, D. Micro-wave-Assisted Extraction of Bioactive Compounds from Mandarin Peel: A Compre-hensive Biorefinery Strategy. Antioxidants 2025, 14, 722. [Google Scholar] [CrossRef]
- Eroglu, E. Investigation of phenolic profile and in vitro bioactivities of DMSO extract from clove oil. S. Afr. J. Bot. 2025, 184, 761–768. [Google Scholar] [CrossRef]
- Tapia-Quiros, P.; Granados, M.; Sentellas, S.; Saurina, J. Microwave-assisted extraction with natural deep eutectic solvents for polyphenol recovery from agrifood waste: Mature for scaling-up? Sci. Total Environ. 2024, 912, 168716. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, P.; Sivashanmugam, P. Studies on the extraction of polyphenolic compounds from pre-consumer organic solid waste. J. Ind. Eng. Chem. 2020, 82, 130–137. [Google Scholar] [CrossRef]
- Aspé, E.; Fernández, K. The effect of different extraction techniques on extraction yield, total phenolic, and anti-radical capacity of extracts from Pinus radiata Bark. Ind. Crops Prod. 2011, 34, 838–844. [Google Scholar] [CrossRef]
- Oroian, M.; Escriche, I. Antioxidants: Characterization, natural sources, extraction and analysis. Food Res. Int. 2015, 74, 10–36. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Barros, L.; Oliveira, S.; Carvalho, A.M.; Ferreira, I.C.F.R. In vitro antioxidant properties and characterization in nutrients and phytochemicals of six medicinal plants from the Portuguese folk medicine. Ind. Crops Prod. 2010, 32, 572–579. [Google Scholar] [CrossRef]
- Lee, J.H.; Woo, K.S.; Lee, H.U.; Nam, S.S.; Lee, B.W.; Lee, Y.Y.; Lee, B.; Kim, H.J. Intracellular Reactive Oxygen Species (ROS) Removal and Cytoprotection Effects of Sweet Potatoes of Various Flesh Colors and Their Polyphenols, Including Anthocyanin. Prev. Nutr. Food Sci. 2019, 24, 293–298. [Google Scholar] [CrossRef]
- Lang, Y.; Gao, N.; Zang, Z.; Meng, X.; Lin, Y.; Yang, S.; Yang, Y.; Jin, Z.; Li, B. Classification and antioxidant assays of polyphenols: A review. J. Future Foods 2024, 4, 193–204. [Google Scholar] [CrossRef]
- Coscueta, E.R.; Reis, C.A.; Pintado, M. Phenylethyl Isothiocyanate Extracted from Watercress By-Products with Aqueous Micellar Systems: Development and Optimisation. Antioxidants 2020, 9, 698. [Google Scholar] [CrossRef]
- Rothe, J.; Fischer, R.; Cotterchio, C.; Gastl, M.; Becker, T. Analytical determination of antioxidant capacity of hop-derived compounds in beer using specific rapid assays (ORAC, FRAP) and ESR-spectroscopy. Eur. Food Res. Technol. 2023, 249, 81–93. [Google Scholar] [CrossRef]
- Vázquez, G.; Antorrena, G.; Parajó, J.C. Studies on the utilization of Pinus pinaster bark. Wood Sci. Technol. 1987, 21, 65–74. [Google Scholar] [CrossRef]
- Santos, J.; Pereira, J.; Ferreira, N.; Paiva, N.; Ferra, J.; Magalhães, F.D.; Martins, J.M.; Dulyanska, Y.; Carvalho, L.H. Valorisation of non-timber by-products from maritime pine (Pinus pinaster, Ait) for particleboard production. Ind. Crops Prod. 2021, 168, 113581. [Google Scholar] [CrossRef]
- Maekawa, E.; Ichizawa, T.; Koshijima, T. An Evaluation of the Acid-Soluble Lignin Determination in Analyses of Lignin by the Sulfuric Acid Method. J. Wood Chem. Technol. 1989, 9, 549–567. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, T.; Van Den Bosch, S.; Koelewijn, S.F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef]
- Fradinho, D.M.; Neto, C.P.; Evtuguin, D.; Jorge, F.C.; Irle, M.A.; Gil, M.H.; Pedrosa de Jesus, J. Chemical characterisation of bark and of alkaline bark extracts from maritime pine grown in Portugal. Ind. Crops Prod. 2002, 16, 23–32. [Google Scholar] [CrossRef]
- Nunes, E.; Quilhó, T.; Pereira, H. Anatomy and Chemical Composition of Pinus pinaster Bark. IAWA J. 1996, 17, 141–150. [Google Scholar] [CrossRef]
- Willför, S.M.; Smeds, A.I.; Holmbom, B.R. Chromatographic analysis of lignans. J. Chromatogr. A 2006, 1112, 64–77. [Google Scholar] [CrossRef]
- Bożym, M.; Gendek, A.; Siemiątkowski, G.; Aniszewska, M.; Malaťák, J. Assessment of the Composition of Forest Waste in Terms of Its Further Use. Materials 2021, 14, 973. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2010; pp. 1–520. [Google Scholar]
- Narayan, O.P.; Kumar, P.; Yadav, B.; Dua, M.; Johri, A.K. Sulfur nutrition and its role in plant growth and development. Plant Signal. Behav. 2022, 18, 2030082. [Google Scholar] [CrossRef]
- Zhao, F.-j.; Tausz, M.; De Kok, L.J. Role of Sulfur for Plant Production in Agricultural and Natural Ecosystems. In Sulfur Metabolism in Phototrophic Organisms; Hell, R., Dahl, C., Knaff, D., Leustek, T., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 417–435. [Google Scholar]
- Chorianopoulou, S.N.; Bouranis, D.L. The Role of Sulfur in Agronomic Biofortification with Essential Micronutrients. Plants 2022, 11, 1979. [Google Scholar] [CrossRef]
- Sukhdolgor, J.; Badamtsetseg, S.; Adyakhuu, D. Chemical Composition and Amount of Macro and Microelements of Pine (Pinus silvestris L) and Larch (Larix sibirica Ldb) Trees in Mongolia. Mong. J. Biol. Sci. 2003, 1, 81–83. [Google Scholar] [CrossRef]
- Kirchner, P.; Biondi, F.; Edwards, R.; McConnell, J.R. Variability of trace metal concentrations in Jeffrey pine (Pinus jeffreyi) tree rings from the Tahoe Basin, California, USA. J. For. Res. 2008, 13, 347–356. [Google Scholar] [CrossRef]
- Poikolainen, J. Sulphur and heavy metal concentrations in Scots pine bark in northern Finland and the Kola peninsula. Water Air Soil Pollut. 1997, 93, 395–408. [Google Scholar] [CrossRef]
- Nunes, E.; Quilhó, T.; Pereira, H. Anatomy and chemical composition of Pinus pinea L. bark. Ann. For. Sci. 1999, 56, 479–484. [Google Scholar] [CrossRef]
- Pals, M.; Lauberte, L.; Ponomarenko, J.; Lauberts, M.; Arshanitsa, A. Microwave-Assisted Water Extraction of Aspen (Populus tremula) and Pine (Pinus sylvestris L.) Barks as a Tool for Their Valorization. Plants 2022, 11, 1544. [Google Scholar] [CrossRef] [PubMed]
- Galili, S.; Hovav, R. Chapter 16—Determination of Polyphenols, Flavonoids, and Antioxidant Capacity in Dry Seeds. In Polyphenols in Plants; Watson, R.R., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 305–323. [Google Scholar]
- Chupin, L.; Motillon, C.; Charrier-El Bouhtoury, F.; Pizzi, A.; Charrier, B. Characterisation of maritime pine (Pinus pinaster) bark tannins extracted under different conditions by spectroscopic methods, FTIR and HPLC. Ind. Crops Prod. 2013, 49, 897–903. [Google Scholar] [CrossRef]
- Chupin, L.; Maunu, S.L.; Reynaud, S.; Pizzi, A.; Charrier, B.; Charrier-El Bouhtoury, F. Microwave assisted extraction of maritime pine (Pinus pinaster) bark: Impact of particle size and characterization. Ind. Crops Prod. 2015, 65, 142–149. [Google Scholar] [CrossRef]
- Olszowy, M. What is responsible for antioxidant properties of polyphenolic compounds from plants? Plant Physiol. Biochem. 2019, 144, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Korkalo, P.; Korpinen, R.; Beuker, E.; Sarjala, T.; Hellström, J.; Kaseva, J.; Lassi, U.; Jyske, T. Clonal Variation in the Bark Chemical Properties of Hybrid Aspen: Potential for Added Value Chemicals. Molecules 2020, 25, 4403. [Google Scholar] [CrossRef] [PubMed]
- Amarowicz, R.; Pegg, R.B. Chapter One—Natural antioxidants of plant origin. In Advances in Food and Nutrition Research; Ferreira, I.C.F.R., Barros, L., Eds.; Academic Press: New York, NY, USA, 2019; Volume 90, pp. 1–81. [Google Scholar]
- Dudonné, S.; Xavier, V.; Coutière, P.; Woillez, M.; Merillon, J.-M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Ustun, O.; Senol, F.S.; Kurkcuoglu, M.; Orhan, I.E.; Kartal, M.; Baser, K.H.C. Investigation on chemical composition, anticholinesterase and antioxidant activities of extracts and essential oils of Turkish Pinus species and pycnogenol. Ind. Crops Prod. 2012, 38, 115–123. [Google Scholar] [CrossRef]
- Vieito, C.; Pires, P.; Fernandes, É.; Vaz-Velho, M. Chemical characterization of pine bark (Pinus pinaster Aiton subsp. atlantica), antioxidant properties and phenolic profile of its extracts. Milleninum 2019, 2, 79–87. [Google Scholar] [CrossRef]
- Sillero, L.; Prado, R.; Welton, T.; Labidi, J. Extraction of flavonoid compounds from bark using sustainable deep eutectic solvents. Sustain. Chem. Pharm. 2021, 24, 100544. [Google Scholar] [CrossRef]
- Hamad, A.; Ates, S.; Olgun, Ç.; Gür, M. Chemical composition and antioxidant properties of some industrial tree bark extracts. BioResources 2019, 14, 15. [Google Scholar] [CrossRef]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.M.; Yoga Latha, L. Extraction, isolation and characterization of bioactive compounds from plants? Extracts. Afr. J. Tradit. Complement Altern. Med. 2011, 8. [Google Scholar] [CrossRef]
- Cuevas-Valenzuela, J.; González-Rojas, Á.; Wisniak, J.; Apelblat, A.; Pérez-Correa, J.R. Solubility of (+)-catechin in water and water-ethanol mixtures within the temperature range 277.6–331.2K: Fundamental data to design polyphenol extraction processes. Fluid Phase Equilibria 2014, 382, 279–285. [Google Scholar] [CrossRef]
- Ko, M.-J.; Cheigh, C.-I.; Chung, M.-S. Relationship analysis between flavonoids structure and subcritical water extraction (SWE). Food Chem. 2014, 143, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Gorinstein, S.; Böhm, V.; Schaich, K.M.; Özyürek, M.; Güçlü, K. Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC Technical Report). Pure Appl. Chem. 2013, 85, 957–998. [Google Scholar] [CrossRef]
- Venkatesan, T.; Choi, Y.W.; Kim, Y.K. Impact of Different Extraction Solvents on Phenolic Content and Antioxidant Potential of Pinus densiflora Bark Extract. BioMed Res. Int. 2019, 2019, 3520675. [Google Scholar] [CrossRef]
- Nhu-Trang, T.-T.; Nguyen, Q.-D.; Cong-Hau, N.; Anh-Dao, L.-T.; Behra, P. Characteristics and Relationships between Total Polyphenol and Flavonoid Contents, Antioxidant Capacities, and the Content of Caffeine, Gallic Acid, and Major Catechins in Wild/Ancient and Cultivated Teas in Vietnam. Molecules 2023, 28, 3470. [Google Scholar] [CrossRef]
- Kim, Y.J.; Nam, T.G.; Lee, I.; Heo, H.J.; Kim, D.O. Development and validation of ana-lytical HPLC for phenolics in Pinus densiflora bark extract. Food Sci. Biotechnol. 2025, 34, 1139–1148. [Google Scholar] [CrossRef]
- Karaçelik, A.A.; Şeker, M.E.; Karaköse, M. Determination of Antioxidant Activity of Different Extracts from Bark of Pinus spp. grown in Giresun (Turkey) Province—Phenolic analysis by RP-HPLC-DAD. Ksu Tarim Ve Doga Derg. Ksu J. Agric. Nat. 2022, 25, 10–18. [Google Scholar] [CrossRef]
- Yesil-Celiktas, O.; Otto, F.; Parlar, H. A comparative study of flavonoid contents and anti-oxidant activities of supercritical CO2 extracted pine barks grown in different regions of Turkey and Germany. Eur. Food Res. Technol. 2009, 229, 671–677. [Google Scholar] [CrossRef]
- Nisca, A.; Ștefănescu, R.; Stegăruș, D.I.; Mare, A.D.; Farczadi, L.; Tanase, C. Comparative Study Regarding the Chemical Composition and Biological Activity of Pine (Pinus nigra and P. sylvestris) Bark Extracts. Antioxidants 2021, 10, 327. [Google Scholar] [CrossRef]
- Popescu, D.I.; Frum, A.; Dobrea, C.M.; Cristea, R.; Gligor, F.G.; Vicas, L.G.; Ionete, R.E.; Sutan, N.A.; Georgescu, C. Comparative Antioxidant and Antimicrobial Activities of Several Conifer Needles and Bark Extracts. Pharmaceutics 2024, 16, 52. [Google Scholar] [CrossRef]
- Mármol, I.; Vieito, C.; Andreu, V.; Levert, A.; Amiot, A.; Bertrand, C.; Rodríguez-Yoldi, M.J.; Santos, J.; Vaz-Velho, M. Influence of extraction solvent on the biological properties of maritime pine bark (Pinus pinaster). Int. J. Food Stud. 2022, 11, 51–62. [Google Scholar] [CrossRef]
- Douglas, T.; Heredia, M.; Pulczynska, A.; Lapa, A.; Pietryga, K.; Schaubroeck, D.; Santos, S.; Pais, A.; Brackman, G.; De Scham-phelaere, K.; et al. Phenolic plant extract enrichment of enzymatically mineralized hydrogels. Eng. Biomater. 2019, 149, 2–9. [Google Scholar]
- Torras, M.A.; Faura, C.A.; Schönlau, F.; Rohdewald, P. Antimicrobial activity of Pycnogenol. Phytother. Res. 2005, 19, 647–648. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices: Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization (ISO): Geneva, Switzerland, 2009; Reviewed and Confirmed in 2022.
- Abraham, M.; Augustine, D.; Rao, R.S.; Sowmya, S.V.; Haragannavar, V.C.; Nambiar, S.; Prasad, K.; Awan, K.H.; Patil, S. Naturally Available Extracts Inhibiting Cancer Progression: A Systematic Review. J. Evid.-Based Complement. Altern. Med. 2017, 22, 870–878. [Google Scholar] [CrossRef]
- Weichmann, F.; Rohdewald, P. Pycnogenol® French maritime pine bark extract in randomized, double-blind, placebo-controlled human clinical studies. Front. Nutr. 2024, 11, 1389374. [Google Scholar] [CrossRef]
- Sánchez-Moya, T.; López-Nicolás, R.; Peso-Echarri, P.; González-Bermúdez, C.A.; Frontela-Saseta, C. Effect of pine bark extract and its phenolic compounds on selected pathogenic and probiotic bacterial strains. Front. Nutr. 2024, 11, 1381125. [Google Scholar] [CrossRef]
- Xu, W.; Lin, Z.; Cortez-Jugo, C.; Qiao, G.G.; Caruso, F. Antimicrobial Phenolic Materials: From Assembly to Function Angew. Chem. Int. Ed. 2025, 64, e202423654. [Google Scholar] [CrossRef]
- Dembińska, K.; Shinde, A.H.; Pejchalová, M.; Richert, A.; Swiontek Brzezinska, M. The Ap-plication of Natural Phenolic Substances as Antimicrobial Agents in Agriculture and Food In-dustry. Foods 2025, 14, 1893. [Google Scholar] [CrossRef]
- Chen, X.; Lan, W.; Xie, J. Natural phenolic compounds: Antimicrobial properties, antimicrobial mechanisms, and potential utilization in the preservation of aquatic products. Food Chem. 2024, 440, 138198. [Google Scholar] [CrossRef] [PubMed]
- Ajiboye, T.O.; Aliyu, M.; Isiaka, I.; Haliru, F.Z.; Ibitoye, O.B.; Uwazie, J.N.; Muritala, H.F.; Bello, S.A.; Yusuf, I.I.; Mohammed, A.O. Contribution of reactive oxygen species to (+)-catechin-mediated bacterial lethality. Chem. Biol. Interact. 2016, 258, 276–287. [Google Scholar] [CrossRef]
- Mayer, R.; Stecher, G.; Wuerzner, R.; Silva, R.C.; Sultana, T.; Trojer, L.; Feuerstein, I.; Krieg, C.; Abel, G.; Popp, M.; et al. Proanthocyanidins: Target compounds as antibacterial agents. J. Agric. Food Chem. 2008, 56, 6959–6966. [Google Scholar] [CrossRef] [PubMed]
- Lobiuc, A.; Pavăl, N.-E.; Mangalagiu, I.I.; Gheorghiță, R.; Teliban, G.-C.; Amăriucăi-Mantu, D.; Stoleru, V. Future Antimicrobials: Natural and Functionalized Phenolics. Molecules 2023, 28, 1114. [Google Scholar] [CrossRef] [PubMed]




| Compound | Hill Notation | Wavelength (nm) 1 | R2 | LoD (mg/L) |
|---|---|---|---|---|
| Cinnamic acid | C9H8O2 | 280 | 0.9996 | 0.079 |
| Catechin | C15H14O6 | 280 | 0.9995 | 0.197 |
| Gallocatechin | C15H14O7 | 280 | 0.9956 | 0.995 |
| Epicatechin | C15H14O6 | 280 | 0.9997 | 0.671 |
| Quercetin | C15H10O7 | 320 | 0.9993 | 0.070 |
| Gallic acid | C7H6O5 | 280 | 0.9998 | 0.248 |
| Syringic acid | C9H10O5 | 280 | 0.9997 | 0.342 |
| Caffeic acid | C9H8O4 | 320 | 0.9992 | 0.143 |
| Taxifolin | C15H12O7 | 280 | 0.9992 | 0.383 |
| Ferulic acid | C10H10O4 | 320 | 0.9999 | 0.051 |
| Ellagic acid | C14H6O8 | 280 | 0.9984 | 0.556 |
| Protocatechuic acid | C7H6O4 | 280 | 0.9990 | 0.378 |
| Vanillin | C8H8O3 | 280 | 0.9998 | 0.185 |
| Resveratrol | C14H12O3 | 320 | 0.9999 | 0.059 |
| o-coumaric acid | C9H8O3 | 280 | 0.9996 | 0.079 |
| Parameter | (% w/w) |
|---|---|
| Moisture | 6.67 ± 0.01 |
| Total protein | 1.94 ± 0.22 |
| Total fat | 0.98 ± 0.00 |
| Ash | 0.44 ± 0.01 |
| Holocellulose | 46.09 ± 0.48 |
| Cellulose | 23.21 ± 0.57 |
| α-Cellulose | 22.88 ± 0.02 |
| Hemicellulose a | 23.21 ± 0.02 a |
| Lignins | 51.15 ± 0.35 |
| Acid soluble | 2.85 ± 0.35 |
| Klason | 48.31 ± 0.31 |
| Total extractives | 9.75 ± 0.13 |
| Toluene–ethanol (T:E) | 5.61 ± 0.05 |
| Ethanol (E) | 1.54 ± 0.06 |
| Water (W) | 2.59 ± 0.10 |
| Minerals | |
| Minerals, Content > 3.4 × 10−3% b | 1.52 × 10−1 ± 1.91 × 10−3 |
| Minerals, Content < 3.2 × 10−4% c | 5.01 × 10−4 ± 1.12 × 10−5 |
| DPPH | ABTS | ORAC | FRAP | ||
|---|---|---|---|---|---|
| mg TE/g PBE | DPPH | - | 2.17 × 10−2 | 1.10 × 10−5 | 7.95 × 10−4 |
| (PBE—100% Water) | ABTS | 2.17 × 10−2 | - | 1.30 × 10−5 | 6.10 × 10−5 |
| ORAC | 1.10 × 10−5 | 1.30 × 10−5 | - | 5.00 × 10−6 | |
| FRAP | 7.95 × 10−4 | 6.10 × 10−5 | 5.00 × 10−6 | - | |
| mg TE/g PBE | DPPH | - | 2.05 × 10−4 | 5.00 × 10−5 | 8.00 × 10−6 |
| (PBE—1% DMSO) | ABTS | 2.05 × 10−4 | - | 6.60 × 10−5 | 3.00 × 10−6 |
| ORAC | 5.00 × 10−5 | 6.60 × 10−5 | - | 3.10 × 10−5 | |
| FRAP | 8.00 × 10−6 | 3.00 × 10−6 | 3.10 × 10−5 | - |
| Water | 1%DMSO | p-Value * | |||
|---|---|---|---|---|---|
| Extraction yield | mg/mL PBE | 2.91 ± 0.02 | 3.31 ± 0.01 | p < 0.05 | p = 1.81 × 10−3 |
| g PBE/100 g PB | 5.77 ± 0.04 | 6.55 ± 0.03 | p < 0.05 | p = 1.92 × 10−3 | |
| TPC | mg GAE/g PBE | 397.4 ± 5.7 | 401.2 ± 8.1 | p > 0.05 | p = 0.540 |
| mg GAE/mL PBE | 1.155 ± 0.017 | 1.328 ± 0.027 | p < 0.001 | p = 6.83 × 10−4 | |
| mg GAE/g PB | 22.93 ± 0.33 | 26.27 ± 0.53 | p < 0.001 | p = 7.59 × 10−4 | |
| TFC | mg CE/g PBE | 90.4 ± 2.0 | 93.3 ± 2.3 | p > 0.05 | p = 0.100 |
| mg CE/mL PBE | 0.263 ± 0.006 | 0.309 ± 0.008 | p < 0.001 | p = 7.00 × 10−5 | |
| mg CE/g PB | 5.21 ± 0.11 | 6.11 ± 0.15 | p < 0.001 | p = 7.90 × 10−5 | |
| DPPH | mg TE/g PBE | 881 ± 55 | 875 ± 13 | p > 0.05 | p = 0.855 |
| mg TE/mL PBE | 2.56 ± 0.16 | 2.896 ± 0.043 | p < 0.05 | p = 2.43 × 10−2 | |
| mg TE/g PB | 50.8 ± 3.2 | 57.27 ± 0.86 | p < 0.05 | p = 2.70 × 10−2 | |
| ABTS | mg TE/g PBE | 1025 ± 41 | 1034 ± 17 | p > 0.05 | p = 0.743 |
| mg TE/mL PBE | 2.98 ± 0.12 | 3.422 ± 0.055 | p < 0.05 | p = 4.16 × 10−3 | |
| mg TE/g PB | 59.1 ± 2.3 | 67.7 ± 1.1 | p < 0.05 | p = 4.62 × 10−3 | |
| ORAC | mg TE/g PBE | 3188 ± 137 | 3227.0 ± 301 | p > 0.05 | p = 0.806 |
| mg TE/mL PBE | 9.26 ± 0.40 | 10.7 ± 1.0 | p < 0.05 | p = 4.12 × 10−2 | |
| mg TE/g PB | 183.9 ± 7.9 | 211 ± 20 | p < 0.05 | p = 4.44 × 10−2 | |
| FRAP—TE | mg TE/g PBE | 581 ± 16 | 588 ± 11 | p > 0.05 | p = 0.530 |
| mg TE/mL PBE | 1.688 ± 0.047 | 1.948 ± 0.036 | p < 0.05 | p = 1.62 × 10−3 | |
| mg TE/g PB | 33.51 ± 0.93 | 38.52 ± 0.72 | p < 0.05 | p = 1.79 × 10−3 | |
| FRAP—Fe2+ | mg Fe2+/g PBE | 265 ± 12 | 265.8 ± 2.0 | p > 0.05 | p = 0.879 |
| mg Fe2+/mL PBE | 0.769 ± 0.036 | 0.880 ± 0.007 | p < 0.05 | p = 6.10 × 10−3 | |
| mg Fe2+/g PB | 15.27 ± 0.71 | 17.40 ± 0.13 | p < 0.05 | p = 6.81 × 10−3 | |

| Compound | Water | 1%DMSO | p-Value * | ||
|---|---|---|---|---|---|
| Cinnamic acid (C9H8O2) | mg PC 1/g PBE | 1.47 ± 0.10 | 1.495 ± 0.033 | p > 0.05 | p = 0.886 |
| mg PC/L PBE | 4.27 ± 0.30 | 4.95 ± 0.11 | p < 0.05 | p = 2.08 × 10−2 | |
| mg PC/Kg PB | 84.7 ± 5.9 | 98.0 ± 2.2 | p < 0.05 | p = 2.18 × 10−2 | |
| Catechin (C15H14O6) | mg PC/g PBE | 21.3 ± 1.5 | 21.77 ± 0.48 | p > 0.05 | p = 0.883 |
| mg PC/L PBE | 62.1 ± 4.3 | 72.1 ± 1.6 | p < 0.05 | p = 2.05 × 10−2 | |
| mg PC/Kg PB | 1232 ± 86 | 1426 ± 31 | p < 0.001 | p = 2.15 × 10−2 | |
| Gallocatechin (C15H14O7) | mg PC/g PBE | 3.89 ± 0.27 | 3.991 ± 0.088 | p > 0.05 | p = 0.841 |
| mg PC/L PBE | 11.31 ± 0.79 | 13.21 ± 0.29 | p < 0.05 | p = 1.7 × 10−2 | |
| mg PC/Kg PB | 224 ± 16 | 261.4 ± 5.8 | p < 0.05 | p = 1.83 × 10−2 | |
| Quercetin (C15H10O7) | mg PC/g PBE | 2.825 ± 0.085 | 2.96 ± 0.21 | p > 0.05 | p = 0.758 |
| mg PC/L PBE | 8.22 ± 0.25 | 9.81 ± 0.69 | p < 0.001 | p = 1.95 × 10−2 | |
| mg PC/Kg PB | 163.0 ± 4.9 | 194.1 ± 4.2 | p < 0.001 | p = 2.02 × 10−2 | |
| Caffeic acid (C9H8O4) | mg PC/g PBE | 1.251 ± 0.088 | 1.320 ± 0.029 | p > 0.05 | p = 0.682 |
| mg PC/L PBE | 3.64 ± 0.25 | 4.370 ± 0.096 | p < 0.001 | p = 9.71 × 10−3 | |
| mg PC/Kg PB | 72.2 ± 5.1 | 86.5 ± 1.9 | p < 0.001 | p = 1.01 × 10−2 | |
| Taxifolin (C15H12O7) | mg PC/g PBE | 18.59 ± 0.93 | 19.32 ± 0.62 | p > 0.05 | p = 0.728 |
| mg PC/L PBE | 54.1 ± 2.7 | 63.9 ± 2.0 | p < 0.001 | p = 7.33 × 10−3 | |
| mg PC/Kg PB | 1073 ± 54 | 1265 ± 28 | p < 0.001 | p = 7.66 × 10−3 | |
| Ferulic acid (C10H10O4) | mg PC/g PBE | 2.001 ± 0.068 | 2.009 ± 0.052 | p > 0.05 | p = 0.961 |
| mg PC/L PBE | 5.82 ± 0.20 | 6.65 ± 0.17 | p < 0.05 | p = 5.51 × 10−3 | |
| mg PC/Kg PB | 115.5 ± 3.9 | 131.6 ± 3.4 | p < 0.05 | p = 5.82 × 10−3 | |
| Ellagic acid (C14H6O8) | mg PC/g PBE | 4.69 ± 0.33 | 4.87 ± 0.11 | p > 0.05 | p = 0.780 |
| mg PC/L PBE | 13.66 ± 0.96 | 16.12 ± 0.35 | p < 0.05 | p = 1.39 × 10−2 | |
| mg PC/Kg PB | 271 ± 19 | 319.0 ± 7.0 | p < 0.001 | p = 1.46 × 10−2 | |
| Protocatechuic acid (C7H6O4) | mg PC/g PBE | 5.92 ± 0.18 | 5.97 ± 0.34 | p > 0.05 | p = 0.942 |
| mg PC/L PBE | 17.22 ± 0.52 | 19.8 ± 1.1 | p < 0.05 | p = 2.35 × 10−2 | |
| mg PC/Kg PB | 341 ± 10 | 391.2 ± 8.6 | p < 0.05 | p = 2.46 × 10−2 | |
| o-coumaric acid (C9H8O3) | mg PC/g PBE | 1.93 ± 0.14 | 2.030 ± 0.045 | p > 0.05 | p = 0.714 |
| mg PC/L PBE | 5.63 ± 0.39 | 6.72 ± 0.15 | p < 0.05 | p = 1.09 × 10−2 | |
| mg PC/Kg PB | 111.6 ± 7.8 | 133.0 ± 2.9 | p < 0.05 | p = 1.14 × 10−2 | |
| Total | mg PC/g PBE | 63.91 | 65.74 | ||
| mg PC/L PBE | 185.98 | 217.59 | |||
| mg PC/Kg PB | 3687.7 | 4305.8 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barros, D.; Grenho, L.; Fernandes, M.H.; Gomes, P.S.; Fernandes, É. Correlation of Antioxidant and Antibacterial Activities of the Aqueous Pinus pinaster Aiton Bark Extract Within a Cytocompatible Concentration Range. Antioxidants 2025, 14, 1377. https://doi.org/10.3390/antiox14111377
Barros D, Grenho L, Fernandes MH, Gomes PS, Fernandes É. Correlation of Antioxidant and Antibacterial Activities of the Aqueous Pinus pinaster Aiton Bark Extract Within a Cytocompatible Concentration Range. Antioxidants. 2025; 14(11):1377. https://doi.org/10.3390/antiox14111377
Chicago/Turabian StyleBarros, Diana, Liliana Grenho, Maria Helena Fernandes, Pedro Sousa Gomes, and Élia Fernandes. 2025. "Correlation of Antioxidant and Antibacterial Activities of the Aqueous Pinus pinaster Aiton Bark Extract Within a Cytocompatible Concentration Range" Antioxidants 14, no. 11: 1377. https://doi.org/10.3390/antiox14111377
APA StyleBarros, D., Grenho, L., Fernandes, M. H., Gomes, P. S., & Fernandes, É. (2025). Correlation of Antioxidant and Antibacterial Activities of the Aqueous Pinus pinaster Aiton Bark Extract Within a Cytocompatible Concentration Range. Antioxidants, 14(11), 1377. https://doi.org/10.3390/antiox14111377








