Dysfunction of the ABCA1 and ABCG1 Transporters and Their Impact on HDL Metabolism
Abstract
1. Introduction
2. ABC Transporters
| Transporter | ABCA1 | ABCG1 |
|---|---|---|
| Type of transporter | Full transporter [40,48] | Half transporter |
| Molecular Mass | 254 kDa [40] | 75.69 kDa [40] |
| Tissue expressed | Ubiquitous, highly expressed in hepatocytes, macrophages, and smooth muscle cells [67,68] | High expression levels in macrophages, lymphocytes, epithelial cells, endothelial cells, vascular smooth muscle cells, liver, and intestine, brain and placenta [60,62,69,70,71] |
| Cell localization | Plasma membrane, endosome, peroxisomes, mitochondria, endoplasmic reticulum, or lamellar bodies [40] | Plasma membrane, endosomes, peroxisomes, mitochondria, endoplasmic reticulum, Golgi apparatus, lamellar bodies and endocytic vesicles [40,72] |
| Function | HDL biogenesis, cholesterol efflux, insulin secretion, microvesicle formation in platelets, glucose uptake in skeletal muscle, apolipoprotein ApoE secretion, and lipidation in astrocytes [67] | Transport of cholesterol, phosphatidylcholine, sphingomyelin, oxysterol and participates in RCT [47,63,73] |
| Human Genetic disease | Tangier disease [65] | There are no reports [40] |
| Associated diseases | Dyslipidemia, atherosclerosis, inflammation, coronary heart disease, type 2 diabetes, thrombosis, neurological disorders, age-related macular degeneration, glaucoma, viral infections, and cancer progression [32,67,74,75] | Atherosclerosis, inflammation, Alzheimer’s disease (AD), type 2 diabetes, cancer, immune disorders, obesity, and age-related macular degeneration [47,60,63,71] |
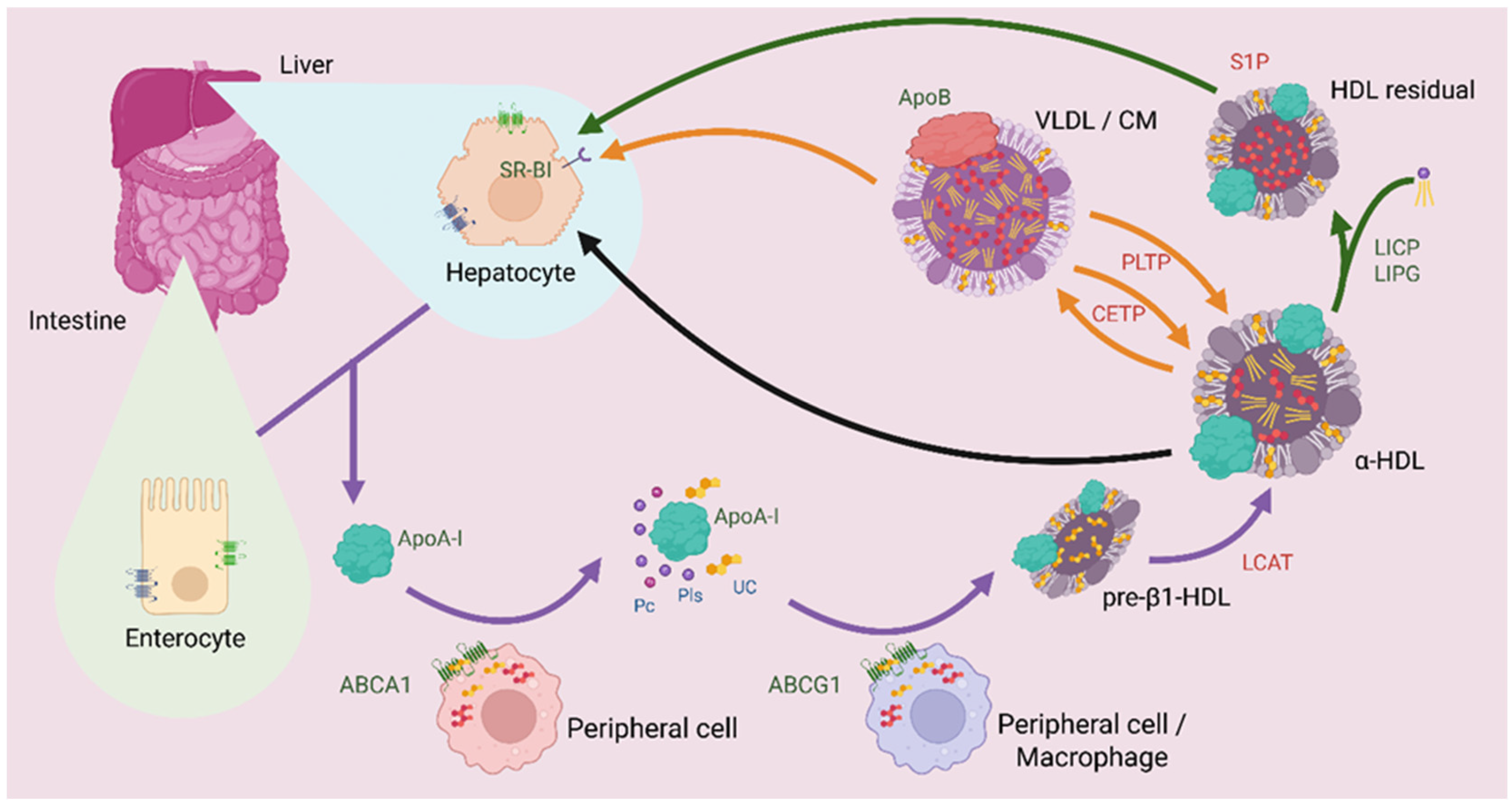
3. HDL Metabolism
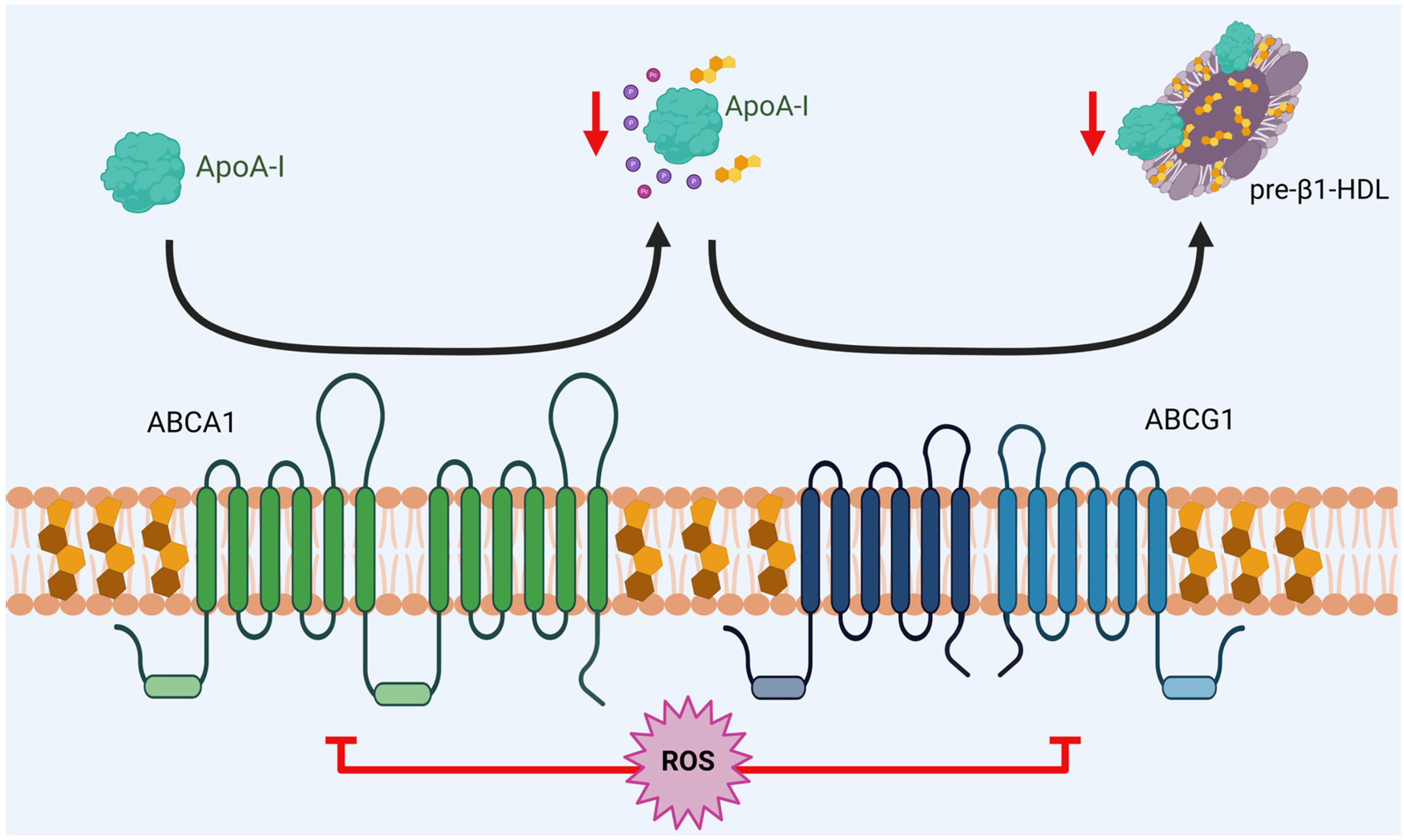
4. Alterations in HDL Metabolism
5. Potential Treatments
| Compound | Status | Nature | Target | Effect | Mechanism | Model System | Adverse Effects | Reference |
|---|---|---|---|---|---|---|---|---|
| Antagomir | Preclinic | Synthetic | ABCA1 & ABCG1 | ↑ | ↓ miR-23a-5p | ApoE−/− mice | ND | [105] |
| Cilostazol | Clinic | Synthetic | ABCA1 | ↑ | ↑ LXR/ABCA1/SREBP-1 | Human hepatoma cell line HepG2 | ND | [106] |
| CoQ | Preclinic | Natural | ABCG1 | ↑ | ↑ Activator protein-1, ↑ miR-378 | C57BL/6J mouse peritoneal macrophages, J774. A1, THP-1, HEK293 cells & ApoE−/− mice | No found | [107] |
| Diosgenin | Preclinic | Natural | ABCA1 | ↑ | ↓ miR-19b, | THP-1 macrophages/MPM-derived foam cells & ApoE−/− mice | ND | [108] |
| Dihydrogen | Clinic | Synthetic | ABCA1 | ↑ | ND | Potential metabolic syndrome patients | No found | [109] |
| Hydrogen sulfide | Preclinic | Synthetic | ABCA1 | ↑ | ↑ Peroxisome proliferator-activated receptor α (PPARα) translocation | Human hepatoma cell line HepG2 & ApoE−/− mice | ND | [110] |
| Paeonol | Preclinic | Natural | ABCA1 | ↑ | ↓ Calpain-related pathway, ↓ CD36 (platelet glycoprotein-4), ↑ Heme oxygenase-1 | RAW264.7 macrophages & ApoE−/− mice | ND | [104] |
| Qingre Sanjie Formula | Preclinic | Natural | ABCA1 | ↑ | ↑ LXRα/ABCG5/G8 pathway | ApoE−/− mice | No found | [111] |
| Statins (Atorvastatin & pitavastatin) | Clinic | Synthetic | ABCA1 | ↓ | ↓ Protein kinase B phosphorylation, ↑ miR-33 levels | RAW264.7 cells & bone marrow-derived macrophages | Risk of hyperglycemia and new-onset diabetes | [112] |
| Triptolide | Preclinic | Natural | ABCA1 | ↑ | May mediate expression through LPS/TLR4/GPS2 pathway | Male Sprague Dawley rats | ND | [113] |
6. Limitations and Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HDLs | High-density lipoproteins |
| ABC | ATP-binding cassette |
| LDLs | Low-density lipoproteins |
| CMs | Chylomicrons |
| VLDL | Very-low-density lipoprotein |
| RCT | Reverse cholesterol transport |
| ROS | Reactive oxygen species |
| NBDs | Nucleotide-binding domains |
| TMDs | Transmembrane domains |
| ECDs | Extracellular domains |
| LXR | Liver X receptor |
| RXR | Retinoid X receptor |
| PPAR α and γ | Peroxisome proliferator-activated receptors alpha and gamma |
| SREBF1 | Sterol regulatory element binding factors 1 |
| SREBF2 | Sterol regulatory element binding factors 2 |
| AD | Alzheimer’s disease |
| miRNAs/miR | MicroRNAs |
| LCAT | Lecithin cholesterol acyltransferase |
| PC | Phosphatidylcholine |
| UC | Unesterified cholesterol |
| PLs | Phospholipids |
| SR-B1 | Scavenger receptor class B type 1 |
| CETP | Cholesteryl ester transfer protein |
| PLTP | Phospholipid transfer protein |
| LIPC | Hepatic lipase |
| LIPG | Endothelial lipase |
| SREBP-2 | Sterol regulatory element-binding protein 2 |
| HMG-CoA | β-hydroxy β-methylglutaryl-CoA |
| HMGR | HMG-CoA reductase |
| HMGS | HMG-CoA synthase |
| Sqs | Squalene synthase |
| Fpps | Farnesyl diphosphate synthase |
| LDLR | Low-density lipoprotein receptor |
| INSIG1 | Insulin-induced gene 1 protein S |
| StarD4 | StAR-related lipid transfer protein 4 |
| Nrf2 | Nuclear factor erythroid 2 |
| SR-A1 | Scavenger Receptor Class A type 1 |
| CD36 | Platelet glycoprotein 4 |
| NPC1L1 | Niemann-Pick-C1 protein |
| MsrA | Methionine sulfoxide reductase A |
| PON1 | Paraoxonase 1 |
| GPX4/xCT | Phospholipid hydroperoxide glutathione peroxidase 4/Cystine/glutamate transporter |
References
- Avery, S.V. Molecular targets of oxidative stress. Biochem. J. 2011, 434, 201–210. [Google Scholar] [CrossRef]
- Iqbal, J.; Hussain, M.M. Intestinal lipid absorption. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1183–E1194. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and Nutritional Antioxidants in Human Diseases. Front. Physiol. 2018, 9, 477. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Pineiro, J.A.; Gonzalez-Rovira, A.; Sanchez-Gomar, I.; Moreno, J.A.; Duran-Ruiz, M.C. Nrf2 and Heme Oxygenase-1 Involvement in Atherosclerosis Related Oxidative Stress. Antioxidants 2021, 10, 1463. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [PubMed]
- Matuz-Mares, D.; Gonzalez-Andrade, M.; Araiza-Villanueva, M.G.; Vilchis-Landeros, M.M.; Vazquez-Meza, H. Mitochondrial Calcium: Effects of Its Imbalance in Disease. Antioxidants 2022, 11, 801. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Álvarez-López, H.; Ruiz-Gastélum, E.; Díaz-Aragón, A. Conociendo los mecanismos básicos del metabolismo de los lípidos. Cardiovasc. Metab. Sci. 2021, 32, 147–152. [Google Scholar] [CrossRef]
- Havel, R.J.; Eder, H.A.; Bragdon, J.H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Investig. 1955, 34, 1345–1353. [Google Scholar] [CrossRef]
- Ansell, B.J.; Navab, M.; Hama, S.; Kamranpour, N.; Fonarow, G.; Hough, G.; Rahmani, S.; Mottahedeh, R.; Dave, R.; Reddy, S.T.; et al. Inflammatory/antiinflammatory properties of high-density lipoprotein distinguish patients from control subjects better than high-density lipoprotein cholesterol levels and are favorably affected by simvastatin treatment. Circulation 2003, 108, 2751–2756. [Google Scholar] [CrossRef]
- Chapman, M.J. Animal lipoproteins: Chemistry, structure, and comparative aspects. J. Lipid Res. 1980, 21, 789–853. [Google Scholar] [CrossRef]
- Franczyk, B.; Rysz, J.; Lawinski, J.; Rysz-Gorzynska, M.; Gluba-Brzozka, A. Is a High HDL-Cholesterol Level Always Beneficial? Biomedicines 2021, 9, 1083. [Google Scholar] [CrossRef]
- Ginsberg, H.N. Lipoprotein physiology and its relationship to atherogenesis. Endocrinol. Metab. Clin. N. Am. 1990, 19, 211–228. [Google Scholar] [CrossRef]
- Jonas, A.P.; Michael, C. Lipoprotein structure. In Biochemistry of Lipids, Lipoproteins and Membranes, 5th ed.; Ennis, E., Vance, J.E.V., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 485–506. [Google Scholar]
- Khosravi, M.; Hosseini-Fard, R.; Najafi, M. Circulating low density lipoprotein (LDL). Horm. Mol. Biol. Clin. Investig. 2018, 35, 20180024. [Google Scholar] [CrossRef] [PubMed]
- Kindel, T.; Lee, D.M.; Tso, P. The mechanism of the formation and secretion of chylomicrons. Atheroscler. Suppl. 2010, 11, 11–16. [Google Scholar] [CrossRef] [PubMed]
- van Zwol, W.; van de Sluis, B.; Ginsberg, H.N.; Kuivenhoven, J.A. VLDL Biogenesis and Secretion: It Takes a Village. Circ. Res. 2024, 134, 226–244. [Google Scholar] [CrossRef]
- Errico, T.L.; Chen, X.; Martin Campos, J.M.; Julve, J.; Escola-Gil, J.C.; Blanco-Vaca, F. Basic mechanisms: Structure, function and metabolism of plasma lipoproteins. Clin. Investig. Arterioscler. 2013, 25, 98–103. [Google Scholar] [CrossRef]
- Hernández Puga, G.; Laguna Maldonado, K.D.; Reyes Galindo, M.; Moreno Piña, J.R.; Matuz Mares, D. Lipoproteínas de Alta Densidad y Riesgo Cardiovascular. Rev. Educ. Bioquíim. 2019, 4, 93–99. [Google Scholar]
- Chen, J.; Fang, Z.; Luo, Q.; Wang, X.; Warda, M.; Das, A.; Oldoni, F.; Luo, F. Unlocking the mysteries of VLDL: Exploring its production, intracellular trafficking, and metabolism as therapeutic targets. Lipids Health Dis. 2024, 23, 14. [Google Scholar] [CrossRef]
- Olofsson, S.O.; Asp, L.; Boren, J. The assembly and secretion of apolipoprotein B-containing lipoproteins. Curr. Opin. Lipidol. 1999, 10, 341–346. [Google Scholar] [CrossRef]
- Jomard, A.; Osto, E. High Density Lipoproteins: Metabolism, Function, and Therapeutic Potential. Front. Cardiovasc. Med. 2020, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Parks, J.S. ATP-binding cassette transporter AI and its role in HDL formation. Curr. Opin. Lipidol. 2005, 16, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.C. Molecular mechanisms of cellular cholesterol efflux. J. Biol. Chem. 2014, 289, 24020–24029. [Google Scholar] [CrossRef] [PubMed]
- Rozhkova, A.V.; Dmitrieva, V.G.; Nosova, E.V.; Dergunov, A.D.; Limborska, S.A.; Dergunova, L.V. Genomic Variants and Multilevel Regulation of ABCA1, ABCG1, and SCARB1 Expression in Atherogenesis. J. Cardiovasc. Dev. Dis. 2021, 8, 170. [Google Scholar] [CrossRef]
- Zannis, V.I.; Kurnit, D.M.; Breslow, J.L. Hepatic apo-A-I and apo-E and intestinal apo-A-I are synthesized in precursor isoprotein forms by organ cultures of human fetal tissues. J. Biol. Chem. 1982, 257, 536–544. [Google Scholar] [CrossRef]
- Guerin, M. Reverse Cholesterol Transport in HDL Metabolism. In The HDL Handbook; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Voloshyna, I.; Reiss, A.B. The ABC transporters in lipid flux and atherosclerosis. Prog. Lipid Res. 2011, 50, 213–224. [Google Scholar] [CrossRef]
- Schumacher, T.; Benndorf, R.A. ABC Transport Proteins in Cardiovascular Disease—A Brief Summary. Molecules 2017, 22, 589. [Google Scholar] [CrossRef]
- Vilchis-Landeros, M.M.; Vazquez-Meza, H.; Vazquez-Carrada, M.; Uribe-Ramirez, D.; Matuz-Mares, D. Antioxidant Enzymes and Their Potential Use in Breast Cancer Treatment. Int. J. Mol. Sci. 2024, 25, 5675. [Google Scholar] [CrossRef]
- Chen, M.; Li, W.; Wang, N.; Zhu, Y.; Wang, X. ROS and NF-kappaB but not LXR mediate IL-1beta signaling for the downregulation of ATP-binding cassette transporter A1. Am. J. Physiol. Cell Physiol. 2007, 292, C1493–C1501. [Google Scholar] [CrossRef]
- Wang, J.; Xiao, Q.; Wang, L.; Wang, Y.; Wang, D.; Ding, H. Role of ABCA1 in Cardiovascular Disease. J. Pers. Med. 2022, 12, 1010. [Google Scholar] [CrossRef]
- An, Y.; Xu, B.T.; Wan, S.R.; Ma, X.M.; Long, Y.; Xu, Y.; Jiang, Z.Z. The role of oxidative stress in diabetes mellitus-induced vascular endothelial dysfunction. Cardiovasc. Diabetol. 2023, 22, 237. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Pu, J.X.; Yang, X.; Liu, M.C.; Zhang, P.; Cao, J.; Du, F.; Wu, D.F.; Lu, Z.B.; Yu, H. ApoE Mimetic Peptide-MsrA Fusion Protein Restores HDL Function and Ameliorates Atherosclerosis via Circulatory Redox Remodeling in SR-BI Deficient Mice. FASEB J. 2025, 39, e70999. [Google Scholar] [CrossRef] [PubMed]
- Matuz-Mares, D.; Riveros-Rosas, H.; Vilchis-Landeros, M.M.; Vazquez-Meza, H. Glutathione Participation in the Prevention of Cardiovascular Diseases. Antioxidants 2021, 10, 1220. [Google Scholar] [CrossRef]
- Panth, N.; Paudel, K.R.; Parajuli, K. Reactive Oxygen Species: A Key Hallmark of Cardiovascular Disease. Adv. Med. 2016, 2016, 9152732. [Google Scholar] [CrossRef]
- Ranneh, Y.; Ali, F.; Akim, A.M.; Hamid, H.A.; Khazaai, H.; Fadel, A. Crosstalk between reactive oxygen species and pro-inflammatory markers in developing various chronic diseases: A review. Appl. Biol. Chem. 2017, 60, 327–338. [Google Scholar] [CrossRef]
- Seyffer, F.; Tampe, R. ABC transporters in adaptive immunity. Biochim. Biophys. Acta 2015, 1850, 449–460. [Google Scholar] [CrossRef]
- Alam, A.; Locher, K.P. Structure and Mechanism of Human ABC Transporters. Annu. Rev. Biophys. 2023, 52, 275–300. [Google Scholar] [CrossRef]
- Falguieres, T. ABC Transporters in Human Diseases: Future Directions and Therapeutic Perspectives. Int. J. Mol. Sci. 2022, 23, 4250. [Google Scholar] [CrossRef]
- Thomas, C.; Tampe, R. Structural and Mechanistic Principles of ABC Transporters. Annu. Rev. Biochem. 2020, 89, 605–636. [Google Scholar] [CrossRef]
- Dean, M.; Moitra, K.; Allikmets, R. The human ATP-binding cassette (ABC) transporter superfamily. Hum. Mutat. 2022, 43, 1162–1182. [Google Scholar] [CrossRef]
- Duvivier, L.; Gerard, L.; Diaz, A.; Gillet, J.P. Linking ABC transporters to the hallmarks of cancer. Trends Cancer 2024, 10, 124–134. [Google Scholar] [CrossRef]
- Stefan, K.Y.; Leck, L.Y.W.; Namasivayam, V.; Bascuñana, P.; Huang, M.L.H.; Riss, P.J.; Pahnke, J.; Jansson, P.J.; Stefan, S.M. Vesicular ATP-binding cassette transporters in human disease: Relevant aspects of their organization for future drug development. Future Drug Discov. 2020, 4, FDD51. [Google Scholar] [CrossRef]
- Szakacs, G.; Abele, R. An inventory of lysosomal ABC transporters. FEBS Lett. 2020, 594, 3965–3985. [Google Scholar] [CrossRef]
- Kotlyarov, S.; Kotlyarova, A. Biological Functions and Clinical Significance of the ABCG1 Transporter. Biology 2024, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, M. ABCA1 and ABCG1 as potential therapeutic targets for the prevention of atherosclerosis. J. Pharmacol. Sci. 2022, 148, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Liu, X. ABC Family Transporters. Adv. Exp. Med. Biol. 2019, 1141, 13–100. [Google Scholar] [CrossRef] [PubMed]
- Beis, K. Structural basis for the mechanism of ABC transporters. Biochem. Soc. Trans. 2015, 43, 889–893. [Google Scholar] [CrossRef]
- Vasiliou, V.; Vasiliou, K.; Nebert, D.W. Human ATP-binding cassette (ABC) transporter family. Hum. Genom. 2009, 3, 281–290. [Google Scholar] [CrossRef]
- Nguyen, J.P.; Kim, Y.; Cao, Q.; Hirota, J.A. Interactions between ABCC4/MRP4 and ABCC7/CFTR in human airway epithelial cells in lung health and disease. Int. J. Biochem. Cell Biol. 2021, 133, 105936. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, F.; Ren, Q.; Zhao, Q.; Ren, H.; Lu, S.; Zhang, L.; Han, Z. Suppression of ABCG2 inhibits cancer cell proliferation. Int. J. Cancer 2010, 126, 841–851. [Google Scholar] [CrossRef]
- Polireddy, K.; Chavan, H.; Abdulkarim, B.A.; Krishnamurthy, P. Functional significance of the ATP-binding cassette transporter B6 in hepatocellular carcinoma. Mol. Oncol. 2011, 5, 410–425. [Google Scholar] [CrossRef] [PubMed]
- Mantel, I.; Sadiq, B.A.; Blander, J.M. Spotlight on TAP and its vital role in antigen presentation and cross-presentation. Mol. Immunol. 2022, 142, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R. Introduction to Lipids and Lipoproteins. In Endotext; Feingold, K.R., Ahmed, S.F., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Mineo, C.; Shaul, P.W. Regulation of signal transduction by HDL. J. Lipid Res. 2013, 54, 2315–2324. [Google Scholar] [CrossRef] [PubMed]
- Kerr, I.D.; Hutchison, E.; Gerard, L.; Aleidi, S.M.; Gelissen, I.C. Mammalian ABCG-transporters, sterols and lipids: To bind perchance to transport? Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158860. [Google Scholar] [CrossRef]
- Choi, H.Y.; Choi, S.; Iatan, I.; Ruel, I.; Genest, J. Biomedical Advances in ABCA1 Transporter: From Bench to Bedside. Biomedicines 2023, 11, 561. [Google Scholar] [CrossRef]
- Dahik, V.D.; Kc, P.; Materne, C.; Reydellet, C.; Lhomme, M.; Cruciani-Guglielmacci, C.; Denom, J.; Bun, E.; Ponnaiah, M.; Deknuydt, F.; et al. ABCG1 orchestrates adipose tissue macrophage plasticity and insulin resistance in obesity by rewiring saturated fatty acid pools. Sci. Transl. Med. 2024, 16, eadi6682. [Google Scholar] [CrossRef]
- Wang, H.J.; Wang, J.H.; Xu, X.N.; Zhao, X.S.; Liu, W. ABCG1 is Expressed in an LXR-Independent Manner in Patients with Type 2 Diabetes Mellitus. Curr. Mol. Med. 2023, 23, 815–824. [Google Scholar] [CrossRef]
- Wang, N.; Yvan-Charvet, L.; Lutjohann, D.; Mulder, M.; Vanmierlo, T.; Kim, T.W.; Tall, A.R. ATP-binding cassette transporters G1 and G4 mediate cholesterol and desmosterol efflux to HDL and regulate sterol accumulation in the brain. FASEB J. 2008, 22, 1073–1082. [Google Scholar] [CrossRef]
- Xue, J.; Fan, J.; Li, Y.; Wu, W.; Yan, Q.; Zheng, Q. ABCG1 Attenuates Oxidative Stress Induced by H(2)O(2) through the Inhibition of NADPH Oxidase and the Upregulation of Nrf2-Mediated Antioxidant Defense in Endothelial Cells. Anal. Cell. Pathol. 2020, 2020, 2095645. [Google Scholar] [CrossRef]
- Sunidhi, S.; Sacher, S.; Atul; Garg, P.; Ray, A. Elucidating the Structural Features of ABCA1 in its Heterogeneous Membrane Environment. Front. Mol. Biosci. 2021, 8, 803078. [Google Scholar] [CrossRef] [PubMed]
- Rust, S.; Rosier, M.; Funke, H.; Real, J.; Amoura, Z.; Piette, J.C.; Deleuze, J.F.; Brewer, H.B.; Duverger, N.; Denefle, P.; et al. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat. Genet. 1999, 22, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.A.; Barrera, G.C.; Nakamura, K.; Baldan, A.; Tarr, P.; Fishbein, M.C.; Frank, J.; Francone, O.L.; Edwards, P.A. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 2005, 1, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Jacobo-Albavera, L.; Dominguez-Perez, M.; Medina-Leyte, D.J.; Gonzalez-Garrido, A.; Villarreal-Molina, T. The Role of the ATP-Binding Cassette A1 (ABCA1) in Human Disease. Int. J. Mol. Sci. 2021, 22, 1593. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, M.; Tang, C.K. History and Development of ABCA1. Curr. Probl. Cardiol. 2024, 49, 102036. [Google Scholar] [CrossRef]
- Kober, A.C.; Manavalan, A.P.C.; Tam-Amersdorfer, C.; Holmer, A.; Saeed, A.; Fanaee-Danesh, E.; Zandl, M.; Albrecher, N.M.; Bjorkhem, I.; Kostner, G.M.; et al. Implications of cerebrovascular ATP-binding cassette transporter G1 (ABCG1) and apolipoprotein M in cholesterol transport at the blood-brain barrier. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 573–588. [Google Scholar] [CrossRef]
- Stefulj, J.; Panzenboeck, U.; Becker, T.; Hirschmugl, B.; Schweinzer, C.; Lang, I.; Marsche, G.; Sadjak, A.; Lang, U.; Desoye, G.; et al. Human endothelial cells of the placental barrier efficiently deliver cholesterol to the fetal circulation via ABCA1 and ABCG1. Circ. Res. 2009, 104, 600–608. [Google Scholar] [CrossRef]
- Zeng, G.G.; Lei, Q.; Jiang, W.L.; Zhang, X.X.; Nie, L.; Gong, X.; Zheng, K. A new perspective on the current and future development potential of ABCG1. Curr. Probl. Cardiol. 2024, 49, 102161. [Google Scholar] [CrossRef]
- Rezaei, F.; Farhat, D.; Gursu, G.; Samnani, S.; Lee, J.Y. Snapshots of ABCG1 and ABCG5/G8: A Sterol’s Journey to Cross the Cellular Membranes. Int. J. Mol. Sci. 2022, 24, 484. [Google Scholar] [CrossRef]
- Kobayashi, A.; Takanezawa, Y.; Hirata, T.; Shimizu, Y.; Misasa, K.; Kioka, N.; Arai, H.; Ueda, K.; Matsuo, M. Efflux of sphingomyelin, cholesterol, and phosphatidylcholine by ABCG1. J. Lipid Res. 2006, 47, 1791–1802. [Google Scholar] [CrossRef]
- Paseban, T.; Alavi, M.S.; Etemad, L.; Roohbakhsh, A. The role of the ATP-Binding Cassette A1 (ABCA1) in neurological disorders: A mechanistic review. Expert Opin. Ther. Targets 2023, 27, 531–552. [Google Scholar] [CrossRef]
- Wu, K.; Zou, L.; Lei, X.; Yang, X. Roles of ABCA1 in cancer. Oncol. Lett. 2022, 24, 349. [Google Scholar] [CrossRef]
- Lewandowski, C.T.; Laham, M.S.; Thatcher, G.R.J. Remembering your A, B, C’s: Alzheimer’s disease and ABCA1. Acta Pharm. Sin. B 2022, 12, 995–1018. [Google Scholar] [CrossRef]
- Akiyama, T.E.; Sakai, S.; Lambert, G.; Nicol, C.J.; Matsusue, K.; Pimprale, S.; Lee, Y.H.; Ricote, M.; Glass, C.K.; Brewer, H.B., Jr.; et al. Conditional disruption of the peroxisome proliferator-activated receptor gamma gene in mice results in lowered expression of ABCA1, ABCG1, and apoE in macrophages and reduced cholesterol efflux. Mol. Cell. Biol. 2002, 22, 2607–2619. [Google Scholar] [CrossRef] [PubMed]
- Ozasa, H.; Ayaori, M.; Iizuka, M.; Terao, Y.; Uto-Kondo, H.; Yakushiji, E.; Takiguchi, S.; Nakaya, K.; Hisada, T.; Uehara, Y.; et al. Pioglitazone enhances cholesterol efflux from macrophages by increasing ABCA1/ABCG1 expressions via PPARgamma/LXRalpha pathway: Findings from in vitro and ex vivo studies. Atherosclerosis 2011, 219, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Abildayeva, K.; Jansen, P.J.; Hirsch-Reinshagen, V.; Bloks, V.W.; Bakker, A.H.; Ramaekers, F.C.; de Vente, J.; Groen, A.K.; Wellington, C.L.; Kuipers, F.; et al. 24(S)-hydroxycholesterol participates in a liver X receptor-controlled pathway in astrocytes that regulates apolipoprotein E-mediated cholesterol efflux. J. Biol. Chem. 2006, 281, 12799–12808. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wei, Z.; Ma, X.; Yang, X.; Chen, Y.; Sun, L.; Ma, C.; Miao, Q.R.; Hajjar, D.P.; Han, J.; et al. 25-Hydroxycholesterol activates the expression of cholesterol 25-hydroxylase in an LXR-dependent mechanism. J. Lipid Res. 2018, 59, 439–451. [Google Scholar] [CrossRef]
- Aryal, B.; Singh, A.K.; Rotllan, N.; Price, N.; Fernandez-Hernando, C. MicroRNAs and lipid metabolism. Curr. Opin. Lipidol. 2017, 28, 273–280. [Google Scholar] [CrossRef]
- Najafi-Shoushtari, S.H.; Kristo, F.; Li, Y.; Shioda, T.; Cohen, D.E.; Gerszten, R.E.; Naar, A.M. MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science 2010, 328, 1566–1569. [Google Scholar] [CrossRef]
- Hori, N.; Hayashi, H.; Sugiyama, Y. Calpain-mediated cleavage negatively regulates the expression level of ABCG1. Atherosclerosis 2011, 215, 383–391. [Google Scholar] [CrossRef]
- Ogura, M.; Ayaori, M.; Terao, Y.; Hisada, T.; Iizuka, M.; Takiguchi, S.; Uto-Kondo, H.; Yakushiji, E.; Nakaya, K.; Sasaki, M.; et al. Proteasomal inhibition promotes ATP-binding cassette transporter A1 (ABCA1) and ABCG1 expression and cholesterol efflux from macrophages in vitro and in vivo. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1980–1987. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhao, Z.W.; Zeng, P.H.; Zhou, Y.J.; Yin, W.J. Molecular mechanisms for ABCA1-mediated cholesterol efflux. Cell Cycle 2022, 21, 1121–1139. [Google Scholar] [CrossRef] [PubMed]
- Dergunov, A.D.; Baserova, V.B. Different Pathways of Cellular Cholesterol Efflux. Cell Biochem. Biophys. 2022, 80, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Laurenzi, T.; Parravicini, C.; Palazzolo, L.; Guerrini, U.; Gianazza, E.; Calabresi, L.; Eberini, I. rHDL modeling and the anchoring mechanism of LCAT activation. J. Lipid Res. 2021, 62, 100006. [Google Scholar] [CrossRef]
- Singh, S.A.; Andraski, A.B.; Higashi, H.; Lee, L.H.; Ramsaroop, A.; Sacks, F.M.; Aikawa, M. Metabolism of PLTP, CETP, and LCAT on multiple HDL sizes using the Orbitrap Fusion Lumos. JCI Insight 2021, 6, e143526. [Google Scholar] [CrossRef]
- von Eckardstein, A.; Nordestgaard, B.G.; Remaley, A.T.; Catapano, A.L. High-density lipoprotein revisited: Biological functions and clinical relevance. Eur. Heart J. 2023, 44, 1394–1407. [Google Scholar] [CrossRef]
- Chandrasekaran, P.; Weiskirchen, R. The Role of SCAP/SREBP as Central Regulators of Lipid Metabolism in Hepatic Steatosis. Int. J. Mol. Sci. 2024, 25, 1109. [Google Scholar] [CrossRef]
- Huang, L.; Fan, B.; Ma, A.; Shaul, P.W.; Zhu, H. Inhibition of ABCA1 protein degradation promotes HDL cholesterol efflux capacity and RCT and reduces atherosclerosis in mice. J. Lipid Res. 2015, 56, 986–997. [Google Scholar] [CrossRef]
- Wang, B.; Chen, R.; Yin, T.; Li, Y.; Xi, Z.; Li, B.; Chu, X.M. Fucoidan: A marine-derived polysaccharide with therapeutic potential in atherosclerotic cardiovascular diseases: A review. Int. J. Biol. Macromol. 2025, 321, 146055. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, J. Deciphering Oxidative Stress in Cardiovascular Disease Progression: A Blueprint for Mechanistic Understanding and Therapeutic Innovation. Antioxidants 2024, 14, 38. [Google Scholar] [CrossRef]
- Jebari-Benslaiman, S.; Galicia-Garcia, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martin, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef] [PubMed]
- Casula, M.; Colpani, O.; Xie, S.; Catapano, A.L.; Baragetti, A. HDL in Atherosclerotic Cardiovascular Disease: In Search of a Role. Cells 2021, 10, 1869. [Google Scholar] [CrossRef] [PubMed]
- Vaisar, T.; Pennathur, S.; Green, P.S.; Gharib, S.A.; Hoofnagle, A.N.; Cheung, M.C.; Byun, J.; Vuletic, S.; Kassim, S.; Singh, P.; et al. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J. Clin. Investig. 2007, 117, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Habash, N.; Abdul-Rasheed, O.; Salman, M. The Association of Single Nucleotide Polymorphism rs5883 in The CETP Gene with Oxidized-LDL Level in Coronary Atherosclerosis Patients: CETP gene and Coronary Atherosclerosis. Iraqi J. Cancer Med. Genet. 2023, 16, 93–99. [Google Scholar] [CrossRef]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef]
- Munno, M.; Mallia, A.; Greco, A.; Modafferi, G.; Banfi, C.; Eligini, S. Radical Oxygen Species, Oxidized Low-Density Lipoproteins, and Lectin-like Oxidized Low-Density Lipoprotein Receptor 1: A Vicious Circle in Atherosclerotic Process. Antioxidants 2024, 13, 583. [Google Scholar] [CrossRef]
- Packard, C.J.; Demant, T.; Stewart, J.P.; Bedford, D.; Caslake, M.J.; Schwertfeger, G.; Bedynek, A.; Shepherd, J.; Seidel, D. Apolipoprotein B metabolism and the distribution of VLDL and LDL subfractions. J. Lipid Res. 2000, 41, 305–318. [Google Scholar] [CrossRef]
- Gu, H.M.; Li, G.; Gao, X.; Berthiaume, L.G.; Zhang, D.W. Characterization of palmitoylation of ATP binding cassette transporter G1: Effect on protein trafficking and function. Biochim. Biophys. Acta 2013, 1831, 1067–1078. [Google Scholar] [CrossRef]
- Singaraja, R.R.; Kang, M.H.; Vaid, K.; Sanders, S.S.; Vilas, G.L.; Arstikaitis, P.; Coutinho, J.; Drisdel, R.C.; El-Husseini Ael, D.; Green, W.N.; et al. Palmitoylation of ATP-binding cassette transporter A1 is essential for its trafficking and function. Circ. Res. 2009, 105, 138–147. [Google Scholar] [CrossRef]
- Ye, Z.; Lu, Y.; Wu, T. The impact of ATP-binding cassette transporters on metabolic diseases. Nutr. Metab. 2020, 17, 61. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Y.; Yu, C.; Yang, H.; Zhang, C.; Ye, Y.; Xiao, S. Paeonol suppresses lipid accumulation in macrophages via upregulation of the ATP—binding cassette transporter A1 and downregulation of the cluster of differentiation 36. Int. J. Oncol. 2015, 46, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Ye, Z.M.; Chen, S.; Luo, X.Y.; Chen, S.L.; Mao, L.; Li, Y.; Jin, H.; Yu, C.; Xiang, F.X.; et al. MicroRNA-23a-5p promotes atherosclerotic plaque progression and vulnerability by repressing ATP-binding cassette transporter A1/G1 in macrophages. J. Mol. Cell. Cardiol. 2018, 123, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Jeon, B.H.; Lee, Y.H.; Yun, M.R.; Kim, S.H.; Lee, B.W.; Kang, E.S.; Lee, H.C.; Cha, B.S. Increased expression of ATP-binding cassette transporter A1 (ABCA1) as a possible mechanism for the protective effect of cilostazol against hepatic steatosis. Metabolism 2015, 64, 1444–1453. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yan, X.; Xia, M.; Yang, Y.; Li, D.; Li, X.; Song, F.; Ling, W. Coenzyme Q10 promotes macrophage cholesterol efflux by regulation of the activator protein-1/miR-378/ATP-binding cassette transporter G1-signaling pathway. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1860–1870. [Google Scholar] [CrossRef]
- Lv, Y.C.; Yang, J.; Yao, F.; Xie, W.; Tang, Y.Y.; Ouyang, X.P.; He, P.P.; Tan, Y.L.; Li, L.; Zhang, M.; et al. Diosgenin inhibits atherosclerosis via suppressing the MiR-19b-induced downregulation of ATP-binding cassette transporter A1. Atherosclerosis 2015, 240, 80–89. [Google Scholar] [CrossRef]
- Song, G.; Lin, Q.; Zhao, H.; Liu, M.; Ye, F.; Sun, Y.; Yu, Y.; Guo, S.; Jiao, P.; Wu, Y.; et al. Hydrogen Activates ATP-Binding Cassette Transporter A1-Dependent Efflux Ex Vivo and Improves High-Density Lipoprotein Function in Patients With Hypercholesterolemia: A Double-Blinded, Randomized, and Placebo-Controlled Trial. J. Clin. Endocrinol. Metab. 2015, 100, 2724–2733. [Google Scholar] [CrossRef]
- Li, D.; Xiong, Q.; Peng, J.; Hu, B.; Li, W.; Zhu, Y.; Shen, X. Hydrogen Sulfide Up-Regulates the Expression of ATP-Binding Cassette Transporter A1 via Promoting Nuclear Translocation of PPARalpha. Int. J. Mol. Sci. 2016, 17, 635. [Google Scholar] [CrossRef]
- Li, X.; Huang, X.; Song, K.; Liu, J.; Jin, Y.; Li, T.; Zhang, L.; Zhang, H. Qingre Sanjie Formula alleviates atherosclerosis by promoting LXR-alpha/ABCG5/G8-mediated reverse cholesterol transport and bile acid synthesis. Phytomedicine 2025, 142, 156691. [Google Scholar] [CrossRef]
- Chen, W.M.; Sheu, W.H.; Tseng, P.C.; Lee, T.S.; Lee, W.J.; Chang, P.J.; Chiang, A.N. Modulation of microRNA Expression in Subjects with Metabolic Syndrome and Decrease of Cholesterol Efflux from Macrophages via microRNA-33-Mediated Attenuation of ATP-Binding Cassette Transporter A1 Expression by Statins. PLoS ONE 2016, 11, e0154672. [Google Scholar] [CrossRef]
- Chen, J.; Gao, J.; Yang, J.; Zhang, Y.; Wang, L. Effect of triptolide on the regulation of ATP—binding cassette transporter A1 expression in lipopolysaccharide—induced acute lung injury of rats. Mol. Med. Rep. 2014, 10, 3015–3020. [Google Scholar] [CrossRef][Green Version]
- Katz, A.; Udata, C.; Ott, E.; Hickey, L.; Burczynski, M.E.; Burghart, P.; Vesterqvist, O.; Meng, X. Safety, pharmacokinetics, and pharmacodynamics of single doses of LXR-623, a novel liver X-receptor agonist, in healthy participants. J. Clin. Pharmacol. 2009, 49, 643–649. [Google Scholar] [CrossRef]
- Tardif, J.C.; Gregoire, J.; L’Allier, P.L.; Ibrahim, R.; Lesperance, J.; Heinonen, T.M.; Kouz, S.; Berry, C.; Basser, R.; Lavoie, M.A.; et al. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: A randomized controlled trial. JAMA 2007, 297, 1675–1682. [Google Scholar] [CrossRef]
- Bloomfield, D.; Carlson, G.L.; Sapre, A.; Tribble, D.; McKenney, J.M.; Littlejohn, T.W., 3rd; Sisk, C.M.; Mitchel, Y.; Pasternak, R.C. Efficacy and safety of the cholesteryl ester transfer protein inhibitor anacetrapib as monotherapy and coadministered with atorvastatin in dyslipidemic patients. Am. Heart J. 2009, 157, 352–360.e2. [Google Scholar] [CrossRef] [PubMed]
- Stein, E.A.; Stroes, E.S.; Steiner, G.; Buckley, B.M.; Capponi, A.M.; Burgess, T.; Niesor, E.J.; Kallend, D.; Kastelein, J.J. Safety and tolerability of dalcetrapib. Am. J. Cardiol. 2009, 104, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Moriarty, P.M.; Thompson, P.D.; Cannon, C.P.; Guyton, J.R.; Bergeron, J.; Zieve, F.J.; Bruckert, E.; Jacobson, T.A.; Kopecky, S.L.; Baccara-Dinet, M.T.; et al. Efficacy and safety of alirocumab vs ezetimibe in statin-intolerant patients, with a statin rechallenge arm: The ODYSSEY ALTERNATIVE randomized trial. J. Clin. Lipidol. 2015, 9, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Zhang, W.; Yang, X.; Liu, Y.; Liu, L.; Feng, K.; Zhang, X.; Yang, S.; Sun, L.; Yu, M.; et al. Functional interplay between liver X receptor and AMP-activated protein kinase alpha inhibits atherosclerosis in apolipoprotein E-deficient mice—A new anti-atherogenic strategy. Br. J. Pharmacol. 2018, 175, 1486–1503. [Google Scholar] [CrossRef]
- Fitzgerald, M.L.; Mujawar, Z.; Tamehiro, N. ABC transporters, atherosclerosis and inflammation. Atherosclerosis 2010, 211, 361–370. [Google Scholar] [CrossRef]
- Cao, J.; Xu, Y.; Shang, L.; Liu, H.-M.; Du, F.; Yu, H. Effect of The Apolipoprotein E Mimetic Peptide EpK on Atherosclerosis in apoE(−/−) Mice. Prog. Biochem. Biophys. 2015, 42, 833–842. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Ono, K.; Horie, T.; Nishino, T.; Baba, O.; Kuwabara, Y.; Kimura, T. MicroRNAs and High-Density Lipoprotein Cholesterol Metabolism. Int. Heart J. 2015, 56, 365–371. [Google Scholar] [CrossRef]
- Goedeke, L.; Aranda, J.F.; Fernandez-Hernando, C. microRNA regulation of lipoprotein metabolism. Curr. Opin. Lipidol. 2014, 25, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ma, S.; Hu, Y.; Ling, J.; Wang, Z.; Wang, J.; Chen, J.; Zhang, Y. Auricularia heimuer Ameliorates Oxidative Stress and Inflammation to Inhibit Atherosclerosis Development in ApoE(−/−) Mice. Nutrients 2025, 17, 2799. [Google Scholar] [CrossRef] [PubMed]
- Feng, J. Role of curcumin in altering gut microbiota for anti-obesity and anti-hyperlipidemic effects. Front. Microbiol. 2025, 16, 1625098. [Google Scholar] [CrossRef] [PubMed]
- Ekawa, K.; Marumo, M.; Wakabayashi, I. Antithrombotic Action of Resveratrol: Particularly Regarding Inhibition of Platelet Aggregation. Yakugaku Zasshi 2025, 145, 765–776. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, Y.; Xu, B.; Mou, J.; Wang, M.; Gu, Q.; Sun, Q.; Li, M.; Zhao, C.; Zeng, M.; et al. Targeting KEAP1/NRF2 interaction with oleuropein ameliorates atherosclerosis by inhibiting macrophage ferroptosis. Free Radic. Biol. Med. 2025, 240, 566–582. [Google Scholar] [CrossRef]
- Jim, E.L.; Jim, E.L.; Surya, R.; Permatasari, H.K.; Nurkolis, F. Marine-Derived Polymers-Polysaccharides as Promising Natural Therapeutics for Atherosclerotic Cardiovascular Disease. Mar. Drugs 2025, 23, 325. [Google Scholar] [CrossRef]
- Duan, Y.; Gong, K.; Xu, S.; Zhang, F.; Meng, X.; Han, J. Regulation of cholesterol homeostasis in health and diseases: From mechanisms to targeted therapeutics. Signal Transduct. Target. Ther. 2022, 7, 265. [Google Scholar] [CrossRef]
- Ma, C.; Feng, K.; Yang, X.; Yang, Z.; Wang, Z.; Shang, Y.; Fan, G.; Liu, L.; Yang, S.; Li, X.; et al. Targeting macrophage liver X receptors by hydrogel-encapsulated T0901317 reduces atherosclerosis without effect on hepatic lipogenesis. Br. J. Pharmacol. 2021, 178, 1620–1638. [Google Scholar] [CrossRef]
- Ouimet, M.; Barrett, T.J.; Fisher, E.A. HDL and Reverse Cholesterol Transport. Circ. Res. 2019, 124, 1505–1518. [Google Scholar] [CrossRef]
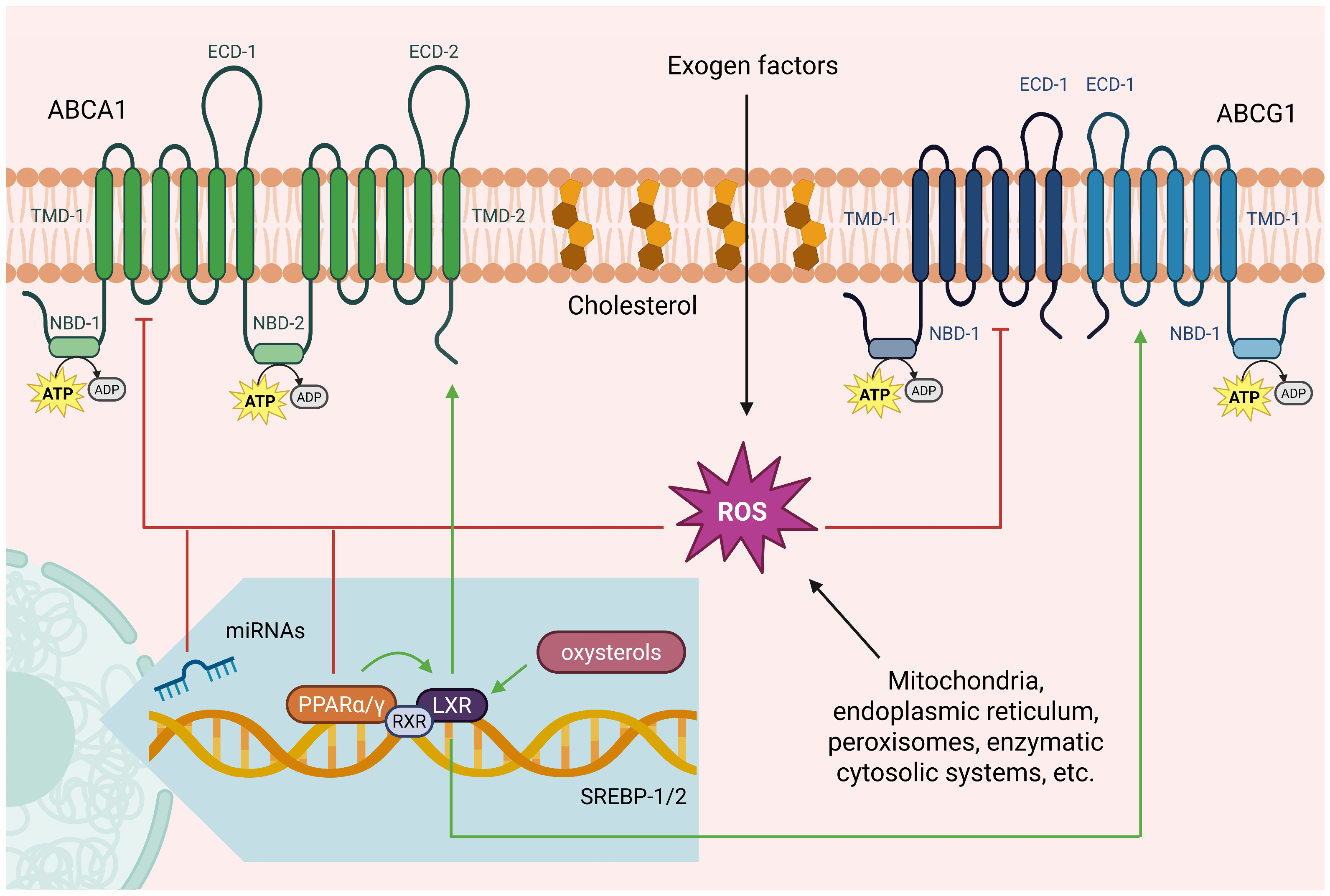
| Transporter | ABCA1 | ABCG1 | ||
|---|---|---|---|---|
| Effect | Upregulation | Downregulation | Upregulation | Downregulation |
| miRNA | miR-28 | miR-10b, miR-17, miR-19b, miR-20, miR-23a-5p, miR-26, miR-27, miR-30e, miR-33, miR-34a, miR92a, miR-93, miR-101, miR-106b, miR-128, miR-130, miR-143, miR-144, miR-145, miR-183, miR-301b, miR302a, miR-361-5p, miR-613, miR-758 * miR-1, * miR-128, * miR-155, * miR-212, * miR-223, * +miR-206, * miR-486 | ND | miR-10b, miR-23a-5p, miR-33, miR-34a, miR378 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laguna-Maldonado, K.D.; Uribe-Ramírez, D.; Vázquez-Carrada, M.; Matuz-Mares, D.; Vilchis-Landeros, M.M. Dysfunction of the ABCA1 and ABCG1 Transporters and Their Impact on HDL Metabolism. Antioxidants 2025, 14, 1362. https://doi.org/10.3390/antiox14111362
Laguna-Maldonado KD, Uribe-Ramírez D, Vázquez-Carrada M, Matuz-Mares D, Vilchis-Landeros MM. Dysfunction of the ABCA1 and ABCG1 Transporters and Their Impact on HDL Metabolism. Antioxidants. 2025; 14(11):1362. https://doi.org/10.3390/antiox14111362
Chicago/Turabian StyleLaguna-Maldonado, Kevin David, Daniel Uribe-Ramírez, Melissa Vázquez-Carrada, Deyamira Matuz-Mares, and María Magdalena Vilchis-Landeros. 2025. "Dysfunction of the ABCA1 and ABCG1 Transporters and Their Impact on HDL Metabolism" Antioxidants 14, no. 11: 1362. https://doi.org/10.3390/antiox14111362
APA StyleLaguna-Maldonado, K. D., Uribe-Ramírez, D., Vázquez-Carrada, M., Matuz-Mares, D., & Vilchis-Landeros, M. M. (2025). Dysfunction of the ABCA1 and ABCG1 Transporters and Their Impact on HDL Metabolism. Antioxidants, 14(11), 1362. https://doi.org/10.3390/antiox14111362








