Exploring the Cytotoxic and Redox-Modulatory Effects of Nanoceria in MCF7 Breast Cancer Cells Using Integrated Molecular and Proteomic Analyses
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. MTT Assay for Assessing Cell Toxicity
2.3. ROS Production Calculation
2.4. RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction
2.5. Measurement of Mitochondrial Potential
2.6. Sample Preparation for Proteomics
2.6.1. Protein Extraction and Digestion
2.6.2. Peptide Processing and Liquid Chromatography
2.6.3. Peptide Separation
2.6.4. Mass Spectrometry Analysis
2.7. Data Processing
3. Results
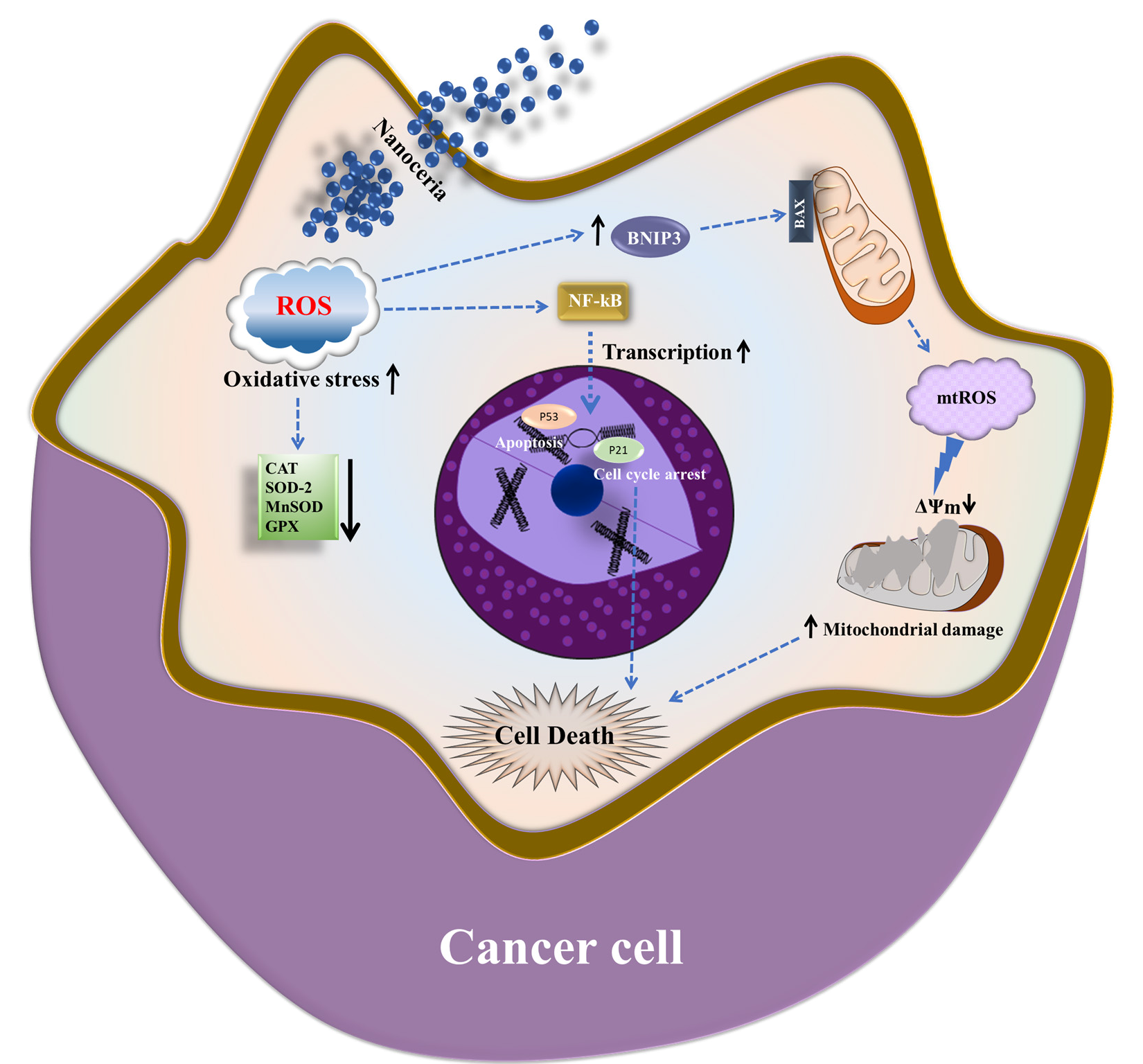
3.1. Cytotoxic and Pro-Oxidant Effects of Nanoceria on MCF7 Breast Cancer Cells
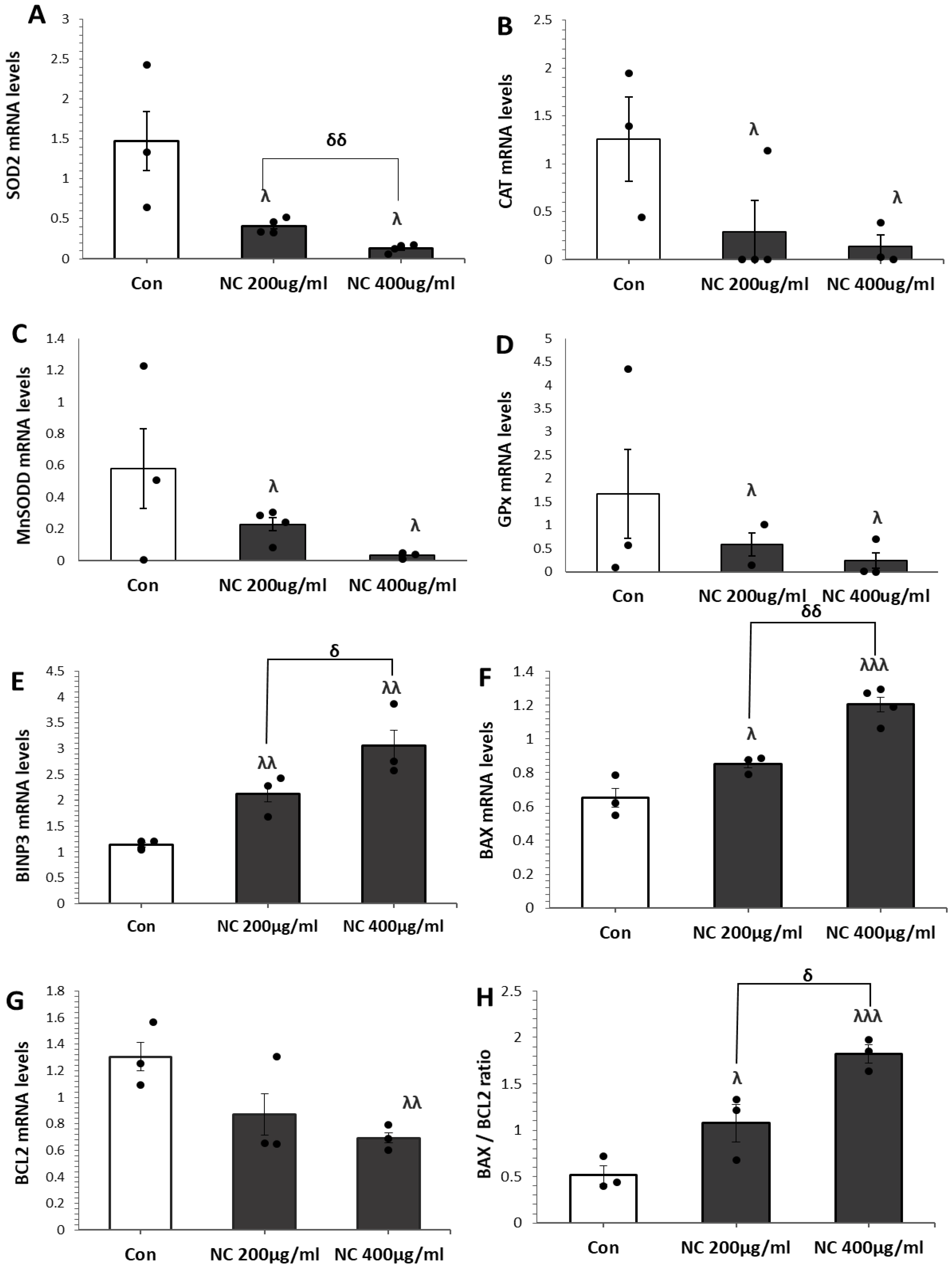
3.2. Nanoceria Downregulates Antioxidant Defense and Induces Apoptosis in MCF7 Cells
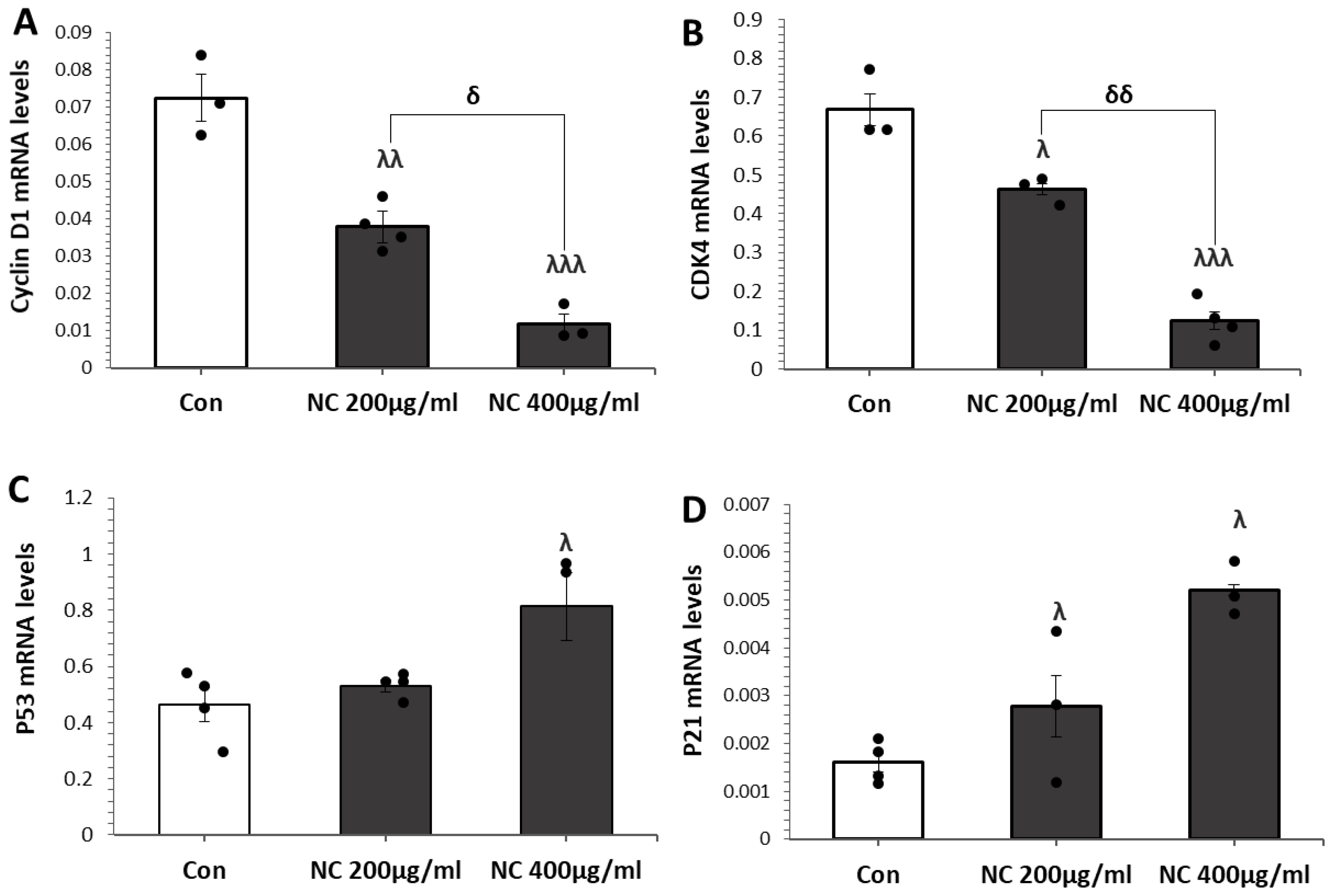
3.3. Nanoceria Induces G1 Phase Arrest via p53–p21 Pathway in MCF Cells
3.4. Nanoceria Inhibits Mitochondrial Biogenesis and Induces Mitochondrial Dysfunction in a Dose-Dependent Manner in MCF7 Cells
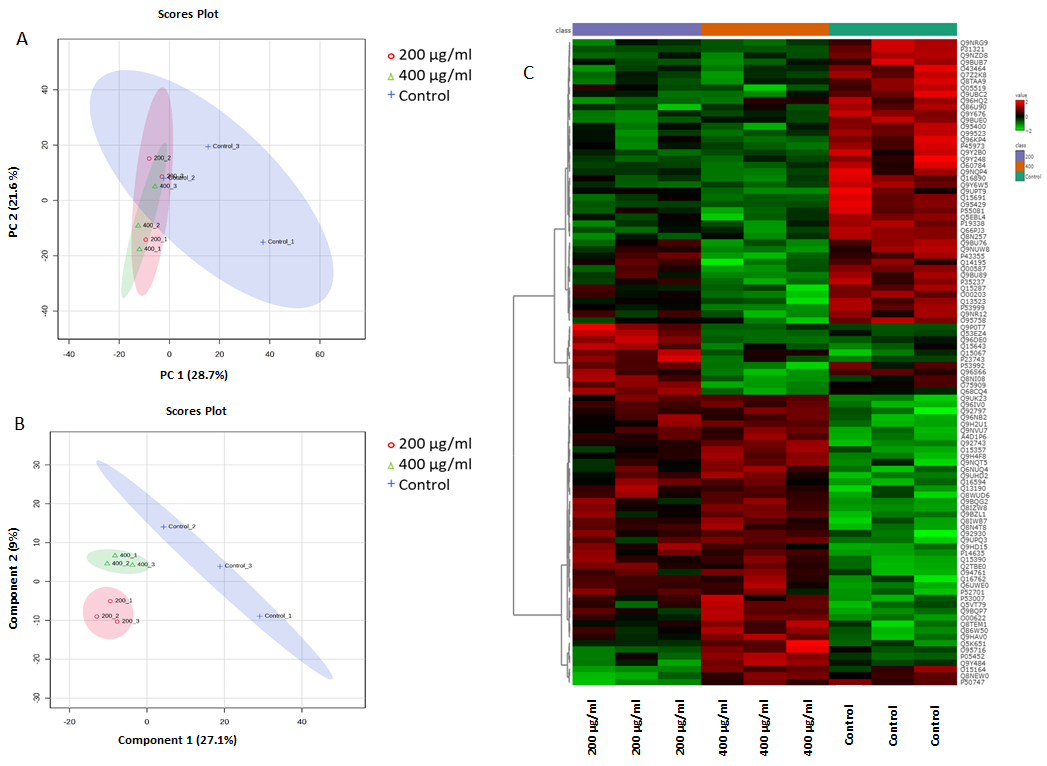
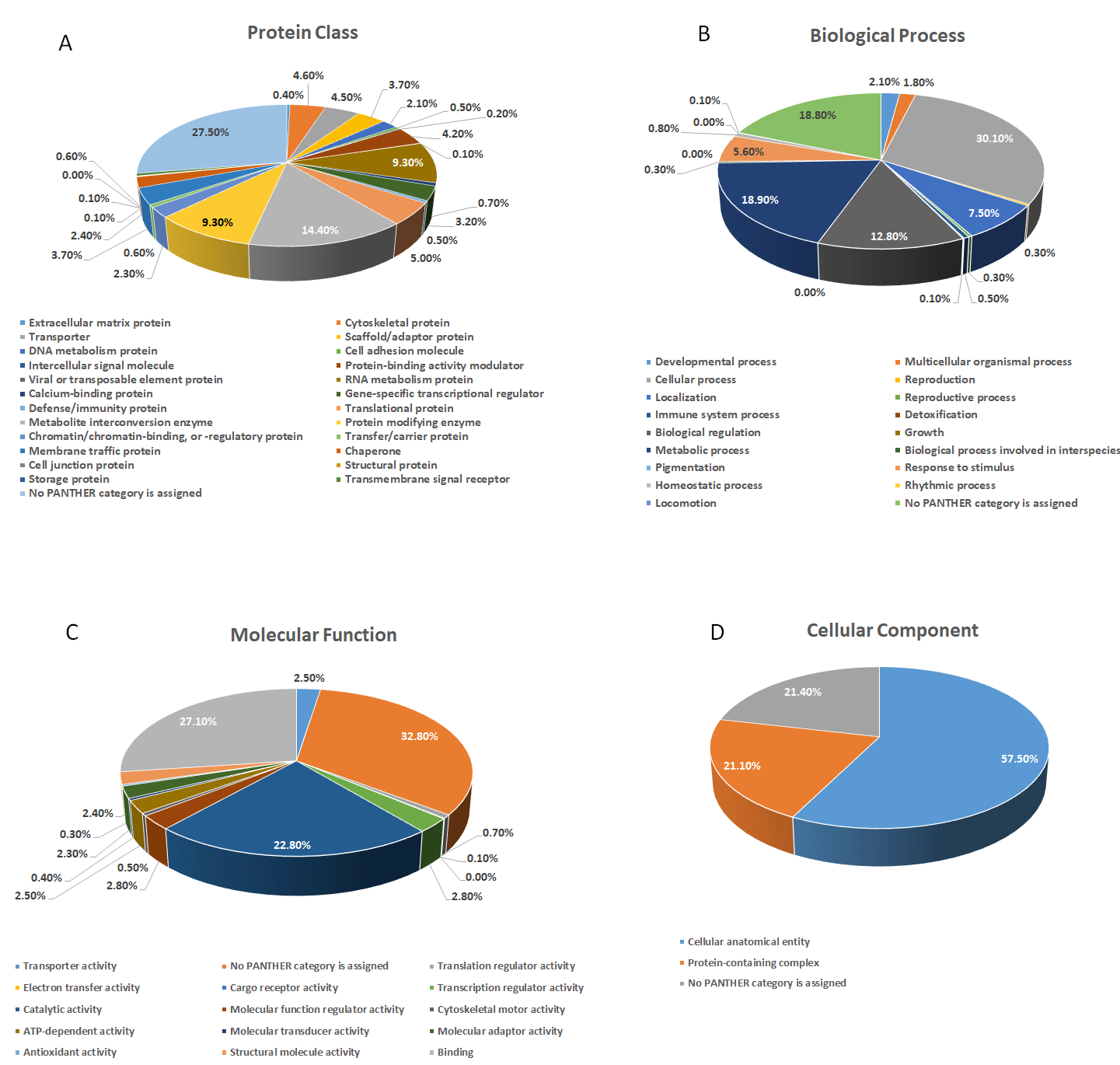
3.5. Label-Free Quantitative Proteomics Analysis
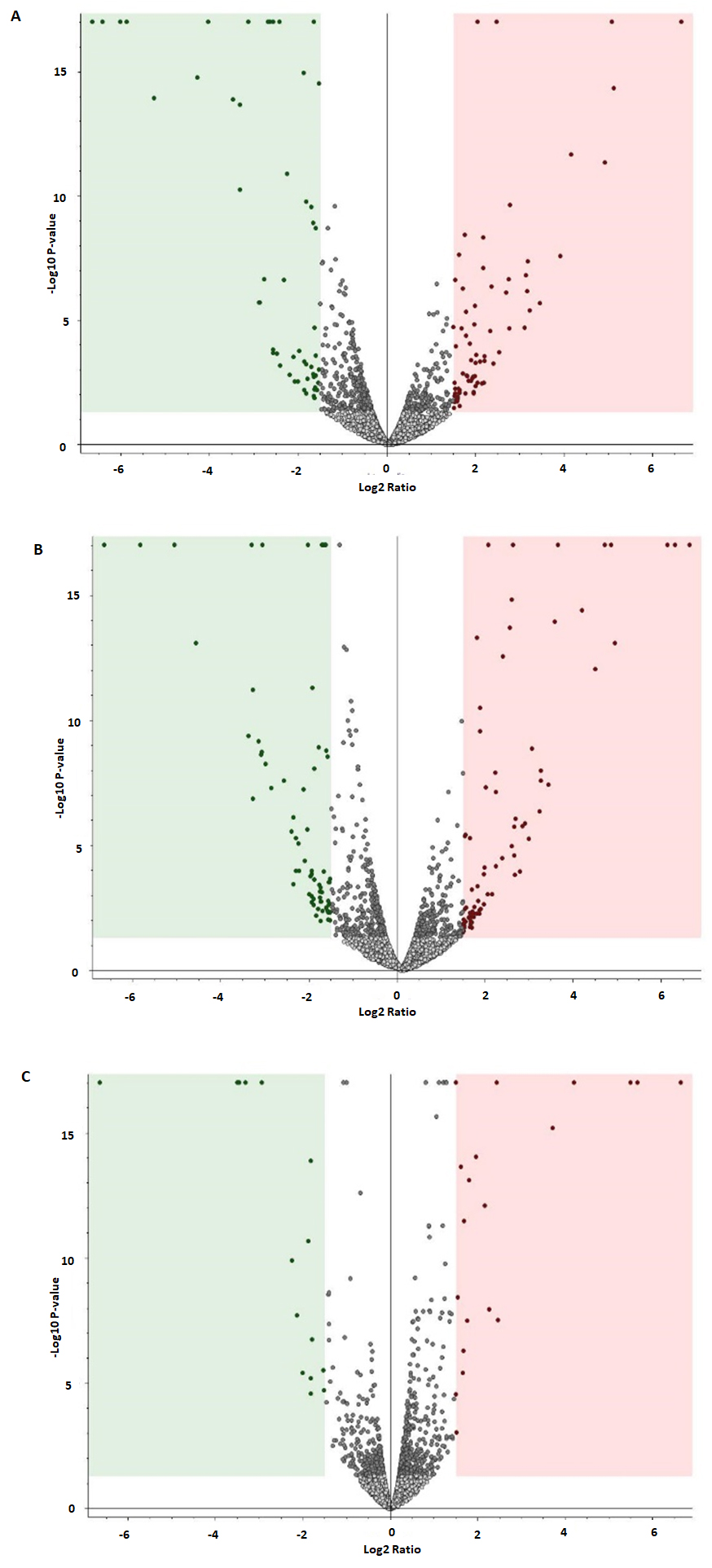
3.6. Analysis of Differentially Expressed Proteins in Untreated Control and 200 µg/mL and 400 µg/mL Nanoceria-Treated Samples
3.7. Protein Dysregulation in Response to Altered Nanoceria Treatment Concentration
3.8. Interaction Network Analysis of Differentially Expressed Proteins
4. Discussion
5. Conclusions
Strengths and Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilkinson, L.; Gathani, T. Understanding breast cancer as a global health concern. Br. J. Radiol. 2022, 95, 20211033. [Google Scholar] [CrossRef]
- WHO. Breast Cancer; WHO: Geneva, Switzerland, 2025. [Google Scholar]
- IARC. Breast Cancer; IARC: Lyon, France, 2025. [Google Scholar]
- Łukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast Cancer-Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies-An Updated Review. Cancers 2021, 13, 4287. [Google Scholar] [CrossRef] [PubMed]
- Midlenko, A.; Mussina, K.; Zhakhina, G.; Sakko, Y.; Rashidova, G.; Saktashev, B.; Adilbay, D.; Shatkovskaya, O.; Gaipov, A. Prevalence, incidence, and mortality rates of breast cancer in Kazakhstan: Data from the Unified National Electronic Health System, 2014–2019. Front Public Health 2023, 11, 1132742. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Xu, B. Breast cancer: Epidemiology, risk factors and screening. Chin. J. Cancer Res. 2023, 35, 565–583. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ji, Y.; Liu, S.; Li, J.; Wu, J.; Jin, Q.; Liu, X.; Duan, H.; Feng, Z.; Liu, Y.; et al. Global burden of female breast cancer: New estimates in 2022, temporal trend and future projections up to 2050 based on the latest release from GLOBOCAN. J. Natl. Cancer Cent. 2025, 5, 287–296. [Google Scholar] [CrossRef]
- Xiong, X.; Zheng, L.-W.; Ding, Y.; Chen, Y.-F.; Cai, Y.-W.; Wang, L.-P.; Huang, L.; Liu, C.-C.; Shao, Z.-M.; Yu, K.-D. Breast cancer: Pathogenesis and treatments. Signal Transduct. Target. Ther. 2025, 10, 49. [Google Scholar] [CrossRef]
- Xiong, N.; Wu, H.; Yu, Z. Advancements and challenges in triple-negative breast cancer: A comprehensive review of therapeutic and diagnostic strategies. Front. Oncol. 2024, 14, 1405491. [Google Scholar] [CrossRef]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef]
- Zafar, A.; Khatoon, S.; Khan, M.J.; Abu, J.; Naeem, A. Advancements and limitations in traditional anti-cancer therapies: A comprehensive review of surgery, chemotherapy, radiation therapy, and hormonal therapy. Discov. Oncol. 2025, 16, 607. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, H.; Tan, L.; Siu, K.T.H.; Guan, X.-Y. Exploring treatment options in cancer: Tumor treatment strategies. Signal Transduct. Target. Ther. 2024, 9, 175. [Google Scholar] [CrossRef]
- Tang, D.-D.; Ye, Z.-J.; Liu, W.-W.; Wu, J.; Tan, J.-Y.; Zhang, Y.; Xu, Q.; Xiang, Y.-B. Survival feature and trend of female breast cancer: A comprehensive review of survival analysis from cancer registration data. Breast 2025, 79, 103862. [Google Scholar] [CrossRef] [PubMed]
- Alwehaibi, H.F.; Ayash, F.M.; Alyousef, H.S.; Almusallam, M.I.; Alharbi, A.M.; Alaqeel, R.A.; Kharaba, A.M. Regional Disparities and Socioeconomic Determinants of Cancer Survival in Saudi Arabia: A Comprehensive Population-based Analysis of 26,487 Patients from 2010 to 2024. J. Adv. Trends Med. Res. 2025, 2, 361–371. [Google Scholar] [CrossRef]
- Clarke, R.; Jones, B.C.; Sevigny, C.M.; Hilakivi-Clarke, L.A.; Sengupta, S. Experimental models of endocrine responsive breast cancer: Strengths, limitations, and use. Cancer Drug Resist. 2021, 4, 762–783. [Google Scholar] [CrossRef] [PubMed]
- Vantangoli, M.M.; Madnick, S.J.; Huse, S.M.; Weston, P.; Boekelheide, K. MCF-7 Human Breast Cancer Cells Form Differentiated Microtissues in Scaffold-Free Hydrogels. PLoS ONE 2015, 10, e0135426. [Google Scholar] [CrossRef]
- Hanker, A.B.; Sudhan, D.R.; Arteaga, C.L. Overcoming Endocrine Resistance in Breast Cancer. Cancer Cell 2020, 37, 496–513. [Google Scholar] [CrossRef]
- Langeh, U.; Kumar, V.; Ahuja, P.; Singh, C.; Singh, A. An update on breast cancer chemotherapy-associated toxicity and their management approaches. Health Sci. Rev. 2023, 9, 100119. [Google Scholar] [CrossRef]
- Cheng, G.J.; Leung, E.Y.; Singleton, D.C. In vitro breast cancer models for studying mechanisms of resistance to endocrine therapy. Explor. Target. Anti-Tumor Ther. 2022, 3, 297–320. [Google Scholar] [CrossRef]
- Minh Hoang, C.N.; Nguyen, S.H.; Tran, M.T. Nanoparticles in cancer therapy: Strategies to penetrate and modulate the tumor microenvironment—A review. Smart Mater. Med. 2025, 6, 270–284. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, P.; Zhao, E.; Xiong, J.; Wang, S.; Lv, Z. Potential application and prospects of ROS-sensitive biomaterials in cancer therapy: A immune microenvironment triggered nanomaterial. Discov. Oncol. 2025, 16, 185. [Google Scholar] [CrossRef]
- Li, Y.; Yang, J.; Sun, X. Reactive Oxygen Species-Based Nanomaterials for Cancer Therapy. Front. Chem. 2021, 9, 650587. [Google Scholar] [CrossRef]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. BioMed Res. Int. 2013, 2013, 942916. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Torres, A.C.; Lorenzo-Anota, H.Y.; García-Juárez, M.G.; Zarate-Triviño, D.G.; Rodríguez-Padilla, C. Chitosan gold nanoparticles induce different ROS-dependent cell death modalities in leukemic cells. Int. J. Nanomed. 2019, 14, 7173–7190. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.T.; Li, K.Y.; Meng, F.Q.; Lin, J.F.; Young, I.C.; Ivkov, R.; Lin, F.H. ROS-induced HepG2 cell death from hyperthermia using magnetic hydroxyapatite nanoparticles. Nanotechnology 2018, 29, 375101. [Google Scholar] [CrossRef] [PubMed]
- Gul, R.; Dar, M.A.; Nawaz, S.; Alfadda, A.A. Protective Effects of Nanoceria against Mitochondrial Dysfunction and Angiotensin II-Induced Hypertrophy in H9c2 Cardiomyoblasts. Antioxidants 2023, 12, 877. [Google Scholar] [CrossRef]
- Gul, R.; Benabdelkamel, H.; Dar, M.A.; Masood, A.; Okla, M.; Alanazi, I.O.; Alfadda, A.A. Assessing the protective effects of nanoceria on angiotensin II-induced cardiac injury in H9c2 cardiomyoblasts using proteomic analysis. J. Pharm. Biomed. Anal. 2025, 263, 116835. [Google Scholar] [CrossRef]
- Dar, M.; Gul, R.; Alfadda, A.; Karim, M.; Kim, D.W.; Cheung, C.L.; Almajid, A.; Alharthi, N.H.; Pulakat, L. Size-Dependent Effect of Nanoceria on Their Antibacterial Activity Towards Escherichia coli. Sci. Adv. Mater. 2017, 9, 1248–1253. [Google Scholar] [CrossRef]
- Saifi, M.A.; Seal, S.; Godugu, C. Nanoceria, the versatile nanoparticles: Promising biomedical applications. J. Control. Release 2021, 338, 164–189. [Google Scholar] [CrossRef]
- Aplak, E.; von Montfort, C.; Haasler, L.; Stucki, D.; Steckel, B.; Reichert, A.S.; Stahl, W.; Brenneisen, P. CNP mediated selective toxicity on melanoma cells is accompanied by mitochondrial dysfunction. PLoS ONE 2020, 15, e0227926. [Google Scholar] [CrossRef]
- Tang, J.L.Y.; Moonshi, S.S.; Ta, H.T. Nanoceria: An innovative strategy for cancer treatment. Cell Mol. Life Sci. 2023, 80, 46. [Google Scholar] [CrossRef]
- Datta, A.; Mishra, S.; Manna, K.; Saha, K.D.; Mukherjee, S.; Roy, S. Pro-Oxidant Therapeutic Activities of Cerium Oxide Nanoparticles in Colorectal Carcinoma Cells. ACS Omega 2020, 5, 9714–9723. [Google Scholar] [CrossRef]
- Baskar, G.; Lalitha, K.; Aiswarya, R.; Naveenkumar, R. Synthesis, characterization and synergistic activity of cerium-selenium nanobiocomposite of fungal l-asparaginase against lung cancer. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 93, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Pešić, M.; Podolski-Renić, A.; Stojković, S.; Matović, B.; Zmejkoski, D.; Kojić, V.; Bogdanović, G.; Pavićević, A.; Mojović, M.; Savić, A.; et al. Anti-cancer effects of cerium oxide nanoparticles and its intracellular redox activity. Chem. Biol. Interact. 2015, 232, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Adebayo, I.A.; Arsad, H.; Samian, M.R. Chapter 19—The inhibitory role of the metabolites of Moringa oleifera seeds in cancer cells by apoptosis and cell cycle arrest activation. In Phytomedicine; Bhat, R.A., Hakeem, K.R., Dervash, M.A., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 533–554. [Google Scholar]
- Gul, R.; Benabdelkamel, H.; Dar, M.A.; Masood, A.; Alanazi, I.O.; Alfadda, A.A. Exploring the Protective Mechanisms of Nanoceria Against Angiotensin II-Induced Cardiac Damage in H9c2 Cardiomyoblasts through Proteomic Analysis. J. Pharmacol. Exp. Ther. 2024, 389, 263. [Google Scholar] [CrossRef]
- Gul, R.; Alsalman, N.; Bazighifan, A.; Alfadda, A.A. Comparative beneficial effects of nebivolol and nebivolol/valsartan combination against mitochondrial dysfunction in angiotensin II-induced pathology in H9c2 cardiomyoblasts. J. Pharm. Pharmacol. 2021, 73, 1520–1529. [Google Scholar] [CrossRef]
- Kashoob, M.; Masood, A.; Alfadda, A.A.; Joy, S.S.; Alluhaim, W.; Nawaz, S.; Abaalkhail, M.; Alotaibi, O.; Alsaleh, S.; Benabdelkamel, H. Label-Free Quantitative Proteomics Analysis of Nasal Lavage Fluid in Chronic Rhinosinusitis with Nasal Polyposis. Biology 2024, 13, 887. [Google Scholar] [CrossRef]
- Masood, A.; Benabdelkamel, H.; Joy, S.S.; Alhossan, A.; Alsuwayni, B.; Abdeen, G.; Aldhwayan, M.; Alfadda, N.A.; Miras, A.D.; Alfadda, A.A. Label-free quantitative proteomic profiling reveals differential plasma protein expression in patients with obesity after treatment with liraglutide. Front. Mol. Biosci. 2024, 11, 1458675. [Google Scholar] [CrossRef]
- Perez, J.M.; Asati, A.; Nath, S.; Kaittanis, C. Synthesis of biocompatible dextran-coated nanoceria with pH-dependent antioxidant properties. Small 2008, 4, 552–556. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, K.; Ma, J.-l.; Gao, F. Cerium oxide nanoparticles in cancer. OncoTargets Ther. 2014, 7, 835–840. [Google Scholar] [CrossRef]
- Hao, G.; Xu, Z.P.; Li, L. Manipulating extracellular tumour pH: An effective target for cancer therapy. RSC Adv. 2018, 8, 22182–22192. [Google Scholar] [CrossRef]
- Tian, Z.; Liu, H.; Guo, Z.; Gou, W.; Liang, Z.; Qu, Y.; Han, L.; Liu, L. A pH-Responsive Polymer-CeO2 Hybrid to Catalytically Generate Oxidative Stress for Tumor Therapy. Small 2020, 16, e2004654. [Google Scholar] [CrossRef]
- Atlı Şekeroğlu, Z.; Şekeroğlu, V.; Aydın, B.; Kontaş Yedier, S. Cerium oxide nanoparticles exert antitumor effects and enhance paclitaxel toxicity and activity against breast cancer cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2023, 111, 579–589. [Google Scholar] [CrossRef]
- Chen, D.; Guo, Z.; Yao, L.; Sun, Y.; Dian, Y.; Zhao, D.; Ke, Y.; Zeng, F.; Zhang, C.; Deng, G.; et al. Targeting oxidative stress-mediated regulated cell death as a vulnerability in cancer. Redox Biol. 2025, 84, 103686. [Google Scholar] [CrossRef]
- Nakamura, H.; Takada, K. Reactive oxygen species in cancer: Current findings and future directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef] [PubMed]
- Dash, U.C.; Bhol, N.K.; Swain, S.K.; Samal, R.R.; Nayak, P.K.; Raina, V.; Panda, S.K.; Kerry, R.G.; Duttaroy, A.K.; Jena, A.B. Oxidative stress and inflammation in the pathogenesis of neurological disorders: Mechanisms and implications. Acta Pharm. Sin. B 2025, 15, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Xu, Z.; Bambrick, H.; Prescott, V.; Wang, N.; Zhang, Y.; Su, H.; Tong, S.; Hu, W. Cardiorespiratory effects of heatwaves: A systematic review and meta-analysis of global epidemiological evidence. Environ. Res. 2019, 177, 108610. [Google Scholar] [CrossRef] [PubMed]
- Corsi, F.; Caputo, F.; Traversa, E.; Ghibelli, L. Not Only Redox: The Multifaceted Activity of Cerium Oxide Nanoparticles in Cancer Prevention and Therapy. Front. Oncol. 2018, 8, 309. [Google Scholar] [CrossRef]
- Abedi Tameh, F.; Mohamed, H.E.A.; Aghababaee, L.; Akbari, M.; Alikhah Asl, S.; Javadi, M.H.; Aucamp, M.; Cloete, K.J.; Soleimannejad, J.; Maaza, M. In-vitro cytotoxicity of biosynthesized nanoceria using Eucalyptus camaldulensis leaves extract against MCF-7 breast cancer cell line. Sci. Rep. 2024, 14, 17465. [Google Scholar] [CrossRef]
- Panda, S.R.; Singh, R.K.; Priyadarshini, B.; Rath, P.P.; Parhi, P.K.; Sahoo, T.; Mandal, D.; Sahoo, T.R. Nanoceria: A rare-earth nanoparticle as a promising anti-cancer therapeutic agent in colon cancer. Mater. Sci. Semicond. Process. 2019, 104, 104669. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Wei, S.; Nguyen, T.H.; Jo, Y.; Zhang, Y.; Park, W.; Gariani, K.; Oh, C.-M.; Kim, H.H.; Ha, K.-T.; et al. Mitochondria-associated programmed cell death as a therapeutic target for age-related disease. Exp. Mol. Med. 2023, 55, 1595–1619. [Google Scholar] [CrossRef]
- Moris, D.; Spartalis, M.; Tzatzaki, E.; Spartalis, E.; Karachaliou, G.S.; Triantafyllis, A.S.; Karaolanis, G.I.; Tsilimigras, D.I.; Theocharis, S. The role of reactive oxygen species in myocardial redox signaling and regulation. Ann. Transl. Med. 2017, 5, 324. [Google Scholar] [CrossRef]
- Arfin, S.; Jha, N.K.; Jha, S.K.; Kesari, K.K.; Ruokolainen, J.; Roychoudhury, S.; Rathi, B.; Kumar, D. Oxidative Stress in Cancer Cell Metabolism. Antioxidants 2021, 10, 642. [Google Scholar] [CrossRef]
- An, X.; Yu, W.; Liu, J.; Tang, D.; Yang, L.; Chen, X. Oxidative cell death in cancer: Mechanisms and therapeutic opportunities. Cell Death Dis. 2024, 15, 556. [Google Scholar] [CrossRef]
- Nelson, V.K.; Nuli, M.V.; Mastanaiah, J.; Saleem, T.S.M.; Birudala, G.; Jamous, Y.F.; Alshargi, O.; Kotha, K.K.; Sudhan, H.H.; Mani, R.R.; et al. Reactive oxygen species mediated apoptotic death of colon cancer cells: Therapeutic potential of plant derived alkaloids. Front. Endocrinol. 2023, 14, 1201198. [Google Scholar] [CrossRef]
- Moulder, D.E.; Hatoum, D.; Tay, E.; Lin, Y.; McGowan, E.M. The Roles of p53 in Mitochondrial Dynamics and Cancer Metabolism: The Pendulum between Survival and Death in Breast Cancer? Cancers 2018, 10, 189. [Google Scholar] [CrossRef]
- Shafagh, M.; Rahmani, F.; Delirezh, N. CuO nanoparticles induce cytotoxicity and apoptosis in human K562 cancer cell line via mitochondrial pathway, through reactive oxygen species and P53. Iran. J. Basic Med. Sci. 2015, 18, 993–1000. [Google Scholar] [PubMed]
- Ma, D.D.; Yang, W.X. Engineered nanoparticles induce cell apoptosis: Potential for cancer therapy. Oncotarget 2016, 7, 40882–40903. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gul, R.; Benabdelkamel, H.; Dar, M.A.; Bazighifan, A.; Masood, A.; Joy, S.S.; Ousman, O.M.; Alfadda, A.A. Exploring the Cytotoxic and Redox-Modulatory Effects of Nanoceria in MCF7 Breast Cancer Cells Using Integrated Molecular and Proteomic Analyses. Antioxidants 2025, 14, 1361. https://doi.org/10.3390/antiox14111361
Gul R, Benabdelkamel H, Dar MA, Bazighifan A, Masood A, Joy SS, Ousman OM, Alfadda AA. Exploring the Cytotoxic and Redox-Modulatory Effects of Nanoceria in MCF7 Breast Cancer Cells Using Integrated Molecular and Proteomic Analyses. Antioxidants. 2025; 14(11):1361. https://doi.org/10.3390/antiox14111361
Chicago/Turabian StyleGul, Rukhsana, Hicham Benabdelkamel, Mushtaq Ahmad Dar, Arwa Bazighifan, Afshan Masood, Salini Scaria Joy, Ousman Mahmood Ousman, and Assim A. Alfadda. 2025. "Exploring the Cytotoxic and Redox-Modulatory Effects of Nanoceria in MCF7 Breast Cancer Cells Using Integrated Molecular and Proteomic Analyses" Antioxidants 14, no. 11: 1361. https://doi.org/10.3390/antiox14111361
APA StyleGul, R., Benabdelkamel, H., Dar, M. A., Bazighifan, A., Masood, A., Joy, S. S., Ousman, O. M., & Alfadda, A. A. (2025). Exploring the Cytotoxic and Redox-Modulatory Effects of Nanoceria in MCF7 Breast Cancer Cells Using Integrated Molecular and Proteomic Analyses. Antioxidants, 14(11), 1361. https://doi.org/10.3390/antiox14111361







