Abstract
Food antioxidant supplementation has been widely proposed for cancer prevention and adjuvant therapy due to the pleiotropic role of antioxidants. Herein, particular attention is given to recent clinical trials based on the use of dietary supplements in cancer patients, both as monotherapy and in combination with standard treatments, exploring both their potential benefits and risks. This review focuses on the efficacy of the most important food antioxidants, highlighting how their action may change depending on different factors such as cancer type, dose, timing of administration and antioxidant status of the patient. The results of clinical trials are often contradictory, and the clinical benefit of dietary antioxidants appears more consistent in patients with a baseline antioxidant deficiency. Furthermore, by analyzing the mechanisms underlying the contradictory clinical evidence and critically addressing the issues related to the methodologies used in preclinical models, this review could be helpful in guiding the personalized use of antioxidant supplementation in cancer patients.
1. Introduction
Genetic, epigenetic, and lifestyle-related factors may promote tumorigenesis and malignant transformation as a consequence of aberrant redox homeostasis, leading to the impairment of antioxidant defenses [1]. Therefore, the use of antioxidants has been proposed as a possible approach to counteracting the onset of the carcinogenesis process [2]. In fact, several in vitro and in vivo findings and epidemiological studies have demonstrated that antioxidants, by either quenching Reactive Oxygen Species (ROS) or enhancing DNA repair enzyme activity, can help prevent certain kinds of cancer [3]. However, evidence from randomized controlled trials often conflicts due to the heterogeneity of types and doses of antioxidants tested and the timing of supplementation [4,5,6,7,8]. Moreover, another critical yet often overlooked factor contributing to the inconsistent and sometimes conflicting results regarding the efficacy of antioxidant supplementation in cancer treatment is related to the experimental conditions under which in vitro studies are conducted. In fact, the composition of standard culture media differs substantially from that of human plasma, both in nutrient concentration and in the presence of metabolites. These non-physiological conditions can significantly alter cellular responses, including redox balance and metabolism, thereby potentially confounding the observed effects of antioxidant compounds. Therefore, the use of conventional media without consideration of their physiological relevance may limit the translational value of in vitro findings and contribute to the observed discrepancies in preclinical and clinical research.
Indeed, many studies have demonstrated that people receiving antioxidant supplementation have developed cancer at a higher rate or the same rate than those treated with a placebo, raising the possibility that these supplements may be harmful rather than beneficial [9,10,11,12]. One possible explanation is that antioxidants can change their biological function based on the lifestyle habits (e.g., smoking) of the subjects involved, paradoxically promoting instead of reducing pro-oxidative products, which can lead to a higher risk of developing cancer [13].
To date, many studies concerning these compounds have been published, but, to the best of our knowledge, none of these studies have examined all of them together. The primary aim of this review is to critically evaluate the role of pleiotropic antioxidant supplements in modulating cancer development and progression. Particular attention is given to recent clinical studies assessing the efficacy of these supplements, both as standalone interventions and in combination with conventional therapies. To provide a comprehensive perspective, we have also considered relevant preclinical in vitro and in vivo studies that help elucidate the underlying molecular mechanisms and cellular responses induced by antioxidant treatment. Furthermore, by integrating clinical evidence with mechanistic insights and methodological considerations, this review aims to offer a more accurate and translationally relevant understanding of antioxidant supplementation in cancer therapy.
2. Effects of Food Supplementation in Cancer Treatment: An Update on Clinical Trials
Identification of the real potential and limitations of antioxidant food supplementation from a clinical point of view is essential. To provide such information, this section has been focused on examining clinical trials conducted from 1999 on cancer patients treated with antioxidants and whose results have been published in high-impact journals. For completeness, two case studies were also included.
Based on the results, these studies were divided into three subgroups: (1) those in which the approach is effective in counteracting cancer progression (Table 1); (2) those in which the approach is not able to counteract cancer progression (Table 2); and (3) those in which the supplementation, despite not reducing tumor mass, can improve the patients’ quality of life (Table 3).

Table 1.
Clinical trials in which antioxidant supplementation was effective in counteracting cancer progression. The efficacy of supplemental antioxidants was evaluated and quantified by comparing clinical outcomes between patients treated with antioxidants and those receiving placebo or standard care, as reported in the original clinical studies.
By analyzing the first and the second group of studies, it is evident that the response to antioxidant supplementation is closely specific to cancer type (Table 1 and Table 2). Moreover, although the studies reporting an effective action of supplementation on cancer remission are promising for some types of cancer, the small number of patients treated negatively impacts their clinical value (Table 1).
However, it is important to consider that most of the supplements leading to a positive effect on cancer remission may, under other conditions (depending not only on cancer type/cancer stage but also on the dose and the time of antioxidant administration), show null or sometimes even negative effects (Table 2).

Table 2.
Clinical trials in which antioxidant supplementation was ineffective in counteracting cancer progression. The efficacy of supplemental antioxidants was evaluated and quantified by comparing clinical outcomes between patients treated with the antioxidants and those receiving placebo or standard care, as reported in the original clinical studies.
Table 2.
Clinical trials in which antioxidant supplementation was ineffective in counteracting cancer progression. The efficacy of supplemental antioxidants was evaluated and quantified by comparing clinical outcomes between patients treated with the antioxidants and those receiving placebo or standard care, as reported in the original clinical studies.
| Cancer Type | Antioxidants | Conventional Therapy | Patients Enrolled (No.) | Ref. |
|---|---|---|---|---|
| Breast cancer |
| RT | 79 (early-stage or ductal carcinoma in situ) | [38] |
| Biphosphonate | 40 (bone metastatic breast cancer) | [39] | |
| Docetaxel (100 mg/m2) every 3 weeks for six cycles with methylprednisolone (50 mg, six times in 3 days) | 42 (HER2-negative metastatic or loco-regionally recurrent or inoperable patients) | [40] | |
| Not specified | 76 (T1-3, No-2, non-metastatic) | [41] | |
| Colorectal cancer |
| - | 409 (I–IV) | [42] |
| - | 72 (metastatic colorectal cancer) | [43] | |
| Head and neck cancer |
| CT | 40 (locally advanced cancer | [44] |
| - | 2592 (non-small-cell lung cancer, stages pT1-2, N0-1, and T3N0; cancer of larynx, stages Tis, T1-3, N0-1; cancer of the oral cavity, stages T1-2 and N0-1) | [45] | |
| RT | 540 (stage I–II) | [46] | |
| Myeloma |
| Bortezomib (1, 3 mg/m2 on days 1, 4, 8, and 11) | 24 (multiple myeloma) | [47] |
| - | 44 (multiple myeloma or lymphoma) | [48] | |
| Non-acute promyelocytic leukemia |
| Arsenic trioxide (0.25 mg/kg/day over 1–4 h, for 5 days a week for 5 weeks) | 10 (relapsed or refractory acute myeloid leukemia, excluding acute promyelocytic leukemia) | [49] |
| Nonmelanoma skin cancer |
| Surgery | 60 (stage n.d.) | [50] |
| Non–muscle-invasive bladder cancer (NMIBC) |
| - | 270 (Ta, Tis, T1 at baseline) | [51] |
| Prostatic cancer |
| - | 23 (chemotherapy-naive metastatic castration-resistant cancer) | [52] |
| - | 60 (multifocal high-grade intraepithelial neoplasia and/or atypical small acinar proliferation) | [10] | |
| - | 29,133 (stage n.d.) | [53] | |
| Other malignancy |
| Not specified | 24 (stage n.d.) | [54] |
| Imatinib (400 mg once daily) | 17 (refractory to standard therapy) | [55] | |
| - | 62 (chronic-phase chronic myeloid leukemia) | [56] | |
| - | 720 (non-muscle-invasive bladder cancer) | [51] |
Considering the clinical trials reported in Table 1 and Table 2, antioxidant supplementation appears to have a beneficial effect in patients with antioxidant deficiencies, while it appears to have a detrimental effect in patients with adequate antioxidant status. Therefore, the evaluation of blood antioxidant levels could be helpful in identifying patients who could be potentially treated with antioxidant supplements.
However, the results obtained from clinical studies conducted to date are extremely variable because of the heterogeneity among the populations examined, the variability in the measurement of analytes, and the poor standardization of the experimental methods used. Therefore, the interaction between the multiple cofactors makes it essential to apply more complex statistical analyses, such as multivariate analysis, to more correctly identify the possible relationships between dietary supplementation and the patient’s response to cancer. A further factor that could justify the high heterogeneity of the results is the fact that dietary supplements are considered to be as cofactors indirectly involved in carcinogenesis.
Furthermore, unbalanced antioxidant supplementation, interfering with overlapping metabolic pathways, could make it difficult to identify a single causal agent since it adds many variables that could explain the discrepancies in the results obtained.
Similar doubts arise about the beneficial effect of administering antioxidants in combination with anti-cancer therapies. In fact, although several pre-clinical studies have demonstrated that antioxidant supplementation may potentiate the effect of anti-cancer therapies, the results obtained by clinical trials are often conflicting [1]. Indeed, on one hand, the administration of supplements has been found to counteract the effects of chemo- and radiotherapy, which exert their cytotoxic action by inducing oxidative stress through the depletion of antioxidant reserves [57]. On the other hand, high doses of supplements often determine a pro-oxidant effect rather than an antioxidant one [58]. In addition, since therapy-resistant tumors are characterized by increased antioxidant levels, their supplementation could promote therapy refractoriness [57,59,60,61,62,63,64]. Furthermore, supplements can interfere with the physiological action of ROS and with the biodistribution of chemotherapeutic drugs [58].
In a limited number of clinical studies, the combination of chemo-/radiotherapy and antioxidants has been tested, and the current evidence indicates that it is better to administer antioxidants only at the end of therapy. However, it is necessary for these food supplements to selectively interfere with ROS production by cancer cells without altering their physiological role as cell signaling molecules.
Moreover, while antioxidant supplementation may not always lead to measurable improvements in tumor response or survival outcomes, a growing body of evidence suggests that these compounds can play a valuable supportive role by alleviating the debilitating side effects associated with conventional cancer therapies (Table 3). These adverse effects—often underestimated—can significantly impact patient quality of life, reduce treatment adherence, and compromise overall therapeutic success. By mitigating toxicity and improving tolerability, antioxidants may enhance patient resilience, psychological well-being, and motivation to complete intensive treatment protocols, thereby indirectly contributing to better clinical outcomes.

Table 3.
Clinical trials in which antioxidant supplements have a positive (+) or negative (-) impact on therapy side effects and patients’ quality of life. The efficacy of supplemental antioxidants was evaluated and quantified by comparing the results obtained in antioxidant-treated patients with those obtained in placebo groups.
Table 3.
Clinical trials in which antioxidant supplements have a positive (+) or negative (-) impact on therapy side effects and patients’ quality of life. The efficacy of supplemental antioxidants was evaluated and quantified by comparing the results obtained in antioxidant-treated patients with those obtained in placebo groups.
| Cancer Type | Antioxidants | Conventional Therapy | Patients Enrolled (No.) | Effect | Ref. |
|---|---|---|---|---|---|
| Acute lymphoblastic leukemia |
| Vincristine | 141 pediatric patients | + | [65] |
| None; candidate for mastectomy | 39 (Tis-IIb) | + | [15] | |
| RT and CT | 572 (0-IIb) | + | [17] | |
| Breast cancer |
| RT | 52 | + | [66] |
| CT and RT | 53 (stages Iia–IIIb) | + | [67] | |
| Fluorouracil, doxorubicin, cyclophosphamide | 40 (stage II) | + | [68] | |
| RT | 79 (early stage or ductal carcinoma in situ) | - | [38] | |
| - | 95 (stages 0–III) | + | [69] | |
| CT | 49 (stage n.d.) | + | [70] | |
| - | 40 (bone metastatic breast cancer) | - | [39] | |
| Doxorubicin (60 mg/m2) + Cyclophosphamide (600 mg/m2) every 21 days | 150 (stage n.d.) | + | [71] | |
| Letrozole | 82 (stage n.d.) | + | [72] | |
| Colorectal cancer |
| FOLFOX | 27 (metastatic colon cancer) | + | [22] |
| - | 2552 (stage I–III) | + | [73] | |
| CT | 81 (stage II–III) | + | [74] | |
| Hepatocellular carcinoma |
| Surgery | 41 (primary hepatocellular carcinoma) | + | [75] |
| Leukemia |
| DCAG (15 mg/m2 of decitabine (days 1–5) and 300 μg/day of granulocyte colony-stimulating factor (G-CSF, days 0–9) for priming its combination with 10 mg/m2 of cytarabine (q12h, days 3–9), 8 mg/m2 of aclarubicin (days 3–6) | 73 (acute myeloid leukemia) | + | [33] |
| Methotrexate (3 g/m2 (leukemia) and 5 g/m2 (Lymphoma) | 35 (leukemia and lymphoma) | + | [76] | |
| LHNC (locally advanced head and neck cancer) |
| EBRT | 60 (stage III–IVB) | + | [30] |
| Melanoma |
| Surgery | 500 (stage IB–III) | + | [24] |
| Multiple myeloma |
| CT | 36 (patients in complete response status and candidates for autologous hematopoietic stem cell transplantation) | + | [77] |
| Nonmelanoma Skin cancer |
| Surgery | 60 (stage n.d.) | - | [50] |
| Ovarian cancer |
| neoadjuvant platinum-taxane, surgery, adjuvant platinum-taxane | 284 (stage III–IV) | + | [34] |
| Thoracic cancer (breast, lung, esophageal) |
| RT | 19 (severe radio-induced dermatitis in cancers stage II–IV) | + | [37] |
| Other malignancies |
| - | 39 (terminal patients) | + | [78] |
| Paclitaxel | 32 (stage n.d). | + | [79] | |
| Cisplatin | 30 (stage n.d) | + | [80] | |
| Cisplatin (300 mg/m2) | 23 (stage n.d.) | + | [81] | |
| Cisplatin | 108 (stage n.d.) | + | [82] | |
| Surgery | 33 (stage n.d.) | + | [83] |
2.1. Curcumin
Several studies have been conducted to investigate the effect of curcumin on different solid tumors: breast, prostate, colorectal, head and neck cancer, and hepatocellular carcinoma.
The mechanisms through which curcumin seems to be a good support in anti-cancer treatment vary from its direct effects on the tumor to its coadjutant role in radio- or chemo-therapy as an antioxidant and anti-inflammation agent. Furthermore, curcumin administration seems to reduce the side effects of therapies, also improving patients’ compliance.
A prospective randomized study conducted on LHNC (locally advanced head and neck cancer) patients showed that curcumin is able to decrease chemo-radiation therapy-induced hematological, renal, skin, and mucosal toxicities resulting in improvement of disease control (Table 1) [30].
Ávila-Gálvez et al. demonstrated that curcuminoids directly affect breast cancer growth by inducing cell-cycle arrest, senescence, and apoptosis via a p53/p21-dependent pathway, while isoflavone-derived metabolites exert estrogenic-like activity, mainly in p53-wild-type MCF-7 cells, suggesting that the consumption of 3 capsules/day (turmeric, red clover, and flaxseed extracts plus resveratrol; 296.4 mg phenolics/capsule) could be helpful in fighting breast cancer [15].
Curcumin is also a safe and tolerable adjunct to chemotherapy (FOLFOX ± bevacizumab vs. FOLFOX ± bevacizumab plus curcumin, CUFOX) in patients with metastatic colorectal cancer. Despite the low number of patients included in the study, CUFOX patients had smaller negative changes to their functional, symptom, and global health scores. In addition, curcumin has been found to prevent oxaliplatin-induced neuropathy in CUFOX patients (Table 1) [22].
Moreover, a study carried out on HCC patients treated with trans-arterial chemoembolization (TACE) demonstrated that the combination of curcumin, piperine, and taurine (CPT) significantly increased IFN-γ serum levels (Table 1) [28], downregulated the expression of PD-1, and induced a significant reduction in Treg lymphocytes. Moreover, the CPT approach is clinicopathologically relevant, since it has been found to exert a high impact on aspartate aminotransferase (AST), lactate dehydrogenase (LDH), and alpha fetoprotein (AFP) levels, which significantly declined after treatment.
A randomized, double-blind, placebo-controlled, phase II presurgical trial conducted by Macis et al. on patients with adenomatous polyps demonstrated that the combination of anthocyanins and curcumin did not directly modulate the circulating biomarkers of inflammation and metabolism, but it influenced the metabolic biomarkers involved in colon carcinogenesis via adiponectin and IL-6 levels, since these molecules influence the tumor microenvironment and its energetic balance (Table 1) [14].
2.2. Trans-Resveratrol
Trans-resveratrol is present in high concentrations in the skin of red grapes and red wine and has been advertised as a chemopreventive agent, since it prevents the formation of mammary tumors and has a dose-dependent antiproliferative effect in vitro. A study by Zhu et al. [84] showed the effects of trans-resveratrol on women with increased breast cancer risk vs. a placebo-treated group. Significant increases were found in serum total resveratrol and its glucuronide metabolites (p < 0.001), while a reduction in breast NAF PGE2 levels correlated with RASSF-1α demethylation (p = 0.045). Trans-resveratrol supplementation can affect epigenetic regulation and inflammatory markers in breast tissue, suggesting a promising chemopreventive mechanism. Further large-scale studies are recommended.
On the other hand, in a phase II clinical trial conducted in patients with relapsed/refractory multiple myeloma, it was observed that the combined therapy of resveratrol plus bortezomib had an unacceptable safety profile and minimal efficacy [47].
Recently, in an open-label study conducted in patients with head and neck cancer and receiving home enteral nutrition, it was reported that resveratrol supplementation enhanced antioxidant defenses by increasing glutathione peroxidase activity and superoxide dismutase protein levels [27].
2.3. Selenium and Vitamin E
Selenium supplementation (SS) was investigated for the prevention of prostate cancer, while selenium and vitamin E supplementation was investigated for the prevention of non-muscle-invasive bladder cancer (NMIBC) relapse.
A subsequent report analyzed the correlation between prostate-specific antigen (PSA) and selenium concentrations, showing that SS significantly reduced the incidence of prostate cancer and that this effect was greater in men with the lowest baseline plasma selenium concentrations (Table 1) [35].
A multi-center, prospective, double-blinded, placebo-controlled clinical trial including patients with newly diagnosed NMIBC was carried out by Bryan et al. [51]. The results showed that SS did not reduce the risk of recurrence in NMIBC patients, while vitamin E supplementation was associated with an increased risk of recurrence. No effect due to selenium or vitamin E supplementation was observed on cancer progression or overall survival, even if vitamin E supplementation appeared to be harmful for NMIBC patients.
The results obtained with vitamin E supplementation are extremely heterogeneous (Table 1, Table 2 and Table 3). In fact, for example, in a randomized controlled trial on patients affected by head and neck cancer and treated with α-tocopherol and β-carotene during radiation therapy, vitamin E was demonstrated to be harmful [46]. On the other hand, it was reported that vitamin E administration, given alone or in combination with β-carotene, increased the overall survival of prostate cancer patients [53].
2.4. Vitamin A
In a study conducted in patients with advanced cervical cancer, vitamin A supplementation was found to increase the clinical response to cisplatin and paclitaxel (Table 1) [20]. Furthermore, it was interesting to note that vitamin A administration, before methotrexate treatment, appeared to be effective in protecting children affected by leukemia and lymphoma from drug-induced D-xylose malabsorption (Table 3) [76].
A robust randomized clinical trial was performed in 18,314 subjects at risk for lung cancer, treating them with vitamin A for 4 years (Beta Carotene and Retinol Efficacy Trial). The results provided evidence that no benefit was obtained in terms of cancer prevention. Furthermore, vitamin A supplementation was associated with increased mortality from lung cancer and cardiovascular disease. In addition, beta carotene appeared to alter the redox balance, acting as a pro-oxidant agent in non-physiologic conditions [85].
2.5. Vitamin D
Contradictory results have also been observed in clinical trials in which the efficacy of vitamin D was tested. In fact, as reported in Table 1, vitamin D administration to patients with cervical intraepithelial neoplasia was found to have beneficial effects on CIN1/2/3 recurrence and metabolic status [21]. Similar positive effects have also been detected in patients with gastric cancer [25] and melanoma [24]. Positive results have also been observed in clinical studies in which vitamin D was combined with conventional therapy. In fact, in a phase II study conducted on breast cancer patients, vitamin D at high doses was found to enhance the action of conventional chemotherapy [18]. Similarly, vitamin D supplementation was observed to increase the progression-free survival rate in colorectal cancer patients treated with mFOLFOX6 plus bevacizumab [23].
In contrast, other studies have reported the ineffectiveness of vitamin D supplementation (Table 2). In fact, treatment with vitamin D3 and calcium in patients with bone-metastatic breast cancer did not produce significant beneficial effects [39]. A similar situation was described in patients with colorectal cancer treated with cholecalciferol [43] and in patients with chronic myeloid leukemia treated with Vitamin D in combination with imatinib [56]. Moreover, in a recently published study, it was found that the treatment of vitamin D increased IL-6 serum levels and, when it was administered together with symbiotic supplements, markedly enhanced the anti-inflammatory index, suggesting a potential anti-inflammatory function of this combination [41].
Furthermore, several studies have shown that vitamin D supplementation is able to improve the quality of life of different oncologic patients and to attenuate the side effects of conventional therapies (Table 3).
2.6. Vitamin C
The effects of vitamin C supplementation on cancer are also contradictory and complex. As reported by Zhao, the addition of vitamin C to low-dose decitabine before aclarubicin and cytarabine (DCAG) increased the rate of complete remissions and was associated with prolonged overall survival in patients with acute myeloid leukemia (Table 1) [33]. In addition, vitamin C supplementation was also shown to potentiate the biological effects of DNA methyltransferase inhibitors in patients with myeloid cancer (Table 1) [32].
In contrast, several studies have reported the ineffectiveness of vitamin C supplementation. In this regard, a study conducted on patients with prostate cancer demonstrated that intravenous administration of vitamin C did not induce disease remission and, therefore, its supplementation was not recommended (Table 2) [52]. Similar results were obtained in patients with nonmelanoma skin cancer treated with a cocktail containing vitamin C, vitamin E, and zinc, where no significant reduction in oxidative stress biomarkers was observed (Table 2) [50]. Limited clinical efficacy was also reported in patients with acute myeloid leukemia treated with a combined therapy of vitamin C and arsenic trioxide (Table 2) [49]. Other studies have demonstrated that, although vitamin C was well tolerated, it failed to demonstrate anticancer efficacy (Table 2) [54,55].
Better results have been obtained in terms of the improvement in quality of life and reduction of side effects of conventional therapy (Table 3). In several studies conducted in patients with breast cancer [68], nonmelanoma skin cancer [50], leukemia [33], and other neoplasia [78], it has been reported that vitamin C supplementation was well tolerated and led to a significant reduction in disease-induced and chemo/radiotherapy-promoted disorders.
These contradictory results are related to the fact that vitamin C behaves either as an antioxidant or pro-oxidant depending on the dose and the administration route.
Vitamin C acts as an antioxidant when administered at low doses by the oral route, where it is metabolized as ascorbic acid. This is currently the most used manner of administration and appear to be ineffective, if not detrimental, in cancer patients.
Conversely, vitamin C acts as pro-oxidant when used at high doses through blood infusion, where it is metabolized as the oxidant ossalic acid [86]. Indeed, when high doses of vitamin C are administered intravenously, most of it is metabolized to oxalic acid. Under these conditions, clinical effects in cancer patients are promising and warrant further clinical studies.
2.7. Flavonoids
The effects of epigallocatechin-3-gallate (EGCG) solution can be useful to treat the acute severe dermatitis that may occur after radiotherapy. A phase I study on radiotherapy-treated patients with thoracic cancer, grade III RID (Regional or Distant Involvement), showed that three days of continuous EGCG administration (sprayed in the radiation field) induced the regression of lesions to grade I or grade II RID and significantly reduced associated symptoms after 15 days of EGCG treatment (Table 1 and Table 3) [37].
Among flavonoids, phytoestrogens have been suggested to have an anti-proliferative role in prostate cancer. Ahlin et al. [87] carried out a randomized controlled trial involving men with low- to intermediate-risk prostate cancer scheduled for radical prostatectomy. Participants were randomized either to an intervention group who received ~200 mg/day of phytoestrogens (derived from soybeans and flaxseeds), added to their usual diet for approximately 6 weeks, or a control group following standard dietary advice without supplementation. Dietary supplementation with soybeans and flaxseeds (~200 mg phytoestrogens/day) did not affect circulating levels of testosterone, IGF-1, or SHBG in the perioperative setting, even though a high intake of phytoestrogens was hypothesized to reduce the concentration of estradiol in prostate cancer patients with a specific genetic profile of Erβ.
Anticancer effects of flavonoids are known, but little information about the influence of flavonoid intake on colorectal cancer (CRC)-related mortality is available. A prospective study investigated the association of post-diagnostic flavonoid intake with CRC-specific and all-cause mortality in 2552 patients diagnosed with stage I-III CRC. Total flavonoid intake was not associated with mortality, except for flavan-3-ols, a component of tea, where higher intake showed a linear relationship with lower CRC-specific and all-cause mortality (Table 3) [73].
Another study investigated the effects of flavonoid and lignan intakes on the risk of CRC recurrence and overall survival in CRC patients. The results did not demonstrate a role of flavonoid or lignan intake on CRC prognosis nor mortality, since after 8.6 years of follow-up, 32.5% participants died, and 24.1% had CRC recurrence (Table 2) [42].
While the association between flavonoids and the incidence of breast cancer is clear, less is known about the association between the intake of flavonoids and cancer recurrence. A study by Cheon et al. showed that the intake of flavonoid-rich food improved disease-free survival among overweight and obese patients, suggesting that the intake of flavonoids could have beneficial effects on cancer recurrence in these patients (Table 1 and Table 3) [17].
2.8. Indole-3-Carbinol (I3C)
The results obtained by a five-year study on patients with stage III-IV serous ovarian cancer demonstrated that the combination of I3C and EGCG was able to prolong progression-free survival and overall survival of patients, suggesting that long-term usage of this approach represents a promising maintenance therapy in the treatment of patients with advanced ovarian cancer (Table 1 and Table 3) [34].
2.9. Butyric Acid and Short-Chain Fatty Acids
There are 12 registered clinical trials (clinicaltrials.gov) currently underway, investigating the administration of butyric acid and short-chain fatty acids to cancer patients for therapeutic purposes or to cancer survivors for preventive purposes. Butyrate is generated by gut microbial fermentation of dietary fibers. Its antineoplastic effects are exerted via the activation of G protein-coupled receptors and the inhibition of histone deacetylases [88]. Despite the strong experimental and mechanistic evidence, robust results of large-sized clinical trials are not yet available.
2.10. Ozonated Fatty Acids
The oxidation of cancer cells has been proposed as a possible radical therapy to treat cancer cells and prevent cancer relapses [89]. Indeed, the chemo- and radioresistance of cancer cells, particularly of stem cancer cells, is due to the high amounts of their antioxidants [90]. Oxidizing agents, such as ozone, have been demonstrated to selectively inhibit the growth of human cancer cells [91]. However, a clinical study using ozone blood perfusion or insufflation obtained only limited clinical benefit, mainly focused on the attenuation of fatigue syndrome in cancer patients [92]. This limited clinical result has been attributed to the fact that when administered as gas, the persistence and penetration of ozone inside the body is limited. Indeed, the amount of gas dissolved in a body fluid is inversely related to the temperature (Henry’s law), which is relatively high in the human body (37 °C). To address this problem, the binding of ozone to a lipid carrier was proposed [93]. This approach resulted in the production of ozonated fatty acids or ozonated oils. However, anticancer properties are exerted by ozonated oils only when a sufficient amount of ozone (i.e., >800 mEq/l) is bound to the lipid carrier [94]. Furthermore, the direct oral administration of ozonated oil is not feasible as it results in poor absorption and side effects such as nausea and diarrhea. These side effects may be avoided by properly adsorbing the ozonated oil onto an adsorbing powder under slow release. High-ozonide oils were demonstrated to be effective in killing cancer cells by inducing apoptosis through oxidative damage to the mitochondrial membrane [94]. Clinical application in cancer patients has been very promising, resulting in a dramatic decrease in the risk of cancer relapses and improving clinical outcomes in a clinical study performed in 115 patients affected by solid tumors [94]. Furthermore, oxygenation of healthy tissue improves the quality of life of cancer patients and attenuates side effects of chemotherapy, such as fatigue syndrome and bone marrow suppression [94].
3. Mechanisms Involved in the Effects of Food Supplementation in Cancer Prevention and Therapy
Although the consumption of dietary antioxidants is increasingly encouraged, only a limited number of them can be considered for their therapeutic efficacy, and the collected results are often contradictory.
Furthermore, as emphasized by Saeidnia and Abdollahi [95], natural antioxidants may provide potential benefits in cancer chemoprevention, primarily by counteracting oxidative stress and inflammation. However, their role in cancer therapy, especially during the early stages of cancer development, remains controversial due to inconsistent and often inconclusive evidence. Therefore, rigorous and well-designed clinical trials are essential to determine the safety and therapeutic relevance of various phytochemicals in both cancer prevention and treatment [96].
Several factors may account for the disappointing outcomes of many clinical studies involving antioxidant supplementation, such as (i) the use of non-specific and poorly characterized compounds, which limits the ability to target defined molecular pathways; (ii) the administration of pharmacological doses rather than physiologically relevant dietary levels, which may alter expected biological effects; (iii) short durations of administration, which are insufficient to observe long-term preventive or therapeutic effects; (iv) poor patient compliance to supplementation protocols, which reduces statistical power; and (v) inadequate limited follow-up periods, which hinder the assessment of sustained clinical outcomes or late-onset effects. Indeed, this lack of success should encourage us to look for new ways to prevent and/or treat cancer by modulating oxidative balance. In fact, this “push” is justified by the promising results obtained in several in vitro and in vivo studies, which demonstrate a strong link between oxidative stress and cancer.
However, it is necessary to underline that the action of food antioxidants is extremely complex, and the effects are not predictable considering only the oxidative mechanisms. As summarized in Figure 1, this treatment can result in metabolic and phenotypic changes due to their effects on (i) microbiota; (ii) the immune system; (iii) ROS-sensitive signaling pathways; (iv) cell metabolism; and (v) the epigenome.
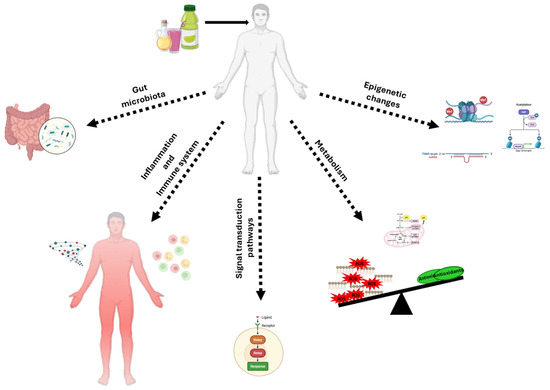
Figure 1.
Mechanisms of action of antioxidant supplementation in cancer prevention and therapy. This figure summarizes the most described biological mechanisms through which antioxidant supplementation may influence cancer development, progression, and therapy response. Pleiotropic antioxidant compounds can modulate the following: (i) gut microbiota composition through the production of anticancer metabolites and the reinforcement of intestinal homeostasis, particularly in colorectal cancer; (ii) immune system responses, potentially enhancing anti-tumor immunity or reducing inflammation-induced tumor promotion; (iii) redox-sensitive signaling pathway activity, regulating cell proliferation, apoptosis, autophagy, angiogenesis, and metastasis formation; (iv) cell metabolism, by enhancing oxidative stress and influencing glycolytic metabolism of cancer cells; (v) epigenetic regulation, including modulation of DNA methylation, histone modifications, and particularly miRNA expression, leading to changes in oncogene/oncosuppressor gene activity.
3.1. Effects on Microbiota
The intricate relationship between dietary antioxidants and gut microbiota unveils a novel frontier in cancer treatment, offering both opportunities and challenges for precision nutrition interventions [97]. Among these antioxidants, resveratrol and curcumin stand out for their impact on gut microbial composition and functionality, thereby influencing cancer progression and relapse risk [98,99].
Resveratrol exerts potent anticarcinogenic effects by reshaping the gut microbiota landscape. Clinical trials performed by Singh and Zhu et al. reported the therapeutic outcomes of resveratrol in breast, colorectal cancer, and multiple myeloma. Resveratrol was found to suppress methylation of the RASSF-1α gene and to lower breast cancer-promoting PGE2 levels. In contrast, in colon cancer patients, micronized resveratrol (SRT501) was found to increase the expression of cleaved caspase-3 in malignant hepatic tissue, indicating potential apoptosis in colorectal cancer patients. The 39% increase in cleaved caspase-3 strongly indicates enhanced apoptosis in malignant hepatic cells [84,100]. Resveratrol has been found to create a gut environment conducive to cancer prevention and therapy by promoting the growth of beneficial microbial species while inhibiting the proliferation of pathogenic bacteria. Moreover, its anti-inflammatory properties mitigate tumor-promoting inflammation, further attenuating cancer relapse risk [101].
EGCG has excellent antioxidant properties, and it also maintains gut microbiota stability and health by inhibiting pathogenic bacteria proliferation and by increasing the number of probiotic bacteria [102].
Genistein belongs to the isoflavone class and can modulate the abundance of phenolic metabolites produced by gut microbes, modifying the metabolism of gut microbiota [103]. Another source of phenolic compounds is Hibiscus sabdariffa L., which was recently found to modulate the intestinal microbiota (by increasing the amount of Lachnospiraceae, Ruminococcaceae, Clostridiaceae, and the genus Clostridum, important producers of butyrate) and to induce apoptosis during the initial phase of an in vivo model of colorectal cancer [104].
Brazil nuts are a rich source of selenium, but their effect on gut health is still not clear. A systematic review with meta-analysis reported randomized controlled trials on nut consumption, and significant increases in the gut contents of Dialister, Clostridium, Roseburia, and Lachnospira, as well as a significant decrease in Parabacteroides, were described [105].
Similarly, curcumin, derived from turmeric, modulates the gut microbiota composition, offering promising avenues for cancer management. Through its antimicrobial activity, curcumin suppresses the growth of harmful bacteria implicated in carcinogenesis while fostering the proliferation of beneficial strains, such as Ruminococcin [106,107]. This rebalancing of the gut microbiota ecosystem aligns with reduced inflammation and enhanced immune surveillance against cancer cells, underscoring curcumin’s potential as an adjuvant therapy in cancer treatment [108,109]. A similar study performed by Liu et al., 2022, also observed a sensitization response of curcumin in acute myeloid leukemia through the regulation of intestinal microbiota [110].
Furthermore, the symbiotic relationship between dietary antioxidants and the gut microbiota extends beyond mere compositional changes. These antioxidants, by promoting microbial metabolism of bioactive compounds, generate metabolites with potent anticancer properties in colorectal cancer [111]. Flavonoids, a subclass of dietary antioxidants, undergo microbial metabolism in the gut, yielding metabolites with demonstrated anticarcinogenic effects. Through the modulation of gut microbial activity, flavonoids contribute to the maintenance of gut homeostasis, thereby mitigating cancer progression and relapse [84]. In addition, it has been recently demonstrated that the combined treatment of sulforaphane (an antioxidant extracted from broccoli sprouts) and inulin successfully inhibited in vivo breast cancer growth and increased tumor onset latency through several mechanisms, including changes in gut microbiota composition, by increasing Ruminococcus, Muribaculaceae, and Faecalibaculum and decreasing the abundance of Blautia, Turicibacter, and Clostridium sensu stricto 1 [112].
However, while the potential of dietary antioxidants in cancer treatment is promising, challenges persist in understanding the precise mechanisms underlying their interactions with the gut microbiota. Unravelling the complex interplay between dietary antioxidants, gut microbial dynamics, and cancer treatment requires comprehensive research efforts. Addressing critical questions regarding the optimal dosage, timing, and specific microbial targets of dietary antioxidants will be paramount for advancing precision nutrition strategies in cancer management [97,113].
3.2. Effects on Immune System
Unfortunately, studies reporting the relationships between immune system activity and antioxidant supplementation are limited. The scarcity of such data derives from multiple factors including the high time and financial costs of conducting analyses and the lack of standardized tests capable of evaluating these supplements in a specific and reliable manner.
Since micronutrient deficiencies result in deleterious outcomes in chronic diseases such as cancer, antioxidant supplementation may play a crucial role in the response to metabolic and immunological stress conditions of oncologic patients. However, to clarify the potential beneficial effects of such supplementation, it is urgently necessary to understand the specific mechanisms of action.
Several studies have investigated the role of antioxidant supplementation in cancer prevention, although the findings are often contradictory. Therefore, it is crucial to consider the diverse array of antioxidants and their multifaceted effects on the immune system and cancer relapse risk in order to determine a correct approach to cancer management.
3.2.1. In Vitro Studies
Artemisinin, a derivative of Artemisia annua Linnè, is considered an antioxidant with antitumor properties [114,115]. Recently, it has been shown that artemisinin exerts its antitumor action by upregulating, on the surface of endometrial carcinoma cells, the expression of CD155, a potent immune ligand capable of stimulating the cytotoxic activity of NK cells [116].
Studies by Yan and collaborators demonstrated that hepatocarcinoma cells treated with resveratrol showed reduced expression of IL-6 and CXCR4 receptor due to the inhibitory action of resveratrol on the expression of GLI-1 (glioma-associated oncogene 1) [117]. In this context, it was shown that both CXCR4, a receptor implicated in the stimulation of chemotaxis, and IL-6, associated with the inflammatory response, are downstream effectors of GLI-1 [118]. These in vitro studies suggest that GLI-1 may be a therapeutic target useful for counteracting angiogenesis and the metastatic potential of cancer cells.
Interestingly, in a study carried out through label-free quantitative proteomic methods and real-time cellular analyses, co-treatment of breast cancer cells with vitamin C and doxorubicin was able to reduce the expression of HSP90AA1. This heat shock protein, being able to influence both the innate and adaptive immune responses, could potentially impact the clinical response to immunotherapy [119].
Finally, it has also been shown that vitamin E can induce changes in immune responses. For example, vitamin E has been found to (i) activate naïve CD8+ T cells against the antigens exposed on breast cancer and lung carcinoma cells [120]; (ii) stimulate the activation of T cells and decrease the expression of PD-L1 (programmed cell death ligand 1), a protein frequently overexpressed in cancer cells and responsible for the evasion of immune surveillance [121,122]; (iii) downregulate the expression of LCK (lymphocyte-specific kinase) involved in the activation of T-cell or B-cell receptors [123].
3.2.2. Pre-Clinical In Vivo Studies
Even more interesting and promising results were observed in in vivo studies carried out to reproduce several kinds of human cancers.
In this context, it was observed that the administration of melatonin to mice with lung cancer was able to reduce metastasis formation and restore thymic efficiency [124].
Interestingly, in mice bearing melanoma lung metastasis, treatment with resveratrol was found to increase the expressions of IFN-γ and CXCL-10 [125] by activating cytotoxic T lymphocytes and NK cells, amplifying the expression of Class I MHC, inducing chemotaxis, and controlling the inflammation response. Altering the balance between regulatory T cells (Tregs) and effector T cells (Teffs) critically influences cancer progression. A higher Treg-to-Teff ratio suppresses antitumor immunity and promotes tumor growth, whereas restoring this balance in favor of effector T cells enhances immune-mediated tumor control. Since the immune system’s ability to modulate tumor growth is closely related to this immunological equilibrium, the evidence that resveratrol significantly increases activated T cells while decreasing Treg cells further supports its potential role as a useful supplement in the anticancer strategies.
Intriguingly, several studies have attributed a crucial role to vitamins in modulating innate and adaptive immune response. For example, it was found that vitamin A supplementation reduced the growth of colorectal cancer by upregulating CD8+ T cells [126]. Similar effects were observed following the administration of vitamin C to mice bearing breast, colorectal, pancreatic cancer, melanoma, and lymphoma, where reductions in tumor growth and potentiation of immune checkpoint therapy (ICT) based on anti-PD-1/PD-L1 or anti-CTLA-a antibodies were observed. Moreover, it has been shown that these responses are due to the over-production of IFN-γ and increased tumor infiltration by CD8+ and CD4+ lymphocytes [127,128]. Similarly, vitamin C counteracted ovarian cancer growth by reducing the amount of M2 macrophages in the tumoral microenvironment [129]. Likewise, vitamin D administration has been shown to reduce breast cancer growth by favoring CD8+ T cell tumor infiltration and reducing the levels of pro-inflammatory cytokines [130,131].
3.3. Effects on Redox-Sensitive Signaling Pathways Involved in Cell Death and Proliferation
A high number of studies have suggested that many antitumor effects of antioxidant supplementation are related to the modulation of several redox-modulated signal transduction pathways involved in cell cycle progression, cell proliferation and differentiation, apoptosis, autophagy, invasion, angiogenesis, and gap junction intercellular communication. Since the activity of these signaling pathways is altered during cancer progression, understanding the mechanisms underlying antioxidant supplementation has a crucial clinical role in the fight against cancer.
However, since a comprehensive discussion concerning the signaling pathways modulated by antioxidant supplementation is beyond the specific scope of this review, the most important studies on this topic are summarized in Table 4 (in vitro studies) and Table 5 (in vivo studies).

Table 4.
Redox signaling pathways modulated by antioxidant supplementation: in vitro studies.

Table 5.
Redox signaling pathways modulated by antioxidant supplementation: in vivo studies.
3.4. Effects on Metabolism
Several studies support the common hypothesis that the different metabolism displayed by cancer cells compared to healthy cells can be considered an “Achilles’ heel” that must be considered to develop new therapeutic strategies. In fact, one of the hallmarks of carcinogenesis is that cancer cells have basal increased levels of ROS with respect to their healthy counterparts [63,211,212,213,214,215,216,217]. Consequently, it is conceivable that antioxidant supplementation, by targeting metabolic atypia, could be useful as a therapy or as an adjuvant in anticancer treatment.
3.4.1. In Vitro Studies
Melatonin is a compound useful for treating cancer, as it is able to modulate different signaling pathways involved in tumor metabolism. In fact, it has been observed that melatonin induces the inhibition of glycolysis and the stimulation of OXPHOS in prostate cancer [218] and in head and neck carcinoma cells [219].
Similar results have been observed in papillary thyroid [220], breast [162], pancreatic [163], and colorectal [166,221] cancer cells exposed to vitamin C.
A marked decrease in glycolytic enzymes and GLUT-1 receptor, with a consequent decrease in glucose uptake, was found in colorectal cancer cells [173] exposed to vitamin D and in breast cancer cells exposed to vitamin D [171] or vitamin E [183].
3.4.2. In Vivo Studies
The effects that melatonin and vitamins have on metabolism have also been reported in several in vivo studies. In fact, it has been demonstrated that the administration of melatonin to mice bearing colon cancer potentiates the cytotoxic action of radiotherapy. This event is accompanied by a reduction in SOD (superoxide dismutase) and glutathione peroxidase activities and in MDA (malondialdehyde) levels [222].
Regarding vitamins, it has been shown that the treatment of mice with colon cancer using vitamin D or vitamin C induced a marked reduction of GLUT1 and PKM2 activity and expression, leading to the suppression of glycolysis [166,173].
Recently, it was found that exposure to aspirin and vitamin D, by counteracting DNA damage, was able to reduce the formation of aberrant crypts foci (ACF) in rat colon, while this did not occur following vitamin C exposure. Moreover, aspirin stimulated catalase activity, but no further activation was induced by vitamin co-treatments. On the other hand, aspirin and both vitamins markedly decreased hepatic SOD levels, but this was not accompanied by alterations in glutathione (GSH) levels [223]. Interestingly, in regard to GSH, it has been demonstrated that GSH and selenium supplementation markedly reduced the diethylnitrosamine-induced DNA damage by reducing malondialdehyde levels and by increasing GSH levels and the activities of GSH-related enzymes, such as glutathione peroxidase and glutathione- S-transferase [224].
3.5. Epigenetic Effects
Epigenetic changes do not induce alterations in DNA sequences, are potentially reversible, and consist of three mechanisms: DNA methylation, histone modifications, and miRNAs. miRNAs are short double-stranded RNAs that can regulate gene expression by binding to complementary mRNAs [225]. Depending on their role in cancer, miRNAs can target unsuppressed genes (oncogenic miRNAs) and oncogenes (tumor-suppressive (TS)-miRNAs).
Increasing evidence from in vitro and in vivo studies indicates that antioxidant food supplementation can influence miRNA expression. For example, melatonin was found to effectively counteract the viability and growth of thyroid cancer cells both in vitro and in vivo by reducing the expression of miRNA-21 and miRNA-30e [196]. Further studies have demonstrated that melatonin also influences the in vitro and in vivo growth of (i) hepatocellular carcinoma by inducing the expression of mi-RNA Let7i-3p [139]; (ii) triple-negative breast cancer by upregulating miRNA-152-3p [140], (iii) oral squamous cell carcinoma by over-expressing miRNA-892 a and miRNA-34b-5p [197], and (iv) glioblastoma by increasing the expression of miRNA-6858-5p [138].
Regarding the role played by vitamins in modulating miRNA expression, it was demonstrated that vitamin C inhibited colon cancer growth both in vitro and in vivo, by increasing miRNA-627 expression [226].
Furthermore, several in vitro studies have shown that resveratrol [227], vitamin A [157], vitamin D [228,229], and vitamin E [190,191] are also able of reducing the survival and inhibiting the invasion and migration of several cancer cell lines.
Among antioxidants, curcumin emerges as a standout contender due to its remarkable ability to regulate microRNAs implicated in cancer metastases [230].
Moreover, resveratrol shows a capacity to modulate histone acetylation and DNA methylation, which can potentially reprogram gene expression profiles associated with cancer relapse [99,231], presenting an attractive strategy for adjuvant cancer therapy [232].
4. Future Perspectives
The studies presented and discussed here highlight the clinical need to move beyond the current approach to antioxidant administration, recommending a tailored approach for each patient. This need arises from analyses of clinical trials conducted over the past 25 years, which demonstrate that antioxidant supplementation does not produce the same effects, positive or negative, in all patients. Therefore, this awareness highlights the importance of categorizing patients into those who may benefit from and those who may experience negative consequences from such supplementation. In this context, it is essential to identify valid biomarkers able to predict the patient’s response to these supplements. This goal will be achievable only through multi-center clinical trials in which nutritional and genomic data will be integrated with the patients’ lifestyles and compliance to their therapeutic plan.
Furthermore, from this perspective, it is necessary to shed light on molecular mechanisms and to focus the attention mainly on redox, immune, and metabolic mechanisms through which exogenous antioxidants can interact with therapy and the tumor microenvironment. This objective can be reached by means of proteomic, genomic, and metabolomic studies and also by 3D in vitro assays capable of mimicking the pathophysiological conditions of cancer patients.
5. Conclusions
The roles of nutrition and food antioxidant supplementation in cancer management is increasingly recognized, although not yet fully understood. The set of results obtained from the clinical trials reported here provides evidence that antioxidant supplementation is not always an effective strategy to counteract cancer progression and relapses. In fact, since many of these food supplements can exert a dual antioxidant/pro-oxidant role, it is necessary to balance these effects to optimize efficacy and limit the toxicity of current antitumoral approaches.
The scientific data collected in this review explain why a fundamental change in the use of supplements is necessary in the treatment of cancer patients. In fact, clinical evidence has shown that the administration of antioxidants in cancer patients may also induce adverse effects in terms of cancer progression.
Based on these considerations, this adjuvant approach represents an important component in the fight against cancer, and greater integration of nutritional, clinical, and molecular studies will be essential to develop more effective preventive and therapeutic strategies.
Author Contributions
Conceptualization, A.I. and C.D.; methodology, A.P., O.F., Z.K., B.M. and S.V.; validation, A.P., A.I., C.D., B.M., O.F., S.V. and N.R.; formal analysis and resources, A.I.; data curation, A.P., O.F., Z.K., B.M. and S.V.; writing—original draft preparation, A.P., Z.K., B.M., C.D., S.V. and O.F.; writing—review and editing, A.I., C.D., B.M. and O.F.; visualization, A.P., Z.K., B.M., C.D., S.V. and N.R.; supervision, A.P., A.I., and C.D.; project administration, A.P., A.I. and C.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Oxidative stress and cancer: Have we moved forward? Biochem. J. 2007, 401, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hazafa, A.; Rehman, K.U.; Jahan, N.; Jabeen, Z. The Role of Polyphenol (Flavonoids) Compounds in the Treatment of Cancer Cells. Nutr. Cancer 2020, 72, 386–397. [Google Scholar] [CrossRef]
- Sayin, V.I.; Ibrahim, M.X.; Larsson, E.; Nilsson, J.A.; Lindahl, P.; Bergo, M.O. Antioxidants accelerate lung cancer progression in mice. Sci. Transl. Med. 2014, 6, 221ra15. [Google Scholar] [CrossRef] [PubMed]
- Le Gal, K.; Ibrahim, M.X.; Wiel, C.; Sayin, V.I.; Akula, M.K.; Karlsson, C.; Dalin, M.G.; Akyürek, L.M.; Lindahl, P.; Nilsson, J.; et al. Antioxidants can increase melanoma metastasis in mice. Sci. Transl. Med. 2015, 7, 308re8. [Google Scholar] [CrossRef]
- Zou, Z.V.; Le Gal, K.; El Zowalaty, A.E.; Pehlivanoglu, L.E.; Garellick, V.; Gul, N.; Ibrahim, M.X.; Bergh, P.O.; Henricsson, M.; Wiel, C.; et al. Antioxidants Promote Intestinal Tumor Progression in Mice. Antioxidants 2021, 10, 241. [Google Scholar] [CrossRef]
- Wiel, C.; Le Gal, K.; Ibrahim, X.; Jahangir, C.A.; Kashif, M.; Yao, H.; Ziegler, D.V.; Xu, X.; Ghosh, T.; Mondal, T.; et al. BACH1 Stabilization by Antioxidants Stimulates Lung Cancer Metastasis. Cell 2019, 178, 330–345.e22. [Google Scholar] [CrossRef]
- Kashif, M.; Yao, H.; Schmidt, S.; Chen, X.; Truong, M.; Tüksammel, E.; Liu, Y.; Bergo, M.O. ROS-lowering doses of vitamins C and A accelerate malignant melanoma metastasis. Redox Biol. 2023, 60, 102619. [Google Scholar] [CrossRef]
- Goodman, M.; Bostick, R.M.; Kucuk, O.; Jones, D.P. Clinical trials of antioxidants as cancer prevention agents: Past, present, and future. Free Radic. Biol. Med. 2011, 51, 1068–1084. [Google Scholar] [CrossRef]
- Gontero, P.; Marra, G.; Soria, F.; Oderda, M.; Zitella, A.; Baratta, F.; Chiorino, G.; Gregnanin, I.; Daniele, L.; Cattel, L.; et al. A randomized double-blind placebo controlled phase I-II study on clinical and molecular effects of dietary supplements in men with precancerous prostatic lesions. Chemoprevention or “chemopromotion”? Prostate 2015, 75, 1177–1186. [Google Scholar] [CrossRef]
- Klein, E.A.; Thompson, I.M., Jr.; Tangen, C.M.; Crowley, J.J.; Lucia, M.S.; Goodman, P.J.; Minasian, L.M.; Ford, L.G.; Parnes, H.L.; Gaziano, J.M.; et al. Vitamin E and the risk of prostate cancer: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011, 306, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- The Alpha-Tocopherol Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N. Engl. J. Med. 1994, 330, 1029–1035. [Google Scholar] [CrossRef]
- Kordiak, J.; Bielec, F.; Jabłoński, S.; Pastuszak-Lewandoska, D. Role of Beta-Carotene in Lung Cancer Primary Chemoprevention: A Systematic Review with Meta-Analysis and Meta-Regression. Nutrients 2022, 14, 1361. [Google Scholar] [CrossRef]
- Macis, D.; Briata, I.M.; D’Ecclesiis, O.; Johansson, H.; Aristarco, V.; Buttiron Webber, T.; Oppezzi, M.; Gandini, S.; Bonanni, B.; DeCensi, A. Inflammatory and Metabolic Biomarker Assessment in a Randomized Presurgical Trial of Curcumin and Anthocyanin Supplements in Patients with Colorectal Adenomas. Nutrients 2023, 15, 3894. [Google Scholar] [CrossRef]
- Ávila-Gálvez, M.Á.; González-Sarrías, A.; Martínez-Díaz, F.; Abellán, B.; Martínez-Torrano, A.J.; Fernández-López, A.J.; Giménez-Bastida, J.A.; Espín, J.C. Disposition of Dietary Polyphenols in Breast Cancer Patients’ Tumors, and Their Associated Anticancer Activity: The Particular Case of Curcumin. Mol. Nutr. Food Res. 2021, 65, e2100163. [Google Scholar] [CrossRef]
- Hemati, S.; Mehrabinejad, F.; Elhaie, M.; Najafizade, N. Curcumin Supplementation as a Preventive Strategy Against Tamoxifen-Induced Nonalcoholic Fatty Liver Disease in ER + Breast Cancer Patients: A Triple-Blind Randomized Placebo-Controlled Trial. J. Diet. Suppl. 2025, 22, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Cheon, M.; Chung, M.; Park, Y. Association between Dietary Intake of Flavonoids and Cancer Recurrence among Breast Cancer Survivors. Nutrients 2021, 13, 3049. [Google Scholar] [CrossRef] [PubMed]
- Chartron, E.; Firmin, N.; Touraine, C.; Chapelle, A.; Legouffe, E.; Rifai, L.; Pouderoux, S.; Roca, L.; D’Hondt, V.; Jacot, W. A Phase II Multicenter Trial on High-Dose Vitamin D Supplementation for the Correction of Vitamin D Insufficiency in Patients with Breast Cancer Receiving Adjuvant Chemotherapy. Nutrients 2021, 13, 4429. [Google Scholar] [CrossRef]
- Jansen, F.H.; Adoubi, I.; J C, K.C.; DE Cnodder, T.; Jansen, N.; Tschulakow, A.; Efferth, T. First study of oral Artenimol-R in advanced cervical cancer: Clinical benefit, tolerability and tumor markers. Anticancer Res. 2011, 31, 4417–4422. [Google Scholar]
- Sanusi, R.S. Outcome of Combined Neoadjuvant Chemotherapy and Vitamin A in Advanced Cervical Carcinoma: A Randomized Double-Blind Clinical Trial. Asian Pac. J. Cancer Prev. 2019, 20, 2213–2218. [Google Scholar] [CrossRef]
- Vahedpoor, Z.; Mahmoodi, S.; Samimi, M.; Gilasi, H.R.; Bahmani, F.; Soltani, A.; Sharifi Esfahani, M.; Asemi, Z. Long-Term Vitamin D Supplementation and the Effects on Recurrence and Metabolic Status of Cervical Intraepithelial Neoplasia Grade 2 or 3: A Randomized, Double-Blind, Placebo-Controlled Trial. Ann. Nutr. Metab. 2018, 72, 151–160. [Google Scholar] [CrossRef]
- Howells, L.M.; Iwuji, C.O.O.; Irving, G.R.B.; Barber, S.; Walter, H.; Sidat, Z.; Griffin-Teall, N.; Singh, R.; Foreman, N.; Patel, S.R.; et al. Curcumin Combined with FOLFOX Chemotherapy Is Safe and Tolerable in Patients with Metastatic Colorectal Cancer in a Randomized Phase IIa Trial. J. Nutr. 2019, 149, 1133–1139. [Google Scholar] [CrossRef]
- Ng, K.; Nimeiri, H.S.; McCleary, N.J.; Abrams, T.A.; Yurgelun, M.B.; Cleary, J.M.; Rubinson, D.A.; Schrag, D.; Miksad, R.; Bullock, A.J.; et al. Effect of High-Dose vs. Standard-Dose Vitamin D3 Supplementation on Progression-Free Survival Among Patients With Advanced or Metastatic Colorectal Cancer: The SUNSHINE Randomized Clinical Trial. JAMA 2019, 321, 1370–1379. [Google Scholar] [CrossRef]
- De Smedt, J.; Van Kelst, S.; Boecxstaens, V.; Stas, M.; Bogaerts, K.; Vanderschueren, D.; Aura, C.; Vandenberghe, K.; Lambrechts, D.; Wolter, P.; et al. Vitamin D supplementation in cutaneous malignant melanoma outcome (ViDMe): A randomized controlled trial. BMC Cancer 2017, 17, 562. [Google Scholar] [CrossRef]
- Morita, M.; Okuyama, M.; Akutsu, T.; Ohdaira, H.; Suzuki, Y.; Urashima, M. Vitamin D Supplementation Regulates Postoperative Serum Levels of PD-L1 in Patients with Digestive Tract Cancer and Improves Survivals in the Highest Quintile of PD-L1: A Post Hoc Analysis of the AMATERASU Randomized Controlled Trial. Nutrients 2021, 13, 1987. [Google Scholar] [CrossRef] [PubMed]
- Strik, H.; Efferth, T.; Kaina, B. Artesunate in glioblastoma therapy: Case reports and review of clinical studies. Phytomedicine 2024, 123, 155274. [Google Scholar] [CrossRef] [PubMed]
- Ławiński, M.; Zadka, K.; Ksepka, N.; Matin, M.; Wysocki, K.; Karkocha, D.; Gradowska, A.; Atanasov, A.G.; Słodkowski, M.; Wierzbicka, A.; et al. Does Resveratrol Impact Oxidative Stress Markers in Patients with Head and Neck Cancer Receiving Home Enteral Nutrition? Nutrients 2025, 17, 504. [Google Scholar] [CrossRef] [PubMed]
- Abd, E.l.; Rahiem, R.; Ibrahim, S.A.; Effat, H.; El-Houseini, M.E.; Osman, R.A.; Abdelraouf, A.; Elzayat, E.M. Curcumin, Piperine and Taurine Combination Enhances the Efficacy of Transarterial Chemoembolization Therapy in patients with Intermediate Stage Hepatocellular Carcinoma: A Pilot Study. Asian Pac. J. Cancer Prev. 2024, 25, 1589–1598. [Google Scholar] [CrossRef]
- Nair, P.M.; Palanisamy, A.; Ramalakshmi, R.; Devibala, M.; Saranya, M.; Sivaranjini, S.; Thangavelu, R.; Mahalingam, M. Oncothermia and Integrative Medicine-A Novel Paradigm for Infratentorial Meningioma Management: A Case Report With One-Year Follow-Up. Cureus 2025, 17, e77005. [Google Scholar] [CrossRef]
- Kumar, D.; Paramjeet, K.; Nupur, B.; Kumar, J.V.; Khurana, A.; Chauhan, A. A Prospective Randomized Study Evaluating the Role of Oral Curcumin Along With Chemoradiationin Management of Locally Advanced Head and Neck Carcinoma. Int. J. Trop. Dis. Health 2016, 16, 1–7. [Google Scholar] [CrossRef]
- Mack, E.; Rau, C.; Otet, C.; Schäfer, J.A.; Brendel, C.; Grass, A.; Bredow, P.; Hohl, C.; Denkert, C.; Willeke, F.; et al. High-dose vitamin C as a targeted treatment for KRAS-driven cancers? Redox Biol. 2025, 85, 103726. [Google Scholar] [CrossRef]
- Gillberg, L.; Ørskov, A.D.; Nasif, A.; Ohtani, H.; Madaj, Z.; Hansen, J.W.; Rapin, N.; Mogensen, J.B.; Liu, M.; Dufva, I.H.; et al. Oral vitamin C supplementation to patients with myeloid cancer on azacitidine treatment: Normalization of plasma vitamin C induces epigenetic changes. Clin. Epigenetics 2019, 11, 143. [Google Scholar] [CrossRef]
- Zhao, H.; Zhu, H.; Huang, J.; Zhu, Y.; Hong, M.; Zhu, H.; Zhang, J.; Li, S.; Yang, L.; Lian, Y.; et al. The synergy of Vitamin C with decitabine activates TET2 in leukemic cells and significantly improves overall survival in elderly patients with acute myeloid leukemia. Leuk. Res. 2018, 66, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kiselev, V.I.; Ashrafyan, L.A.; Muyzhnek, E.L.; Gerfanova, E.V.; Antonova, I.B.; Aleshikova, O.I.; Sarkar, F.H. A new promising way of maintenance therapy in advanced ovarian cancer: A comparative clinical study. BMC Cancer 2018, 18, 904. [Google Scholar] [CrossRef] [PubMed]
- Duffield-Lillico, A.J.; Dalkin, B.L.; Reid, M.E.; Turnbull, B.W.; Slate, E.H.; Jacobs, E.T.; Marshall, J.R.; Clark, L.C. Nutritional Prevention of Cancer Study Group. Selenium supplementation, baseline plasma selenium status and incidence of prostate cancer: An analysis of the complete treatment period of the Nutritional Prevention of Cancer Trial. BJU Int. 2003, 91, 608–612. [Google Scholar] [CrossRef]
- Kim, J.; Sun, P.; Lam, Y.W.; Troncoso, P.; Sabichi, A.L.; Babaian, R.J.; Pisters, L.L.; Pettaway, C.A.; Wood, C.G.; Lippman, S.M.; et al. Changes in serum proteomic patterns by presurgical alpha-tocopherol and L-selenomethionine supplementation in prostate cancer. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1697–1702. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xie, J.; Jia, L.; Xie, P.; Yin, X.; Zhu, W.; Zhao, H.; Wang, X.; Meng, X.; Xing, L.; Zhao, H.; et al. Efficacy and safety of epigallocatechin-3-gallate in treatment acute severe dermatitis in patients with cancer receiving radiotherapy: A phase I clinical trial. Sci. Rep. 2023, 13, 13865. [Google Scholar] [CrossRef]
- Mukhopadhyay, N.D.; Khorasanchi, A.; Pandey, S.; Nemani, S.; Parker, G.; Deng, X.; Arthur, D.W.; Urdaneta, A.; Del Fabbro, E. Melatonin Supplementation for Cancer-Related Fatigue in Patients With Early Stage Breast Cancer Receiving Radiotherapy: A Double-Blind Placebo-Controlled Trial. Oncologist 2024, 29, e206–e212. [Google Scholar] [CrossRef]
- Amir, E.; Simmons, C.E.; Freedman, O.C.; Dranitsaris, G.; Cole, D.E.; Vieth, R.; Ooi, W.S.; Clemons, M. A phase 2 trial exploring the effects of high-dose (10,000 IU/day) vitamin D(3) in breast cancer patients with bone metastases. Cancer 2010, 116, 284–291. [Google Scholar] [CrossRef]
- Judith, P.J.; Maureen, B.; Mélanie, P.; Fabrice, K.; Isabelle, V.D.; Pascale, D.L.; Catherine, A.; Jean-Marc, N.; Hervé, C.; Valérie, D.; et al. Curcumin’s effect in advanced and metastatic breast cancer patients treated with first or second-line docetaxel: A randomized trial. Health Sci. Rep. 2024, 7, e70052. [Google Scholar] [CrossRef]
- Tirgar, A.; Rezaei, M.; Ehsani, M.; Salmani, Z.; Rastegari, A.; Jafari, E.; Khandani, B.K.; Nakhaee, N.; Khaksari, M.; Moazed, V. Exploring the synergistic effects of vitamin D and synbiotics on cytokines profile, and treatment response in breast cancer: A pilot randomized clinical trial. Sci. Rep. 2024, 14, 21372. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Guinó, E.; Alonso, M.H.; Vidal, C.; Barenys, M.; Soriano, A.; Moreno, V. Dietary flavonoids, lignans and colorectal cancer prognosis. Sci. Rep. 2015, 5, 14148. [Google Scholar] [CrossRef]
- Antunac Golubić, Z.; Baršić, I.; Librenjak, N.; Pleština, S. Vitamin D Supplementation and Survival in Metastatic Colorectal Cancer. Nutr. Cancer 2018, 70, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, T.; Wasa, M.; Inohara, H.; Ito, T. L-Glutamine and Survival of Patients with Locally Advanced Head and Neck Cancer Receiving Chemoradiotherapy. Nutrients 2023, 15, 4117. [Google Scholar] [CrossRef]
- van Zandwijk, N.; Dalesio, O.; Pastorino, U.; de Vries, N.; van Tinteren, H. EUROSCAN, a randomized trial of vitamin A and N-acetylcysteine in patients with head and neck cancer or lung cancer. For the EUropean Organization for Research and Treatment of Cancer Head and Neck and Lung Cancer Cooperative Groups. J. Natl. Cancer Inst. 2000, 92, 977–986. [Google Scholar] [CrossRef]
- Bairati, I.; Meyer, F.; Jobin, E.; Gélinas, M.; Fortin, A.; Nabid, A.; Brochet, F.; Têtu, B. Antioxidant vitamins supplementation and mortality: A randomized trial in head and neck cancer patients. Int. J. Cancer 2006, 119, 2221–2224. [Google Scholar] [CrossRef]
- Popat, R.; Plesner, T.; Davies, F.; Cook, G.; Cook, M.; Elliott, P.; Jacobson, E.; Gumbleton, T.; Oakervee, H.; Cavenagh, J. A phase 2 study of SRT501 (resveratrol) with bortezomib for patients with relapsed and or refractory multiple myeloma. Br. J. Haematol. 2013, 160, 714–717. [Google Scholar] [CrossRef]
- van Gorkom, G.N.Y.; Boerenkamp, L.S.; Gijsbers, B.L.M.G.; van Ojik, H.H.; Wodzig, W.K.W.H.; Wieten, L.; van Elssen, C.H.M.J.; Bos, G.M.J. No Effect of Vitamin C Administration on Neutrophil Recovery in Autologous Stem Cell Transplantation for Myeloma or Lymphoma: A Blinded, Randomized Placebo-Controlled Trial. Nutrients 2022, 14, 4784. [Google Scholar] [CrossRef]
- Aldoss, I.; Mark, L.; Vrona, J.; Ramezani, L.; Weitz, I.; Mohrbacher, A.M.; Douer, D. Adding ascorbic acid to arsenic trioxide produces limited benefit in patients with acute myeloid leukemia excluding acute promyelocytic leukemia. Ann. Hematol. 2014, 93, 1839–1843. [Google Scholar] [CrossRef] [PubMed]
- Freitas Bde, J.; Lloret, G.R.; Visacri, M.B.; Tuan, B.T.; Amaral, L.S.; Baldini, D.; de Sousa, V.M.; de Castro, L.L.; Aguiar, J.R.; Pincinato Ede, C.; et al. High 15-F2t-Isoprostane Levels in Patients with a Previous History of Nonmelanoma Skin Cancer: The Effects of Supplementary Antioxidant Therapy. Biomed. Res. Int. 2015, 2015, 963569. [Google Scholar] [CrossRef]
- Bryan, R.T.; Pirrie, S.J.; Abbotts, B.; Maycock, S.; During, V.; Lewis, C.; Grant, M.; Bird, D.; Devall, A.J.; Wallace, D.M.A.; et al. Selenium and Vitamin E for Prevention of Non-Muscle-Invasive Bladder Cancer Recurrence and Progression: A Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e2337494. [Google Scholar] [CrossRef]
- Nielsen, T.K.; Højgaard, M.; Andersen, J.T.; Jørgensen, N.R.; Zerahn, B.; Kristensen, B.; Henriksen, T.; Lykkesfeldt, J.; Mikines, K.J.; Poulsen, H.E. Weekly ascorbic acid infusion in castration-resistant prostate cancer patients: A single-arm phase II trial. Transl. Androl. Urol. 2017, 6, 517–528. [Google Scholar] [CrossRef]
- Watters, J.L.; Gail, M.H.; Weinstein, S.J.; Virtamo, J.; Albanes, D. Associations between alpha-tocopherol, beta-carotene, and retinol and prostate cancer survival. Cancer Res. 2009, 69, 3833–3841. [Google Scholar] [CrossRef] [PubMed]
- Hoffer, L.J.; Levine, M.; Assouline, S.; Melnychuk, D.; Padayatty, S.J.; Rosadiuk, K.; Rousseau, C.; Robitaille, L.; Miller, W.H., Jr. Phase I clinical trial of i.v. ascorbic acid in advanced malignancy. Ann. Oncol. 2008, 19, 1969–1974. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, C.M.; Levin, R.D.; Spector, T.; Lis, C.G. Phase I clinical trial to evaluate the safety, tolerability, and pharmacokinetics of high-dose intravenous ascorbic acid in patients with advanced cancer. Cancer Chemother. Pharmacol. 2013, 72, 139–146. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Palepu, S.; Dhamija, P.; Nath, U.K.; Chetia, R.; Bakliwal, A.; Vaniyath, S.; Chattopadhyay, D.; Handu, S. Safety and efficacy of Vitamin D3 supplementation with Imatinib in Chronic Phase- Chronic Myeloid Leukaemia: An Exploratory Randomized Controlled Trial. BMJ Open 2023, 13, e066361. [Google Scholar] [CrossRef] [PubMed]
- Panieri, E.; Santoro, M.M. ROS homeostasis and metabolism: A dangerous liason in cancer cells. Cell Death Dis. 2016, 7, e2253. [Google Scholar] [CrossRef]
- Halliwell, B. Understanding mechanisms of antioxidant action in health and disease. Nat. Rev. Mol. Cell Biol. 2024, 25, 13–33. [Google Scholar] [CrossRef]
- Marengo, B.; Friedmann Angeli, J.P.; Domenicotti, C. Editorial: Redox metabolism: A double edge sword sustaining the adaptive resistance to therapy in cancer. Front. Oncol. 2023, 13, 1260233. [Google Scholar] [CrossRef] [PubMed]
- Garbarino, O.; Valenti, G.E.; Monteleone, L.; Pietra, G.; Mingari, M.C.; Benzi, A.; Bruzzone, S.; Ravera, S.; Leardi, R.; Farinini, E.; et al. PLX4032 resistance of patient-derived melanoma cells: Crucial role of oxidative metabolism. Front. Oncol. 2023, 13, 1210130. [Google Scholar] [CrossRef]
- Colla, R.; Izzotti, A.; De Ciucis, C.; Fenoglio, D.; Ravera, S.; Speciale, A.; Ricciarelli, R.; Furfaro, A.L.; Pulliero, A.; Passalacqua, M.; et al. Glutathione-mediated antioxidant response and aerobic metabolism: Two crucial factors involved in determining the multi-drug resistance of high-risk neuroblastoma. Oncotarget 2016, 7, 70715–70737. [Google Scholar] [CrossRef]
- Marengo, B.; Nitti, M.; Furfaro, A.L.; Colla, R.; De Ciucis, C.; Marinari, U.M.; Pronzato, M.A.; Traverso, N.; Domenicotti, C. Redox Homeostasis and Cellular Antioxidant Systems: Crucial Players in Cancer Growth and Therapy. Oxid. Med. Cell. Longev. 2016, 2016, 6235641. [Google Scholar] [CrossRef] [PubMed]
- Traverso, N.; Ricciarelli, R.; Nitti, M.; Marengo, B.; Furfaro, A.L.; Pronzato, M.A.; Marinari, U.M.; Domenicotti, C. Role of glutathione in cancer progression and chemoresistance. Oxid. Med. Cell. Longev. 2013, 2013, 972913. [Google Scholar] [CrossRef]
- Dawood, S.; Austin, L.; Cristofanilli, M. Cancer stem cells: Implications for cancer therapy. Oncology 2014, 28, 1101–1107, 1110. [Google Scholar]
- Eghbali, A.; Adibifar, M.; Ghasemi, A.; Afzal, R.R.; Moradi, K.; Eghbali, A.; Faress, F.; Ghaffari, K. The effect of oral curcumin on vincristine-induced neuropathy in pediatric acute lymphoblastic leukemia: A double-blind randomized controlled clinical trial. BMC Cancer 2025, 25, 344. [Google Scholar] [CrossRef]
- Heydari, B.; Sheikhalishahi, S.; Hoseinzade, F.; Shabani, M.; Ramezani, V.; Saghafi, F. Topical Curcumin for Prevention of Radiation-Induced Dermatitis: A Pilot Double-Blind, Placebo-Controlled Trial. Cancer Invest. 2025, 43, 173–182. [Google Scholar] [CrossRef]
- Vollbracht, C.; Schneider, B.; Leendert, V.; Weiss, G.; Auerbach, L.; Beuth, J. Intravenous vitamin C administration improves quality of life in breast cancer patients during chemo-/radiotherapy and aftercare: Results of a retrospective, multicentre, epidemiological cohort study in Germany. In Vivo 2011, 25, 983–990. [Google Scholar] [PubMed]
- Suhail, N.; Bilal, N.; Khan, H.Y.; Hasan, S.; Sharma, S.; Khan, F.; Mansoor, T.; Banu, N. Effect of vitamins C and E on antioxidant status of breast-cancer patients undergoing chemotherapy. J. Clin. Pharm. Ther. 2012, 37, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Y.; Giobbie-Hurder, A.; Gantman, K.; Savoie, J.; Scheib, R.; Parker, L.M.; Schernhammer, E.S. A randomized, placebo-controlled trial of melatonin on breast cancer survivors: Impact on sleep, mood, and hot flashes. Breast Cancer Res. Treat. 2014, 145, 381–388. [Google Scholar] [CrossRef]
- Nimee, F.; Gioxari, A.; Papandreou, P.; Amerikanou, C.; Karageorgopoulou, S.; Kaliora, A.C.; Skouroliakou, M. The Effect of Melatonin Supplementation on Cancer-Related Fatigue during Chemotherapy Treatment of Breast Cancer Patients: A Double-Blind, Randomized Controlled Study. Cancers 2024, 16, 802. [Google Scholar] [CrossRef]
- El-Bassiouny, N.A.; Helmy, M.W.; Hassan, M.A.E.; Khedr, G.A. The Cardioprotective Effect of Vitamin D in Breast Cancer Patients Receiving Adjuvant Doxorubicin Based Chemotherapy. Clin. Breast Cancer 2022, 22, 359–366. [Google Scholar] [CrossRef]
- Arul Vijaya Vani, S.; Ananthanarayanan, P.H.; Kadambari, D.; Harichandrakumar, K.T.; Niranjjan, R.; Nandeesha, H. Effects of vitamin D and calcium supplementation on side effects profile in patients of breast cancer treated with letrozole. Clin. Chim. Acta 2016, 459, 53–56. [Google Scholar] [CrossRef]
- Shi, S.; Wang, K.; Zhong, R.; Cassidy, A.; Rimm, E.B.; Nimptsch, K.; Wu, K.; Chan, A.T.; Giovannucci, E.L.; Ogino, S.; et al. Flavonoid intake and survival after diagnosis of colorectal cancer: A prospective study in 2 US cohorts. Am. J. Clin. Nutr. 2023, 117, 1121–1129. [Google Scholar] [CrossRef]
- Haidari, F.; Abiri, B.; Iravani, M.; Ahmadi-Angali, K.; Vafa, M. Effects of Vitamin D and Omega-3 Fatty Acids Co-Supplementation on Inflammatory Factors and Tumor Marker CEA in Colorectal Cancer Patients Undergoing Chemotherapy: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutr. Cancer 2020, 72, 948–958. [Google Scholar] [CrossRef]
- Liu, H.T.; Huang, Y.C.; Cheng, S.B.; Huang, Y.T.; Lin, P.T. Effects of coenzyme Q10 supplementation on antioxidant capacity and inflammation in hepatocellular carcinoma patients after surgery: A randomized, placebo-controlled trial. Nutr. J. 2016, 15, 85. [Google Scholar] [CrossRef]
- Dagdemir, A.; Yildirim, H.; Aliyazicioglu, Y.; Kanber, Y.; Albayrak, D.; Acar, S. Does vitamin A prevent high-dose-methotrexate-induced D-xylose malabsorption in children with cancer? Support. Care Cancer 2004, 12, 263–267. [Google Scholar] [CrossRef]
- Jahankhani, K.; Taghipour, N.; Nikoonezhad, M.; Behboudi, H.; Mehdizadeh, M.; Kadkhoda, D.; Hajifathali, A.; Mosaffa, N. Adjuvant therapy with zinc supplementation; anti-inflammatory and anti-oxidative role in multiple myeloma patients receiving autologous hematopoietic stem cell transplantation: A randomized controlled clinical trial. Biometals 2024, 37, 1609–1627. [Google Scholar] [CrossRef] [PubMed]
- Yeom, C.H.; Jung, G.C.; Song, K.J. Changes of terminal cancer patients’ health-related quality of life after high dose vitamin C administration. J. Korean Med. Sci. 2007, 22, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Argyriou, A.A.; Chroni, E.; Koutras, A.; Iconomou, G.; Papapetropoulos, S.; Polychronopoulos, P.; Kalofonos, H.P. Preventing paclitaxel-induced peripheral neuropathy: A phase II trial of vitamin E supplementation. J. Pain Symptom Manag. 2006, 32, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Argyriou, A.A.; Chroni, E.; Koutras, A.; Iconomou, G.; Papapetropoulos, S.; Polychronopoulos, P.; Kalofonos, H. A randomized controlled trial evaluating the efficacy and safety of vitamin E supplementation for protection against cisplatin-induced peripheral neuropathy: Final results. Support. Care Cancer 2006, 14, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Villani, V.; Zucchella, C.; Cristalli, G.; Galiè, E.; Bianco, F.; Giannarelli, D.; Carpano, S.; Spriano, G.; Pace, A. Vitamin E neuroprotection against cisplatin ototoxicity: Preliminary results from a randomized, placebo-controlled trial. Head Neck 2016, 38 (Suppl. S1), E2118–E2121. [Google Scholar] [CrossRef] [PubMed]
- Pace, A.; Giannarelli, D.; Galiè, E.; Savarese, A.; Carpano, S.; Della Giulia, M.; Pozzi, A.; Silvani, A.; Gaviani, P.; Scaioli, V.; et al. Vitamin E neuroprotection for cisplatin neuropathy: A randomized, placebo-controlled trial. Neurology 2010, 74, 762–766. [Google Scholar] [CrossRef] [PubMed]
- Kuyumcu, A.; Akyol, A.; Buyuktuncer, Z.; Ozmen, M.M.; Besler, H.T. Improved oxidative status in major abdominal surgery patients after N-acetyl cystein supplementation. Nutr. J. 2015, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Qin, W.; Zhang, K.; Rottinghaus, G.E.; Chen, Y.C.; Kliethermes, B.; Sauter, E.R. Trans-resveratrol alters mammary promoter hypermethylation in women at increased risk for breast cancer. Nutr. Cancer 2012, 64, 393–400. [Google Scholar] [CrossRef]
- Omenn, G.S.; Goodman, G.E.; Thornquist, M.D.; Balmes, J.; Cullen, M.R.; Glass, A.; Keogh, J.P.; Meyskens, F.L.; Valanis, B.; Williams, J.H.; et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N. Engl. J. Med. 1996, 334, 1150–1155. [Google Scholar] [CrossRef]
- Robitaille, L.; Mamer, O.A.; Miller, W.H., Jr.; Levine, M.; Assouline, S.; Melnychuk, D.; Rousseau, C.; Hoffer, L.J. Oxalic acid excretion after intravenous ascorbic acid administration. Metabolism 2009, 58, 263–269. [Google Scholar] [CrossRef]
- Ahlin, R.; Nørskov, N.P.; Nybacka, S.; Landberg, R.; Skokic, V.; Stranne, J.; Josefsson, A.; Steineck, G.; Hedelin, M. Effects on Serum Hormone Concentrations after a Dietary Phytoestrogen Intervention in Patients with Prostate Cancer: A Randomized Controlled Trial. Nutrients 2023, 15, 1792. [Google Scholar] [CrossRef]
- Sun, J.; Chen, S.; Zang, D.; Sun, H.; Sun, Y.; Chen, J. Butyrate as a promising therapeutic target in cancer: From pathogenesis to clinic (Review). Int. J. Oncol. 2024, 64, 44. [Google Scholar] [CrossRef]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug. Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef]
- Diehn, M.; Cho, R.W.; Lobo, N.A.; Kalisky, T.; Dorie, M.J.; Kulp, A.N.; Qian, D.; Lam, J.S.; Ailles, L.E.; Wong, M.; et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 2009, 458, 780–783. [Google Scholar] [CrossRef]
- Sweet, F.; Kao, M.S.; Lee, S.C.; Hagar, W.L.; Sweet, W.E. Ozone selectively inhibits growth of human cancer cells. Science 1980, 209, 931–933. [Google Scholar] [CrossRef]
- Tirelli, U.; Valdenassi, L.; Franzini, M.; Pandolfi, S.; Fisichella, R.; Chirumbolo, S. Oxygen-ozone autohemotherapy in breast cancer patients suffering from fatigue and musculoskeletal pain upon aromatase inhibitors treatment: A case-series study. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 11643–11652. [Google Scholar] [CrossRef]
- Izzotti, A. Oxidative Drugs and microRNA: New Opportunities for Cancer Prevention. Cancers 2022, 15, 132. [Google Scholar] [CrossRef]
- Izzotti, A.; Fracchia, E.; Rosano, C.; Comite, A.; Belgioia, L.; Sciacca, S.; Khalid, Z.; Congiu, M.; Colarossi, C.; Blanco, G.; et al. Efficacy of High-Ozonide Oil in Prevention of Cancer Relapses Mechanisms and Clinical Evidence. Cancers 2022, 14, 1174. [Google Scholar] [CrossRef] [PubMed]
- Saeidnia, S.; Abdollahi, M. Antioxidants: Friends or foe in prevention or treatment of cancer: The debate of the century. Toxicol. Appl. Pharmacol. 2013, 271, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Marino, P.; Pepe, G.; Basilicata, M.G.; Vestuto, V.; Marzocco, S.; Autore, G.; Procino, A.; Gomez-Monterrey, I.M.; Manfra, M.; Campiglia, P. Potential Role of Natural Antioxidant Products in Oncological Diseases. Antioxidants 2023, 12, 704. [Google Scholar] [CrossRef] [PubMed]
- Greathouse, K.L.; Wyatt, M.; Johnson, A.J.; Toy, E.P.; Khan, J.M.; Dunn, K.; Clegg, D.J.; Reddy, S. Diet-microbiome interactions in cancer treatment: Opportunities and challenges for precision nutrition in cancer. Neoplasia 2022, 29, 100800. [Google Scholar] [CrossRef]
- Flemer, B.; Herlihy, M.; O’Riordain, M.; Shanahan, F.; O’Toole, P.W. Tumour-associated and non-tumour-associated microbiota: Addendum. Gut Microbes 2018, 9, 369–373. [Google Scholar] [CrossRef]
- Li, C.; Xu, Y.; Zhang, J.; Zhang, Y.; He, W.; Ju, J.; Wu, Y.; Wang, Y. The effect of resveratrol, curcumin and quercetin combination on immuno-suppression of tumor microenvironment for breast tumor-bearing mice. Sci. Rep. 2023, 13, 13278. [Google Scholar] [CrossRef]
- Singh, A.P.; Singh, R.; Verma, S.S.; Rai, V.; Kaschula, C.H.; Maiti, P.; Gupta, S.C. Health benefits of resveratrol: Evidence from clinical studies. Med. Res. Rev. 2019, 39, 1851–1891. [Google Scholar] [CrossRef]
- Wang, P.; Song, M.; Eliassen, A.H.; Wang, M.; Fung, T.T.; Clinton, S.K.; Rimm, E.B.; Hu, F.B.; Willett, W.C.; Tabung, F.K.; et al. Optimal dietary patterns for prevention of chronic disease. Nat. Med. 2023, 29, 719–728. [Google Scholar] [CrossRef]
- Wang, X.; Ye, T.; Chen, W.J.; Lv, Y.; Hao, Z.; Chen, J.; Zhao, J.Y.; Wang, H.P.; Cai, Y.K. Structural shift of gut microbiota during chemo-preventive effects of epigallocatechin gallate on colorectal carcinogenesis in mice. World J. Gastroenterol. 2017, 23, 8128–8139. [Google Scholar] [CrossRef]
- Guevara-Cruz, M.; Godinez-Salas, E.T.; Sanchez-Tapia, M.; Torres-Villalobos, G.; Pichardo-Ontiveros, E.; Guizar-Heredia, R.; Arteaga-Sanchez, L.; Gamba, G.; Mojica-Espinosa, R.; Schcolnik-Cabrera, A.; et al. Genistein stimulates insulin sensitivity through gut microbiota reshaping and skeletal muscle AMPK activation in obese subjects. BMJ Open Diabetes Res. Care 2020, 8, e000948. [Google Scholar] [CrossRef] [PubMed]
- Ladeira Bernardes, A.; Albuquerque Pereira, M.F.; Xisto Campos, I.; Ávila, L.; Dos Santos Cruz, B.C.; Duarte Villas Mishima, M.; Maciel Dos Santos Dias, M.; de Oliveira Mendes, T.A.; Gouveia Peluzio, M.D.C. Oral intake of Hibiscus sabdariffa L. increased c-Myc and caspase-3 gene expression and altered microbial population in colon of BALB/c mice induced to preneoplastic lesions. Eur. J. Nutr. 2025, 64, 109. [Google Scholar] [CrossRef] [PubMed]
- Creedon, A.C.; Hung, E.S.; Berry, S.E.; Whelan, K. Nuts and their Effect on Gut Microbiota, Gut Function and Symptoms in Adults: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients 2020, 12, 2347. [Google Scholar] [CrossRef]
- Weng, W.; Goel, A. Curcumin and colorectal cancer: An update and current perspective on this natural medicine. Semin. Cancer Biol. 2022, 80, 73–86. [Google Scholar] [CrossRef]
- Li, S.; Fu, C.; Zhao, Y.; He, J. Intervention with α-Ketoglutarate Ameliorates Colitis-Related Colorectal Carcinoma via Modulation of the Gut Microbiome. Biomed. Res. Int. 2019, 2019, 8020785. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jiang, Q. Roles of the Polyphenol-Gut Microbiota Interaction in Alleviating Colitis and Preventing Colitis-Associated Colorectal Cancer. Adv. Nutr. 2021, 12, 546–565. [Google Scholar] [CrossRef]
- McFadden, R.M.; Larmonier, C.B.; Shehab, K.W.; Midura-Kiela, M.; Ramalingam, R.; Harrison, C.A.; Besselsen, D.G.; Chase, J.H.; Caporaso, J.G.; Jobin, C.; et al. The Role of Curcumin in Modulating Colonic Microbiota During Colitis and Colon Cancer Prevention. Inflamm. Bowel Dis. 2015, 21, 2483–2494. [Google Scholar] [CrossRef]
- Liu, J.; Luo, W.; Chen, Q.; Chen, X.; Zhou, G.; Sun, H. Curcumin sensitizes response to cytarabine in acute myeloid leukemia by regulating intestinal microbiota. Cancer Chemother. Pharmacol. 2022, 89, 243–253. [Google Scholar] [CrossRef]
- Appunni, S.; Rubens, M.; Ramamoorthy, V.; Tonse, R.; Saxena, A.; McGranaghan, P.; Kaiser, A.; Kotecha, R. Emerging Evidence on the Effects of Dietary Factors on the Gut Microbiome in Colorectal Cancer. Front. Nutr. 2021, 8, 718389. [Google Scholar] [CrossRef]
- Wu, H.; Witt, B.L.; van der Pol, W.J.; Morrow, C.D.; Duck, L.W.; Tollefsbol, T.O. Combined Phytochemical Sulforaphane and Dietary Fiber Inulin Contribute to the Prevention of ER-Negative Breast Cancer via PI3K/AKT/MTOR Pathway and Modulating Gut Microbial Composition. Nutrients 2025, 17, 2023. [Google Scholar] [CrossRef]
- Wang, M.; Yu, F.; Zhang, Y.; Chang, W.; Zhou, M. The Effects and Mechanisms of Flavonoids on Cancer Prevention and Therapy: Focus on Gut Microbiota. Int. J. Biol. Sci. 2022, 18, 1451–1475. [Google Scholar] [CrossRef]
- Lai, H.C.; Singh, N.P.; Sasaki, T. Development of artemisinin compounds for cancer treatment. Invest. New Drugs 2013, 31, 230–246. [Google Scholar] [CrossRef]
- Efferth, T. From ancient herb to modern drug: Artemisia annua and artemisinin for cancer therapy. Semin. Cancer Biol. 2017, 46, 65–83. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, L.; Xiang, J.D.; Jin, C.S.; Li, M.Q.; He, Y.Y. Artesunate-induced ATG5-related autophagy enhances the cytotoxicity of NK92 cells on endometrial cancer cells via interactions between CD155 and CD226/TIGIT. Int. Immunopharmacol. 2021, 97, 107705. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhou, C.; Li, J.; Chen, K.; Wang, G.; Wei, G.; Chen, M.; Li, X. Resveratrol inhibits hepatocellular carcinoma progression driven by hepatic stellate cells by targeting Gli-1. Mol. Cell. Biochem. 2017, 434, 17–24. [Google Scholar] [CrossRef]
- Lei, J.; Huo, X.; Duan, W.; Xu, Q.; Li, R.; Ma, J.; Li, X.; Han, L.; Li, W.; Sun, H.; et al. α-Mangostin inhibits hypoxia-driven ROS-induced PSC activation and pancreatic cancer cell invasion. Cancer Lett. 2014, 347, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Bober, P.; Alexovic, M.; Talian, I.; Tomkova, Z.; Viscorova, Z.; Benckova, M.; Andrasina, I.; Ciccocioppo, R.; Petrovic, D.; Adamek, M.; et al. Proteomic analysis of the vitamin C effect on the doxorubicin cytotoxicity in the MCF-7 breast cancer cell line. J. Cancer Res. Clin. Oncol. 2017, 143, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hahn, T.; Garrison, K.; Cui, Z.H.; Thorburn, A.; Thorburn, J.; Hu, H.M.; Akporiaye, E.T. The vitamin E analogue α-TEA stimulates tumor autophagy and enhances antigen cross-presentation. Cancer Res. 2012, 72, 3535–3545. [Google Scholar] [CrossRef]
- Sun, Z.; Yin, S.; Zhao, C.; Fan, L.; Hu, H. Inhibition of PD-L1-mediated tumor-promoting signaling is involved in the anti-cancer activity of β-tocotrienol. Biochem. Biophys. Res. Commun. 2022, 617, 33–40. [Google Scholar] [CrossRef]
- Sun, Z.; Ma, X.; Zhao, C.; Fan, L.; Yin, S.; Hu, H. Delta-tocotrienol disrupts PD-L1 glycosylation and reverses PD-L1-mediated immune suppression. Biomed. Pharmacother. 2024, 170, 116078. [Google Scholar] [CrossRef]
- Wang, H.; Hong, J.; Yang, C.S. δ-Tocopherol inhibits receptor tyrosine kinase-induced AKT activation in prostate cancer cells. Mol. Carcinog. 2016, 55, 1728–1738. [Google Scholar] [CrossRef]
- Mocchegiani, E.; Perissin, L.; Santarelli, L.; Tibaldi, A.; Zorzet, S.; Rapozzi, V.; Giacconi, R.; Bulian, D.; Giraldi, T. Melatonin administration in tumor-bearing mice (intact and pinealectomized) in relation to stress, zinc, thymulin and IL-2. Int. J. Immunopharmacol. 1999, 21, 27–46. [Google Scholar] [CrossRef] [PubMed]
- Davoodvandi, A.; Darvish, M.; Borran, S.; Nejati, M.; Mazaheri, S.; Reza Tamtaji, O.; Hamblin, M.R.; Masoudian, N.; Mirzaei, H. The therapeutic potential of resveratrol in a mouse model of melanoma lung metastasis. Int. Immunopharmacol. 2020, 88, 106905. [Google Scholar] [CrossRef]
- Bhattacharya, N.; Yuan, R.; Prestwood, T.R.; Penny, H.L.; DiMaio, M.A.; Reticker-Flynn, N.E.; Krois, C.R.; Kenkel, J.A.; Pham, T.D.; Carmi, Y.; et al. Normalizing Microbiota-Induced Retinoic Acid Deficiency Stimulates Protective CD8(+) T Cell-Mediated Immunity in Colorectal Cancer. Immunity 2016, 45, 641–655. [Google Scholar] [CrossRef] [PubMed]
- Magrì, A.; Germano, G.; Lorenzato, A.; Lamba, S.; Chilà, R.; Montone, M.; Amodio, V.; Ceruti, T.; Sassi, F.; Arena, S.; et al. High-dose vitamin C enhances cancer immunotherapy. Sci. Transl. Med. 2020, 12, 532. [Google Scholar] [CrossRef] [PubMed]
- Luchtel, R.A.; Bhagat, T.; Pradhan, K.; Jacobs, W.R., Jr.; Levine, M.; Verma, A.; Shenoy, N. High-dose ascorbic acid synergizes with anti-PD1 in a lymphoma mouse model. Proc. Natl. Acad. Sci. USA 2020, 117, 1666–1677. [Google Scholar] [CrossRef]
- Xu, Y.; Guo, X.; Wang, G.; Zhou, C. Vitamin C Inhibits Metastasis of Peritoneal Tumors By Preventing Spheroid Formation in ID8 Murine Epithelial Peritoneal Cancer Model. Front. Pharmacol. 2020, 11, 645. [Google Scholar] [CrossRef]
- Karkeni, E.; Morin, S.O.; Bou Tayeh, B.; Goubard, A.; Josselin, E.; Castellano, R.; Fauriat, C.; Guittard, G.; Olive, D.; Nunès, J.A. Vitamin D Controls Tumor Growth and CD8+ T Cell Infiltration in Breast Cancer. Front. Immunol. 2019, 10, 1307. [Google Scholar] [CrossRef]
- Radhakrishnan, A.K.; Anandha Rao, J.S.; Subramaniam, S.; Ramdas, P. Gamma-tocotrienol modifies methylation of HOXA10, IRF4 and RORα genes in CD4+ T-lymphocytes: Evidence from a syngeneic mouse model of breast cancer. Curr. Res. Immunol. 2021, 2, 169–174. [Google Scholar] [CrossRef]
- Kim, E.J.; Kim, G.T.; Kim, B.M.; Lim, E.G.; Kim, S.Y.; Kim, Y.M. Apoptosis-induced effects of extract from Artemisia annua Linné by modulating PTEN/p53/PDK1/Akt/ signal pathways through PTEN/p53-independent manner in HCT116 colon cancer cells. BMC Complement. Altern. Med. 2017, 17, 236. [Google Scholar] [CrossRef]
- Sarkar, E.; Kotiya, A.; Bhuyan, R.; Raza, S.T.; Misra, A.; Ahmad, R.; Mahdi, A.A. Curcumin chemo-sensitizes intrinsic apoptosis through ROS-mediated mitochondrial hyperpolarization and DNA damage in breast cancer cells. Cell. Signal. 2025, 128, 111637. [Google Scholar] [CrossRef]
- Dash, P.; Nayak, S.; Parida, P.K. The Efficacy of Curcumin in Reducing Immunosuppressive States of Peripheral Blood Mononuclear Cells Extracted From Oral Squamous Cell Carcinoma Patients: An In Vitro Study. Cureus 2025, 17, e77899. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Yuan, L.; Slakey, L.M.; Jones, F.E.; Burow, M.E.; Hill, S.M. Inhibition of breast cancer cell invasion by melatonin is mediated through regulation of the p38 mitogen-activated protein kinase signaling pathway. Breast Cancer Res. 2010, 12, R107. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves Ndo, N.; Colombo, J.; Lopes, J.R.; Gelaleti, G.B.; Moschetta, M.G.; Sonehara, N.M.; Hellmén, E.; Zanon Cde, F.; Oliani, S.M.; Zuccari, D.A. Effect of Melatonin in Epithelial Mesenchymal Transition Markers and Invasive Properties of Breast Cancer Stem Cells of Canine and Human Cell Lines. PLoS ONE 2016, 11, e0150407. [Google Scholar] [CrossRef] [PubMed]
- Jung-Hynes, B.; Schmit, T.L.; Reagan-Shaw, S.R.; Siddiqui, I.A.; Mukhtar, H.; Ahmad, N. Melatonin, a novel Sirt1 inhibitor, imparts antiproliferative effects against prostate cancer in vitro in culture and in vivo in TRAMP model. J. Pineal Res. 2011, 50, 140–149. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, Z.; Qi, Q.; Wang, J.; Kong, Y.; Feng, Z.; Chen, A.; Li, W.; Zhang, Q.; Wang, J.; et al. miR-6858 plays a key role in the process of melatonin inhibition of the malignant biological behavior of glioma. J. Clin. Neurosci. 2021, 87, 137–146. [Google Scholar] [CrossRef]
- Wang, T.H.; Hsueh, C.; Chen, C.C.; Li, W.S.; Yeh, C.T.; Lian, J.H.; Chang, J.L.; Chen, C.Y. Melatonin Inhibits the Progression of Hepatocellular Carcinoma through MicroRNA Let7i-3p Mediated RAF1 Reduction. Int. J. Mol. Sci. 2018, 19, 2687. [Google Scholar] [CrossRef]
- Marques, J.H.M.; Mota, A.L.; Oliveira, J.G.; Lacerda, J.Z.; Stefani, J.P.; Ferreira, L.C.; Castro, T.B.; Aristizábal-Pachón, A.F.; Zuccari, D.A.P.C. Melatonin restrains angiogenic factors in triple-negative breast cancer by targeting miR-152-3p: In vivo and in vitro studies. Life Sci. 2018, 208, 131–138. [Google Scholar] [CrossRef]
- Cucielo, M.S.; Freire, P.P.; Emílio-Silva, M.T.; Romagnoli, G.G.; Carvalho, R.F.; Kaneno, R.; Hiruma-Lima, C.A.; Delella, F.K.; Reiter, R.J.; Chuffa, L.G.A. Melatonin enhances cell death and suppresses the metastatic capacity of ovarian cancer cells by attenuating the signaling of multiple kinases. Pathol. Res. Pract. 2023, 248, 154637. [Google Scholar] [CrossRef]
- Akbarzadeh, M.; Movassaghpour, A.A.; Ghanbari, H.; Kheirandish, M.; Fathi Maroufi, N.; Rahbarghazi, R.; Nouri, M.; Samadi, N. The potential therapeutic effect of melatonin on human ovarian cancer by inhibition of invasion and migration of cancer stem cells. Sci. Rep. 2017, 7, 17062. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Xiao, X.; Zhang, C.; Yu, W.; Guo, W.; Zhang, Z.; Li, Z.; Feng, X.; Hao, J.; Zhang, K.; et al. Melatonin synergizes the chemotherapeutic effect of 5-fluorouracil in colon cancer by suppressing PI3K/AKT and NF-κB/iNOS signaling pathways. J. Pineal Res. 2017, 62, e1238. [Google Scholar] [CrossRef]
- Sun, X.; Fu, P.; Xie, L.; Chai, S.; Xu, Q.; Zeng, L.; Wang, X.; Jiang, N.; Sang, M. Resveratrol inhibits the progression of cervical cancer by suppressing the transcription and expression of HPV E6 and E7 genes. Int. J. Mol. Med. 2021, 47, 335–345. [Google Scholar] [CrossRef]
- Li, J.; Fan, Y.; Zhang, Y.; Liu, Y.; Yu, Y.; Ma, M. Resveratrol Induces Autophagy and Apoptosis in Non-Small-Cell Lung Cancer Cells by Activating the NGFR-AMPK-mTOR Pathway. Nutrients 2022, 14, 2413. [Google Scholar] [CrossRef]
- Innets, B.; Thongsom, S.; Petsri, K.; Racha, S.; Yokoya, M.; Moriue, S.; Chaotham, C.; Chanvorachote, P. Akt/mTOR Targeting Activity of Resveratrol Derivatives in Non-Small Lung Cancer. Molecules 2022, 27, 8268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, J.; Zeng, J.; Li, Z.; Zuo, H.; Huang, C.; Zhao, X. Nano-Gold Loaded with Resveratrol Enhance the Anti-Hepatoma Effect of Resveratrol In Vitro and In Vivo. J. Biomed. Nanotechnol. 2019, 15, 288–300. [Google Scholar] [CrossRef]
- Xu, J.; Liu, D.; Niu, H.; Zhu, G.; Xu, Y.; Ye, D.; Li, J.; Zhang, Q. Resveratrol reverses Doxorubicin resistance by inhibiting epithelial-mesenchymal transition (EMT) through modulating PTEN/Akt signaling pathway in gastric cancer. J. Exp. Clin. Cancer Res. 2017, 36, 19. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Mao, Q.Q.; Qin, J.; Zheng, X.Y.; Wang, Y.B.; Yang, K.; Shen, H.F.; Xie, L.P. Resveratrol induces apoptosis and cell cycle arrest of human T24 bladder cancer cells in vitro and inhibits tumor growth in vivo. Cancer Sci. 2010, 101, 488–493. [Google Scholar] [CrossRef]
- Ferraresi, A.; Phadngam, S.; Morani, F.; Galetto, A.; Alabiso, O.; Chiorino, G.; Isidoro, C. Resveratrol inhibits IL-6-induced ovarian cancer cell migration through epigenetic up-regulation of autophagy. Mol. Carcinog. 2017, 56, 1164–1181. [Google Scholar] [CrossRef]
- Cheng, L.; Yan, B.; Chen, K.; Jiang, Z.; Zhou, C.; Cao, J.; Qian, W.; Li, J.; Sun, L.; Ma, J.; et al. Resveratrol-Induced Downregulation of NAF-1 Enhances the Sensitivity of Pancreatic Cancer Cells to Gemcitabine via the ROS/Nrf2 Signaling Pathways. Oxid. Med. Cell. Longev. 2018, 2018, 9482018. [Google Scholar] [CrossRef]
- Bhal, S.; Das, B.; Sinha, S.; Das, C.; Acharya, S.S.; Maji, J.; Kundu, C.N. Resveratrol nanoparticles induce apoptosis in oral cancer stem cells by disrupting the interaction between β-catenin and GLI-1 through p53-independent activation of p21. Med. Oncol. 2024, 41, 167. [Google Scholar] [CrossRef] [PubMed]
- Farhan, M. Cytotoxic Activity of the Red Grape Polyphenol Resveratrol against Human Prostate Cancer Cells: A Molecular Mechanism Mediated by Mobilization of Nuclear Copper and Generation of Reactive Oxygen Species. Life 2024, 14, 611. [Google Scholar] [CrossRef]
- Ding, X.; Wang, W.; Wang, M.; Wu, J.; Yao, F. DOK1/PPARgamma pathway mediates anti-tumor ability of all-trans retinoic acid in breast cancer MCF-7 cells. Biochem. Biophys. Res. Commun. 2017, 487, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Yang, X.; Lu, M.; Hu, R.; Zhu, H.; Zhang, S.; Zhou, Q.; Chen, F.; Gui, S.; Wang, Y. All-Trans Retinoic Acid Inhibits Human Colorectal Cancer Cells RKO Migration via Downregulating Myosin Light Chain Kinase Expression through MAPK Signaling Pathway. Nutr. Cancer 2016, 68, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Aouad, P.; Saikali, M.; Abdel-Samad, R.; Fostok, S.; El-Houjeiri, L.; Pisano, C.; Talhouk, R.; Darwiche, N. Antitumor activities of the synthetic retinoid ST1926 in two-dimensional and three-dimensional human breast cancer models. Anticancer Drugs 2017, 28, 757–770. [Google Scholar] [CrossRef]
- Cui, J.; Gong, M.; Fang, S.; Hu, C.; Wang, Y.; Zhang, J.; Tang, N.; He, Y. All-trans retinoic acid reverses malignant biological behavior of hepatocarcinoma cells by regulating miR-200 family members. Genes Dis. 2020, 8, 509–520. [Google Scholar] [CrossRef]
- Halakos, E.G.; Connell, A.J.; Glazewski, L.; Wei, S.; Mason, R.W. Bottom up proteomics reveals novel differentiation proteins in neuroblastoma cells treated with 13-cis retinoic acid. J. Proteom. 2019, 209, 103491. [Google Scholar] [CrossRef]
- Yang, H.; Tao, Y.; Zhang, M.; Ma, P.; Li, L.; Diao, Q. Effects of 9-cis-retinoic acid on the proliferation and apoptosis of cutaneous T-cell lymphoma cells. Anticancer Drugs 2019, 30, 56–64. [Google Scholar] [CrossRef]
- Zito, G.; Naselli, F.; Saieva, L.; Raimondo, S.; Calabrese, G.; Guzzardo, C.; Forte, S.; Rolfo, C.; Parenti, R.; Alessandro, R. Retinoic Acid affects Lung Adenocarcinoma growth by inducing differentiation via GATA6 activation and EGFR and Wnt inhibition. Sci. Rep. 2017, 7, 4770. [Google Scholar] [CrossRef]
- Long, B.; Shan, Y.; Sun, Y.; Wang, T.; Li, X.; Huang, K.; Zhang, W.; He, Y.; Wen, R.; Li, Y.; et al. Vitamin C promotes anti-leukemia of DZNep in acute myeloid leukemia. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166357. [Google Scholar] [CrossRef]
- Ghanem, A.; Melzer, A.M.; Zaal, E.; Neises, L.; Baltissen, D.; Matar, O.; Glennemeier-Marke, H.; Almouhanna, F.; Theobald, J.; Abu El Maaty, M.A.; et al. Ascorbate kills breast cancer cells by rewiring metabolism via redox imbalance and energy crisis. Free Radic. Biol. Med. 2021, 163, 196–209. [Google Scholar] [CrossRef]
- Kim, J.H.; Hwang, S.; Lee, J.H.; Im, S.S.; Son, J. Vitamin C Suppresses Pancreatic Carcinogenesis through the Inhibition of Both Glucose Metabolism and Wnt Signaling. Int. J. Mol. Sci. 2022, 23, 12249. [Google Scholar] [CrossRef]
- Wu, T.M.; Liu, S.T.; Chen, S.Y.; Chen, G.S.; Wu, C.C.; Huang, S.M. Mechanisms and Applications of the Anti-cancer Effect of Pharmacological Ascorbic Acid in Cervical Cancer Cells. Front. Oncol. 2020, 10, 1483. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Chapman, J.; Levine, M.; Polireddy, K.; Drisko, J.; Chen, Q. High-dose parenteral ascorbate enhanced chemosensitivity of ovarian cancer and reduced toxicity of chemotherapy. Sci. Transl. Med. 2014, 6, 222ra18. [Google Scholar] [CrossRef]
- Aguilera, O.; Muñoz-Sagastibelza, M.; Torrejón, B.; Borrero-Palacios, A.; Del Puerto-Nevado, L.; Martínez-Useros, J.; Rodriguez-Remirez, M.; Zazo, S.; García, E.; Fraga, M.; et al. Vitamin C uncouples the Warburg metabolic switch in KRAS mutant colon cancer. Oncotarget 2016, 7, 47954–47965. [Google Scholar] [CrossRef]
- Shen, X.; Wang, J.; Kong, W.; John, C.; Deng, B.; Chen, S.; Zhang, H.; Haag, J.; Sinha, N.; Sun, W.; et al. High-dose Ascorbate Exhibits Anti-proliferative and Anti-invasive Effects Dependent on PTEN/AKT/mTOR Pathway in Endometrial Cancer in vitro and in vivo. Int. J. Biol. Sci. 2025, 21, 1545–1565. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, Y.; Takeda, K.; Okawachi, K.; Tanimura, Y. High dose of ascorbic acid induces selective cell growth inhibition and cell death in human gastric signet-ring cell carcinoma-derived NUGC-4 cells. Biochim. Biophys. Acta Gen. Subj. 2025, 1869, 130738. [Google Scholar] [CrossRef] [PubMed]
- Shariev, A.; Painter, N.; Reeve, V.E.; Haass, N.K.; Rybchyn, M.S.; Ince, F.A.; Mason, R.S.; Dixon, K.M. PTEN: A novel target for vitamin D in melanoma. J. Steroid Biochem. Mol. Biol. 2022, 218, 106059. [Google Scholar] [CrossRef]
- Bajbouj, K.; Al-Ali, A.; Shafarin, J.; Sahnoon, L.; Sawan, A.; Shehada, A.; Elkhalifa, W.; Saber-Ayad, M.; Muhammad, J.S.; Elmoselhi, A.B.; et al. Vitamin D Exerts Significant Antitumor Effects by Suppressing Vasculogenic Mimicry in Breast Cancer Cells. Front. Oncol. 2022, 12, 918340. [Google Scholar] [CrossRef]
- Santos, J.M.; Khan, Z.S.; Munir, M.T.; Tarafdar, K.; Rahman, S.M.; Hussain, F. Vitamin D3 decreases glycolysis and invasiveness, and increases cellular stiffness in breast cancer cells. J. Nutr. Biochem. 2018, 53, 111–120. [Google Scholar] [CrossRef]
- Veeresh, P.K.M.; Basavaraju, C.G.; Dallavalasa, S.; Anantharaju, P.G.; Natraj, S.M.; Sukocheva, O.A.; Madhunapantula, S.V. Vitamin D3 Inhibits the Viability of Breast Cancer Cells In Vitro and Ehrlich Ascites Carcinomas in Mice by Promoting Apoptosis and Cell Cycle Arrest and by Impeding Tumor Angiogenesis. Cancers 2023, 15, 4833. [Google Scholar] [CrossRef]
- Huang, C.Y.; Weng, Y.T.; Li, P.C.; Hsieh, N.T.; Li, C.I.; Liu, H.S.; Lee, M.F. Calcitriol Suppresses Warburg Effect and Cell Growth in Human Colorectal Cancer Cells. Life 2021, 11, 963. [Google Scholar] [CrossRef]
- Li, Z.; Jia, Z.; Gao, Y.; Xie, D.; Wei, D.; Cui, J.; Mishra, L.; Huang, S.; Zhang, Y.; Xie, K. Activation of vitamin D receptor signaling downregulates the expression of nuclear FOXM1 protein and suppresses pancreatic cancer cell stemness. Clin. Cancer Res. 2015, 21, 844–853. [Google Scholar] [CrossRef]
- Chiang, K.C.; Yeh, T.S.; Chen, S.C.; Pang, J.H.; Yeh, C.N.; Hsu, J.T.; Chen, L.W.; Kuo, S.F.; Takano, M.; Kittaka, A.; et al. The Vitamin D Analog, MART-10, Attenuates Triple Negative Breast Cancer Cells Metastatic Potential. Int. J. Mol. Sci. 2016, 17, 606. [Google Scholar] [CrossRef]
- Chiang, K.C.; Yeh, C.N.; Hsu, J.T.; Jan, Y.Y.; Chen, L.W.; Kuo, S.F.; Takano, M.; Kittaka, A.; Chen, T.C.; Chen, W.T.; et al. The vitamin D analog, MART-10, represses metastasis potential via downregulation of epithelial-mesenchymal transition in pancreatic cancer cells. Cancer Lett. 2014, 354, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Chiang, K.C.; Kuo, S.F.; Chen, C.H.; Ng, S.; Lin, S.F.; Yeh, C.N.; Chen, L.W.; Takano, M.; Chen, T.C.; Juang, H.H.; et al. MART-10, the vitamin D analog, is a potent drug to inhibit anaplastic thyroid cancer cell metastatic potential. Cancer Lett. 2015, 369, 76–85. [Google Scholar] [CrossRef]
- Montagnani Marelli, M.; Marzagalli, M.; Moretti, R.M.; Beretta, G.; Casati, L.; Comitato, R.; Gravina, G.L.; Festuccia, C.; Limonta, P. Vitamin E δ-tocotrienol triggers endoplasmic reticulum stress-mediated apoptosis in human melanoma cells. Sci. Rep. 2016, 6, 30502. [Google Scholar] [CrossRef]
- Fontana, F.; Moretti, R.M.; Raimondi, M.; Marzagalli, M.; Beretta, G.; Procacci, P.; Sartori, P.; Montagnani Marelli, M.; Limonta, P. δ-Tocotrienol induces apoptosis, involving endoplasmic reticulum stress and autophagy, and paraptosis in prostate cancer cells. Cell Prolif. 2019, 52, e12576. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Prasadam, I.; Yu, M.; Zhang, F.; Ling, P.; Xiao, Y.; Yu, C. Gamma tocotrienol targets tyrosine phosphatase SHP2 in mammospheres resulting in cell death through RAS/ERK pathway. BMC Cancer 2015, 15, 609. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.; Palau, V.E.; Mahboob, R.; Lightner, J.; Stone, W.; Krishnan, K. Upregulation of pERK and c-JUN by γ-tocotrienol and not α-tocopherol are essential to the differential effect on apoptosis in prostate cancer cells. BMC Cancer 2020, 20, 428. [Google Scholar] [CrossRef]
- Idriss, M.; Hodroj, M.H.; Fakhoury, R.; Rizk, S. Beta-Tocotrienol Exhibits More Cytotoxic Effects than Gamma-Tocotrienol on Breast Cancer Cells by Promoting Apoptosis via a P53-Independent PI3-Kinase Dependent Pathway. Biomolecules 2020, 10, 577. [Google Scholar] [CrossRef]
- Dronamraju, V.; Ibrahim, B.A.; Briski, K.P.; Sylvester, P.W. γ-Tocotrienol Suppression of the Warburg Effect Is Mediated by AMPK Activation in Human Breast Cancer Cells. Nutr. Cancer 2019, 71, 1214–1228. [Google Scholar] [CrossRef]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K.; Aggarwal, B.B. γ-Tocotrienol suppresses growth and sensitises human colorectal tumours to capecitabine in a nude mouse xenograft model by down-regulating multiple molecules. Br. J. Cancer 2016, 115, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Husain, K.; Centeno, B.A.; Coppola, D.; Trevino, J.; Sebti, S.M.; Malafa, M.P. δ-Tocotrienol, a natural form of vitamin E, inhibits pancreatic cancer stem-like cells and prevents pancreatic cancer metastasis. Oncotarget 2017, 8, 31554–31567. [Google Scholar] [CrossRef] [PubMed]
- Siveen, K.S.; Ahn, K.S.; Ong, T.H.; Shanmugam, M.K.; Li, F.; Yap, W.N.; Kumar, A.P.; Fong, C.W.; Tergaonkar, V.; Hui, K.M.; et al. Y-tocotrienol inhibits angiogenesis-dependent growth of human hepatocellular carcinoma through abrogation of AKT/mTOR pathway in an orthotopic mouse model. Oncotarget 2014, 5, 1897–1911. [Google Scholar] [CrossRef] [PubMed]
- Manu, K.A.; Shanmugam, M.K.; Ramachandran, L.; Li, F.; Fong, C.W.; Kumar, A.P.; Tan, P.; Sethi, G. First evidence that γ-tocotrienol inhibits the growth of human gastric cancer and chemosensitizes it to capecitabine in a xenograft mouse model through the modulation of NF-κB pathway. Clin. Cancer Res. 2012, 18, 2220–2229. [Google Scholar] [CrossRef]
- Patacsil, D.; Tran, A.T.; Cho, Y.S.; Suy, S.; Saenz, F.; Malyukova, I.; Ressom, H.; Collins, S.P.; Clarke, R.; Kumar, D. Gamma-tocotrienol induced apoptosis is associated with unfolded protein response in human breast cancer cells. J. Nutr. Biochem. 2012, 23, 93–100. [Google Scholar] [CrossRef]
- Ye, C.; Zhao, W.; Li, M.; Zhuang, J.; Yan, X.; Lu, Q.; Chang, C.; Huang, X.; Zhou, J.; Xie, B.; et al. δ-tocotrienol induces human bladder cancer cell growth arrest, apoptosis and chemosensitization through inhibition of STAT3 pathway. PLoS ONE 2015, 10, e0122712. [Google Scholar] [CrossRef]
- Wang, C.; Ju, H.; Shen, C.; Tong, Z. miR-429 mediates δ-tocotrienol-induced apoptosis in triple-negative breast cancer cells by targeting XIAP. Int. J. Clin. Exp. Med. 2015, 8, 15648–15656. [Google Scholar]
- Rajasinghe, L.D.; Pindiprolu, R.H.; Gupta, S.V. Delta-tocotrienol inhibits non-small-cell lung cancer cell invasion via the inhibition of NF-κB, uPA activator, and MMP-9. Onco. Targets Ther. 2018, 11, 4301–4314. [Google Scholar] [CrossRef]
- Qanungo, S.; Uys, J.D.; Manevich, Y.; Distler, A.M.; Shaner, B.; Hill, E.G.; Mieyal, J.J.; Lemasters, J.J.; Townsend, D.M.; Nieminen, A.L. N-acetyl-L-cysteine sensitizes pancreatic cancers to gemcitabine by targeting the NFκB pathway. Biomed. Pharmacother. 2014, 68, 855–864. [Google Scholar] [CrossRef]
- Deng, J.; Liu, A.D.; Hou, G.Q.; Zhang, X.; Ren, K.; Chen, X.Z.; Li, S.S.C.; Wu, Y.S.; Cao, X. N-acetylcysteine decreases malignant characteristics of glioblastoma cells by inhibiting Notch2 signaling. J. Exp. Clin. Cancer Res. 2019, 38, 2. [Google Scholar] [CrossRef] [PubMed]
- Jardim-Perassi, B.V.; Arbab, A.S.; Ferreira, L.C.; Borin, T.F.; Varma, N.R.; Iskander, A.S.; Shankar, A.; Ali, M.M.; de Campos Zuccari, D.A. Effect of melatonin on tumor growth and angiogenesis in xenograft model of breast cancer. PLoS ONE 2014, 9, e85311. [Google Scholar] [CrossRef]
- Paroni, R.; Terraneo, L.; Bonomini, F.; Finati, E.; Virgili, E.; Bianciardi, P.; Favero, G.; Fraschini, F.; Reiter, R.J.; Rezzani, R.; et al. Antitumour activity of melatonin in a mouse model of human prostate cancer: Relationship with hypoxia signalling. J. Pineal Res. 2014, 57, 43–52. [Google Scholar] [CrossRef]
- Jia, H.; Sun, W.; Li, X.; Xu, W. Melatonin promotes apoptosis of thyroid cancer cells via regulating the signaling of microRNA-21 (miR-21) and microRNA-30e (miR-30e). Bioengineered 2022, 13, 9588–9601. [Google Scholar] [CrossRef]
- Hsieh, M.J.; Lin, C.W.; Su, S.C.; Reiter, R.J.; Chen, A.W.; Chen, M.K.; Yang, S.F. Effects of miR-34b/miR-892a Upregulation and Inhibition of ABCB1/ABCB4 on Melatonin-Induced Apoptosis in VCR-Resistant Oral Cancer Cells. Mol. Ther. Nucleic Acids 2020, 19, 877–889. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.; Dauchy, R.T.; Hauch, A.; Mao, L.; Yuan, L.; Wren, M.A.; Belancio, V.P.; Mondal, D.; Frasch, T.; Blask, D.E.; et al. Doxorubicin resistance in breast cancer is driven by light at night-induced disruption of the circadian melatonin signal. J. Pineal Res. 2015, 59, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Tanriover, G.; Dilmac, S.; Aytac, G.; Farooqi, A.A.; Sindel, M. Effects of Melatonin and Doxorubicin on Primary Tumor And Metastasis in Breast Cancer Model. Anticancer Agents Med. Chem. 2022, 22, 1970–1983. [Google Scholar] [CrossRef]
- Zhao, Y.N.; Cao, Y.N.; Sun, J.; Liang, Z.; Wu, Q.; Cui, S.H.; Zhi, D.F.; Guo, S.T.; Zhen, Y.H.; Zhang, S.B. Anti-breast cancer activity of resveratrol encapsulated in liposomes. J. Mater. Chem. B 2020, 8, 27–37. [Google Scholar] [CrossRef]
- Singh, B.; Shoulson, R.; Chatterjee, A.; Ronghe, A.; Bhat, N.K.; Dim, D.C.; Bhat, H.K. Resveratrol inhibits estrogen-induced breast carcinogenesis through induction of NRF2-mediated protective pathways. Carcinogenesis 2014, 35, 1872–1880. [Google Scholar] [CrossRef]
- Samadi, M.; Daryanoosh, F.; Mojtahedi, Z.; Samsamy Pour, A.; Nobari, H.; Zarifkar, A.H.; Khoramipour, K. Resistance Training and Resveratrol Supplementation Improve Cancer Cachexia and Tumor Volume in Muscle Tissue of Male Mice Bearing Colon Cancer CT26 Cell Tumors. Cell Biochem. Biophys. 2025, 83, 619–631. [Google Scholar] [CrossRef]
- Williams, D.E. Indoles Derived From Glucobrassicin: Cancer Chemoprevention by Indole-3-Carbinol and 3,3′-Diindolylmethane. Front. Nutr. 2021, 8, 734334. [Google Scholar] [CrossRef]
- El Sadda, R.R.; Elshahawy, Z.R.; Saad, E.A. Biochemical and pathophysiological improvements in rats with thioacetamide induced-hepatocellular carcinoma using aspirin plus vitamin C. BMC Cancer 2023, 23, 175. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Zhou, J.; Fu, L.; Li, Y.; Zeng, H.; Xu, X.; Lv, C.; Jin, H. Ascorbic Acid Inhibits Liver Cancer Growth and Metastasis in vitro and in vivo, Independent of Stemness Gene Regulation. Front. Pharmacol. 2021, 12, 726015. [Google Scholar] [CrossRef] [PubMed]
- Polireddy, K.; Dong, R.; Reed, G.; Yu, J.; Chen, P.; Williamson, S.; Violet, P.C.; Pessetto, Z.; Godwin, A.K.; Fan, F.; et al. High Dose Parenteral Ascorbate Inhibited Pancreatic Cancer Growth and Metastasis: Mechanisms and a Phase I/IIa study. Sci. Rep. 2017, 7, 17188. [Google Scholar] [CrossRef] [PubMed]
- Refaat, B.; El-Shemi, A.G.; Kensara, O.A.; Mohamed, A.M.; Idris, S.; Ahmad, J.; Khojah, A. Vitamin D3 enhances the tumouricidal effects of 5-Fluorouracil through multipathway mechanisms in azoxymethane rat model of colon cancer. J. Exp. Clin. Cancer Res. 2015, 34, 71. [Google Scholar] [CrossRef]
- Maj, E.; Filip-Psurska, B.; Świtalska, M.; Kutner, A.; Wietrzyk, J. Vitamin D Analogs Potentiate the Antitumor Effect of Imatinib Mesylate in a Human A549 Lung Tumor Model. Int. J. Mol. Sci. 2015, 16, 27191–27207. [Google Scholar] [CrossRef]
- Swami, S.; Krishnan, A.V.; Williams, J.; Aggarwal, A.; Albertelli, M.A.; Horst, R.L.; Feldman, B.J.; Feldman, D. Vitamin D mitigates the adverse effects of obesity on breast cancer in mice. Endocr. Relat. Cancer 2016, 23, 251–264. [Google Scholar] [CrossRef]
- Husain, K.; Centeno, B.A.; Chen, D.T.; Hingorani, S.R.; Sebti, S.M.; Malafa, M.P. Vitamin E δ-tocotrienol prolongs survival in the LSL-KrasG12D/+;LSL-Trp53R172H/+;Pdx-1-Cre (KPC) transgenic mouse model of pancreatic cancer. Cancer Prev. Res. 2013, 6, 1074–1083. [Google Scholar] [CrossRef]
- Aykin-Burns, N.; Ahmad, I.M.; Zhu, Y.; Oberley, L.W.; Spitz, D.R. Increased levels of superoxide and H2O2 mediate the differential susceptibility of cancer cells versus normal cells to glucose deprivation. Biochem. J. 2009, 418, 29–37. [Google Scholar] [CrossRef]
- Nitti, M.; Marengo, B.; Furfaro, A.L.; Pronzato, M.A.; Marinari, U.M.; Domenicotti, C.; Traverso, N. Hormesis and Oxidative Distress: Pathophysiology of Reactive Oxygen Species and the Open Question of Antioxidant Modulation and Supplementation. Antioxidants 2022, 11, 1613. [Google Scholar] [CrossRef]
- Marengo, B.; Garbarino, O.; Speciale, A.; Monteleone, L.; Traverso, N.; Domenicotti, C. MYC Expression and Metabolic Redox Changes in Cancer Cells: A Synergy Able to Induce Chemoresistance. Oxid. Med. Cell. Longev. 2019, 2019, 7346492. [Google Scholar] [CrossRef] [PubMed]
- Marengo, B.; Raffaghello, L.; Pistoia, V.; Cottalasso, D.; Pronzato, M.A.; Marinari, U.M.; Domenicotti, C. Reactive oxygen species: Biological stimuli of neuroblastoma cell response. Cancer Lett. 2005, 228, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Domenicotti, C.; Marengo, B. Paradox Role of Oxidative Stress in Cancer: State of the Art. Antioxidants 2022, 11, 1027. [Google Scholar] [CrossRef]
- Liou, G.Y.; Storz, P. Reactive oxygen species in cancer. Free Rad. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef]
- Misra, S.; Boylan, M.; Selvam, A.; Spallholz, J.E.; Björnstedt, M. Redox-active selenium compounds—from toxicity and cell death to cancer treatment. Nutrients 2015, 7, 3536–3556. [Google Scholar] [CrossRef]
- Hevia, D.; Gonzalez-Menendez, P.; Fernandez-Fernandez, M.; Cueto, S.; Rodriguez-Gonzalez, P.; Garcia-Alonso, J.I.; Mayo, J.C.; Sainz, R.M. Melatonin Decreases Glucose Metabolism in Prostate Cancer Cells: A 13C Stable Isotope-Resolved Metabolomic Study. Int. J. Mol. Sci. 2017, 18, 1620. [Google Scholar] [CrossRef]
- Guerra-Librero, A.; Fernandez-Gil, B.I.; Florido, J.; Martinez-Ruiz, L.; Rodríguez-Santana, C.; Shen, Y.Q.; García-Verdugo, J.M.; López-Rodríguez, A.; Rusanova, I.; Quiñones-Hinojosa, A.; et al. Melatonin Targets Metabolism in Head and Neck Cancer Cells by Regulating Mitochondrial Structure and Function. Antioxidants 2021, 10, 603. [Google Scholar] [CrossRef]
- Tronci, L.; Serreli, G.; Piras, C.; Frau, D.V.; Dettori, T.; Deiana, M.; Murgia, F.; Santoru, M.L.; Spada, M.; Leoni, V.P.; et al. Vitamin C Cytotoxicity and Its Effects in Redox Homeostasis and Energetic Metabolism in Papillary Thyroid Carcinoma Cell Lines. Antioxidants 2021, 10, 809. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Mullarky, E.; Lu, C.; Bosch, K.N.; Kavalier, A.; Rivera, K.; Roper, J.; Chio, I.I.; Giannopoulou, E.G.; Rago, C.; et al. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science 2015, 350, 1391–1396. [Google Scholar] [CrossRef]
- Abadi, S.H.M.H.; Shirazi, A.; Alizadeh, A.M.; Changizi, V.; Najafi, M.; Khalighfard, S.; Nosrati, H. The Effect of Melatonin on Superoxide Dismutase and Glutathione Peroxidase Activity, and Malondialdehyde Levels in the Targeted and the Non-targeted Lung and Heart Tissues after Irradiation in Xenograft Mice Colon Cancer. Curr. Mol. Pharmacol. 2018, 11, 326–335. [Google Scholar] [CrossRef]
- Pereira, M.; Brandão Ostermann, R.A.; de Fáveri, W.; Damiani, A.P.; Magenis, M.L.; de Oliveira Monteiro, I.; Longaretti, L.M.; Zaccaron, R.P.; Lock Silveira, P.C.; Bazo, A.P.; et al. Vitamin C and D do not increase the chemopreventive effect of aspirin on colon carcinogenesis in a mouse model. Food Chem. Toxicol. 2025, 200, 115400. [Google Scholar] [CrossRef]
- Hsiao, Y.F.; Huang, S.C.; Cheng, S.B.; Hsu, C.C.; Huang, Y.C. Glutathione and Selenium Supplementation Attenuates Liver Injury in Diethylnitrosamine-Induced Hepatocarcinogenic Mice by Enhancing Glutathione-Related Antioxidant Capacities. Int. J. Mol. Sci. 2024, 25, 11339. [Google Scholar] [CrossRef]
- Almeida, M.I.; Reis, R.M.; Calin, G.A. MicroRNA history: Discovery, recent applications, and next frontiers. Mutat. Res. 2011, 717, 1–8. [Google Scholar] [CrossRef]
- Padi, S.K.; Zhang, Q.; Rustum, Y.M.; Morrison, C.; Guo, B. MicroRNA-627 mediates the epigenetic mechanisms of vitamin D to suppress proliferation of human colorectal cancer cells and growth of xenograft tumors in mice. Gastroenterology 2013, 145, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jiang, H.; Chen, Y.; Ren, F. Resveratrol chemosensitizes adriamycin-resistant breast cancer cells by modulating miR-122-5p. J. Cell. Biochem. 2019, 120, 16283–16292. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Díaz, S.; Valle, N.; Ferrer-Mayorga, G.; Lombardía, L.; Herrera, M.; Domínguez, O.; Segura, M.F.; Bonilla, F.; Hernando, E.; Muñoz, A. MicroRNA-22 is induced by vitamin D and contributes to its antiproliferative, antimigratory and gene regulatory effects in colon cancer cells. Hum. Mol. Genet. 2012, 21, 2157–2165. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Gao, L.; Yang, Y.; Tong, D.; Guo, B.; Liu, L.; Li, Z.; Song, T.; Huang, C. miR-145 mediates the antiproliferative and gene regulatory effects of vitamin D3 by directly targeting E2F3 in gastric cancer cells. Oncotarget 2015, 6, 7675–7685. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Tian, J.; Jin, L.; Liu, H.; Hua, Z. Stilbenes: A promising small molecule modulator for epigenetic regulation in human diseases. Front. Pharmacol. 2023, 14, 1326682. [Google Scholar] [CrossRef] [PubMed]
- Farhan, M.; Ullah, M.F.; Faisal, M.; Farooqi, A.A.; Sabitaliyevich, U.Y.; Biersack, B.; Ahmad, A. Differential Methylation and Acetylation as the Epigenetic Basis of Resveratrol’s Anticancer Activity. Medicines 2019, 6, 24. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).