Counteracting Roles of Lipidic Aldehydes and Phenolic Antioxidants on Soy Protein Oxidation Defined by a Chemometric Survey of Solvent and Mechanically Extracted Soybean Meals
Abstract
1. Introduction
2. Materials and Methods
2.1. Soybean Meal Samples
2.2. Chemicals and Reagents
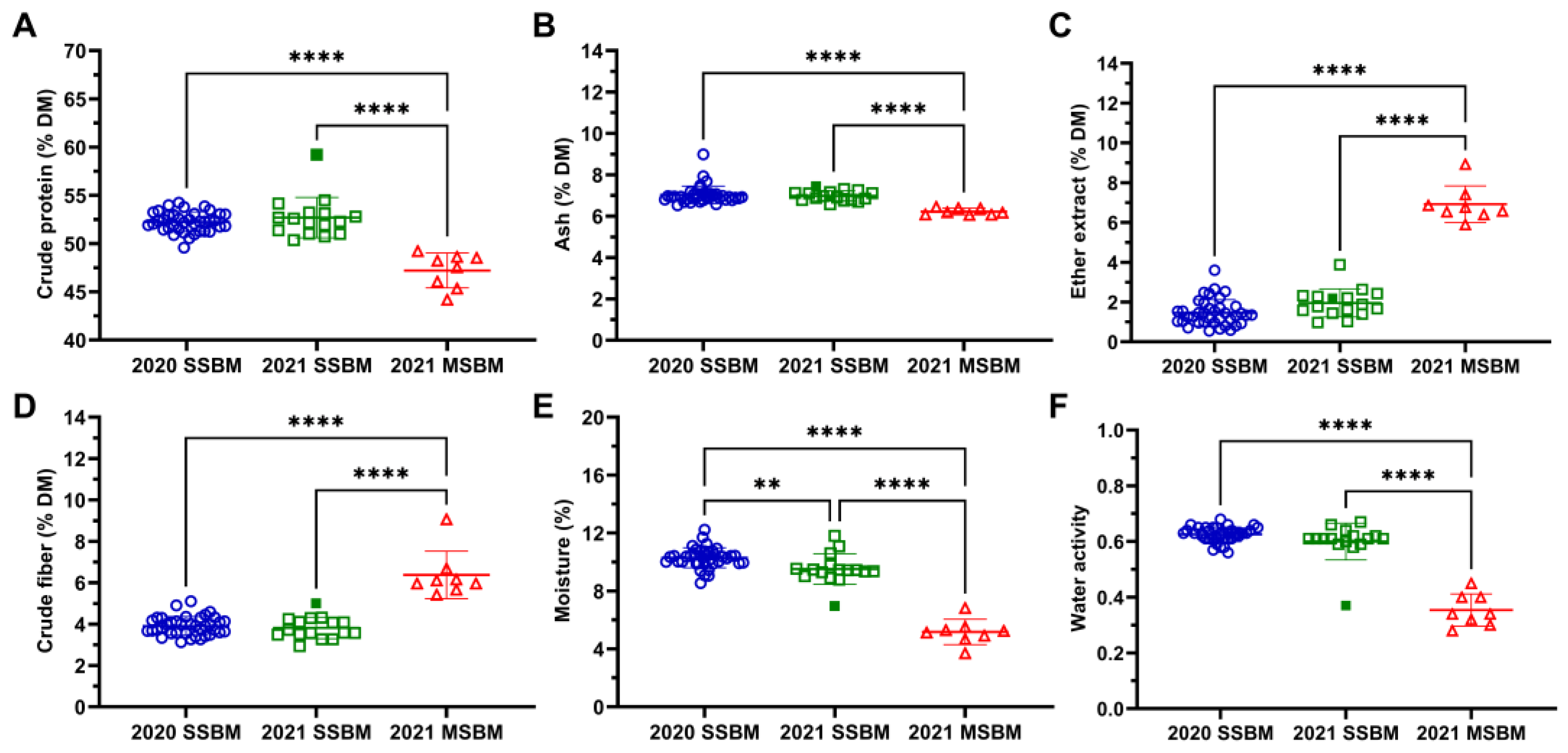
2.3. Proximate Analysis
2.4. Water Activity
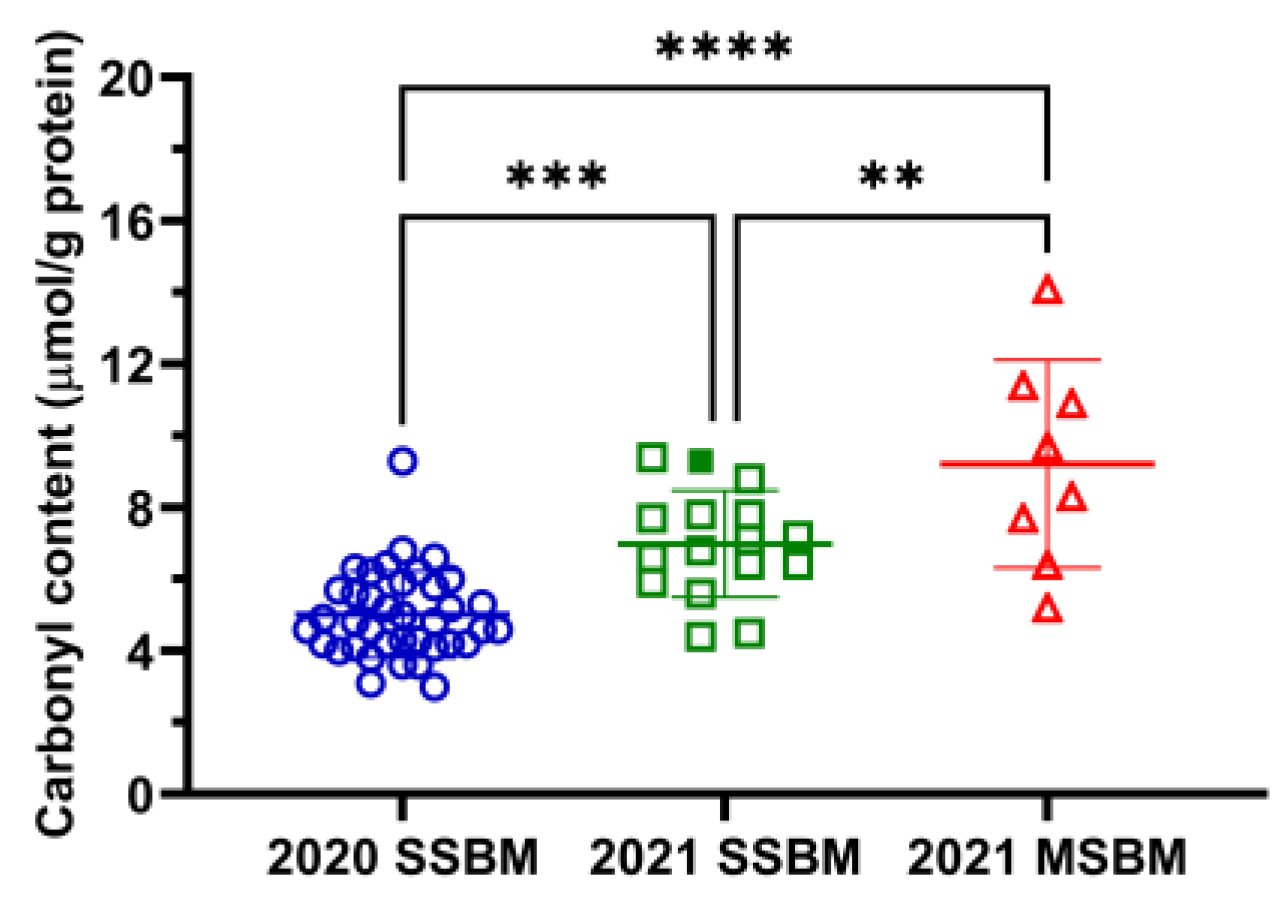
2.5. Protein Carbonyl Content
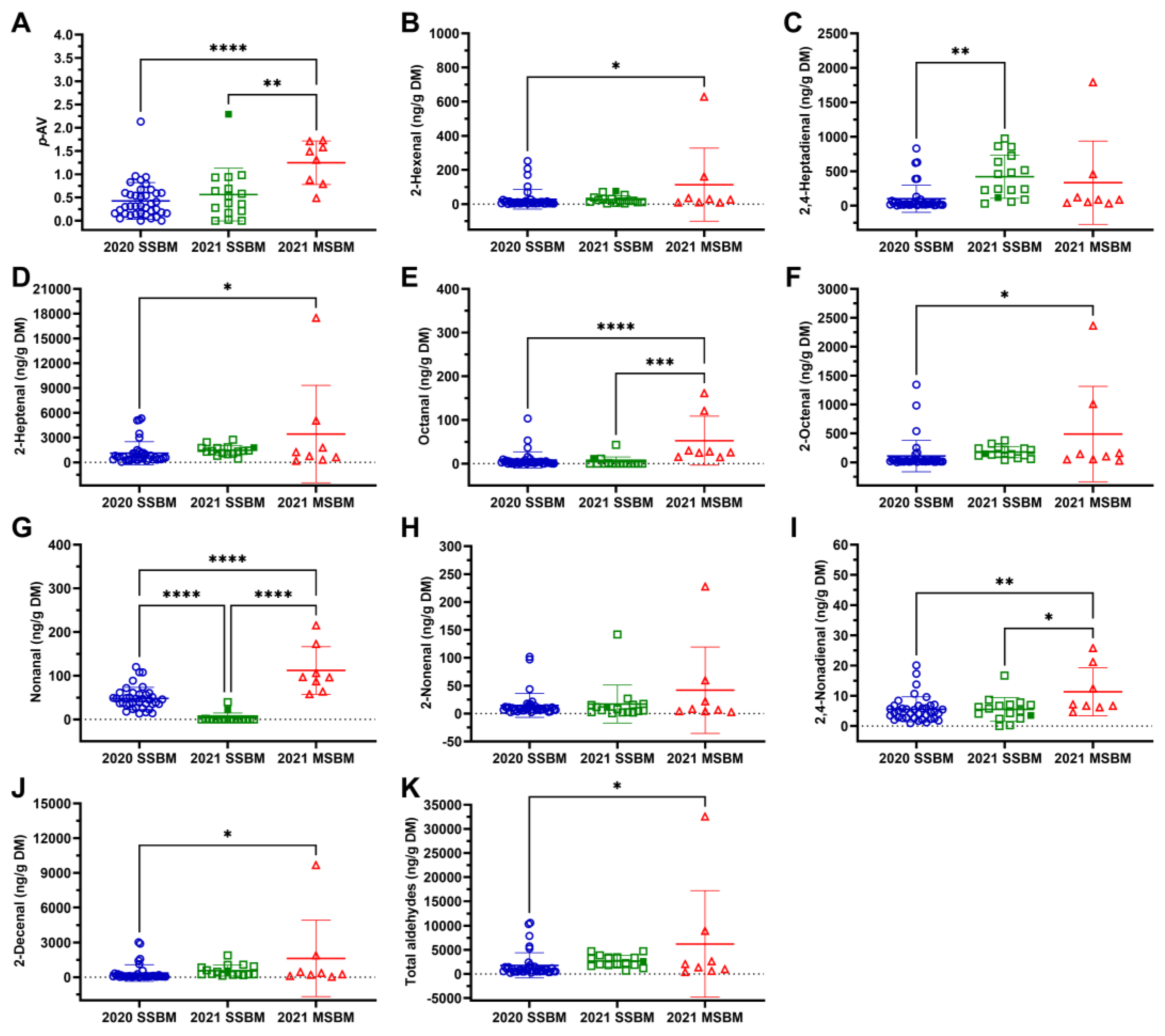
2.6. p-Anisidine Value (p-AV)
2.7. Aldehydes
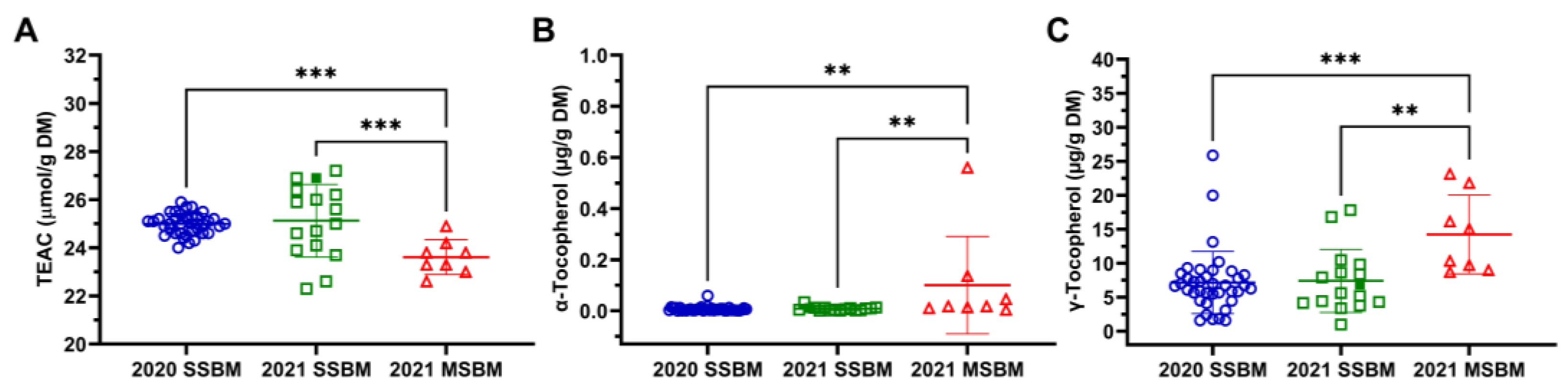
2.8. Trolox Equivalent Antioxidant Capacity (TEAC)
2.9. Tocopherols
2.10. Total Phenolic Content
2.11. Isoflavones
2.12. Statistical Analysis
3. Results
3.1. Proximate Analysis and Water Activity of SSBM and MSBM
3.2. Protein Oxidation of SSBM and MSBM
3.3. Lipid Oxidation of SSBM and MSBM
3.4. Total Antioxidant Capacity and Tocopherol Concentrations of SSBM and MSBM
3.5. Total Phenolic and Isoflavone Concentrations of SSBM and MSBM
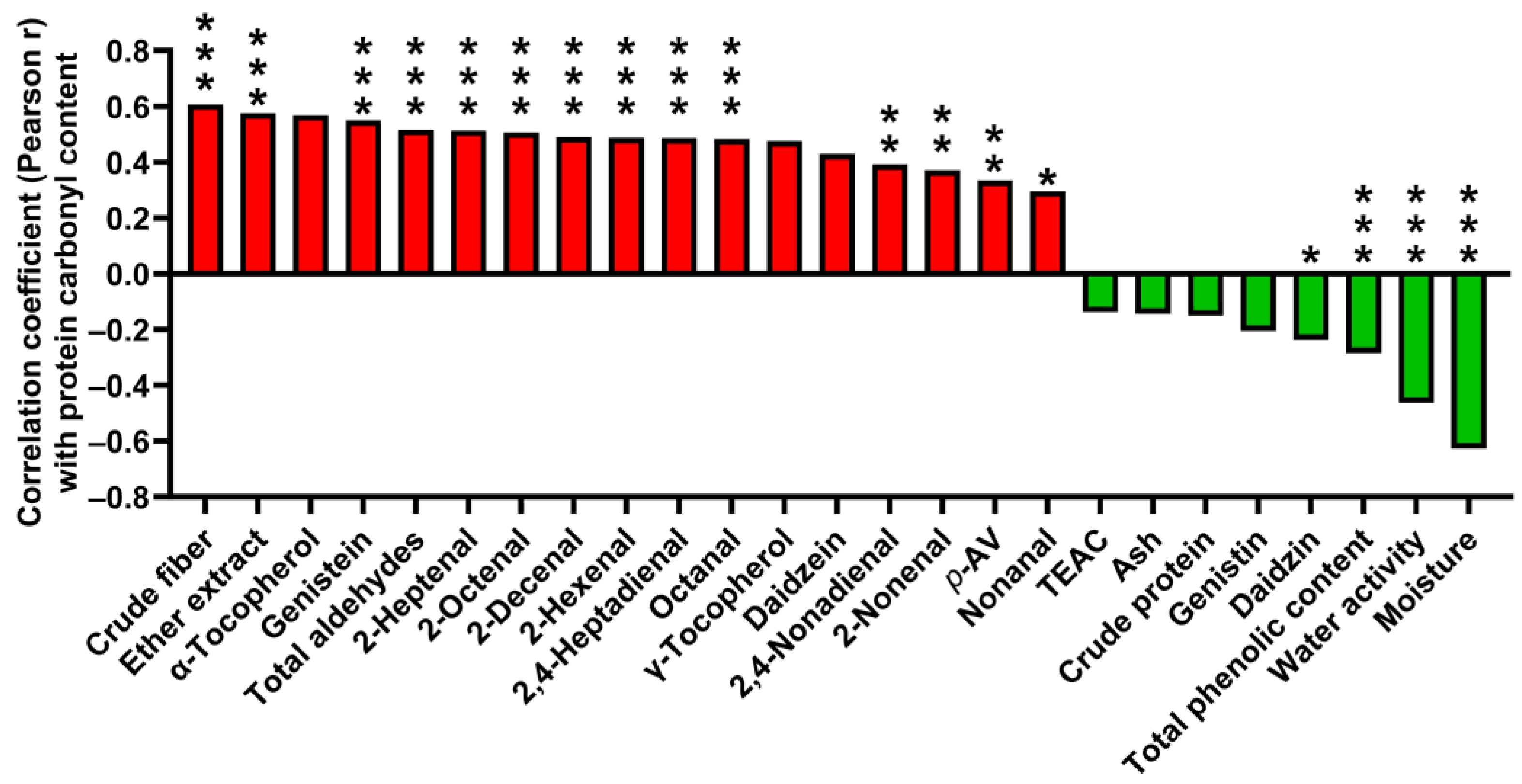
3.6. Correlations of Proximate Analysis Composition, Lipid Oxidation, and Antioxidants with Protein Oxidation in SBM
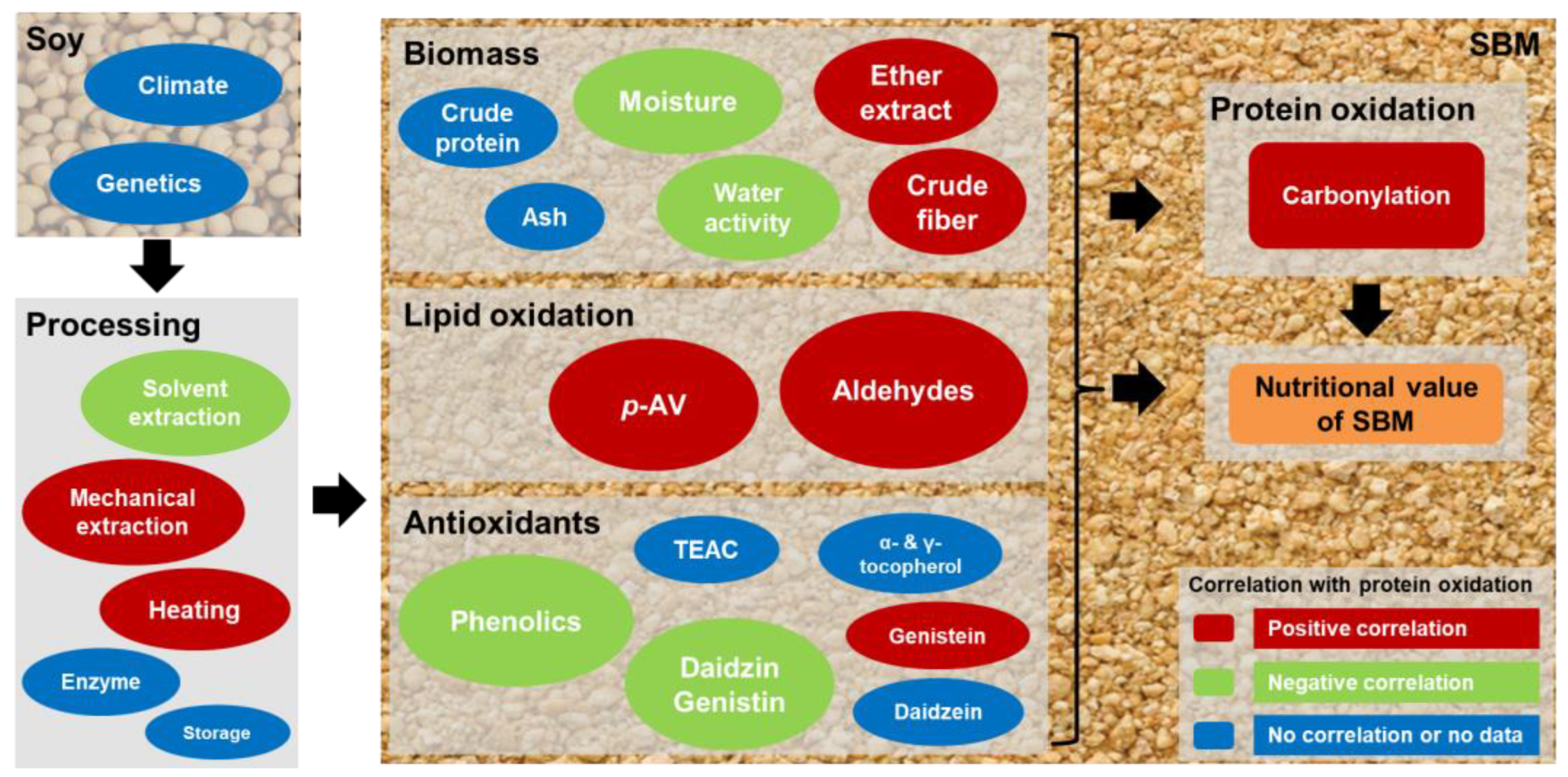
4. Discussion
4.1. Protein Oxidation Status in SSBM and MSBM
4.2. Correlations of Lipid Oxidation with Protein Oxidation in SSBM and MSBM
4.3. Correlations of Antioxidants with Protein Oxidation in SSBM and MSBM
4.4. Correlations of Proximate Analysis Components with Protein Oxidation in SBM
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oilseeds: World Markets and Trade. United States Department of Agriculture, 2023; p. 15. Available online: https://apps.fas.usda.gov/psdonline/circulars/oilseeds.pdf (accessed on 13 May 2023).
- Ruiz, N.; Parsons, C.; Stein, H.; Coon, C.; Eys, J.; Miles, R. A Review: 100 Years of Soybean Meal; ADM: Chicago, IL, USA, 2020. [Google Scholar]
- Erickson, D.R. Practical Handbook of Soybean Processing and Utilization; AOCS Press: Urbana, IL, USA, 1995. [Google Scholar]
- Johnson, L.A. 11-Oil Recovery from Soybeans. In Soybeans; Johnson, L.A., White, P.J., Galloway, R., Eds.; AOCS Press: Urbana, IL, USA, 2008; pp. 331–375. [Google Scholar]
- Li, H.; Yin, J.; He, X.; Li, Z.; Tan, B.; Jiang, Q.; Chen, J.; Ma, X. Enzyme-Treated Soybean Meal Replacing Extruded Full-Fat Soybean Affects Nitrogen Digestibility, Cecal Fermentation Characteristics and Bacterial Community of Newly Weaned Piglets. Front. Vet. Sci. 2021, 8, 639039. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.K.; Shang, Q.H.; Wang, Q.Q.; Hu, J.X.; Piao, X.S. Comparative effects of enzymolytic soybean meal and antibiotics in diets on growth performance, antioxidant capacity, immunity, and intestinal barrier function in weaned pigs. Anim. Feed Sci. Technol. 2019, 248, 47–58. [Google Scholar] [CrossRef]
- Zhou, S.F.; Sun, Z.W.; Ma, L.Z.; Yu, J.Y.; Ma, C.S.; Ru, Y.J. Effect of Feeding Enzymolytic Soybean Meal on Performance, Digestion and Immunity of Weaned Pigs. Asian-Australas. J. Anim. Sci. 2011, 24, 103–109. [Google Scholar] [CrossRef]
- González-Vega, J.C.; Kim, B.G.; Htoo, J.K.; Lemme, A.; Stein, H.H. Amino acid digestibility in heated soybean meal fed to growing pigs1. J. Anim. Sci. 2011, 89, 3617–3625. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Zhang, X.L.; Xue, W.Y.; Wu, D.W.; Ding, L.R.; Wen, C.; Zhou, Y.M. The protein oxidation of soybean meal induced by heating decreases its protein digestion in vitro and impairs growth performance and digestive function in broilers. Br. Poult. Sci. 2017, 58, 704–711. [Google Scholar] [CrossRef]
- Lu, P.; Xue, W.; Zhang, X.; Wu, D.; Ding, L.; Wen, C.; Zhou, Y. Heat-induced protein oxidation of soybean meal impairs growth performance and antioxidant status of broilers. Poult. Sci. 2019, 98, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Ye, J.; Tang, X.; Huang, Y. Determination of soybean isoflavones in soybean meal and fermented soybean meal by micellar electrokinetic capillary chromatography (MECC). Food Chem. 2011, 126, 1488–1492. [Google Scholar] [CrossRef]
- Freitas, C.S.; Alves da Silva, G.; Perrone, D.; Vericimo, M.A.; Dos S. Baião, D.; Pereira, P.R.; Paschoalin, V.M.F.; Del Aguila, E.M. Recovery of antimicrobials and bioaccessible isoflavones and phenolics from soybean (Glycine max) meal by aqueous extraction. Molecules 2018, 24, 74. [Google Scholar] [CrossRef]
- Liener, I.E. Implications of antinutritional components in soybean foods. Crit. Rev. Food Sci. Nutr. 1994, 34, 31–67. [Google Scholar] [CrossRef] [PubMed]
- Purushotham, B.; Radhakrishna, P.M.; Sherigara, B.S. Effects of Steam Conditioning and Extrusion Temperature on Some Anti-nutritional Factors of Soyabean (Glycine max) for Pet Food Applications. Am. J. Anim. Vet. Sci. 2007, 2, 1–5. [Google Scholar] [CrossRef]
- Wang, L.; Csallany, A.S.; Kerr, B.J.; Shurson, G.C.; Chen, C. Kinetics of Forming Aldehydes in Frying Oils and Their Distribution in French Fries Revealed by LC-MS-Based Chemometrics. J. Agric. Food Chem. 2016, 64, 3881–3889. [Google Scholar] [CrossRef]
- Yuan, J.; Kerr, B.J.; Curry, S.M.; Chen, C. Identification of C9-C11 unsaturated aldehydes as prediction markers of growth and feed intake for non-ruminant animals fed oxidized soybean oil. J. Anim. Sci. Biotechnol. 2020, 11, 49. [Google Scholar] [CrossRef]
- Anthonsen, T.; Sjursnes, B.J. Importance of Water Activity for Enzyme Catalysis in Non-Aqueous Organic Systems. In Methods in Non-Aqueous Enzymology; Gupta, M.N., Ed.; Birkhäuser Basel: Basel, Switzerland, 2000; pp. 14–35. [Google Scholar]
- Hemmler, D.; Roullier-Gall, C.; Marshall, J.W.; Rychlik, M.; Taylor, A.J.; Schmitt-Kopplin, P. Insights into the Chemistry of Non-Enzymatic Browning Reactions in Different Ribose-Amino Acid Model Systems. Sci. Rep. 2018, 8, 16879. [Google Scholar] [CrossRef] [PubMed]
- Petkar, A.; Alali, W.; Harrison, M.; Beuchat, L. Survival of Salmonella in organic and conventional broiler feed as affected by temperature and water activity. Agric. Food Anal. Bacteriol. 2011, 1, 175–185. [Google Scholar]
- Wu, W.; Zhang, C.; Hua, Y. Structural modification of soy protein by the lipid peroxidation product malondialdehyde. J. Sci. Food Agric. 2009, 89, 1416–1423. [Google Scholar] [CrossRef]
- Cucu, T.; Devreese, B.; Kerkaert, B.; Mestdagh, F.; Sucic, M.; Van De Perre, I.; De Meulenaer, B. A comparative study of lipid and hypochlorous acid induced oxidation of soybean proteins. LWT-Food Sci. Technol. 2013, 50, 451–458. [Google Scholar] [CrossRef]
- Wang, T.; Johnson, L.A. Survey of soybean oil and meal qualities produced by different processes. J. Am. Oil Chem. Soc. 2001, 78, 311–318. [Google Scholar] [CrossRef]
- Karr-Lilienthal, L.K.; Grieshop, C.M.; Merchen, N.R.; Mahan, D.C.; Fahey, G.C. Chemical Composition and Protein Quality Comparisons of Soybeans and Soybean Meals from Five Leading Soybean-Producing Countries. J. Agric. Food Chem. 2004, 52, 6193–6199. [Google Scholar] [CrossRef] [PubMed]
- Hemmingsen, A.K.T.; Stevik, A.M.; Claussen, I.C.; Lundblad, K.K.; Prestl⊘kken, E.; S⊘rensen, M.; Eikevik, T.M. Water Adsorption in Feed Ingredients for Animal Pellets at Different Temperatures, Particle Size, and Ingredient Combinations. Dry. Technol. 2008, 26, 738–748. [Google Scholar] [CrossRef]
- Estrada, P.D.; Berton-Carabin, C.C.; Schlangen, M.; Haagsma, A.; Pierucci, A.P.T.R.; van der Goot, A.J. Protein Oxidation in Plant Protein-Based Fibrous Products: Effects of Encapsulated Iron and Process Conditions. J. Agric. Food Chem. 2018, 66, 11105–11112. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Yuan, J.; Yao, D.; Chen, C. Fingerprinting triacylglycerols and aldehydes as identity and thermal stability indicators of camellia oil through chemometric comparison with olive oil. Food Sci. Nutr. 2021, 9, 2561–2575. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Tao, G.; Wang, Y.; Liu, Y.; Li, J. Identification of α-Tocopherol and Its Oxidation Products by Ultra-Performance Liquid Chromatography Coupled with Quadrupole Time-of-Flight Mass Spectrometry. J. Agric. Food Chem. 2020, 68, 669–677. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kim, B.; Hwang, S.R.; Kim, K.; Lee, J.H. Rapid characterization of metabolites in soybean using ultra high performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight mass spectrometry (UPLC-ESI-Q-TOF-MS/MS) and screening for α-glucosidase inhibitory and antioxidant properties through different solvent systems. J. Food Drug Anal. 2018, 26, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Rosero, D.S.; Odle, J.; Moeser, A.J.; Boyd, R.D.; van Heugten, E. Peroxidised dietary lipids impair intestinal function and morphology of the small intestine villi of nursery pigs in a dose-dependent manner. Br. J. Nutr. 2015, 114, 1985–1992. [Google Scholar] [CrossRef] [PubMed]
- Shurson, G.C.; Hung, Y.-T.; Jang, J.C.; Urriola, P.E. Measures Matter—Determining the True Nutri-Physiological Value of Feed Ingredients for Swine. Animals 2021, 11, 1259. [Google Scholar] [CrossRef]
- Davies, M.J. Protein oxidation and peroxidation. Biochem. J. 2016, 473, 805–825. [Google Scholar] [CrossRef] [PubMed]
- Gillery, P.; Jaisson, S. Usefulness of non-enzymatic post-translational modification derived products (PTMDPs) as biomarkers of chronic diseases. J. Proteom. 2013, 92, 228–238. [Google Scholar] [CrossRef]
- Gu, Y.; Chen, Y.; Jin, R.; Wang, C.; Wen, C.; Zhou, Y. Protective effects of curcumin on laying hens fed soybean meal with heat-induced protein oxidation. Ital. J. Anim. Sci. 2021, 20, 1069–1078. [Google Scholar] [CrossRef]
- Estévez, M.; Díaz-Velasco, S.; Martínez, R. Protein carbonylation in food and nutrition: A concise update. Amino Acids 2022, 54, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Duque-Estrada, P.; Kyriakopoulou, K.; de Groot, W.; van der Goot, A.J.; Berton-Carabin, C.C. Oxidative stability of soy proteins: From ground soybeans to structured products. Food Chem. 2020, 318, 126499. [Google Scholar] [CrossRef]
- Webster, M.J.; Goodband, R.D.; Tokach, M.D.; Nelssen, J.L.; Dritz, S.S.; Woodworth, J.C.; De La Llata, M.; Said, N.W. Evaluating processing temperature and feeding value of extruded-expelled soybean meal on nursery and finishing pig growth performance1,2. J. Anim. Sci. 2003, 81, 2032–2040. [Google Scholar] [CrossRef] [PubMed]
- Opapeju, F.O.; Golian, A.; Nyachoti, C.M.; Campbell, L.D. Amino acid digestibility in dry extruded-expelled soybean meal fed to pigs and poultry1. J. Anim. Sci. 2006, 84, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Pahm, S.K.; Stein, H.H. Ileal digestibility of amino acids in conventional, fermented, and enzyme-treated soybean meal and in soy protein isolate, fish meal, and casein fed to weanling pigs1. J. Anim. Sci. 2010, 88, 2674–2683. [Google Scholar] [CrossRef]
- Goebel, K.P.; Stein, H.H. Phosphorus digestibility and energy concentration of enzyme-treated and conventional soybean meal fed to weanling pigs1. J. Anim. Sci. 2011, 89, 764–772. [Google Scholar] [CrossRef]
- Fernandez, S.R.; Parsons, C.M. Bioavailability of digestible lysine in heat-damaged soybean meal for chick growth. Poult. Sci. 1996, 75, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.d.O.; Perrone, D. Characterization and stability of bioactive compounds from soybean meal. LWT-Food Sci. Technol. 2015, 63, 992–1000. [Google Scholar] [CrossRef]
- Zhu, Y.L.; Zhang, H.S.; Zhao, X.S.; Xue, H.H.; Xue, J.; Sun, Y.H. Composition, Distribution, and Antioxidant Activity of Phenolic Compounds in 18 Soybean Cultivars. J. AOAC Int. 2018, 101, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Mahungu, S.M.; Diaz-Mercado, S.; Li, J.; Schwenk, M.; Singletary, K.W.; Faller, J.Y. Stability of isoflavones during extrusion processing of corn/soy mixture. J. Agric. Food Chem. 1999, 47, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Stein, H.H.; Berger, L.L.; Drackley, J.K.; Fahey, G.C.; Hernot, D.C.; Parsons, C.M. 18-Nutritional Properties and Feeding Values of Soybeans and Their Coproducts. In Soybeans; Johnson, L.A., White, P.J., Galloway, R., Eds.; AOCS Press: Urbana, IL, USA, 2008; pp. 613–660. [Google Scholar]
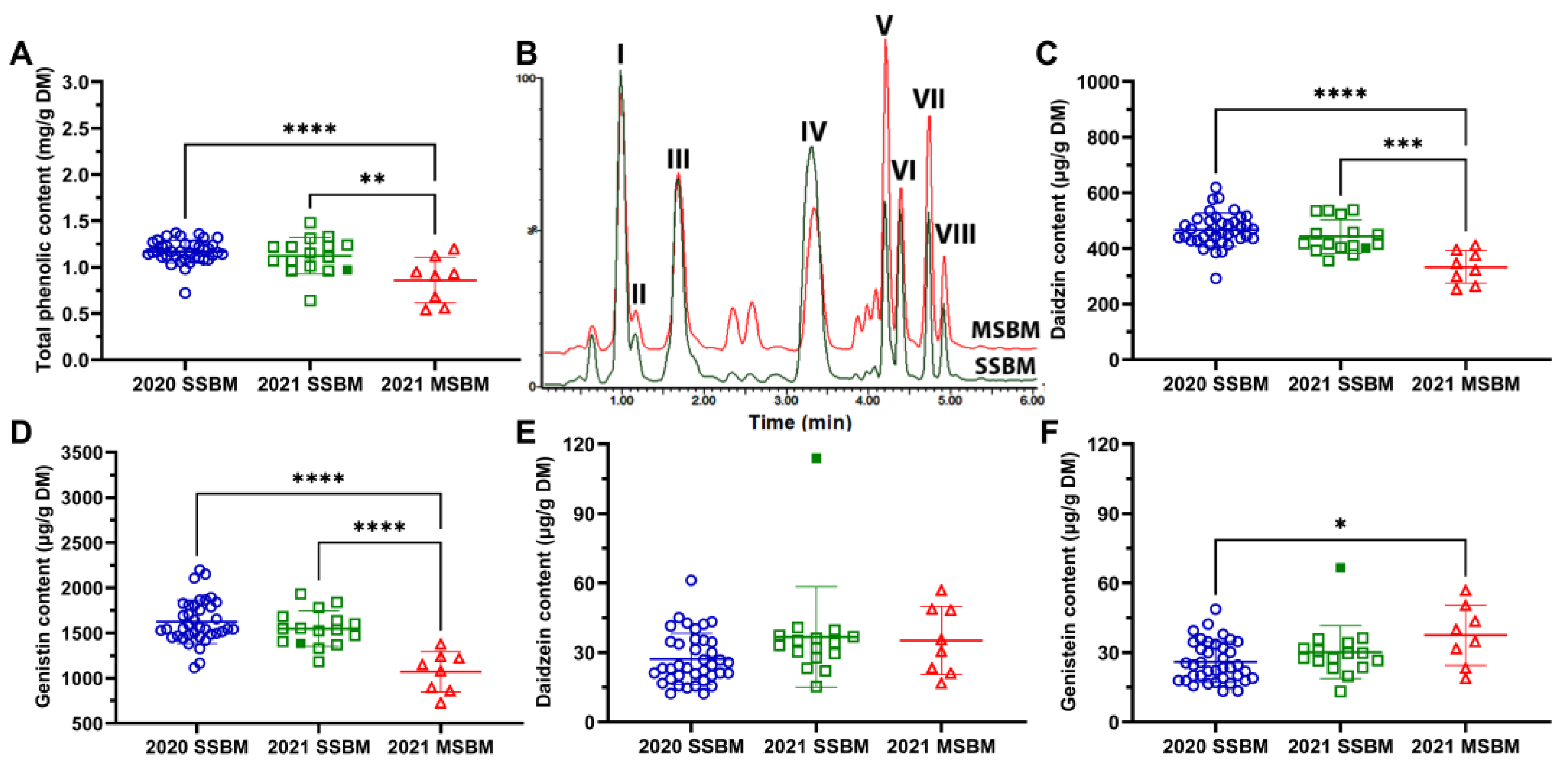
| Peak ID | RT (min) | Identity | Formula | Mode of Ion Detection | m/z of Detection | Δppm | Database ID |
|---|---|---|---|---|---|---|---|
| I | 0.98 | Daidzin * | C21H20O9 | M+H | 417.1180 | 0 | HMDB0033991 |
| II | 1.17 | Glycitin # | C22H22O10 | M+H | 447.1286 | 0 | HMDB0002219 |
| III | 1.69 | Genistin * | C21H20O10 | M+H | 433.1129 | 1 | HMDB0033988 |
| IV | 3.30 | 6″-O-Malonyldaidzin # | C24H22O12 | M+H | 503.1184 | 1 | HMDB0041263 |
| V | 4.19 | 6″-O-Acetyldaidzin # | C23H22O10 | M+H | 459.1286 | 1 | HMDB0030689 |
| VI | 4.38 | Daidzein * | C15H10O4 | M+H | 255.0652 | 4 | HMDB0003312 |
| VII | 4.73 | 6″-O-Acetylgenistin # | C23H22O11 | M+H | 475.1235 | 1 | HMDB0029528 |
| VIII | 4.91 | Genistein * | C15H10O5 | M+H | 271.0601 | 4 | HMDB0003217 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Urriola, P.E.; Naeve, S.L.; Shurson, G.C.; Chen, C. Counteracting Roles of Lipidic Aldehydes and Phenolic Antioxidants on Soy Protein Oxidation Defined by a Chemometric Survey of Solvent and Mechanically Extracted Soybean Meals. Antioxidants 2023, 12, 1419. https://doi.org/10.3390/antiox12071419
Zhang J, Urriola PE, Naeve SL, Shurson GC, Chen C. Counteracting Roles of Lipidic Aldehydes and Phenolic Antioxidants on Soy Protein Oxidation Defined by a Chemometric Survey of Solvent and Mechanically Extracted Soybean Meals. Antioxidants. 2023; 12(7):1419. https://doi.org/10.3390/antiox12071419
Chicago/Turabian StyleZhang, Junwei, Pedro E. Urriola, Seth L. Naeve, Gerald C. Shurson, and Chi Chen. 2023. "Counteracting Roles of Lipidic Aldehydes and Phenolic Antioxidants on Soy Protein Oxidation Defined by a Chemometric Survey of Solvent and Mechanically Extracted Soybean Meals" Antioxidants 12, no. 7: 1419. https://doi.org/10.3390/antiox12071419
APA StyleZhang, J., Urriola, P. E., Naeve, S. L., Shurson, G. C., & Chen, C. (2023). Counteracting Roles of Lipidic Aldehydes and Phenolic Antioxidants on Soy Protein Oxidation Defined by a Chemometric Survey of Solvent and Mechanically Extracted Soybean Meals. Antioxidants, 12(7), 1419. https://doi.org/10.3390/antiox12071419








