Lentinula edodes, a Novel Source of Polysaccharides with Antioxidant Power
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Polysaccharides from the Fruiting Bodies of L. edodes
2.3. Characterization of LECP
2.3.1. Sugar and Glucans Composition
2.3.2. Protein and Phenol Composition
2.3.3. Ultraviolet and FT-IR Spectroscopy Analysis
2.3.4. Monosaccharide Composition
2.3.5. NMR Analysis
2.3.6. Preliminary Triple Helix of the Conformation by Congo Red Analysis
2.4. Antiradical Activity
2.4.1. DPPH Radical Scavenging Assay
2.4.2. ABTS Radical Scavenging Assay
2.4.3. Hydroxyl Radical Scavenging Assay
2.4.4. Superoxide Radical Scavenging Activity
2.4.5. Reducing Power Assay
2.5. Antioxidant Activity
2.5.1. Conjugated Diene Hydroperoxide (CDH)
2.5.2. Thiobarbituric Acid Reactive Substances (TBARS)
2.6. Statistical Analysis
3. Results and Discussion
3.1. Extraction and Chemical Characterization of LECP
3.1.1. Chemical Composition and Monosaccharide Composition
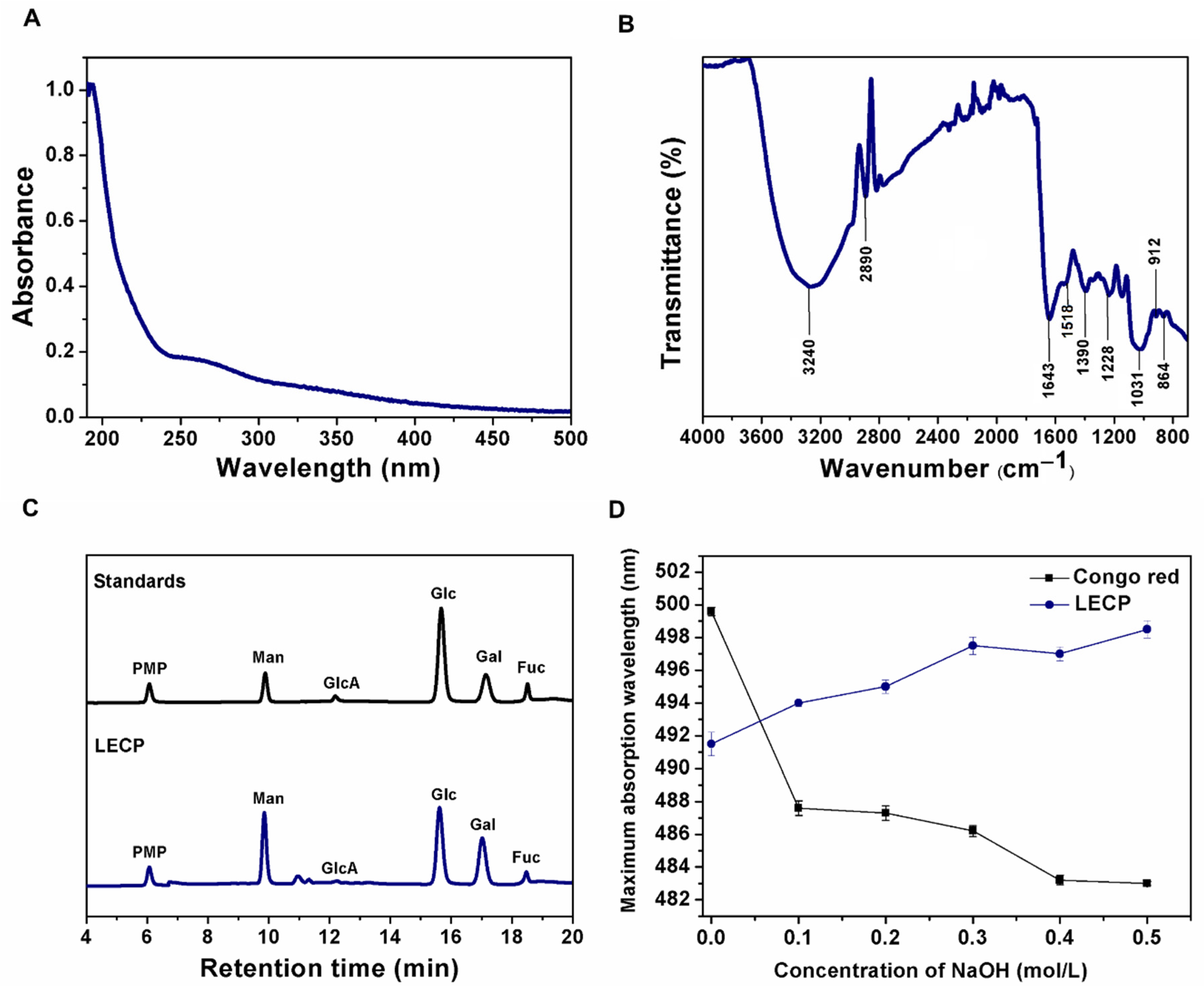
3.1.2. UV-Vis Spectra and FT-IR Spectra Analysis of LECP
3.1.3. NMR Analysis
3.1.4. Preliminary Results on Triple-Helix Conformation
3.2. Antiradical Activity
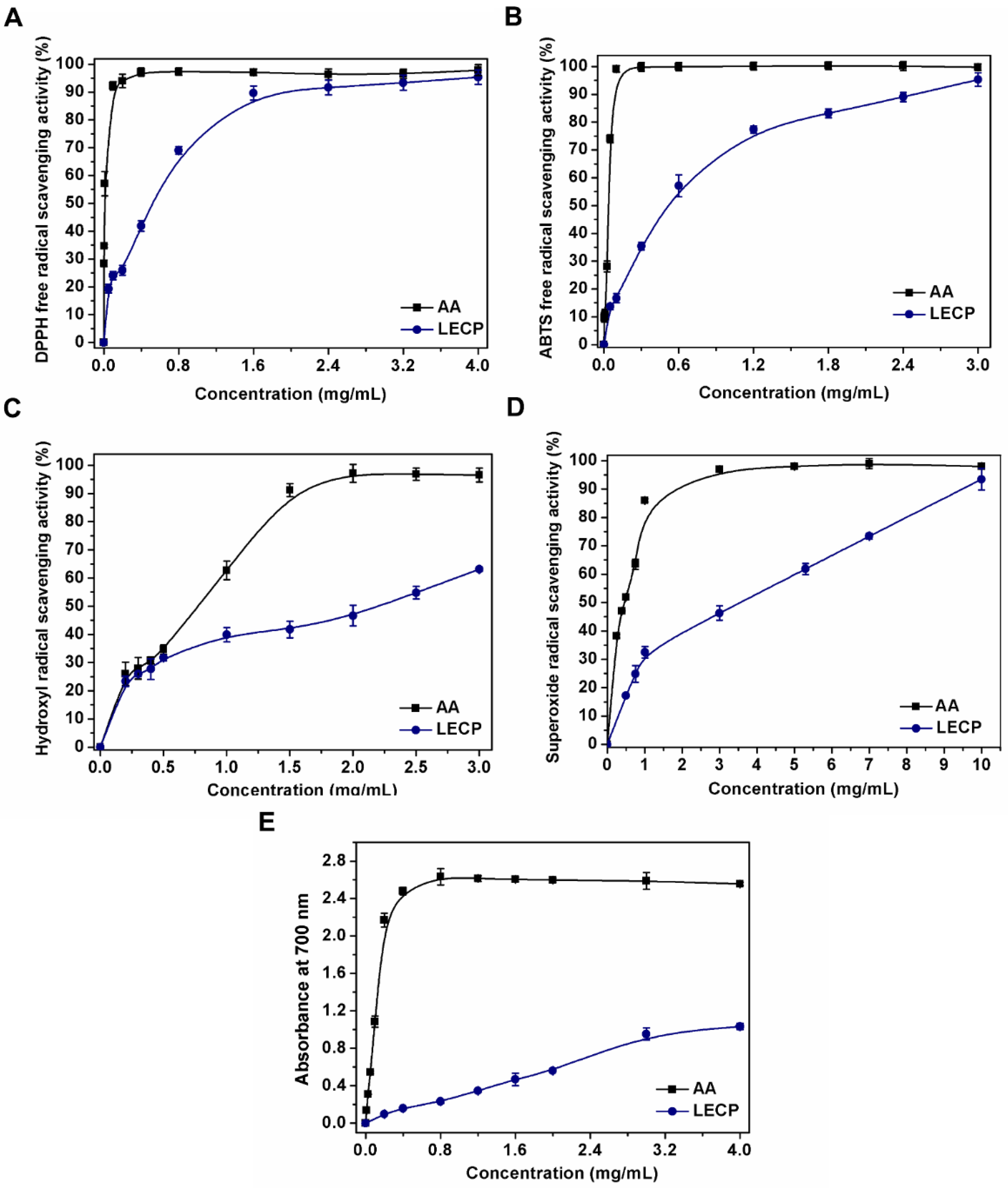
3.2.1. DPPH Radical Scavenging Ability
3.2.2. ABTS Radical Scavenging Ability
3.2.3. Hydroxyl Radical Scavenging Ability
3.2.4. Superoxide Radical Scavenging Ability
3.2.5. Reducing Power Assay
3.3. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hyder, M.S.; Dutta, S.D. Mushroom-derived polysaccharides as antitumor and anticancer agent: A concise review. Biocatal. Agric. Biotechnol. 2021, 35, 102085. [Google Scholar] [CrossRef]
- Kuang, M.T.; Xu, J.Y.; Li, J.Y.; Yang, L.; Hou, B.; Zhao, Q.; Hu, J.M. Purification, structural characterization and immunomodulatory activities of a polysaccharide from the fruiting body of Morchella sextelata. Int. J. Biol. Macromol. 2022, 213, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Niño-Fernandez, Y.M.; Henao, L.G.; Peña, C.E.R.; Enao, L.G. Aislamiento y producción de semilla de Auricularia fuscosuccinea (Mont.) Henn. y Crepidotus palmarum Sing. usados tradicionalmente en Pauna (Boyacá, Colombia). Rev. Colomb. Ciencias Hortic. 2017, 11, 151–158. [Google Scholar] [CrossRef]
- Roncero-Ramos, I.; Delgado-Andrade, C. The beneficial role of edible mushrooms in human health. Curr. Opin. Food Sci. 2017, 14, 122–128. [Google Scholar] [CrossRef]
- Maity, P.; Sen, I.K.; Chakraborty, I.; Mondal, S.; Bar, H.; Bhanja, S.K.; Mandal, S.; Maity, G.N. Biologically active polysaccharide from edible mushrooms: A review. Int. J. Biol. Macromol. 2021, 172, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Siu, K.-C.; Chen, X.; Wu, J.-Y. Constituents actually responsible for the antioxidant activities of crude polysaccharides isolated from mushrooms. J. Funct. Foods 2014, 11, 548–556. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, M.; Liu, F.; Feng, X.; Ibrahim, S.A.; Cheng, L.; Huang, W. Effects of freeze drying and hot-air drying on the physicochemical properties and bioactivities of polysaccharides from Lentinula edodes. Int. J. Biol. Macromol. 2020, 145, 476–483. [Google Scholar] [CrossRef]
- Fimbres-Olivarria, D.; Carvajal-Millan, E.; Lopez-Elias, J.A.; Martinez-Robinson, K.G.; Miranda-Baeza, A.; Martinez-Cordova, L.R.; Enriquez-Ocaña, F.; Valdez-Holguin, J.E. Chemical characterization and antioxidant activity of sulfated polysaccharides from Navicula sp. Food Hydrocoll. 2018, 75, 229–236. [Google Scholar] [CrossRef]
- Zhu, H.; Tian, L.; Zhang, L.; Bi, J.; Song, Q.; Yang, H.; Qiao, J. Preparation, characterization and antioxidant activity of polysaccharide from spent Lentinus edodes substrate. Int. J. Biol. Macromol. 2018, 112, 976–984. [Google Scholar] [CrossRef]
- Sun, Z.; Huang, K.; Li, H.; Fu, X.; Cui, Y.; Zhou, Z. A chemically sulfated polysaccharide derived from Ganoderma lucidum induces mitochondrial-mediated apoptosis in human osteosarcoma MG63 cells. Tumor Biol. 2014, 35, 9919–9926. [Google Scholar] [CrossRef]
- Zhao, X.; Ma, S.; Liu, N.; Liu, J.; Wang, W. A polysaccharide from Trametes robiniophila Murrill induces apoptosis through intrinsic mitochondrial pathway in human osteosarcoma (U-2 OS) cells. Tumor Biol. 2015, 36, 5255–5263. [Google Scholar] [CrossRef] [PubMed]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Li, J.; Kisara, K.; Danielsson, S.; Lindström, M.E.; Gellerstedt, G. An improved methodology for the quantification of uronic acid units in xylans and other polysaccharides. Carbohydr. Res. 2007, 342, 1442–1449. [Google Scholar] [CrossRef] [PubMed]
- Monsalve-Bustamante, Y.; Rincón-Valencia, S.; Mejía-Giraldo, J.; Moreno-Tirado, D.; Puertas-Mejía, M. Screening of the UV absorption capacity, proximal and chemical characterization of extracts, and polysaccharide fractions of the Gracilariopsis tenuifrons cultivated in Colombia. J. Appl. Pharm. Sci. 2019, 9, 103–109. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Liu, J.; Shang, F.; Yang, Z.; Wu, M.; Zhao, J. Structural analysis of a homogeneous polysaccharide from Achatina fulica. Int. J. Biol. Macromol. 2017, 98, 786–792. [Google Scholar] [CrossRef]
- Dong, X.; Zhu, C.P.; Huang, G.Q.; Xiao, J.X. Fractionation and structural characterization of polysaccharides derived from red grape pomace. Process Biochem. 2021, 109, 37–45. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, H.; Wen, C.; Zhang, J.; He, Y.; Ma, H.; Duan, Y. Purification, characterization, antioxidant and immunological activity of polysaccharide from Sagittaria sagittifolia L. Food Res. Int. 2020, 136, 109345. [Google Scholar] [CrossRef]
- Shang, X.L.; Liu, C.Y.; Dong, H.Y.; Peng, H.H.; Zhu, Z.Y. Extraction, purification, structural characterization, and antioxidant activity of polysaccharides from Wheat Bran. J. Mol. Struct. 2021, 1233, 130096. [Google Scholar] [CrossRef]
- Hamed, M.; Bougatef, H.; Karoud, W.; Krichen, F.; Haddar, A.; Bougatef, A.; Sila, A. Polysaccharides extracted from pistachio external hull: Characterization, antioxidant activity and potential application on meat as preservative. Ind. Crops Prod. 2020, 148, 112315. [Google Scholar] [CrossRef]
- Mejía-Giraldo, J.C.; Winkler, R.; Puertas-Mejía, M. Novel UV filters from Pentacalia pulchella extracts with photoprotective properties and antioxidant activity. Photochem. Photobiol. Sci. 2021, 20, 1585–1597. [Google Scholar] [CrossRef] [PubMed]
- Bak, W.C.; Park, J.H.; Park, Y.A.; Ka, K.H. Determination of glucan contents in the fruiting bodies and mycelia of Lentinula edodes cultivars. Mycobiology 2014, 42, 301–304. [Google Scholar] [CrossRef]
- Morales, D.; Piris, A.J.; Ruiz-Rodriguez, A.; Prodanov, M.; Soler-Rivas, C. Extraction of bioactive compounds against cardiovascular diseases from Lentinula edodes using a sequential extraction method. Biotechnol. Prog. 2018, 34, 746–755. [Google Scholar] [CrossRef]
- Ruthes, A.C.; Smiderle, F.R.; Iacomini, M. Mushroom heteropolysaccharides: A review on their sources, structure and biological effects. Carbohydr. Polym. 2016, 136, 358–375. [Google Scholar] [CrossRef] [PubMed]
- López-Legarda, X.; Arboleda-Echavarría, C.; Parra-Saldívar, R.; Rostro-Alanis, M.; Alzate, J.F.; Villa-Pulgarín, J.A.; Segura-Sánchez, F. Biotechnological production, characterization and in vitro antitumor activity of polysaccharides from a native strain of Lentinus crinitus. Int. J. Biol. Macromol. 2020, 164, 3133–3144. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Chisti, Y. Polysaccharopeptides of Coriolus versicolor: Physiological activity, uses, and production. Biotechnol. Adv. 2003, 21, 109–122. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Wang, X.; Zhang, L.; Cheung, P.C.K. Advances in lentinan: Isolation, structure, chain conformation and bioactivities. Food Hydrocoll. 2011, 25, 196–206. [Google Scholar] [CrossRef]
- Amirullah, N.A.; Zainal Abidin, N.; Abdullah, N. The potential applications of mushrooms against some facets of atherosclerosis: A review. Food Res. Int. 2018, 105, 517–536. [Google Scholar] [CrossRef]
- Siriamornpun, S.; Kaewseejan, N.; Chumroenphat, T.; Inchuen, S. Characterization of polysaccharides from Gynura procumbens with relation to their antioxidant and anti-glycation potentials. Biocatal. Agric. Biotechnol. 2021, 32, 101957. [Google Scholar] [CrossRef]
- Vojvodić Cebin, A.; Komes, D.; Ralet, M.C. Development and Validation of HPLC-DAD Method with Pre-Column PMP Derivatization for monomeric profile analysis of polysaccharides from agro-industrial wastes. Polymers 2022, 14, 544. [Google Scholar] [CrossRef]
- Trabelsi, L.; M’sakni, N.H.; Ouada, H.B.; Bacha, H.; Roudesli, S. Partial characterization of extracellular polysaccharides produced by cyanobacterium Arthrospira platensis. Biotechnol. Bioprocess Eng. 2009, 14, 27–31. [Google Scholar] [CrossRef]
- Feriani, A.; Tir, M.; Hamed, M.; Sila, A.; Nahdi, S.; Alwasel, S.; Harrath, A.H.; Tlili, N. Multidirectional insights on polysaccharides from Schinus terebinthifolius and Schinus molle fruits: Physicochemical and functional profiles, in vitro antioxidant, anti-genotoxicity, antidiabetic, and antihemolytic capacities, and in vivo anti-inflammator. Int. J. Biol. Macromol. 2020, 165, 2576–2587. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, A.; Liu, L.; Tian, G.; Xu, F. Effect of deproteinization methods on the antioxidant activity of polysaccharides extracted from Lentinus edodes stipe. J. Food Meas. Charact. 2019, 13, 1382–1389. [Google Scholar] [CrossRef]
- Kozarski, M.; Klaus, A.; Nikšić, M.; Vrvić, M.M.; Todorović, N.; Jakovljević, D.; Van Griensven, L.J.L.D. Antioxidative activities and chemical characterization of polysaccharide extracts from the widely used mushrooms Ganoderma applanatum, Ganoderma lucidum, Lentinus edodes and Trametes versicolor. J. Food Compos. Anal. 2012, 26, 144–153. [Google Scholar] [CrossRef]
- Li, Y.-M.; Zhong, R.-F.; Chen, J.; Luo, Z.-G. Structural characterization, anticancer, hypoglycemia and immune activities of polysaccharides from Russula virescens. Int. J. Biol. Macromol. 2021, 184, 380–392. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Li, F.; Huang, L.; Xue, D.; Liu, W.; Xu, C. Chemical characterization and antioxidant activity of polysaccharide extract from spent mushroom substrate of Pleurotus eryngii. J. Taiwan Inst. Chem. Eng. 2016, 69, 48–53. [Google Scholar] [CrossRef]
- Tang, W.; Liu, C.; Liu, J.; Hu, L.; Huang, Y.; Yuan, L.; Liu, F.; Pan, S.; Chen, S.; Bian, S.; et al. Purification of polysaccharide from Lentinus edodes water extract by membrane separation and its chemical composition and structure characterization. Food Hydrocoll. 2020, 105, 105851. [Google Scholar] [CrossRef]
- Agrawal, P.K. NMR Spectroscopy in the structural elucidation of oligosaccharides and glycosides. Phytochemistry 1992, 31, 3307–3330. [Google Scholar] [CrossRef]
- Jeff, I.B.; Yuan, X.; Sun, L.; Kassim, R.M.R.; Foday, A.D.; Zhou, Y. Purification and in vitro anti-proliferative effect of novel neutral polysaccharides from Lentinus edodes. Int. J. Biol. Macromol. 2013, 52, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Kar Mandal, E.; Maity, K.; Bhunia, S.K.; Behera, B.; Maiti, T.K.; Mallick, P.; Sikdar, S.R.; Islam, S.S. Structural study of an immunoenhancing polysaccharide isolated from an edible hybrid mushroom of Pleurotus florida and Lentinula edodes. Bioact. Carbohydr. Diet. Fibre 2013, 1, 72–80. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; Huo, D.; Cao, C.; Li, Y.; Liang, Y.; Li, B.; Li, L. Preliminary characterization, antioxidant and α-glucosidase inhibitory activities of polysaccharides from Mallotus furetianus. Carbohydr. Polym. 2019, 215, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Niu, L.L.; Liu, H.P.; Wu, Y.R.; Li, M.Y.; Jia, Q. Structural characterization of a novel polysaccharide from Pleurotus citrinopileatus and its antitumor activity on H22 tumor-bearing mice. Int. J. Biol. Macromol. 2021, 168, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Qi, J.; Ho, C.T.; Li, B.; Mu, J.; Zhang, Y.; Hu, H.; Mo, W.; Chen, Z.; Xie, Y. Structural characterization and immunomodulatory activity of a water-soluble polysaccharide from Ganoderma leucocontextum fruiting bodies. Carbohydr. Polym. 2020, 249, 116874. [Google Scholar] [CrossRef]
- Sánchez, C. Reactive oxygen species and antioxidant properties from mushrooms. Synth. Syst. Biotechnol. 2017, 2, 13–22. [Google Scholar] [CrossRef]
- Wang, W.; Li, X.; Chen, K.; Yang, H.; Jialengbieke, B.; Hu, X. Extraction optimization, characterization and the antioxidant activities in vitro and in vivo of polysaccharide from Pleurotus ferulae. Int. J. Biol. Macromol. 2020, 160, 380–389. [Google Scholar] [CrossRef]
- Qu, Y.; Yan, J.; Zhang, X.; Song, C.; Zhang, M.; Mayo, K.H.; Sun, L.; Cheng, H.; Zhou, Y. Structure and antioxidant activity of six mushroom-derived heterogalactans. Int. J. Biol. Macromol. 2022, 209, 1439–1449. [Google Scholar] [CrossRef] [PubMed]
- Maity, G.N.; Maity, P.; Khatua, S.; Acharya, K.; Dalai, S.; Mondal, S. Structural features and antioxidant activity of a new galactoglucan from edible mushroom Pleurotus djamor. Int. J. Biol. Macromol. 2021, 168, 743–749. [Google Scholar] [CrossRef]
- Hong, Y.; Ying, T. Isolation, molecular characterization and antioxidant activity of a water-soluble polysaccharide extracted from the fruiting body of Termitornyces albuminosus (Berk.) Heim. Int. J. Biol. Macromol. 2019, 122, 115–126. [Google Scholar] [CrossRef]
- Hammami, N.; Gara, A.B.; Bargougui, K.; Ayedi, H.; Abdalleh, F.B.; Belghith, K. Improved in vitro antioxidant and antimicrobial capacities of polysaccharides isolated from Salicornia arabica. Int. J. Biol. Macromol. 2018, 120, 2123–2130. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, Y.; Song, H.; Zhou, H.; Zhong, F.; Hu, H.; Feng, Y. Extraction optimization, characterization and antioxidant activity of polysaccharide from Gentiana scabra bge. Int. J. Biol. Macromol. 2016, 93, 369–380. [Google Scholar] [CrossRef]
- Liu, J.; Pu, Q.; Qiu, H.; Di, D. Polysaccharides isolated from Lycium barbarum L. by integrated tandem hybrid membrane technology exert antioxidant activities in mitochondria. Ind. Crops Prod. 2021, 168, 113547. [Google Scholar] [CrossRef]
- Tian, L.; Zhao, Y.; Guo, C.; Yang, X. A comparative study on the antioxidant activities of an acidic polysaccharide and various solvent extracts derived from herbal Houttuynia cordata. Carbohydr. Polym. 2011, 83, 537–544. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, M.; Xie, B. Quantification of uronic acids in Tea polysaccharide conjugates and their antioxidant properties. J. Agric. Food Chem. 2004, 52, 3333–3336. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhu, M.; Geng, X.; Wang, H.; Ng, T.B. Characterization of polysaccharides with antioxidant and hepatoprotective activities from the edible mushroom Oudemansiella radicata. Molecules 2017, 22, 234. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Wang, L.; Walid, E.; Zhang, H. In vitro antioxidant and anti-proliferation activities of polysaccharides from various extracts of different mushrooms. Int. J. Mol. Sci. 2012, 13, 5801–5817. [Google Scholar] [CrossRef] [PubMed]
- Cheung, L.M.; Cheung, P.C.K. Mushroom extracts with antioxidant activity against lipid peroxidation. Food Chem. 2005, 89, 403–409. [Google Scholar] [CrossRef]
- Quan, H.; Qiong-Yao, Y.; Jiang, S.; Chang-Yun, X.; Ze-Jie, L.; Pu-Ming, H. Structural characterization and antioxidant activities of 2 water-soluble polysaccharide fractions purified from tea (Camellia sinensis) flower. J. Food Sci. 2011, 76, 462–471. [Google Scholar] [CrossRef]

| Parameter, %, w/w | Reference Substance | Regression Equation, Correlation Coefficient |
|---|---|---|
| Neutral sugar | Glucose | y = 0.010x − 0.006, R2 = 0.999 |
| Uronic sugar | Glucuronic acid | y = 0.008x + 0.027, R2 = 0.996 |
| Sulfated sugar | Chondroitin sulfate | y = 0.006x + 0.011, R2 = 0.997 |
| Protein | Bovine serum albumin | y = 0.002x + 0.001, R2 = 0.998 |
| Total phenols | Gallic acid | y = 0.088x − 0.012, R2 = 0.996 |
| Glycosyl Residues | Chemical Shifts (ppm) | ||||||
|---|---|---|---|---|---|---|---|
| H1/C1 | H2/C2 | H3/C3 | H4/C4 | H5/C5 | H6a, H6b/C6 | ||
| A | α-d-Glc | 5.31 | 3.56 | 3.78 | 3.48 | 3.89 | 3.78 |
| 99.29 | 71.61 | 70.83 | 72.78 | 73.17 | 60.3 | ||
| B | →6)-β-d-Glcp-(1→ | 4.46 | 3.26 | 3.45 | 3.39 | 3.59 | 4.16, 3.8 |
| 102.8 | 73.17 | 75.51 | 69.66 | 74.76 | 68.88 | ||
| C | →6)-α-d-Galp-(1→ | 5.06 | 3.83 | 3.97 | 3.83 | 3.89 | 3.69 |
| 98.12 | 69.66 | 71.61 | 66.54 | 77.07 | 60.69 | ||
| D | β-d-Manp-(1→ | 4.74 | 4.05 | 3.61 | 3.64 | 3.34 | 3.78 |
| 101.63 | 70.05 | 70.05 | 66.54 | 76.29 | 67.71 | ||
| Crude Extract | EC50 (mg/mL) | ||||
|---|---|---|---|---|---|
| DPPH | ABTS | Hydroxyl Radical | Superoxide Radical | Reducing Power | |
| LECP | 0.51 ± 0.05 | 0.52 ± 0.02 | 2.19 ± 0.18 | 3.59 ± 0.06 | 1.73 ± 0.02 |
| AA | 0.010 ± 0.001 | 0.040 ± 0.001 | 0.79 ± 0.02 | 0.46 ± 0.03 | 0.042 ± 0.002 |
| CDH (mmol/Kg MeLo) | MDA (mmol/Kg MeLo) | |
|---|---|---|
| LECP | 14.36 ± 2.41 | 0.08 ± 0.02 |
| BHT | 30.68 ± 3.43 | 0.06 ± 0.01 |
| MeLo | 163.72 ± 10.16 | 10.42 ± 0.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Castiblanco, T.; Mejía-Giraldo, J.C.; Puertas-Mejía, M.Á. Lentinula edodes, a Novel Source of Polysaccharides with Antioxidant Power. Antioxidants 2022, 11, 1770. https://doi.org/10.3390/antiox11091770
Muñoz-Castiblanco T, Mejía-Giraldo JC, Puertas-Mejía MÁ. Lentinula edodes, a Novel Source of Polysaccharides with Antioxidant Power. Antioxidants. 2022; 11(9):1770. https://doi.org/10.3390/antiox11091770
Chicago/Turabian StyleMuñoz-Castiblanco, Tatiana, Juan Camilo Mejía-Giraldo, and Miguel Ángel Puertas-Mejía. 2022. "Lentinula edodes, a Novel Source of Polysaccharides with Antioxidant Power" Antioxidants 11, no. 9: 1770. https://doi.org/10.3390/antiox11091770
APA StyleMuñoz-Castiblanco, T., Mejía-Giraldo, J. C., & Puertas-Mejía, M. Á. (2022). Lentinula edodes, a Novel Source of Polysaccharides with Antioxidant Power. Antioxidants, 11(9), 1770. https://doi.org/10.3390/antiox11091770






